Triboelectric Charging Behaviors of Polyester Films Doped with Titanium Dioxide Nanoparticles of Various Crystal Structures
Abstract
Featured Application
Abstract
1. Introduction
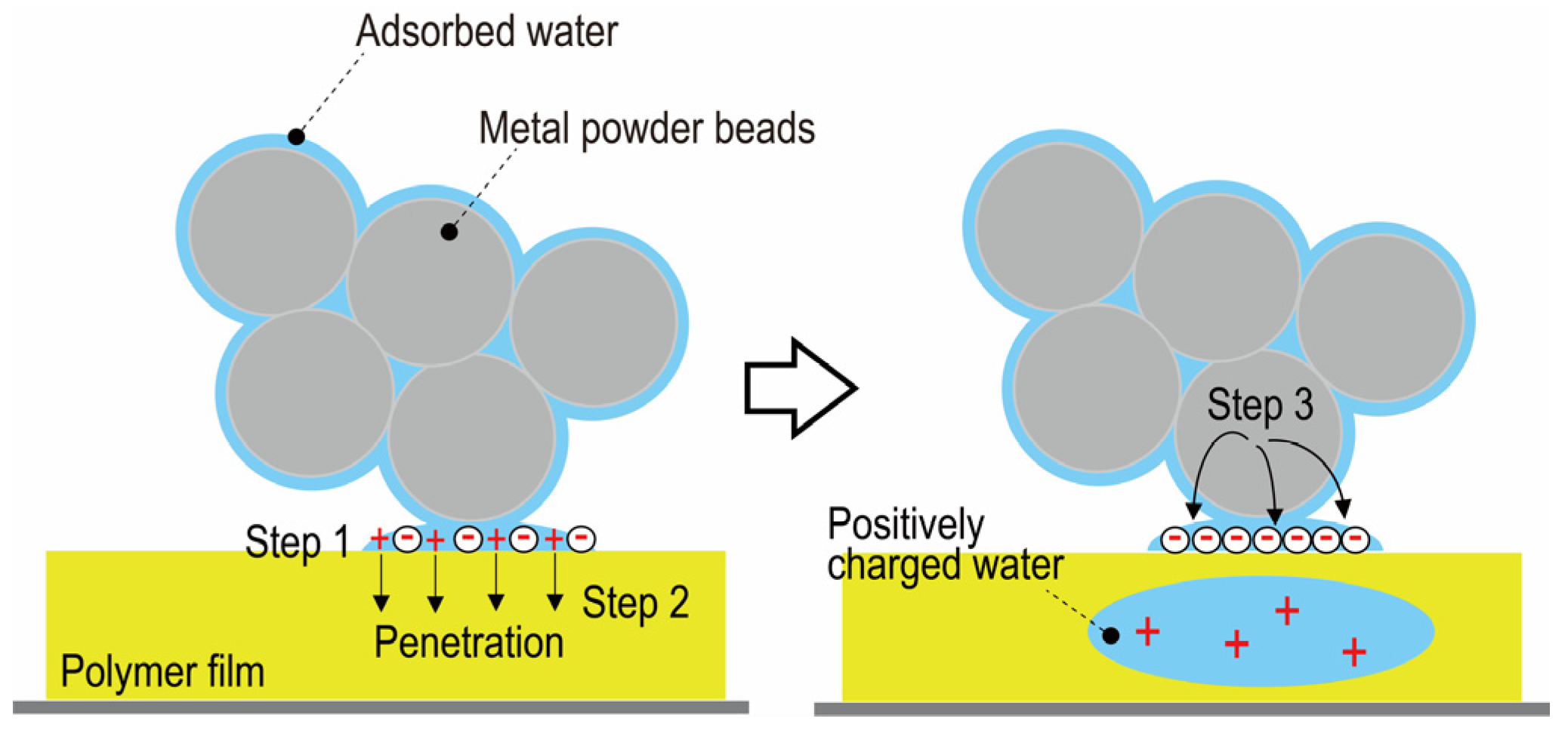
2. CWP Model
3. Materials and Methods
3.1. Tribocharging Apparatus
3.2. Materials
3.3. Preparation of a Layered Film
3.4. Preparation of Films for the Measurement of Water Content
3.5. Measurements
4. Results and Discussion
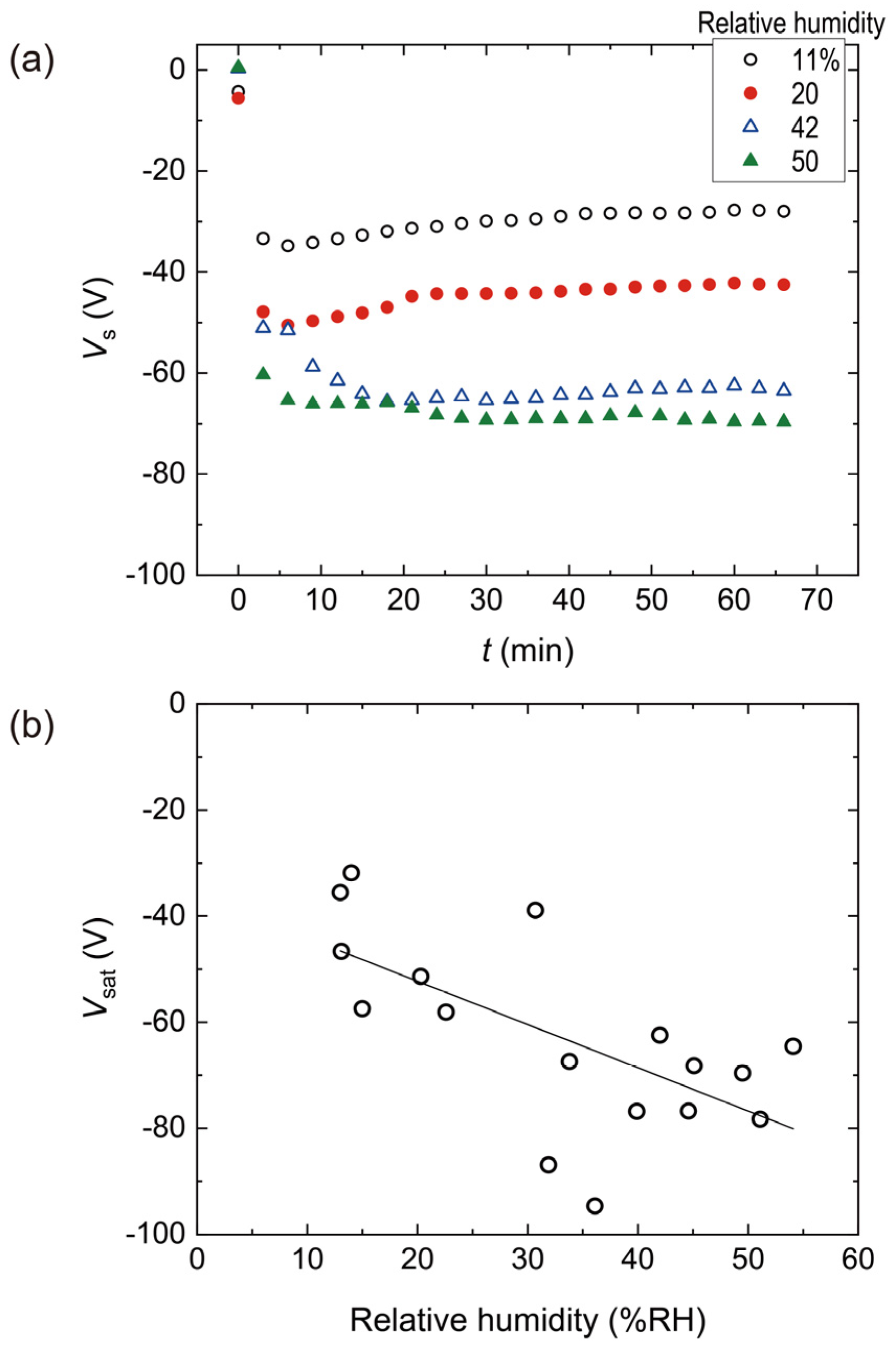
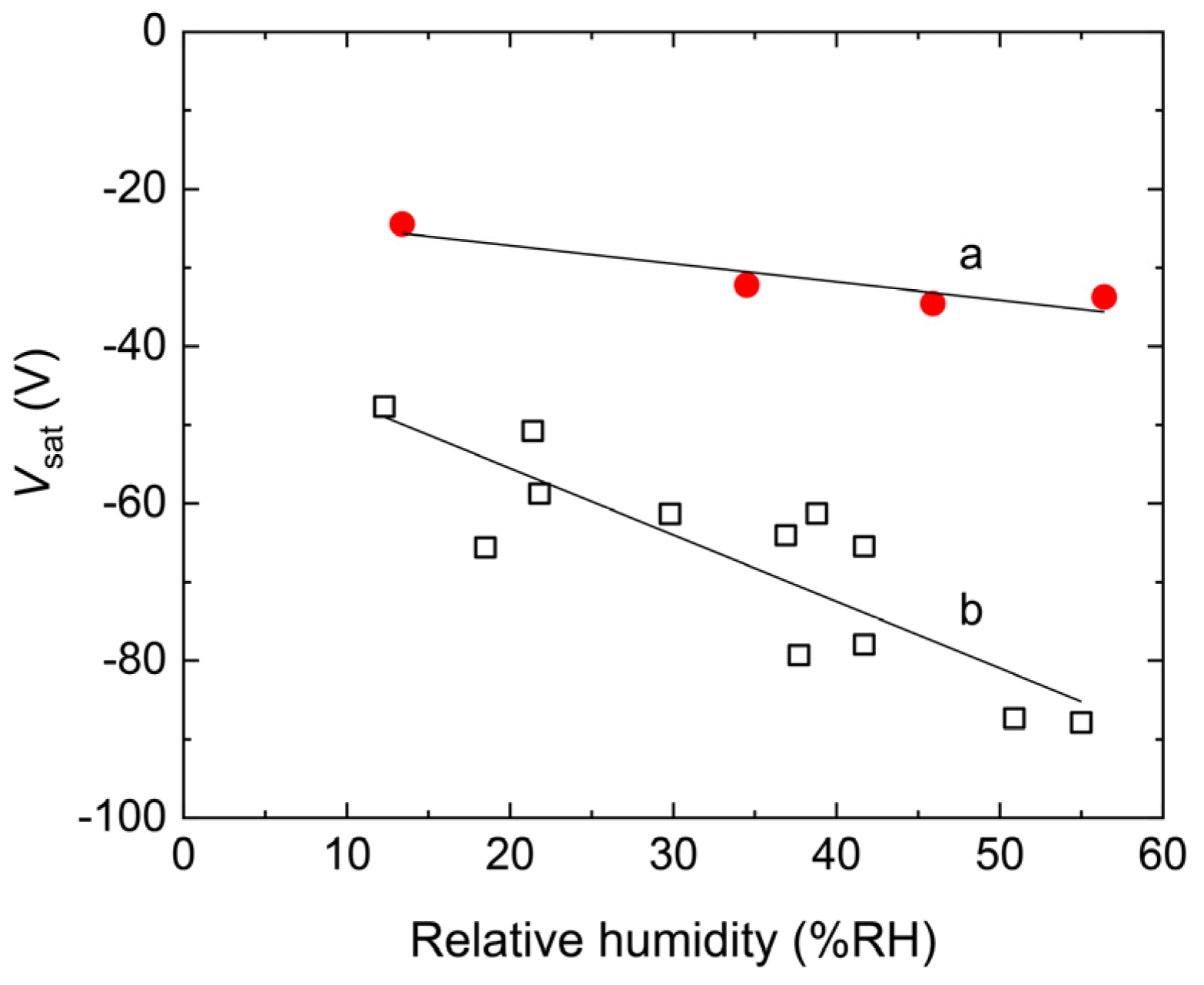
4.1. Triboelectric Charging Behaviors of a PES Single Film
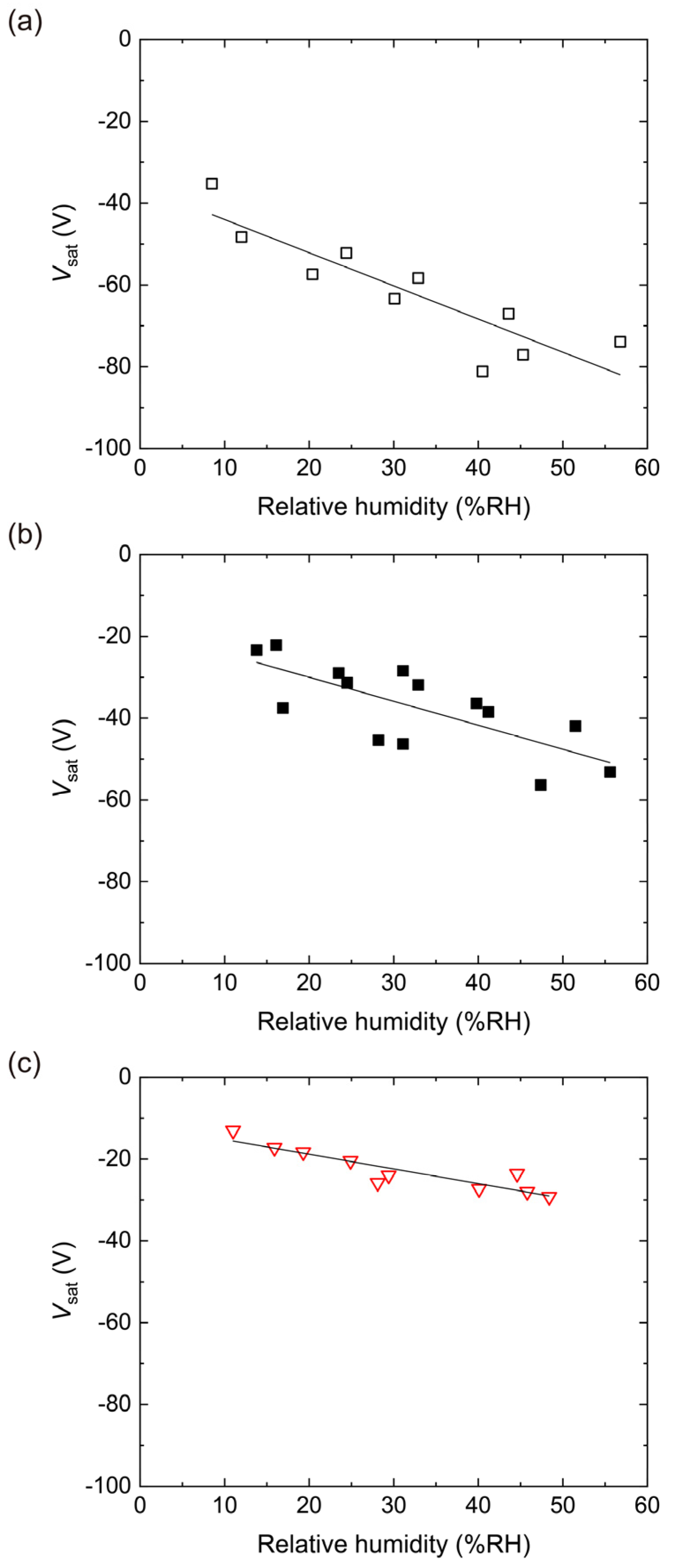
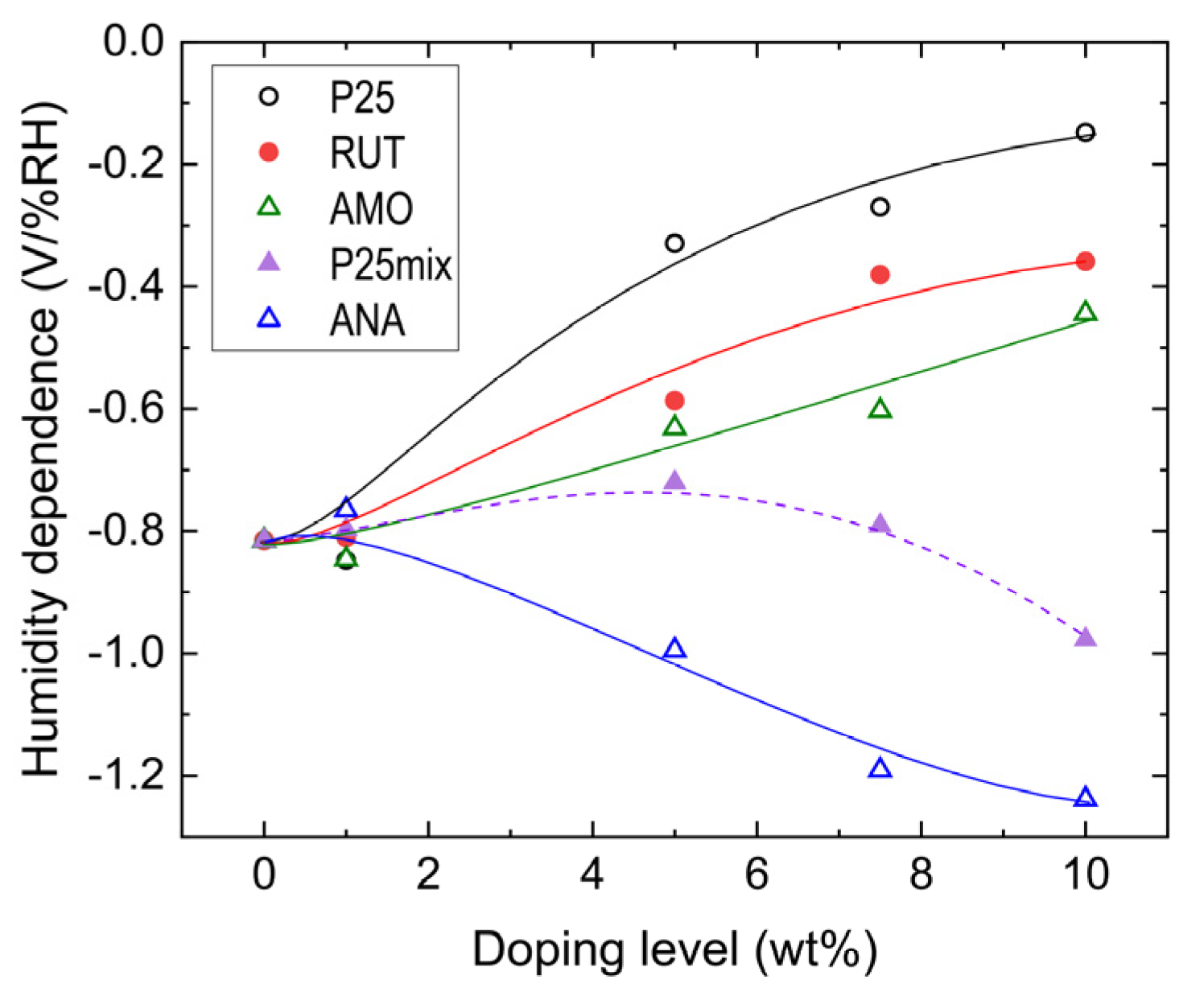
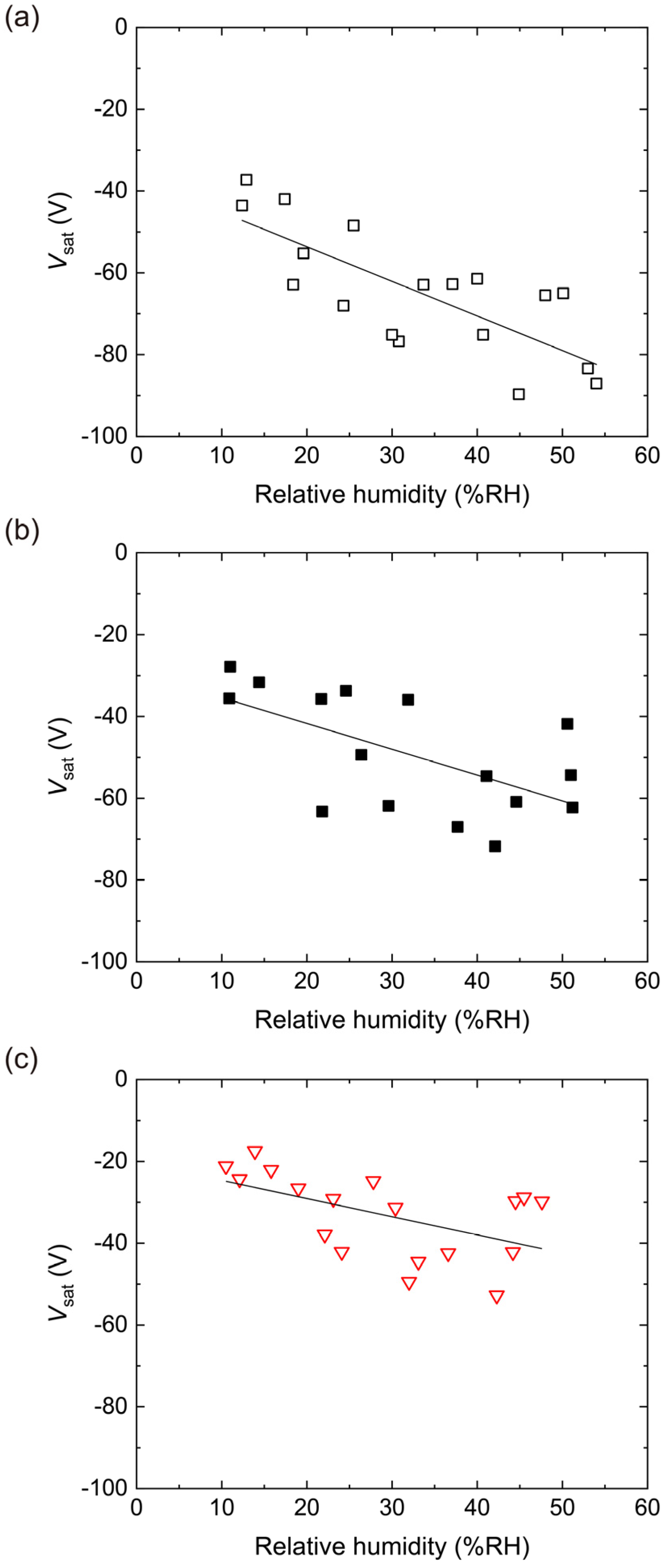
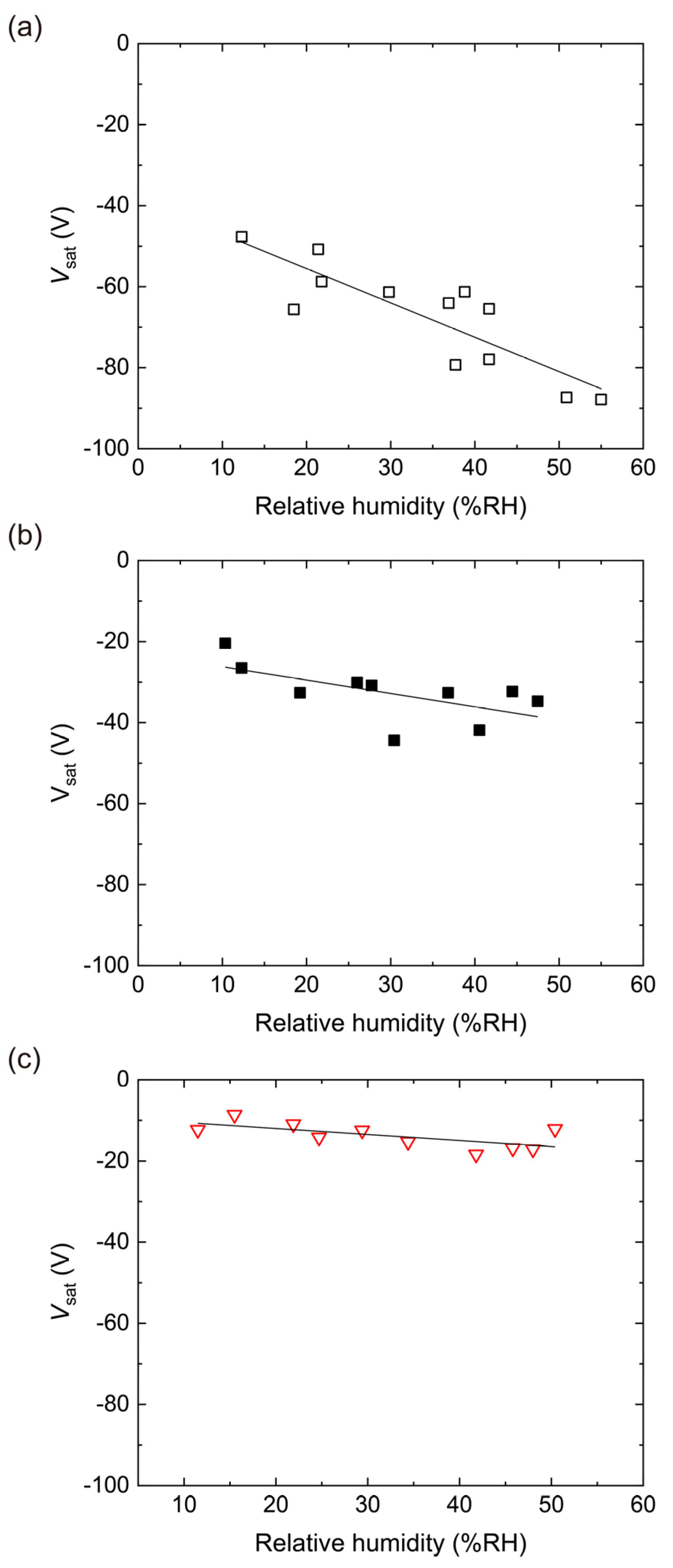
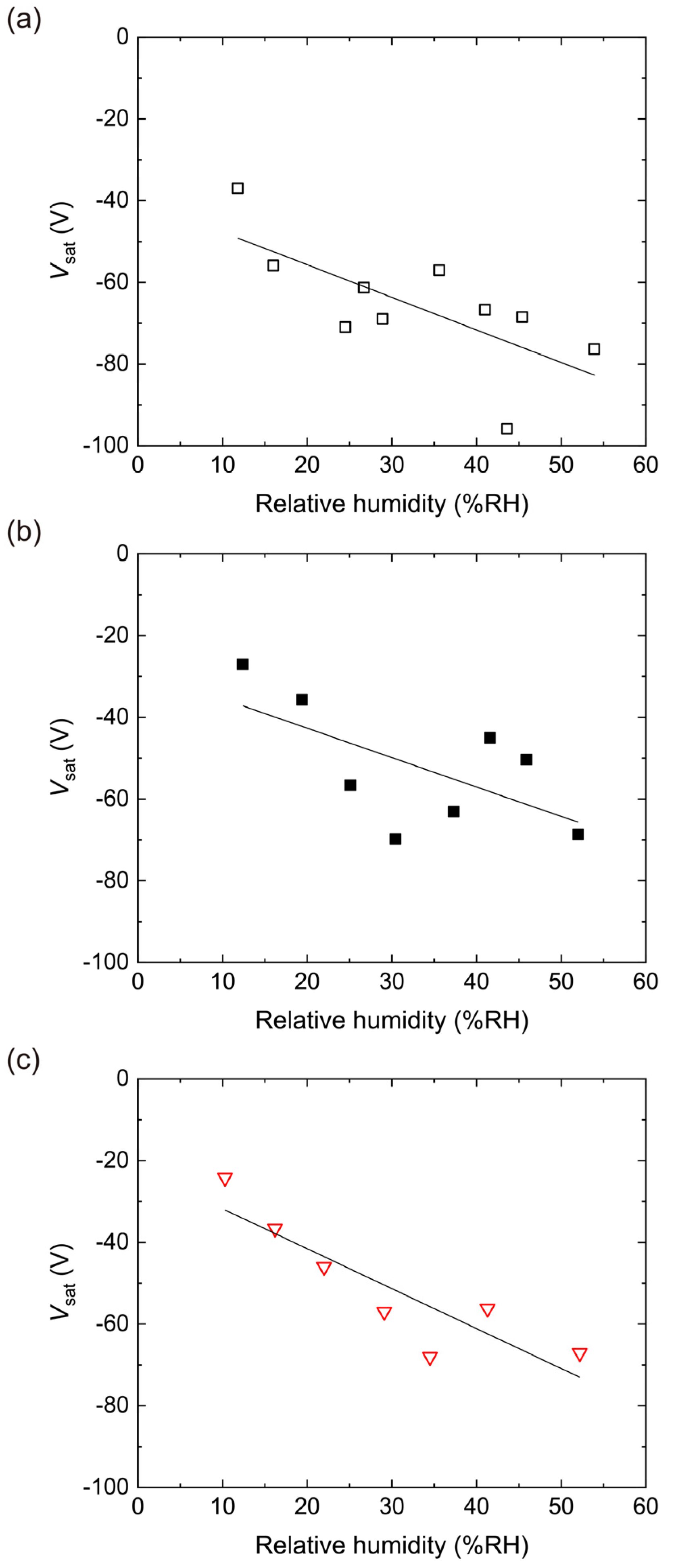
4.2. Triboelectric Charging of PES Films Doped with RUT
4.3. Triboelectric Charging of PES Films Doped with ANA
4.4. Triboelectric Charging of PES Films Doped with AMO
4.5. Triboelectric Charging of PES Films Doped with P25
4.6. Measurements of the Water Contents in Films before and after Frictional Electrification
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davis, K.A. Titanium dioxide. J. Chem. Educ. 1982, 59, 158–159. [Google Scholar] [CrossRef]
- Singh, P.; Nanda, A. Enhanced sun protection of nano-sized metal oxide particles over conventional metal oxide particles: An in vitro comparative study. Int. J. Cosmetic Sci. 2014, 36, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Ali, T.; Hunge, Y.M.; Venkatraman, A. UV assisted photoelectrocatalytic degradation of reactive red 152 dye using spray deposited TiO2 thin films. J. Mater. Sci.-Mater. Electron. 2018, 29, 1209–1215. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Yadav, A.A.; Mathe, V.L. Photocatalytic hydrogen production using TiO2 nanogranules prepared by hydrothermal route. Chem. Phys. Lett. 2019, 731, 136582. [Google Scholar] [CrossRef]
- Wang, R.; Hashimoto, K.; Fujishima, A.; Chikuni, M.; Kojima, E.; Kitamura, A.; Shimohigoshi, M.; Watanabe, T. Light-induced amphiphilic surfaces. Nature 1997, 388, 431–432. [Google Scholar] [CrossRef]
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Cummins, D.; Boschloo, G.; Ryan, M.; Corr, D.; Rao, S.N.; Fitzmaurice, D. Ultrafast electrochromic windows based on redox-chromophore modified nanostructured semiconducting and conducting films. J. Phys. Chem. B 2000, 104, 11449–11459. [Google Scholar] [CrossRef]
- Grätzel, M. Ultrafast colour displays. Nature 2001, 409, 575–576. [Google Scholar] [CrossRef]
- Comiskey, B.; Albert, J.D.; Yoshizawa, H.; Jacobson, J. An electrophoretic ink for all-printed reflective electronic displays. Nature 1998, 394, 253–255. [Google Scholar] [CrossRef]
- Ni, W.; Wu, S.; Ren, Q. Silanized TiO2 nanoparticles and their application in toner as charge control agents: Preparation and characterization. Chem. Eng. J. 2013, 214, 272–277. [Google Scholar] [CrossRef]
- Ni, W.; Wu, S.; Ren, Q. Preparation and Characterization of silanized TiO2 nanoparticles and their application in toner. Ind. Eng. Chem. Res. 2012, 51, 13157–13163. [Google Scholar] [CrossRef]
- Piccinno, F.; Gottschalk, F.; Seeger, S.; Nowack, B. Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. J. Nanopart. Res. 2012, 14, 1109. [Google Scholar] [CrossRef]
- Sun, C.; Liu, L.-M.; Selloni, A.; Lua, G.Q.; Smith, S.C. Titania-water interactions: A review of theoretical studies. J. Mater. Chem. 2010, 20, 10287–10554. [Google Scholar] [CrossRef]
- Huang, L.; Gubbins, K.E.; Li, L.; Lu, X. Water on titanium dioxide surface: A revisiting by reactive molecular dynamics simulations. Langmuir 2014, 30, 14832–14840. [Google Scholar] [CrossRef] [PubMed]
- Veregin, R.P.N.; Powell, D.; Tripp, C.R.; McDougall, M.N.V.; Mahon, M. Kelvin potential measurement of insulative particles. 1. Mechanism of metal oxide triboelectric charging and relative humidity sensitivity. J. Imaging Sci. Technol. 1997, 41, 192–196. [Google Scholar]
- Asada, Y. Micro TiO2 for toner additives. J. Imaging Soc. Jpn. 2008, 47, 532–535. [Google Scholar]
- Pan, S.; Zhang, Z. Fundamental theories and basic principles of triboelectric effect: A review. Friction 2019, 7, 2–17. [Google Scholar] [CrossRef]
- Baytekin, H.T.; Patashinski, A.Z.; Branicki, M.; Baytekin, B.; Soh, S.; Grzybowski, B.A. The mosaic of surface charge in contact electrification. Science 2011, 333, 308–312. [Google Scholar] [CrossRef]
- Apodaca, M.M.; Wesson, P.J.; Bishop, K.J.M.; Ratner, M.A.; Grzybowski, B.A. Contact electrification between identical materials. Angew. Chem. Int. Ed. 2010, 49, 946–949. [Google Scholar] [CrossRef]
- Takahashi, M.; Hoshino, K.; Kokado, H. Dynamic aspect of triboelectric charging of some toner materiaIs. Electrophotography 1995, 34, 173–180. [Google Scholar]
- Kayahara, S.; Hoshino, K.; Kokado, H. Dynamic aspect of triboelectric charging of poIystyrene films doped with electron-donative and electron-acceptive dopants. Electrophotography 1996, 35, 304–314. [Google Scholar]
- Koike, T.; Ooka, H.; Hoshino, K.; Kokado, H. TriboeIectrification of poIymer films in contact with metaI-plated carriers—Mechanistic study on humidity-dependent charging. J. Imaging Soc. Jpn. 1998, 37, 225–233. [Google Scholar]
- Hiraga, Y.; Teramoto, Y.; Miyagawa, N.; Hoshino, K. Unexpected and unusual triboelectrification behavior of polymer films containing carboxyl group. Langmuir 2013, 29, 6903–6910. [Google Scholar] [CrossRef] [PubMed]
- Hatae, Y.; Tomura, K.; Hiraga, Y.; Hoshino, K. Mechanistic study on triboelectrification behavior of polymer films in contact with iron carrier beads. J. Inst. Electrost. Jpn. 2014, 38, 260–266. [Google Scholar]
- Miyamoto, K.; Hatae, Y.; Tomura, K.; Hoshino, K. Investigation of triboelectric charging behaviors of some polymer films using charged-water penetration model. J. Inst. Electrost. Jpn. 2015, 39, 216–220. [Google Scholar]
- Teramoto, Y.; Miyazaki, N.; Ando, K.; Tsukada, S.; Hoshino, K. Triboelectrification behaviors of polyester films containing hindered amines ―Mechanistic Study on Electret Filters. J. Imaging Soc. Jpn. 2022, 61, 466–478. [Google Scholar]
- Bowden, F.P.; Throssell, W.R. Adsorption of water vapour on solid surfaces. Nature 1951, 167, 601–602. [Google Scholar] [CrossRef]
- Mizuguchi, J.; Hitachi, A.; Sato, Y.; Uta, K. Study on the charge control mechanism of charge control agents. J. Imaging Sci. Technol 2008, 52, 030506. [Google Scholar] [CrossRef]
- Wang, Z.L.; Wang, A.C. On the origin of contact-electrification. Mater. Today 2019, 30, 34–51. [Google Scholar] [CrossRef]
- Shirai, Y.; Tsubokawa, N. Grafting of polymers onto ultrafine inorganic particle surface: Graft polymerization of vinyl monomers initiated by the system consisting of trichloroacetyl groups on the surface and molybdenum hexacarbonyl. React. Funct. Polym. 1997, 32, 153–160. [Google Scholar] [CrossRef]
- Kawase, S.; Sugimoto, K.; Fujiwara, R.; Ono, S. Quantitative analysis of amorphous contents in photocatalytic titania powders. Bunseki Kagaku 2010, 59, 921–926. [Google Scholar] [CrossRef]
- Bachina; Almjasheva, O.V.; Popkov, V.I.; Nevedomskiy, V.N.; Gusarov, V.V. Heat-stimulated crystallization and phase transformation of titania nanoparticles. J. Cryst. Growth 2021, 576, 126371. [Google Scholar] [CrossRef]
- Chao, H.E.; Yun, Y.U.; Xingfang, H.U.; Larbot, A. Effect of silver doping on the phase transformation and grain growth of sol-gel titania powder. J. Eur. Ceram. Soc. 2003, 23, 1457–1464. [Google Scholar] [CrossRef]
- Lowell, J.; Rose-Innes, A.C. Contact electrification. Adv. Phys. 1980, 29, 947–1023. [Google Scholar] [CrossRef]
- Castle, G.S.P. Contact charging between insulators. J. Electrost. 1997, 40–41, 13–20. [Google Scholar] [CrossRef]
- Musa, U.G.; Cezan, S.D.; Baytekin, B.; Baytekin, H.T. The charging events in contact-separation electrification. Sci. Rep. 2018, 8, 2472. [Google Scholar] [CrossRef]
- Alimohammadi, M.; Fichthorn, K.A. A force field for the interaction of water with TiO2 surfaces. J. Phys. Chem. C 2011, 115, 24206–24214. [Google Scholar] [CrossRef]
- Lindan, P.J.D.; Zhang, C. Exothermic water dissociation on the rutile TiO2(110) surface. Phys. Rev. B 2005, 72, 075439. [Google Scholar] [CrossRef]
- Goniakowski, J.; Bouette-Russo, S.; Noguera, Q. Acido-basic properties of simple oxide surfaces: III. Systematics of H+ and OH− adsorption. Surf. Sci. 1993, 284, 315–327. [Google Scholar] [CrossRef]
- Lindan, P.J.D.; Harrison, N.M.; Gillan, M.J. Mixed Dissociative and molecular adsorption of water on the rutile (110) surface. Phys. Rev. Lett. 1998, 80, 762. [Google Scholar] [CrossRef]
- Diebold, U. The surface science of titanium dioxide. Surf. Sci. Rep. 2003, 48, 53–229. [Google Scholar] [CrossRef]
- Wendt, S.; Matthiesen, J.; Schaub, R.; Vestergaard, E.K.; Lægsgaard, E.; Besenbacher, F.; Hammer, B. Formation and splitting of paired hydroxyl groups on reduced TiO2(110). Phy. Rev. Lett. 2006, 96, 066107. [Google Scholar] [CrossRef] [PubMed]
- Bikondoa, D.O.; Pang, C.L.; Ithnin, R.; Muryn, C.A.; Onishi, H.; Thornton, G. Direct visualization of defect-mediated dissociation of water on TiO2(110). Nat. Mater. 2006, 5, 189–192. [Google Scholar] [CrossRef]
- Ketteler, G.; Yamamoto, S.; Bluhm, H.; Andersson, K.; Starr, D.E.; Ogletree, D.F.; Ogasawara, H.; Nilsson, A.; Salmeron, M. The nature of water nucleation sites on TiO2(110) surfaces revealed by ambient pressure X-ray photoelectron spectroscopy. J. Phys. Chem. C 2007, 111, 8278–8282. [Google Scholar] [CrossRef]
- Sarkar, A.; Khan, G.G. The formation and detection techniques of oxygen vacancies in titanium oxide-based nanostructures. Nanoscale 2019, 11, 3414–3444. [Google Scholar] [CrossRef]
- Kim, S.; Ko, K.C.; Lee, J.Y.; Illas, F. Single oxygen vacancies of (TiO2)35 as a prototype reduced nanoparticle: Implication for photocatalytic activity. Phys. Chem. Chem. Phys. 2016, 18, 23755–23762. [Google Scholar] [CrossRef]
- Selloni, A.; Vittadini, A.; Grätzel, M. The adsorption of small molecules on the TiO2 anatase (101) surface by first-principles molecular dynamics. Surf. Sci. 1998, 402–404, 219–222. [Google Scholar] [CrossRef]
- Vittadini, A.; Selloni, A.; Rotzinger, F.P.; Grätzel, M. Structure and energetics of water adsorbed at TiO2 anatase 101 and 001 surfaces. Phys. Rev. Lett. 1998, 81, 2954–2957. [Google Scholar] [CrossRef]
- Herman, G.S.; Dohna´lek, Z.; Ruzycki, N.; Diebold, U. Experimental investigation of the interaction of water and methanol with anatase−TiO2(101). J. Phys. Chem. B 2003, 107, 2788–2795. [Google Scholar] [CrossRef]
- Yan, Y.; Shi, W.; Peng, W.; Lin, Y.; Zhang, C.; Li, L.; Sun, Y.; Ju, H.; Zhu, J.; Ma, W.; et al. Proton-free electron-trapping feature of titanium dioxide nanoparticles without the characteristic blue color. Comm. Chem. 2019, 2, 88. [Google Scholar] [CrossRef]
- Setvin, M.; Franchini, C.; Hao, X.; Schmid, M.; Janotti, A.; Kaltak, M.; Van de Walle, C.G.; Kresse, G.; Diebold, U. Direct view at excess electrons in TiO2 rutile and anatase. Phys. Rev. Lett. 2014, 113, 086402. [Google Scholar] [CrossRef] [PubMed]
- Deskins, N.A.; Rousseau, R.; Dupuis, M. Distribution of Ti3+ surface sites in reduced TiO2. J. Phys. Chem. C 2011, 115, 7562–7572. [Google Scholar] [CrossRef]
- Li, G.; Dimitrijevic, N.M.; Chen, L.; Nichols, J.M.; Rajh, T.; Gray, K.A. The important role of tetrahedral Ti4+ sites in the phase transformation and photocatalytic activity of TiO2 nanocomposites. J. Am. Chem. Soc. 2008, 130, 5402–5403. [Google Scholar] [CrossRef] [PubMed]
- Vequizo, J.J.M.; Matsunaga, H.; Ishiku, T.; Kamimura, S.; Ohno, T.; Yamakata, A. Trapping-induced enhancement of photocatalytic activity on brookite TiO2 powders: Comparison with anatase and rutile TiO2 powders. ACS Catal. 2017, 7, 2644–2651. [Google Scholar] [CrossRef]
- Ghuman, K.K. Mechanistic insights into water adsorption and dissociation on amorphous TiO2-based catalysts. Sci. Technol. Adv. Mater. 2018, 19, 44–52. [Google Scholar] [CrossRef]
- Anantharaj, S.; Noda, S. Amorphous catalysts and electrochemical water splitting: An untold story of harmony. Small 2019, 16, 1905779. [Google Scholar] [CrossRef]
- Xu, Z.; Liang, J.; Wang, Y.; Dong, K.; Shi, X.; Liu, Q.; Luo, Y.; Li, T.; Jia, Y.; Asiri, A.M.; et al. Enhanced electrochemical H2O2 production via two-electron oxygen reduction enabled by surface-derived amorphous oxygen-deficient TiO2–x. ACS Appl. Mater. Interfaces 2021, 13, 33182–33187. [Google Scholar] [CrossRef]
- Ohtani, B.; Prieto-Mahaney, O.O.; Li, D.; Abe, R. What is Degussa (Evonik) P25? Crystalline composition analysis, reconstruction from isolated pure particles and photocatalytic activity test. J. Photochem. Photobiol. A-Chem. 2010, 216, 179–182. [Google Scholar] [CrossRef]
- Wang, K.; Wei, Z.; Colbeau-Justin, C.; Nitta, A.; Kowalska, E. P25 and its components–Electronic properties and photocatalytic activities. Surf. Interfaces 2022, 31, 102057. [Google Scholar] [CrossRef]
- Jianga, X.; Manawan, M.; Feng, T.; Qian, R.; Zhao, T.; Zhou, G.; Kong, F.; Wang, Q.; Dai, S.; Pan, J.H. Anatase and rutile in evonik aeroxide P25: Heterojunctioned or individual nanoparticles? Catal. Today 2018, 300, 12–17. [Google Scholar] [CrossRef]
- Bickley, R.I.; Gonzalezcarreno, T.; Lees, J.S.; Palmisano, L.; Tilley, R.J.D. A structural investigation of titanium dioxide photocatalysts. J. Solid State Chem. 1991, 92, 178–190. [Google Scholar] [CrossRef]
- Budavari, S.; O’Neil, M.J.; Smith, A.; Heckelman, P.E.; Kinneary, J.F. The Merk Index. An Encyclopedia of Chemicals, Drugs and Biologicals, 12th ed.; Merck and Co. Inc.: Whitehouse Station, NJ, USA, 1996. [Google Scholar]
- TOYOBO. Available online: https://www.toyobo.co.jp/products/xi/vylon/cg/index.html (accessed on 23 December 2022).
- Schein, L.B. Electrophotography and Development Physics; Springer-Verlag: Berlin, Germany, 1988; pp. 63–93. [Google Scholar]
- Gibson, N. Static electricity—An industrial hazard under control? J. Electrostat. 1997, 40–41, 2l–30. [Google Scholar] [CrossRef]
- Kilic, A.; Shim, E.; Pourdeyhimi, B. Electrostatic capture efficiency enhancement of polypropylene electret filters with barium titanate. Aerosol Sci. Technol. 2015, 49, 666–673. [Google Scholar] [CrossRef]



| Doping Level (wt%) | 95% CI | |||
|---|---|---|---|---|
| RUT | ANA | AMO | P25 | |
| 0 | 0.50 | 0.50 | 0.50 | 0.50 |
| 1 | 0.30 | 0.48 | 0.36 | 0.32 |
| 5 | 0.30 | 0.44 | 0.44 | 0.30 |
| 10 | 0.12 | 0.42 | 0.36 | 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teramoto, Y.; Ando, K.; Tsukada, S.; Hoshino, K. Triboelectric Charging Behaviors of Polyester Films Doped with Titanium Dioxide Nanoparticles of Various Crystal Structures. Appl. Sci. 2023, 13, 1468. https://doi.org/10.3390/app13031468
Teramoto Y, Ando K, Tsukada S, Hoshino K. Triboelectric Charging Behaviors of Polyester Films Doped with Titanium Dioxide Nanoparticles of Various Crystal Structures. Applied Sciences. 2023; 13(3):1468. https://doi.org/10.3390/app13031468
Chicago/Turabian StyleTeramoto, Yudai, Keita Ando, Satoru Tsukada, and Katsuyoshi Hoshino. 2023. "Triboelectric Charging Behaviors of Polyester Films Doped with Titanium Dioxide Nanoparticles of Various Crystal Structures" Applied Sciences 13, no. 3: 1468. https://doi.org/10.3390/app13031468
APA StyleTeramoto, Y., Ando, K., Tsukada, S., & Hoshino, K. (2023). Triboelectric Charging Behaviors of Polyester Films Doped with Titanium Dioxide Nanoparticles of Various Crystal Structures. Applied Sciences, 13(3), 1468. https://doi.org/10.3390/app13031468






