An Automatic ECG Signal Quality Assessment Method Based on Resnet and Self-Attention
Abstract
1. Introduction
2. Background
2.1. Resnet Based ECG Classification
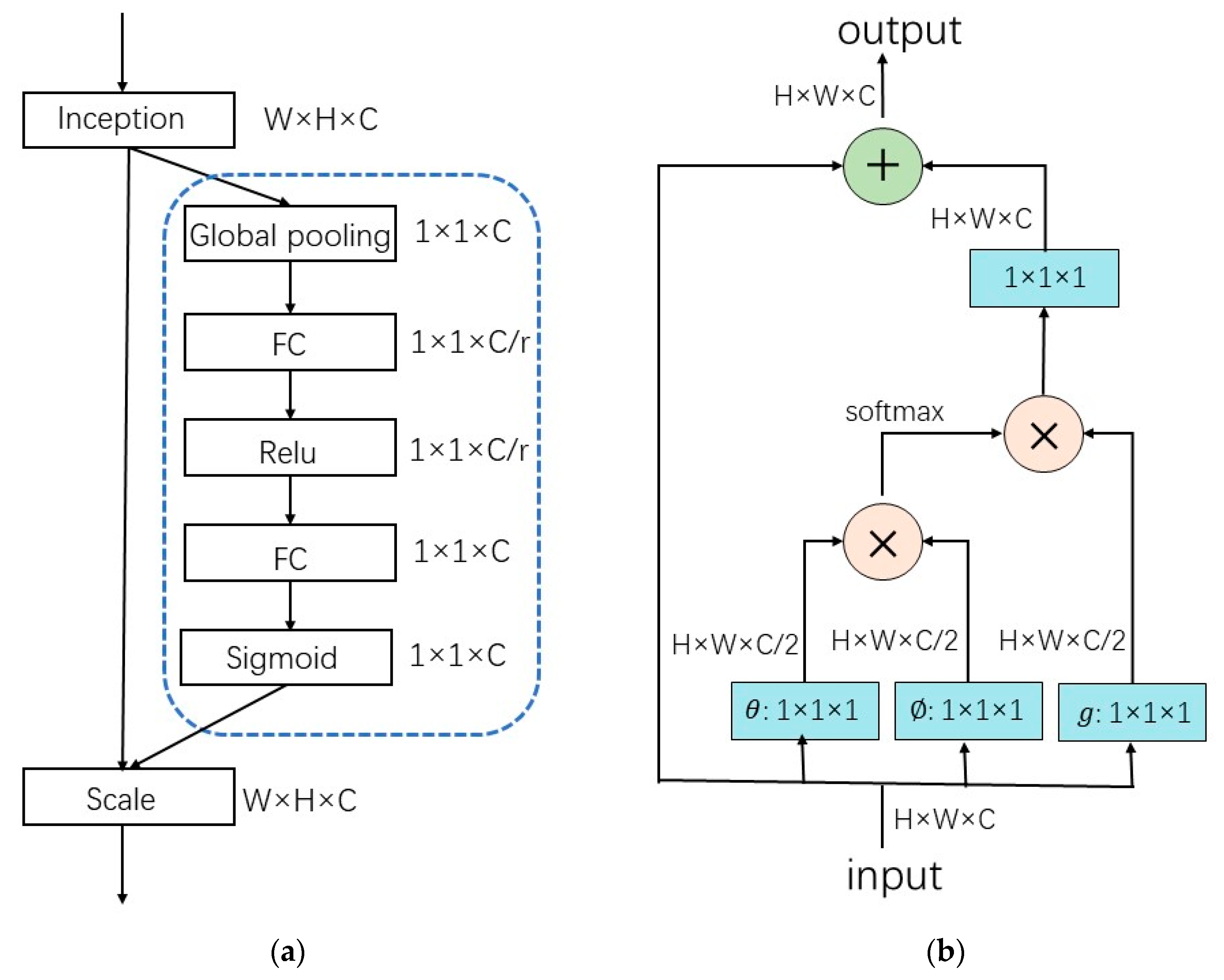
2.2. Attention Mechanism in ECG Signal Processing
3. Materials and Methods
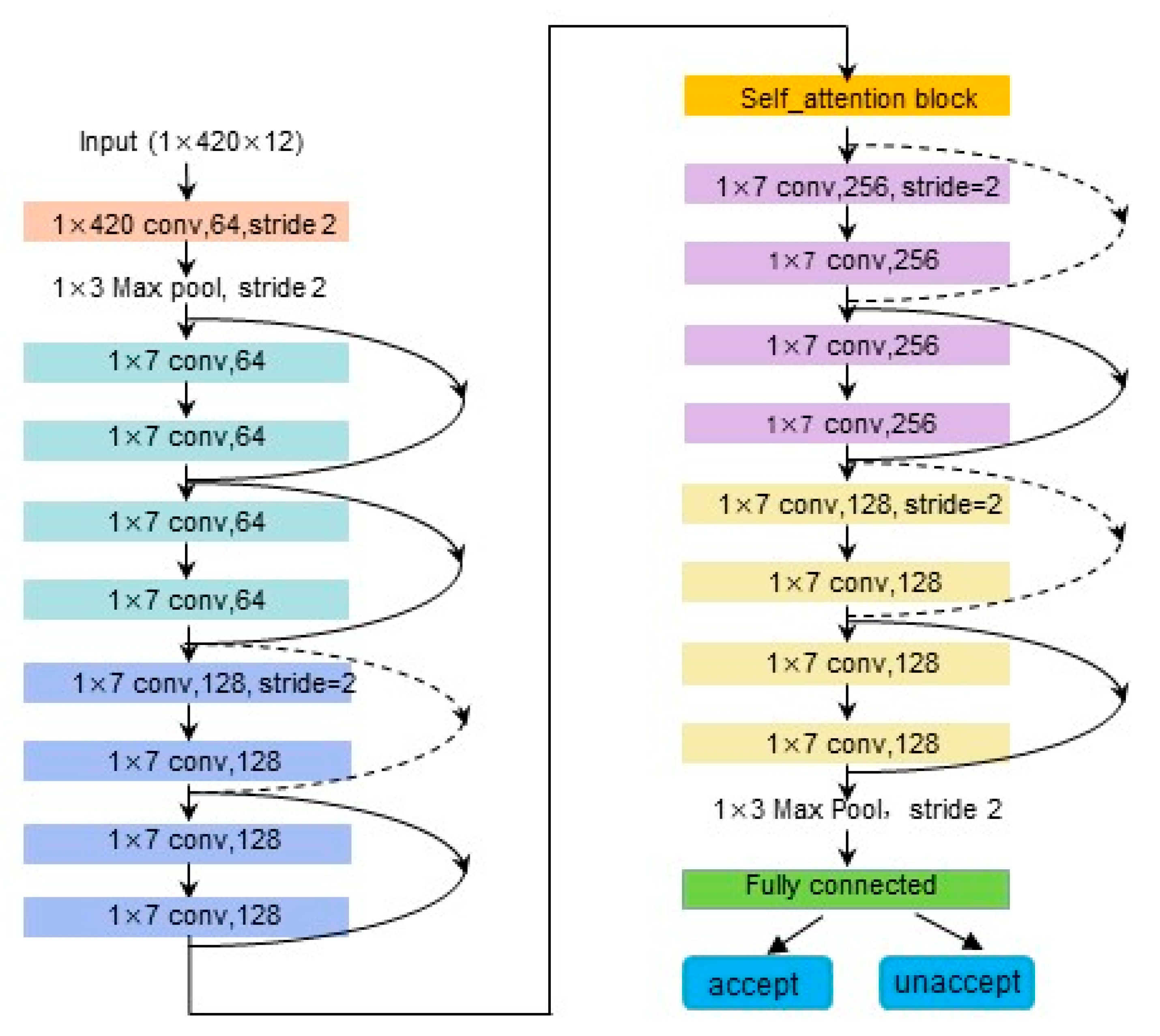
3.1. Proposed Signal Quality Assessment Model Framework
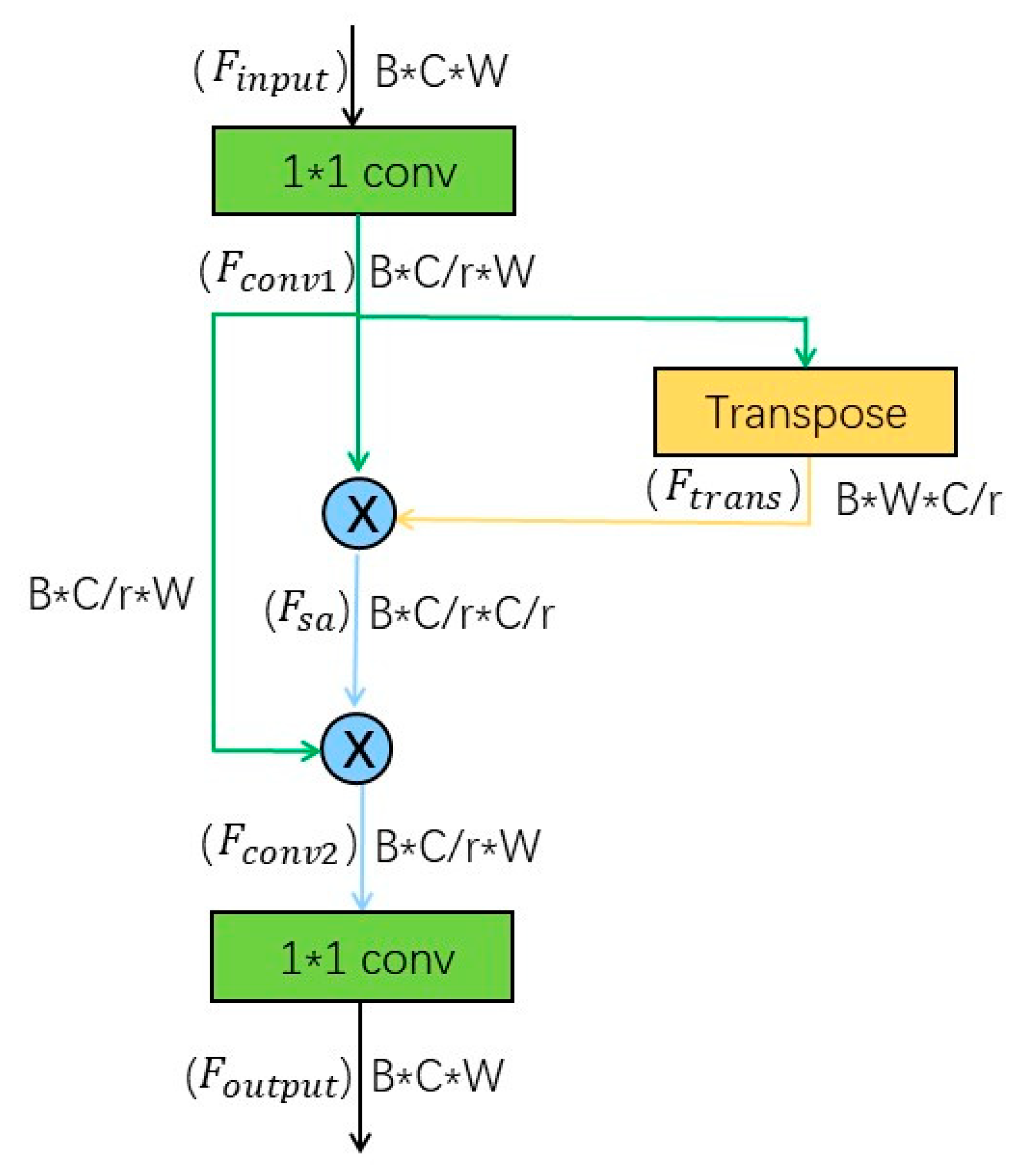
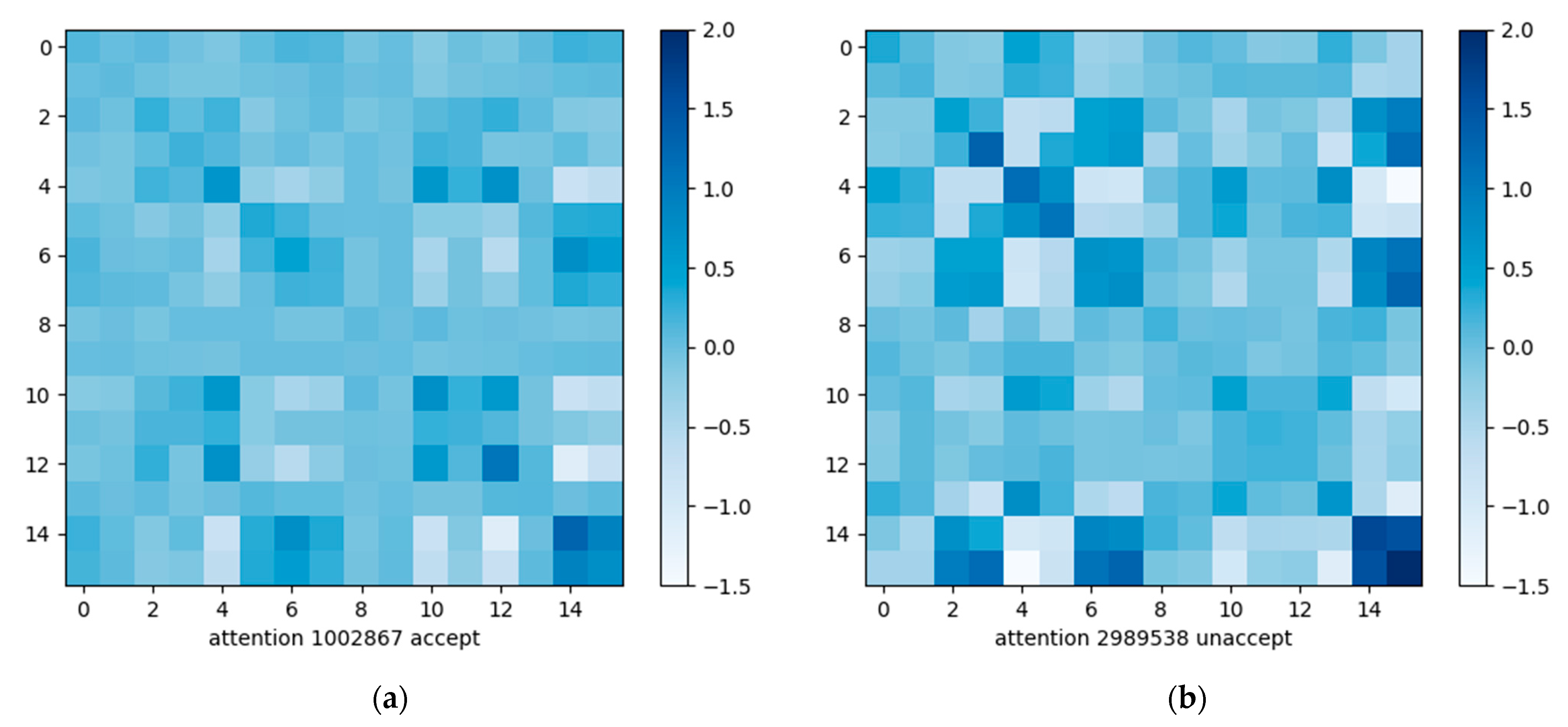
3.2. Proposed Self-Attention Module
3.3. Comparison of Parameters and Calculations of Attention Modules
4. Results
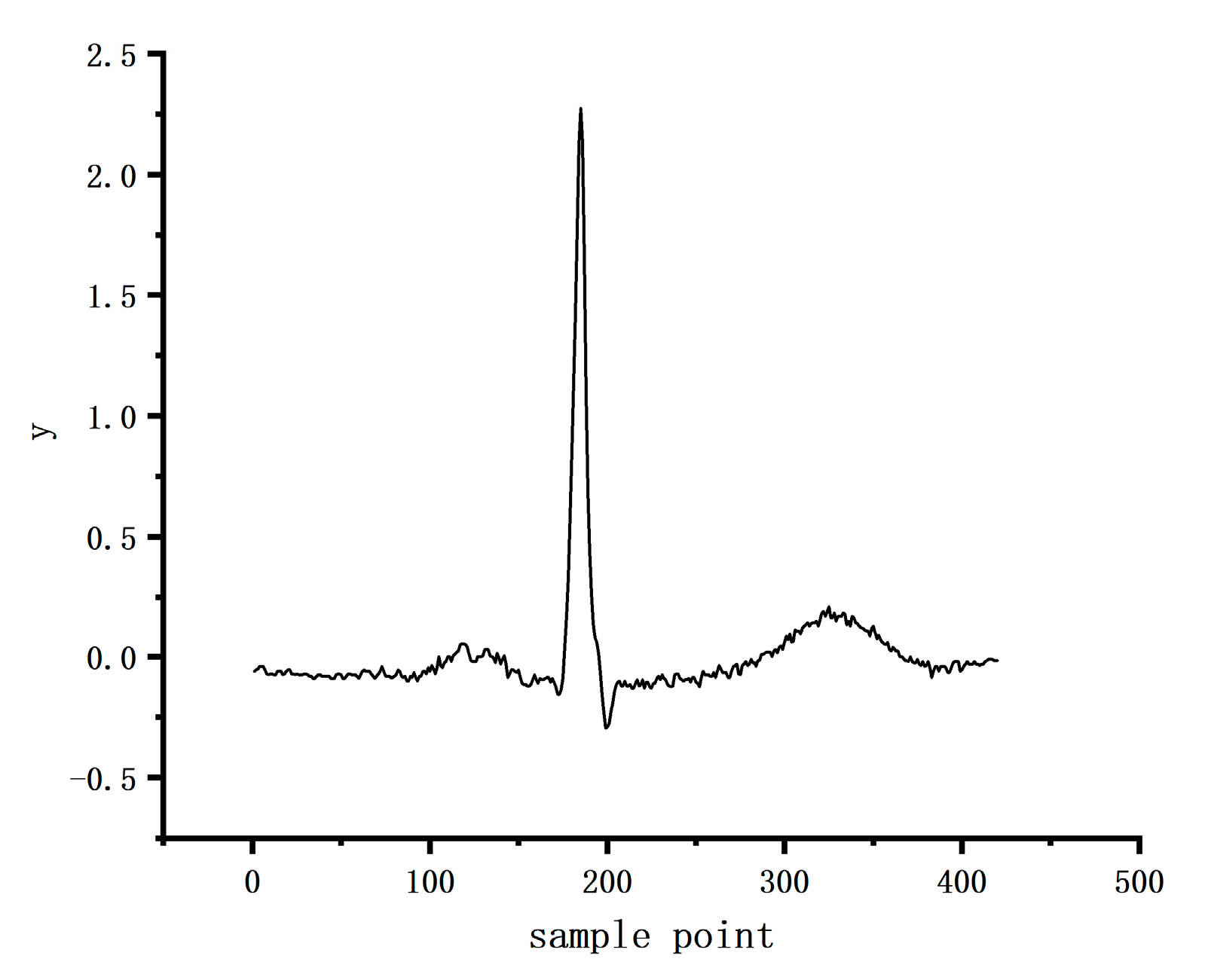
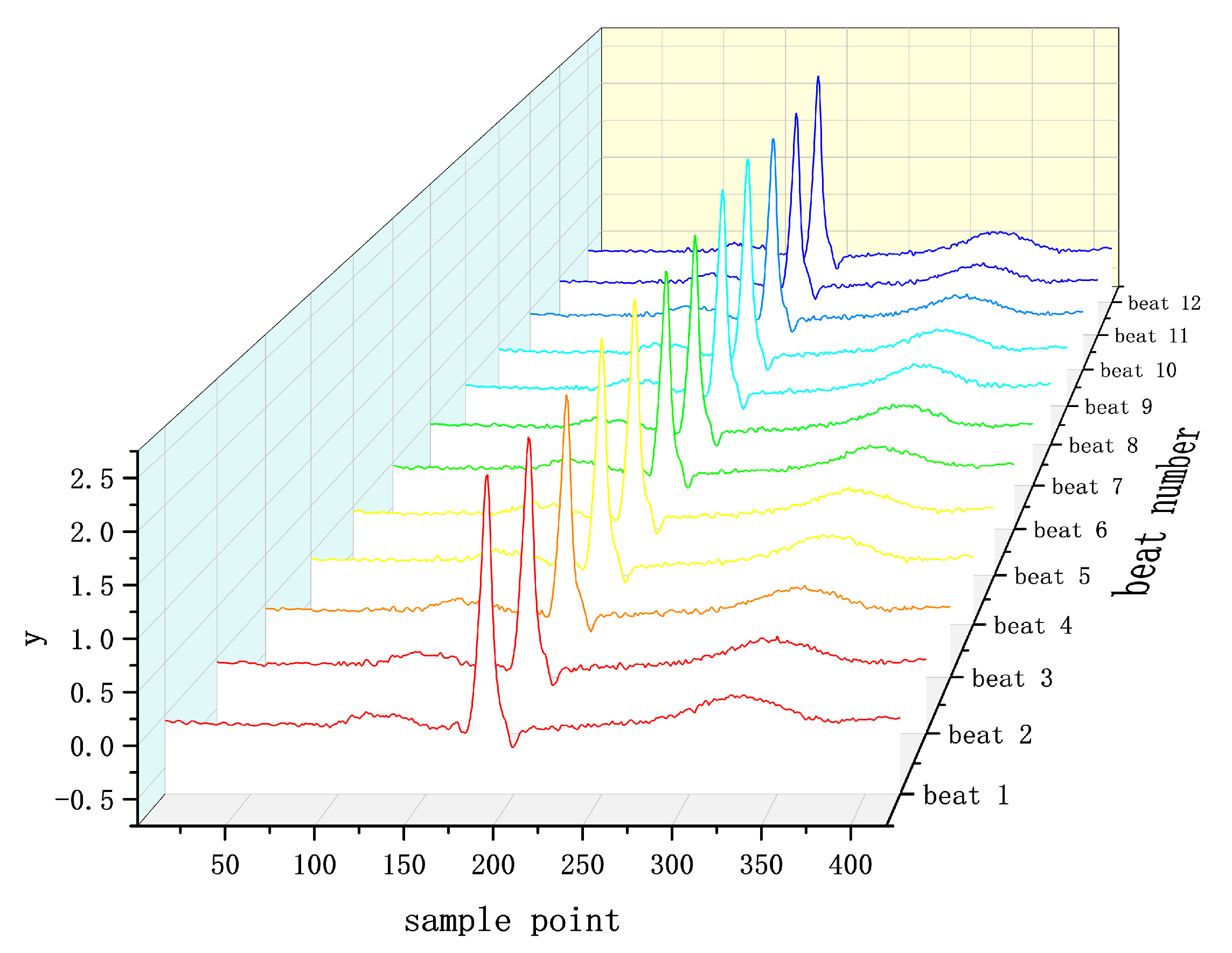
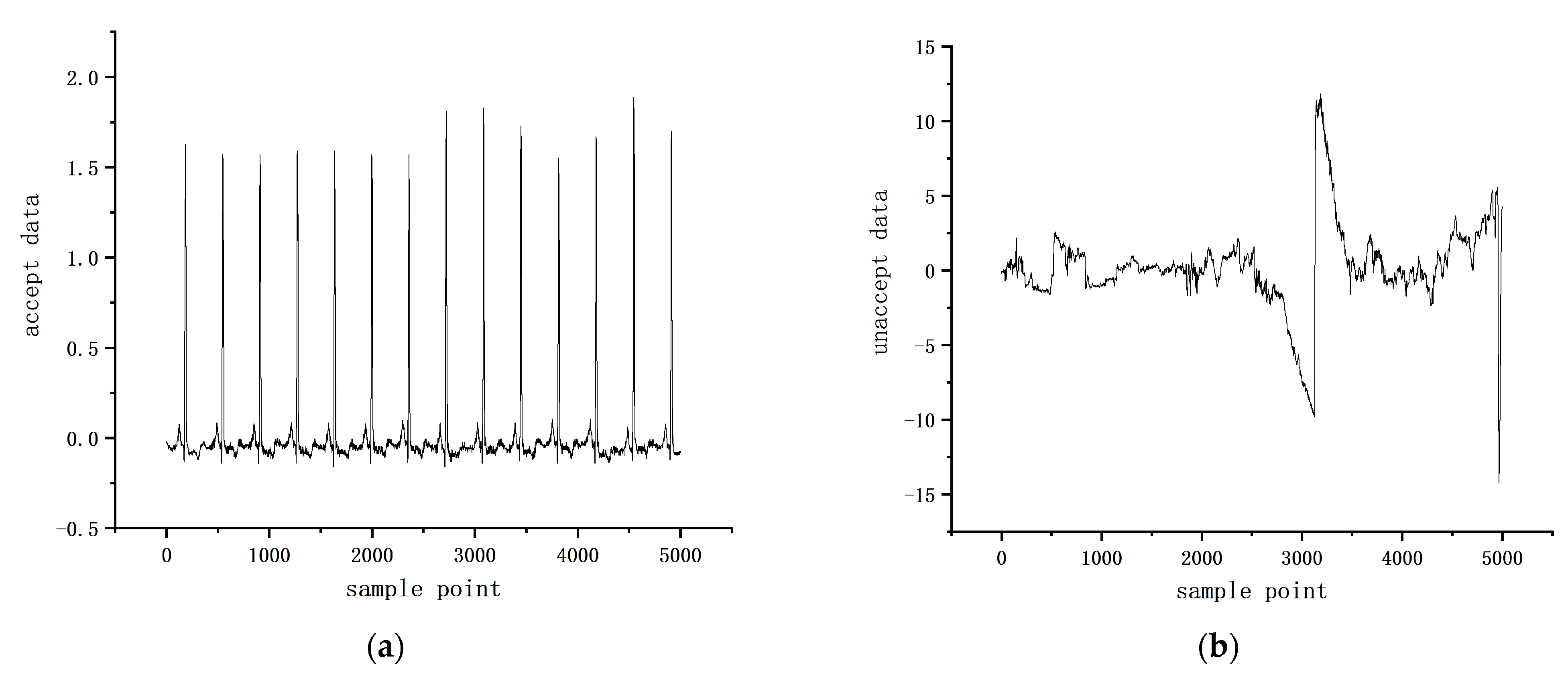
4.1. Datasets
4.2. Data Preprocessing
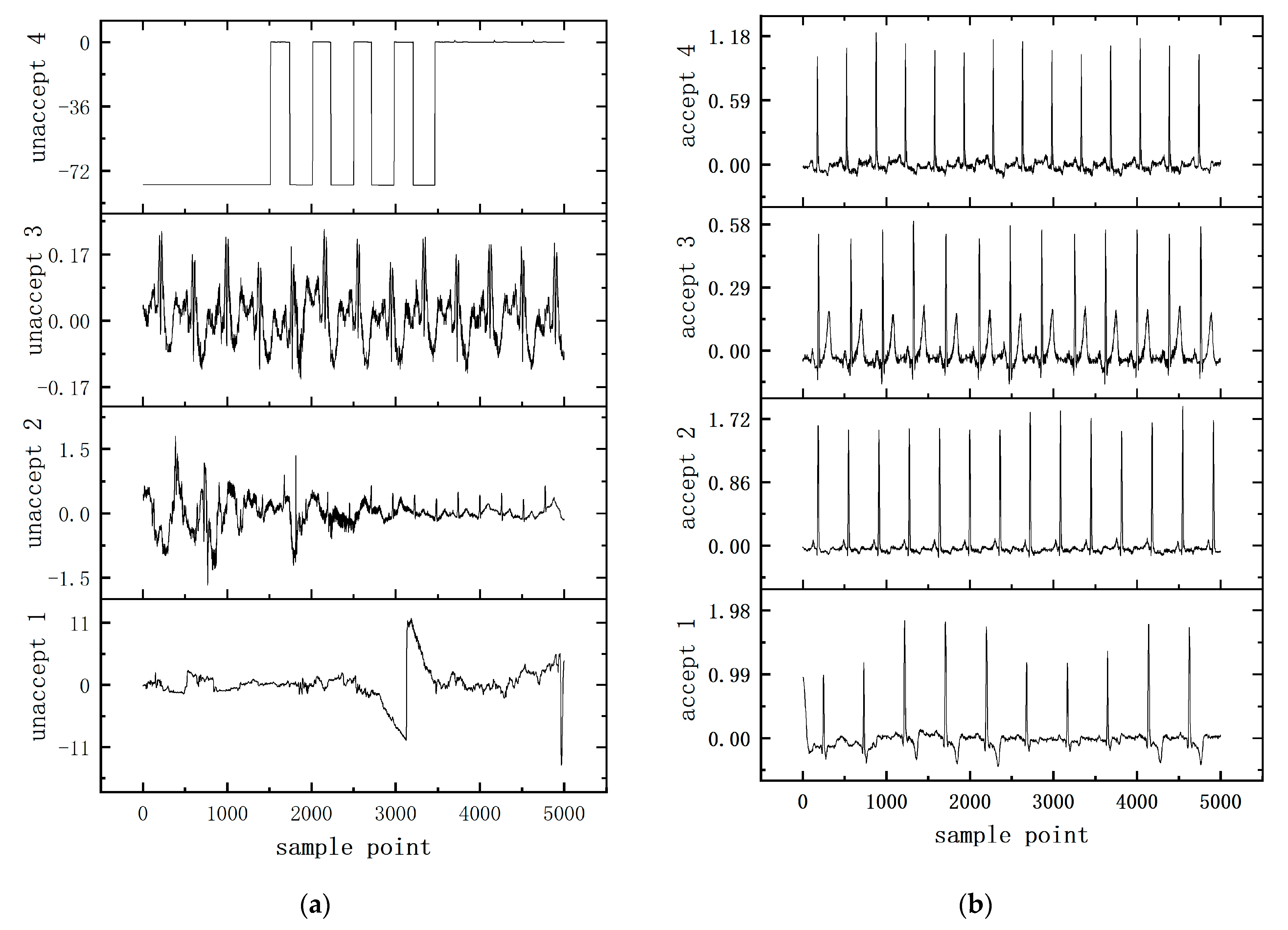
4.3. Experiment
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cardiovascular Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 8 October 2022).
- Mendis, S.; Puska, P.; Norrving, B.; World Health Organization. Global Atlas on Cardiovascular Disease Prevention and Control; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Kumar, P.; Sharma, V.K. Cardiac Signals Based Methods For Recognizing Heart Disease: A Review. In Proceedings of the 2021 Third International Conference on Intelligent Communication Technologies and Virtual Mobile Networks (ICICV), Tirunelveli, India, 4–6 February 2021; pp. 1375–1377. [Google Scholar]
- Islam, M.R.; Ahmad, S.; Hirose, K.; Molla, M.K.I. Data adaptive analysis of ECG signals for cardiovascular disease diagnosis. In Proceedings of the 2010 IEEE International Symposium on Circuits and Systems, Paris, France, 30 May–2 June 2010; pp. 2243–2246. [Google Scholar]
- Isin, A.; Ozdalili, S. Cardiac arrhythmia detection using deep learning. Procedia Comput. Sci. 2017, 120, 268–275. [Google Scholar] [CrossRef]
- Hao, P.; Gao, X.; Li, Z.; Zhang, J.; Wu, F.; Bai, C. Multi-branch fusion network for Myocardial infarction screening from 12-lead ECG images. Comput. Methods Programs Biomed. 2020, 184, 105286. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Rahman, T.; Ghani, N.H.; Hossain, S.; Uddin, J. IoT Based Patient Monitoring System Using ECG Sensor. In Proceedings of the 2019 International Conference on Robotics, Electrical and Signal Processing Techniques (ICREST), Dhaka, Bangladesh, 10–12 January 2019; pp. 378–382. [Google Scholar]
- Lobodzinski, S.S.; Laks, M.M. New devices for very long-term ECG monitoring. Cardiol. J. 2012, 19, 210–214. [Google Scholar] [CrossRef] [PubMed]
- van der Bijl, K.; Elgendi, M.; Menon, C. Automatic ECG Quality Assessment Techniques: A Systematic Review. Diagnostics 2022, 12, 2578. [Google Scholar] [CrossRef]
- Satija, U.; Ramkumar, B.; Manikandan, M.S. Automated ECG Noise Detection and Classification System for Unsupervised Healthcare Monitoring. IEEE J. Biomed. Health Inform. 2018, 22, 722–732. [Google Scholar] [CrossRef]
- Cioffi, R.; Travaglioni, M.; Piscitelli, G.; Petrillo, A.; De Felice, F. Artificial Intelligence and Machine Learning Applications in Smart Production: Progress, Trends, and Directions. Sustainability 2020, 12, 492. [Google Scholar] [CrossRef]
- El Naqa, I.; Murphy, M.J. What Is Machine Learning? In Machine Learning in Radiation Oncology: Theory and Applications; El Naqa, I., Li, R., Murphy, M.J., Eds.; Springer International Publishing: Cham, The Netherlands, 2015; pp. 3–11. [Google Scholar]
- Satija, U.; Ramkumar, B.; Manikandan, M.S. A Review of Signal Processing Techniques for Electrocardiogram Signal Quality Assessment. IEEE Rev. Biomed. Eng. 2018, 11, 36–52. [Google Scholar] [CrossRef]
- Li, L. A Quality Assessment Method of Single-Lead ECG Signal Based on Spectral Analysis. In Proceedings of the 2016 8th International Conference on Information Technology in Medicine and Education (ITME), Fuzhou, China, 23–25 December 2016; pp. 35–38. [Google Scholar]
- Agrawal, A.; Dash, A.; Ghosh, N.; Patra, A. Morphological Event Based Signal Quality Assessment of Electrocardiogram. In Proceedings of the 2022 44th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Glasgow, UK, 11–15 July 2022; pp. 2021–2024. [Google Scholar]
- Behar, J.; Oster, J.; Li, Q.; Clifford, G.D. ECG signal quality during arrhythmia and its application to false alarm reduction. IEEE Trans. Biomed. Eng. 2013, 60, 1660–1666. [Google Scholar] [CrossRef]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef]
- Schmidhuber, J. Deep learning in neural networks: An overview. Neural Netw. 2015, 61, 85–117. [Google Scholar] [CrossRef]
- Bengio, Y. Learning Deep Architectures for AI. In Foundations and Trends® in Machine Learning; Now Publishers: Norwell, MA, USA, 2009; Volume 2, pp. 1–127. [Google Scholar]
- Guo, J.; Zhu, X.; Xiao, J.; Lei, Z.; Wan, G.; Li, S.Z. Improving Face Anti-Spoofing by 3D Virtual Synthesis. In Proceedings of the 2019 International Conference on Biometrics (ICB), Crete, Greece, 4–7 June 2019; pp. 1–8. [Google Scholar]
- Liu, J.; Zhang, C.; Sun, Y.; Han, J.; Ding, E. Detecting Text in the Wild with Deep Character Embedding Network. arXiv 2019, arXiv:1901.00363. [Google Scholar]
- Elola, A.; Aramendi, E.; Irusta, U.; Picon, A.; Alonso, E.; Owens, P.; Idris, A. Deep Neural Networks for ECG-Based Pulse Detection during Out-of-Hospital Cardiac Arrest. Entropy 2019, 21, 305. [Google Scholar] [CrossRef] [PubMed]
- Á, H.; Martínez-Rodrigo, A.; Puchol, A.; Pachón, M.I.; Rieta, J.J.; Alcaraz, R. Comparison of Pre-Trained Deep Learning Algorithms for Quality Assessment of Electrocardiographic Recordings. In Proceedings of the 2020 International Conference on e-Health and Bioengineering (EHB), Iasi, Romania, 29–30 October 2020; pp. 1–4. [Google Scholar]
- Zhou, X.; Zhu, X.; Nakamura, K.; Mahito, N. ECG Quality Assessment Using 1D-Convolutional Neural Network. In Proceedings of the 2018 14th IEEE International Conference on Signal Processing (ICSP), Beijing, China, 12–16 August 2018; pp. 780–784. [Google Scholar]
- Bortolan, G.; Christov, I.; Simova, I. Rule-Based methods and Deep Learning Networks for Automatic Classification of ECG. In Proceedings of the 2020 Computing in Cardiology Conference (CinC), Rimini, Italy, 13–16 September 2020; pp. 1–4. [Google Scholar]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep Residual Learning for Image Recognition. In Proceedings of the 2016 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Las Vegas, NV, USA, 27–30 June 2016; pp. 770–778. [Google Scholar]
- Fang, S.; Xie, H.; Wang, Y.; Mao, Z.; Zhang, Y. Read Like Humans: Autonomous, Bidirectional and Iterative Language Modeling for Scene Text Recognition. In Proceedings of the 2021 IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR), Nashville, TN, USA, 20–25 June 2021; pp. 7094–7103. [Google Scholar]
- Lanchantin, J.; Wang, T.; Ordonez, V.; Qi, Y. General Multi-label Image Classification with Transformers. In Proceedings of the 2021 IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR), Nashville, TN, USA, 20–25 June 2021; pp. 16473–16483. [Google Scholar]
- Ramirez-Villegas, J.F.; Lam-Espinosa, E.; Ramirez-Moreno, D.F.; Calvo-Echeverry, P.C.; Agredo-Rodriguez, W. Heart rate variability dynamics for the prognosis of cardiovascular risk. PLoS ONE 2011, 6, e17060. [Google Scholar] [CrossRef]
- Holst, H.; Ohlsson, M.; Peterson, C.; Edenbrandt, L. A confident decision support system for interpreting electrocardiograms. Clin. Physiol. 1999, 19, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Monasterio, V.; Laguna, P.; Martinez, J.P. Multilead analysis of T-wave alternans in the ECG using principal component analysis. IEEE Trans. Biomed. Eng. 2009, 56, 1880–1890. [Google Scholar] [CrossRef]
- de Chazal, P.; O’Dwyer, M.; Reilly, R.B. Automatic classification of heartbeats using ECG morphology and heartbeat interval features. IEEE Trans. Biomed. Eng. 2004, 51, 1196–1206. [Google Scholar] [CrossRef]
- Ye, C.; Kumar, B.V.; Coimbra, M.T. Heartbeat classification using morphological and dynamic features of ECG signals. IEEE Trans. Biomed. Eng. 2012, 59, 2930–2941. [Google Scholar]
- Osowski, S.; Hoai, L.T.; Markiewicz, T. Support vector machine-based expert system for reliable heartbeat recognition. IEEE Trans. Biomed. Eng. 2004, 51, 582–589. [Google Scholar] [CrossRef]
- Gyawali, P.K.; Horacek, B.M.; Sapp, J.L.; Wang, L. Sequential Factorized Autoencoder for Localizing the Origin of Ventricular Activation From 12-Lead Electrocardiograms. IEEE Trans. Biomed. Eng. 2020, 67, 1505–1516. [Google Scholar] [CrossRef]
- Xu, S.S.; Mak, M.W.; Cheung, C.C. Towards End-to-End ECG Classification With Raw Signal Extraction and Deep Neural Networks. IEEE J. Biomed. Health Inform. 2019, 23, 1574–1584. [Google Scholar] [CrossRef]
- Nurmaini, S.; Umi Partan, R.; Caesarendra, W.; Dewi, T.; Naufal Rahmatullah, M.; Darmawahyuni, A.; Bhayyu, V.; Firdaus, F. An Automated ECG Beat Classification System Using Deep Neural Networks with an Unsupervised Feature Extraction Technique. Appl. Sci. 2019, 9, 2921. [Google Scholar] [CrossRef]
- Niu, L.; Chen, C.; Liu, H.; Zhou, S.; Shu, M. A Deep-Learning Approach to ECG Classification Based on Adversarial Domain Adaptation. Healthcare 2020, 8, 437. [Google Scholar] [CrossRef] [PubMed]
- Byeon, Y.H.; Pan, S.B.; Kwak, K.C. Intelligent Deep Models Based on Scalograms of Electrocardiogram Signals for Biometrics. Sensors 2019, 19, 935. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Lee, M.; Song, H.S.; Lee, S.-W. Automatic Cardiac Arrhythmia Classification Using Residual Network Combined With Long Short-Term Memory. IEEE Trans. Instrum. Meas. 2022, 71, 1–17. [Google Scholar] [CrossRef]
- Liu, F.; Zhou, X.; Wang, T.; Cao, J.; Wang, Z.; Wang, H.; Zhang, Y. An Attention-based Hybrid LSTM-CNN Model for Arrhythmias Classification. In Proceedings of the 2019 International Joint Conference on Neural Networks (IJCNN), Budapest, Hungary, 14–19 July 2019; pp. 1–8. [Google Scholar]
- Mnih, V.; Heess, N.; Graves, A.; Kavukcuoglu, K. Recurrent Models of Visual Attention. arXiv 2014, arXiv:1406.6247. [Google Scholar]
- Rafi, T.H.; Woong Ko, Y. HeartNet: Self Multihead Attention Mechanism via Convolutional Network With Adversarial Data Synthesis for ECG-Based Arrhythmia Classification. IEEE Access 2022, 10, 100501–100512. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, H.; Cao, Q.; Yan, J.; Wu, F.; Zhu, H.; Pan, Y. Multi-Label Classification of Multi-lead ECG Based on Deep 1D Convolutional Neural Networks With Residual and Attention Mechanism. In Proceedings of the 2021 Computing in Cardiology (CinC), Brno, Czech Republic, 13–15 September 2021; pp. 1–4. [Google Scholar]
- Wang, X.; He, Z.; Lin, Z.; Han, Y.; Liu, T.; Lu, J.; Xie, S. PA2Net: Period-Aware Attention Network for Robust Fetal ECG Detection. IEEE Trans. Instrum. Meas. 2022, 71, 1–12. [Google Scholar]
- Wang, X.; Girshick, R.; Gupta, A.; He, K. Non-local Neural Networks. In Proceedings of the 2018 IEEE/CVF Conference on Computer Vision and Pattern Recognition, Salt Lake City, UT, USA, 18–23 June 2018; pp. 7794–7803. [Google Scholar]
- Vaswani, A.; Shazeer, N.; Parmar, N.; Uszkoreit, J.; Jones, L.; Gomez, A.N.; Kaiser, L.; Polosukhin, I. Attention Is All You Need. arXiv 2017, arXiv:1706.03762. [Google Scholar]
- PhysioNet. Available online: https://www.physionet.org/content/challenge-2011/1.0.0/ (accessed on 19 September 2022).
- Hamilton, P.S.; Tompkins, W.J. Quantitative Investigation of QRS Detection Rules Using the MIT/BIH Arrhythmia Database. IEEE Trans. Biomed. Eng. 1986, BME-33, 1157–1165. [Google Scholar] [CrossRef]
- Hermawan, I.; Ma’sum, M.A.; Intan, P.R.D.; Jatmiko, W.; Wiweko, B.; Boediman, A.; Pradekso, B.K. Temporal feature and heuristics-based Noise Detection over Classical Machine Learning for ECG Signal Quality Assessment. In Proceedings of the 2019 International Workshop on Big Data and Information Security (IWBIS), Bali, Indonesia, 11 October 2019; pp. 1–8. [Google Scholar]
- Liu, G.; Han, X.; Tian, L.; Zhou, W.; Liu, H. ECG quality assessment based on hand-crafted statistics and deep-learned S-transform spectrogram features. Comput. Methods Programs Biomed. 2021, 208, 106269. [Google Scholar] [CrossRef]
- Athif, M.; Daluwatte, C. Combination of rule based classification and decision trees to identify low quality ECG. In Proceedings of the 2017 IEEE International Conference on Industrial and Information Systems (ICIIS), Peradeniya, Sri Lanka, 15–16 December 2017; pp. 1–4. [Google Scholar]
- Morgado, E.; Alonso-Atienza, F.; Santiago-Mozos, R.; Barquero-Perez, O.; Silva, I.; Ramos, J.; Mark, R. Quality estimation of the electrocardiogram using cross-correlation among leads. Biomed. Eng. Online 2015, 14, 59. [Google Scholar] [CrossRef] [PubMed]
| True | |||
|---|---|---|---|
| Predicted | accepted | unaccepted | |
| accepted | TP | FP | |
| unaccepted | FN | TN | |
| Category | SE-Net | Non-Local | Self-Attention |
|---|---|---|---|
| Calculation volume | 8192 | 8256 | 4128 |
| Number of participants | 8192 | 5312 | 4208 |
| Category | Diagnosis | Count |
|---|---|---|
| 1 | accepted | 706 |
| 2 | unaccepted | 157 |
| Total | 863 |
| Accuracy | Recall | Specificity | Precision | F1-Score | |
|---|---|---|---|---|---|
| Resnet18 | 91.43% | 98.32% | 52.87% | 90.51% | 94.26% |
| SA-Resnet18 | 92.82% | 99.17% | 60.51% | 91.92% | 95.40% |
| References | Methods | Datasets | Lead Number | Accuracy | F1-Score |
|---|---|---|---|---|---|
| Hermawan et al., 2019 [50] | Machine learning | Cinc2011 | 2 | 85.7% | Unknown |
| Huerta et al., 2020 [23] | GoogLeNet Combining the deep-learned Stockwell | Cinc2017 | 12 | 91.5% | Unknown |
| Liu et al., 2021 [51] | Transform (S-Transform) spectrogram features and hand-crafted statistical features | Cinc2011 | 12 | 93.1% | 84.7% |
| Athif et al., 2018 [52] | Decision tree | Cinc2011 | 12 | 91.1% | Unknown |
| Morgado et al., 2015 [53] | Covariance characteristic matrix and machine learning classifier | Cinc2011 | 12 | 89.8% | Unknown |
| Our proposed method | Resnet18+Self-Attention | Cinc2011 | 1 | 92.8% | 95.4% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhang, H.; Zhao, K.; Liu, H.; Long, F.; Chen, L.; Yang, Y. An Automatic ECG Signal Quality Assessment Method Based on Resnet and Self-Attention. Appl. Sci. 2023, 13, 1313. https://doi.org/10.3390/app13031313
Liu Y, Zhang H, Zhao K, Liu H, Long F, Chen L, Yang Y. An Automatic ECG Signal Quality Assessment Method Based on Resnet and Self-Attention. Applied Sciences. 2023; 13(3):1313. https://doi.org/10.3390/app13031313
Chicago/Turabian StyleLiu, Yuying, Hao Zhang, Kun Zhao, Haiyang Liu, Fei Long, Liping Chen, and Yaguang Yang. 2023. "An Automatic ECG Signal Quality Assessment Method Based on Resnet and Self-Attention" Applied Sciences 13, no. 3: 1313. https://doi.org/10.3390/app13031313
APA StyleLiu, Y., Zhang, H., Zhao, K., Liu, H., Long, F., Chen, L., & Yang, Y. (2023). An Automatic ECG Signal Quality Assessment Method Based on Resnet and Self-Attention. Applied Sciences, 13(3), 1313. https://doi.org/10.3390/app13031313





