Modelling Nannochloropsis gaditana Growth in Reactors with Different Geometries, Determination of Kinetic Parameters and Biochemical Analysis in Response to Light Intensity
Abstract
:1. Introduction
2. Mathematical Modelling
2.1. Flat-Plate Geometry
2.2. Annular Geometry
3. Materials and Methods
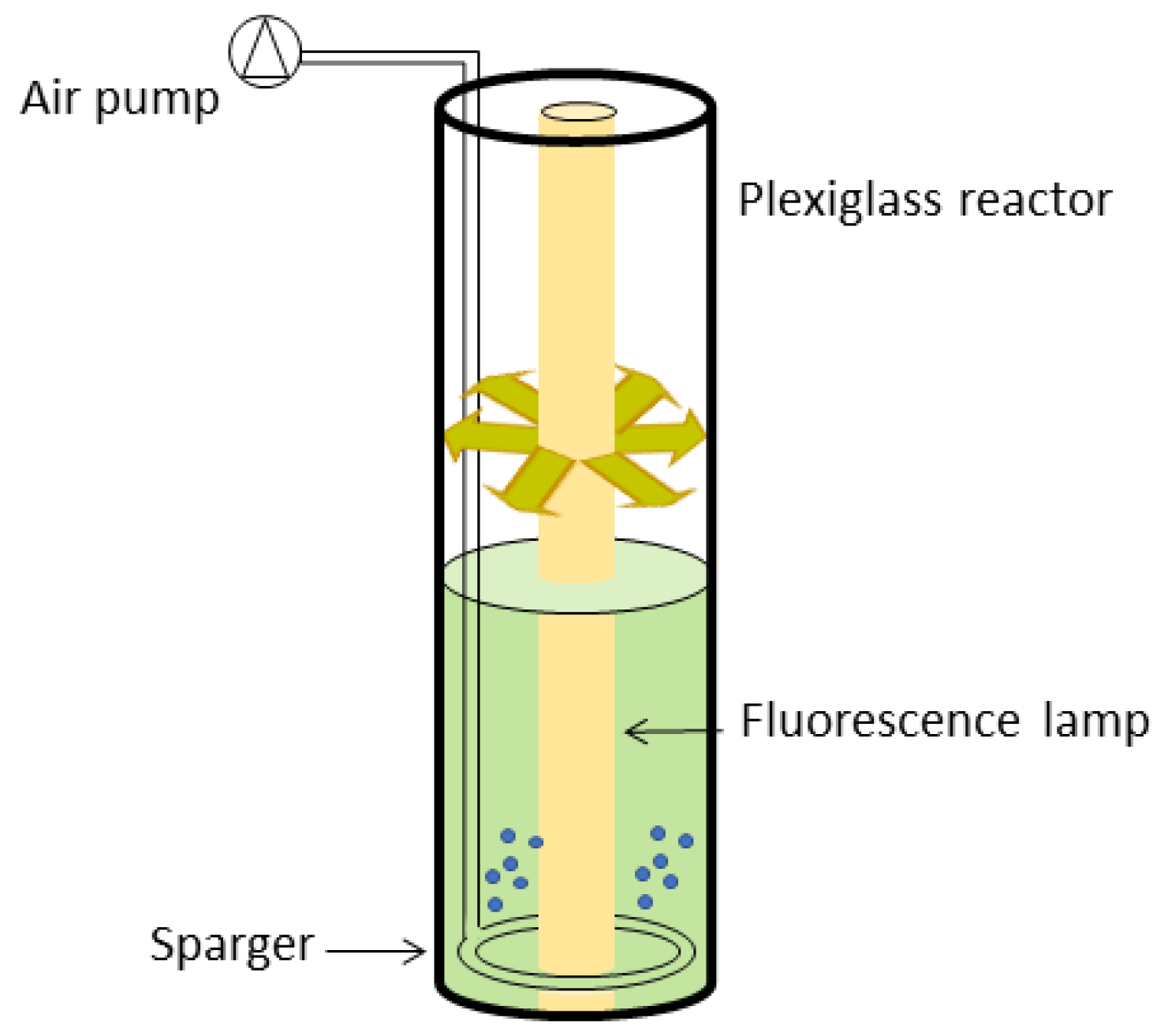
3.1. Quasi-Isoactinic Photobioreactor
3.2. Flat-Plate Photobioreactors
3.3. Annular Column Photobioreactor
3.4. Algal Growth
3.5. Harvesting of Microalgal Biomass
3.6. Extraction and Analysis of Fatty Acids
3.7. Total Carbohydrates
3.8. Total Proteins
3.9. Carotenoids
4. Results and Discussion
4.1. Kinetic Parameters
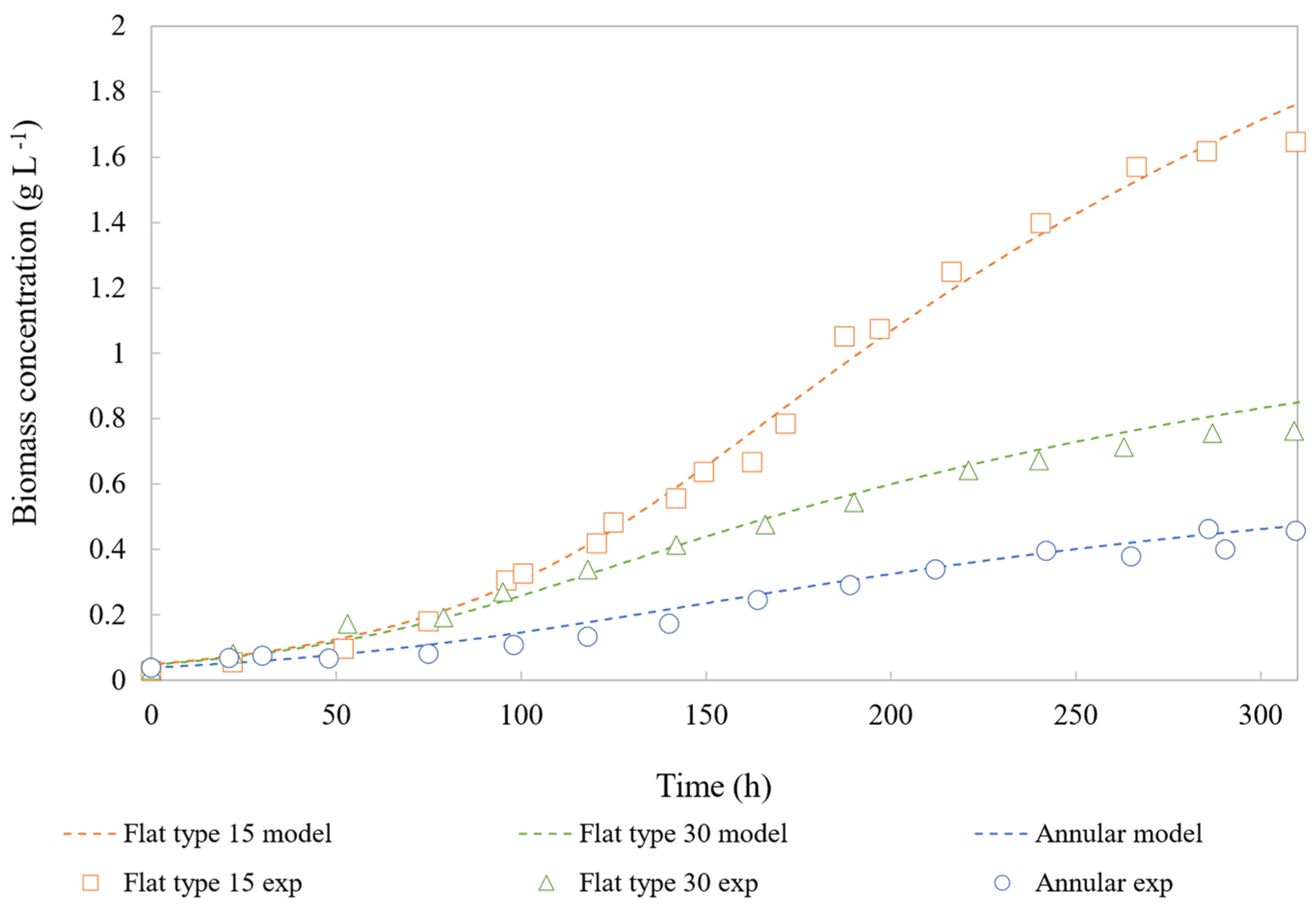
4.2. Model Validation
4.3. Biochemical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Con sent Statement
Conflicts of Interest
Nomenclature
| I = local light intensity, µE s−1m−2 | µ = growth rate, h−1 |
| I0 = incident light intensity, µE s−1m−2 | µmax = maximum growth rate, h−1 |
| ka = attenuation constant, m2 g−1 | Ik = light saturation constant, µE s−1m−2 |
| X = biomass concentration, g L−1 | V = volume of the reactor, m3 |
| z = distance from the surface to the considered position, m | A = enlightened surface of the reactor, m2 |
| Acapt = area of the cell capturing light, m2 | L = depth of the reactor, m |
| dcapt = diameter of the transversal section of light capture, m | kd = constant of cellular death, h−1 |
| dcell = cell diameter, m | rx = rate of biomass production, g m−3 h−1 |
| ρcell = cell density, g m−3 | r = radius of the annular section, m |
| h = height of the reactor, m |
References
- Richmond, A.; Hu, Q. (Eds.) Handbook of Microalgal Culture; John Wiley & Sons, Ltd.: Oxford, UK, 2013; ISBN 9781118567166. [Google Scholar]
- Kitada, K.; Machmudah, S.; Sasaki, M.; Goto, M.; Nakashima, Y.; Kumamoto, S.; Hasegawa, T. Supercritical CO2 Extraction of Pigment Components with Pharmaceutical Importance from Chlorella Vulgaris. J. Chem. Technol. Biotechnol. 2009, 84, 657–661. [Google Scholar] [CrossRef]
- Lima, S.; Villanova, V.; Grisafi, F.; Caputo, G.; Brucato, A.; Scargiali, F. Autochthonous Microalgae Grown in Municipal Wastewaters as a Tool for Effectively Removing Nitrogen and Phosphorous. J. Water Process Eng. 2020, 38, 101647. [Google Scholar] [CrossRef]
- Sanchez, A.; Gonzalez, A.; Maceiras, R.; Cancela, A.; Urrejola, S. Raceway Pond Design for Micoalgae Culture for Biodiesel. Chem. Eng. Trans. 2011, 25, 845–850. [Google Scholar] [CrossRef]
- Slade, R.; Bauen, A. Micro-Algae Cultivation for Biofuels: Cost, Energy Balance, Environmental Impacts and Future Prospects. Biomass Bioenergy 2013, 53, 29–38. [Google Scholar] [CrossRef] [Green Version]
- Molina, E.; Fernández, J.; Acién, F.G.; Chisti, Y. Tubular Photobioreactor Design for Algal Cultures. J. Biotechnol. 2001, 92, 113–131. [Google Scholar] [CrossRef]
- Fernandes, B.D.; Mota, A.; Ferreira, A.; Dragone, G.; Teixeira, J.A.; Vicente, A.A. Characterization of Split Cylinder Airlift Photobioreactors for Efficient Microalgae Cultivation. Chem. Eng. Sci. 2014, 117, 445–454. [Google Scholar] [CrossRef] [Green Version]
- Simionato, D.; Basso, S.; Giacometti, G.M.; Morosinotto, T. Optimization of Light Use Efficiency for Biofuel Production in Algae. Biophys. Chem. 2013, 182, 71–78. [Google Scholar] [CrossRef]
- Sabri, L.S.; Ojha, A.; Sultan, A.J.; Aldahhan, M.H. Integration of Dynamic Growth Modeling and Hydrodynamics in an Internal-loop Split Photobioreactor. J. Chem. Technol. Biotechnol. 2022, 97, 1112–1127. [Google Scholar] [CrossRef]
- Ojah, A.; Sabri, L.S.; Aldahhan, M.H. Local Volumetric Mass Transfer Coefficient Estimation for Scenedesmus Microalgae Culture in a Cylindrical Airlift Photobioreactor. J. Chem. Technol. Biotechnol. 2021, 96, 764–774. [Google Scholar] [CrossRef]
- Béchet, Q.; Shilton, A.; Guieysse, B. Modeling the Effects of Light and Temperature on Algae Growth: State of the Art and Critical Assessment for Productivity Prediction during Outdoor Cultivation. Biotechnol. Adv. 2013, 31, 1648–1663. [Google Scholar] [CrossRef] [PubMed]
- Lima, S.; Grisafi, F.; Scargiali, F.; Caputo, G.; Brucato, A. Growing Microalgae in a “Quasi-Isoactinic” Photobioreactor. Chem. Eng. Trans. 2018, 64, 673–678. [Google Scholar] [CrossRef]
- Li, S.; Huang, J.; Ji, L.; Chen, C.; Wu, P.; Zhang, W.; Tan, G.; Wu, H.; Fan, J. Assessment of Light Distribution Model for Marine Red Microalga Porphyridium Purpureum for Sustainable Production in Photobioreactor. Algal Res. 2021, 58, 102390. [Google Scholar] [CrossRef]
- Pfaffinger, C.E.; Schöne, D.; Trunz, S.; Löwe, H.; Weuster-Botz, D. Model-Based Optimization of Microalgae Areal Productivity in Flat-Plate Gas-Lift Photobioreactors. Algal Res. 2016, 20, 153–163. [Google Scholar] [CrossRef]
- Zittelli, G.C.; Rodolfi, L.; Tredici, M.R. Mass cultivation of Nannochloropsis sp. in annular reactors. J. Appl. Phycol. 2003, 15, 107–114. [Google Scholar] [CrossRef]
- Ranglová, K.; Bureš, M.; Manoel, J.C. Efficient microalgae feed production for fish hatcheries using an annular column photobioreactor characterized by a short light path and central LED illumination. J. Appl. Phycol. 2022, 34, 31–41. [Google Scholar] [CrossRef]
- Guillard, R.R.L. Culture of Phytoplankton for Feeding Marine Invertebrates. In Culture of Marine Invertebrate Animals; Smith, W.L., Chanley, M.H., Eds.; Springer: Boston, MA, USA, 1975; pp. 29–60. ISBN 978-1-4615-8714-9. [Google Scholar]
- Trevelyan, W.E.; Forrest, R.S.; Harrison, J.S. Determination of Yeast Carbohydrates with the Anthrone Reagent. Nature 1952, 170, 626–627. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of Total Carotenoids and Chlorophylls a and b of Leaf Extracts in Different Solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef] [Green Version]
- Doran, P.M. Bioprocess Engineering Principles, 2nd ed.; Academic Press: London, UK, 2013; ISBN 978-0-12-220851-5. [Google Scholar]
- Simionato, D.; Sforza, E.; Corteggiani Carpinelli, E.; Bertucco, A.; Giacometti, G.M.; Morosinotto, T. Acclimation of Nannochloropsis gaditana to Different Illumination Regimes: Effects on Lipids Accumulation. Bioresour. Technol. 2011, 102, 6026–6032. [Google Scholar] [CrossRef]
- Lima, S.; Villanova, V.; Grisafi, F.; Brucato, A.; Scargiali, F. Combined Effect of Nutrient and Flashing Light Frequency for a Biochemical Composition Shift in Nannochloropsis gaditana Grown in a Quasi-isoactinic Reactor. Can. J. Chem. Eng. 2020, 98, 1944–1954. [Google Scholar] [CrossRef]
- Polishchuk, A.; Valev, D.; Tarvainen, M.; Mishra, S.; Kinnunen, V.; Antal, T.; Yang, B.; Rintala, J.; Tyystjärvi, E. Cultivation of Nannochloropsis for Eicosapentaenoic Acid Production in Wastewaters of Pulp and Paper Industry. Bioresour. Technol. 2015, 193, 469–476. [Google Scholar] [CrossRef]
- Huesemann, M.; Crowe, B.; Waller, P.; Chavis, A.; Hobbs, S.; Edmundson, S.; Wigmosta, M. A Validated Model to Predict Microalgae Growth in Outdoor Pond Cultures Subjected to Fluctuating Light Intensities and Water Temperatures. Algal Res. 2016, 13, 195–206. [Google Scholar] [CrossRef] [Green Version]
- Gharat, K.; Agarwal, A.; Pandit, R.A.; Lali, A.M. Development of Fed Batch Strategies to Improve the Production of Eicosapentaenoic Acid from a Marine Microalga Nannochloropsis oculata. Bioresour. Technol. Rep. 2018, 4, 193–201. [Google Scholar] [CrossRef]
- Devasya, R.R.P.; Bassi, A.S. Effect of Nitrate Feeding Strategies on Lipid and Biomass Productivities in Fed-Batch Cultures of Nannochloropsis gaditana. Biotechnol. Prog. 2021, 37, e3120. [Google Scholar] [CrossRef] [PubMed]
- Lima, S.; Schulze, P.S.C.; Schüler, L.M.; Rautenberger, R.; Morales-Sánchez, D.; Santos, T.F.; Pereira, H.; Varela, J.C.S.; Scargiali, F.; Wijffels, R.H.; et al. Flashing Light Emitting Diodes (LEDs) Induce Proteins, Polyunsaturated Fatty Acids and Pigments in Three Microalgae. J. Biotechnol. 2021, 325, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Rodolfi, L.; Chini Zittelli, G.; Bassi, N.; Padovani, G.; Biondi, N.; Bonini, G.; Tredici, M.R. Microalgae for Oil: Strain Selection, Induction of Lipid Synthesis and Outdoor Mass Cultivation in a Low-Cost Photobioreactor. Biotechnol. Bioeng. 2009, 102, 100–112. [Google Scholar] [CrossRef]
- Pal, D.; Khozin-Goldberg, I.; Cohen, Z.; Boussiba, S. The Effect of Light, Salinity, and Nitrogen Availability on Lipid Production by Nannochloropsis sp. Appl. Microbiol. Biotechnol. 2011, 90, 1429–1441. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.-N.; Chen, T.-P.; Yang, B.; Liu, J.; Chen, F. Lipid Production from Nannochloropsis. Mar. Drugs 2016, 14, 61. [Google Scholar] [CrossRef] [Green Version]
- Renaud, S.M.; Parry, D.L.; Thinh, L.-V.; Kuo, C.; Padovan, A.; Sammy, N. Effect of Light Intensity on the Proximate Biochemical and Fatty Acid Composition of Isochrysis Sp. and Nannochloropsis oculata for Use in Tropical Aquaculture. J. Appl. Phycol. 1991, 3, 43–53. [Google Scholar] [CrossRef]
- He, Q.; Yang, H.; Wu, L.; Hu, C. Effect of Light Intensity on Physiological Changes, Carbon Allocation and Neutral Lipid Accumulation in Oleaginous Microalgae. Bioresour. Technol. 2015, 191, 219–228. [Google Scholar] [CrossRef]
- Wang, B.; Jia, J. Photoprotection Mechanisms of Nannochloropsis oceanica in Response to Light Stress. Algal Res. 2020, 46, 101784. [Google Scholar] [CrossRef]
- Arena, R.; Lima, S.; Villanova, V.; Moukri, N.; Curcuraci, F.; Messina, C.; Santulli, A.; Scargiali, F. Cultivation and biochemical characterization of isolated Sicilian microalgal species in salt and temperature stress conditions. Algal Res. 2021, 59, 102430. [Google Scholar] [CrossRef]



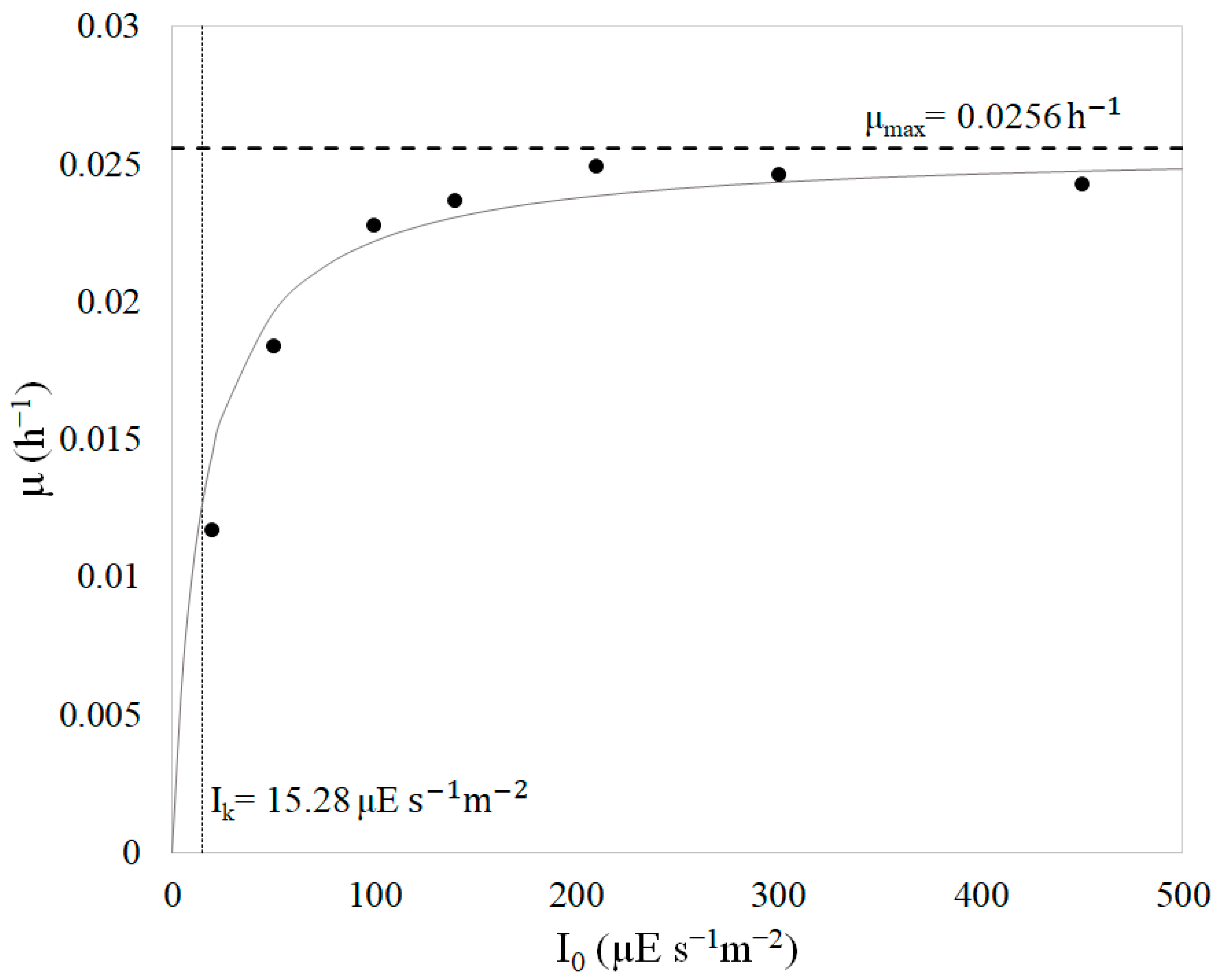
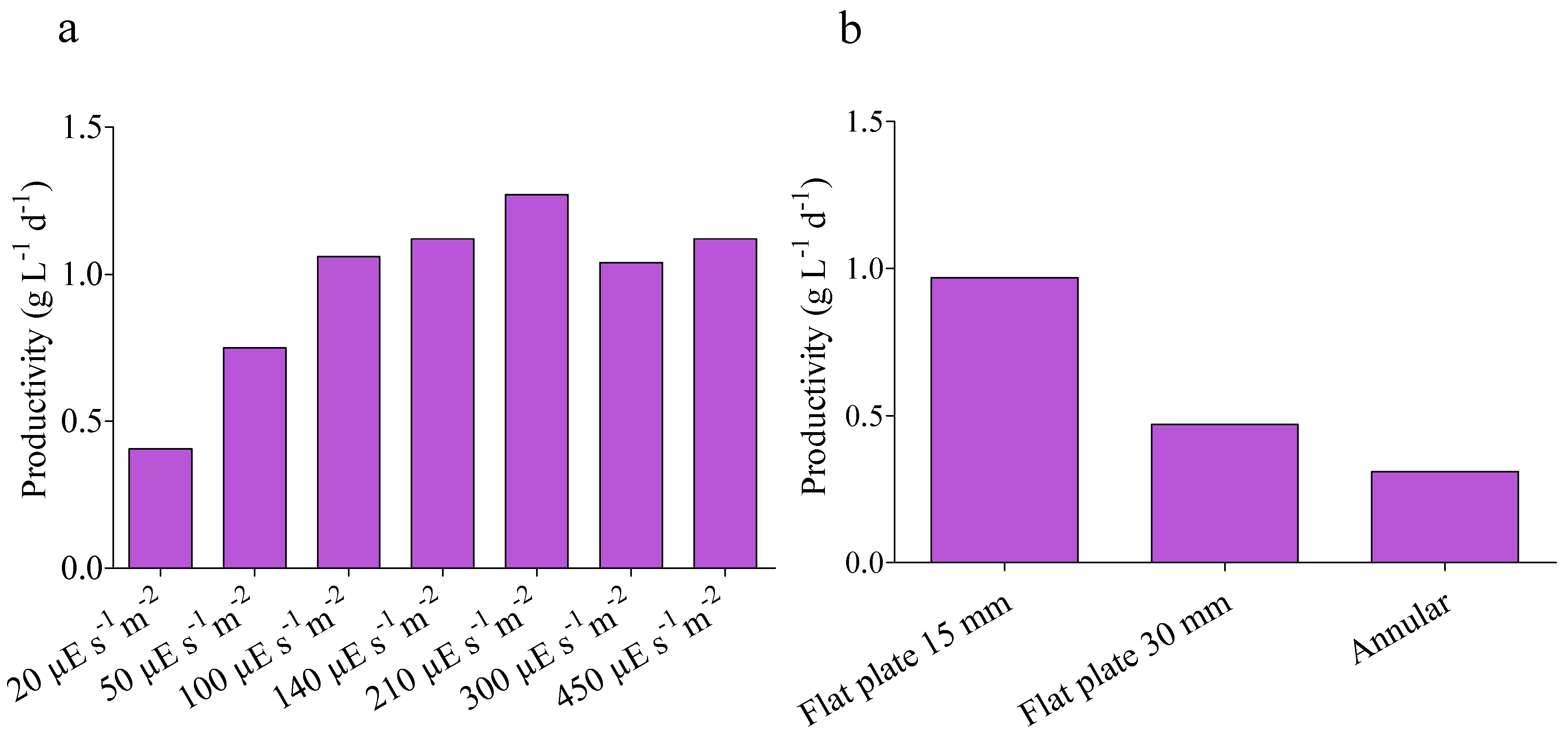
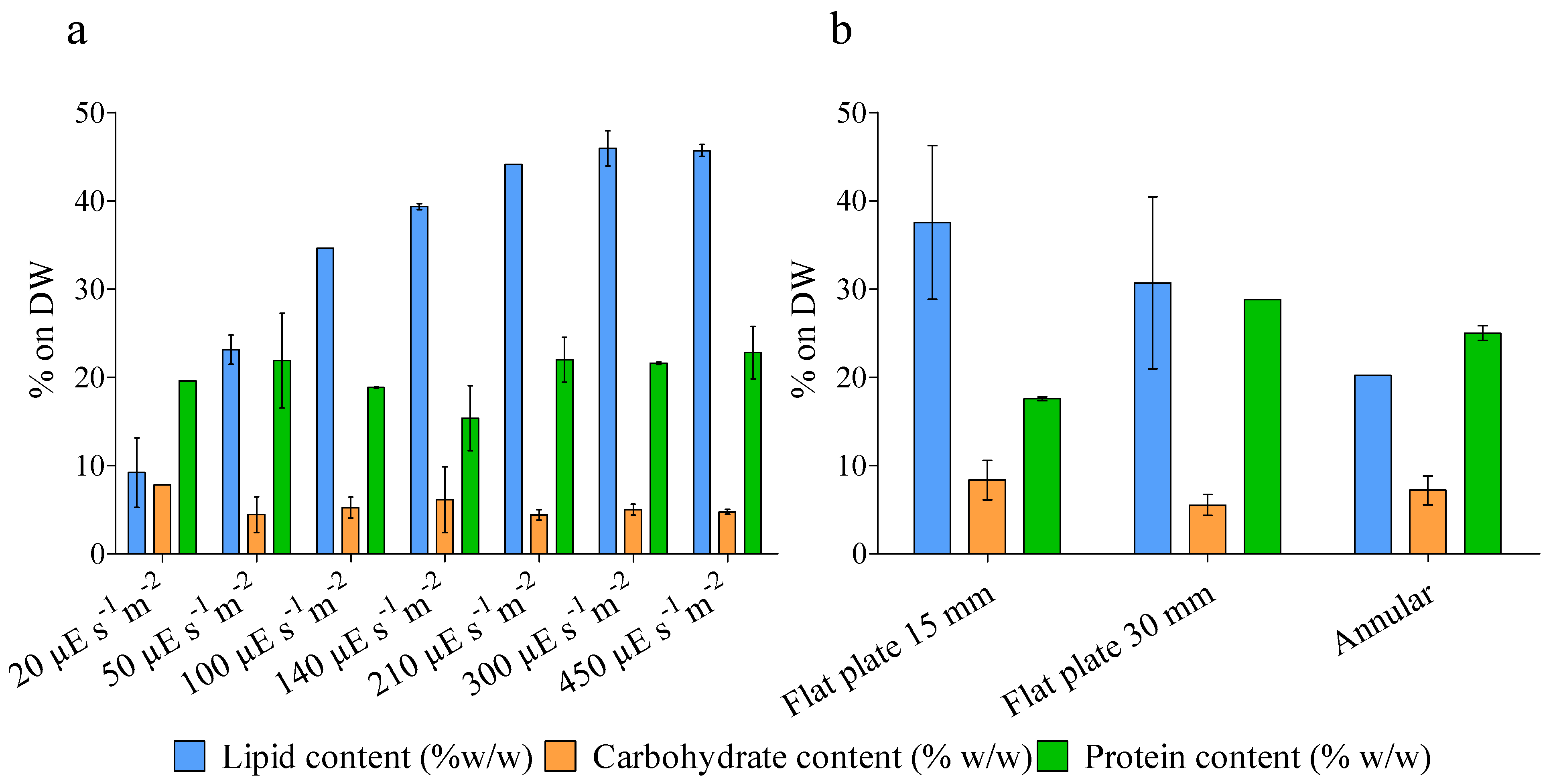
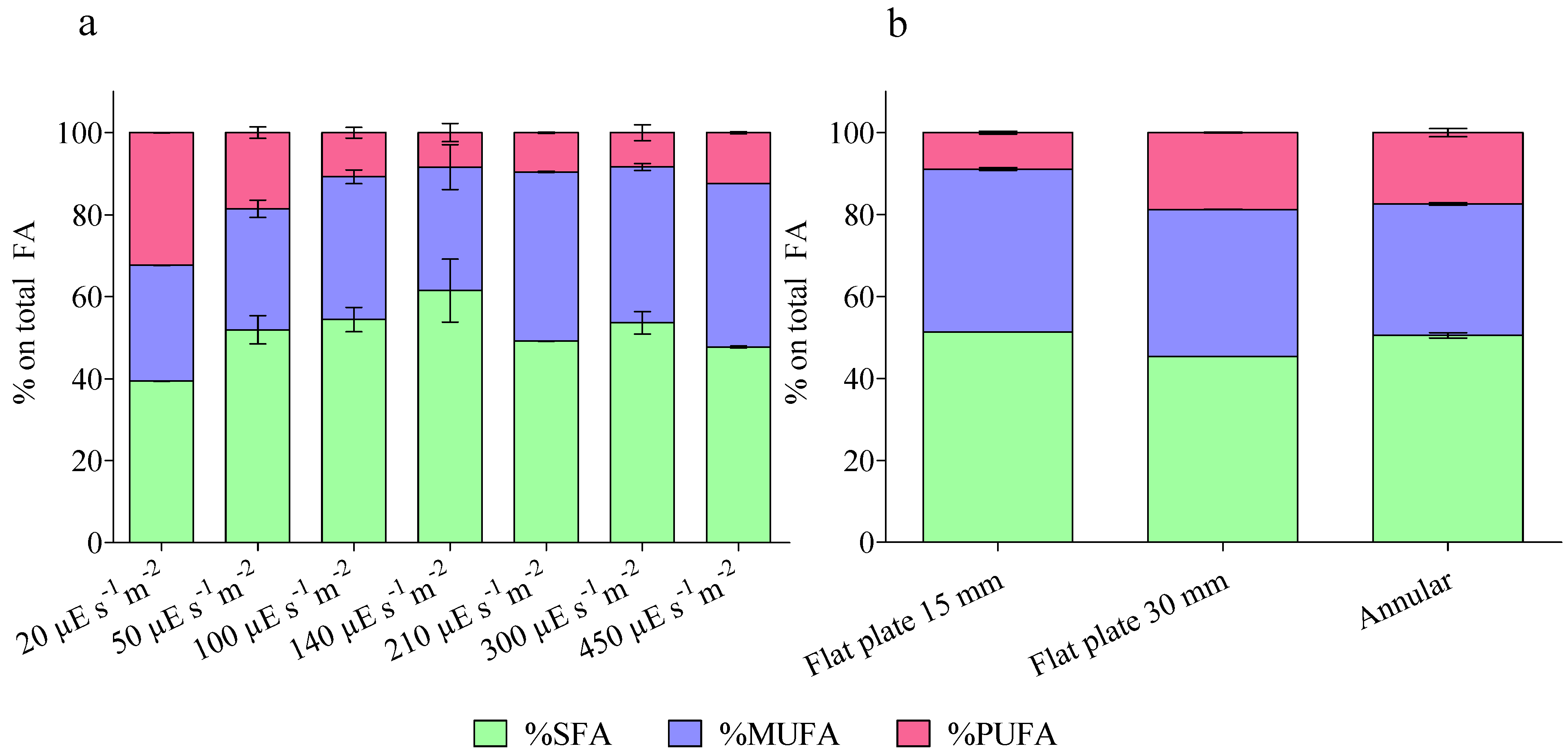
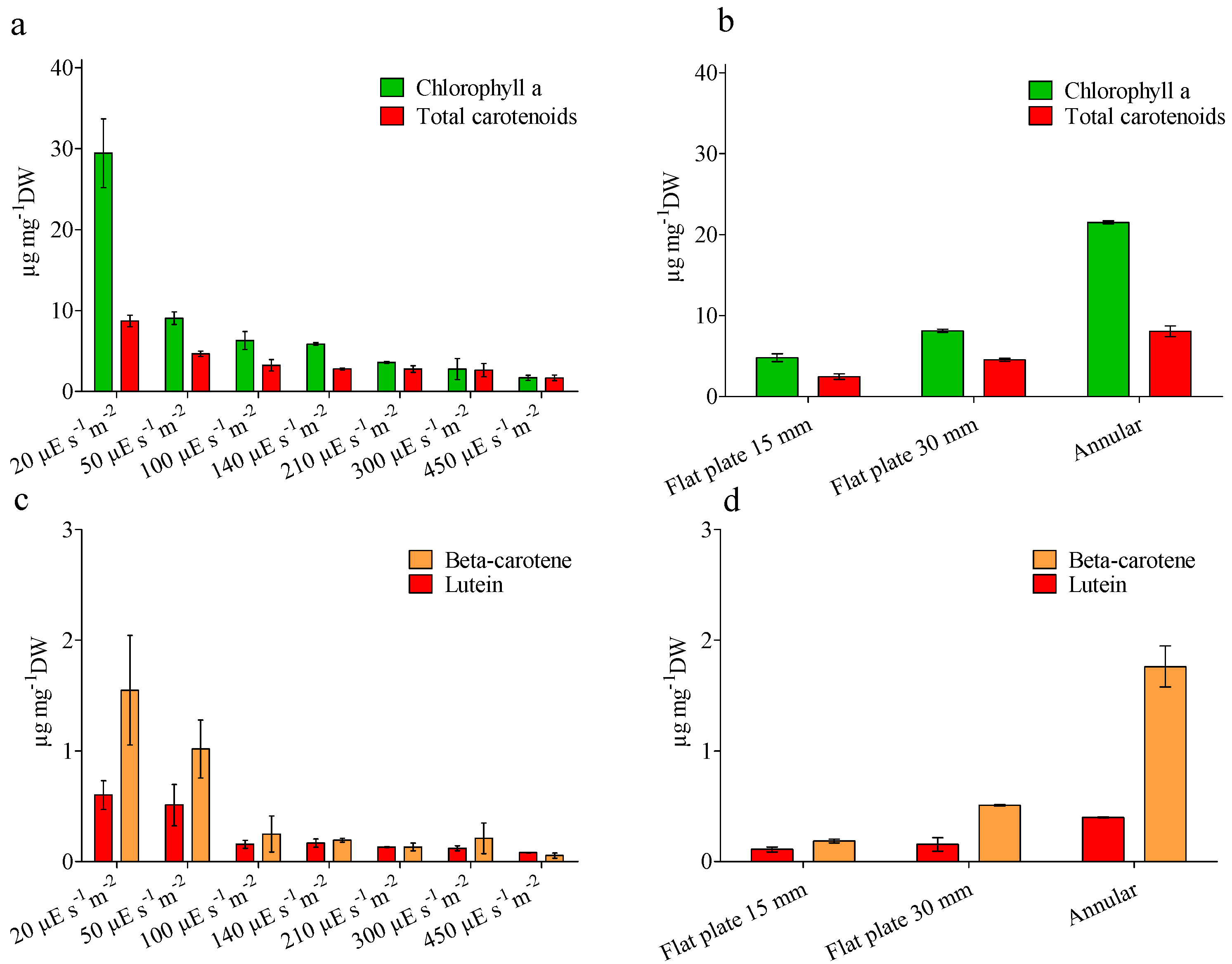
| I0 (µE s−1m−2) | µ (h−1) | I0/µ (µE h s−1m−2) | R2 |
|---|---|---|---|
| 20 | 0.0117 | 1709 | 0.9956 |
| 50 | 0.0184 | 2717 | 0.9912 |
| 100 | 0.0228 | 4386 | 0.9817 |
| 140 | 0.0237 | 5907 | 0.9976 |
| 210 | 0.0249 | 8434 | 0.9891 |
| 300 | 0.0246 | 12195 | 0.9859 |
| 450 | 0.0243 | 18519 | 0.9988 |
| Parameter | Value | Unit | ||
|---|---|---|---|---|
| Flat type 15 | Flat type 30 | Annular | ||
| L | 15 | 30 | 35.5 | mm |
| µmax | 0.0256 | 0.0256 | 0.0256 | h−1 |
| IK | 15.28 | 15.28 | 15.28 | µE s−1m−2 |
| kd | 0.0046 | 0.0046 | 0.0046 | h−1 |
| ka | 0.38 | 0.38 | 0.20 | m2 g−1 |
| 20 µE s−1m−2 | 50 µE s−1m−2 | 100 µE s−1m−2 | 140 µE s−1m−2 | 210 µE s−1m−2 | 300 µE s−1m−2 | 450 µE s−1m−2 | 15 mm | 30 mm | Annular | |
|---|---|---|---|---|---|---|---|---|---|---|
| % of n-3 on TFAs | 29 ± 0.1 | 15.9 ± 0.9 | 7 ± 0.3 | 6 ± 1.6 | 6.1 ± 1.6 | 8.3 ± 0.3 | 5.9 ± 0.3 | 6.4 ± 0.1 | 15.6 ± 0.9 | 12.7 ± 0 |
| % of n-6 on TFAs | 3.4 ± 0.1 | 2.7 ± 0.5 | 3.2 ± 0.8 | 2.4 ± 0.6 | 2.3 ± 0.3 | 4.1 ± 0 | 3.6 ± 0.1 | 2.5 ± 0 | 3.1 ± 0 | 4.6 ± 0 |
| n3/n6 ratio | 8.5 ± 0.2 | 6 ± 0.8 | 2.3 ± 0.6 | 2.4 ± 0.1 | 2.6 ± 0.4 | 2 ± 0.1 | 1.6 ± 0 | 2.5 ± 0 | 5 ± 0.2 | 2.7 ± 0 |
| % of C20:5 n-3 (EPA) on TFAs | 26 ± 0.1 | 13.3 ± 0.5 | 4.9 ± 0.3 | 4 ± 1.1 | 4.2 ± 1 | 5.6 ± 0.2 | 0 ± 0 | 4.4 ± 0 | 10.4 ± 0.6 | 9 ± 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, S.; Brucato, A.; Caputo, G.; Schembri, L.; Scargiali, F. Modelling Nannochloropsis gaditana Growth in Reactors with Different Geometries, Determination of Kinetic Parameters and Biochemical Analysis in Response to Light Intensity. Appl. Sci. 2022, 12, 5776. https://doi.org/10.3390/app12125776
Lima S, Brucato A, Caputo G, Schembri L, Scargiali F. Modelling Nannochloropsis gaditana Growth in Reactors with Different Geometries, Determination of Kinetic Parameters and Biochemical Analysis in Response to Light Intensity. Applied Sciences. 2022; 12(12):5776. https://doi.org/10.3390/app12125776
Chicago/Turabian StyleLima, Serena, Alberto Brucato, Giuseppe Caputo, Luca Schembri, and Francesca Scargiali. 2022. "Modelling Nannochloropsis gaditana Growth in Reactors with Different Geometries, Determination of Kinetic Parameters and Biochemical Analysis in Response to Light Intensity" Applied Sciences 12, no. 12: 5776. https://doi.org/10.3390/app12125776
APA StyleLima, S., Brucato, A., Caputo, G., Schembri, L., & Scargiali, F. (2022). Modelling Nannochloropsis gaditana Growth in Reactors with Different Geometries, Determination of Kinetic Parameters and Biochemical Analysis in Response to Light Intensity. Applied Sciences, 12(12), 5776. https://doi.org/10.3390/app12125776









