1. Introduction
Aluminium and aluminium alloys, as foils or sheets, are increasingly used in several fields. The main markets where aluminium laminates are used are rigid, semi-rigid and flexible packaging (
Figure 1), heating, automotive, construction, aeronautics, pharmaceutical sector, etc. For instance, household foils, rigid and semi-rigid food containers, bottle caps, packs for pills, car bodies, doors and roofs of some cars, cooling fins of air conditioners, aircraft fuselages and their floorings, etc., are made of aluminium. In 2021, global aluminium consumption reached approximately 64.2 million tons. The market for flat-rolled aluminium products exceeded 28.1 million tons in the same year. In particular, the foil-stock and packaging sectors constitute 9% and 8%, respectively, of the total aluminium market. It is expected that the annual growth rate in this sector will be 2.6%. It has been estimated that 40% of the total world-aluminium-production is in the form of rolled products. Due to the large quantity of material utilised, the waste production of the industrial processes employed to treat/modify the Al-alloy items’ surface, is heavy. At the same time, to reduce the environmental impact, companies are also interested in reducing the amount of natural resources consumed in their processes, ensuring the quality of the marketed products.
It is known that primary aluminium production is energy-intensive: 1 kg of primary aluminium production needs 15 kWh, while 0.75 kWh is needed to produce secondary aluminium. Thus, the companies use waste aluminium from their production and, when possible, materials from waste sorting. In addition, the need to decrease production costs has pushed the aluminium industry toward reducing sheet thickness when possible. For this reason, the aluminium alloy must have specific chemical/physical characteristics; in particular, it must be able to undergo large plastic deformations without presenting surface defects or breaks in the film. One of the most used aluminium series to make laminates is the 8000 one. It belongs to the “strain-hardenable” type, which includes alloys containing several types of alloying elements. In particular, in this experimental campaign, the AA8079 alloy was used, in which the chemical composition is characterised by the presence of Fe and Si [
1,
2], even if, sometimes, lithium is indicated as the characterising element [
3].
Relatively high iron-levels in the alloy determine combinations of strength and ductility. These properties are due to the fine grain-size stabilised by the finely dispersed iron-rich second phase. The alloying elements improving the mechanical properties (i.e., ductility) allow the obtaining of extremely thin films (up to 10 µm thick) without showing any defect such as streaks on the film surface or tears in the laminated material. Unfortunately, some alloying elements, such as Fe and Cu, reduce the material corrosion-resistance. From an electrochemical point of view, they are nobler than Al, therefore promoting the formation of local corrosion cells by galvanic coupling between the cathodic intermetallic particles formed between the Al and the alloying elements and the anodic aluminium matrix. Corrosion develops at the grain boundary where intermetallic particles are segregated, leading to various types of corrosion [
4]. Several surface treatments (anodisation, conversion coating, painting) are used to increase the item’s service life. The degradation rate of the material is even faster when in contact with acidic and/or chloride-containing environments. These conditions are frequently found when the aluminium items are used as food containers or heat-exchanger fins. In addition, special attention must be paid when the products come into contact with food, to avoid their contamination with substances harmful to health.
Structural alloys, such as AA2024 and AA7075, are probably the most studied aluminium alloys [
5,
6,
7]. In the same manner, several papers deal with the improvement in corrosion resistance of the 3000- and 6000-series alloys [
6,
8,
9]; in contrast, very few papers focussed their attention on the AA8079 alloy, which shows several remarkable properties, making it suitable to be used in the numerous applications mentioned before [
10,
11]. As well as this, the scientific literature presents many papers dealing with the formation and characterisation of so-called “Cr-free” rinse conversion-layers of aluminium alloys (containing CrIII [
12,
13], while very few address their attention to the no-rinse “true-Cr-free” process [
14]. Although the idea of using no-rinse “true-Cr-free” processes has been patented since 1988 [
15], costs and product performances have delayed their industrial use until now.
This work aimed to study the protective properties of a “true” chromium-free, no-rinse surface-treatment of the AA8079 to be used as a replacement for rinse chromium III-based processes, taking into account also the natural-resource consumption, and comparing the process costs. It will be highlighted that the no-rinse, “true” chromium-free conversion-coating industrial process exhibits similar, if not better, protective properties when compared to the rinse CrIII-based one, allowing for the reduction in the environmental impact and production costs.
2. The Conversion-Coating Process
To enhance the corrosion resistance of aluminium alloys and increase organic coatings’ adhesion to the surface, aluminium parts are subjected to a chemical or electrochemical treatment, to form protective inorganic films. When chemical treatments are employed, the most widely used method involves depositing “conversion coatings” on the surface to be protected [
16].
Efficient protective systems have been developed over the years by using chromium-based solutions. The best results were obtained employing CrVI-based acidic aqueous solutions, which, on the other hand, proved to be carcinogenic and harmful to the environment. More recently, the so-called “Cr-free” rinse treatments have become commercially available, showing a lower environmental impact and being less harmful to humans. These products are CrIII or phosphate-chromate aqueous-solutions-based, still causing heavy environmental impact, due to the use of a massive quantity of water needed in the process and the need for waste disposal. The latter contains CrIII, CrVI or chromate-phosphate salts, among other chemicals, dissolved in acidic aqueous solutions that must be appropriately disposed of at the end of the industrial process. Therefore, the need to use eco-friendly goods for the corrosion protection of aluminium alloys, eliminating the Cr from the chemical formulation, has led to the development of products containing Zr, Ti, Y, Ce, etc., showing reduced environmental impact [
17].
The conversion coatings should guarantee high corrosion-resistance, delaying the oxidation of the metal substrate and allowing good adhesion of paints or organic films. An ideal conversion layer, therefore, should have characteristics such as:
Continuity and impermeability to gas and liquids;
Inertia, or at least insolubility, in the environment in which it operates;
Inhibition of metal-substrate attack by electrolytes;
High resistance to mechanical damage from abrasion, scratching, or, if weak and of reduced thickness, they should exhibit self-repairing properties;
Promoting the adhesion of paints or other organic coatings.
3. Comparison of Rinse and No-Rinse Processes
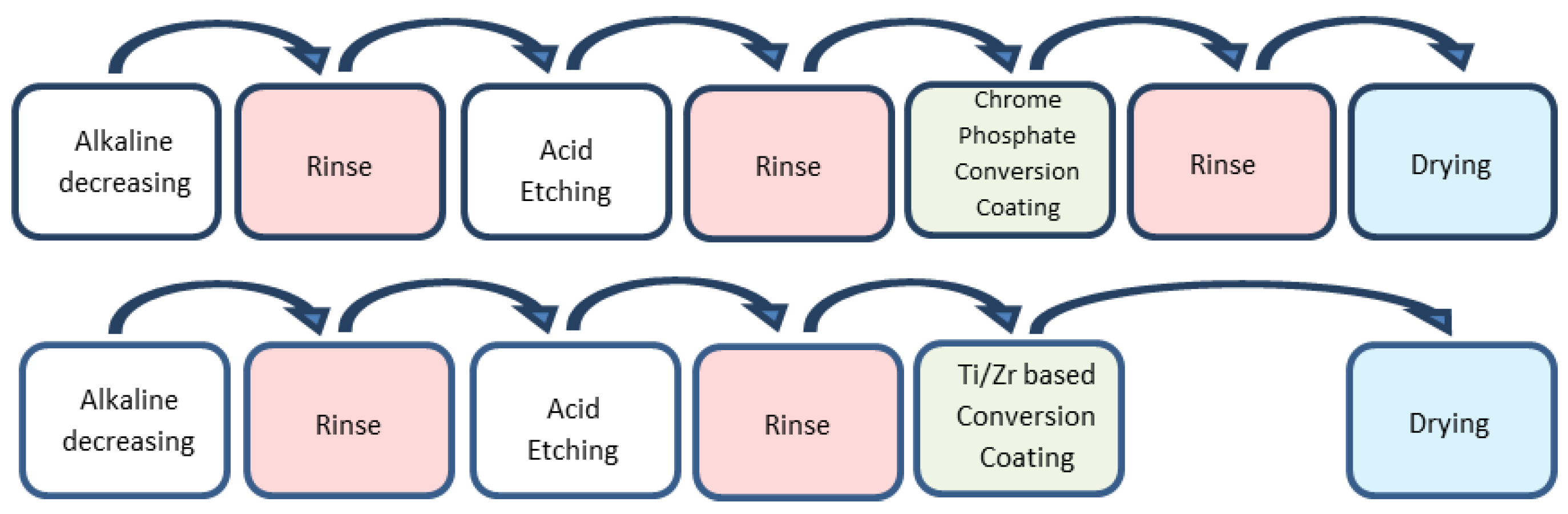
Substantial differences exist between the rinse and no-rinse conversion-coating systems, regardless of the presence of heavy metals in the process fluids. Both conversion treatments are carried out using multiple-process steps (
Figure 2).
In the case of the rinse process, the metal sheet/foil is immersed in a degreasing bath (often an alkaline aqueous solution) to remove oil and grease due to rolling, followed by subsequent washing with demineralised water. After this, an acid etching is required to “desmut” the metal surface; then, after another rinsing, it is possible to treat the metal by applying the conversion coating by dipping the sheet/foil in an aqueous solution containing chromic acid (CrVI) or chromate-phosphate acidic aqueous solution (containing CrIII), followed by the last rinsing-bath. Usually, each washing step is performed by using two washing tanks, separated from each other, to ensure the cleaning of the rolled parts. The active ingredients react with the metal surface, forming the protective saline deposit. Then, the laminate has to be dried in an oven to form a solid layer on the surface. In this process, reaction products are also formed in the bulk of the conversion bath. They do not deposit on the laminate and pollute the solution, which is therefore subject to ageing and must be frequently renewed. Therefore, the rinse process leads to the formation of sludge to be disposed of as special waste, as well as larger water consumption.
The first four steps (alkaline degreasing, rinse, acid etching and rinse) of the no-rinse treatments are carried out following the same steps as in the rinse process. The deposition/formation of the conversion layer differs greatly, as the last phase of the material washing is absent. Furthermore, the no-rinse treatments differ from the conventional ones also for the following reasons: a) the solutions used are much more concentrated, so they are usually diluted before use; b) they can be spread, and this implies a different mechanism of film formation, if compared to the rinse process; c) the no-rinse product contains an organic film-forming part (typically consisting of a polyvinyl-phenolic resin), allowing a uniform film-thickness of the deposited layer; d) in addition, a better dosage of the active ingredients allows for reducing the amount of product used in the process. For those reasons, no-rinse products are called “for total consumption”, meaning that the product utilised in the treatment plant is fully deposited on the substrate. In this way, it is possible to avoid the ageing of the treatment bath and the production of waste. Therefore, the no-rinse products, unlike the rinse ones, do not require a washing downstream to the pre-treatment. Their use allows the elimination of wastewater polluted by the active ingredients and then the subsequent sludge-disposal, thus reducing costs and carrying out the process with a lower environmental-impact.
Figure 3 shows a picture of the no-rinse conversion-coating plant.
In conclusion, the no-rinse process shows several benefits and few drawbacks, compared to the rinse one, as reported in
Table 1.
The rinse process takes place by immersing the aluminium-alloy sheets in the treatment solution, ensuring that both sides of the foils are treated in the same manner, showing the same amount of deposited product. When using the no-rinse process, it could occur that, due to non-perfect spray nozzles or roller-coater alignment, the amount of material deposited could differ between the two faces of the sheet. Both processes are continuous, showing treatment speeds of up to 200 m/min. The no-rinse process allows the use of smaller-sized treatment plants, due to the lack of at least two washing-tanks. It is estimated that the no-rinse plants take up approximately 30% less surface than the rinse ones. However, it is necessary to consider that rinse-type processes ensure an easier execution than the no-rinse one; the treatment products have lower costs-per-unit of mass than those used in the no-rinse treatment, but the aqueous no-rinse solution is more diluted if compared to the rinse one. Moreover, if there is a need to change the product used for the surface treatment, the tank containing it must be emptied (with the consequent associated costs) when using the rinse treatment, while there is no need for this when utilising the no-rinse one.
4. Environmental Impact and Cost Comparison
As already stated, the no-rinse processes, unlike the rinse ones, do not require the washing downstream of the pre-treatment phase, but only the drying. It has been estimated that the no-rinse process uses approximately 40% less water; accordingly, 40% more water must be treated/purified in order to be disposed of when using the rinse-type process. This allows the elimination of the production of polluted wastewater and the disposal of sludge, carrying out the process with a lower environmental-impact, and reducing costs.
“True-Cr-free” no-rinse conversion-coatings are not widely utilised in industries, because they are believed to exhibit poor performances and to be expensive compared to CrIII-based rinse-type treatments. This paper aims to demonstrate that some of no-rinse “true Cr-free” products possess performances similar to, if not better, than the rinse CrIII-based one, highlighting the use of “true Cr-free” no-rinse conversion coatings permitting the reduction of the process’s environmental impact and diminishing the production costs.
5. Materials and Methods
The aluminium-alloy-8079 nominal chemical-composition used in this work is reported in
Table 2.
Samples (150 × 100 × 0.2 mm
3 in size) were taken from a coil treated in the Laminazione Sottile S.p.A industrial plant, which supplied all materials used. The composition and the name of all commercial products used to treat/coat the metallic substrates are held as a trade secret. The corrosion behaviour of bare samples, conversion-coated samples and samples painted after the chemical-conversion process was studied.
Table 3 reports the acronyms, materials and processes used in the experimental procedure.
The conversion-layers deposition was carried out by using two types of commercial products: the first containing chromium and phosphorus, which provides a washing cycle at the end of the treatment, and the second based on titanium and zirconium salts, which, does not require it.
The CrIII-containing product forms a chrome-phosphate chemical conversion-coating on the aluminium substrate. The conversion-coating colour ranges from light blue-green to a darker green. It is a well-known water-based solution, utilised for many years, and showing remarkable performances in slowing down the corrosion rate of organic-coated aluminium alloys. The no-rinse conversion solution, free of chromium, containing a polyvinyl phenol-based resin, forms a thin conversion-layer (<20 nm) of Ti and Zr salts. It possesses one more attractive property, as declared by the manufacturer, which consists of a significant increase in adhesion for organic finishes, paint, sealers and most adhesives.
The organic coating used in this study consisted of a polyester-based system, an aromatic polyester-based resin with melamine containing a non-volatile fraction of 52 wt%. The thickness of the deposited organic-layer was 25 µm. All coated samples were produced using the same batch of paint and cured in an oven to avoid any possible differences between samples caused by the different procedures or materials used.
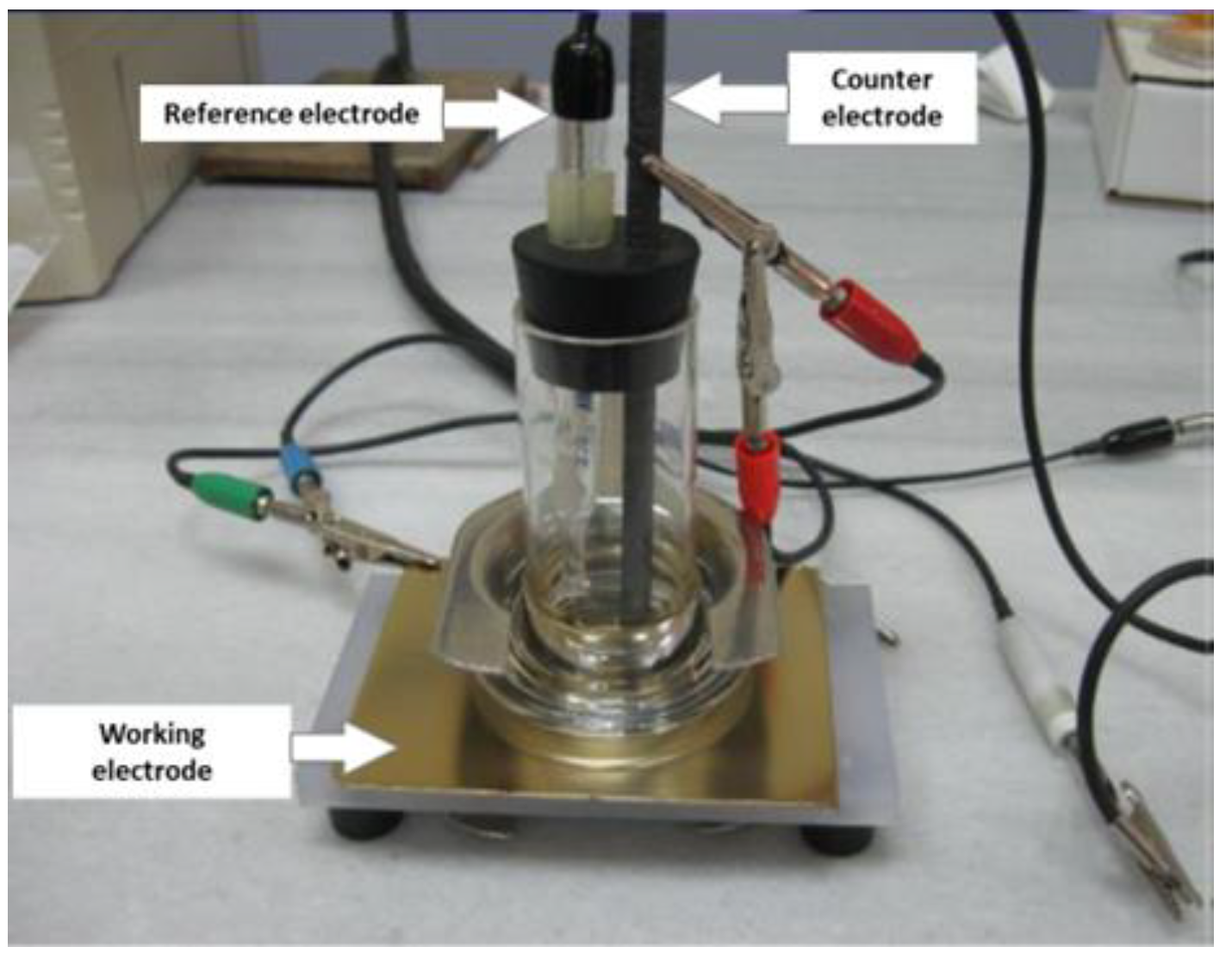
The electrochemical analysis was carried out through potentiodynamic-polarisation and electrochemical-impedance-spectroscopy (EIS) tests, using the Gamry Reference 600-05123 potentiostat (Gamry, Warminster, PA, USA), coupled to a conventional three-electrode electrolytic cell (
Figure 4), made of a platinum counter electrode, a saturated calomel electrode (SCE) as a reference electrode, and the sample under test as the working electrode. The electrolytic solution used was an aerated aqueous 3.5 wt.% of NaCl solution. The tests were carried out at room temperature, under static conditions, exposing an area of 13.8 cm
2. The open-circuit-potential (OCP) measurement was carried out for 30 min before the potentiodynamic curves were recorded at a scanning rate of 0.167 mV/s. The EIS tests were performed under the same experimental conditions as the potentiodynamic-polarisation, in the 2 × 10
−2–1 × 10
5 Hz frequency range. At least three tests were carried out for each sample, to verify the repeatability of the tests.
6. Results and Discussion
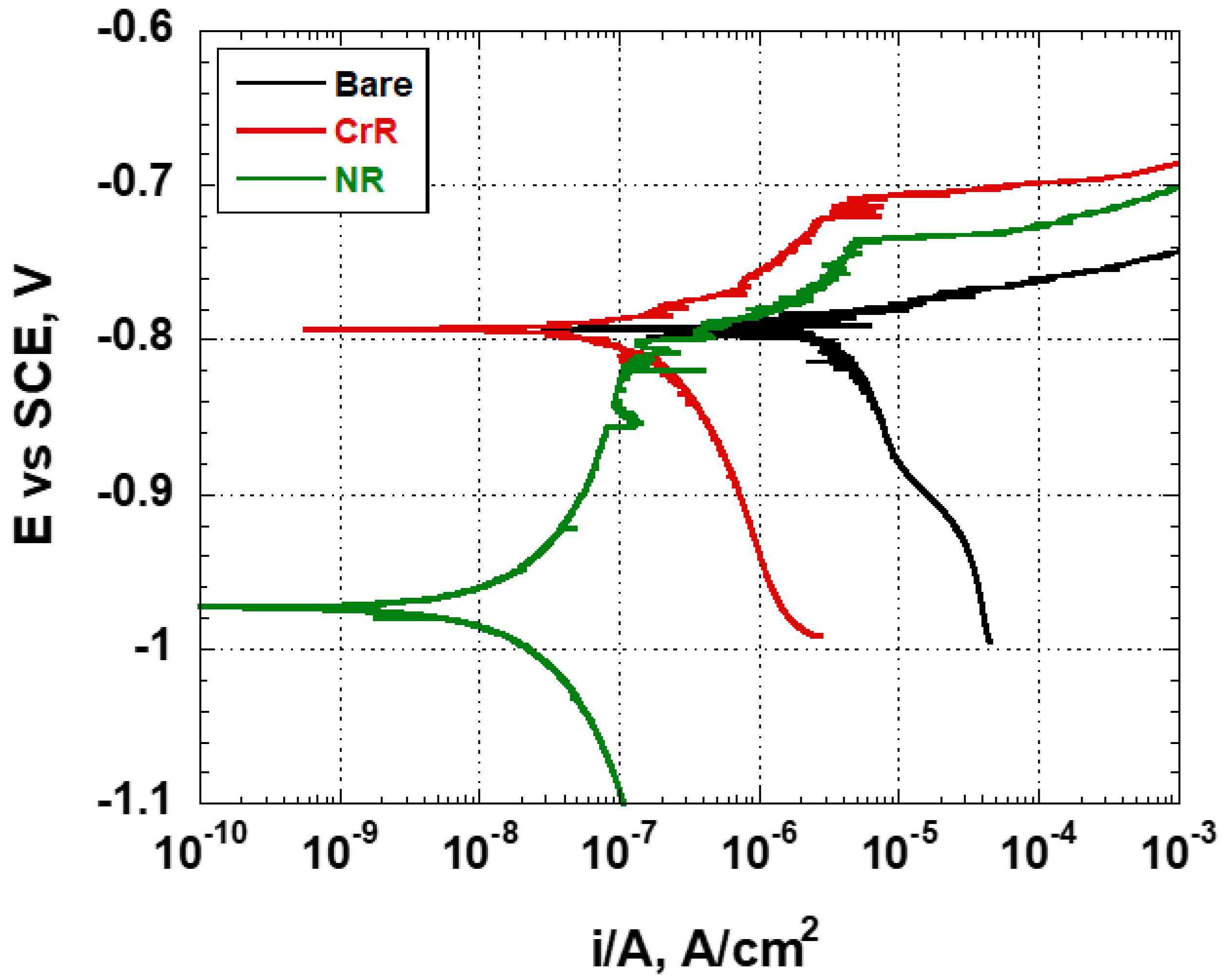
The potentiodynamic-polarisation curves recorded on the bare, CrR and NR samples are shown in
Figure 5.
All specimens show the expected behaviour of Al alloys when in contact with an aqueous solution containing chlorides. The picture shows that the NR sample exhibited a quite different corrosion potential from the other two samples, while all samples were affected by pitting corrosion. In more detail, the curves analysis highlights the fact that the sample NR stands apart, with a corrosion potential equal to −0.975 V vs. SCE, significantly different from that shown by the bare and CrR samples for which, in both cases, the corrosion potential (E
corr) is equal to −0.793 V vs. SCE, as reported in
Table 4.
Generally speaking, when the corrosion mechanism is of the activated type, the sample’ anodic and cathodic branches of the potentiodynamic curves allow for an estimating of the samples’ corrosion current-density using the Tafel approximation. In the case under study, the corrosion current-densities were evaluated in different modes in relationship to the recorded potentiodynamic-curves shape. Analysing the potentiodynamic curves, it is clear that the bare sample is affected by pitting corrosion, due to the presence of the aluminium oxide that forms spontaneously when the material is exposed to air. The sample CrR, on the other hand, has a layer of chromium oxide that protects it, and it shows a metastable pitting between the corrosion potential and approximately −0.710 V vs. SCE, due to the self-healing action of the CrIII-based conversion layer. The NR sample is coated with the conversion layer containing titanium- and zirconium-fluoride salts; it exhibits an activated corrosion-mechanism between the corrosion potential and −0.793 V vs. SCE, and then it shows a metastable-pitting formation between −0.850 and −0.800 V vs. SCE; after that, it shows the setting-off of pitting between −0.800 and −0.740 V vs. SCE, which is counteracted by the action of the conversion layer deposited on the specimen, which tends to prevent its development. The pitting definitively stabilises when the potential of −0.740 V vs. SCE is reached. It should anyway be noted that samples showed a cathodic-reduction activated-type mechanism. Starting from these observations, the corrosion current-density was estimated using the following criteria. In the case of the NR sample, both anodic and cathodic branches of the potentiodynamic curves were used to determine the Tafel lines and then the current density of the sample. For the other two coupons, only the cathodic branch was used. In this way, the corrosion current-density of the NR sample was evaluated as approximately equal to 2 × 10−8 A/cm2. It is approximately one order of magnitude lower than that exhibited by the sample CrR (equal to 2 × 10−7 A/cm2) and two orders of magnitude lower than that exhibited by the bare sample (equal to 3 × 10−6 A/cm2). These results highlight the effectiveness of the Ti-Zr-based conversion coating, even when compared to the CrIII-based phospho-chromic treatment. The performances of the NR sample can be attributed to the effect of the Ti- and Zr-containing chemicals deposited on the sample surface and to the presence of the polymeric base, which induces relative stability into the conversion layer, while the CrIII-based treatment is more soluble in water. This aspect will be clearly demonstrated by discussing the electrochemical-impedance-spectroscopy results.
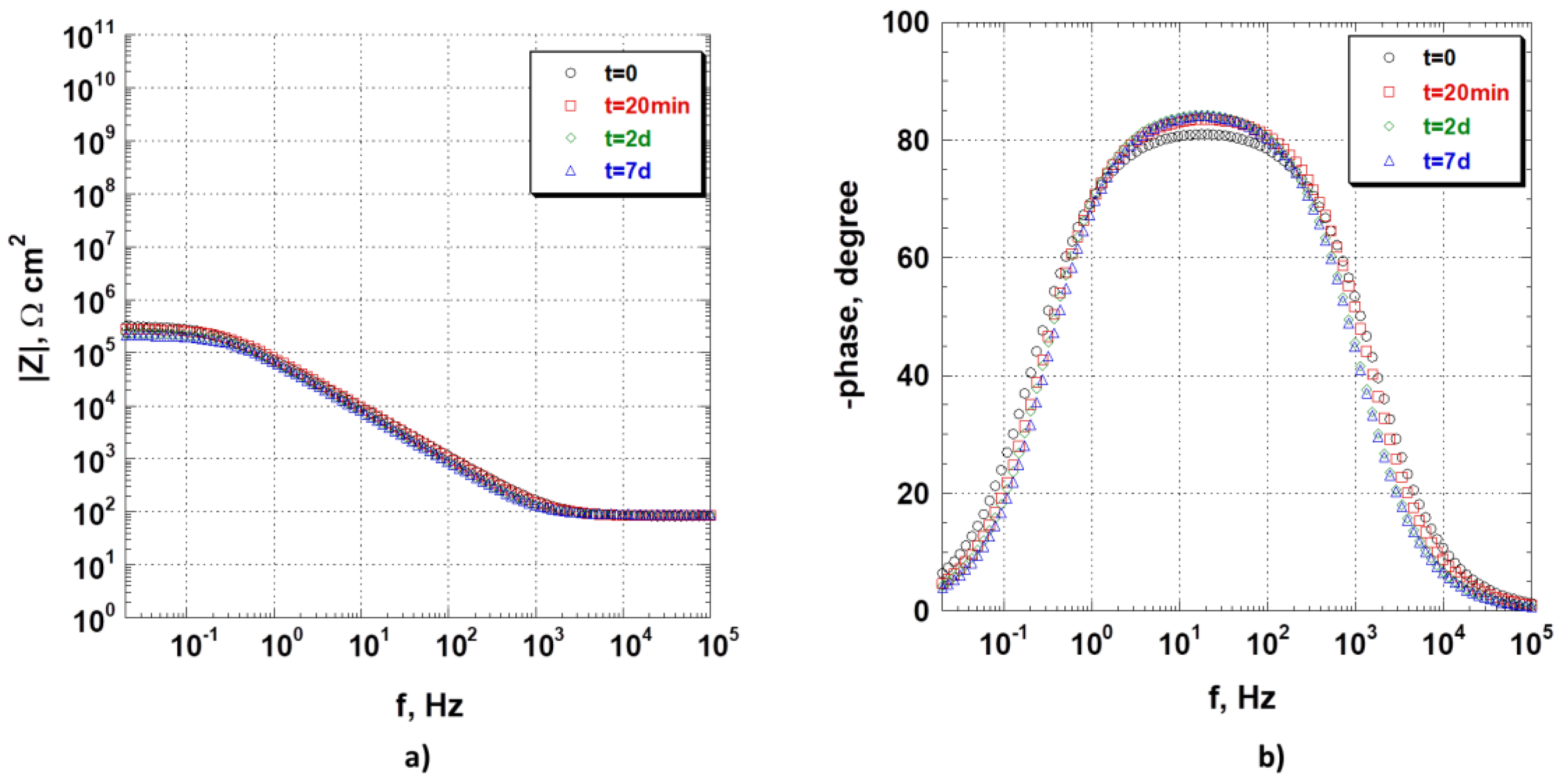
Figure 6 shows EIS spectra as a function of time for the bare 8079 alloy when immersed in the NaCl 3.5 wt.% aerated aqueous solution. The impedance responses are similar at all times of sample exposition to the test solution. This demonstrates that the sample quickly reaches a steady-state regime, and its behaviour does not change during the test duration. The impedance-modulus plot,
Figure 6a, in which the modulus of impedance as a function of frequency is reported, shows a quasi-resistive behaviour at low frequencies (1 × 10
−2–3 × 10
−1 Hz), a capacitive behaviour in the range 3 × 10
−1–1 × 10
3 Hz, due to the presence of the double layer and the electrochemical reactions developed at the interface, determining the corrosion of the sample, and a resistive behaviour in the range 1 × 10
3–1 × 10
5 Hz, due to the solution resistance. The impedance modulus at 1 × 10
−2 Hz is approximately equal to 3.2 × 10
5 Ω·cm
2, due to the active behaviour of the interface which, as demonstrated by the potentiodynamic tests, is affected by pitting corrosion. The phase-angle data, reported in
Figure 6b, confirm this interpretation by exhibiting a resistive behaviour at low frequencies (phase-angle tending towards 0) and a capacitive range (phase-angle tending towards 80°) between 3 × 10
−1 and 1 × 10
3 Hz; after that, the phase angle decreases, reaching 0° at high frequencies.
Figure 7 shows results obtained from carrying out the EIS measurements, as a function of time, for the CCrR sample when immersed in the 3.5 wt.% NaCl aerated aqueous solution. The results clearly demonstrate the very high quality of the products used. The impedance modulus (
Figure 7a), which is one of the most important parameters and among others is used to identify the quality of a coating system, reaches, at the lowest frequency and the beginning of the test campaign, (t = 0), the value of approximately 7 × 10
10 Ω·cm
2. This value is generally considered very high, demonstrating the remarkable corrosion resistance of the entire protective-layer [
18]. Correspondingly, the phase angle (
Figure 7b) remains equal to a value of approximately 90° throughout the frequencies range. After 20 min of the sample exposition to the test solution, the shape of the curves and the sample behaviour did not change significantly, but after 2 days a sharp decrease in phase angle at low frequencies (approaching 0°) can be seen, as well as the modulus of impedance showing a resistive behaviour between 10 and 10
−2 Hz, reaching a value of approximately 4 × 10
9 Ω·cm
2. This means that the electrolyte has reached the interface, diffusing through the organic coating, and has begun to corrode the substrate [
18].
The self-healing properties of the CrIII contained in the conversion coating show their effect after 7 days of sample exposure to the aggressive environment. In fact, the impedance modulus reaches approximately the same values as those obtained after 20 min of the test, and the phase-angle increases [
19]. At the same time, the data show that both the impedance modulus and the phase-angle curves are shifted downwards in the entire frequency range, demonstrating that the electrolyte continues to penetrate the organic coating. These results confirm the high effectiveness of the CrIII–based conversion layer in protecting the aluminium substrate from degradation.
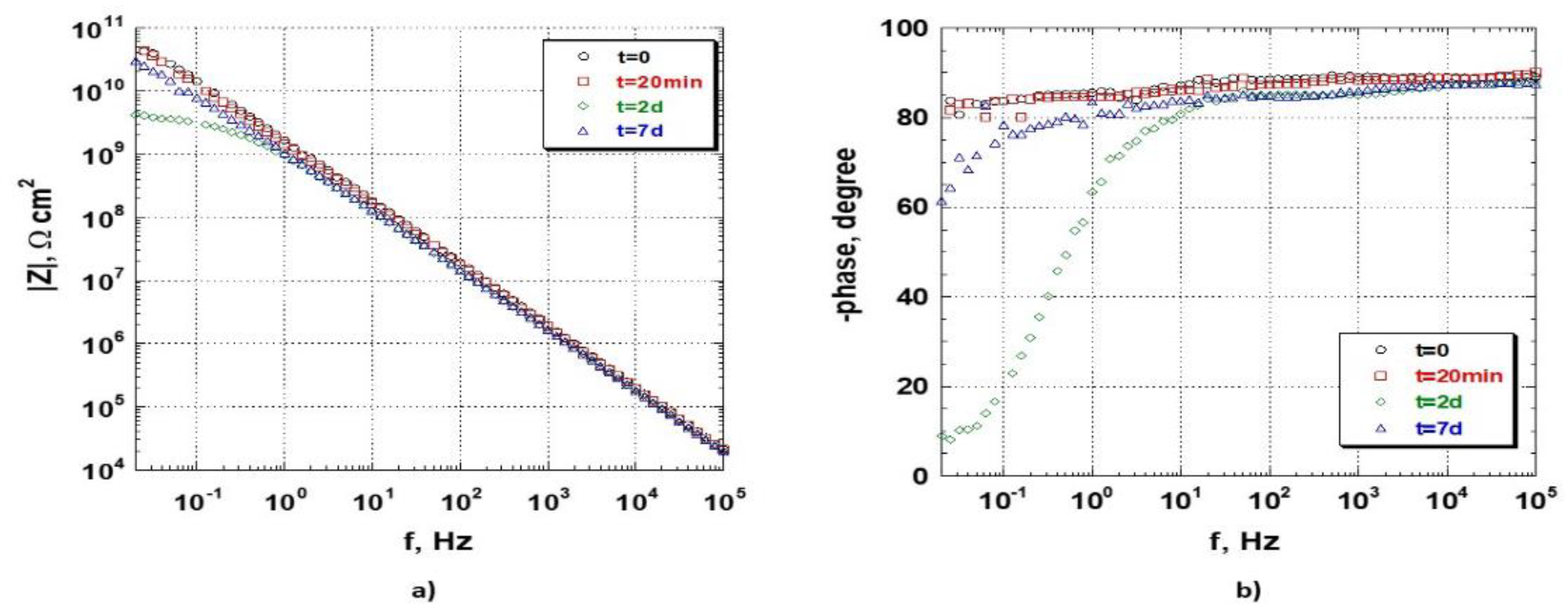
Figure 8 shows the electrochemical behaviour as a function of the time of the CNR sample when immersed in NaCl 3.5 wt.% aerated aqueous solution. The data highlights the stability of the sample throughout the observation period. The impedance modulus (
Figure 8a) reaches the value of approximately 6 × 10
10 Ω·cm
2 at the lowest frequency; this value remains practically constant until the end of the observation period. The shape of the curves demonstrates a capacitive behaviour of the sample, exhibiting a slope −1 straight line in the entire frequency-range studied. As for the CCrR sample, the data demonstrates the good quality of the organic coating used, which absorbs a very small quantity of the electrolyte during the immersion in the test solution, proved by the almost unnoticeable variation of the modulus of the impedance in the range 10
−1–10
4 Hz. These observations are confirmed by analysing the phase-angle data (
Figure 8b), which remain constant throughout the frequency range and for the entire sample observation-period, at a value between 76 and 86 degrees.
It is interesting to discuss the experimental results by comparing the data obtained by studying the CCrR and the CNR samples. As can be seen from
Figure 7 and
Figure 8, the samples highlighted a significant difference in their electrochemical response at low frequencies. It is appropriate to describe the physical meaning of such differences by looking at the phase-angle plot of the samples which is known to be more “sensitive” to some kinds of the sample’s characteristics variations than the modulus of impedance. As said before, the phase-angle variations exhibited by the CCrR sample during the sample test period are due to the electrolyte penetration through the coating, the subsequent corrosion of the substrate and the protective action played by the conversion coating (the self-healing properties of CrIII). The CNR sample, instead, showed a practically constant phase-angle in the same observation period. As the two samples were “fabricated” in such a way as to minimise (if not eliminate) the possibility of differences between the samples caused by the deposition and cure of the organic coating, it is possible, therefore, to charge the observed difference in the behaviour of the two samples exclusively to the different characteristics existing between the two conversion-coatings used. The experimental results highlighted the fact that the Zr/Ti-based one seems to be “very fast” in blocking the deleterious effect of the electrolyte penetration. This effect could be addressed by the presence of the thin organic-layer in the conversion coating and the faster-reaction kinetics at the interface performed by the conversion layer. This hypothesis should be verified by more in-depth analysis, which is beyond the aim of this paper.
7. Cost Comparison
The cost comparison of the two different processes is not easy. The purchasing, planting and setting up costs of an industrial production plant depend on multiple factors that make the cost analysis complex. In this approach, it was taken into account the fact that the company defined its turnover and productivity annual-target, and therefore purchased the two conversion-coating systems, the rinse and the no-rinse one, of comparable productivity. Considering that the no-rinse system has a roll-coater or a sprayer, which, therefore, is more complex than the rinse one, but that the latter requires at least two more tanks and ancillary equipment, it was assumed that the two plants had similar costs, and so this was not considered as a variable to discriminate between the two processes. In addition, the costs of piece handling, stops for the renewal of the tanks, maintenance, etc., were estimated to be similar, and not considered. It should be noted that the no-rinse raw material is offered at a price per unit of mass approximately double the price of the rinse one (
Table 5).
Furthermore, the no-rinse product is used by depositing a wet film of thickness equal to approximately 2–2.5 times that used for the rinse product (0.7–0.8 g/m2 vs. 1.4–2.0 g/m2). At the same time, however, the no-rinse product is diluted up to 30–40% to obtain an adequate product-distribution on the foil surface. In the end, the raw materials’ costs differ slightly if quantified in terms of cost per square-meter of treated foil.
In light of the recent findings relating to climate changes caused by the anthropic and industrial impact on the world around us, the prerogatives of no-rinse products reside, mainly, in the reduced environmental impact and the decrease in the exploitation of water resources. The water quantity in the no-rinse process is equal to the amount necessary to dilute the product to the concentrations indicated (a part of the water used in the cleaning and the etching steps that is the same amount in both processes). The last step of the no-rinse conversion-coating process does not require the washing of the metallic film; therefore, there is no need to process the mains water to produce demineralised water, purify it at the end of the conversion process, and dispose of it in the ways permitted by current laws. Taking into account (
Table 6) the company’s annual average-production (40 × 10
6 m with the standard width of 1.80 m), it has been evaluated that by using the no-rinse conversion-coating process, the cost of the entire production is approximately EUR 65,000, while using the CrIII rinse is EUR 45,000, representing a cost increase of approximately 31%. At the same time, the consumption of water was reduced by 13,000 tons/year, and the sludge to be disposed of was decreased by approximately 130 tons/year. Considering that the company produces the demineralised water estimating its cost to be 1 EUR/ton and the cost of the sludge disposal can be evaluated at approximately 235 EUR/ton, the overall saving can be estimated at approximately EUR 43,000 per year. Ultimately, the company, using the no-rinse product, achieves a net saving on production costs of around EUR 23,000.
8. Conclusions
The results obtained in the test campaign using aluminium-laminate samples made of the 8079 alloys have shown that excellent no-rinse commercial products that are totally chromium-free exist and offer protective properties comparable to those exhibited by rinse products containing CrIII. No-rinse “true Cr-free” products are approximately 30% more expensive than products containing chromium, but they are used diluted. Nevertheless, the absence of the last phase of rinsing in the production process implicates a decrease in the process costs and a profit for the company.
However, the economic profit takes a back seat if one considers that the “true Cr-free” no-rinse-based process determines a drastic decrease in the amount of water used and the lack of production of significant quantities of chromium-containing waste. These circumstances determine a noteworthy reduction in the environmental impact of the entire production-cycle.