Impact of Perfluorocarbons with Gas Transport Function on Growth of Phototrophic Microorganisms in a Free and Immobilized State and in Consortia with Bacteria
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Microorganisms and Cultivation Condition
2.3. Biomass Separation and Mechanical Disintegration
2.4. Analytical Methods and Calculated Parameters
3. Results
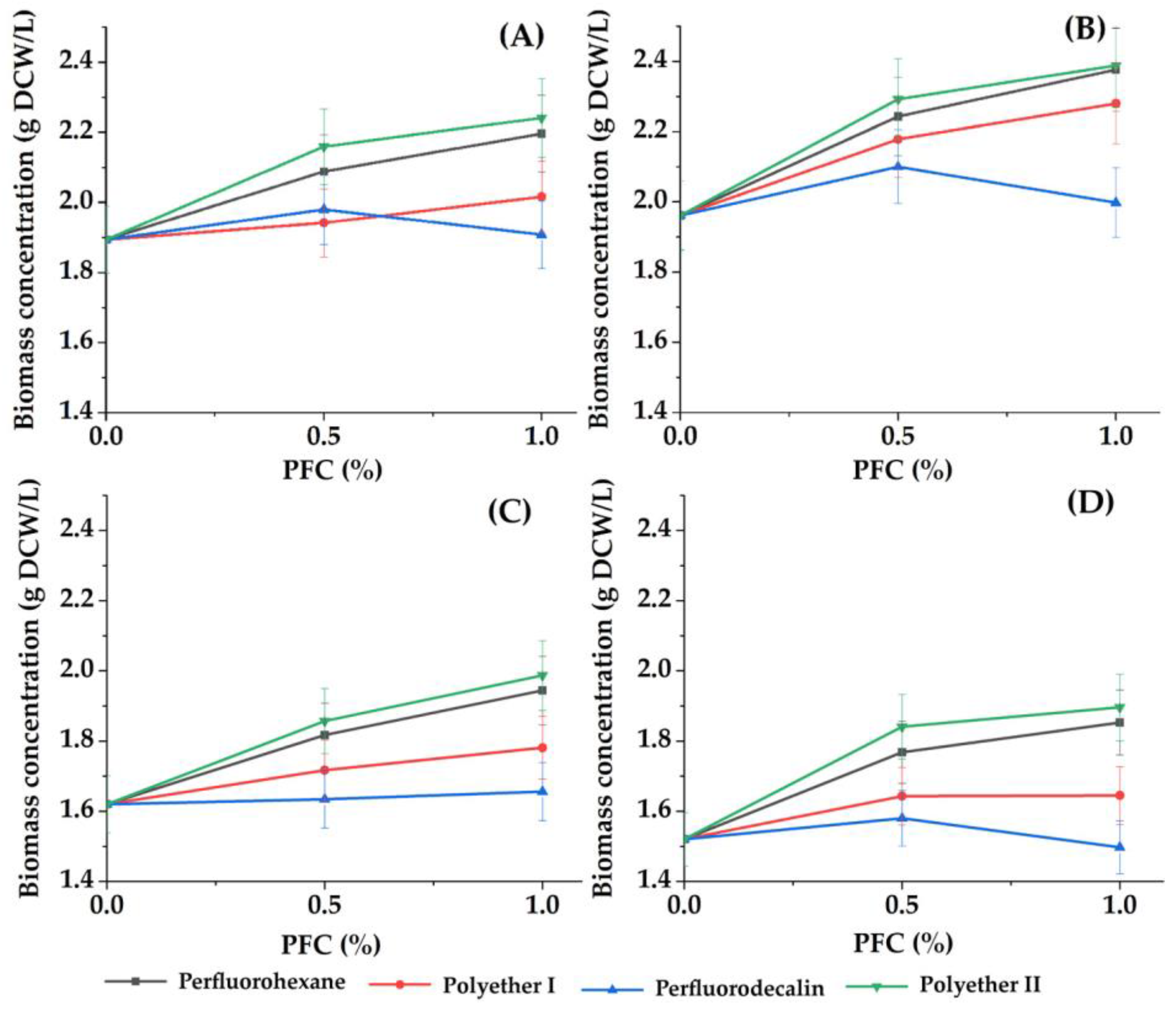
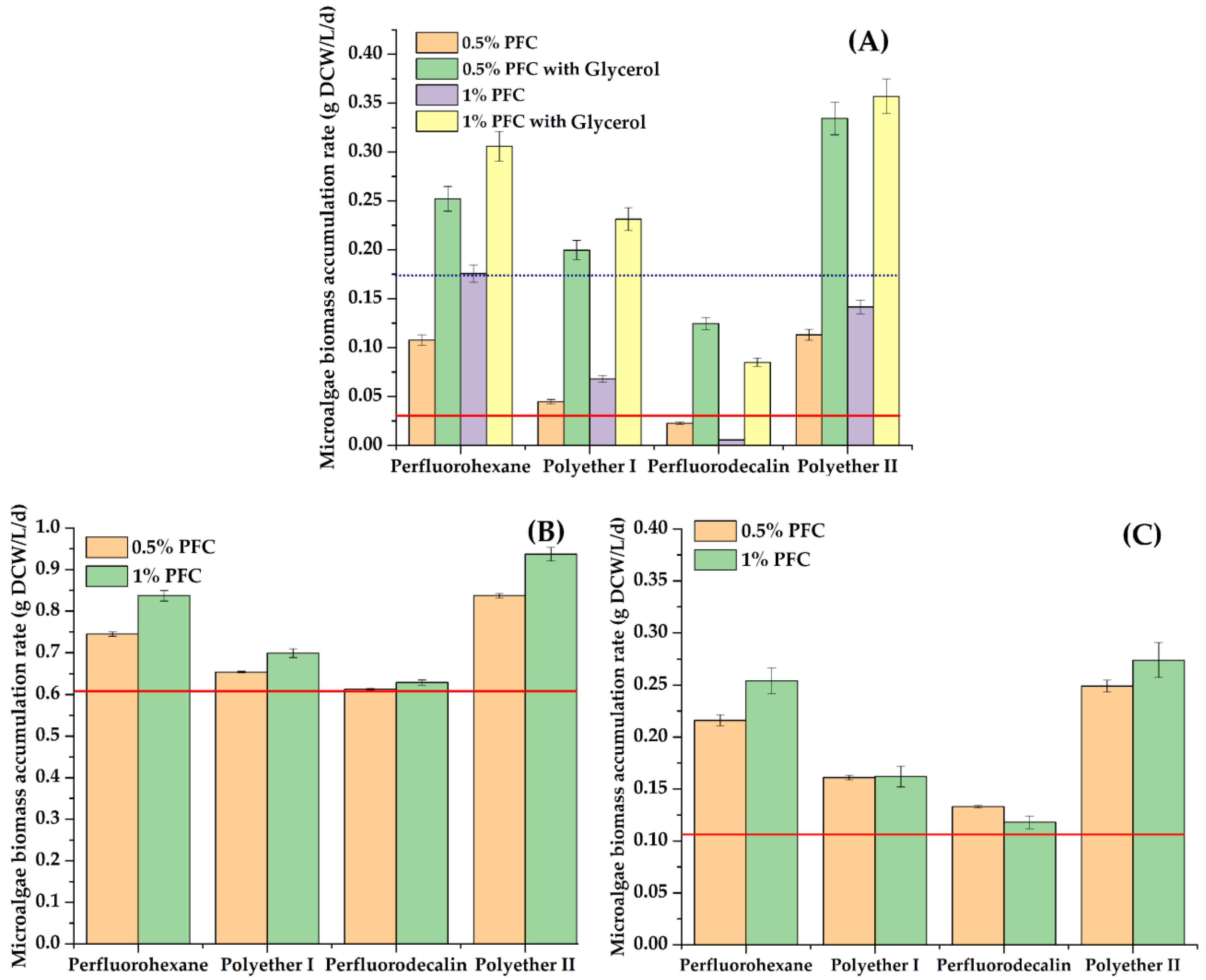
3.1. Effect of Perfluorocarbon Compounds on the Growth and ATP Concentration of Phototrophic Microorganisms
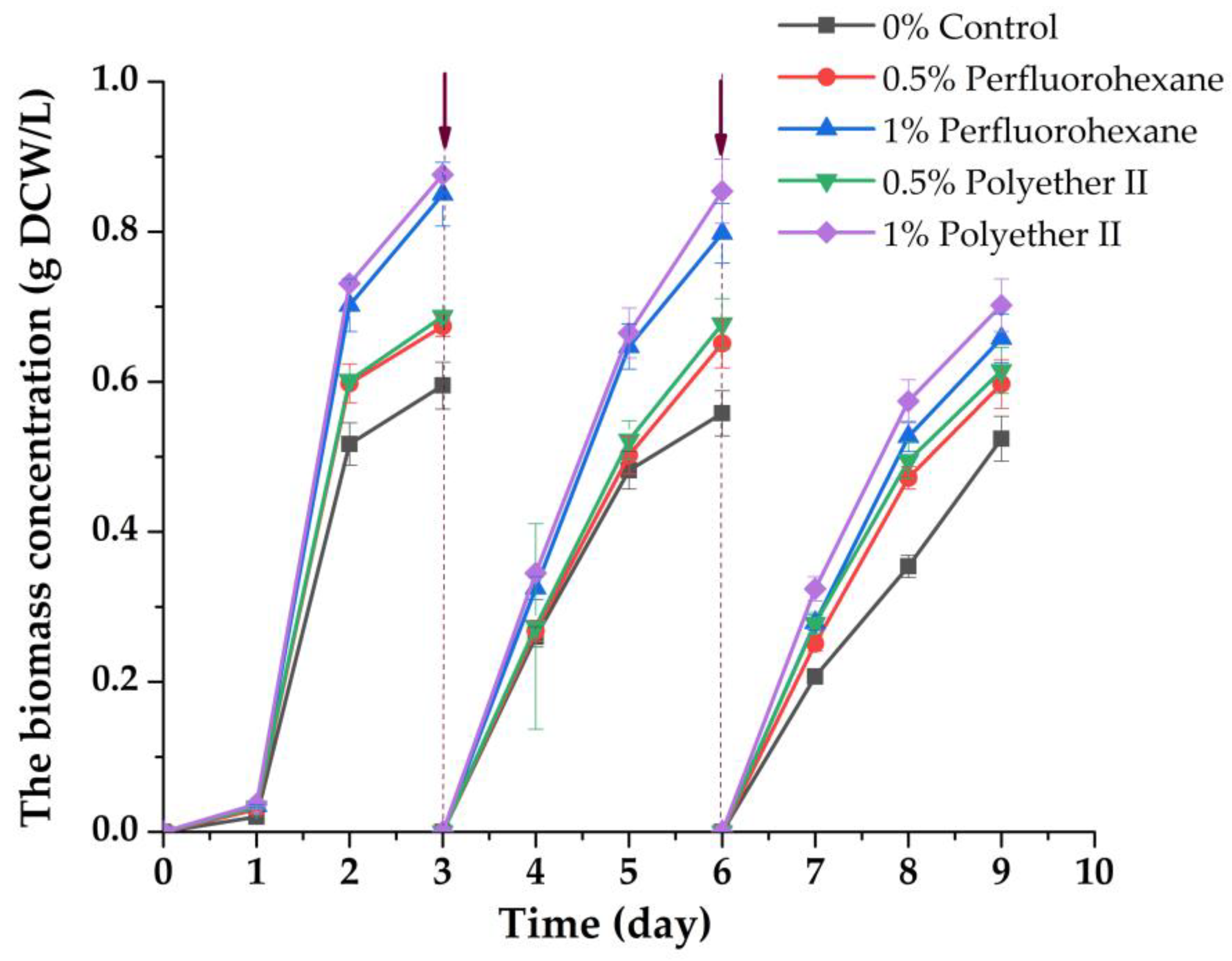
3.2. Effect of PFC on the Characteristics of Chlorella Biomass Accumulation in Presence of Immobilized Inoculum under Conditions of Periodic Cultivation in Wastewater
3.3. Effect of Joint Presence of PFCs and OPCs on Consortia of Bacterial and Microalgae Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Magdalena, J.A.; Gonzalez-Fernandez, S. Microalgae biomass as a potential raw material for a carboxylate platform. Molecules 2019, 24, 4404. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Hu, K.S.; Chu, K.V.; Selvaraju, A.; Chen, V.H.; Chang, J.S.; Shaw, P.L. Microalgae: A future source of biohydrogen and biogas. Front. Energy Res. 2021, 9, 660399. [Google Scholar] [CrossRef]
- Ansari, F.A.; Singh, P.; Guldhe, A.; Bux, F. Microalgal cultivation using aquaculture wastewater: Integrated biomass generation and nutrient remediation. Algal Res. 2017, 21, 169–177. [Google Scholar] [CrossRef]
- Efremenko, E.N.; Nikolskaya, A.B.; Lyagin, I.V.; Senko, O.V.; Makhlis, T.A.; Stepanov, N.A.; Maslova, O.V.; Mamedova, F.; Varfolomeev, S.D. Production of biofuels from pretreated microalgae biomass by anaerobic fermentation with immobilized Clostridium acetobutylicum cells. Bioresour. Technol. 2012, 114, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Limongi, A.R.; Viviano, E.; De Luca, M.; Radice, R.P.; Bianco, G.; Martelli, G. Biohydrogen from microalgae: Production and application. Appl. Sci. 2021, 11, 1616. [Google Scholar] [CrossRef]
- Schiano di Visconte, G.; Spicer, A.; Chuck, K.J.; Allen, M.J. Bio-purification of microalgae: A look at the current state and future possibilities using genetic modification. Appl. Sci. 2019, 9, 4793. [Google Scholar] [CrossRef]
- Maslova, O.; Stepanov, N.; Senko, O.; Efremenko, E. Preparation of various organic acids from various renewable sources by immobilized cells in the modes of separate hydrolysis and fermentation (microwave) and simultaneous saccharification and fermentation (SFF). Bioresour. Technol. 2019, 272, 1–9. [Google Scholar] [CrossRef]
- Efremenko, E.; Senko, O.; Maslova, O.; Stepanov, N.; Aslanli, A.; Lyagin, I. Biocatalysts in the synthesis of microbial polysaccharides: Properties and development trends. Catalysts 2022, 12, 1377. [Google Scholar] [CrossRef]
- Stepanov, N.; Efremenko, E. “Deceived” concentrated immobilized cells as a biocatalyst for intensive production of bacterial cellulose from various sources. Catalysts 2018, 8, 33. [Google Scholar] [CrossRef]
- Martin, N.; Bernat, T.; Dinasquet, J.; Stofko, A.; Damon, A.; Deheyn, D.D.; Azam, F.; Smith, J.E.; Davey, M.P.; Smith, A.G.; et al. Synthetic consortia of algae and bacteria for the economical cultivation of microalgae in a simple hydrogel system. J. Appl. Physiol. 2021, 33, 2805–2815. [Google Scholar] [CrossRef]
- Senko, O.; Stepanov, N.; Maslova, O.; Efremenko, E. Transformation of enzymatic hydrolysates of Chlorella–fungus mixed biomass into poly(hydroxyalkanoates). Catalysts 2023, 13, 118. [Google Scholar] [CrossRef]
- Maslova, O.; Senko, O.; Stepanov, N.; Gladchenko, M.; Gaydamaka, S.; Akopyan, A.; Yeseva, E.; Anisimov, A.; Efremenko, E. Sulfur-containing mixed waste during anaerobic processing by new immobilized synthetic consortia. Bioresour. Technol. 2022, 362, 127794. [Google Scholar] [CrossRef] [PubMed]
- Senko, O.; Stepanov, N.; Maslova, O.; Efremenko, E. “Nature-like” cryoimmobilization of phototrophic microorganisms: New opportunities for their long-term storage and sustainable use. Sustainability 2022, 14, 661. [Google Scholar] [CrossRef]
- Johnson, D.B.; Schideman, L.C.; Canam, T.; Hudson, R.J. Pilot-scale demonstration of efficient ammonia removal from a high-strength municipal wastewater treatment sidestream by algal-bacterial biofilms affixed to rotating contactors. Algal Res. 2018, 34, 143–153. [Google Scholar] [CrossRef]
- Wollmann, F.; Dietze, S.; Ackermann, J.U.; Bley, T.; Walther, T.; Steingroewer, J.; Krujatz, F. Microalgae wastewater treatment: Biological and technological approaches. Eng. Life Sci. 2019, 19, 860–871. [Google Scholar] [CrossRef]
- Chan, S.S.; Khoo, K.S.; Chew, K.W.; Ling, T.C.; Show, P.L. Recent advances biodegradation and biosorption of organic compounds from wastewater: Microalgae-bacteria consortium—A review. Bioresour. Technol. 2022, 344, 126159. [Google Scholar] [CrossRef]
- Wang, S.; Ji, B.; Zhang, M.; Ma, Y.; Gu, J.; Liu, Y. Defensive responses of microalgal-bacterial granules to tetracycline in municipal wastewater treatment. Bioresour. Technol. 2020, 312, 123605. [Google Scholar] [CrossRef]
- Vasseghian, Y.; Alimohamadi, M.; Khataee, A.; Dragoi, E. A global systematic review on the concentration of organophosphate esters in water resources: Meta-analysis, and probabilistic risk assessment. Sci. Total Environ. 2021, 807 Pt 2, 150876. [Google Scholar] [CrossRef]
- Tang, K.H.D.; Kristanti, R.A. Bioremediation of perfluorochemicals: Current state and the way forward. Bioprocess Biosyst. Eng. 2022, 45, 1–17. [Google Scholar] [CrossRef]
- Eriksson, U.; Haglund, P.; Kärrman, A. Contribution of precursor compounds to the release of per-and polyfluoroalkyl substances (PFASs) from waste water treatment plants (WWTPs). J. Environ. Sci. 2017, 61, 80–90. [Google Scholar] [CrossRef]
- Wu, J.Y.; Hua, Z.L.; Gu, L. Planktonic microbial responses to perfluorinated compound (PFC) pollution: Integrating PFC distributions with community coalescence and metabolism. Sci. Total Environ. 2021, 788, 147743. [Google Scholar] [CrossRef] [PubMed]
- Presentato, A.; Lampis, S.; Vantini, A.; Manea, F.; Daprà, F.; Zuccoli, S.; Vallini, G. On the ability of perfluorohexane sulfonate (PFHxS) bioaccumulation by two Pseudomonas sp. strains isolated from PFAS-contaminated environmental matrices. Microorganisms 2020, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Ofrydopoulou, A.; Nannou, C.; Evgenidou, E.; Christodoulou, A.; Lambropoulou, D. Assessment of a wide array of organic micropollutants of emerging concern in wastewater treatment plants in Greece: Occurrence, removals, mass loading and potential risks. Sci. Total Environ. 2022, 802, 149860. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, K.; Kim, D.; Moon, H.B.; Jeon, J. Ny-Ålesund-oriented organic pollutants in sewage effluent and receiving seawater in the Arctic region of Kongsfjorden. Environ. Pollut. 2020, 258, 113792. [Google Scholar] [CrossRef]
- Lourthuraj, A.A.; Hatshan, M.R.; Hussein, D.S. Biocatalytic degradation of organophosphate pesticide from the wastewater and hydrolytic enzyme properties of consortium isolated from the pesticide contaminated water. Environ. Res. 2022, 205, 112553. [Google Scholar] [CrossRef] [PubMed]
- Petromelidou, S.; Margaritis, D.; Nannou, C.; Keramydas, C.; Lambropoulou, D.A. HRMS screening of organophosphate flame retardants and poly-perfluorinated substances in dust from cars and trucks: Occurrence and human exposure implications. Sci. Total Environ. 2022, 848, 157696. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, D.; Lee, D.J.; Varjani, S.; Lam, S.S.; Allakhverdiev, S.I.; Chang, J.S. Microalgae-based wastewater treatment–microalgae-bacteria consortia, multi-omics approaches and algal stress response. Sci. Total Environ. 2022, 845, 157110. [Google Scholar] [CrossRef] [PubMed]
- Touliabah, H.E.S.; El-Sheekh, M.M.; Ismail, M.M.; El-Kassas, H. A review of microalgae-and cyanobacteria-based biodegradation of organic pollutants. Molecules 2022, 27, 1141. [Google Scholar] [CrossRef]
- Abdel-Razek, M.A.; Abozeid, A.M.; Eltholth, M.M.; Abouelenien, F.A.; El-Midany, S.A.; Moustafa, N.Y.; Mohamed, R.A. Bioremediation of a pesticide and selected heavy metals in wastewater from various sources using a consortium of microalgae and cyanobacteria. Slov. Vet. 2019, 56, 61–73. [Google Scholar] [CrossRef]
- Jägers, J.; Wrobeln, A.; Ferenz, K.B. Perfluorocarbon-based oxygen carriers: From physics to physiology. Pflüg. Arch. Eur. J. Phy. 2021, 473, 139–150. [Google Scholar] [CrossRef]
- Senko, O.; Stepanov, N.; Tyutyunov, A.; Sterlin, S.; Grinberg, V.; Makhlis, T.; Efremenko, E. Intensification of organophosphorus hydrolase synthesis by using substances with gas-transport function. Appl.Sci. 2017, 7, 1305. [Google Scholar] [CrossRef]
- Vandermies, M.; Fickers, P. Bioreactor-scale strategies for the production of recombinant protein in the yeast Yarrowia lipolytica. Microorganisms 2019, 7, 40. [Google Scholar] [CrossRef]
- Costa Gomes, M.F.; Pádua, A.A.H. Interactions of carbon dioxide with liquid fluorocarbons. J. Phys. Chem. B. 2003, 107, 14020–14024. [Google Scholar] [CrossRef]
- Rajapitamahuni, S.; Bachani, P.; Sardar, R.K.; Mishra, S. Co-cultivation of siderophore-producing bacteria Idiomarina loihiensis RS14 with Chlorella variabilis ATCC 12198, evaluation of micro-algal growth, lipid, and protein content under iron starvation. J. Appl. Phycol. 2019, 31, 29–39. [Google Scholar] [CrossRef]
- Han, P.; Lu, Q.; Fan, L.; Zhou, W. A review on the use of microalgae for sustainable aquaculture. Appl. Sci. 2019, 9, 2377. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, G.; Wang, H.; Cheng, Y.; Liu, H.; Jiang, Z.; Li, P.; Wang, Y. Effects of different dissolved organic matter on microbial communities and arsenic mobilization in aquifers. J. Hazard. Mater. 2021, 411, 125146. [Google Scholar] [CrossRef]
- Philippot, L.; Griffiths, B.S.; Langenheder, S. Microbial community resilience across ecosystems and multiple disturbances. Microbiol. Molecul. Biol. Rev. 2021, 85, e00026-20. [Google Scholar] [CrossRef]
- Tawfik, A.; Niaz, H.; Qadeer, K.; Qyyum, M.A.; Liu, J.J.; Lee, M. Valorization of algal cells for biomass and bioenergy production from wastewater: Sustainable strategies, challenges, and techno-economic limitations. Renew. Sust. Energ. Rev. 2022, 157, 112024. [Google Scholar] [CrossRef]
- Fallahi, A.; Rezvani, F.; Asgharnejad, H.; Nazloo, E.K.; Hajinajaf, N.; Higgins, B. Interactions of microalgae-bacteria consortia for nutrient removal from wastewater: A review. Chemosphere 2021, 272, 129878. [Google Scholar] [CrossRef]
- Chai, W.S.; Tan, W.G.; Munawaroh, H.S.H.; Gupta, V.K.; Ho, S.H.; Show, P.L. Multifaceted roles of microalgae in the application of wastewater biotreatment: A review. Environ. Pollut. 2021, 269, 116236. [Google Scholar] [CrossRef]
- Razaviarani, V.; Arab, G.; Lerdwanawattana, N.; Gadia, Y. Algal biomass dual roles in phycoremediation of wastewater and production of bioenergy and value-added products. Int. J. Environ. Sci. Technol. 2022. [Google Scholar] [CrossRef]
- Efremenko, E.; Stepanov, N.; Maslova, O.; Senko, O.; Aslanli, A.; Lyagin, I. “Unity and struggle of opposites” as a basis for the functioning of synthetic bacterial immobilized consortium that continuously degrades organophosphorus pesticides. Microorganisms 2022, 10, 1394. [Google Scholar] [CrossRef] [PubMed]
- Dubber, D.; Gray, N.F. Replacement of chemical oxygen demand (COD) with total organic carbon (TOC) for monitoring wastewater treatment performance to minimize disposal of toxic analytical waste. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2010, 45, 1595–1600. [Google Scholar] [CrossRef] [PubMed]
- Efremenko, E.N.; Nikolskaya, A.B.; Mamedova, F.T.; Senko, O.V.; Trusov, L.I. Semicontinuous and continuous process of accumulation of microalgae biomass of Chlorella vulgaris cells. ISJAEE 2013, 2, 44–49. [Google Scholar]
- Bharte, S.; Krutika, D. Techniques for harvesting, cell disruption and lipid extraction of microalgae for biofuel production. Biofuels 2021, 12, 285–305. [Google Scholar] [CrossRef]
- Efremenko, E.N.; Maslova, O.V.; Kholstov, A.V.; Senko, O.V.; Ismailov, A.D. Biosensitive element in the form of immobilized luminescent photobacteria for detecting ecotoxicants in aqueous flow-through systems. Luminescence 2016, 31, 283–1289. [Google Scholar] [CrossRef]
- Yu, H.; Jia, S.; Dai, Y. Growth characteristics of the cyanobacterium Nostoc flagelliforme in photoautotrophic, mixotrophic and heterotrophic cultivation. J. Appl. Phycol. 2009, 21, 127–133. [Google Scholar] [CrossRef]
- Xie, Y.; Jin, Y.; Zeng, X.; Chen, J.; Lu, Y.; Jing, K. Fed-batch strategy for enhancing cell growth and C-phycocyanin production of Arthrospira (Spirulina) platensis under phototrophic cultivation. Bioresour. Technol. 2015, 180, 281–287. [Google Scholar] [CrossRef]
- Coronado-Reyes, J.A.; Salazar-Torres, J.A.; Juarez-Campos, B.; Gonzalez-Hernandez, J.C. Chlorella vulgaris, a microalgae important to be used in Biotechnology: A review. Food Sci. Technol. 2022, 42, e37320. [Google Scholar] [CrossRef]
- Gao, K.; Köster, A.; Thol, M.; Wu, J.; Lemmon, E.W. Equations of state for the thermodynamic properties of n-Perfluorobutane, n-Perfluoropentane, and n-Perfluorohexane. Ind. Eng. Chem. Res. 2021, 60, 17207–17227. [Google Scholar] [CrossRef]
- Menz, D.H.; Feltgen, N.; Menz, H.; Müller, B.K.; Lechner, T.; Dresp, J.; Hoerauf, H. How to ward off retinal toxicity of perfluorooctane and other perfluorocarbon liquids? Invest. Ophthalmol. Vis. Sci. 2018, 59, 4841–4846. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Ma, Q.; Cao, J.; Shi, Y.; Wang, J.; Ma, H.; Song, Y. Bifunctional alginate/chitosan stabilized perfluorohexane nanodroplets as smart vehicles for ultrasound and pH responsive delivery of anticancer agents. Int. J. Biol. Macromol. 2021, 191, 1068–1078. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yan, K.; Fu, C.; Peng, H.; Hawker, C.J.; Whittaker, A.K. Biological utility of fluorinated compounds: From materials design to molecular imaging, therapeutics and environmental remediation. Chem. Rev. 2021, 122, 167–208. [Google Scholar] [CrossRef] [PubMed]
- Efremenko, E.N. (Ed.) Immobilized Cells: Biocatalysts and Processes; RIOR: Moscow, Russia, 2018; p. 524. ISBN 978-5-369-02004-3. [Google Scholar] [CrossRef]
- Rana, M.S.; Prajapati, S.K. Stimulating effects of glycerol on the growth, phycoremediation and biofuel potential of Chlorella pyrenoidosa cultivated in wastewater. Environ. Technol. Innov. 2021, 24, 102082. [Google Scholar] [CrossRef]
- Rattanapoltee, P.; Dujjanutat, P.; Muanruksa, P.; Kaewkannetra, P. Biocircular platform for third generation biodiesel production: Batch/fed batch mixotrophic cultivations of microalgae using glycerol waste as a carbon source. Biochem. Eng. J. 2021, 175, 108128. [Google Scholar] [CrossRef]
- Zhang, B.; Sun, W.; Su, Y.; Ren, Q.; Ji, Z.; Zhang, A. Utilization of N-Acyl homoserine lactone-secreting bacteria in algal environment to increase biomass accumulation of Chlorella. BioEnergy Res. 2022, 15, 2111–2121. [Google Scholar] [CrossRef]
- Qixin, L.; Xuan, F.; Zhiya, S.; Wenxin, S.; Shuo, W.; Ji, L. Enhanced wastewater treatment performance by understanding the interaction between algae and bacteria based on quorum sensing. Bioresour. Technol. 2022, 354, 127161. [Google Scholar] [CrossRef]
- Chetverikov, S.P.; Sharipov, D.A.; Korshunova, T.Y.; Loginov, O. Degradation of perfluorooctanyl sulfonate by strain Pseudomonas plecoglossicida 2.4-D. Appl. Biochem. Microbiol. 2017, 53, 533–538. [Google Scholar] [CrossRef]
- Del Rio-Chanona, E.A.; Cong, X.; Bradford, E.; Zhang, D.; Jing, K. Review of advanced physical and data-driven models for dynamic bioprocess simulation: Case study of algae–bacteria consortium wastewater treatment. Biotechnol. Bioeng. 2019, 116, 342–353. [Google Scholar] [CrossRef]
- Rezvani, F.; Sarrafzadeh, M.H.; Oh, H.M. Hydrogen producer microalgae in interaction with hydrogen consumer denitrifiers as a novel strategy for nitrate removal from groundwater and biomass production. Algal Res. 2020, 45, 101747. [Google Scholar] [CrossRef]
- Subashchandrabose, S.R.; Venkateswarlu, K.; Venkidusamy, K.; Palanisami, T.; Naidu, R.; Megharaj, M. Bioremediation of soil long-term contaminated with PAHs by algal–bacterial synergy of Chlorella sp. MM3 and Rhodococcus wratislaviensis strain 9 in slurry phase. Sci. Total Environ. 2019, 659, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Dow, L. How do quorum-sensing signals mediate algae–bacteria interactions? Microorganisms 2021, 9, 1391. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Lens, P.N.L.; Shi, W.; Zhang, R.; Zhang, Z.; Guo, Y.; Bao, X.; Cui, F. The attachment potential and N-acyl-homoserine lactone-based quorum sensing in aerobic granular sludge and algal-bacterial granular sludge. Appl. Microbiol.Biotechnol. 2018, 102, 5343–5353. [Google Scholar] [CrossRef]
- Stock, F.; Bilcke, G.; De Decker, S.; Osuna-Cruz, C.M.; Van den Berge, K.; Vancaester, E.; De Veylder, L.; Vandepoele, K.; Mangelinckx, S.; Vyverman, W. Distinctive growth and transcriptional changes of the diatom seminavis robusta in response to quorum sensing related compounds. Front. Microbiol. 2020, 11, 1240. [Google Scholar] [CrossRef]
- Zhou, D.; Zhang, C.; Fu, L.; Xu, L.; Gui, X.; Li, Q.; Crittenden, J.C. Responses of the microalga Chlorophyta sp. to bacterial quorum sensing molecules (N-acylhomoserine lactones): Aromatic protein-induced self-aggregation. Environ. Sci. Technol. 2017, 51, 3490–3498. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, W.; Guo, Y.; Zhang, Z.; Shi, W.; Gui, F.; Lens, P.N.L.; Tay, J.H. Microalgal-bacterial consortia: From interspecies interactions to biotechnological applications. Renew. Sustain. Energy Rev. 2020, 118, 109563. [Google Scholar] [CrossRef]
- Efremenko, E.; Senko, O.; Stepanov, N.; Maslova, O.; Lomakina, G.Y.; Ugarova, N. Luminescent analysis of ATP: Modern objects and processes for sensing. Chemosensors 2022, 10, 493. [Google Scholar] [CrossRef]
- Berman, M.C.; O’Farrell, I.; Huber, P.; Marino, D.; Zagarese, H. A large-scale geographical coverage survey reveals a pervasive impact of agricultural practices on plankton primary producers. Agric. Ecosyst. Environ. 2022, 325, 107740. [Google Scholar] [CrossRef]
- Verasoundarapandian, G.; Lim, Z.S.; Radziff, S.B.M.; Taufik, S.H.; Puasa, N.A.; Shaharuddin, N.A.; Merican, F.; Wong, C.-Y.; Lalung, J.; Ahmad, S.A. Remediation of pesticides by microalgae as feasible approach in agriculture: Bibliometric strategies. Agronomy 2022, 12, 117. [Google Scholar] [CrossRef]
- Goh, P.S.; Lau, W.J.; Ismail, A.F.; Samawati, Z.; Liang, Y.Y.; Kanakaraju, D. Microalgae-enabled wastewater treatment: A sustainable strategy for bioremediation of pesticides. Water 2023, 15, 70. [Google Scholar] [CrossRef]
- Nicodemus, T.J.; DiRusso, C.C.; Wilson, M.; Black, P.N. Reactive oxygen species (ROS) mediated degradation of organophosphate pesticides by the green microalgae Coccomyxa subellipsoidea. Bioresour. Technol. Rep. 2020, 11, 100461. [Google Scholar] [CrossRef]
- Llorca, M.; Farré, M.; Sànchez-Melsió, A.; Villagrasa, M.; Knepper, T.P.; Barceló, D. Perfluoroalkyl phosphonic acids adsorption behaviour and removal by wastewater organisms. Sci. Total Environ. 2018, 636, 273–281. [Google Scholar] [CrossRef] [PubMed]
| Microalgae | PFC (% v/v) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Control ** | Perfluorohexane | Polyether I | Perfluorodecalin | Polyether II | |||||
| 0 | 0.5 | 1 | 0.5 | 1 | 0.5 | 1 | 0.5 | 1 | |
| C. vulgaris | 33 ± 1 | 22 ± 1 | 24 ± 1 | 18 ± 0.9 | 26 ± 1 | 30 ± 1 | 24 ± 1 | 26 ± 1 | 22 ± 1 |
| C. vulgaris *** | 57 ± 2 | 32 ± 1 | 57 ± 2 | 37 ± 1 | 66 ± 3 | 57 ± 2 | 55 ± 2 | 62 ± 3 | 89 ± 4 |
| Arthrospira platensis | 27 ± 1 | 17 ± 1 | 18 ± 1 | 15 ± 1 | 21 ± 1 | 26 ± 1 | 20 ± 1 | 20 ± 1 | 18 ± 1 |
| Nostoc sp. | 63 ± 3 | 40 ± 2 | 41 ± 2 | 33 ± 1 | 50 ± 2 | 60 ± 3 | 44 ± 2 | 48 ± 2 | 42 ± 2 |
| Studied Parameter | Biomass Ratio “Microalgae:Bacteria” | |||||
|---|---|---|---|---|---|---|
| 6:1 | 12:1 | 25:1 | 6:1 | 12:1 | 25:1 | |
| Perfluorohexane | Polyether II | |||||
| 0% v/v | ||||||
| ATPin granules (nmol/g) | 0.4 ± 0.0 | 0.8 ± 0.0 | 15.2 ± 0.7 | 0.4 ± 0.0 | 0.8 ± 0.0 | 15.2 ± 0.7 |
| ATPin medium (nmol/mL) | 33 ± 0.1 | 37 ± 1 | 80 ± 3 | 33 ± 0.1 | 37 ± 1 | 80 ± 3 |
| The average rate of biomass accumulation (g DCW/L/d) | 0.25 ± 0.0 | 0.35 ± 0.0 | 0.41 ± 0.0 | 0.25 ± 0.0 | 0.35 ± 0.0 | 0.41 ± 0.0 |
| 0.5% v/v | ||||||
| ATPin granules (nmol/g) * | 0.8 ± 0.0 | 4.3 ± 0.2 | 17.7 ± 0.9 | 0.8 ± 0.0 | 4.32 ± 0.21 | 17.7 ± 0.9 |
| ATPin medium (nmol/mL) | 34 ± 1 | 38 ± 1 | 87 ± 4 | 35 ± 1 | 38 ± 1 | 88 ± 4 |
| The average rate of biomass accumulation (g DCW/L/d) | 0.32 ± 0.01 | 0.36 ± 0.01 | 0.44 ± 0.02 | 0.3 ± 0.01 | 0.35 ± 0.01 | 0.48 ± 0.02 |
| 1% v/v | ||||||
| ATPin granules (nmol/g) * | 1.5 ± 0.1 | 2.5 ± 0.1 | 19.8 ± 0.9 | 1.6 ± 0.1 | 2.5 ± 0.11 | 22.7 ± 0.8 |
| ATPin medium (nmol/mL) | 34 ± 1 | 38 ± 1 | 89 ± 4 | 35 ± 1 | 40 ± 2 | 90 ± 4 |
| The average rate of biomass accumulation (g DCW/L/d) | 0.36 ± 0.01 | 0.38 ± 0.01 | 0.53 ± 0.02 | 0.37 ± 0.01 | 0.37 ± 0.01 | 0.54 ± 0.02 |
| PFC (% v/v) | Biomass Ratio “Microalgae:Bacteria” | |||||
|---|---|---|---|---|---|---|
| 6:1 | 12:1 | 25:1 | 6:1 | 12:1 | 25:1 | |
| Perfluorohexane | Polyether II | |||||
| 0 | 78.1 ± 3.7 | 89.1 ± 4.1 | 92.7 ± 4.3 | 80.4 ± 2.5 | 95.1 ± 4.1 | 95.7 ± 3.3 |
| 0.5 | 6.8 ± 0.3 | 8.0 ± 0.3 | 22.4 ± 1.1 | 6.4 ± 0.2 | 8.3 ± 0.3 | 20.4 ± 1.1 |
| 1 | 3.0 ± 0.1 | 6.0 ± 0.2 | 15.0 ± 1.7 | 2.0 ± 0.1 | 5.0 ± 0.1 | 14.0 ± 1.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senko, O.; Maslova, O.; Aslanli, A.; Efremenko, E. Impact of Perfluorocarbons with Gas Transport Function on Growth of Phototrophic Microorganisms in a Free and Immobilized State and in Consortia with Bacteria. Appl. Sci. 2023, 13, 1868. https://doi.org/10.3390/app13031868
Senko O, Maslova O, Aslanli A, Efremenko E. Impact of Perfluorocarbons with Gas Transport Function on Growth of Phototrophic Microorganisms in a Free and Immobilized State and in Consortia with Bacteria. Applied Sciences. 2023; 13(3):1868. https://doi.org/10.3390/app13031868
Chicago/Turabian StyleSenko, Olga, Olga Maslova, Aysel Aslanli, and Elena Efremenko. 2023. "Impact of Perfluorocarbons with Gas Transport Function on Growth of Phototrophic Microorganisms in a Free and Immobilized State and in Consortia with Bacteria" Applied Sciences 13, no. 3: 1868. https://doi.org/10.3390/app13031868
APA StyleSenko, O., Maslova, O., Aslanli, A., & Efremenko, E. (2023). Impact of Perfluorocarbons with Gas Transport Function on Growth of Phototrophic Microorganisms in a Free and Immobilized State and in Consortia with Bacteria. Applied Sciences, 13(3), 1868. https://doi.org/10.3390/app13031868






