Abstract
Ulcerative colitis is a chronic inflammatory bowel disease characterized by colonic mucosal inflammation, intestinal microflora imbalance, and intestinal permeability. It is essential to develop natural compounds with anti-inflammatory and intestinal bacterial imbalance correction properties. The brown alga Sargassum horneri is rich in polyphenols, such as fucoxanthin and chromene, which have antioxidant, anti-inflammatory, and anti-cancer properties. In results, S. horneri ethanol extract (SHE) reduced TNF-α and IL-6 levels as well as Pi3k/Mtor/S6k mRNA expression in LPS-treated RAW264.7 and Caco-2 cells. In addition, SHE treatment decreased the expression of genes associated with inflammation and the mTOR axis in the co-culture system while increasing the expression of tight junction factors. In a mouse model of dextran sulfate sodium (DSS)-induced colitis, SHE treatment improved intestinal length, histological scores, and the expression of genes related to tight junctions while decreasing the expression of genes related to inflammatory markers and the mTOR axis. The gut microbiota of mice treated with SHE exhibited a decrease in the ratio of Firmicutes to Bacteroidota, which had been increased by DSS treatment and an increase in beneficial bacteria. Therefore, SHE consumption may be a useful natural alternative, as it improves gut microbiota, alleviates colitis symptoms, and prevents their onset.
1. Introduction
Inflammatory bowel disease (IBD) is a chronic inflammatory disease characterized by diarrhea, bloody stools, and abdominal pain [1]. There are two major types of IBD, namely Crohn’s disease (CD) and ulcerative colitis (UC), which are correlated to the gut microbiota due to a combination of genetic, immunological, and environmental factors [2]. Chronic idiopathic IBD of the colon, also known as UC, is characterized by varying degrees of superficial mucosal inflammation that extends continuously from the rectum to the proximal colon intestine and is associated with an increased risk of colorectal cancer [3,4]. The most common treatment for UC is 5-aminosalicylate, while acute patients are administered methylprednisolone or hydrocortisone [5]. In addition to renal and hepatic toxicity, these medications can cause side effects, such as nausea, fever, and gastrointestinal issues [6]. Therefore, it is essential to develop natural anti-inflammatory substances without side effects.
Seaweeds are commonly consumed and used medicinally in East Asia due to their anti-inflammatory, anti-cancer, hepatoprotective, anti-diabetic, and antihypertensive qualities [7,8]. Numerous bioactive substances, including vitamins, minerals, flavonoids, phenolic compounds, and polysaccharides, have been found in seaweed [9]. Sargassum horneri, a member of the family Sargassaceae, is a perennial brown alga that grows beneath reefs in the ocean and is primarily found along Asian coastlines [10,11]. Currently, active research is being conducted on S. horneri, which has been found to have anti-inflammatory, anti-tumor, and antioxidant properties [7,10,12]. S. horneri has been reported to contain polysaccharides, such as fucoidan; and polyphenols, including gallic acid, catechin, chlorogenic acid, and quercetin; chromenes; and carotenoids, such as fucoxanthin [7,11]. Fucoxanthin, a carotenoid derived from brown seaweeds, possesses various health-promoting properties, including anti-inflammatory, anti-cancer, anti-obesity, and anti-diabetic effects [13]. Due to its potential to decrease COX-2 and iNOS levels, fucoxanthin decreased the expression of pro-inflammatory mediators such as PGE2, IL-1β, IL-6, and TNF-α in lipopolysaccharide (LPS)-induced in vitro and in vivo models [14,15]. In particular, in experiments related to the efficacy of fucoxanthin in UC, fucoxanthin treatment attenuated the dextran sulfate sodium (DSS)-induced increase of disease activity index and histological damage in the colon by inhibiting COX-2 and NF-κB expressions [16]. Chromenes isolated from seaweeds have also attracted attention due to their antioxidant and anti-inflammatory properties [17]. In particular, sargachromenol, isolated from Sargassum, showed potent anti-inflammatory properties via the activation of Nrf2/HO-1 signaling [18,19]. However, it remains unknown the underlying mechanism by which S. horneri, a mixture of these substances, regulates inflammation.
The PI3K/Akt/mTOR pathway is essential for various cellular processes and is involved in inflammatory processes through modulation of the NF-κB and STAT3 pathways [20]. Inhibition of the PI3K/Akt/mTOR pathway may be regarded as a key regulator of intestinal inflammation in colitis [21], and several dietary polyphenols exert anti-inflammatory effects by modulating the mTOR signaling pathway [22]. Fucoxanthin has previously demonstrated a potential to regulate the PI3K/Akt/mTOR pathway [23]; however, no studies involving S. horneri have been conducted.
The gut microbiota of healthy individuals maintains gut homeostasis by fermenting indigestible polysaccharides to produce short-chain fatty acids (SCFAs), protecting the intestinal mucosa, and inhibiting pathogenic microorganisms [24,25]. However, patients with IBD experience leaky gut and gut dysbiosis, and the most consistent observation of intestinal flora abnormalities in IBD patients is a decrease in the diversity of the gut microbiota and the ratio of Firmicutes to Bacteroidetes (F/B) [26,27,28]. Polyphenols have recently demonstrated their potential as prebiotics by increasing the diversity of the gut microbiota, promoting the growth of beneficial bacteria (Akkermansia, Bifidobacterium, and Lactobacillus), and inhibiting the growth of pathogenic bacteria (Clostridium and Staphlococcus) [29,30]. Moreover, polyphenols have been demonstrated to have a positive effect on the intestinal lumen barrier by affecting tight junction cells [31]. Previously, fucoxanthin, abundant in brown seaweed, prevented obesity and obesity-induced gut dysbiosis by inducing the growth of beneficial bacteria Lactobacillus, Lactococcus, and Bifidobacterium, and suppressing the growth of inflammation-related bacteria Lachnospiraceae and Erysipelotrichaceae in mice fed a high-fat diet [10]. S. horneri also decreased the F/B ratio in mice fed a high-sucrose and low-fiber diet [32]. These previous studies investigated the modulation of gut dysbiosis in an obesity model by S. horneri or its components, but the effects of S. horneri on the gut microbiota in colitis have not yet been investigated.
This study aimed at determining the anti-inflammatory and preventative effects of S. horneri ethanol extract on UC. By modifying the mTOR axis and the make-up of the gut microbiota, S. horneri extract helps to reduce inflammation in UC, according to our theory.
2. Materials and Methods
2.1. Extract Preparation and LC-MS Analysis
S. horneri extracted with 70% ethanol was obtained from the National Marine Biodiversity Institute of Korea (Chungchungnam-do, Republic of Korea) and ParaJeju (Jeju-si, Republic of Korea). Fucoxanthin extraction, which is abundant in S. horneri, was previously shown to be better extracted using organic solvent than water [33], and the maximum fucoxanthin extraction rate from S. horneri was obtained using ethanol [34]. Moreover, according to prior studies, 70% ethanol extract of S. horneri has an anti-inflammatory impact on RAW264.7 cells [35]. Therefore, 70% ethanol was used for the extraction process in this investigation. The levels of total phenolics and flavonoids were measured using the Folin–Ciocalteu assay [36] and diethylene glycol colorimetric method [37]. Liquid chromatography–mass spectrometry (LC-MS) analysis was performed using an Agilent 6530 Infinity HPLC system (Agilent Technologies, Santa Clara, CA, USA) coupled with a hybrid quadrupole time-of-flight mass spectrometer [38].
2.2. Cell Culture and Cell Viability
Caco-2 and RAW264.7 were purchased from the Korean Cell Line Bank (Seoul, Republic of Korea) and incubated at 37 °C with 5% CO2. Caco-2 was cultured in modified Eagle’s medium containing 25 mM HEPES, 0.1 mM non-essential amino acids, 1.0 mM sodium pyruvate, 20% fetal bovine serum (FBS), and 100 U/mL penicillin/streptomycin (P/S), while RAW264.7 was cultured in RPMI1640 containing 10% FBS, 100 U/mL P/S (all purchased from Hyclone, Logan, UT, USA). The medium was changed every 2–3 days until 80–90% confluency. The CellTiter 96® Aqueous One-Solution Cell Proliferation Assay (MTS; Promega, Madison, WI, USA) was used to examine the viability of Caco-2 and RAW264.7 cells. The cells (1 × 104 cells/well) were seeded in 96-well plates and treated with S. horneri ethanol extract (SHE) at concentrations ranging from 0 to 50 mg/L after 24 h. After 48 h, the cell proliferation (MTS) solution was added and incubated at 37 °C for 2 h, after which viable cells were detected using a microplate reader (Spectrophotometer, Thermo Fisher, Waltham, MA, USA) at 490 nm [38]. To evaluate the anti-inflammatory effects of SHE, RAW264.7 macrophages were seeded for 24 h at a density of 1 × 104 cells/well in a 96-well plate. The following day, SHE was added at concentrations ranging from 0 to 40 mg/L for 1 h, followed by LPS (50 ng/mL, Escherichia coli O55:B5; Sigma-Aldrich, St. Louis, MO, USA) with SHE. The conditioned medium was collected at 4 h for the TNF-α measurement and at 20 h for the IL-6 measurement. To investigate the underlying mechanisms of SHE, Caco-2 cells were seeded at a density of 5 × 104 cells/well for 24 h in 6-well plates. The next day, SHE (25 and 50 mg/L) was added for 1 h, followed by 2 µg/mL of LPS with SHE for 3 h for RNA extraction. The LPS dosage that causes considerable inflammation in RAW264.7 and Caco-2 cells was selected by consulting previous studies (50 ng/mL and 2 µg/mL for RAW264.7 and Caco-2 cells, respectively) [39,40].
2.3. Co-Culture System with Intestinal Epithelial Caco-2 Cells and RAW264.7 Macrophages
Caco-2 cells were seeded at density of 1 × 105 cells/insert on the apical side of a 24-mm transparent polyester cell culture plate and were differentiated for 21 days while the media was changed every 2–3 days. The TEER readings were monitored using an EndOhm volt ohmmeter (World Precision Instruments Inc., Sarasota, FL, USA). The day before the experiment, RAW264.7 cells were seeded at a density of 1 × 106 cells per well on the basolateral side of a new 6-well plate. When the TEER values of the Caco-2 monolayer reached 800 Ω·cm2, the RAW264.7 cell-containing plates were combined with the insert with the Caco-2 cells. The apical side received SHE (25 and 50 mg/L) for 2h, while the basolateral side received LPS (final con: 100 ng/mL) for an additional 3 h. All conditioned media and cells from the apical and basolateral chambers were collected [41].
2.4. DSS Model of Colitis
Six–seven-week-old Balb/c male mice were purchased from OrientBio (Seongnam-si, Gyeong gi-do, Republic of Korea) and housed in a temperature-controlled facility with a 12 h light/dark cycle. One week after adaptation, the mice were randomly assigned to one of four groups (n = 8/group): Control, DSS, DSS + SHE-low (DSS-SHE-L), and DSS + SHE-high (DSS-SHE-H). All groups were given normal drinking water for three weeks. In the final week, the DSS, DSS-SHE-L, and DSS-SHE-H groups were administered water containing 2.5% (w/v) DSS (MW: 36,000–50,000 Da; MP Biomedicals, Solon, OH, USA) to induce colitis for one week. During the experiment, the DSS-SHE-L received 100 mg/kg body weight (bw)/day of SHE, while the DSS-SHE-H received 200 mg/kg bw/day. The other two groups received PBS orally once per day for four weeks [42]. SHE has never been utilized in the DSS colitis model, however both 100 and 300 mg/kg bw of SHE demonstrated anti-obesity effects in animal models fed a high-fat diet [43]. And 150 mg/kg bw of SHE improved immunological function in an immune-suppressing mouse model [44] On the basis of these results, the concentration of SHE was determined to be between 100 and 200 mg/kg bw. Body weights were recorded weekly; and then daily after DSS administration during the experiment. All mice were euthanized on day 28, and the severity of colitis was evaluated based on changes in body weight, colon length, and histopathology. The Institutional Animal Care and Use Committee of the Pusan National University approved the animal protocol (PNU-2022-0119).
2.5. Histopathology
Sections (5 μm) of paraffin-embedded blocks were stained with hematoxylin and eosin (H&E). For each slide, three images were acquired using a BX50 Fluorescence Microscope (Olympus, Tokyo, Japan) in order to score the level of inflammation. Histological scores were evaluated by the following criteria: inflammation and epithelial hyperplasia, 0–3 (0, normal; 1, mild; 2, moderate; 3, pseudopolyps); mucosal crypt loss and ulceration, 0–3 (0, normal; 1, surface epithelial inflammation; 2, erosions; 3, ulcerations) [45,46].
2.6. ELISA Assay
The concentrations of cytokines released by RAW264.7 macrophages were determined using a commercial enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN, USA). Colon tissues were homogenized in lysis buffer, centrifuged at 10,000× g for 5 min, and the upper phase was collected. According to the manufacturer’s instructions, the levels of inflammatory regulators, MPO, IL-6, IFN-γ, and TNF-α were measured using the ELISA method (R&D Systems). The levels of colonic cytokines were expressed as ng of cytokines per mg of colonic protein (ng/mg). The collected serum was used to measure the level of lipocalin 2 (R&D Systems).
2.7. RT-PCR, DNA Extraction from Bacteria, and 16s rRNA Gene Sequencing
Total RNA was extracted from RAW264.7, Caco-2 cells, and colon tissues using TRIzol (Thermo Fisher) and RNeasy mini kit (QIAGEN, Hilden, Germany) according to the manufacturers’ instructions. The concentration was determined using a spectrophotometer (Nabi, Seongnam-si, Gyeonggi-do, Republic of Korea), 1 μg of RNA was reverse transcribed into cDNA using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA), and gene amplification was detected using the CFX Connect Real-Time PCR Detection System (Bio-Rad). The primer designs are listed in Supplementary Table S1 (Macrogen, Seoul, Republic of Korea). The relative gene expression was normalized to that of β-actin. The composition of the gut microbiota was determined as follows. Microbial DNA was extracted from mouse feces using a PowerFecal Pro DNA Kit (QIAGEN) according to the manufacturer’s instructions. DNA from two mice (n = 3–4/group) was mixed in equal proportions for 16S rRNA sequencing using the Illumina iSeq platform (Illumina, San Diego, CA, USA). The following primers were designed for the v4 region of 16s metagenomic sequencing: forward primer: TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG and reverse primer: GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVG-GGTATCTAATCC. Trimmomatic (version 0.39) was utilized to eliminate the adaptor sequence, and the results were grouped into operational taxonomic units (OTUs). α- and β-diversity analyses were performed using QIIME2 (version 1.9.5) (https://qiime2.org, accessed on 30 September 2022) [38]. To analyze the composition of specific intestinal microorganisms, 2 μg of DNA extracted from bacteria was used for quantitative PCR and amplified using primers (Supplementary Table S2, Macrogen, Seoul, Republic of Korea). The CFX Connect Real-Time PCR Detection System (Bio-Rad) was used for quantitative PCR. The relative abundance of bacterial groups in each fecal sample was calculated as the percentage of total bacteria (F341/R518) [47].
2.8. Western Blotting
Colonic tissue protein was extracted with T-PER™ tissue protein extraction reagent (Pierce, Rockford, IL, USA) containing the Halt protease inhibitor cocktail (Thermo Fisher). The homogenate was centrifuged (14,000× g for 5 min at 4 °C) and the supernatant was collected for Bradford assay quantification (Bio-Rad). Protein samples were separated using SDS-PAGE gels and transferred to PVDF membranes. The membranes were blocked with 5% milk for 1 h at room temperature before overnight incubation at 4 °C with the indicated primary antibodies against mTOR, p-mTOR, S6K, p-S6K and β-actin (Cell Signaling Technology, Danvers, MA, USA). Subsequently, a secondary antibody conjugated with HRP was added. The western blotting bands were analyzed using a Chemiluminescence Imaging System (Davinch K, Seoul, Republic of Korea), and the band intensities were normalized to those of β-actin using ImageJ software (NIH, Bethesda, MD, USA).
2.9. Statistical Analysis
Data were analyzed using Prism 6 (GraphPad Software, La Jolla, CA, USA). The results are presented as mean ± SD. The p-values were calculated using one-way ANOVA (Dunnett’s test), and the abundance of bacteria was determined using the nonparametric Kruskal–Wallis test. p < 0.05 was considered statistically significant.
3. Results
3.1. Identification of Bioactive Compounds in SHE
The total phenolic content of the SHE was 19.51 ± 0.24 mg gallic acid equivalent (GAE)/g extract, while the total flavonoid content was 54.65 ± 15.61 mg catechin equivalent (CE)/g extract. LC-MS analysis of the bioactive substances in SHE revealed a high concentration of fucoxanthin (MF: C42H58O6, MW: 658.9, calculated MW: 659.3), similar to that in previous studies. In addition, several phenols, including salicortin (MF: C20H24O10, MW: 424.4, calculated MW: 425.3), feruloylquinic acid (MF: C17H20O9, MW: 368.3, calculated MW: 369.2), vitexin (MF: C21H20O10, MW: 432.4, calculated MW: 433.2), and loliolide (MF: C11H16O3, MW: 196.2, calculated MW: 197.1), were detected (Figure 1, Table 1). The highest concentration of SHE constituent was loliolide (16.52 μg fucoxanthin/mg extract), followed by fucoxanthin, salicortin, feruloylquinic acid, and vitexin.
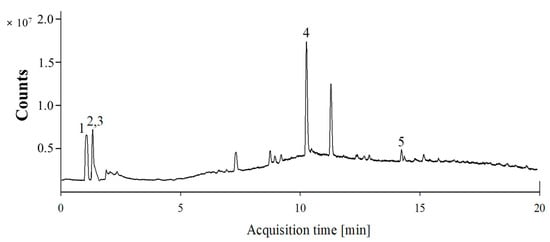
Figure 1.
Representative LC-MS chromatogram of S. horneri extract. 1. Fucoxanthin, 2. Salicortin, 3. Feruloylquinic acid, 4. Loliolide, and 5. Vitexin.

Table 1.
Representative LC-MS chromatogram of S. horneri extracts.
3.2. Effects of SHE on LPS-Treated RAW264.7 and Caco-2 Cells
The cytotoxicity of SHE was evaluated in RAW264.7 and Caco-2 cells. After 48 h, SHE exhibited survival rates of 74.2% and 72.3% for RAW264.7 cells at 25 and 50 mg/L, and 78.3% and 77.6% for Caco-2 cells at 25 and 50 mg/L, respectively (Figure 2A). TNF-α and IL-6 inflammatory markers were analyzed in RAW264.7 macrophages treated with SHE for 4 and 20 h. LPS treatment significantly increased the levels of TNF-α and IL-6 compared to those in the control, whereas SHE demonstrated an anti-inflammatory effect by lowering the levels of TNF-α and IL-6 by 67.2% and 51.9%, respectively, compared to those in the LPS control at 40 mg/L (Figure 2B,C). Caco-2 cells treated with LPS were used to examine the expression of genes involved in the tight junction and mTOR axis to determine the underlying mechanisms of SHE (Figure 2D–J). LPS significantly decreased Occludin mRNA level, and increased Mtor mRNA level compared to those in the control (Figure 2D,I), whereas SHE significantly decreased Pi3k, Mtor, and S6k mRNA expressions compared to those in the LPS control at 50 mg/L (Figure 2G,I,J). There were no significant differences in the mRNA levels of Claudin1, Zo-1, and Akt (Figure 2E,F,H).
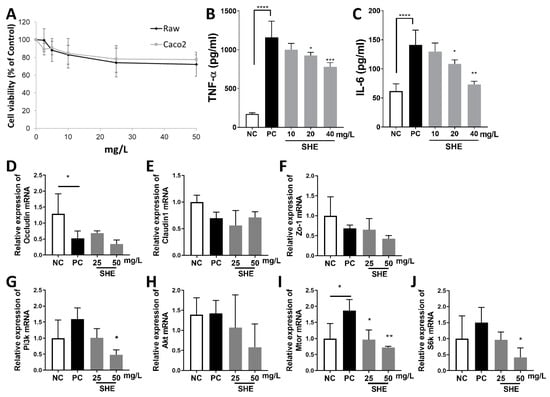
Figure 2.
Effects of S. horneri extract (SHE) on LPS-treated RAW264.7 and Caco-2 cells. (A) The cytotoxicity of SHE (0–50 mg/L) on RAW264.7 and Caco-2 cells was estimated. (B) Levels of TNF-α and (C) IL-6 production in conditioned medium of RAW264.7 macrophages stimulated with LPS (50 ng/mL) for 4 h (TNF-α) and 20 h (IL-6) with SHE (0–40 mg/L). Gene expressions of (D) Occludin, (E) Claudin1, (F) Zo-1, (G) Pi3k, (H) Akt, (I) Mtor, and (J) S6k mRNA in LPS (2 µg/mL)-treated Caco-2 cells with SHE (0–50 mg/L). All data are expressed as means ± SD (n = 4). * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, significantly different compared with PC by one way ANOVA followed by Dunnett’s test. NC: negative control; PC: positive control.
3.3. Effects of SHE in a Co-Culture Model of Caco-2 and RAW264.7 Cells
The Caco-2 and RAW264.7 co-culture system, a well-established in vitro colitis model (Figure 3A), was used to assess treatment with SHE [41]. Compared to that in the control, LPS tended to increase the gene expression of inflammatory markers in RAW264.7 from the co-culture system. However, SHE (25 and 50 mg/L) significantly decreased Nfkb p65, Cox2, and Inos gene expression (Figure 3A–C). Caco-2 cells from the co-culture system were examined for gene expression to determine the effect of SHE on tight junctions and the mTOR axis (Figure 3E–K). LPS decreased the expression of genes involved in tight junction formation (Figure 3D–F), whereas SHE (50 mg/L) increased the expression of Zo-1 mRNA. LPS treatment increased the expression of genes involved in the mTOR axis compared to those in the control, but SHE significantly decreased the levels of Pi3k, Akt, Mtor, and S6k mRNA compared to those in the LPS control at 50 mg/L (Figure 3H–K).
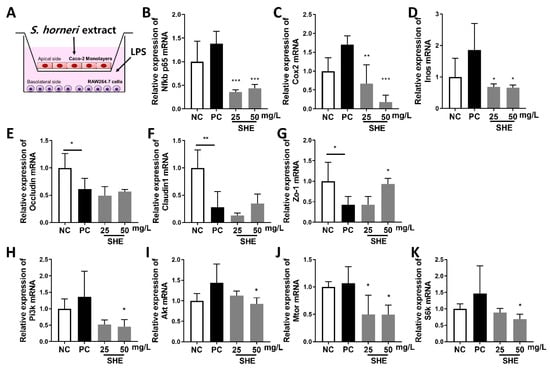
Figure 3.
Effects of S. horneri extract (SHE) in a co-culture model of Caco-2 and RAW264.7 cells. (A) Co-culture system. Caco-2 cells were pre-seeded on the apical side for 21 days. The day after RAW264.7 cells were pre-seeded, they were incorporated into transwell plates containing Caco-2 cells. After 2 h of SHE treatment (25 and 50 mg/L) to the apical side, RAW264.7 cells on the basolateral side were then stimulated with 100 ng/mL LPS for 3 h. Changes in genes associated with inflammation in RAW264.7 cells and tight junction and mTOR axis genes in Caco-2 cells were measured. Gene expression of (B) Nfkb p65, (C) Cox2, (D) Inos mRNA in RAW264.7 cells (basolateral side) in the co-culture system. Gene expression of (E) Occludin, (F) Claudin1, (G) Zo1, (H) Pi3k, (I) Akt, (J) Mtor, (K) S6k mRNA in Caco-2 cells (apical side) in the co-culture system. All data are expressed as means ± SD (n = 4). * p < 0.05, ** p < 0.01, *** p < 0.001, significantly different compared with PC by one way ANOVA followed by Dunnett’s test. NC: negative control; PC: positive control.
3.4. Effects SHE on the Weight Loss, Gut Length, Spleen, and Histological Score of DSS-Treated Mice
The therapeutic effects of low- and high-dose SHE intake on experimental colitis were evaluated (Figure 4A). The experimental treatments were administered to mice for 4 weeks, and 2.5% DSS administration began on the fourth week (Day 22). Mice treated with DSS experienced weight loss, a short colon, diarrhea, and bloody stools at the onset of colitis. The second day after DSS administration, one of the mice had diarrhea. On Day seven, seven out of eight mice in the DSS group developed diarrhea, and four had bloody stool. The body weight of mice in the control group increased, whereas that of mice in the groups receiving DSS significantly decreased (Figure 4B). The weight change was calculated by dividing the weight gain from Day 0 to Day 28 by the initial body weight. At 4 weeks, mice in the control group gained 106.16% of their body weight, whereas mice in the DSS group gained only 102.19%, indicating a significant difference (Figure 4C). DSS-SHE-L and DSS-SHE-H increased by 99.43% and 104.26%, respectively, indicative of the severity of colonic inflammation. Colon length was shorter in the DSS group compared to that in the control group but increased by 28.19% in the DSS-SHE-H group compared to that in the DSS group (Figure 4D,E). The weight of the spleen and degree of inflammation did not change significantly (Figure 4F). Histological images of the colon tissue were graded on a 0–6 point scale after staining with H&E. DSS-SHE-H scored considerably lower than did the DSS group (DSS, 5.81; DSS-SHE-L, 4.85; DSS-SHE-H, 4.61; Figure 4G). In DSS mice, crypts of epithelial cells were damaged, resulting in extensive ulceration and infiltration of inflammatory cell (neutrophil). In contrast, mice treated with SHE showed gradual repair of the damaged crypts and decreased inflammation (Figure 4H).
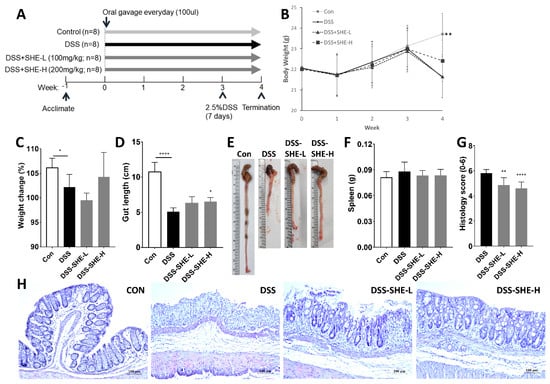
Figure 4.
Effects of S. horneri extract (SHE) on DSS-induced colitis. (A) Experimental timeline for the mouse model of DSS. (B) Body weight was measured weekly throughout the experiment, and (C) body weight changes (%) for four weeks were calculated. Measurements included (D) colon lengths, (E) a representative image of colon lengths, and (F) spleen weights. (G) Histological alterations of colon tissue were determined. The score was based on a 0–6 scale. Colon images stained with hematoxylin and eosin (H&E) were used to assess the level of inflammation and ulceration. (H) Representative photographs of H&E-stained colonic tissues (magnification ×400). All data are expressed as means ± SD (n = 8). * p < 0.05, ** p < 0.01, **** p < 0.0001, significantly different compared with DSS group by one way ANOVA followed by Dunnett’s test. CON: control group; DSS: 2.5% DSS in drinking water for the last 7 days; DSS-SHE-L: DSS + S. horneri extract low dose group (100 mg/kg bw/day); DSS-SHE-H: DSS + S. horneri extract high dose group (200 mg/kg bw/day).
3.5. Effects of SHE on Inflammation Markers in DSS-Treated Mice
Lipocalin 2 is an immune protein that increases in response to intestinal inflammation and mucosal damage [48]. DSS treatment significantly increased the level of lipocalin 2, whereas SHE treatment had no effect when compared to the lipocalin 2 level of the DSS group (Figure 5A). MPO is a marker of neutrophil infiltration [49] that indicates the intensity of inflammation. MPO activity was higher in DSS-induced UC mice than in control mice but significantly decreased in the DSS-SHE-H group (Figure 5B). Inflammatory cytokines, such as TNF-α, IL-6, and IFN-γ, are produced by immune cells (T cells, dendritic cells and intestinal epithelial cells) and are important factors of inflammation in UC. In the DSS-treated group, TNF-α, IL-6, and IFN-γ levels were significantly elevated, whereas they were significantly decreased in the DSS-SHE-H group (Figure 5C–E).
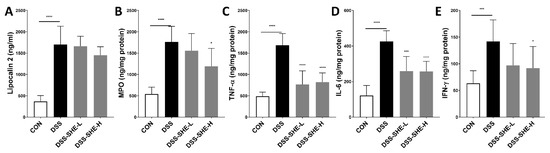
Figure 5.
Effects of S. horneri extract (SHE) on levels of inflammatory markers in DSS-treated mice. (A) Lipocalin-2 in serum, (B) myeloperoxidase (MPO), (C) TNF-α, (D) IL-6, (E) IFN-γ levels in the colons of mice were measured by ELISA. All data are expressed as means ± SD (n = 8). * p < 0.05, *** p < 0.001, **** p < 0.0001, significantly different compared with DSS group by one way ANOVA followed by Dunnett’s test. CON: control group; DSS: 2.5% DSS in drinking water for the last 7 days; DSS-SHE-L: DSS + S. horneri extract low dose group (100 mg/kg bw/day); DSS-SHE-H: DSS + S. horneri extract high dose group (200 mg/kg bw/day).
3.6. Effects of SHE on the Gene and Protein Expressions Involved in Inflammatory Markers, Tight Junctions, and the mTOR Axis in DSS-Treated Mice
The expression levels of the genes involved in inflammatory markers, tight junctions, and the mTOR axis were measured (Figure 6A–J). For the inflammatory markers, the DSS group showed increased Nfkb p65 mRNA (Figure 6A) levels compared to those in the control group, whereas the DSS-SHE-H group showed decreased Nfkb p65 and Cox2 mRNA levels compared to those in the DSS group. DSS treatment significantly decreased the expression of all tight junction-related genes, whereas the DSS-SHE-H group showed increased Occludin and Claudin1 mRNA levels compared to those in the DSS group (Figure 6D–F). To investigate the underlying mechanisms of SHE, mRNA and protein expressions associated with the mTOR axis were investigated. DSS treatment significantly increased Akt and Mtor mRNA expressions compared to those in the control; however, DSS-SHE-L significantly decreased the gene expression of Pi3k and Akt mRNA, and DSS-SHE-H significantly decreased the gene expression of Pi3k and Mtor mRNA compared to those in the DSS group (Figure 6G–J). Additionally, protein levels of the mTOR axis were measured. DSS treatment significantly increased the relative ratios of p-mTOR/mTOR and p-S6K/S6K compared to those in the control, whereas SHE-H significantly decreased these ratios compared to those in the DSS group (Figure 6K–M).
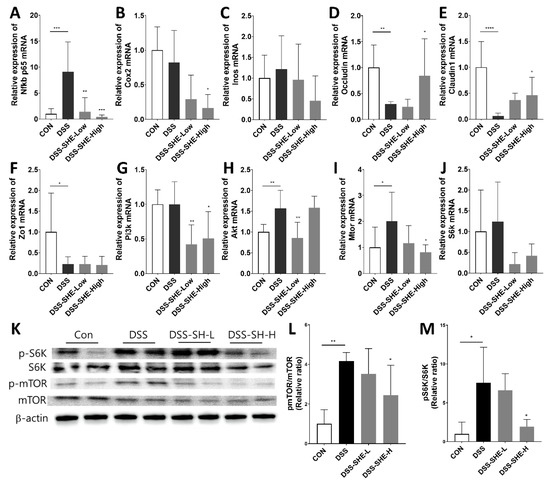
Figure 6.
Effects of S. horneri extract (SHE) on inflammatory markers, tight junctions, and mTOR axis in DSS-treated mice. Gene expression of (A) Nfkb p65, (B) Cox2, (C) Inos, (D) Occludin, (E) Claudin1, (F) Zo1, (G) Pi3k, (H) Akt, (I) Mtor, and (J) S6k mRNA in the colon of DSS-treated mice. As a reference gene, β-actin was utilized. (K) Western blotting was used to determine the expression of p-mTOR, mTOR, p-S6K, S6K, and β-actin in colonic tissues, as well as the relative ratios of (L) p-mTOR/mTOR and (M) p-S6K/S6K were calculated. All data are expressed as means ± SD (n = 8). * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, significantly different compared with DSS group by one way ANOVA followed by Dunnett’s test. CON: control group; DSS: 2.5% DSS in drinking water for the last 7 days; DSS-SHE-L: DSS + S. horneri extract low dose group (100 mg/kg bw/day); DSS-SHE-H: DSS + S. horneri extract high dose group (200 mg/kg bw/day).
3.7. Effects of SHE on Fecal Microbiota Composition in DSS-Treated Mice
The abundance and consistency of the gut microbiota were evaluated. There was no significant variation in the Shannon diversity index (Figure 7A). In contrast, for Faith’s phylogenetic diversity (Faith’s PD), the DSS group exhibited a higher tendency than the control group, whereas the SHE-treated group showed significantly reduced diversity compared to that of the DSS group (Figure 7B). Analysis based on weighted UniFrac distances revealed different bacterial distributions between the DSS and SHE-treated groups (R = 1, P = 0.021) (Figure 7C). According to the bacterial classification, phyla and genera were analyzed (Figure 7D). F/B ratio increased significantly in the DSS group compared to that in the control group but decreased significantly in the SHE-treated group compared to that in the DSS group (Figure 7E). The taxa whose relative abundance at the genus level changed due to DSS or DSS-SHE treatment were investigated (Figure 7F–J). Bacteroides spp. and GCA-900066575 spp. were significantly less prevalent in the DSS group, whereas Bacteroides spp., GCA-900066575 spp., and Erysipelatoclostridium spp. were significantly more prevalent in the SHE-treated group (Figure 7F–H). However, Anaerotruncus spp. were significantly greater in the DSS group, but significantly lower in the SHE-treated group (Figure 7J). The SHE-treated group showed significantly increased levels of Akkermansia muciniphila, Bifidobacterium bifidum, and Lactococcus lactis (Figure 7K–M), as measured by qPCR. Spearman’s correlation between gut microbial species and UC-related factors was investigated (Figure 7N). GCA-900066575 spp. correlated negatively with inflammatory factors including lipocalin 2, MPO, IFN-γ, TNF-α, and IL-6. The Anaerotruncus genus, in contrast, exhibited positive correlations with UC-related factors, including histology score, MPO, and IFN-γ.
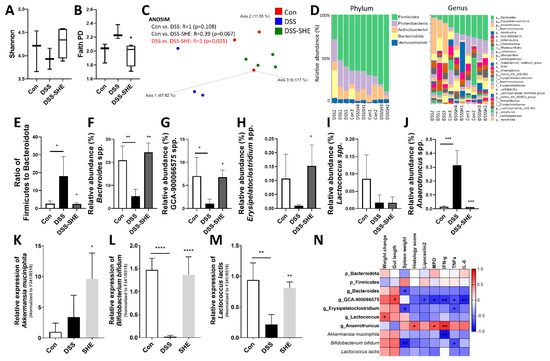
Figure 7.
Effects of S. horneri extract (SHE) on the composition of the gut microbiota in DSS-treated mice. (A) Shannon index, and (B) Faith’s phylogenetic diversity (PD). (C) Principal coordinate analysis (PCoA) of weighted UniFrac distances. (D) Analysis of the taxonomy community at the phylum and genus levels. (E) The Firmicutes to Bacteroidota ratio. (F–J) Taxa whose relative abundance changed as a result of DSS or DSS-SHE treatment. (n = 3 or 4; fecal DNA from 2 mice was combined for 16S sequencing in a single sample). (K) Quantitative PCR for Akkermansia muciniphila, (L) Bifidobacterium bifidum, and (M) Lactococcus lactis. The abundance of bacterial groups was quantified as a ratio of total bacteria (F341/R518). All data are expressed as means ± SD (n = 8/group). * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 using a nonparametric Kruskal–Wallis test. (N) Spearman correlation analysis of gut microbial species and inflammation-related index (* p < 0.05, ** p < 0.01). CON: control group; DSS: 2.5% DSS in drinking water for the last 7 days; DSS-SHE: DSS + S. horneri extract high dose group (200 mg/kg bw/day).
4. Discussion
In this study, we demonstrated that SHE has anti-inflammatory properties by inhibiting the inflammatory markers MPO, TNF-α, IL-6, and IFN-γ, and mRNA expression of Nfkb p65 and Cox2 and the mTOR axis and inducing the mRNA expression of tight junction factors, including Occludin and Claudin1, and beneficial bacteria, Bacteroides spp., in in vitro and in vivo colitis models. We confirmed our hypothesis that SHE modulates the mTOR axis and the gut microbiota composition to reduce inflammation in intestinal colitis.
UC is a chronic inflammatory bowel disease with increasing prevalence worldwide [50]. Multiple studies have shown that dietary polyphenols may help colitis by modulating the NF-κB and MAPK pathways and improving the Nrf2 pathway and intestinal dysbiosis [51,52]. This study identified the carotenoid fucoxanthin, phenol salicortin, and flavonoid vitexin in SHE. Fucoxanthin, mainly found in brown seaweeds, has demonstrated antioxidant and anti-inflammatory properties in inflammatory diseases via inhibition of the NF-κB/COX-2/PGE2 pathway and downregulation of pro-inflammatory cytokines such as IL-6 and TNF-α [53]. In a previous study, fucoxanthin treatment specifically protected DSS-induced mice by preventing body weight loss, increase in DAI, and shortening of the colon, and by reducing the expression of COX-2 and NF-κB [16]. Salicortin is well-known for its anti-obesity properties, but it also demonstrates anti-inflammatory properties by inhibiting NF-κb/JNK expression [54]. Vitexin, an apigenin glycoside found in bamboo and mung beans, reportedly possesses antioxidant, anti-inflammatory, and anti-cancer properties [55]. NF-κB is significantly activated in patients with IBD and has a potent effect on mucosal inflammatory processes [56]. Therapeutic agents used to treat IBD, such as anti-TNF-α antibodies, sulfasalazine, and methotrexate, are known to inhibit NF-κB activity [57]. Consequently, this experiment was conducted with the hypothesis that S. horneri, with a combination of these substances, would be therapeutic for ulcerative colitis via the suppression of NF-κB and pro-inflammatory cytokines, and the results were consistent with this hypothesis. We confirmed that SHE reduced Nfkb and Inos mRNA levels and pro-inflammatory cytokines including TNF-α, both in vivo and in vitro.
The mTOR pathway is involved in the production of inflammatory cytokines and promotes the progression of colitis [58], which regulates intestinal barrier integrity and plays an essential role in UC remission [59]. Therefore, one of the targets of IBD prevention and treatment is to preserve the intestinal barrier function by upregulating tight junction proteins and inhibiting mTOR signaling [21]. Fucoxanthin and salicortin demonstrated the ability to inhibit the mTOR pathway [23,60]; therefore, S. horneri is a potential mTOR inhibitor that can be used to treat and/or prevent UC. In this study, SHE decreased mTOR and its downstream S6K in cell and animal experiments. In addition, ZO-1, a tight junction factor, was significantly elevated in cell experiments, whereas Claudin-1 and occludin were elevated in animal experiments. This is likely due to the fact that animals exhibited a greater variety of variables.
The gut microbiota and immune system interact to maintain intestinal homeostasis; when this balance is disrupted, gut dysbiosis results in gut bacterial overgrowth, leading to intestinal barrier disruption and systemic translocation of pathogens [61]. Recent advances in IBD research have focused on modifying gut microbiota composition and gut dysbiosis, which is regulated by diet but not inheritance [62,63]. According to previous studies, fucoidan improved dysbiosis by decreasing harmful bacteria such as Peptococcus and increasing beneficial bacteria such as Lactobacillus and Ruminococcaceae [64], and S. horneri decreased the F/B ratio in high-fat-fed mice [32]. In our study, there was no difference in the Shannon index between the groups, and the DSS group showed an increased tendency in Faith’s PD richness compared with that in the control group, whereas the α diversity in the SHE treatment group was lower than that of the DSS group. The results of another study involving polyphenol-rich extracts and a DSS colitis model were comparable to our results. The Shannon index was unaffected by DSS treatment or the anti-inflammatory bioactive compound, but DSS increased Faith’s PD richness, and the polyphenol-rich extract decreased it [65]. β-diversity analysis showed that SHE treatment regulated the gut microbiota composition differently than that of DSS treatment. At the phylum level, the F/B ratio is an indicator of normal intestinal homeostasis. An abnormal balance of the ratio indicates gut dysbiosis, and it has been reported that IBD patients have a lower F/B ratio than healthy individuals because Faecalibacterium spp. are lowered and Bacteroides spp. induced [66]. However, in our study, the F/B ratio increased with DSS treatment but decreased with SHE treatment. It was reported that the distribution of gut microbiota in DSS-induced colitis in mice differs from that of IBD patients [67]. The F/B ratio fluctuated weekly at 7, 14, 21 days after DSS administration, and especially the F/B ratio increased 7 days after DSS administration [67], consistent with our findings and those of other researches [68,69]. Bacteroides spp. and Erysipelatoclostridium spp. are lactic acid bacteria and SCFA producers [70,71]; their abundances decreased in the DSS-treated group but increased significantly in the SHE-treated group. In addition, the SHE-treated group had elevated levels of beneficial probiotics for maintaining intestinal homeostasis, including Akkermansia muciniphila, Bifidobacterium bifidum, and Lactococcus lactis [72,73]. In conclusion, SHE treatment increased the abundance of beneficial bacteria. Although the benefits of SHE for improving intestinal health, reducing obesity, and acting as an antioxidant are well-established, this is the first study to examine how SHE affects UC while observing changes in the intestinal microbiome.
One limitation of this experiment is that only male mice were used in this experiment because the prevalence of IBD is gender-specific. In the DSS-induced colitis model, female mice exhibited significantly fewer inflammatory symptoms than male mice (longer colon, lower body weight loss, lower TNF-α level) and were partially protected against chemically induced colitis due to estradiol [74]. Therefore, female mice will exhibit different tendencies, and additional research with female mice needs to be conducted. As another limitation, the study did not investigate the toxicity of SHE. All the extracts should be tested for safety, but there is minimal information regarding the use of SHE. In experiments utilizing similar concentrations of 70% ethanol extract of S. horneri (50–400 mg/kg bw), no toxicity was observed [43,44,75,76]. In addition, for the toxicity test of SHE on Sprague–Dawley rats, 2000 mg/kg of SHE administered for 14 days exhibited no signs of toxicity, and all blood parameters were within the normal range [77]. Consequently, it is thought that the concentration utilized in this experiment (100–200 mg/kg bw) was not detrimental; nonetheless, additional SHE toxicity testing needs be conducted. To analyze the effects of SHE on gut microbiota-derived metabolites, the amount of short chain fatty acids (SCFAs) must be determined in future studies, and the cross-talk between the gut microbiota, SCFAs, and the mTOR pathway should be explored.
5. Conclusions
We suggest that SHE suppresses inflammation in animals with colitis produced by DSS. The protective effect of SHE may reduce the DSS-induced inflammatory response by inhibiting the mTOR axis, inducing tight junction protein, and modulating the gut microbiota. Therefore, SHE consumption may be a viable option for preventing IBD symptoms and other gastrointestinal problems.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app13031742/s1.
Author Contributions
Conceptualization and supervision, H.K.; methodology, Y.I. and Q.W.; investigation, Y.I., Q.W., J.P., H.L. and H.K.; data curation, Y.I.; writing—original draft preparation, Y.I.; writing—review and editing, Y.I. and H.K.; funding acquisition, H.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2021R1F1A1055822) and Pusan National University Research Grant, 2020.
Institutional Review Board Statement
The animal protocol was approved by the Animal Ethics Committee of Pusan National University (PNU-2022-0119).
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author H.K. on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cardozo, W.S.; Sobrado, C.W. Clinical manifestations in inflammatory bowel disease. In Inflammatory Bowel Disease; River Publishers: Aalborg, Denmark, 2022; pp. 81–100. [Google Scholar]
- Guzzo, G.L.; Andrews, J.M.; Weyrich, L.S. The Neglected Gut Microbiome: Fungi, Protozoa, and Bacteriophages in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2022, 28, 1112–1122. [Google Scholar] [CrossRef] [PubMed]
- Rubin, D.T.; Ananthakrishnan, A.N.; Siegel, C.A.; Sauer, B.G.; Long, M.D. ACG clinical guideline: Ulcerative colitis in adults. Off. J. Am. Coll. Gastroenterol. ACG 2019, 114, 384–413. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.-H.; Zhu, C.-X.; Quan, Y.-S.; Yang, Z.-Y.; Wu, S.; Luo, W.-W.; Tan, B.; Wang, X.-Y. Relationship between intestinal microbiota and ulcerative colitis: Mechanisms and clinical application of probiotics and fecal microbiota transplantation. World J. Gastroenterol. 2018, 24, 5. [Google Scholar] [CrossRef] [PubMed]
- Borum, M.L.; Ginsberg, A. Hypersensitivity to 5-ASA suppositories. Dig. Dis. Sci. 1997, 42, 1076. [Google Scholar] [CrossRef]
- Shao, P.; Chen, X.; Sun, P. Chemical characterization, antioxidant and antitumor activity of sulfated polysaccharide from Sargassum horneri. Carbohydr. Polym. 2014, 105, 260–269. [Google Scholar] [CrossRef]
- Gomez-Zavaglia, A.; Prieto Lage, M.A.; Jimenez-Lopez, C.; Mejuto, J.C.; Simal-Gandara, J. The potential of seaweeds as a source of functional ingredients of prebiotic and antioxidant value. Antioxidants 2019, 8, 406. [Google Scholar] [CrossRef]
- Kim, H.-S.; Sanjeewa, K.; Fernando, I.; Ryu, B.; Yang, H.-W.; Ahn, G.; Kang, M.C.; Heo, S.-J.; Je, J.-G.; Jeon, Y.-J. A comparative study of Sargassum horneri Korea and China strains collected along the coast of Jeju Island South Korea: Its components and bioactive properties. Algae 2018, 33, 341–349. [Google Scholar] [CrossRef]
- Choudhary, B.; Chauhan, O.; Mishra, A. Edible seaweeds: A potential novel source of bioactive metabolites and nutraceuticals with human health benefits. Front. Mar. Sci. 2021, 8, 740054. [Google Scholar] [CrossRef]
- Andrade, P.B.; Barbosa, M.; Matos, R.P.; Lopes, G.; Vinholes, J.; Mouga, T.; Valentão, P. Valuable compounds in macroalgae extracts. Food Chem. 2013, 138, 1819–1828. [Google Scholar] [CrossRef]
- Menshova, R.V.; Shevchenko, N.M.; Imbs, T.I.; Zvyagintseva, T.N.; Malyarenko, O.S.; Zaporoshets, T.S.; Besednova, N.N.; Ermakova, S.P. Fucoidans from brown alga Fucus evanescens: Structure and biological activity. Front. Mar. Sci. 2016, 3, 129. [Google Scholar] [CrossRef]
- Di, T.; Chen, G.; Sun, Y.; Ou, S.; Zeng, X.; Ye, H. In vitro digestion by saliva, simulated gastric and small intestinal juices and fermentation by human fecal microbiota of sulfated polysaccharides from Gracilaria rubra. J. Funct. Foods 2018, 40, 18–27. [Google Scholar] [CrossRef]
- Bae, M.; Kim, M.-B.; Park, Y.-K.; Lee, J.-Y. Health benefits of fucoxanthin in the prevention of chronic diseases. BBA-Mol. Cell Biol. Lipids 2020, 1865, 158618. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.-J.; Yoon, W.-J.; Kim, K.-N.; Ahn, G.-N.; Kang, S.-M.; Kang, D.-H.; Oh, C.; Jung, W.-K.; Jeon, Y.-J. Evaluation of anti-inflammatory effect of fucoxanthin isolated from brown algae in lipopolysaccharide-stimulated RAW 264.7 macrophages. Food Chem. Toxicol. 2010, 48, 2045–2051. [Google Scholar] [CrossRef] [PubMed]
- Shiratori, K.; Ohgami, K.; Ilieva, I.; Jin, X.-H.; Koyama, Y.; Miyashita, K.; Yoshida, K.; Kase, S.; Ohno, S. Effects of fucoxanthin on lipopolysaccharide-induced inflammation in vitro and in vivo. Exp. Eye Res. 2005, 81, 422–428. [Google Scholar] [CrossRef]
- Yang, Y.-P.; Tong, Q.-Y.; Zheng, S.-H.; Zhou, M.-D.; Zeng, Y.-M.; Zhou, T.-T. Anti-inflammatory effect of fucoxanthin on dextran sulfate sodium-induced colitis in mice. Nat. Prod. Res. 2020, 34, 1791–1795. [Google Scholar] [CrossRef]
- Iwashima, M.; Mori, J.; Ting, X.; Matsunaga, T.; Hayashi, K.; Shinoda, D.; Saito, H.; Sankawa, U.; Hayashi, T. Antioxidant and antiviral activities of plastoquinones from the brown alga Sargassum micracanthum, and a new chromene derivative converted from the plastoquinones. Biol. Pharm. Bull. 2005, 28, 374–377. [Google Scholar] [CrossRef]
- Yoon, W.-J.; Heo, S.-J.; Han, S.-C.; Lee, H.-J.; Kang, G.-J.; Kang, H.-K.; Hyun, J.-W.; Koh, Y.-S.; Yoo, E.-S. Anti-inflammatory effect of sargachromanol G isolated from Sargassum siliquastrum in RAW 264.7 cells. Arch. Pharm. Res. 2012, 35, 1421–1430. [Google Scholar] [CrossRef]
- Han, E.-J.; Zhang, C.; Kim, H.-S.; Kim, J.-Y.; Park, S.-M.; Jung, W.-K.; Ahn, G.; Cha, S.-H. Sargachromenol Isolated from Sargassum horneri Attenuates Glutamate-Induced Neuronal Cell Death and Oxidative Stress through Inhibition of MAPK/NF-κB and Activation of Nrf2/HO-1 Signaling Pathway. Mar. Drugs 2022, 20, 710. [Google Scholar] [CrossRef]
- Weichhart, T.; Hengstschläger, M.; Linke, M. Regulation of innate immune cell function by mTOR. Nat. Rev. Immunol. 2015, 15, 599–614. [Google Scholar] [CrossRef]
- Gutiérrez-Martínez, I.; Rubio, J.; Piedra-Quintero, Z.; Lopez-Mendez, O.; Serrano, C.; Reyes-Maldonado, E.; Salinas-Lara, C.; Betanzos, A.; Shibayama, M.; Silva-Olivares, A. mTORC1 prevents epithelial damage during inflammation and inhibits colitis-associated colorectal cancer development. Transl. Oncol. 2019, 12, 24–35. [Google Scholar] [CrossRef]
- Cao, Y.; Han, S.; Lu, H.; Luo, Y.; Guo, T.; Wu, Q.; Luo, F. Targeting mTOR Signaling by Dietary Polyphenols in Obesity Prevention. Nutrients 2022, 14, 5171. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zheng, J.; Zhang, Y.; Wang, Z.; Yang, Y.; Bai, M.; Dai, Y. Fucoxanthin activates apoptosis via inhibition of PI3K/Akt/mTOR pathway and suppresses invasion and migration by restriction of p38-MMP-2/9 pathway in human glioblastoma cells. Neurochem. Res. 2016, 41, 2728–2751. [Google Scholar] [CrossRef] [PubMed]
- Tomasello, G.; Mazzola, M.; Leone, A.; Sinagra, E.; Zummo, G.; Farina, F.; Damiani, P.; Cappello, F.; Gerges Geagea, A.; Jurjus, A. Nutrition, oxidative stress and intestinal dysbiosis: Influence of diet on gut microbiota in inflammatory bowel diseases. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech. Repub. 2016, 160, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Ullah, N.; Zha, L.; Bai, Y.; Khan, A.; Zhao, T.; Che, T.; Zhang, C. Alteration of gut microbiota in inflammatory bowel disease (IBD): Cause or consequence? IBD treatment targeting the gut microbiome. Pathogens 2019, 8, 126. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhi, F. Lower level of bacteroides in the gut microbiota is associated with inflammatory bowel disease: A meta-analysis. Biomed Res. Int. 2016, 2016, 5828959. [Google Scholar] [CrossRef]
- Gophna, U.; Sommerfeld, K.; Gophna, S.; Doolittle, W.F.; Veldhuyzen van Zanten, S.J. Differences between tissue-associated intestinal microfloras of patients with Crohn’s disease and ulcerative colitis. J. Clin. Microbiol. 2006, 44, 4136–4141. [Google Scholar] [CrossRef]
- Manichanh, C.; Rigottier-Gois, L.; Bonnaud, E.; Gloux, K.; Pelletier, E.; Frangeul, L.; Nalin, R.; Jarrin, C.; Chardon, P.; Marteau, P. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut 2006, 55, 205–211. [Google Scholar] [CrossRef]
- Corrêa, T.A.F.; Rogero, M.M.; Hassimotto, N.M.A.; Lajolo, F.M. The two-way polyphenols-microbiota interactions and their effects on obesity and related metabolic diseases. Front. Nutr. 2019, 6, 188. [Google Scholar] [CrossRef]
- Lavefve, L.; Howard, L.R.; Carbonero, F. Berry polyphenols metabolism and impact on human gut microbiota and health. Food Funct. 2020, 11, 45–65. [Google Scholar] [CrossRef]
- da Silva-Maia, J.K.; Batista, Â.G.; Cazarin, C.B.B.; Soares, E.S.; Bogusz Junior, S.; Leal, R.F.; da Cruz-Höfling, M.A.; Maróstica Junior, M.R. Aqueous extract of Brazilian berry (Myrciaria jaboticaba) peel improves inflammatory parameters and modulates Lactobacillus and Bifidobacterium in rats with induced-colitis. Nutrients 2019, 11, 2776. [Google Scholar] [CrossRef]
- Lee, G.; Midorikawa, Y.; Kuda, T.; Harada, M.; Fujita, S.; Takahashi, H.; Kimura, B. In vitro antioxidant and anti-glycation properties of Sargassum horneri from golden tides on the South Korean coast and the effect on gut microbiota of mice fed a high-sucrose and low-fibre diet. J. Appl. Phycol. 2022, 34, 2211–2222. [Google Scholar] [CrossRef]
- Kim, S.M.; Jung, Y.-J.; Kwon, O.-N.; Cha, K.H.; Um, B.-H.; Chung, D.; Pan, C.-H. A potential commercial source of fucoxanthin extracted from the microalga Phaeodactylum tricornutum. Appl. Biochem. Biotechnol. 2012, 166, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, G.; Ye, Y.; Zhao, J.; Zhou, C.; Zhu, J.; Li, Y.; Zhang, J.; Yan, X. High-Purity Fucoxanthin Can Be Efficiently Prepared from Isochrysis zhangjiangensis by Ethanol-Based Green Method Coupled with Octadecylsilyl (ODS) Column Chromatography. Mar. Drugs 2022, 20, 510. [Google Scholar] [CrossRef] [PubMed]
- Sanjeewa, K.; Jayawardena, T.U.; Kim, H.-S.; Kim, S.-Y.; Ahn, G.; Kim, H.-J.; Fu, X.; Jee, Y.; Jeon, Y.-J. Ethanol extract separated from Sargassum horneri (Turner) abate LPS-induced inflammation in RAW 264.7 macrophages. Fish. Aquat. Sci. 2019, 22, 6. [Google Scholar] [CrossRef]
- Swain, T.; Hillis, W. The phenolic constituents of Prunus domestica. I.—The quantitative analysis of phenolic constituents. J. Sci. Food Agric. 1959, 10, 63–68. [Google Scholar] [CrossRef]
- Cassano, A.; Conidi, C.; Ruby-Figueroa, R. Recovery of flavonoids from orange press liquor by an integrated membrane process. Membranes 2014, 4, 509–524. [Google Scholar] [CrossRef]
- Kim, D.; Yan, J.; Bak, J.; Park, J.; Lee, H.; Kim, H. Sargassum thunbergii Extract Attenuates High-Fat Diet-Induced Obesity in Mice by Modulating AMPK Activation and the Gut Microbiota. Foods 2022, 11, 2529. [Google Scholar] [CrossRef]
- Kim, H.; Lee, M.J.; Bae, E.-H.; Ryu, J.S.; Kaur, G.; Kim, H.J.; Kim, J.Y.; Barreda, H.; Jung, S.Y.; Choi, J.M. Comprehensive molecular profiles of functionally effective MSC-derived extracellular vesicles in immunomodulation. Mol. Ther. 2020, 28, 1628–1644. [Google Scholar] [CrossRef]
- Wei, C.-X.; Wu, J.-H.; Huang, Y.-H.; Wang, X.-Z.; Li, J.-Y. Lactobacillus plantarum improves LPS-induced Caco2 cell line intestinal barrier damage via cyclic AMP-PKA signaling. PLoS ONE 2022, 17, e0267831. [Google Scholar] [CrossRef]
- Kim, K.-M.; Kim, Y.-S.; Lim, J.Y.; Min, S.J.; Ko, H.-C.; Kim, S.-J.; Kim, Y. Intestinal anti-inflammatory activity of Sasa quelpaertensis leaf extract by suppressing lipopolysaccharide-stimulated inflammatory mediators in intestinal epithelial Caco-2 cells co-cultured with RAW 264.7 macrophage cells. Nutr. Res. Pract. 2015, 9, 3–10. [Google Scholar] [CrossRef]
- Sun, J.; Chen, H.; Kan, J.; Gou, Y.; Liu, J.; Zhang, X.; Wu, X.; Tang, S.; Sun, R.; Qian, C. Anti-inflammatory properties and gut microbiota modulation of an alkali-soluble polysaccharide from purple sweet potato in DSS-induced colitis mice. Int. J. Biol. Macromol. 2020, 153, 708–722. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.-C.; Lee, H.G.; Lee, S.H.; Song, K.-M.; Kim, H.-S.; Kim, S.; Choi, Y.-S.; Jeon, Y.-J. Sargassum horneri inhibits fat accumulation via up-regulation of thermogenesis in obese mice. J. Funct. Foods 2022, 92, 105022. [Google Scholar] [CrossRef]
- Kim, H.I.; Kim, D.-S.; Jung, Y.; Sung, N.-Y.; Kim, M.; Han, I.-J.; Nho, E.Y.; Hong, J.H.; Lee, J.-K.; Boo, M. Immune-Enhancing Effect of Sargassum horneri on Cyclophosphamide-Induced Immunosuppression in BALB/c Mice and Primary Cultured Splenocytes. Molecules 2022, 27, 8253. [Google Scholar] [CrossRef] [PubMed]
- Erben, U.; Loddenkemper, C.; Doerfel, K.; Spieckermann, S.; Haller, D.; Heimesaat, M.M.; Zeitz, M.; Siegmund, B.; Kühl, A.A. A guide to histomorphological evaluation of intestinal inflammation in mouse models. Int. J. Clin. Exp. Pathol. 2014, 7, 4557. [Google Scholar]
- Pandurangan, A.K.; Mohebali, N.; Esa, N.M.; Looi, C.Y.; Ismail, S.; Saadatdoust, Z. Gallic acid suppresses inflammation in dextran sodium sulfate-induced colitis in mice: Possible mechanisms. Int. Immunopharmacol. 2015, 28, 1034–1043. [Google Scholar] [CrossRef]
- Zhu, Y.; Guo, J.; Hu, X.; Liu, J.; Li, S.; Wang, J. Eckol protects against acute experimental colitis in mice: Possible involvement of Reg3g. J. Funct. Foods 2020, 73, 104088. [Google Scholar] [CrossRef]
- Singh, V.; San Yeoh, B.; Chassaing, B.; Zhang, B.; Saha, P.; Xiao, X.; Awasthi, D.; Shashidharamurthy, R.; Dikshit, M.; Gewirtz, A. Microbiota-inducible innate immune siderophore binding protein lipocalin 2 is critical for intestinal homeostasis. Cell. Mol. Gastroenterol. Hepatol. 2016, 2, 482–498.e6. [Google Scholar] [CrossRef]
- Kolli, V.K.; Abraham, P.; Isaac, B.; Selvakumar, D. Neutrophil infiltration and oxidative stress may play a critical role in methotrexate-induced renal damage. Chemotherapy 2009, 55, 83–90. [Google Scholar] [CrossRef]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.; Chan, F.K. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiang, Q. Roles of the polyphenol–gut microbiota interaction in alleviating colitis and preventing colitis-associated colorectal cancer. Adv. Nutr. 2021, 12, 546–565. [Google Scholar] [CrossRef]
- Martin, D.A.; Bolling, B.W. A review of the efficacy of dietary polyphenols in experimental models of inflammatory bowel diseases. Food Funct. 2015, 6, 1773–1786. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.; Chen, K.; Tong, Z.; Chen, L.; Chen, Q.; Su, J. Advances in Fucoxanthin Research for the Prevention and Treatment of Inflammation-Related Diseases. Nutrients 2022, 14, 4768. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.-J.; Bae, Y.-S.; Ju, S.M.; Youn, G.S.; Choi, S.Y.; Park, J. Salicortin suppresses lipopolysaccharide-stimulated inflammatory responses via blockade of NF-κB and JNK activation in RAW 264.7 macrophages. BMB Rep. 2014, 47, 318. [Google Scholar] [CrossRef] [PubMed]
- Babaei, F.; Moafizad, A.; Darvishvand, Z.; Mirzababaei, M.; Hosseinzadeh, H.; Nassiri-Asl, M. Review of the effects of vitexin in oxidative stress-related diseases. Food Sci. Nutr. 2020, 8, 2569–2580. [Google Scholar] [CrossRef]
- Wu, X.-F.; Ouyang, Z.-J.; Feng, L.-L.; Chen, G.; Guo, W.-J.; Shen, Y.; Wu, X.-D.; Sun, Y.; Xu, Q. Suppression of NF-κB signaling and NLRP3 inflammasome activation in macrophages is responsible for the amelioration of experimental murine colitis by the natural compound fraxinellone. Toxicol. Appl. Pharmacol. 2014, 281, 146–156. [Google Scholar] [CrossRef]
- Gwon, W.-G.; Joung, E.-J.; Kwon, M.-S.; Lim, S.-J.; Utsuki, T.; Kim, H.-R. Sargachromenol protects against vascular inflammation by preventing TNF-α-induced monocyte adhesion to primary endothelial cells via inhibition of NF-κB activation. Int. Immunopharmacol. 2017, 42, 81–89. [Google Scholar] [CrossRef]
- Kim, H.; Banerjee, N.; Barnes, R.C.; Pfent, C.M.; Talcott, S.T.; Dashwood, R.H.; Mertens-Talcott, S.U. Mango polyphenolics reduce inflammation in intestinal colitis—Involvement of the miR-126/PI3K/AKT/mTOR axis in vitro and in vivo. Mol. Carcinog. 2017, 56, 197–207. [Google Scholar] [CrossRef]
- Hu, J.E.; Weiß, F.; Bojarski, C.; Branchi, F.; Schulzke, J.D.; Fromm, M.; Krug, S.M. Expression of tricellular tight junction proteins and the paracellular macromolecule barrier are recovered in remission of ulcerative colitis. BMC Gastroenterol. 2021, 21, 141. [Google Scholar] [CrossRef]
- Lee, M.; Lee, S.H.; Kang, J.; Yang, H.; Jeong, E.J.; Kim, H.P.; Kim, Y.C.; Sung, S.H. Salicortin-derivatives from Salix pseudo-lasiogyne twigs inhibit adipogenesis in 3T3-L1 cells via modulation of C/EBPα and SREBP1c dependent pathway. Molecules 2013, 18, 10484–10496. [Google Scholar] [CrossRef]
- Pagliari, D.; Saviano, A.; Newton, E.; Serricchio, M.; Dal Lago, A.; Gasbarrini, A.; Cianci, R. Gut microbiota-immune system crosstalk and pancreatic disorders. Mediators Inflamm. 2018, 2018, 7946431. [Google Scholar] [CrossRef]
- Morgan, X.C.; Tickle, T.L.; Sokol, H.; Gevers, D.; Devaney, K.L.; Ward, D.V.; Reyes, J.A.; Shah, S.A.; LeLeiko, N.; Snapper, S.B. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012, 13, R79. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, K.; Kanai, T. The gut microbiota and inflammatory bowel disease. Semin. Immunopathol. 2015, 37, 47–55. [Google Scholar] [CrossRef]
- Shang, Q.; Shan, X.; Cai, C.; Hao, J.; Li, G.; Yu, G. Dietary fucoidan modulates the gut microbiota in mice by increasing the abundance of Lactobacillus and Ruminococcaceae. Food Funct. 2016, 7, 3224–3232. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.N.; Yu, J.G.; Zhang, D.B.; Zhang, Z.; Ren, L.L.; Li, L.H.; Wang, Z.; Tang, Z.S. Indigo Naturalis Ameliorates Dextran Sulfate Sodium-Induced Colitis in Mice by Modulating the Intestinal Microbiota Community. Molecules 2019, 24, 4086. [Google Scholar] [CrossRef] [PubMed]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [PubMed]
- Gu, W.; Zhang, L.; Han, T.; Huang, H.; Chen, J. Dynamic Changes in Gut Microbiome of Ulcerative Colitis: Initial Study from Animal Model. J. Inflamm. Res. 2022, 15, 2631. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wu, X.; Cao, S.; Wang, L.; Wang, D.; Yang, H.; Feng, Y.; Wang, S.; Li, L. Caffeic acid ameliorates colitis in association with increased Akkermansia population in the gut microbiota of mice. Oncotarget 2016, 7, 31790–31799. [Google Scholar] [CrossRef]
- Tong, L.; Zhang, X.; Hao, H.; Liu, Q.; Zhou, Z.; Liang, X.; Liu, T.; Gong, P.; Zhang, L.; Zhai, Z.; et al. Lactobacillus rhamnosus GG Derived Extracellular Vesicles Modulate Gut Microbiota and Attenuate Inflammatory in DSS-Induced Colitis Mice. Nutrients 2021, 13, 3319. [Google Scholar] [CrossRef]
- Liu, Y.; Li, W.; Yang, H.; Zhang, X.; Wang, W.; Jia, S.; Xiang, B.; Wang, Y.; Miao, L.; Zhang, H.; et al. Leveraging 16S rRNA Microbiome Sequencing Data to Identify Bacterial Signatures for Irritable Bowel Syndrome. Front. Cell. Infect. Microbiol. 2021, 11, 645951. [Google Scholar] [CrossRef]
- Tan, H.; Zhai, Q.; Chen, W. Investigations of Bacteroides spp. towards next-generation probiotics. Food Res. Int. 2019, 116, 637–644. [Google Scholar] [CrossRef]
- Tojo, R.; Suárez, A.; Clemente, M.G.; de los Reyes-Gavilán, C.G.; Margolles, A.; Gueimonde, M.; Ruas-Madiedo, P. Intestinal microbiota in health and disease: Role of bifidobacteria in gut homeostasis. World J. Gastroenterol. 2014, 20, 15163. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; de Vos, W.M. Next-generation beneficial microbes: The case of Akkermansia muciniphila. Front. Microbiol. 2017, 8, 1765. [Google Scholar] [CrossRef] [PubMed]
- Bábíčková, J.; Tóthová, Ľ.; Lengyelová, E.; Bartoňová, A.; Hodosy, J.; Gardlík, R.; Celec, P. Sex differences in experimentally induced colitis in mice: A role for estrogens. Inflammation 2015, 38, 1996–2006. [Google Scholar] [CrossRef] [PubMed]
- Han, E.J.; Fernando, I.P.S.; Kim, H.-S.; Jeon, Y.-J.; Madusanka, D.M.D.; Dias, M.K.H.M.; Jee, Y.; Ahn, G. Oral administration of sargassum horneri improves the HDM/DNCB-induced atopic dermatitis in NC/Nga mice. Nutrients 2020, 12, 2482. [Google Scholar] [CrossRef]
- Herath, K.H.I.N.M.; Mihindukulasooriya, S.P.; Kim, H.J.; Kim, A.; Kim, H.J.; Jeon, Y.-J.; Jee, Y. Oral administration of polyphenol-rich Sargassum horneri suppresses particulate matter exacerbated airway inflammation in murine allergic asthma: Relevance to the TLR mediated NF-κB pathway inhibition. J. Funct. Foods 2020, 71, 103991. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Yeo, M.-H.; Yoon, S.-A.; Hyun, H.-B.; Ham, Y.-M.; Jung, Y.-H.; Chang, K.-S. Effects of Sargassum horneri and Ulva australis extracts on body weight and serum glucose levels of Sprague-Dawley rats. Prev. Nutr. Food Sci. 2021, 26, 307. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).