Abstract
Root canal preparation generates inorganic and organic tissue debris. Various irrigation techniques are used to remove the smear layer from the root canal system. The present study aimed to evaluate canal cleanliness using a novel irrigation device with ultrasonic and manual irrigation in extracted teeth. Twenty-one freshly extracted single-rooted teeth with specified inclusion and exclusion criteria were collected for the present study. The specimens were prepared to a size using a rotary file to size 30 and 6% taper preparation. The specimens were then divided into three groups: group I, Manual irrigation group (n = 7), group II, Automated irrigation (n = 7), group III, Passive ultrasonic irrigation (n = 7). Following the experimental irrigation, the teeth were subjected to the scanning electron microscopy (SEM). Statistically significant reduction (p < 0.05) in both smear layer and debris scores were seen in group III as compared to the other groups. Based on Hülsmann’s scoring method, it was found that the automated root canal irrigation device showed better canal cleanliness when compared with the manual irrigation technique but was less effective than passive ultrasonic irrigation.
1. Introduction
Endodontic therapy aims to achieve an entirely three-dimensional seal of the root canal space after its optimal shaping, cleaning, and disinfection [1]. This goal is not always easily achieved, due to both the anatomical complexity of root canal systems and the nature of the biofilm [2]. Moreover, root canal preparation, independent of the shaping technique, generates debris of a mineralized collagen matrix which clogs the surface, forming the smear layer [3], and gets forced into the dentinal tubules at varied distances, forming smear plugs, which prevent or reduce the antibacterial activity of a chemical disinfectant [1,4]. Furthermore, the presence of the smear layer and plugs contributes to the increased coronal and apical microleakage by limiting root canal sealer penetration [5,6]. Hence, the removal of the adherent smear layer is paramount to obtaining the thorough disinfection of the root canal system, and to improve the fluid-tight seal of the root canal system [7]. Therefore, the use of irrigants, which have the role of chemically and mechanically debriding the smear layer, becomes crucial to reach a good penetrability and bactericidal activity, inactivate the endotoxins, and dissolve the necrotic pulp remnants [8]. Currently, various irrigants are used for root canal irrigation [2,9]; however, clinically, it is recommended to use intermittent irrigation using 5.25% sodium hypochlorite (NaOCl) and 17% ethylene diamine tetra acetic acid (EDTA) solution [8]. Irrigants are generally delivered by various gauge needles using different syringe barrels [10] and activated with a sonic or ultrasonic system to improve irrigant transfer, and the debridement and removal of the smear layer or biofilm [11]. However, these protocols, delivering irrigants with various gauge needles using different syringe barrels and activating with sonic or ultrasonic systems, cannot allow the flow rate to be controlled during the irrigant delivery [12]; this is an essential parameter to be considered to minimize the irrigant extrusions [13]. A recent study in the literature stated the safe optimal flow rates to be 1–4 mL/min to prevent inherent pressures apically during irrigation [14,15]. However, ideally, an operator cannot maintain a constant optimal irrigation flow rate during the irrigation [13]. Hence, the currently developed automated root canal irrigation device could help in maintaining the optimal irrigant flow rates, preventing irrigant extrusions [13]. The automated root canal rrigation device is a modified version of the syringe infusion pump [13] and helps in the delivery of irrigants at a constant flow rate of 0.5 mL/min up to 12 mL/min, allowing the flow rate to be maintained in the optimal range, preventing irrigant extrusions when the needle is tightly bound to canal walls during the irrigation. To date, there is only a single study which has assessed canal cleanliness by comparing the automated device with manual [16]. However, there is no focused study assessing this novel irrigation system with activation strategies. Considering this concept, this study aimed to evaluate the canal cleanliness using a novel irrigation device, comparing it with ultrasonic and manual irrigation in extracted teeth. The null hypothesis states there is no difference in canal cleanliness between the novel irrigation device when compared with manual or ultrasonic irrigation devices.
2. Materials and Methods
Before the research commencement, approval was obtained from Saveetha Dental College and Hospitals, Chennai, Tamil Nadu, India (SRB: SDC/ENDO-1703/20/456). Prior to extraction, patient consent was obtained. PRILE guidelines (Figure 1) were followed for reporting the study [17]. Sample size was based on a previous study, which assessed canal cleanliness using an automated irrigation device [16]. A G* power, version 3.1 (Heinrich Heine University, Universitätsstraße 1, 40225 Düsseldorf, Germany) was used. A total sample size of 21 was obtained (7 per group) at an effect size of 0.98 and alpha set at 0.05 and power at 95%.
Figure 1.
PRILE 2021 flow chart.
Twenty-one freshly extracted single-rooted teeth were collected. The inclusion criteria for the specific teeth were curvatures < 5 degrees, fully matured apex, patent root canals, and teeth undergoing extraction for therapeutic or periodontal reasons. The exclusion criteria for the study were teeth with severe calcifications, teeth with restorations, root caries, and signs of fracture or root resorption.
Immediately after the extraction, the extracted tooth surface was curetted to remove the accumulated debris and soft tissue. Following this, each tooth specimen was ruled out for extra canals using digital radiography taken at multiple angulations. The specimens were then stored in physiological saline at 4 °C until experimentation [16]. All the specimens were then dipped in molten wax forming an approximate 0.2–0.3 mm layer to mimic the periodontal ligament. Alveolar bone was simulated by dipping in wax 1 mm to CEJ. Following this, the molds were dipped in clear self-cure acrylic resin and the wax was removed. Finally, elastomeric impression material was used to fill the mold space [16].
After specimen confirmation, the teeth were decoronated 2 mm coronal to CEJ using a diamond disc to obtain uniform standard length. The access cavity was then prepared by an endodontist who was not involved in the study. Endo-access bur (Dentsply Mallefer, Ballagues, Switzerland) was used to initiate the access and Endo-Z (Dentsply Mallefer, Ballagues, Switzerland) was used to refine the prepared access walls. Following this, a manual glide path was established with standard #10 hand K-file (Dentsply Mallefer, Ballagues, Switzerland). Estimated working length was kept 1 mm short from the visible file tip which was seen through the apical foramen. Once the access cavity was prepared, the teeth were randomly allocated to the second endodontist who was not involved in the study. The instructions on the entire instrumentation and irrigation protocol were provided to the operator, who was blinded until the end of the study. Experimental sample allocation was then carried out [16]:
- Group I: Manual irrigation group (MI) (n = 7).
- Group II: Automated root canal irrigation (AI) (n = 7).
- Group III: Passive ultrasonic irrigation (PUI) (n = 7).
2.1. Group I: Manual Irrigation Group (MI)
The samples in this group were sequentially shaped to a 30 size and 0.6 taper. (Protaper gold, Dentsply Mallefer, Ballagues, Switzerland). In due course of instrumentation, a 30-gauge side-vented needle fixed to a 5 mL barrel syringe was used for irrigation. An amount of 2 mL of 5.25% NaOCl (Parcan, Septodont, Saint Maur-des-Fossés, France) was used after each instrumentation. Alternative irrigation was carried out using 2 mL of 17% EDTA (MD Cleanser, Metabiomed, Cheongju-si, South Korea) after every change of instrument. The needle was oscillated at a frequency of 1 Hz, at amplitude of 3 mm continuously until the end of the irrigant delivery. The final rinse was carried out using 4 mL of 5.25% NaOCl, 5 mL of 17% EDTA, and 5 mL of distilled water. A total of 20 mL of NaOCl was used per specimen.
2.2. Group II: Automated Root Canal Irrigation (AI)
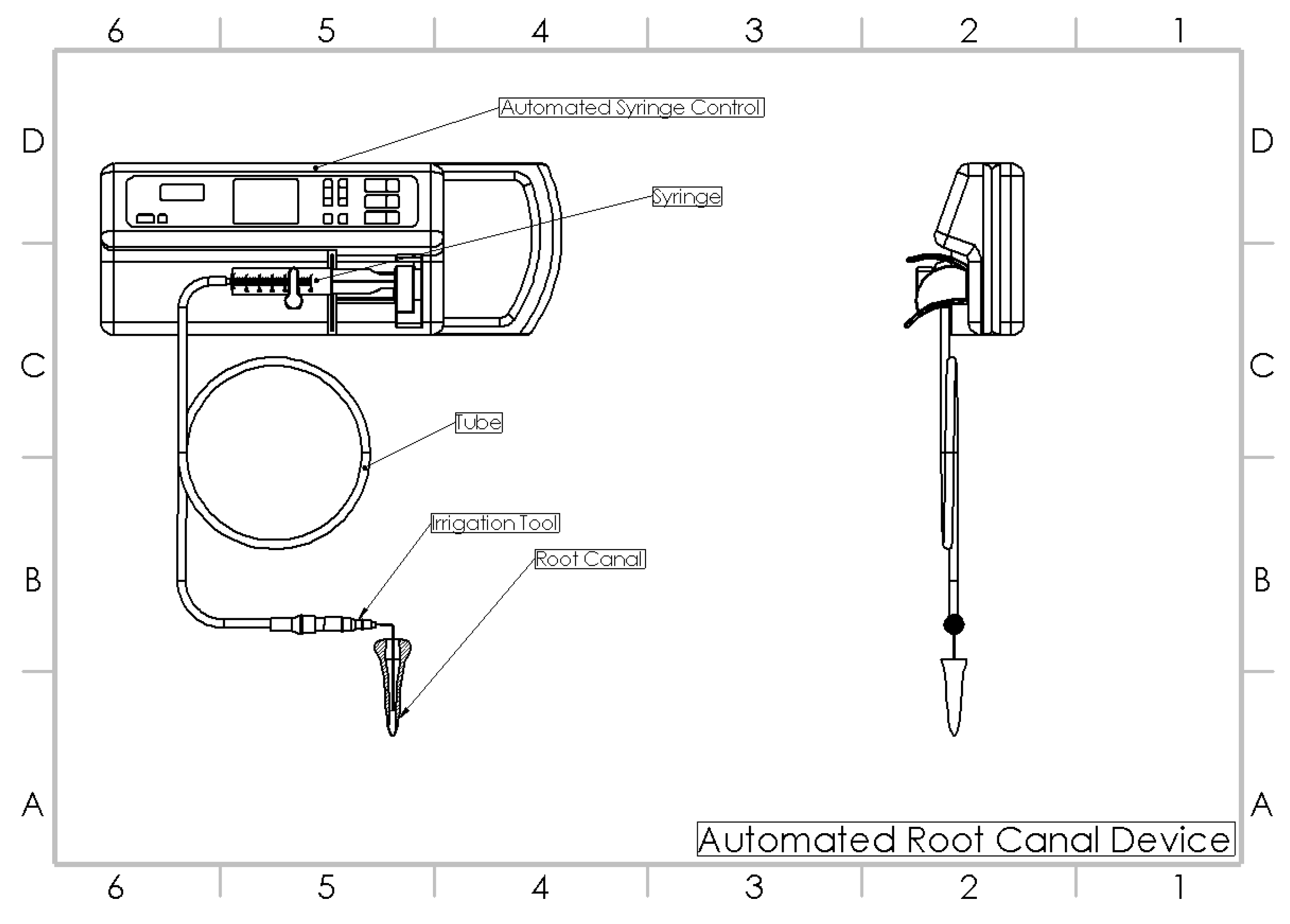
The samples in this group were sequentially shaped to a 30 size and 0.6 taper (Protaper gold, Dentsply Mallefer, Ballagues, Switzerland). However, an automated root canal irrigation device (Figure 2) was used for irrigation. An automated irrigation device is a modified syringe infusion pump, with customized connections allowing the syringe irrigation at a constant flow rate. In this group the flow rate during the entire course of irrigation was maintained at 6 mL/min by oscillating the needle continuously until the end of the experimental irrigation. Separate syringes and barrels were replaced for the respective irrigants [13]. Each barrel syringe was filled with respective irrigants and connected to the fixation slots. Following which, the flow rate was set to 6 mL/min in in the device monitor and the irrigation was initiated. In due course of instrumentation, 2 mL of 5.25% NaOCl (Parcan, Septodont, France) was used after each instrumentation. Alternative irrigation was carried out using 2 mL of 17% EDTA (MD Cleanser, Metabiomed, South Korea) after every change of instrument. The needle was oscillated at a frequency of 1 Hz, at an amplitude of 3 mm continuously until the end of the irrigant delivery. In due course of the entire irrigation, the flow rate was set constant. The final rinse was carried out using 4 mL of 5.25% NaOCl, 5 mL of 17% EDTA and 5 mL of distilled water at a flow rate of 6 mL/min. A total of 20 mL of NaOCl was used per specimen.
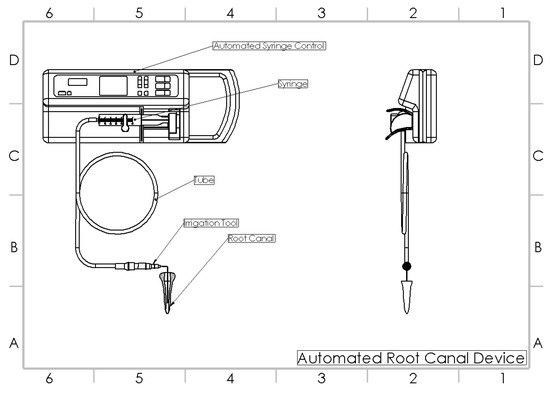
Figure 2.
Automated root canal irrigation device [13].
2.3. Group III: Passive Ultrasonic Irrigation (PUI)
The samples in this group were sequentially shaped to a 30 size and 0.6 taper (Protaper gold, Dentsply Mallefer, Ballagues, Switzerland). In due course of instrumentation, a 30-gauge side-vented needle fixed to a 5 mL barrel syringe was used for irrigation. An amount of 2 mL of 5.25% NaOCl (Parcan, Septodont, France) was used after each instrumentation. Alternative irrigation was carried out using 2 mL of 17% EDTA (MD Cleanser, Metabiomed, South Korea) after every change of instrument. The needle was oscillated at a frequency of 1 Hz, at an amplitude of 3 mm continuously until the end of the irrigant delivery. At the end of instrumentation and manual irrigation, activation was carried out using passive ultrasonic irrigant activation (PUI). An amount of 1 mL of 5.25% NaOCl was activated for 20 s, which was performed for 3 consecutive cycles using an IRRI S ultrasonic tip (VDW), attached to an ultrasonic device (Ultra Device, VDW), at a power setting at 30, placed 1 mm short of the working length. Following the activation, the final rinse was carried out using 4 mL of 5.25% NaOCl, 5 mL of 17% EDTA, and 5 mL of distilled water. A total of 20 mL of NaOCl was used per specimen [18].
Following the experimental instrumentation and irrigation, decoronation was performed until the CEJ. The roots were then split buccolingually using a microtome (Leica SP1600; Leica, Wetzlar, Germany). The roots were then sputter coated with gold and examined under scanning electron microscopy (SEM) (FE-SEM; Sigma, Carl Zeiss, Jena, Germany; secondary electron emission mode, 5 KV accelerated voltage) at 2000×. The Hülsmann scoring system includes a score from 1 to 5 with regard to the residual smear layer and debris [19]. A score of 1–5 was given independently by the examiners using the reference images. Separate evaluations were undertaken for the debris and smear layer with a five score index for each using reference photographs. Debris was defined as dentin chips, pulp remnants, and particles loosely attached to the root canal wall [19]. Below is the reported Hülsmann criteria for debris scoring:
- Score 1: Clean root canal wall, only few small debris particles.
- Score 2: Few small agglomerations of debris.
- Score 3: Many agglomerations of debris covering less than 50% of the root canal wall.
- Score 4: More than 50% of the root canal wall covered by debris.
- Score 5: Complete or nearly complete root canal wall covered by debris.
Smear layer was defined as proposed by the American Association of Endodontists’ glossary “Contemporary Terminology for Endodontics” [19]: a surface film of debris retained on dentin or other surfaces after instrumentation with either rotary instruments or endodontic files; consists of dentin particles, remnants of vital or necrotic pulp tissue, bacterial components, and retained irrigant. Below is the reported Hülsmann criteria for smear layer scoring:
- Score 1: No smear layer, dentinal tubules open.
- Score 2: Small amount of smear layer, some dentinal tubules open.
- Score 3: Homogeneous smear layer covering the root canal wall, only few dentinal tubules open.
- Score 4: Complete root canal wall covered by a homogeneous smear layer, no open dentinal tubules.
- Score 5: Heavy, inhomogeneous smear layer covering the complete root canal wall.
Two independent evaluators (KR, KVT) with calibration on debris and smear layer scores performed the scoring of all the samples. The debris and smear layers were scored in 2000× magnification.
IBM SPSS Statistics for Windows, Version 23.0 (IBM Corp, Armonk, NY, USA) was used to assess the data statistically. Independent groups were assessed using the Kruskal–Wallis test. Dunn’s pairwise test was used for intragroup comparison.
3. Results
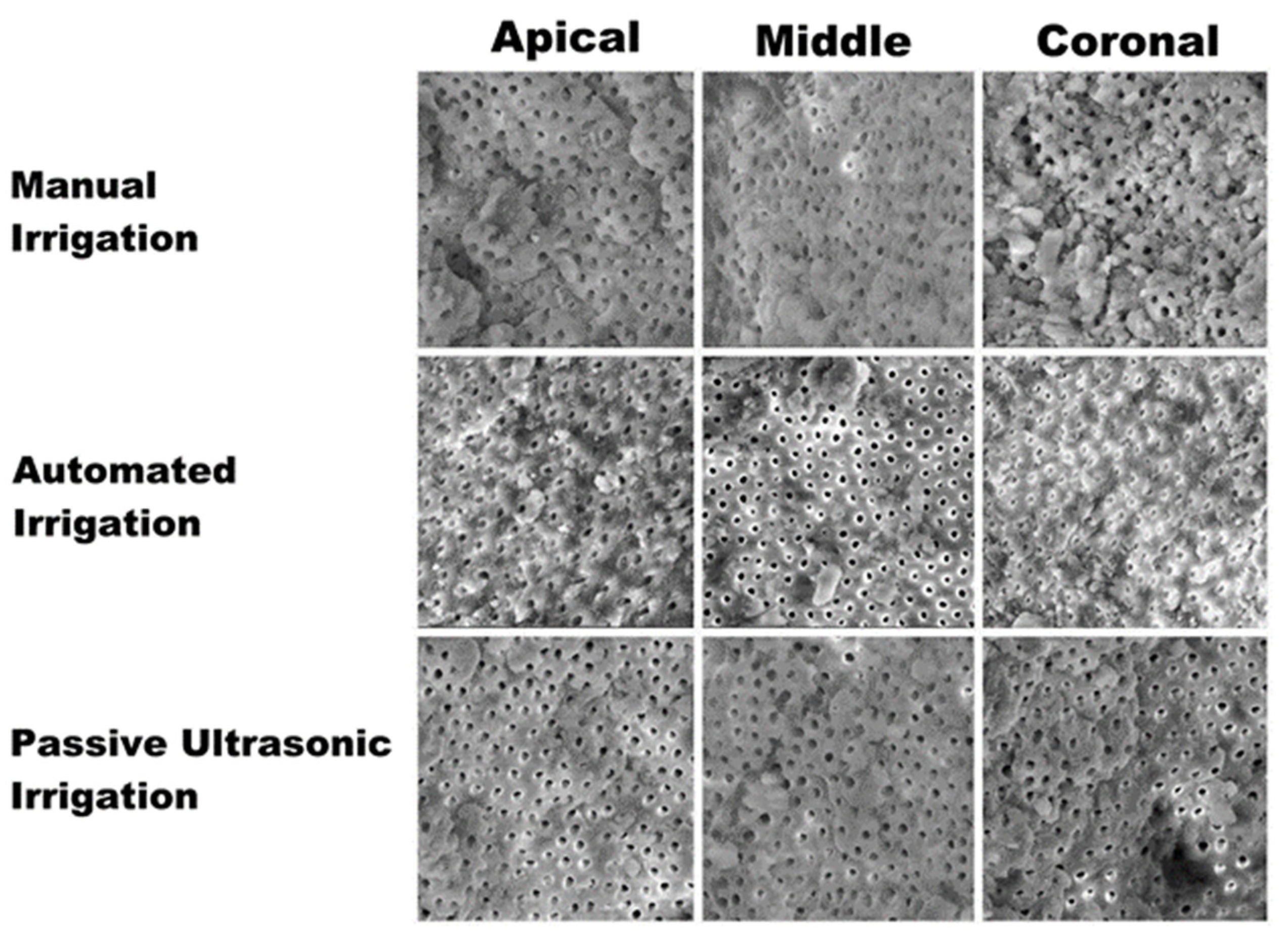
Kruskal–Wallis test revealed a statistical significant difference (p < 0.05) in both the debris and smear removal scores in the groups compared. Among the apical, middle, and coronal sections compared, there was a significant difference (p < 0.05) elicited in different assessed groups. The recorded debris scores were higher at apical levels which were around 3.48 ± 0.36, 2.64 ± 0.32, and 1.22 ± 0.24 in the manual irrigation, automated irrigation, and passive ultrasonic irrigation groups, respectively. The recorded smear scores were higher at the apical level as well. The smear scores were around 3.02 ± 0.25, 2.70 ± 0.21, 1.15 ± 0.19 in the manual irrigation, automated irrigation, and passive ultrasonic irrigation groups, respectively. The study results also showed a significant difference (p < 0.05) in the scores among the groups and also at various levels assessed. Among the various experimental groups assessed, the passive ultrasonic irrigation group showed the least debris and smear scores, whereas higher debris and smear layer was observed in manual irrigation. Among the various sections assessed, the coronal section elicited the least debris and smear scores, whereas the apical section showed a higher accumulation of smear layer and debris (Figure 3).
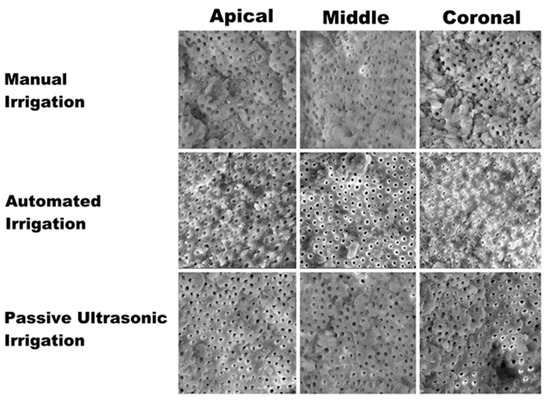
Figure 3.
Scanning electron microscopy images showing the smear layer removal at apical, middle, and coronal sections with regard to different irrigation techniques.
Dunn’s pairwise test was carried out to elicit a significant difference among the various groups compared. On pairwise comparison, there was a statistically significant difference (p < 0.05) elicited in all the groups at various levels assessed.
The current study showed a significant difference (p < 0.05) in the groups (Table 1). The mean and SD values at different sections are presented in Table 1. Pairwise comparison of smear and debris scores showed a significant difference (p < 0.05) among all the groups (Table 2 and Table 3).

Table 1.
Mean distribution of debris and smear scores among different irrigation modes.

Table 2.
Pairwise comparison of mean debris scores.

Table 3.
Pairwise comparison of mean smear scores.
4. Discussion
In recent years, multiple factors and parameters, such as mass flow rate, irrigant delivery time, shear stress, fluid flow velocity, turbulence, and shear wall stress showed to be crucial in altering the clinical irrigant flow rates [20]. Therefore, it should be of relevant importance to use a system that allows these parameters to be constantly controlled to obtain the best cleaning without the risk of being too invasive [13]. Then, the use of an automated root canal irrigation (AI) device was proposed. The AI device helps to deliver irrigants at a constant flow rate of 0.5 mL/min up to 12 mL/min. The fixation slots available in the device help in attaching various volume syringe barrels. The issues of cross-contamination are limited with the device as the separate barrels and irrigation tubes can be connected to the fixation slots during the usage of different irrigating solutions. The programmable modes are inbuilt into the device which helps in delivering the irrigant at a constant set flow rate [16]. Therefore, the potential benefit of using the AI device would be the reduced operator fatigue with enhanced irrigation, especially on using various irrigating solutions in the root canal system.
The present study is the first study in the literature designed to assess root canal cleanliness after irrigation with an automated root canal irrigation device, employing a constant flow rate of 6 mL/min as already reported in the literature [12,16], comparing its ability of smear layer and debrides removal with the most used technique for root canal irrigation, manual needle irrigation, and passive ultrasonic irrigation [12].
The current study showed a significant reduction (p < 0.05) in smear and debris scores in passive ultrasonic irrigation as compared with the automated and manual needle irrigation groups. Pairwise comparison test also showed the passive ultrasonic irrigation group to be better compared to the manual and automated irrigation groups; however, the automated irrigation showed better results compared with the manual needle groups. This result is in contrast from what has previously been reported in the literature by Rajamanickam et al. [16], that showed an insignificant difference by using manual irrigation or an automated root canal irrigation device. This difference in result could be explained by the oscillation of the needle at a frequency of 1 Hz and an amplitude of 3 mm during the course of irrigation as reported in the previous literature [21].
In the current study, authors decided to evaluate smear removal using a 30-gauge side-vented closed-ended needle, because the side-vented needles claimed to cause less irrigant extrusions and apical pressures with better shear wall stress compared to the other needle types [20].
Results demonstrate that smear formation is more significant at the apical one-third of the root canal, and it is quite challenging to clean and disinfect; this is mainly attributed to the inherent anatomical complexities [22] and canal diameter, and as a result there is a reduced irrigant flow in the apical aspect of the canal. Regardless of the use of various irrigant activation techniques, there still remains a smear layer at the apical one-third, hence the apical one-third is the most critical and difficult part to shape and clean [23], and it seems to improve the outcomes of root canal treatment [24]. Indeed, as reported in the result section of the present study, the recorded debris scores were higher at apical levels, which were around 3.48 ± 0.36, 2.64 ± 0.32, and 1.22 ± 0.24 in the manual irrigation, automated irrigation, and passive ultrasonic irrigation groups, respectively, compared with the debris score recorded in the other two-thirds of the teeth. The recorded smear scores were higher at the apical level as well, and it was around 3.02 ± 0.25, 2.70 ± 0.21, 1.15 ± 0.19 in the manual irrigation, automated irrigation, and passive ultrasonic irrigation groups, respectively. SEM analysis of different groups showed the least smear and debris scores with the passive ultrasonic irrigation group. Higher debris and smear layer scores were observed in the manual irrigation group. Among the various sections assessed, the coronal section elicited the least debris and smear scores, whereas the apical section showed a higher accumulation of smear layer and debris.
Evaluating individually the technique compared in this study, the score differences obtained in the three-thirds using the PUI technique are low and not statistically significant (p > 0.05) (Table 1) (Figure 3). This result might suggest that as the technique allows the clinician to clean the coronal third of the root canal system, it guarantees the cleaning of the other two-third, with only few small debris particles and dentinal tubules opened. In contrast, the manual irrigation technique determinates a homogeneous smear layer covering the root canal wall and only few dentinal tubules open, along all the three-thirds of the endodontic canal (p > 0.05) (Table 1) (Figure 3). The automated irrigation (AI), as in the PUI technique, is able to remove debris and smear layers at the coronal and middle third, with several dentin tubules opened (1.42 ± 0.13 and 1.87 ± 0.14, respectively) (Table 1) (Figure 3); however, differently from the PUI, it has a statistically significantly worse ability (p < 0.05) in cleaning the apical third, managing to obtain only a few open dentinal canals (2.70 ± 0.21) (Table 1) (Figure 3).
Studies claim the inefficiency in thoroughly achieving the disinfection at the apical one-third [14,25,26]. When collectively considered, the literature states the inefficiency of the SNI in achieving effective disinfection and debridement [27], smear and debris removal [28], and irrigant replacement [29]. Hence, activation is claimed to be important in achieving better and more effective cleansing of the root canal system [30].
Effective root canal irrigation always depends on the choice of root canal irrigant used along with the mode of usage [31]. Although the chemical properties of the irrigating solution are claimed to be important in enhancing the primary role of the root canal irrigant, various other factors, such as the volume of irrigant used, contact time, temperature, and mode of delivery play a wider role in enhancing the irrigant activity [32].
When the efficacy of root canal irrigation has to be considered, it should effectively remove the organic and inorganic tissue debris from the root canal walls [33], have better microbial and biofilm removal [34] and better removal of the loosely and tightly adhered smear and debris from the root canal surface, and leave canals cleaner and better for effective bonding during the obturation [35].
An enormous body of literature with substantial evidence is available on ultrasonic activation techniques [36,37]. Compared with manual irrigation, the ultrasonic activation promotes the acoustic flow within the root canal system. The forces caused on the root canal walls causes enormous shear wall stress, leading to the dislodgement of bacterial smear from the root canal system. This phenomenon is clearly lacking in the conventional needle-based irrigation. A recent systematic review has highlighted the importance of using an activation device for better root canal cleanliness [28]. However, there was no justification provided on the specific activation device for better smear and debris removal.
Alklaghi et al. [38] assessed the effect of master apical file size and taper on root canal cleanliness in single-rooted specimens. According to their study results, the specimens which have been enlarged to 0.06 taper and a 30 size minimum seemed to achieve an acceptable root canal debridement [38]. The current data also suggest better smear and debris removal when the specimens were shaped to a size greater than 25 [39]. The irrigation protocol, volume, and concentration were constant in all the groups. To date, there is only one study which assessed in vitro smear removal of an automated root canal irrigation device [16] and evidence is also scarce in the literature on the efficacy of this novel irrigation device, which still needs to be explored in future study designs.
Current evidence is always in favor of the use of the activation devices with no justification of the superiority of one device over the others [28]. Overall, analyzing in vitro studies on passive ultrasonic activation, the data showed favorable results on pulp tissue removal [37], hard tissue debris removal [40], better root canal disinfection [36], and overall improvement in root canal debridement [32] on using ultrasonic activation as compared to the other experimental modes used. Current study results also showed better debris and smear removal by using an ultrasonic activation device. Although it is mandatory to apply activation after the complete root canal instrumentation, the usage of activation devices during the preparatory phases causes extensive extrusion of debris produced in the root canal system [41]. Hence, the currently developed automated irrigation device could benefit by delivering the irrigant at a constant flow rate, preventing the irrigant extrusion and reducing operator fatigue.
The limitations of the current study were only considering the constant flow rates. Single-rooted teeth with minimal curvatures with adequate shaping were considered in the present study. So, the real-time benefit of the device could not be explored to its full potential as better smear removal could also have been attributed partly to instrumentation. Therefore, future studies can assess irrigant flow rates and their canal cleaning ability in minimal shapes with acute curvature and assess their efficiency.
5. Conclusions
Based on Hülsmann scoring criteria, although passive ultrasonic activation has shown effective debris and smear removal compared to the other experimental irrigation modes, the novel automated root canal irrigation has proven to show better efficiency than manual irrigation techniques.
Author Contributions
Conceptualization, K.V.T. and S.C.; data curation, K.R., K.V.T., S.C., M.C. and N.G.A.; formal analysis, S.R., N.G.A. and G.S.; investigation, K.R.; methodology, K.R. and S.R.; project administration, S.C. and G.S.; resources, K.V.T. and S.C.; supervision, S.C., M.C., M.M. and G.S.; validation, S.R. and M.M.; visualization, S.R., M.C., N.G.A., M.M. and G.S.; writing—original draft, K.R. and K.V.T.; writing—review and editing, M.C., N.G.A. and G.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Saveetha Dental College & Hospitals (SRB: SDC/ENDO-1703/20/456).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data will be made available on reasonable request from the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tabassum, S.; Khan, F.R. Failure of endodontic treatment: The usual suspects. Eur. J. Dent. 2016, 10, 144–147. [Google Scholar] [CrossRef]
- Neelakantan, P.; Romero, M.; Vera, J.; Daood, U.; Khan, A.U.; Yan, A.; Cheung, G.S.P. Biofilms in Endodontics—Current Status and Future Directions. Int. J. Mol. Sci. 2017, 18, 1748. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Taddei, P.; Pondrelli, A.; Zamparini, F.; Prati, C.; Spagnuolo, G. Demineralization, Collagen Modification and Remineralization Degree of Human Dentin after EDTA and Citric Acid Treatments. Materials 2018, 12, 25. [Google Scholar] [CrossRef]
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence 2017, 9, 522–554. [Google Scholar] [CrossRef]
- Violich, D.R.; Chandler, N.P. The smear layer in endodontics—A review. Int. Endod. J. 2010, 43, 2–15. [Google Scholar] [CrossRef]
- Cameron, J.A. The use of ultrasound for the removal of the smear layer. The effect of sodium hypochlorite concentration; SEM study. Aust. Dent. J. 1988, 33, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Kokkas, A.B.; Boutsioukis, A.C.; Vassiliadis, L.P.; Stavrianos, C.K. The influence of the smear layer on dentinal tubule penetration depth by three different root canal sealers: An in vitro study. J. Endod. 2004, 30, 100–102. [Google Scholar] [CrossRef]
- Cobankara, F.K.; Adanr, N.; Belli, S. Evaluation of the influence of smear layer on the apical and coronal sealing ability of two sealers. J. Endod. 2004, 30, 406–409. [Google Scholar] [CrossRef] [PubMed]
- Niu, W.; Yoshioka, T.; Kobayashi, C.; Suda, H. A scanning electron microscopic study of dentinal erosion by final irrigation with EDTA and NaOCl solutions. Int. Endod. J. 2002, 35, 934–939. [Google Scholar] [CrossRef]
- Topçuoğlu, H.S.; Topçuoğlu, G.; Arslan, H. The Effect of Apical Positive and Negative Pressure Irrigation Methods on Postoperative Pain in Mandibular Molar Teeth with Symptomatic Irreversible Pulpitis: A Randomized Clinical Trial. J. Endod. 2018, 44, 1210–1215. [Google Scholar] [CrossRef] [PubMed]
- Susila, A.; Minu, J. Activated Irrigation vs. Conventional non-activated Irrigation in Endodontics—A Systematic Review. Eur. Endod. J. 2019, 4, 96. [Google Scholar] [CrossRef] [PubMed]
- Teja, K.V.; Ramesh, S.; Choudhari, S.; Janani, K.; Jose, J.; Vasundhara, K.A. A questionnaire-based cross-sectional survey of Indian postgraduates and endodontists on awareness, attitude, and practice of using conventional syringe needle irrigation during root canal treatment. Saudi Endod. J. 2022, 12, 302. [Google Scholar]
- Teja, K.V.; Ramesh, S.; Vasundhara, K.A.; Janani, K.C.; Jose, J.; Battineni, G. A new innovative automated root canal device for syringe needle irrigation. J. Taibah Univ. Med. Sci. 2021, 17, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Shen, Y.; Khakpour, M.; Haapasalo, M. Apical pressure and extent of irrigant flow beyond the needle tip during positive-pressure irrigation in an in vitro root canal model. J. Endod. 2013, 39, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Choudhari, S.; Teja, K.V.; Ramesh, S.; Jose, J.; Janani, K.; Kumar, R. Assessment of apical pressures at different automated irrigant flow rates: An ex vivo study based on computational fluid dynamic analysis. Braz. Dent. Sci. 2022, 25, e3463. [Google Scholar] [CrossRef]
- Rajamanickam, K.; Teja, K.V.; Ramesh, S.; AbuMelha, A.S.; Alkahtany, M.F.; Almadi, K.H.; Bahammam, S.A.; Janani, K.; Choudhari, S.; Jose, J.; et al. Comparative Study Assessing the Canal Cleanliness Using Automated Device and Conventional Syringe Needle for Root Canal Irrigation—An Ex-Vivo Study. Materials 2022, 15, 6184. [Google Scholar] [CrossRef]
- Nagendrababu, V.; Murray, P.E.; Ordinola-Zapata, R.; Peters, O.A.; Rôças, I.N.; Siqueira, J.F., Jr.; Priya, E.; Jayaraman, J.; Pulikkotil, S.J.; Camilleri, J.; et al. PRILE 2021 guidelines for reporting laboratory studies in Endodontology: A consensus-based development. Int. Endod. J. 2021, 54, 1482–1490. [Google Scholar] [CrossRef]
- Plotino, G.; Colangeli, M.; Özyürek, T.; DeDeus, G.; Panzetta, C.; Castagnola, R.; Grande, N.M.; Marigo, L. Evaluation of smear layer and debris removal by stepwise intraoperative activation (SIA) of sodium hypochlorite. Clin. Oral Investig. 2021, 25, 237–245. [Google Scholar] [CrossRef]
- Rödig, T.; Hülsmann, M.; Kahlmeier, C. Comparison of root canal preparation with two rotary NiTi instruments: ProFile .04 and GT Rotary. Int. Endod. J. 2007, 40, 553–562. [Google Scholar] [CrossRef]
- Teja, K.V.; Ramesh, S.; Battineni, G.; Vasundhara, K.A.; Jose, J.; Janani, K. The effect of various in-vitro and ex-vivo parameters on irrigant flow and apical pressure using manual syringe needle irrigation: Systematic review. Saudi Dent. J. 2022, 34, 87–99. [Google Scholar] [CrossRef]
- Hu, S.; Duan, L.; Wan, Q.; Wang, J. Evaluation of needle movement effect on root canal irrigation using a computational fluid dynamics model. BioMed. Eng. OnLine 2019, 18, 52. [Google Scholar] [CrossRef] [PubMed]
- Teja, K.V.; Ramesh, S. Is a filled lateral canal—A sign of superiority? J. Dent. Sci. 2020, 15, 562–563. [Google Scholar] [CrossRef]
- Kanaan, C.G.; Pelegrine, R.A.; da Silveira Bueno, C.E.; Shimabuko, D.M.; Valamatos Pinto, N.M.; Kato, A.S. Can Irrigant Agitation Lead to the Formation of a Smear Layer? J. Endod. 2020, 46, 1120–1124. [Google Scholar] [CrossRef]
- Pintor, A.V.; Dos Santos, M.R.; Ferreira, D.M.; Barcelos, R.; Primo, L.G.; Maia, L.C. Does Smear Layer Removal Influence Root Canal Therapy Outcome? A Systematic Review. J. Clin. Pediatr. Dent. 2016, 40, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Haapasalo, M.; Shen, Y.; Qian, W.; Gao, Y. Irrigation in Endodontics. Dent. Clin. 2010, 54, 291–312. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.C.; Schilder, H. Cleaning and shaping the apical third of a root canal system. Gen. Dent. 2001, 49, 266–270. [Google Scholar] [PubMed]
- Lee, O.Y.S.; Khan, K.; Li, K.Y.; Shetty, H.; Abiad, R.S.; Cheung, G.S.P.; Neelakantan, P. Influence of apical preparation size and irrigation technique on root canal debridement: A histological analysis of round and oval root canals. Int. Endod. J. 2019, 52, 1366–1376. [Google Scholar] [CrossRef] [PubMed]
- Virdee, S.S.; Seymour, D.W.; Farnell, D.; Bhamra, G.; Bhakta, S. Efficacy of irrigant activation techniques in removing intracanal smear layer and debris from mature permanent teeth: A systematic review and meta-analysis. Int. Endod. J. 2018, 51, 605–621. [Google Scholar] [CrossRef]
- Boutsioukis, C.; Lambrianidis, T.; Kastrinakis, E.; Bekiaroglou, P. Measurement of pressure and flow rates during irrigation of a root canal ex vivo with three endodontic needles. Int. Endod. J. 2007, 40, 504–513. [Google Scholar] [CrossRef]
- Gu, L.S.; Kim, J.R.; Ling, J.; Choi, K.K.; Pashley, D.H.; Tay, F.R. Review of contemporary irrigant agitation techniques and devices. J. Endod. 2009, 35, 791–804. [Google Scholar] [CrossRef]
- Zehnder, M. Root Canal Irrigants. J. Endod. 2006, 32, 389–398. [Google Scholar] [CrossRef]
- Torabinejad, M.; Khademi, A.A.; Babagoli, J.; Cho, Y.; Johnson, W.B.; Bozhilov, K.; Kim, J.; Shabahang, S. A New Solution for the Removal of the Smear Layer. J. Endod. 2003, 29, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Kandaswamy, D.; Venkateshbabu, N. Root canal irrigants. J. Conserv. Dent. 2010, 13, 256–264. [Google Scholar] [CrossRef]
- Pereira, T.C.; Boutsioukis, C.; Dijkstra, R.J.B.; Petridis, X.; Versluis, M.; de Andrade, F.B.; van de Meer, W.J.; Sharma, P.K.; van der Sluis, L.W.M.; So, M.V.R. Biofilm removal from a simulated isthmus and lateral canal during syringe irrigation at various flow rates: A combined experimental and Computational Fluid Dynamics approach. Int. Endod. J. 2021, 54, 427–438. [Google Scholar] [CrossRef]
- Shahravan, A.; Haghdoost, A.A.; Adl, A.; Rahimi, H.; Shadifar, F. Effect of smear layer on sealing ability of canal obturation: A systematic review and meta-analysis. J. Endod. 2007, 33, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Nagendrababu, V.; Jayaraman, J.; Suresh, A.; Kalyanasundaram, S.; Neelakantan, P. Effectiveness of ultrasonically activated irrigation on root canal disinfection: A systematic review of in vitro studies. Clin. Oral Investig. 2018, 22, 655–670. [Google Scholar] [CrossRef] [PubMed]
- Căpută, P.E.; Retsas, A.; Kuijk, L.; Chávez de Paz, L.E.; Boutsioukis, C. Ultrasonic Irrigant Activation during Root Canal Treatment: A Systematic Review. J. Endod. 2019, 45, 31–44.e13. [Google Scholar] [CrossRef]
- Akhlaghi, N.M.; Dadresanfar, B.; Darmiani, S.; Moshari, A. Effect of master apical file size and taper on irrigation and cleaning of the apical third of curved canals. J. Dent. 2014, 11, 188–195. [Google Scholar]
- Plotino, G.; Özyürek, T.; Grande, N.M.; Gündoğar, M. Influence of size and taper of basic root canal preparation on root canal cleanliness: A scanning electron microscopy study. Int. Endod. J. 2019, 52, 343–351. [Google Scholar] [CrossRef]
- Barbosa, A.F.A.; Lima, C.O.; Sassone, L.M.; Fares, R.D.; Fidalgo, T.K.D.S.; Silva, E.J.N.L. Effect of passive ultrasonic irrigation on hard tissue debris removal: A systematic review and meta-analysis. Braz. Oral Res. 2021, 35, e123. [Google Scholar] [CrossRef]
- Sujith, I.L.; Teja, K.V.; Ramesh, S. Assessment of irrigant flow and apical pressure in simulated canals of single-rooted teeth with different root canal tapers and apical preparation sizes: An ex vivo study. J. Conserv. Dent. 2021, 24, 314. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).