Oxidation Behaviour of Refractory (HfCo)100−x(NbMo)x High-Entropy Alloys with a bcc+B2 Structure
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Initial Structure
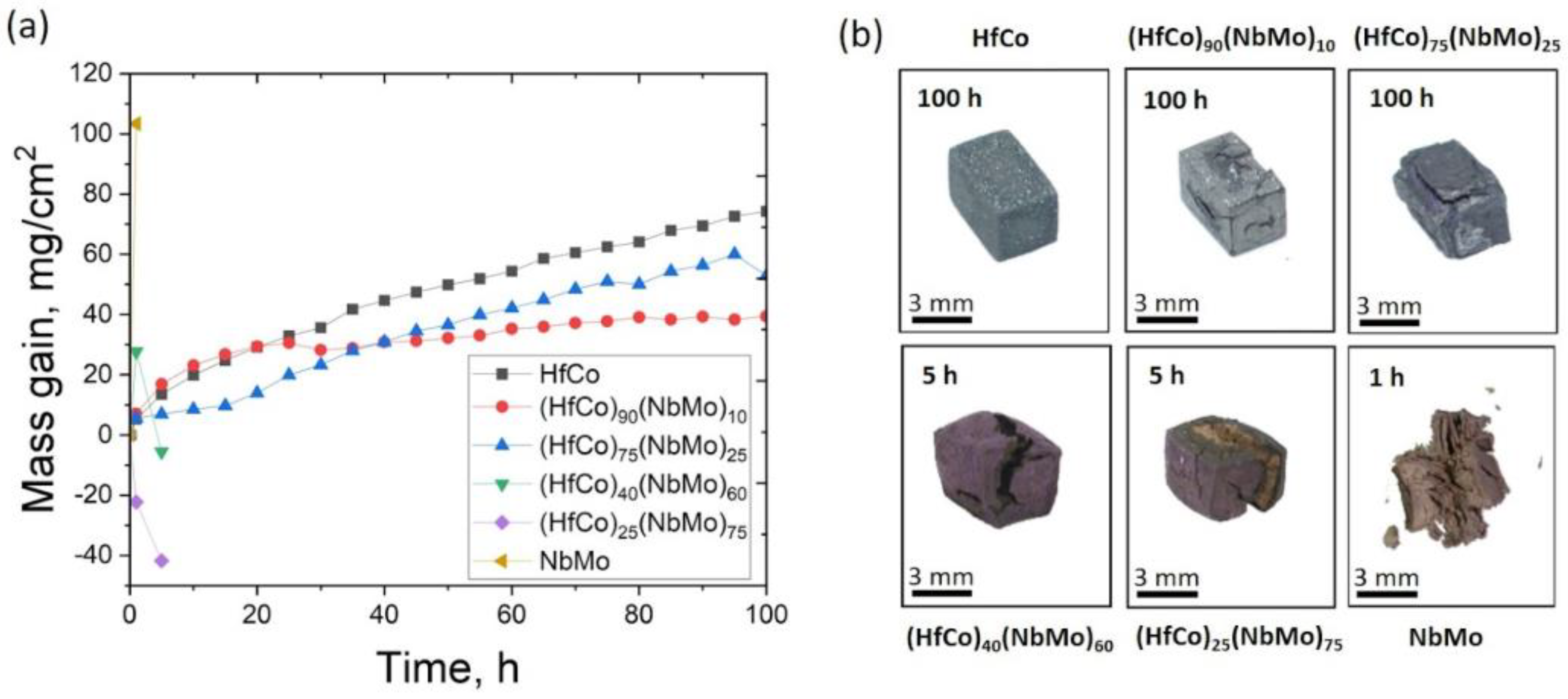
3.2. Oxidation Behaviour
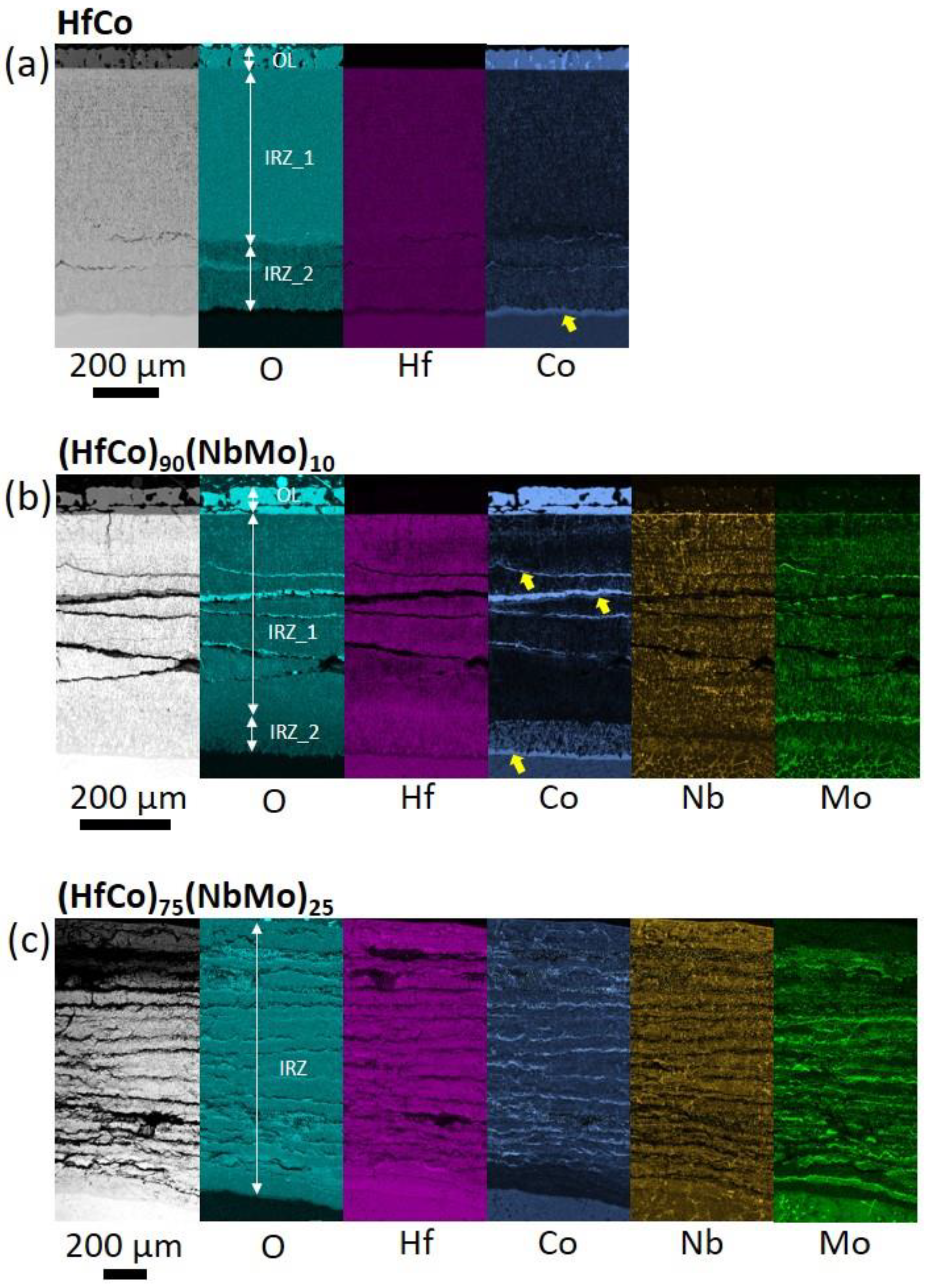
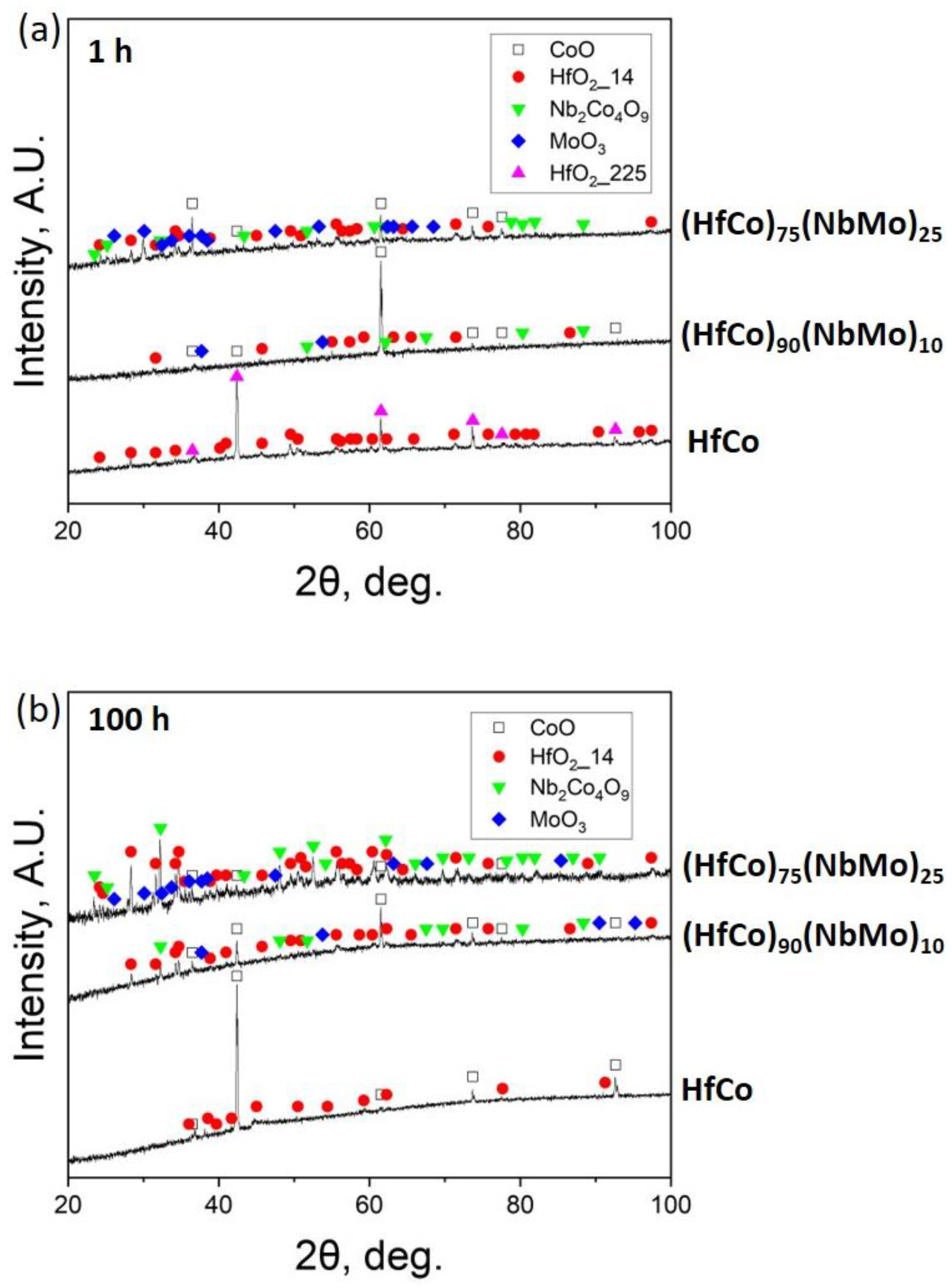
3.3. Structure after Oxidation
3.3.1. Cross-Section Analysis after 1 h
3.3.2. Cross-Section Analysis after 100 h
3.3.3. Surface Analysis
4. Discussion
4.1. Oxidation Behaviour
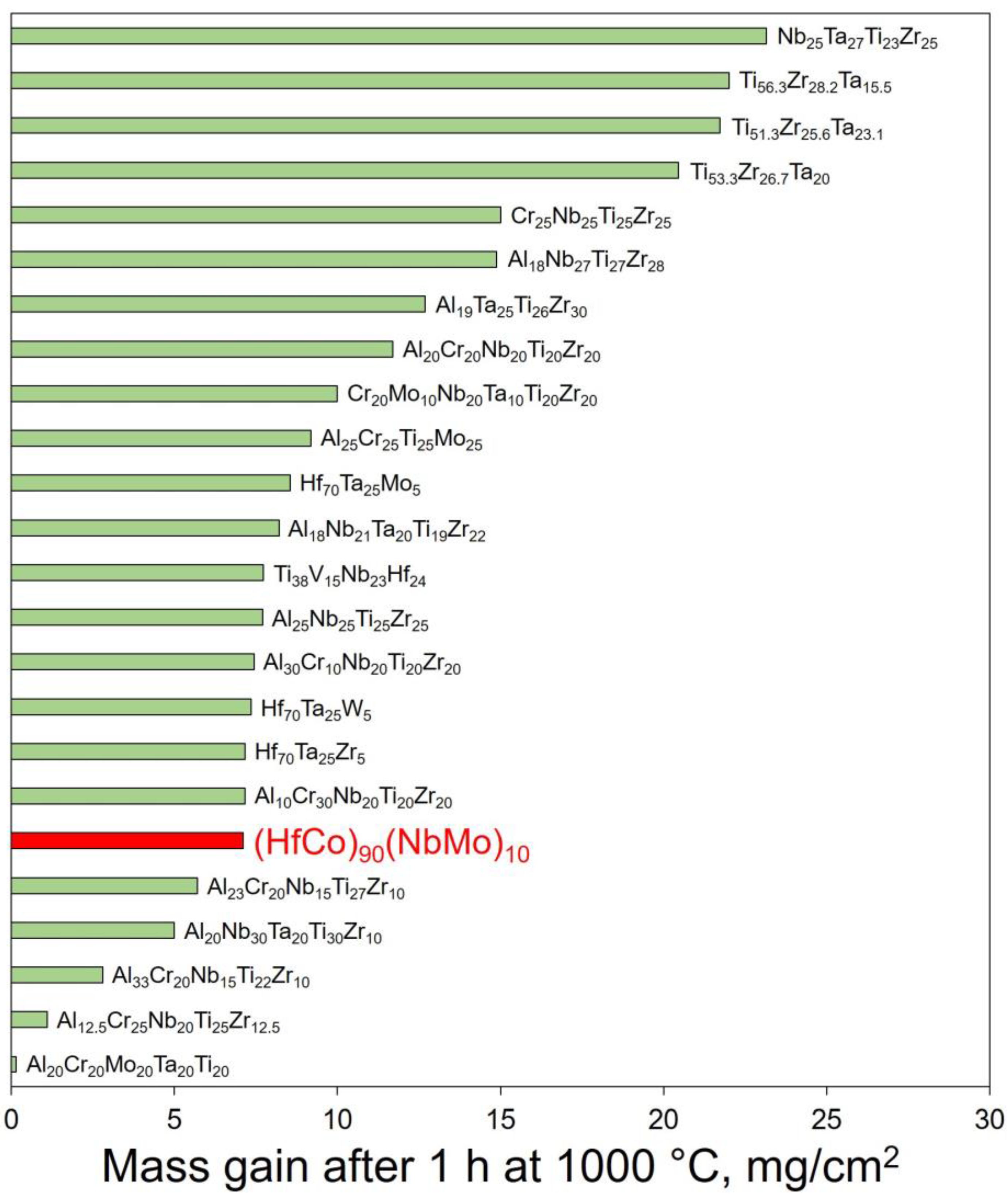
4.2. Comparison with Other RHEAs
5. Conclusions
- (1)
- The single-phase B2-ordered HfCo alloy demonstrated the best spallation resistance and retained a pristine form after 100 h. The near-parabolic oxidation kinetics of the HfCo alloy with n = 0.58 and k = 5.23 mg/cm2 h0.58 was found. The protection was provided by the formation of the external HfO2 or CoO layers after 1 or 100 h, respectively.
- (2)
- In the (NbMo)-containing alloys, the oxidation and spallation/disintegration resistances degraded with an increase in the fraction of the (Nb, Mo)-rich bcc phase that acted as a reservoir for the harmful MoO3. Only the (HfCo)90(NbMo)10 alloy with ~13% of the bcc phase could withstand the spallation up to 15 h and preserved the acceptable integrity after 100 h, while the alloys with >50% of the bcc phase oxidized and disintegrated completely after 1–5 h.
- (3)
- Before spallation, the (HfCo)90(NbMo)10 alloy showed near-parabolic oxidation kinetics with n = 0.46 and k = 7.75 mg/cm2 h0.46. Unlike the HfCo alloy, the protection of the (HfCo)90(NbMo)10 alloy initially relied on the exclusive CoO layer. Due to this, the (HfCo)90(NbMo)10 alloy was more oxidation-resistant compared to many other RHEAs covered by scales composed of complex and/or simple oxides.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Senkov, O.N.; Miracle, D.B.; Chaput, K.J.; Couzinie, J.P. Development and Exploration of Refractory High Entropy Alloys—A Review. J. Mater. Res. 2018, 33, 3092–3128. [Google Scholar] [CrossRef]
- Wei, Q.; Xu, X.; Shen, Q.; Luo, G.; Zhang, J.; Li, J.; Fang, Q.; Liu, C.-T.; Chen, M.; Nieh, T.-G.; et al. Metal-Carbide Eutectics with Multiprincipal Elements Make Superrefractory Alloys. Sci. Adv. 2022, 8, eabo2068. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wei, Q.; Xie, P.; Xu, X. An Ultrastrong Niobium Alloy Enabled by Refractory Carbide and Eutectic Structure. Mater. Res. Lett. 2022, 11, 169–178. [Google Scholar] [CrossRef]
- Sun, B.; Mo, J.; Wang, Q.; Chen, Y.; Zhang, Z.; Shen, B.; Liang, X. Outstanding Specific Yield Strength of a Refractory High-Entropy Composite at an Ultrahigh Temperature of 2273 K. J. Mater. Sci. Technol. 2023, 166, 145–154. [Google Scholar] [CrossRef]
- Liu, C.M.; Wang, H.M.; Zhang, S.Q.; Tang, H.B.; Zhang, A.L. Microstructure and Oxidation Behavior of New Refractory High Entropy Alloys. J. Alloys Compd. 2014, 583, 162–169. [Google Scholar] [CrossRef]
- Chang, C.; Titus, M.S.; Yeh, J. Oxidation Behavior between 700 and 1300 °C of Refractory TiZrNbHfTa High-Entropy Alloys Containing Aluminum. Adv. Eng. Mater. 2018, 20, 1700948. [Google Scholar] [CrossRef]
- Sheikh, S.; Bijaksana, M.K.; Motallebzadeh, A.; Shafeie, S.; Lozinko, A.; Gan, L.; Tsao, T.-K.; Klement, U.; Canadinc, D.; Murakami, H.; et al. Accelerated Oxidation in Ductile Refractory High-Entropy Alloys. Intermetallics 2018, 97, 58–66. [Google Scholar] [CrossRef]
- Yurchenko, N.; Panina, E.; Zherebtsov, S.; Salishchev, G.; Stepanov, N.; Yurchenko, N.; Panina, E.; Zherebtsov, S.; Salishchev, G.; Stepanov, N. Oxidation Behavior of Refractory AlNbTiVZr0.25 High-Entropy Alloy. Materials 2018, 11, 2526. [Google Scholar] [CrossRef]
- He, J.; Tang, Y.; Zhang, Z.; Li, S.; Zhu, L.; Ye, Y.; Luo, H.; Bai, S. Component Fluctuation and Lattice Distortion-Controlled Oxidation Behavior of Refractory Complex Concentrated Alloys. Corros. Sci. 2022, 209, 110778. [Google Scholar] [CrossRef]
- Xu, T.; Chen, Q.; Ji, L.; Wang, H.; Qu, S.; Feng, A. Thermal Stability and Oxidation Resistance of Non-Equimolar Ratio AlTiVCrNb Refractory High-Entropy Alloys. Intermetallics 2023, 160, 107942. [Google Scholar] [CrossRef]
- Lo, K.C.; Chang, Y.J.; Murakami, H.; Yeh, J.W.; Yeh, A.C. An Oxidation Resistant Refractory High Entropy Alloy Protected by CrTaO 4 -Based Oxide. Sci. Rep. 2019, 9, 7266. [Google Scholar] [CrossRef] [PubMed]
- Gorr, B.; Müller, F.; Schellert, S.; Christ, H.-J.; Chen, H.; Kauffmann, A.; Heilmaier, M. A New Strategy to Intrinsically Protect Refractory Metal Based Alloys at Ultra High Temperatures. Corros. Sci. 2020, 166, 108475. [Google Scholar] [CrossRef]
- Lo, K.C.; Murakami, H.; Yeh, J.W.; Yeh, A.C. Oxidation Behaviour of a Novel Refractory High Entropy Alloy at Elevated Temperatures. Intermetallics 2020, 119, 106711. [Google Scholar] [CrossRef]
- Müller, F.; Gorr, B.; Christ, H.J.; Müller, J.; Butz, B.; Chen, H.; Kauffmann, A.; Heilmaier, M. On the Oxidation Mechanism of Refractory High Entropy Alloys. Corros. Sci. 2019, 159, 108161. [Google Scholar] [CrossRef]
- Gorr, B.; Schellert, S.; Müller, F.; Christ, H.J.; Kauffmann, A.; Heilmaier, M. Current Status of Research on the Oxidation Behavior of Refractory High Entropy Alloys. Adv. Eng. Mater. 2021, 23, 2001047. [Google Scholar] [CrossRef]
- Zhang, H.; Du, Y.; Lai, L.; Guo, N.; Li, N.; Guo, S. The As-Cast AlxCrTaTi Refractory Medium Entropy Alloys with Good Room-Temperature Mechanical Properties and High-Temperature Oxidation Resistance. J. Alloys Compd. 2023, 932, 167675. [Google Scholar] [CrossRef]
- Butler, T.M.; Chaput, K.J.; Dietrich, J.R.; Senkov, O.N. High Temperature Oxidation Behaviors of Equimolar NbTiZrV and NbTiZrCr Refractory Complex Concentrated Alloys (RCCAs). J. Alloys Compd. 2017, 729, 1004–1019. [Google Scholar] [CrossRef]
- Waseem, O.A.; Auyeskhan, U.; Lee, H.M.; Ryu, H.J. A Combinatorial Approach for the Synthesis and Analysis of AlxCryMozNbTiZr High-Entropy Alloys: Oxidation Behavior. J. Mater. Res. 2018, 33, 3226–3234. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Y.; Chen, Z.; Zhang, J.; Shen, B. Oxidation Response of a Vacuum Arc Melted NbZrTiCrAl Refractory High Entropy Alloy at 800–1200 °C. Vacuum 2019, 162, 20–27. [Google Scholar] [CrossRef]
- Gawel, R.; Rogal, L.; Przybylski, K. Oxidation Resistance of Ti-Al-Cr-Nb-Based High-Entropy Alloys in Air at 1073 K. J. Mater. Eng. Perform. 2019, 28, 4163–4170. [Google Scholar] [CrossRef]
- Waseem, O.A.; Ryu, H.J. Combinatorial Synthesis and Analysis of AlxTayVz-Cr20Mo20Nb20Ti20Zr10 and Al10CrMoxNbTiZr10 Refractory High-Entropy Alloys: Oxidation Behavior. J. Alloys Compd. 2020, 828, 154427. [Google Scholar] [CrossRef]
- Liu, C.Y.; Ma, Z.L.; Li, H.Y.; Xu, Z.Q.; Cheng, X.W. Designing NbTiTa-Cr/Al Refractory Complex Concentrated Alloys with Balanced Strength-Ductility-Oxidation Resistance Properties: Insights into Oxidation Mechanisms. Int. J. Refract. Met. Hard Mater. 2023, 115, 106308. [Google Scholar] [CrossRef]
- Jayaraj, J.; Thirathipviwat, P.; Han, J.; Gebert, A. Microstructure, Mechanical and Thermal Oxidation Behavior of AlNbTiZr High Entropy Alloy. Intermetallics 2018, 100, 9–19. [Google Scholar] [CrossRef]
- Butler, T.M.; Chaput, K.J. Native Oxidation Resistance of Al20Nb30Ta10Ti30Zr10 Refractory Complex Concentrated Alloy (RCCA). J. Alloys Compd. 2019, 787, 606–617. [Google Scholar] [CrossRef]
- Gorr, B.; Azim, M.; Christ, H.J.; Mueller, T.; Schliephake, D.; Heilmaier, M. Phase Equilibria, Microstructure, and High Temperature Oxidation Resistance of Novel Refractory High-Entropy Alloys. J. Alloys Compd. 2015, 624, 270–278. [Google Scholar] [CrossRef]
- Gorr, B.; Mueller, F.; Christ, H.-J.; Mueller, T.; Chen, H.; Kauffmann, A.; Heilmaier, M. High Temperature Oxidation Behavior of an Equimolar Refractory Metal-Based Alloy 20Nb20Mo20Cr20Ti20Al with and without Si Addition. J. Alloys Compd. 2016, 688, 468–477. [Google Scholar] [CrossRef]
- Gorr, B.; Müller, F.; Azim, M.; Christ, H.-J.; Müller, T.; Chen, H.; Kauffmann, A.; Heilmaier, M. High-Temperature Oxidation Behavior of Refractory High-Entropy Alloys: Effect of Alloy Composition. Oxid. Met. 2017, 88, 339–349. [Google Scholar] [CrossRef]
- Sheikh, S.; Gan, L.; Ikeda, A.; Murakami, H.; Guo, S. Alloying Effect on the Oxidation Behavior of a Ductile Al0.5Cr0.25Nb0.5Ta0.5Ti1.5 Refractory High-Entropy Alloy. Mater. Today Adv. 2020, 7, 100104. [Google Scholar] [CrossRef]
- Ostovari Moghaddam, A.; Sudarikov, M.; Shaburova, N.; Zherebtsov, D.; Zhivulin, V.; Ashurali Solizoda, I.; Starikov, A.; Veselkov, S.; Samoilova, O.; Trofimov, E. High Temperature Oxidation Resistance of W-Containing High Entropy Alloys. J. Alloys Compd. 2022, 897, 162733. [Google Scholar] [CrossRef]
- Lowell, C.E.; Deadmore, D.L. The Role of Thermal Shock in Cyclic Oxidation. Oxid. Met. 1980, 14, 325–336. [Google Scholar] [CrossRef]
- Yurchenko, N.; Panina, E.; Zherebtsov, S.; Stepanov, N. Oxidation Behaviour of Eutectic Refractory High-Entropy Alloys at 800–1000 °C. Corros. Sci. 2022, 205, 110464. [Google Scholar] [CrossRef]
- Panina, E.S.; Yurchenko, N.Y.; Zherebtsov, S.V.; Tikhonovsky, M.A.; Mishunin, M.V.; Stepanov, N.D. Structures and Mechanical Properties of Ti-Nb-Cr-V-Ni-Al Refractory High Entropy Alloys. Mater. Sci. Eng. A 2020, 786, 139409. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y.; Cai, J.; Ji, S.; Geng, J.; Sun, X.; Li, D. High-Strength NbMoTaX Refractory High-Entropy Alloy with Low Stacking Fault Energy Eutectic Phase via Laser Additive Manufacturing. Mater. Des. 2021, 201, 109462. [Google Scholar] [CrossRef]
- Yurchenko, N.; Panina, E.; Shaysultanov, D.; Zherebtsov, S.; Stepanov, N. Refractory High Entropy Alloy with Ductile Intermetallic B2 Matrix/Hard Bcc Particles and Exceptional Strain Hardening Capacity. Materialia 2021, 20, 101225. [Google Scholar] [CrossRef]
- Yurchenko, N.; Panina, E.; Rogal, Ł.; Shekhawat, L.; Zherebtsov, S.; Stepanov, N. Unique Precipitations in a Novel Refractory Nb-Mo-Ti-Co High-Entropy Superalloy. Mater. Res. Lett. 2022, 10, 78–87. [Google Scholar] [CrossRef]
- Panina, E.; Yurchenko, N.; Tojibaev, A.; Mishunin, M.; Zherebtsov, S.; Stepanov, N. Mechanical Properties of (HfCo)100−x(NbMo)x Refractory High-Entropy Alloys with a Dual-Phase Bcc-B2 Structure. J. Alloys Compd. 2022, 927, 167013. [Google Scholar] [CrossRef]
- Xiao, B.; Jia, W.; Wang, J.; Zhou, L. Selective Electron Beam Melting of WMoTaNbVFeCoCrNi Refractory High-Entropy Alloy. Mater. Charact. 2022, 193, 112278. [Google Scholar] [CrossRef]
- Zhang, H.; Cai, J.; Geng, J.; Sun, X.; Zhao, Y.; Guo, X.; Li, D. Study on Annealing Treatment of NbMoTaTiNi High-Entropy Alloy with Ultra-High Strength Disordered-Ordered Transition Structure for Additive Manufacturing. J. Alloys Compd. 2023, 941, 168810. [Google Scholar] [CrossRef]
- Zhang, H.; Cai, J.; Geng, J.; Sun, X.; Zhao, Y.; Guo, X.; Li, D. Development of High Strength High Plasticity Refractory High Entropy Alloy Based on Mo Element Optimization and Advanced Forming Process. Int. J. Refract. Met. Hard Mater. 2023, 112, 106163. [Google Scholar] [CrossRef]
- Panina, E.; Yurchenko, N.; Salishchev, G.; Zherebtsov, S.; Stepanov, N. Thermal Stability of (HfCo)90(NbMo)10 and (HfCo)75(NbMo)25 Refractory High Entropy Alloys with a Bcc + B2 Structure. Int. J. Refract. Met. Hard Mater. 2023, 115, 106297. [Google Scholar] [CrossRef]
- Soni, V.; Gwalani, B.; Senkov, O.N.; Viswanathan, B.; Alam, T.; Miracle, D.B.; Banerjee, R. Phase Stability as a Function of Temperature in a Refractory High-Entropy Alloy. J. Mater. Res. 2018, 33, 3235–3246. [Google Scholar] [CrossRef]
- Soni, V.; Senkov, O.N.; Couzinie, J.-P.; Zheng, Y.; Gwalani, B.; Banerjee, R. Phase Stability and Microstructure Evolution in a Ductile Refractory High Entropy Alloy Al10Nb15Ta5Ti30Zr40. Materialia 2020, 9, 100569. [Google Scholar] [CrossRef]
- Whitfield, T.E.; Pickering, E.J.; Owen, L.R.; Jones, C.N.; Stone, H.J.; Jones, N.G. The Effect of Al on the Formation and Stability of a BCC—B2 Microstructure in a Refractory Metal High Entropy Superalloy System. Materialia 2020, 13, 100858. [Google Scholar] [CrossRef]
- Whitfield, T.E.; Pickering, E.J.; Owen, L.R.; Senkov, O.N.; Miracle, D.B.; Stone, H.J.; Jones, N.G. An Assessment of the Thermal Stability of Refractory High Entropy Superalloys. J. Alloys Compd. 2021, 857, 157583. [Google Scholar] [CrossRef]
- Whitfield, T.E.; Church, N.L.; Stone, H.J.; Jones, N.G. On the Rate of Microstructural Degradation of Al-Ta-Ti-Zr Refractory Metal High Entropy Superalloys. J. Alloys Compd. 2023, 939, 168369. [Google Scholar] [CrossRef]
- Birks, N.; Meier, G.; Pettit, F. Introduction to the High Temperature Oxidation of Metals, 2nd ed.; Cambridge University Press: Cambridge, UK, 2006; ISBN 9781139163903. [Google Scholar]
- HfO2 Crystal Structure—SpringerMaterials. Available online: https://materials.springer.com/isp/crystallographic/docs/sd_0313894 (accessed on 21 July 2023).
- HfO2 Crystal Structure—SpringerMaterials. Available online: https://materials.springer.com/isp/crystallographic/docs/sd_0310050 (accessed on 21 July 2023).
- CoO Crystal Structure—SpringerMaterials. Available online: https://materials.springer.com/isp/crystallographic/docs/sd_0307718 (accessed on 21 July 2023).
- Nb2Co4O9 Crystal Structure—SpringerMaterials. Available online: https://materials.springer.com/isp/crystallographic/docs/sd_0556980 (accessed on 21 July 2023).
- MoO3 Crystal Structure—SpringerMaterials. Available online: https://materials.springer.com/isp/crystallographic/docs/sd_0530932 (accessed on 21 July 2023).
- Robertson, J.; Manning, M.I. Limits to Adherence of Oxide Scales. Mater. Sci. Technol. 1990, 6, 81–92. [Google Scholar] [CrossRef]
- Schütze, M. Mechanical Aspects of High-Temperature Oxidation. Corros. Sci. 1993, 35, 955–963. [Google Scholar] [CrossRef]
- Chan, K.S. A Mechanics-Based Approach to Cyclic Oxidation. Metall. Mater. Trans. 1997, 28, 411–422. [Google Scholar] [CrossRef]
- Su, R.; Zhang, H.; Ouyang, G.; Liu, L.; Nachlas, W.; Cui, J.; Johnson, D.D.; Perepezko, J.H. Enhanced Oxidation Resistance of (Mo95W5)85Ta10(TiZr)5 Refractory Multi-Principal Element Alloy up to 1300 °C. Acta Mater. 2021, 215, 117114. [Google Scholar] [CrossRef]
- Müller, F.; Gorr, B.; Christ, H.J.; Chen, H.; Kauffmann, A.; Heilmaier, M. Effect of Microalloying with Silicon on High Temperature Oxidation Resistance of Novel Refractory High-Entropy Alloy Ta-Mo-Cr-Ti-Al. Mater. High Temp. 2018, 35, 168–176. [Google Scholar] [CrossRef]
- Senkov, O.N.; Daboiku, T.; Butler, T.M.; Rao, S.I.; Payton, E.J. Microstructure, Compression Properties, and Oxidation Behavior of Hf-25Ta-5Me Alloys (Me Is Mo, Nb, W, 0.5Mo + 0.5 W, Cr, or Zr). Int. J. Refract. Met. Hard Mater. 2022, 109, 105968. [Google Scholar] [CrossRef]
- Ouyang, D.; Chen, Z.; Yu, H.; Chan, K.C.; Liu, L. Oxidation Behavior of the Ti38V15Nb23Hf24 Refractory High-Entropy Alloy at Elevated Temperatures. Corros. Sci. 2022, 198, 110153. [Google Scholar] [CrossRef]
- Lo, K.-C.; Murakami, H.; Glatzel, U.; Yeh, J.-W.; Gorsse, S.; Yeh, A.-C. Elemental Effects on the Oxidation of Refractory Compositionally Complex Alloys. Int. J. Refract. Met. Hard Mater. 2022, 108, 105918. [Google Scholar] [CrossRef]
- Zhang, R.; Meng, J.; Han, J.; Tulugan, K.; Zhang, R. Oxidation Resistance Properties of Refractory High-Entropy Alloys with Varied AlxCrTiMo Content. J. Mater. Sci. 2021, 56, 3551–3561. [Google Scholar] [CrossRef]


| Area of EDS Analysis | Elements, at. % | ||||
|---|---|---|---|---|---|
| Hf | Co | Nb | Mo | O | |
| HfCo | |||||
| OL | 48.9 | 10.0 | - | - | 41.1 |
| IRZ | 42.8 | 36.0 | - | - | 21.2 |
| Co-rich layer | 21.7 | 78.3 | - | - | - |
| (HfCo)90(NbMo)10 | |||||
| OL | 2.1 | 41.0 | 0.1 | 0.2 | 57.6 |
| IRZ_1 | 21.5 | 4.4 | 2.7 | 1.0 | 70.4 |
| IRZ_2 | 21.1 | 22.5 | 1.2 | 3.1 | 52.1 |
| Co-rich layer | 21.3 | 61.7 | 1.2 | 1.9 | 13.9 |
| (HfCo)75(NbMo)25 | |||||
| OL | 0.8 | 35.6 | 1.9 | 10.9 | 50.9 |
| IRZ_1 | 42.8 | 5.2 | 5.4 | 0.2 | 46.4 |
| IRZ_2 | 32.1 | 36.8 | 4.0 | 4.5 | 22.7 |
| Area of EDS Analysis | Elements, at. % | ||||
|---|---|---|---|---|---|
| Hf | Co | Nb | Mo | O | |
| HfCo | |||||
| OL | 0.8 | 40.1 | - | - | 59.1 |
| IRZ_1 | 18.2 | 13.8 | - | - | 68.0 |
| IRZ_2 | 21.7 | 22.0 | - | - | 56.3 |
| Co-rich layer | 16.3 | 71.9 | - | - | 11.8 |
| (HfCo)90(NbMo)10 | |||||
| OL | 0.3 | 42.4 | 0.7 | 0.4 | 56.2 |
| IRZ_1 | 14.5 | 6.8 | 2.4 | 1.2 | 75.2 |
| IRZ_2 | 20.0 | 21.0 | 0.9 | 2.2 | 55.9 |
| Co-rich layer | 10.9 | 66.1 | 2.4 | 2.8 | 17.8 |
| (HfCo)75(NbMo)25 | |||||
| Top of the IRZ | 13.6 | 12.6 | 5.4 | 0.9 | 68.6 |
| Middle of the IRZ | 10.2 | 12.7 | 4.2 | 3.3 | 69.6 |
| Bottom of the IRZ | 12.6 | 2.8 | 3.5 | 4.4 | 76.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yurchenko, N.; Panina, E.; Zherebtsov, S.; Stepanov, N. Oxidation Behaviour of Refractory (HfCo)100−x(NbMo)x High-Entropy Alloys with a bcc+B2 Structure. Appl. Sci. 2023, 13, 9336. https://doi.org/10.3390/app13169336
Yurchenko N, Panina E, Zherebtsov S, Stepanov N. Oxidation Behaviour of Refractory (HfCo)100−x(NbMo)x High-Entropy Alloys with a bcc+B2 Structure. Applied Sciences. 2023; 13(16):9336. https://doi.org/10.3390/app13169336
Chicago/Turabian StyleYurchenko, Nikita, Evgeniya Panina, Sergey Zherebtsov, and Nikita Stepanov. 2023. "Oxidation Behaviour of Refractory (HfCo)100−x(NbMo)x High-Entropy Alloys with a bcc+B2 Structure" Applied Sciences 13, no. 16: 9336. https://doi.org/10.3390/app13169336
APA StyleYurchenko, N., Panina, E., Zherebtsov, S., & Stepanov, N. (2023). Oxidation Behaviour of Refractory (HfCo)100−x(NbMo)x High-Entropy Alloys with a bcc+B2 Structure. Applied Sciences, 13(16), 9336. https://doi.org/10.3390/app13169336








