Abstract
In the last decades, marine macroalgae have drawn attention mainly because of their bioactive constituents. Most brown algae are distributed over coastal areas of the Atlantic Ocean, Mediterranean Sea, Aegean Sea and Black Sea, and their composition varies with endogenous and exogenous factors. Phlorotannins, fatty acids, sterols and carbohydrates are some of the compounds responsible for biological activities related to cytotoxic, antiviral, antifungal, antibacterial, antidiabetic, antioxidant and anti-inflammatory potential. In this review we seek to highlight some of the compounds responsible for these last two biological activities, which have enormous importance for the management of neurodegenerative diseases, such as Alzheimer and Parkinson’s, with neuroinflammation and oxidative stress as hallmarks. However, one of the major problems associated with treating these diseases is the highly selective blood-brain-barrier, which can be overcome with nanocarriers used as delivery systems. Weighing the risks, benefits and toxicity of the used nanoparticles is nevertheless important. We also discuss zebrafish as an upcoming adequate biological model for in vivo screening of risks and benefits of such treatment strategies. This review aims to enable researchers working in the exploitation of these macroalgae and in the use of nanocarriers to potentiate the controlled delivery of bioactive compounds.
1. Introduction
In the last decades, marine macroalgae have drawn attention mainly due to their bioactive components, which have a wide range of biological activities [1]. As marine organisms are exposed to extreme environmental conditions, they produce unique secondary metabolites, which have been recognized as important compounds for the development of innovative medicines. Part of the diversity of the oceans comes from algae, which constitute one of the most important groups of organisms, both in number and in diversity of species [2]. Macroalgae, that comprise a varied group of organisms, are eukaryotic, macroscopic and photosynthetic organisms and are among the largest oxygen producers on Earth [2]. Currently, the use of macroalgae is relevant for different areas such as agriculture, aquaculture, food, cosmetics, pharmaceutical industry, and is also widely used as a source of gelling agents, phycocolloids, such as agar, which are usually used as thickeners, and stabilizers for suspensions and emulsions [3].
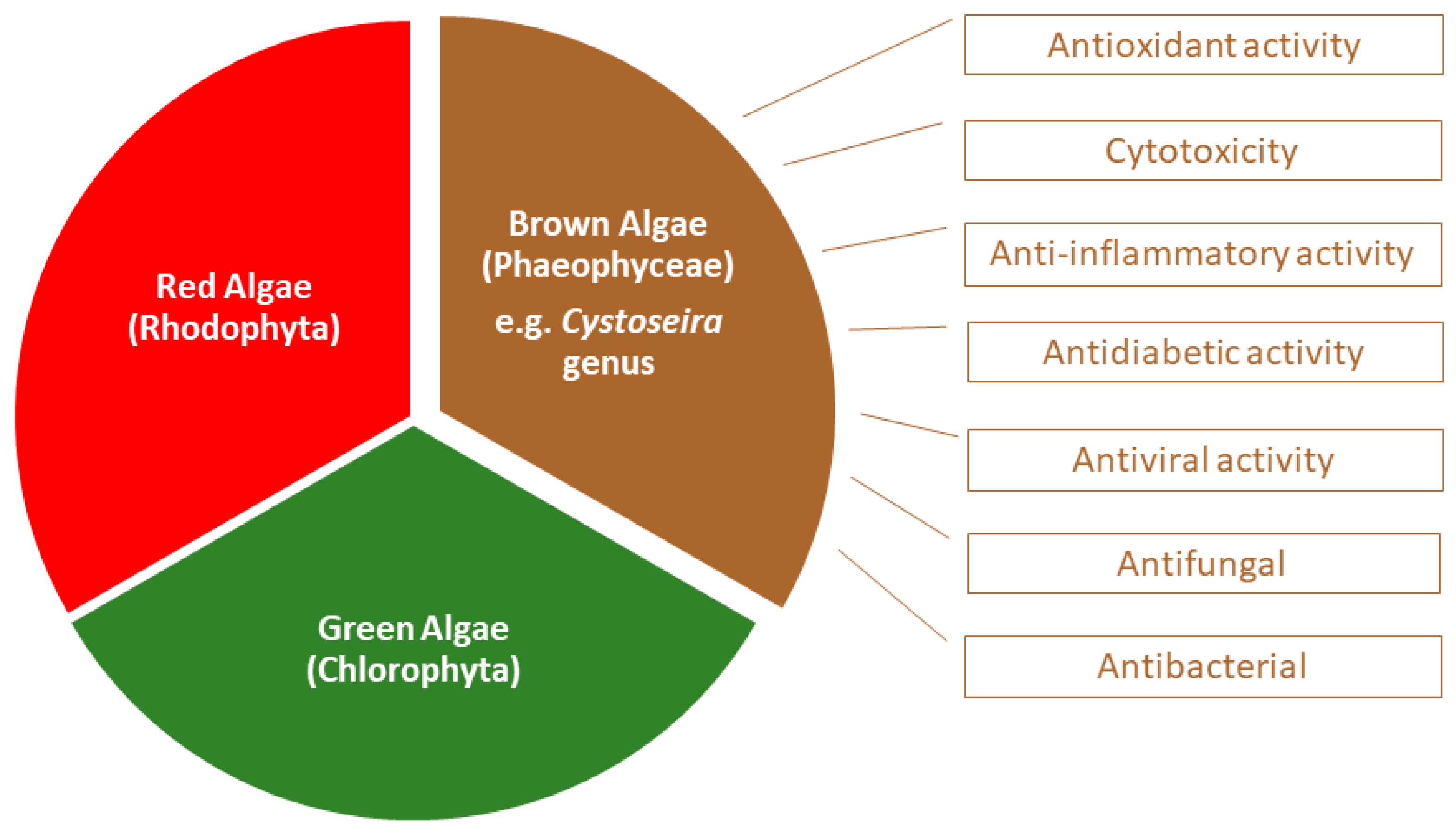
They can be classified as red (phylum Rhodophyta), green (phylum Chlorophyta) or brown macroalgae (phylum Ochrophyta), depending on their pigment composition, which will be responsible for the different colors that characterize them [4] (Figure 1).
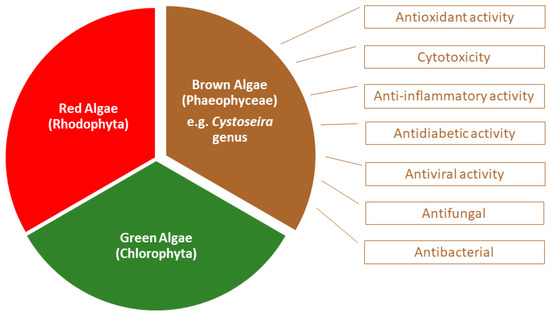
Figure 1.
Biological activities associated to Cystoseira, an important genus belonging to the group of brown algae.
Brown macroalgae are grouped in the Phaeophyceae class and present fucoxanthin as the main carotenoid which gives them a typical brown color. They include macroalgae with a wide range of sizes and shapes, from a few centimeters to tens of meters. The formation of seaweed forests are common, allowing efficient capture of sunlight and serving as habitat for many marine animals [2]. Of the approximately 2000 known species in this class, less than 1% live in fresh water [5]. From the marine macroalgae, the brown algae of the Sargassaceae family have been intensely studied. Chemically, they are essentially composed of water (80–90%) and polysaccharides, namely cellulose, alginic acid, laminarin and fucoidan, the latter being present only in brown algae. Other compounds include polyunsaturated fatty acids, vitamins, proteins, peptides, terpenoids, pigments, and sterols. Furthermore, and among marine algae, brown algae contain the highest levels of phenolic compounds [6]. One of the known genera of this family is the former Cystoseira genus (Figure 1 and Figure 2), which currently comprises 123 species in Algaebase, considering accepted taxonomically species and homotypic or heterotypic synonym (due to the rearrangement of classification systems) [7,8]. Cystoseira spp. is distributed over the Atlantic Ocean, Mediterranean Sea, Aegean Sea and Black Sea [9,10] and their composition varies according to several exogenous factors, such as geographical location, season and environmental factors, or endogenous factors, such as age and species [11,12]. Over the years it has also been discovered that the solvents used in the extraction of these metabolites significantly influence the constitution of the extracts and, consequently, their biological activity [1,9,13,14,15,16].

Figure 2.
Example of specimen Ericaria selaginoides, previously designated as Cystoseira tamariscifolia, (left) collected from the north coast of Portugal (right).
This genus represents one of the most important elements of the marine coast ecosystem [17], being essential for the rocky structure of marine forests and ensuring food to numerous species of organisms that live in the rocky reefs. In addition, it has economic value [18]. It has been widely studied from a chemical and biological point of view, with most studies focusing on species from the coastlines of the Atlantic Ocean and Mediterranean Sea, such as C. tamariscifolia, C. nodicaulis, C. usneoides, C. abies-marina, C. crinite (currently designated as Ericaria selaginoides, Gongolaria nodicaulis, Gongolaria usneoides, Gongolaria abies-marina, Ericaria crinite, respectively), C. sedoides and C. compressa [13,19,20].
Although a huge diversity of compounds has been identified in these algae, making it possible to relate them to the biological activities selected for this review (Figure 1, Table 1), lipid and phenolic compounds are the metabolites that have aroused the most interest due to the various biological properties with which they have been associated [2,21,22,23]. This review focuses on a limited set of biological activities, namely anti-inflammatory and antioxidant potential, and some of the compounds conferring such activities.

Table 1.
Compounds identified in species of the genus Cystoseira and their respective biological activity.
These two biological activities are of great importance for the prevention and treatment of several diseases, some in the field of neurology. Among the increasingly prevalent neurodegenerative diseases in the world, Alzheimer’s and Parkinson’s diseases stand out as two of the most debilitating ones [40], presenting neuroinflammation and oxidative stress as hallmarks [41], and for which there is still no treatment, which means that prevention is a key point. Alzheimer’s diseases is the most common neurodegenerative disorder, occurring mostly in people over 65 years old, mainly in women [42]. It is estimated that in the United States there are about 13.8 million people who suffer from dementia, with Alzheimer’s being the most frequent disease of dementia [42]. These types of illnesses make patients very weak, where caregivers are usually needed both at the hospital level and at home, and such care incurs high costs. This disease is characterized by neuron loss, glia cell proliferation and neurofibrillary tangles (NFT) accumulation. Alzheimer’s has as hallmarks the accumulation of the beta-amyloid protein outside the neurons and twisted strands of the tau protein inside the neurons, that consequently provokes a progressive memory loss [42]. Parkinson’s disease, on the other hand, is a chronic and progressive neurodegenerative disorder caused by various risk factors and genetic mutations. This disease is normally characterized by the loss of dopaminergic cells in the substantia nigra pars compacta with consequent decreased motor function, which leads to resting tremor, bradykinesia and muscular rigidity, also due to the presence of Lewy bodies (cytoplasmic aggregates) that play a role in the neurodegeneration [43].
Although the use of natural compounds of marine origin can be useful in the treatment and/or prevention of certain neurological diseases, it is often difficult to ensure the adequate amount of the desired compound reaches the target site. In this way, the biological activity of macroalgae can be potentiated by nanocarriers that deliver the algal extracts or derived compounds to the desired cells, tissues or organs. Moreover, the properties of some nanoparticles (NPs) can have synergetic activities with the algal extracts, namely anti-inflammatory, antioxidant, antifungal and antibacterial properties. Nanotechnology has grown to the point where its applications reach several areas such as the food industry and agriculture, to the processing and packaging of food products, cosmetics, textile industry, pharmaceutical industry and medicine [44,45,46,47]. In this way, applications of NPs in medicine, diagnosis, imaging and disease therapy will also be addressed. One of the most significant applications of NPs is in drug delivery [48]. These revolutionary carriers come without the aid of conventional drug administration, since it constitutes a major problem in the performance of the drug at target site, especially in the brain.
An important step in the use of NPs is the knowledge of their risks, benefits and toxicity. For that, zebrafish is considered a good biological model for the in vivo tests of neurotoxicity and bioactivity of NPs, since it present a neuronal system similar to that of humans [49], thus allowing to obtain more reliable results. In vivo testing in zebrafish is a very important step before translating any intervention into humans.
This review paper may be a valuable tool for researchers working in the exploitation of brown macroalgae, namely species belonging to the Cystoseira genus, with special focus on compounds responsible for anti-inflammatory and anti-oxidant activities. Furthermore, we reviewed the literature concerning the use of nanocarriers to potentiate the controlled delivery of bioactive compounds for the prevention and treatment of neurodegenerative diseases. The search methodology on PubMed search engine focused on the keywords “Cystoseira”, “bioactivity”, “neurodegenerative diseases” and finally “zebrafish”.
2. Bioactive Compounds Present in Cystoseira Extracts for Neurodegenerative Disease Management
Inflammation is a physiological process in response to invading pathogens or endogenous signals. This process is initiated by the immune cell’s migration from blood vessels and the production of mediators. At this stage, inflammatory cells are recruited and the secretion of greater amounts of growth factors, chemokines, cytokines, and secondary metabolites to eliminate invading pathogens occurs [50,51,52]. Nitric oxide (NO) is as an example of such an inflammatory mediator, being a highly permeable molecule that quickly diffuses through membranes [53,54]. If all these mediators are produced in appropriate amounts, the inflammatory response is helpful. However, deregulation of cytokine expression, especially tumor necrosis factor α (TNF-α), has a role in chronic and autoimmune inflammatory diseases [25,37]. Inflammation is also often associated with oxidative stress, which is accompanied with the production and release of reactive oxygen species (ROS), namely hydrogen peroxide, hydroxyl, superoxide and NO radicals. In this way, ROS overproduction is harmful to body homeostasis, since they can easily react with proteins, lipids, or DNA, causing oxidative damage. In addition, they can be responsible for an inflammatory state and associated to neurodegenerative disorders [55], among others pathogenesis such as coronary heart disease, atherosclerosis, cancer and aging [56].
In this way, compounds that have anti-inflammatory or antioxidant activities can be used in pharmacology, as potential sources in the food and cosmetics industry. Additionally, and bearing in mind that neuroinflammation and oxidative stress are hallmarks of neurodegenerative disorders, such as Alzheimer’s and Parkinson’s diseases [41], these compounds can also be used as potential sources in medicine, to treat these conditions.
2.1. Phlorotannins
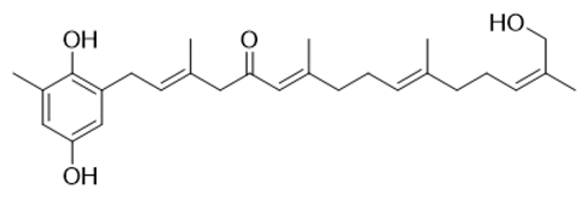
Among marine algae, species of the Phaeophyceae contain the highest levels of phenolic compounds. Of the phenolic compounds present in Cystoseira, the group of tannins reveals the strongest bioactivity [57]. They are considered one of the most widely distributed types of natural plant products and are classified into distinct groups, according to their structure. Phlorotannins are restricted to brown algae and can also be divided into different hydrophilic compound groups (fucols, phlorethols, fucophlorethols, fuhalols, isofuhalols and eckols), with very different molecular weights, ranging from 126 to 650 kDa [11,58]. However, it was found that its percentage in algae is quite variable, depending on factors such as the size of the alga, its age, the season, the light intensity and also the salinity and temperature of the water [2,27]. This may be reflected in differences of anti-inflammatory activity since the potential to reduce inflammatory mediators will be proportional to the content of phlorotannins [59,60]. Over the years, several biological properties associated with phlorotannins have been discovered, highlighting their capability to absorb UV radiation and avoiding the consequent photo-oxidative stress, but also the antioxidant, antimicrobial, antiallergic and anti-inflammatory properties [61,62,63]. The anti-inflammatory activity in vitro of purified extracts of phlorotannins obtained from three different Cystoseira species (C. usneoides, C. nodicaulis and C. tamariscifolia—currently designated as Gongolaria usneoides, Gongolaria nodicaulis and Ericaria selaginoides, respectively)—was demonstrated via an inhibitory effect on the production of NO by RAW 264.7 macrophage cells stimulated by lipopolysaccharides (LPS) [60]. LPS is one of the main components of the membrane of Gram-negative bacteria [64] capable of promoting the secretion of pro-inflammatory cytokines [65] and NO [66]. After the incubation period, the phlorotannins extracts of the three Cystoseira species were able to considerably reduce NO levels produced by cells, especially C. tamariscifolia extract which presented the greatest anti-inflammatory potential [60]. Furthermore, the antioxidant activity of purified phlorotannins extracts was also confirmed in the same Cystoseira species enunciated above [20]. Ferreres and collaborators found that these species could eliminate superoxide radicals, avoiding lipid peroxidation.
Considering these two properties, it would be interesting to use phlorotannins for the treatment of neurodegenerative diseases, and there are already some studies developed for this application. In two different studies, both Ferreres [20] and Barbosa [67] found that these compounds had anti-inflammatory and neuroprotective properties that could slow down the progression of neurodegenerative diseases. Furthermore, it was also proved that Cystoseira species contain compounds that allow it to protect neurons from oxidative stress through DPPH (2,2-diphenyl-1-picrylhydrazyl) capture activity and increasing in SH-SY5Y cell viability after exposure to H2O2 [68], thus evidencing a correlation between antioxidant activity and phenolic content.
2.2. Fatty Acids
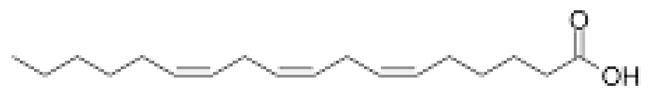
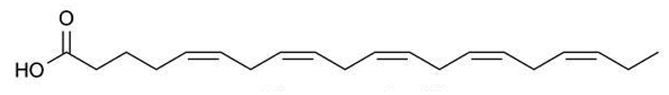
Fatty acids (FA) have been extensively studied, not only for their significant anti-inflammatory effect but, particularly, for their anti-tumor and antimicrobial potential. They are composed of an aliphatic chain and a carboxyl group and can be extracted from Cystoseira [27,30,69,70]. FA can be classified as saturated fatty acids (SFA) when they have no double bond between carbons, or as unsaturated in cases where they have at least one carbon-carbon double bond. FA ω-3 and ω-9 have an excellent anti-inflammatory effect. Regarding FA ω-3, their activity is due to precursors of anti-inflammatory molecules, namely resolvins, docosatrienes and protectins, but also to their ability to replace arachidonic acid in cell membranes, which causes a decrease in the production of pro-inflammatory compounds such as prostaglandins E2 (PGE2), thromboxane B2, among other arachidonic acid derivatives [71]. In addition, FA ω-3 inhibits the activity of nuclear factor kappa B (NFκB), which is a transcription factor with a very important role in many inflammatory signaling pathways since it interferes with the production of several cytokines (IL-1, IL-2, IL-6, IL-12, TNF-α). The production of adhesion molecules and chemokines, such as IL-8, monocyte chemoattractant protein 1, among others, is also affected by FA ω-3. Additionally, this fatty acid also inhibits effector enzymes such as iNOS and cyclooxygenase 2 (COX-2) [69,72]. On the other hand, and although less studied for this purpose, extracts with FA ω 9 demonstrated an inhibitory capacity of COX-2 enzyme and NO production, as well as pro-inflammatory cytokines (TNF-α and IL-1β) [73]. Furthermore, they stimulate the production of anti-inflammatory cytokines and inhibit the migration and accumulation of neutrophils and macrophages at the infection site [74]. Fatty acids can also be part of human diet, providing neuroprotection and reducing the risk of incident Alzheimer’s disease [75,76]. Andrade [27] proved that fatty acids extracted from different species of Cystoseira were able to scavenge DPPH and inhibit enzymes associated with the formation of β-amyloid plaques, the main cause of Alzheimer’s disease.
2.3. Sterols
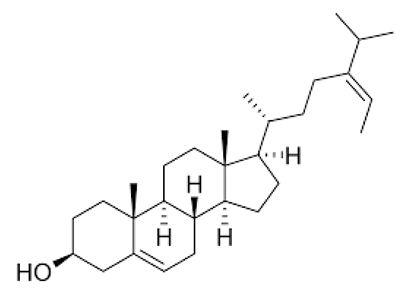
Sterols, which belong to the steroids family, are constituted by a tetracyclic structure and are abundant in species belonging to the genus Cystoseira [27]. Several health benefits have been attributed to these compounds as they were able to reduce low density lipoproteins (LDL) and, consequently, were associated with a reduction in the risk of cardiovascular diseases, representing the principal cause of death globally, according to the world health organization [77]. Phytosterols have been studied for their potential to suppress the secretion of inflammatory factors, such as TNF-α, IL-1β, IL-6, IL-8, NO and ROS. In addition, a partial inhibitory effect of the transcription factor NF-κB on macrophages and the ability to inhibit the expression of the enzymes COX-2 and iNOS have been reported [78,79]. Neuroprotective functions of sterols extracted from marine organisms have already been confirmed [27,80], although there are few studies with seaweed extracts.
2.4. Fucoidans
Fucoidans form a group of sulfated polysaccharides present in brown algae and are generally linear, composed mainly of repeated units of sulfated fucoses in C-2 and/or C-4 with α-(1–3) and/or α-(1–4) bonds [81]. The chemical composition varies according to the species of algae and can vary within the same species [33].
The anti-inflammatory and antioxidant activities of fucoidan extracts from three Mediterranean species of the genus Cystoseira (C. sedoides, C. compressa and C. crinite (currently designated as Ericaria crinite) was demonstrated in vivo [33]. An edema was induced in Wistar rats, and the tested extracts exhibited a significant anti-inflammatory activity with the edema inhibition percentage above 50%. This sulfated polysaccharide has also proved to reduce the inflammatory response in BV2 microglia, and the generation of ROS and inflammatory cytokines in primary microglia [82,83].
3. Macroalgae Compounds Delivery Optimization by Nanoparticles
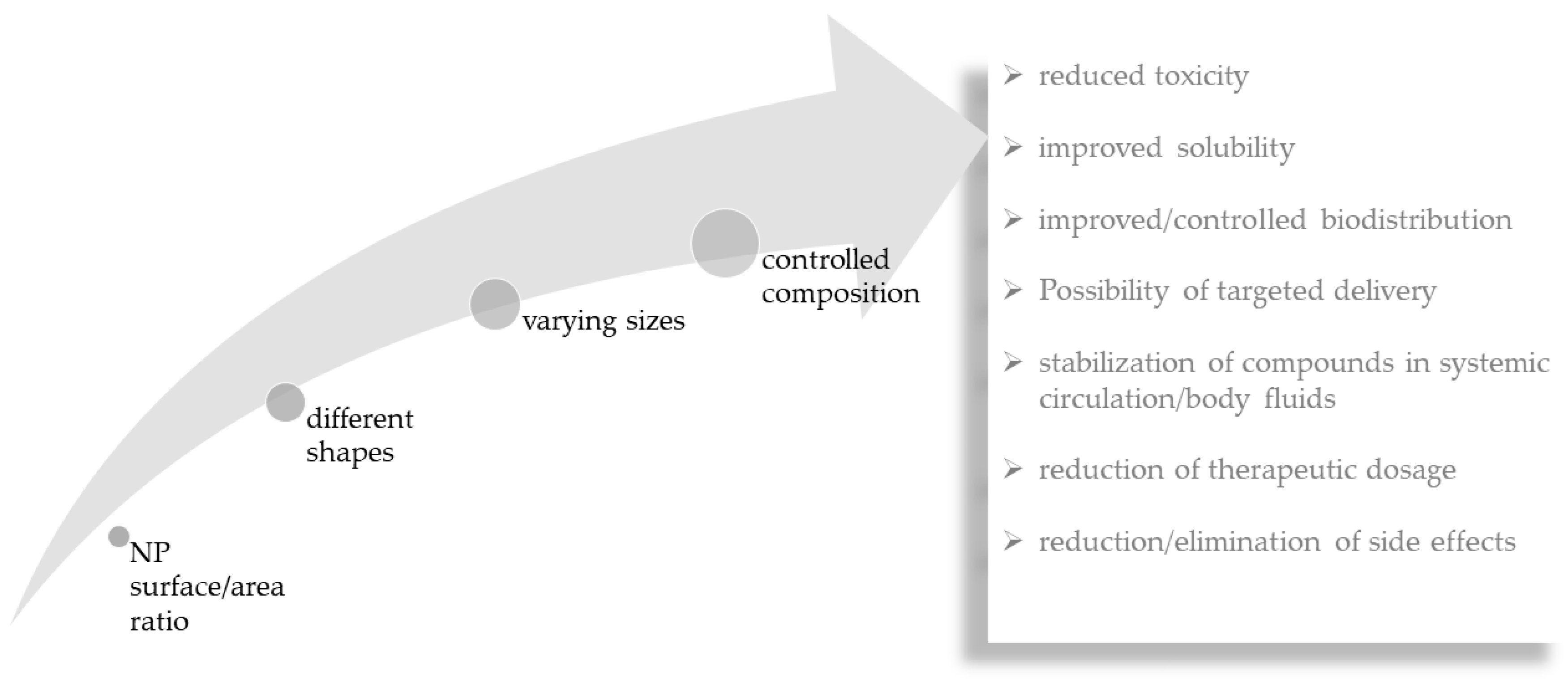
As discussed above, macroalgal compounds have excellent bioactivity against some neurodegenerative diseases. However, in many situations, it is not possible to deliver the bioactive constituents to the desired location. Considering that, one of the objectives of using NPs in nanomedicine is to transport substances, namely bioactive compounds to the targeted tissues and cells, the combination of macroalgae and NPs may potentiate their therapeutic efficiency and reduce even further eventual toxicity of the transported substances [48]. Drug delivery systems can overcome some of the biggest problems in drug administration such as lack of specificity, low biodistribution, reduced efficiency, lack of selectivity and side effects (Figure 3). With enormous potential as drug carriers, the small size of NPs (1 to 100 nm) confers them unique properties such as their surface/area ratio [84]. Additionally, their different shapes, sizes and compositions, have shown enormous importance in medicine, having applicability in the diagnosis and therapy of diseases [85]. They possess advantages in reducing the concentration of drugs, reducing toxicity, improving solubility, providing protection of drugs during circulation and preventing degradation [86,87,88,89].
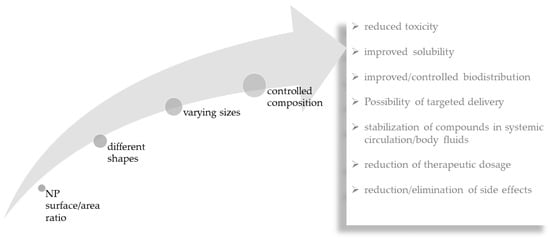
Figure 3.
Relationship between important nanoparticle (NP) features and advantages of nanoparticle-mediated delivery of compounds.
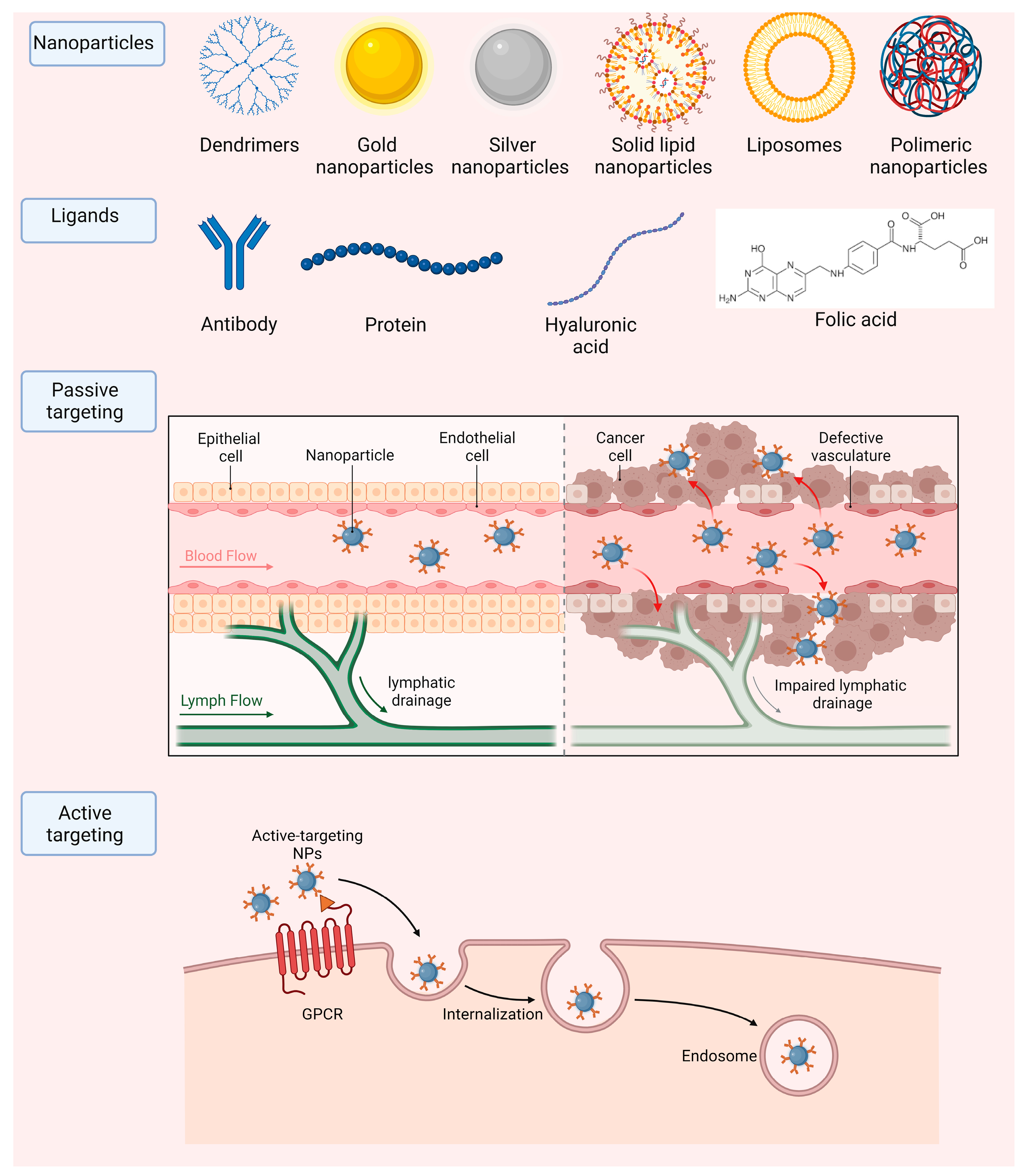
NPs are often divided into two groups: organic and inorganic nanocarriers. Inorganic NPs incorporate mostly metallic particles. In this group we can evidence that some of the most common ones consist in carbon nanotubes, gold, silver and magnetic NPs, among others. Micelles, liposomes, solid lipid NPs and dendrimers fit the organic type of carriers. NPs are very versatile and have numerous applications in the field of biomedicine, which are listed in Table 2 and Figure 4.
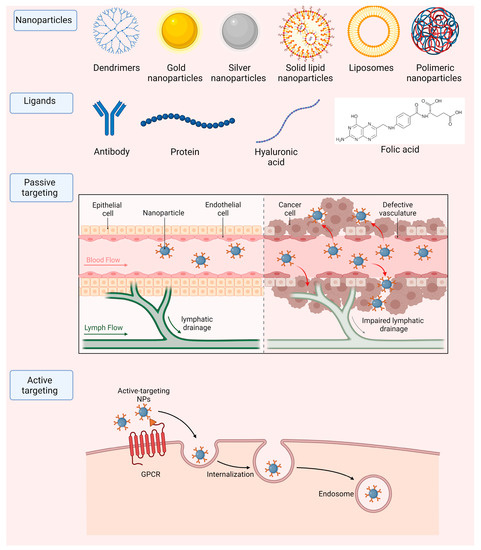
Figure 4.
Main nanoparticle types and strategies to achieve accumulation in target tissues, by passive or active (including ligand-mediated) targeting.
NPs can be directed to the desired local of the treatment either by passive or active targeting. Passive targeting is often associated with the treatment of various types of tumors. This is due to the irregularities that the vascularity around tumors present, having various leakages such as leaky vasculature and defective lymphatic drainage due to the rapid and uncontrolled growth needed to supplement cancer cells, for the growth of the tumor. One can use NPs with a small size that can accumulate specifically in these leakage sites around the tumor and reach their desired therapeutic site [90]. Although this method can be simple and efficient, active targeting is normally preferred since there is no efficient passive method to deliver molecules to other diseases in other parts of the human body. Active targeting consists in the functionalization of the surface of nanoparticles with molecules that are specifically recognized by cells in the local of the treatment. Moreover, this functionalization can improve the nanoparticles pharmacokinetic and pharmacodynamic properties [91]. These can include various types of antibodies, folic acid, proteins, and hyaluronic acid, among others (Figure 4).
Interestingly, algae extracts are often utilized not only as compounds that are encapsulated in NPs for the treatment of various diseases, but also as stabilizers and catalyzers for metallic NPs production [92,93]. In addition, some studies combine the use of chitosan-based NPs with commercially acquired bioactive compounds, such as fucoidan, developing thus chitosan/fucoidan NPs for the delivery of chemicals in breast cancer treatment and nerve regeneration [94,95]. Regarding the last three years, we can find some studies based on fucoidan-based nanoparticles, which allow the enhancement of fucoidan biological activity. In addition, it has already been demonstrated that the synthesis of NPs is promoted by the molecular weight, as well as the structure of fucoidan [96]. Furthermore, the combination of this natural compound with others, or even drugs, may result in the enhancement of the desired effect. As example, Xu Zhang and colleagues have developed fucoidan-coated mesoporous silica NPs to deliver curcumin to the colon tumor site microenvironment [97], which is a polyphenol compound that has also been shown to have neuroprotective effects [98]. The green synthesis of NPs is also a very current topic, which makes it possible to take full advantage of the therapeutic properties, avoiding the use of highly toxic materials and proving to be an ecologically correct and low-cost technique [99,100]. A much smaller number of studies that deliver compounds using NPs are found. Min-Hsuan Tsou and colleagues have developed mesoporous silica NPs to deliver fucoidan to A549 cells [101], and, in line with the main application that we review in this article, PLGA-PEG NPs were developed by Mengxiang Yang and colleagues to facilitate anti-Alzheimer’s effects of fucoxanthin [102].

Table 2.
Examples of nanosystem types, their characteristics and applications in biomedicine.
Table 2.
Examples of nanosystem types, their characteristics and applications in biomedicine.
| Type of Nanosystem | Size (nm) | Characteristics | Applications | Refs. |
|---|---|---|---|---|
| Carbon nanotubes | 0.5–3 nm diameter and 20–1000 nm length | Formed from graphene sheets rolled in cylindrical shape. Classified in single-walled nanotubes (SWNTs) and multiwalled nanotubes (MWNTs). | Cancer photothermal therapy; tissue engineering | [103] |
| Magnetic nanoparticles | 5–50 nm | Normally constituted by iron, cobalt and nickel, with magnetic susceptibility. | Detecting amyloid plaques and biomarkers in Alzheimer’s disease; colon cancer cell theragnostic; treatment of gastric cancer | [104,105,106] |
| Silver nanoparticles | 10–500 nm | Unique optical, electrical and thermal properties. | Antimicrobial properties; anti-proliferative activity; infections of the central nervous system treatment | [39,107,108] |
| Gold nanoparticles | 5–400 nm | Unique optoelectronic properties. | X-ray contrast agent; Alzheimer’s disease diagnostic; HIV diagnostic; tuberculosis diagnostic; angiogenesis therapy; antibacterial therapy | [48,109,110,111,112,113] |
| Polymeric nanoparticles | 10–1000 nm | Solid and spherical particles formed from natural or synthetic polymers. Can be organized into nanospheres and nanocapsules. | Uveitis treatment; treatment of chronic obstructive pulmonary disease; neuroinflammation in Parkinson’s diseases; therapeutic agents for peripheral arterial disease; treatment of periodontal disease | [114,115,116,117] |
| Liposomes | 100–200 nm | Spherical vesicles consisting of phospholipids and other components. Composed of successive bilayers that close on themselves, originating vesicles. | Cancer immunotherapy; antimicrobial therapy; respiratory disorders treatment; arthritis therapy; treatment of Parkinson’s diseases; ocular delivery drugs | [118,119,120] |
| Dendrimers | Up to 10 nm | Polymeric macromolecules in the form of a branched tree. Three fundamental components: the central nucleus, the lower layer where branches linked to the nucleus appear polymerization and the outside region. | Treatment of osteoarthritis; cancer therapy; corneal tissue engineering; antiamyloidogenic agent | [121,122,123] |
| Micelles | 10–100 nm | Lipid aggregates in a globular form. Amphipathic character of these molecules makes them have a natural tendency to aggregate when exposed to water. | Breast cancer therapy; cervical cancer chemotherapy | [124,125] |
| Solid lipid nanoparticles | 50–1000 nm | Composed by solid lipids and surfactants. The surfactants are composed by hydrophilic head and a lipophilic tail. | Glioblastoma treatment; rheumatoid arthritis therapy; topical treatment of pityriasis versicol | [126,127,128] |
Nevertheless, with the growing use of NPs and their application in the biomedical field, which implies exposure and incorporation into the human body, the need for studies to assess their toxicity effects emerges.
Nanoparticle Toxicity and Bioactivity Screening for Neurodegenerative Diseases
Nanotoxicology is the science that studies the toxicity of nanotechnology products, and their interactions in the body, assessing the risks and benefits [129]. This toxicity depends on nanoparticle physicochemical properties, such as composition, size, surface area and charge, among others [130]. These properties also control what will happen to the nanoparticles in vivo and their degradability after excretion from the organism and once in the environment. So, nanomaterial characterization is essential and usually achieved by chromatography techniques, microscopy techniques, and spectroscopic techniques. Some of the techniques are energy dispersive X-ray spectroscopy (EDS); scanning electron microscopy (SEM); dynamic light scattering (DLS); differential scanning microscopy (DSC); inverse gas chromatography (IGC); atomic absorption spectroscopy (AAS), inductively coupled plasma spectroscopy (ICPS); transmission electron microscopy (TEM); atomic force microscopy (AFM); zeta potential and UV-visible spectroscopy and Fourier-transformed infrared spectroscopy (FTIR) [131]. These analytical methods are therefore very important to predict their behavior in storage and during their intended application (in vitro, in vivo, ex vivo) [130]. An upcoming area in recent years implies the use of in silico methods, which will certainly become more potent and useful in their predictive power with the assistance of artificial intelligence.
Regarding nanomaterial degradation, the mechanistic studies will greatly depend on whether it is a natural or synthetic material [130]. The degradation pathways that may be analyzed are: (1) hydrolysis of water-sensitive groups, (2) oxidative degradation, (3) photodegradation, and (4) enzymatic degradation. This assessment will provide crucial understanding on nanoparticle stability, safety, efficacy and potential side effects.
One of the major problems associated with the treatment of neurodegenerative diseases is the blood-brain-barrier (BBB), which separates systemic circulation and central nervous system through highly selective permeability, not allowing the drugs to freely reach and act on the brain [132]. One of the very advantageous characteristics of some NPs is the ability to cross the BBB, revealing a non-invasive alternative path due to their favorable characteristics such as reduced size and low hydrophilicity [133]. Additionally, beyond the good ability to cross the BBB, the nanocarriers must comply some other important parameters: good targeting to the target site, reduced toxicity and high circulation time [134].
A toxicity assessment can be performed in vitro, using cell culture, ex vivo, using cells and tissues collected from humans, or in vivo, using animal models [130]. The in vitro models are simpler biological systems that allow a quick assessment of the effects of NPs. They are low cost and easy to manipulate, making it easy to control and interpret the results. An advantage of these models is that they do not present such ethical restrictions as for in vivo studies [129]. For the in vitro study of the neurotoxicity of NPs, specific brain cell types can be used, namely glial cells, BBB and blood-blood barrier cells and neurons with or without myelin sheath [135]. There are several in vitro techniques usually used to study drug uptake in central nervous system, such as in situ brain perfusion, microdialysis, intravenous injection, brain uptake index, determination of the blood/plasma ratio, cerebrospinal fluid sampling and quantitative autoradiography [136]. The most common nanotoxicity parameter assessed in the brain is related to oxidative stress, resulting from the intense production of ROS, induction of apoptosis that leads to neurons death and neuronal inflammation due to the release and circulation of pro-inflammatory cytokines [137]. However, 2D cell cultures have an important limitation: they do not mimic the 3D microenvironment to which tissues are exposed in organisms. In living organisms, the cells are arranged in a 3D environment, which provide an adequate metabolism, cell-cell and matrix-cell interactions and responses to physiological signals or injuries [38,138,139]. In addition, there are some important factors in assessing the toxicity of NPs that can only be correctly evaluated through in vivo studies, such as biodistribution, biodegradability, route of administration, occurrence of developmental damage, long-term disposition, and activation of the immune system. The correlation between the results of in vitro and in vivo studies of nanotoxicity is scarce, as well as in the correlation between studies in cells and animals [140].
For in vivo toxicology studies, one of the most used animal models are rodents, due to their small size, great similarity of their biochemical processes with humans, easy adaptation to life in the laboratory and short time between generations [133]. However, there are some disadvantages, namely that they are more cumbersome, have a higher cost relatively to cell lines and associated ethical issues. Directive 2010/63/EC on the protection of animals for scientific purposes has pushed laboratories in Europe to actively develop alternatives to animal testing or strategies to greatly reduce them. In this way, zebrafish have been singled out by OECD as a good alternative for toxicology [141]. Furthermore, zebrafish have a cardiovascular, nervous and digestive systems very similar to those of mammals, and signaling pathways present a high level of genomic homology comparing with humans or other mammals [49,142], being possible to evaluate many parameters in preparation for mammal (and human) testing. Most of the toxicity tests are generally carried out in zebrafish embryos until 120 h post-fertilization, which are legally considered as non-animal model, thus not requiring additional ethical permits [143]. This organism has a small size which promotes an easier handling, a very high reproducibility making possible a weekly procedure repetition and a quick development that leaves to faster experiments on zebrafish. At the embryo stage, this organism provides an easier collection of multiple data points by using high-quality imaging. In addition, a low volume of solutions is required, with the possibility of testing various conditions at each experiment. The embryos are transparent which allows to observe the cells since early larval stages and are generally used to assess the development of acute toxicity, while adult fish are used to study chronic toxicity, as well as transport and bioaccumulation of NPs [144,145].
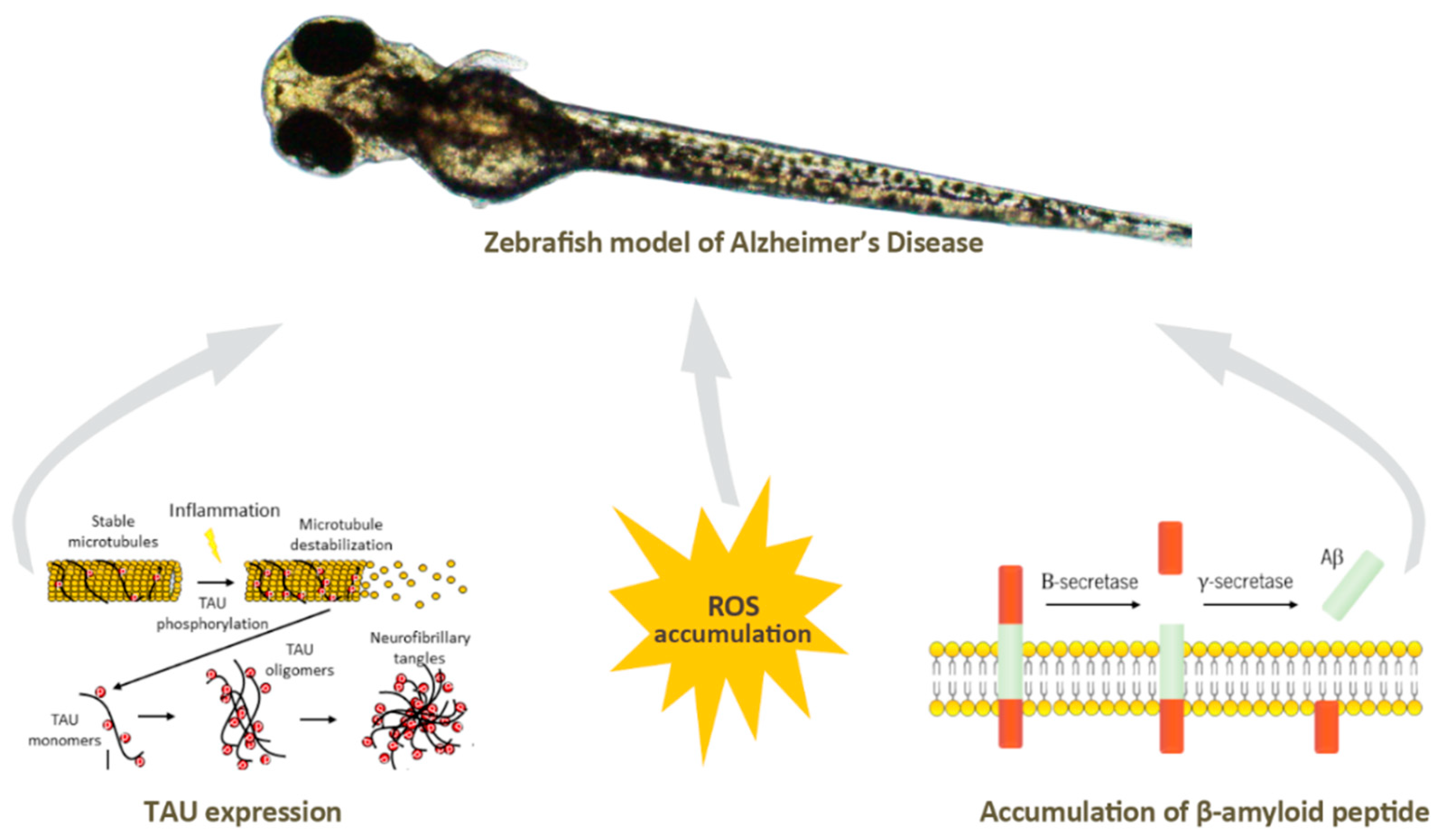
Neurodegenerative diseases such as familial Alzheimer’s disease can be originated through mutations, leading to characteristic hallmarks of this pathology in zebrafish [146,147,148], through addition of various acids to cause oxidative stress [149], which is known to result from a cellular redox state originated inside the cells by complex redox reactions. Oxidative stress causes DNA damage, protein carboxylation, lipid oxidation, and eventually cell death, a mechanism that causes the progression of human neurodegenerative diseases [150]. The induction of phosphorylation of the Tau protein [151], the accumulation of β-amyloid peptides [134], or the formation of neurofibrillary tangles [152], can also be stimulated inducing neuroinflammation, another hallmark of Alzheimer’s disease (Figure 5. Other neurodegenerative diseases such as Parkinsonism may also be induced in zebrafish by exposure to certain toxins or chemicals, such as MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) [153] which leads to loss of dopaminergic neurons in the midbrain with resulting Parkinsonian symptoms.
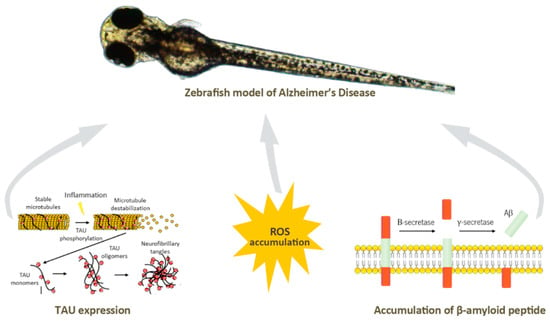
Figure 5.
The main hallmarks of Alzheimer’s disease can be mimicked in zebrafish model, namely the induction of TAU expression, and ROS or β-amyloid peptide accumulation.
Zebrafish disease models are thus upcoming as valid, interesting alternatives for screening both toxicity and bioactivity of new therapeutic compounds and/or formulations currently being developed to tackle neurodegenerative diseases.
4. Conclusions and Future Perspectives
It is recognized that bioactive compounds derived from macroalgae with neuroprotective activity are mostly associated with brown algae (57.6%), followed by red (28.3%) and green (14.1%) algae [154]. Many studies have been performed searching for molecules with neuroprotective effects by acting against oxidative stress, reduction of Aβ-induced cell death, inhibition of pro-inflammatory cytokines production, among others (reviewed in [155]). Additionally, some studies can be found in the literature combining the use of NPs with bioactive compounds, such as fucoidan, most of the time commercially acquired. However, to the best of our knowledge, there is lacking information on the use of NPs for delivery of natural compounds extracted from brown algae, namely Cystoseira spp., alone or in combination with other chemicals, for the prevention and treatment of neurodegenerative diseases. We strongly believe that some of the compounds described in this review present biological activities that can help to reduce neuroinflammation and oxidative stress, both hallmarks of these pathologies. Moreover, the combination of these bioactive compounds with nanotechnology will reduce any associated toxicity or side effects and improve their high circulation time. Furthermore, the use of NPs will also enhance the desired effect by improving the ability of the compounds to reach the target site by crossing the BBB. However, and despite nanotechnology being a solution to overcome some problems in drug delivery, as already mentioned above, this physical barrier remains one of the biggest roadblocks in accelerating of clinical trials, since in vivo, a large percentage of studies fail to show clear therapeutic efficacy.
Author Contributions
A.G.: conceptualization, writing—review and editing, investigation. M.F.: conceptualization, writing—review and editing, investigation. M.L.: writing—original draft preparation, investigation. J.P.G.: writing—original draft preparation, investigation. F.S. (Filipa Silva): writing—original draft preparation, investigation. S.C.: writing—original draft preparation, investigation. F.S. (Filipa Sampaio): writing—original draft preparation, investigation. A.C.G.: conceptualization, writing—review and editing, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the project ATLANTIDA (NORTE-01-0145-FEDER-000040), funded by the Norte Portugal Regional Operational Programme (NORTE 2020) under the Portugal 2020 Partnership Agreement, through the European Regional Development Fund (ERDF), and the strategic programme UID/BIA/04050/2020 funded by National funds through the Fundação para a Ciência e Tecnologia I.P. Mário Fernandes (SFRH/BD/147819/2019) and Anabela Gonçalves (SFRH/BD/146807/2019) hold scholarships from Fundação para a Ciência e a Tecnologia I.P.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Trifan, A.; Vasincu, A.; Luca, S.V.; Neophytou, C.; Wolfram, E.; Opitz, S.E.W.; Sava, D.; Bucur, L.; Cioroiu, B.I.; Miron, A.; et al. Unravelling the potential of seaweeds from the Black Sea coast of Romania as bioactive compounds sources. Part I: Cystoseira barbata (Stackhouse) C. Agardh. Food Chem. Toxicol. 2019, 134, 110820. [Google Scholar] [CrossRef]
- Lopes, G.L.L. Seaweeds from the Portuguese Coast: Chemistry, Antimicrobial and Antiinflammatory Capacity. Ph.D. Thesis, Universidade do Porto, Porto, Portugal, 2014. [Google Scholar]
- Pereira, L. Seaweed: Ecology, Nutrient Composition and Medicinal Uses; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2011. [Google Scholar]
- Pereira, L. Macroalgae. Encyclopedia 2021, 1, 177–188. [Google Scholar] [CrossRef]
- Wilce, R.T. Pleurocladia Lacustris in Arctic America(1). J. Phycol. 1966, 2, 57–66. [Google Scholar] [CrossRef]
- Imbs, T.I.; Zvyagintseva, T.N. Phlorotannins are Polyphenolic Metabolites of Brown Algae. Russ. J. Mar. Biol. 2018, 44, 263–273. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. 2022. Available online: https://www.algaebase.org (accessed on 23 December 2020).
- Molinari Novoa, E.A.; Guiry, M.D. Reinstatement of the genera Gongolaria Boehmer and Ericaria Stackhouse (Sargassaceae, Phaeophyceae). Eur. J. Phycol. 2019, 54, 456. [Google Scholar]
- Guner, A.; Koksal, C.; Erel, S.B.; Kayalar, H.; Nalbantsoy, A.; Sukatar, A.; Karabay Yavasoglu, N.U. Antimicrobial and antioxidant activities with acute toxicity, cytotoxicity and mutagenicity of Cystoseira compressa (Esper) Gerloff & Nizamuddin from the coast of Urla (Izmir, Turkey). Cytotechnology 2015, 67, 135–143. [Google Scholar] [CrossRef]
- Orellana, S.; Hernández, M.; Sansón, M. Diversity of Cystoseira sensu lato (Fucales, Phaeophyceae) in the eastern Atlantic and Mediterranean based on morphological and DNA evidence, including Carpodesmia gen. emend. and Treptacantha gen. emend. Eur. J. Phycol. 2019, 54, 447–465. [Google Scholar] [CrossRef]
- Balboa, E.M.; Conde, E.; Moure, A.; Falque, E.; Dominguez, H. In vitro antioxidant properties of crude extracts and compounds from brown algae. Food Chem. 2013, 138, 1764–1785. [Google Scholar] [CrossRef]
- Čagalj, M.; Skroza, D.; Razola-Díaz, M.D.C.; Verardo, V.; Bassi, D.; Frleta, R.; Generalić Mekinić, I.; Tabanelli, G.; Šimat, V. Variations in the Composition, Antioxidant and Antimicrobial Activities of Cystoseira compressa during Seasonal Growth. Mar. Drugs 2022, 20, 64. [Google Scholar] [CrossRef]
- Mhadhebi, L.; Mhadhebi, A.; Robert, J.; Bouraoui, A. Antioxidant, Anti-inflammatory and Antiproliferative Effects of Aqueous Extracts of Three Mediterranean Brown Seaweeds of the Genus Cystoseira. Iran. J. Pharm. Res. 2014, 13, 207–220. [Google Scholar]
- Mohy El-Din, S.M.; Mohyeldin, M.M. Component Analysis and Antifungal Activity of the Compounds Extracted from Four Brown Seaweeds with Different Solvents at Different Seasons. J. Ocean. Univ. China 2018, 17, 1178–1188. [Google Scholar] [CrossRef]
- Mansur, A.A.; Brown, M.T.; Billington, R.A. The cytotoxic activity of extracts of the brown alga Cystoseira tamariscifolia (Hudson) Papenfuss, against cancer cell lines changes seasonally. J. Appl. Phycol. 2020, 32, 2419–2429. [Google Scholar] [CrossRef]
- Mhadhebi, L.; Laroche-Clary, A.; Robert, J.; Bouraoui, A. Antioxidant, anti-inflammatory, and antiproliferative activities of organic fractions from the Mediterranean brown seaweed Cystoseira sedoides. Can. J. Physiol. Pharmacol. 2011, 89, 911–921. [Google Scholar] [CrossRef]
- Ruberto, G.; Baratta, M.T.; Biondi, D.M.; Amico, V. Antioxidant activity of extracts of the marine algal genus Cystoseira in a micellar model system. J. Appl. Phycol. 2001, 13, 403–407. [Google Scholar] [CrossRef]
- Bermejo, R.; de la Fuente, G.; Ramirez-Romero, E.; Vergara, J.J.; Hernandez, I. Spatial variability and response to anthropogenic pressures of assemblages dominated by a habitat forming seaweed sensitive to pollution (northern coast of Alboran Sea). Mar. Pollut. Bull. 2016, 105, 255–264. [Google Scholar] [CrossRef]
- Montero, L.; Herrero, M.; Ibanez, E.; Cifuentes, A. Separation and characterization of phlorotannins from brown algae Cystoseira abies-marina by comprehensive two-dimensional liquid chromatography. Electrophoresis 2014, 35, 1644–1651. [Google Scholar] [CrossRef]
- Ferreres, F.; Lopes, G.; Gil-Izquierdo, A.; Andrade, P.B.; Sousa, C.; Mouga, T.; Valentao, P. Phlorotannin extracts from fucales characterized by HPLC-DAD-ESI-MSn: Approaches to hyaluronidase inhibitory capacity and antioxidant properties. Mar. Drugs 2012, 10, 2766–2781. [Google Scholar] [CrossRef]
- Rodríguez-Bernaldo de Quirós, A.; Lage-Yusty, M.A.; López-Hernández, J. Determination of phenolic compounds in macroalgae for human consumption. Food Chem. 2010, 121, 634–638. [Google Scholar] [CrossRef]
- Ghannoum, M.A.; Rice, L.B. Antifungal agents: Mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin. Microbiol. Rev. 1999, 12, 501–517. [Google Scholar] [CrossRef]
- Thomas, N.V.; Kim, S.K. Potential pharmacological applications of polyphenolic derivatives from marine brown algae. Environ. Toxicol. Pharmacol. 2011, 32, 325–335. [Google Scholar] [CrossRef]
- Stiger-Pouvreau, V.; Jégou, C.; Cérantola, S.; Guérard, F.; Lann, K.L. Chapter Thirteen—Phlorotannins in Sargassaceae Species from Brittany (France): Interesting Molecules for Ecophysiological and Valorisation Purposes. In Advances in Botanical Research; Bourgougnon, N., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 71, pp. 379–411. [Google Scholar]
- Aggarwal, B.B.; Gupta, S.C.; Kim, J.H. Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey. Blood 2012, 119, 651–665. [Google Scholar] [CrossRef] [PubMed]
- Chkhikvishvili, I.D.; Ramazanov, Z.M. Phenolic substances of brown algae and their antioxidant activity. Appl. Biochem. Microbiol. 2000, 36, 289–291. [Google Scholar] [CrossRef]
- Andrade, P.B.; Barbosa, M.; Matos, R.P.; Lopes, G.; Vinholes, J.; Mouga, T.; Valentão, P. Valuable compounds in macroalgae extracts. Food Chem. 2013, 138, 1819–1828. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, V.L.M.; Seca, A.M.L.; Barreto, M.C.; Neto, A.I.; Kijjoa, A.; Silva, A.M.S. Cytotoxic meroterpenoids from the macroalga Cystoseira abies-marina. Phytochem. Lett. 2013, 6, 593–597. [Google Scholar] [CrossRef]
- Kamenarska, Z.; Yalcin, F.N.; Ersoz, T.; Calis, I.; Stefanov, K.; Popov, S. Chemical composition of Cystoseira crinita Bory from the Eastern Mediterranean. Z. Naturforsch. C J. Biosci. 2002, 57, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Vizetto-Duarte, C.; Pereira, H.; Bruno de Sousa, C.; Pilar Rauter, A.; Albericio, F.; Custódio, L.; Barreira, L.; Varela, J. Fatty acid profile of different species of algae of the Cystoseira genus: A nutraceutical perspective. Nat. Prod. Res. 2015, 29, 1264–1270. [Google Scholar] [CrossRef]
- Ragonese, C.; Tedone, L.; Beccaria, M.; Torre, G.; Cichello, F.; Cacciola, F.; Dugo, P.; Mondello, L. Characterisation of lipid fraction of marine macroalgae by means of chromatography techniques coupled to mass spectrometry. Food Chem. 2014, 145, 932–940. [Google Scholar] [CrossRef]
- Ward, O.P.; Singh, A. Omega-3/6 fatty acids: Alternative sources of production. Process Biochem. 2005, 40, 3627–3652. [Google Scholar] [CrossRef]
- Hadj Ammar, H.; Lajili, S.; Ben Said, R.; Le Cerf, D.; Bouraoui, A.; Majdoub, H. Physico-chemical characterization and pharmacological evaluation of sulfated polysaccharides from three species of Mediterranean brown algae of the genus Cystoseira. Daru J. Fac. Pharm. Tehran Univ. Med. Sci. 2015, 23, 1. [Google Scholar] [CrossRef]
- Hentati, F.; Delattre, C.; Ursu, A.V.; Desbrieres, J.; Le Cerf, D.; Gardarin, C.; Abdelkafi, S.; Michaud, P.; Pierre, G. Structural characterization and antioxidant activity of water-soluble polysaccharides from the Tunisian brown seaweed Cystoseira compressa. Carbohydr. Polym. 2018, 198, 589–600. [Google Scholar] [CrossRef]
- Bahramzadeh, S.; Tabarsa, M.; You, S.; Li, C.; Bita, S. Purification, structural analysis and mechanism of murine macrophage cell activation by sulfated polysaccharides from Cystoseira indica. Carbohydr. Polym. 2019, 205, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Hifney, A.F.; Fawzy, M.A.; Abdel-Gawad, K.M.; Gomaa, M. Upgrading the antioxidant properties of fucoidan and alginate from Cystoseira trinodis by fungal fermentation or enzymatic pretreatment of the seaweed biomass. Food Chem. 2018, 269, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Apostolaki, M.; Armaka, M.; Victoratos, P.; Kollias, G. Cellular mechanisms of TNF function in models of inflammation and autoimmunity. Curr. Dir. Autoimmun. 2010, 11, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Bonnier, F.; Keating, M.E.; Wróbel, T.P.; Majzner, K.; Baranska, M.; Garcia-Munoz, A.; Blanco, A.; Byrne, H.J. Cell viability assessment using the Alamar blue assay: A comparison of 2D and 3D cell culture models. Toxicol. Vitr. 2015, 29, 124–131. [Google Scholar] [CrossRef]
- Asharani, P.V.; Hande, M.P.; Valiyaveettil, S. Anti-proliferative activity of silver nanoparticles. BMC Cell Biol. 2009, 10, 65. [Google Scholar] [CrossRef]
- Kovacs, G.G. Concepts and classification of neurodegenerative diseases. Handb. Clin. Neurol. 2017, 145, 301–307. [Google Scholar] [CrossRef]
- Jorda, A.; Aldasoro, M.; Aldasoro, C.; Guerra-Ojeda, S.; Iradi, A.; Vila, J.M.; Campos-Campos, J.; Valles, S.L. Action of low doses of Aspirin in Inflammation and Oxidative Stress induced by abeta1-42 on Astrocytes in primary culture. Int. J. Med. Sci. 2020, 17, 834–843. [Google Scholar] [CrossRef]
- Association, A.s. 2020 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2020, 16, 391–460. [Google Scholar]
- Sallinen, V.; Torkko, V.; Sundvik, M.; Reenila, I.; Khrustalyov, D.; Kaslin, J.; Panula, P. MPTP and MPP+ target specific aminergic cell populations in larval zebrafish. J. Neurochem. 2009, 108, 719–731. [Google Scholar] [CrossRef]
- Scott, N.R.; Chen, H.; Cui, H. Nanotechnology Applications and Implications of Agrochemicals toward Sustainable Agriculture and Food Systems. J. Agric. Food Chem. 2018, 66, 6451–6456. [Google Scholar] [CrossRef]
- Wissing, S.A.; Muller, R.H. Cosmetic applications for solid lipid nanoparticles (SLN). Int. J. Pharm. 2003, 254, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Reza Mozafari, M.; Johnson, C.; Hatziantoniou, S.; Demetzos, C. Nanoliposomes and Their Applications in Food Nanotechnology. J. Liposome Res. 2008, 18, 309–327. [Google Scholar] [CrossRef] [PubMed]
- Yetisen, A.K.; Qu, H.; Manbachi, A.; Butt, H.; Dokmeci, M.R.; Hinestroza, J.P.; Skorobogatiy, M.; Khademhosseini, A.; Yun, S.H. Nanotechnology in Textiles. ACS Nano 2016, 10, 3042–3068. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, B. Nanosize drug delivery system. Curr. Pharm. Biotechnol. 2013, 14, 1221. [Google Scholar] [CrossRef] [PubMed]
- Paatero, I.; Casals, E.; Niemi, R.; Özliseli, E.; Rosenholm, J.M.; Sahlgren, C. Analyses in zebrafish embryos reveal that nanotoxicity profiles are dependent on surface-functionalization controlled penetrance of biological membranes. Sci. Rep. 2017, 7, 8423. [Google Scholar] [CrossRef]
- Pan, M.H.; Lai, C.S.; Dushenkov, S.; Ho, C.T. Modulation of inflammatory genes by natural dietary bioactive compounds. J. Agric. Food Chem. 2009, 57, 4467–4477. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Lu, H.; Ouyang, W.; Huang, C. Inflammation, a key event in cancer development. Mol. Cancer. Res. 2006, 4, 221–233. [Google Scholar] [CrossRef]
- Palmer, R.M.; Ashton, D.S.; Moncada, S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature 1988, 333, 664–666. [Google Scholar] [CrossRef]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef]
- Feng, W.; Han, X.; Hu, H.; Chang, M.; Ding, L.; Xiang, H.; Chen, Y.; Li, Y. 2D vanadium carbide MXenzyme to alleviate ROS-mediated inflammatory and neurodegenerative diseases. Nat. Commun. 2021, 12, 2203. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Khanbabaee, K.; van Ree, T. Tannins: Classification and definition. Nat. Prod. Rep. 2001, 18, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Jeon, Y.J. Anti-diabetic effects of brown algae derived phlorotannins, marine polyphenols through diverse mechanisms. Fitoterapia 2013, 86, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Zaragoza, M.C.; Lopez, D.; Sáiz, M.P.; Poquet, M.; Perez, J.; Puig-Parellada, P.; Marmol, F.; Simonetti, P.; Gardana, C.; Lerat, Y.; et al. Toxicity and antioxidant activity in vitro and in vivo of two Fucus vesiculosus extracts. J. Agric. Food Chem. 2008, 56, 7773–7780. [Google Scholar] [CrossRef]
- Lopes, G.; Sousa, C.; Silva, L.R.; Pinto, E.; Andrade, P.B.; Bernardo, J.; Mouga, T.; Valentao, P. Can phlorotannins purified extracts constitute a novel pharmacological alternative for microbial infections with associated inflammatory conditions? PLoS ONE 2012, 7, e31145. [Google Scholar] [CrossRef]
- Koivikko, R.; Loponen, J.; Honkanen, T.; Jormalainen, V. Contents of Soluble, Cell-Wall-Bound and Exuded Phlorotannins in the Brown Alga Fucus vesiculosus, with Implications on Their Ecological Functions. J. Chem. Ecol. 2005, 31, 195–212. [Google Scholar] [CrossRef]
- Kim, A.R.; Shin, T.S.; Lee, M.S.; Park, J.Y.; Park, K.E.; Yoon, N.Y.; Kim, J.S.; Choi, J.S.; Jang, B.C.; Byun, D.S.; et al. Isolation and identification of phlorotannins from Ecklonia stolonifera with antioxidant and anti-inflammatory properties. J. Agric. Food Chem. 2009, 57, 3483–3489. [Google Scholar] [CrossRef]
- Li, Y.; Lee, S.H.; Le, Q.T.; Kim, M.M.; Kim, S.K. Anti-allergic effects of phlorotannins on histamine release via binding inhibition between IgE and Fc epsilonRI. J. Agric. Food Chem. 2008, 56, 12073–12080. [Google Scholar] [CrossRef]
- Hasday, J.D.; Bascom, R.; Costa, J.J.; Fitzgerald, T.; Dubin, W. Bacterial Endotoxin Is an Active Component of Cigarette Smoke. Chest 1999, 115, 829–835. [Google Scholar] [CrossRef]
- Liu, X.; Yin, S.; Chen, Y.; Wu, Y.; Zheng, W.; Dong, H.; Bai, Y.; Qin, Y.; Li, J.; Feng, S.; et al. LPSinduced proinflammatory cytokine expression in human airway epithelial cells and macrophages via NFkappaB, STAT3 or AP1 activation. Mol. Med. Rep. 2018, 17, 5484–5491. [Google Scholar] [CrossRef]
- Sosroseno, W.; Barid, I.; Herminajeng, E.; Susilowati, H. Nitric oxide production by a murine macrophage cell line (RAW264.7) stimulated with lipopolysaccharide from Actinobacillus actinomycetemcomitans. Oral Microbiol. Immunol. 2002, 17, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.; Valentao, P.; Ferreres, F.; Gil-Izquierdo, A.; Andrade, P.B. In vitro multifunctionality of phlorotannin extracts from edible Fucus species on targets underpinning neurodegeneration. Food Chem. 2020, 333, 127456. [Google Scholar] [CrossRef] [PubMed]
- Custodio, L.; Silvestre, L.; Rocha, M.I.; Rodrigues, M.J.; Vizetto-Duarte, C.; Pereira, H.; Barreira, L.; Varela, J. Methanol extracts from Cystoseira tamariscifolia and Cystoseira nodicaulis are able to inhibit cholinesterases and protect a human dopaminergic cell line from hydrogen peroxide-induced cytotoxicity. Pharm. Biol. 2016, 54, 1687–1696. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes. Nutrients 2010, 2, 355–374. [Google Scholar] [CrossRef]
- Wall, R.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Fatty acids from fish: The anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr. Rev. 2010, 68, 280–289. [Google Scholar] [CrossRef] [PubMed]
- James, M.J.; Gibson, R.A.; Cleland, L.G. Dietary polyunsaturated fatty acids and inflammatory mediator production. Am. J. Clin. Nutr. 2000, 71, 343s–348s. [Google Scholar] [CrossRef]
- Ghosh, S.; Karin, M. Missing pieces in the NF-kappaB puzzle. Cell 2002, 109 (Suppl. 1), S81–S96. [Google Scholar] [CrossRef]
- Cardoso, C.R.; Souza, M.A.; Ferro, E.A.; Favoreto, S., Jr.; Pena, J.D. Influence of topical administration of n-3 and n-6 essential and n-9 nonessential fatty acids on the healing of cutaneous wounds. Wound Repair Regen. 2004, 12, 235–243. [Google Scholar] [CrossRef]
- Cardoso, C.R.; Favoreto, S., Jr.; Oliveira, L.L.; Vancim, J.O.; Barban, G.B.; Ferraz, D.B.; Silva, J.S. Oleic acid modulation of the immune response in wound healing: A new approach for skin repair. Immunobiology 2011, 216, 409–415. [Google Scholar] [CrossRef]
- Fernandez, R.F.; Kim, S.Q.; Zhao, Y.; Foguth, R.M.; Weera, M.M.; Counihan, J.L.; Nomura, D.K.; Chester, J.A.; Cannon, J.R.; Ellis, J.M. Acyl-CoA synthetase 6 enriches the neuroprotective omega-3 fatty acid DHA in the brain. Proc. Natl. Acad. Sci. USA 2018, 115, 12525–12530. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Evans, D.A.; Bienias, J.L.; Tangney, C.C.; Bennett, D.A.; Wilson, R.S.; Aggarwal, N.; Schneider, J. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch. Neurol. 2003, 60, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Brufau, G.; Canela, M.A.; Rafecas, M. Phytosterols: Physiologic and metabolic aspects related to cholesterol-lowering properties. Nutr. Res. 2008, 28, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.-C.; Lai, M.-H.; Hsu, K.-P.; Kuo, Y.-H.; Chen, J.; Tsai, M.-C.; Li, C.-X.; Yin, X.-J.; Jeyashoke, N.; Chao, L.K.-P. Identification of β-Sitosterol as in Vitro Anti-Inflammatory Constituent in Moringa oleifera. J. Agric. Food Chem. 2018, 66, 10748–10759. [Google Scholar] [CrossRef]
- Jung, H.A.; Jin, S.E.; Ahn, B.R.; Lee, C.M.; Choi, J.S. Anti-inflammatory activity of edible brown alga Eisenia bicyclis and its constituents fucosterol and phlorotannins in LPS-stimulated RAW264.7 macrophages. Food Chem. Toxicol. 2013, 59, 199–206. [Google Scholar] [CrossRef]
- Sun, Y.; Lin, Y.; Cao, X.; Xiang, L.; Qi, J. Sterols from Mytilidae show anti-aging and neuroprotective effects via anti-oxidative activity. Int. J. Mol. Sci. 2014, 15, 21660–21673. [Google Scholar] [CrossRef]
- Rioux, L.E.; Turgeon, S.L.; Beaulieu, M. Characterization of polysaccharides extracted from brown seaweeds. Carbohydr. Polym. 2007, 69, 530–537. [Google Scholar] [CrossRef]
- Park, H.Y.; Han, M.H.; Park, C.; Jin, C.-Y.; Kim, G.-Y.; Choi, I.-W.; Kim, N.D.; Nam, T.-J.; Kwon, T.K.; Choi, Y.H. Anti-inflammatory effects of fucoidan through inhibition of NF-κB, MAPK and Akt activation in lipopolysaccharide-induced BV2 microglia cells. Food Chem. Toxicol. 2011, 49, 1745–1752. [Google Scholar] [CrossRef]
- Cui, Y.Q.; Jia, Y.J.; Zhang, T.; Zhang, Q.B.; Wang, X.M. Fucoidan protects against lipopolysaccharide-induced rat neuronal damage and inhibits the production of proinflammatory mediators in primary microglia. CNS Neurosci. Ther. 2012, 18, 827–833. [Google Scholar] [CrossRef]
- Cabrera Trujillo, L.Y. What is Nanotechnology and why Does it Matter?: From Science to Ethics. NanoEthics 2014, 8, 211–213. [Google Scholar] [CrossRef]
- Marchant, G.E. Small is Beautiful: What Can Nanotechnology Do for Personalized Medicine? Curr. Pharm. Pers. Med. 2009, 7, 231–237. [Google Scholar] [CrossRef]
- Lee, C.C.; Gillies, E.R.; Fox, M.E.; Guillaudeu, S.J.; Frechet, J.M.; Dy, E.E.; Szoka, F.C. A single dose of doxorubicin-functionalized bow-tie dendrimer cures mice bearing C-26 colon carcinomas. Proc. Natl. Acad. Sci. USA 2006, 103, 16649–16654. [Google Scholar] [CrossRef] [PubMed]
- Galbis, E.; Iglesias, N.; Lucas, R.; Tinajero-Díaz, E.; de-Paz, M.V.; Muñoz-Guerra, S.; Galbis, J.A. Validation of Smart Nanoparticles as Controlled Drug Delivery Systems: Loading and pH-Dependent Release of Pilocarpine. ACS Omega 2018, 3, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Dutta, T.; Jain, N.K. Targeting potential and anti-HIV activity of lamivudine loaded mannosylated poly (propyleneimine) dendrimer. Biochim. Biophys. Acta 2007, 1770, 681–686. [Google Scholar] [CrossRef]
- Nooli, M.; Chella, N.; Kulhari, H.; Shastri, N.R.; Sistla, R. Solid lipid nanoparticles as vesicles for oral delivery of olmesartan medoxomil: Formulation, optimization and in vivo evaluation. Drug Dev. Ind. Pharm. 2017, 43, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.K.; Patel, A.P. Passive Targeting of Nanoparticles to Cancer. In Surface Modification of Nanoparticles for Targeted Drug Delivery; Pathak, Y.V., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 125–143. [Google Scholar]
- Menon, I.; Zaroudi, M.; Zhang, Y.; Aisenbrey, E.; Hui, L. Fabrication of active targeting lipid nanoparticles: Challenges and perspectives. Mater. Today Adv. 2022, 16, 100299. [Google Scholar] [CrossRef]
- Arya, A.; Mishra, V.; Chundawat, T.S. Green synthesis of silver nanoparticles from green algae (Botryococcus braunii) and its catalytic behavior for the synthesis of benzimidazoles. Chem. Data Collect. 2019, 20, 100190. [Google Scholar] [CrossRef]
- Mahajan, A.; Arya, A.; Chundawat, T.S. Green synthesis of silver nanoparticles using green alga (Chlorella vulgaris) and its application for synthesis of quinolines derivatives. Synth. Commun. 2019, 49, 1926–1937. [Google Scholar] [CrossRef]
- Huang, Y.C.; Yang, Y.T. Effect of basic fibroblast growth factor released from chitosan-fucoidan nanoparticles on neurite extension. J. Tissue Eng. Regen. Med. 2016, 10, 418–427. [Google Scholar] [CrossRef]
- Oliveira, C.; Neves, N.M.; Reis, R.L.; Martins, A.; Silva, T.H. Gemcitabine delivered by fucoidan/chitosan nanoparticles presents increased toxicity over human breast cancer cells. Nanomedicine 2018, 13, 2037–2050. [Google Scholar] [CrossRef]
- Venkatesan, J.; Murugan, S.S.; Seong, G.H. Fucoidan-based nanoparticles: Preparations and applications. Int. J. Biol. Macromol. 2022, 217, 652–667. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhu, Y.; Fan, L.; Ling, J.; Yang, L.-Y.; Wang, N.; Ouyang, X.-k. Delivery of curcumin by fucoidan-coated mesoporous silica nanoparticles: Fabrication, characterization, and in vitro release performance. Int. J. Biol. Macromol. 2022, 211, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.; Lopes, I.; Magalhães, L.; Sárria, M.P.; Machado, R.; Sousa, J.C.; Botelho, C.; Teixeira, J.; Gomes, A.C. Novel concept of exosome-like liposomes for the treatment of Alzheimer’s disease. J. Control. Release 2021, 336, 130–143. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Aboelfetoh, E.F.; Balusamy, S.R.; Ali, D.; Almarzoug, M.H.A.; Tesfaye, J.L.; Krishnaraj, R. Anticancer, Enhanced Antibacterial, and Free Radical Scavenging Potential of Fucoidan- (Fucus vesiculosus Source) Mediated Silver Nanoparticles. Oxidative Med. Cell. Longev. 2021, 2021, 8511576. [Google Scholar] [CrossRef] [PubMed]
- Machado, S.; González-Ballesteros, N.; Gonçalves, A.; Magalhães, L.; Sárria Pereira de Passos, M.; Rodríguez-Argüelles, M.C.; Castro Gomes, A. Toxicity in vitro and in Zebrafish Embryonic Development of Gold Nanoparticles Biosynthesized Using Cystoseira Macroalgae Extracts. Int. J. Nanomed. 2021, 16, 5017–5036. [Google Scholar] [CrossRef] [PubMed]
- Tsou, M.H.; Lee, C.C.; Wu, Z.Y.; Lee, Z.H.; Lin, H.M. Mesoporous silica nanoparticles with fluorescent and magnetic dual-imaging properties to deliver fucoidan. Int. J. Biol. Macromol. 2021, 188, 870–878. [Google Scholar] [CrossRef]
- Yang, M.; Jin, L.; Wu, Z.; Xie, Y.; Zhang, P.; Wang, Q.; Yan, S.; Chen, B.; Liang, H.; Naman, C.B.; et al. PLGA-PEG Nanoparticles Facilitate In Vivo Anti-Alzheimer’s Effects of Fucoxanthin, a Marine Carotenoid Derived from Edible Brown Algae. J. Agric. Food Chem. 2021, 69, 9764–9777. [Google Scholar] [CrossRef]
- Xue, Y. Chapter 11—Carbon Nanotubes for Biomedical Applications. In Industrial Applications of Carbon Nanotubes; Peng, H., Li, Q., Chen, T., Eds.; Elsevier: Boston, MA, USA, 2017; pp. 323–346. [Google Scholar]
- Cheng, K.K.; Chan, P.S.; Fan, S.; Kwan, S.M.; Yeung, K.L.; Wáng, Y.-X.J.; Chow, A.H.L.; Wu, E.X.; Baum, L. Curcumin-conjugated magnetic nanoparticles for detecting amyloid plaques in Alzheimer’s disease mice using magnetic resonance imaging (MRI). Biomaterials 2015, 44, 155–172. [Google Scholar] [CrossRef]
- Yang, C.C.; Yang, S.Y.; Chieh, J.J.; Horng, H.E.; Hong, C.Y.; Yang, H.C.; Chen, K.H.; Shih, B.Y.; Chen, T.F.; Chiu, M.J. Biofunctionalized magnetic nanoparticles for specifically detecting biomarkers of Alzheimer’s disease in vitro. ACS Chem. Neurosci. 2011, 2, 500–505. [Google Scholar] [CrossRef]
- Jiang, X.; Chan, H.C. Magnetic nanoparticles for treatment of gastric cancer. J. Gastroenterol. Hepatol. 2012, 27, 191–193. [Google Scholar] [CrossRef]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomedicine 2007, 3, 95–101. [Google Scholar] [CrossRef]
- Anwar, A.; Rajendran, K.; Siddiqui, R.; Raza Shah, M.; Khan, N.A. Clinically Approved Drugs against CNS Diseases as Potential Therapeutic Agents To Target Brain-Eating Amoebae. ACS Chem. Neurosci. 2019, 10, 658–666. [Google Scholar] [CrossRef]
- Hainfeld, J.F.; Slatkin, D.N.; Focella, T.M.; Smilowitz, H.M. Gold nanoparticles: A new X-ray contrast agent. Br. J. Radiol. 2006, 79, 248–253. [Google Scholar] [CrossRef]
- Georganopoulou, D.G.; Chang, L.; Nam, J.-M.; Thaxton, C.S.; Mufson, E.J.; Klein, W.L.; Mirkin, C.A. Nanoparticle-based detection in cerebral spinal fluid of a soluble pathogenic biomarker for Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2005, 102, 2273. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, K.A.; Luong, J.H.T. Impedance Method for Detecting HIV-1 Protease and Screening for Its Inhibitors Using Ferrocene−Peptide Conjugate/Au Nanoparticle/Single-Walled Carbon Nanotube Modified Electrode. Anal. Chem. 2008, 80, 7056–7062. [Google Scholar] [CrossRef] [PubMed]
- Baptista, P.V.; Koziol-Montewka, M.; Paluch-Oles, J.; Doria, G.a.; Franco, R. Gold-Nanoparticle-Probe–Based Assay for Rapid and Direct Detection of Mycobacterium tuberculosis DNA in Clinical Samples. Clin. Chem. 2006, 52, 1433–1434. [Google Scholar] [CrossRef] [PubMed]
- Zharov, V.P.; Mercer, K.E.; Galitovskaya, E.N.; Smeltzer, M.S. Photothermal nanotherapeutics and nanodiagnostics for selective killing of bacteria targeted with gold nanoparticles. Biophys. J. 2006, 90, 619–627. [Google Scholar] [CrossRef]
- Sabzevari, A.; Adibkia, K.; Hashemi, H.; Hedayatfar, A.; Mohsenzadeh, N.; Atyabi, F.; Ghahremani, M.H.; Dinarvand, R. Polymeric triamcinolone acetonide nanoparticles as a new alternative in the treatment of uveitis: In vitro and in vivo studies. Eur. J. Pharm. Biopharm. 2013, 84, 63–71. [Google Scholar] [CrossRef]
- Mohamed, A.; Kunda, N.K.; Ross, K.; Hutcheon, G.A.; Saleem, I.Y. Polymeric nanoparticles for the delivery of miRNA to treat Chronic Obstructive Pulmonary Disease (COPD). Eur. J. Pharm. Biopharm. 2019, 136, 1–8. [Google Scholar] [CrossRef]
- Kwon, B.; Kang, C.; Kim, J.; Yoo, D.; Cho, B.-R.; Kang, P.M.; Lee, D. H2O2-responsive antioxidant polymeric nanoparticles as therapeutic agents for peripheral arterial disease. Int. J. Pharm. 2016, 511, 1022–1032. [Google Scholar] [CrossRef]
- Osorio, R.; Alfonso-Rodriguez, C.A.; Medina-Castillo, A.L.; Alaminos, M.; Toledano, M. Bioactive Polymeric Nanoparticles for Periodontal Therapy. PLoS ONE 2016, 11, e0166217. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Liu, S.; Shi, S.; Chen, Y.; Xu, F.; Wei, X.; Xu, Y. Solubilization and delivery of Ursolic-acid for modulating tumor microenvironment and regulatory T cell activities in cancer immunotherapy. J. Control. Release 2020, 320, 168–178. [Google Scholar] [CrossRef]
- Banerjee, R. Liposomes: Applications in medicine. J. Biomater. Appl. 2001, 16, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Lin, Q.; He, S.; Wang, L.; Fu, Y.; Zhang, Z.; Zhang, L. A brain targeting functionalized liposomes of the dopamine derivative N-3,4-bis(pivaloyloxy)-dopamine for treatment of Parkinson’s disease. J. Control. Release 2018, 277, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Geiger, B.C.; Wang, S.; Padera, R.F., Jr.; Grodzinsky, A.J.; Hammond, P.T. Cartilage-penetrating nanocarriers improve delivery and efficacy of growth factor treatment of osteoarthritis. Sci. Transl. Med. 2018, 10, eaat8800. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Yu, T.; Ding, L.; Laurini, E.; Huang, Y.; Zhang, M.; Weng, Y.; Lin, S.; Chen, P.; Marson, D.; et al. A Dual Targeting Dendrimer-Mediated siRNA Delivery System for Effective Gene Silencing in Cancer Therapy. J. Am. Chem. Soc. 2018, 140, 16264–16274. [Google Scholar] [CrossRef]
- Duan, X.; Sheardown, H. Dendrimer crosslinked collagen as a corneal tissue engineering scaffold: Mechanical properties and corneal epithelial cell interactions. Biomaterials 2006, 27, 4608–4617. [Google Scholar] [CrossRef]
- Mathews, A.S.; Ahmed, S.; Shahin, M.; Lavasanifar, A.; Kaur, K. Peptide modified polymeric micelles specific for breast cancer cells. Bioconjug. Chem. 2013, 24, 560–570. [Google Scholar] [CrossRef]
- Niu, K.; Yao, Y.; Xiu, M.; Guo, C.; Ge, Y.; Wang, J. Controlled Drug Delivery by Polylactide Stereocomplex Micelle for Cervical Cancer Chemotherapy. Front. Pharmacol. 2018, 9, 930. [Google Scholar] [CrossRef]
- Grillone, A.; Battaglini, M.; Moscato, S.; Mattii, L.; de Julian Fernandez, C.; Scarpellini, A.; Giorgi, M.; Sinibaldi, E.; Ciofani, G. Nutlin-loaded magnetic solid lipid nanoparticles for targeted glioblastoma treatment. Nanomedicine 2019, 14, 727–752. [Google Scholar] [CrossRef]
- Zhou, M.; Hou, J.; Zhong, Z.; Hao, N.; Lin, Y.; Li, C. Targeted delivery of hyaluronic acid-coated solid lipid nanoparticles for rheumatoid arthritis therapy. Drug Deliv. 2018, 25, 716–722. [Google Scholar] [CrossRef]
- El-Housiny, S.; Shams Eldeen, M.A.; El-Attar, Y.A.; Salem, H.A.; Attia, D.; Bendas, E.R.; El-Nabarawi, M.A. Fluconazole-loaded solid lipid nanoparticles topical gel for treatment of pityriasis versicolor: Formulation and clinical study. Drug Deliv. 2018, 25, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Vranic, S.; Shimada, Y.; Ichihara, S.; Kimata, M.; Wu, W.; Tanaka, T.; Boland, S.; Tran, L.; Ichihara, G. Toxicological Evaluation of SiO(2) Nanoparticles by Zebrafish Embryo Toxicity Test. Int. J. Mol. Sci. 2019, 20, 882. [Google Scholar] [CrossRef] [PubMed]
- Pagar, R.R.; Musale, S.R.; Pawar, G.; Kulkarni, D.; Giram, P.S. Comprehensive Review on the Degradation Chemistry and Toxicity Studies of Functional Materials. ACS Biomater. Sci. Eng. 2022, 8, 2161–2195. [Google Scholar] [CrossRef]
- Schrand, A.M.; Dai, L.; Schlager, J.J.; Hussain, S.M. Toxicity testing of nanomaterials. Adv. Exp. Med. Biol. 2012, 745, 58–75. [Google Scholar] [CrossRef]
- Zhou, Y.; Liyanage, P.Y.; Devadoss, D.; Rios Guevara, L.R.; Cheng, L.; Graham, R.M.; Chand, H.S.; Al-Youbi, A.O.; Bashammakh, A.S.; El-Shahawi, M.S.; et al. Nontoxic amphiphilic carbon dots as promising drug nanocarriers across the blood–brain barrier and inhibitors of β-amyloid. Nanoscale 2019, 11, 22387–22397. [Google Scholar] [CrossRef]
- Sela, H.; Cohen, H.; Elia, P.; Zach, R.; Karpas, Z.; Zeiri, Y. Spontaneous penetration of gold nanoparticles through the blood brain barrier (BBB). J. Nanobiotechnol. 2015, 13, 71. [Google Scholar] [CrossRef]
- Javed, I.; Peng, G.; Xing, Y.; Yu, T.; Zhao, M.; Kakinen, A.; Faridi, A.; Parish, C.L.; Ding, F.; Davis, T.P.; et al. Inhibition of amyloid beta toxicity in zebrafish with a chaperone-gold nanoparticle dual strategy. Nat. Commun. 2019, 10, 3780. [Google Scholar] [CrossRef]
- Xiaoli, F.; Longquan, S. Chapter 20—Neurotoxicity of nanomaterials. In Emerging Nanotechnologies in Dentistry, 2nd ed.; Subramani, K., Ahmed, W., Eds.; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 421–444. [Google Scholar]
- Sharma, B.; Luhach, K.; Kulkarni, G.T. 4—In vitro and in vivo models of BBB to evaluate brain targeting drug delivery. In Brain Targeted Drug Delivery System; Gao, H., Gao, X., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 53–101. [Google Scholar]
- Jampílek, J.; Kráľová, K.; Novák, P.; Novák, M. Nanobiotechnology in Neurodegenerative Diseases. In Nanobiotechnology in Neurodegenerative Diseases; Rai, M., Yadav, A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 65–138. [Google Scholar]
- Stevens, M.M. Toxicology: Testing in the third dimension. Nat. Nanotechnol. 2009, 4, 342–343. [Google Scholar] [CrossRef]
- Lee, J.; Lilly, G.D.; Doty, R.C.; Podsiadlo, P.; Kotov, N.A. In vitro toxicity testing of nanoparticles in 3D cell culture. Small 2009, 5, 1213–1221. [Google Scholar] [CrossRef]
- Rizzo, L.Y.; Golombek, S.K.; Mertens, M.E.; Pan, Y.; Laaf, D.; Broda, J.; Jayapaul, J.; Möckel, D.; Subr, V.; Hennink, W.E.; et al. In Vivo Nanotoxicity Testing using the Zebrafish Embryo Assay. J. Mater. Chem. B 2013, 1, 3918–3925. [Google Scholar] [CrossRef]
- OECD. Test No. 236: Fish Embryo Acute Toxicity (FET) Test; OECD: Paris, France, 2013. [Google Scholar] [CrossRef]
- Chakraborty, C.; Sharma, A.R.; Sharma, G.; Lee, S.S. Zebrafish: A complete animal model to enumerate the nanoparticle toxicity. J. Nanobiotechnol. 2016, 14, 65. [Google Scholar] [CrossRef]
- Strähle, U.; Scholz, S.; Geisler, R.; Greiner, P.; Hollert, H.; Rastegar, S.; Schumacher, A.; Selderslaghs, I.; Weiss, C.; Witters, H.; et al. Zebrafish embryos as an alternative to animal experiments—A commentary on the definition of the onset of protected life stages in animal welfare regulations. Reprod. Toxicol. 2012, 33, 128–132. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, X.; Chen, Y.; Chang, Y. Application of embryonic and adult zebrafish for nanotoxicity assessment. Methods Mol. Biol. 2012, 926, 317–329. [Google Scholar] [CrossRef]
- OECD. Test No. 425: Acute Oral Toxicity: Up-and-Down Procedure; OECD: Paris, France, 2008. [Google Scholar] [CrossRef]
- Leimer, U.; Lun, K.; Romig, H.; Walter, J.; Grunberg, J.; Brand, M.; Haass, C. Zebrafish (Danio rerio) presenilin promotes aberrant amyloid beta-peptide production and requires a critical aspartate residue for its function in amyloidogenesis. Biochemistry 1999, 38, 13602–13609. [Google Scholar] [CrossRef]
- Ramesh, T.; Lyon, A.N.; Pineda, R.H.; Wang, C.; Janssen, P.M.; Canan, B.D.; Burghes, A.H.; Beattie, C.E. A genetic model of amyotrophic lateral sclerosis in zebrafish displays phenotypic hallmarks of motoneuron disease. Dis. Model. Mech. 2010, 3, 652–662. [Google Scholar] [CrossRef]
- Laird, A.S.; Robberecht, W. Modeling neurodegenerative diseases in zebrafish embryos. Methods Mol. Biol. 2011, 793, 167–184. [Google Scholar] [CrossRef]
- Moussavi Nik, S.H.; Croft, K.; Mori, T.A.; Lardelli, M. The comparison of methods for measuring oxidative stress in zebrafish brains. Zebrafish 2014, 11, 248–254. [Google Scholar] [CrossRef]
- Mugoni, V.; Camporeale, A.; Santoro, M.M. Analysis of oxidative stress in zebrafish embryos. J. Vis. Exp. 2014, 89, e51328. [Google Scholar] [CrossRef]
- Shadia, E.N.; Frederick, E.W.; Zahoor, A.S. Development of a Novel and Robust Pharmacological Model of Okadaic Acid-induced Alzheimer’s Disease in Zebrafish. CNS Neurol. Disord.-Drug Targets 2016, 15, 86–94. [Google Scholar] [CrossRef]
- Moussavi Nik, S.H.; Newman, M.; Ganesan, S.; Chen, M.; Martins, R.; Verdile, G.; Lardelli, M. Hypoxia alters expression of Zebrafish Microtubule-associated protein Tau (mapta, maptb) gene transcripts. BMC Res. Notes 2014, 7, 767. [Google Scholar] [CrossRef] [PubMed]
- Nellore, J.; Pauline, C.; Amarnath, K. Bacopa monnieri Phytochemicals Mediated Synthesis of Platinum Nanoparticles and Its Neurorescue Effect on 1-Methyl 4-Phenyl 1,2,3,6 Tetrahydropyridine-Induced Experimental Parkinsonism in Zebrafish. J. Neurodegener. Dis. 2013, 2013, 972391. [Google Scholar] [CrossRef]
- Alghazwi, M.; Kan, Y.Q.; Zhang, W.; Gai, W.P.; Garson, M.J.; Smid, S. Neuroprotective activities of natural products from marine macroalgae during 1999–2015. J. Appl. Phycol. 2016, 28, 3599–3616. [Google Scholar] [CrossRef]
- Pereira, L.; Valado, A. The Seaweed Diet in Prevention and Treatment of the Neurodegenerative Diseases. Mar. Drugs 2021, 19, 128. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).