Physiological and Biochemical Responses of Apple (Malus domestica Borkh.) to Biostimulants Application and Substrate Additives under Salinity Stress
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, M.H.; Cho, E.J.; Wi, S.G. Divergences in morphological changes and antioxidant responses in salt-tolerant and salt-sensitive rice seedlings after salt stress. Plant Physiol. Bioch. 2013, 70, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.M.F. Cell permeability under salt stress. In Strategies for Improving Salt Tolerance in Higher Plants; Jaiwal, P.K., Singh, R.P., Gulati, A., Eds.; Oxford and IBH: New Delhi, India, 1997; pp. 87–110. [Google Scholar]
- Ali, S.; Xie, L. Plant Growth Promoting and Stress mitigating abilities of Soil Born Microorganisms. Recent Pat. Food Nutr. Agric. 2019, 15, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Hasegawa, P.M.; Bressan, R.A. Molecular aspects of osmotic stress in plants. Citri. Rev. Plant Sci. 1997, 16, 253–277. [Google Scholar] [CrossRef]
- Serrano, R.; Mulet, J.M.; Rios, G.; Marquez, J.A.; de Larrinoa, I.F.; Leube, M.P.; Mendizabal, I.; Pascual-Ahuir, A.; Proft, M.; Ros, R.; et al. A glimpse of the mechanism of ion homeostasis during salt stress. J. Exp. Bot. 1999, 50, 1023–1036. [Google Scholar] [CrossRef]
- Flowers, T.J.; Munns, R.; Colmer, T.D. Sodium chloride toxicity and the cellular basis of salt tolerance in halophytes. Ann. Bot. 2015, 115, 419–431. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A.; Babar, M.A. The stimulatory effects of plant growth promoting rhizobacteria and plant growth regulators on wheat physiology grown in sandy soil. Arch. Microbiol. 2019, 201, 769–785. [Google Scholar] [CrossRef]
- Price, A.; Hendry, G. Iron catalysed oxygen radical formation and its possible contribution to drought damage in nine native grasses and three cereals. Plant Cell Environ. 1991, 14, 477–484. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A. Effects of exogenously applied salicylic acid and putrescine alone and in combination with rhizobacteria on the phytoremediation of heavy metals and chickpea growth in sandy soil. Int. J. Phytoremed. 2018, 16, 405–414. [Google Scholar] [CrossRef]
- Dobrota, C. Energy dependant plant stress acclimation. Rev. Environ. Sci. 2006, 5, 243–251. [Google Scholar] [CrossRef]
- Yeo, A.R.; Capron, S.J.M.; Flowers, T.J. The effect of salinity upon photosynthesis in rice (Oryza sativa L.) Gas exchange by individual leaves relation to their salt content. J. Exp. Bot. 1985, 36, 1240–1248. [Google Scholar] [CrossRef]
- Muradoglu, F.; Gundogdu, M.; Ercisli, S.; Encu, T.; Balta, F.; Jaafar, H.Z.; Zia-Ul-Haq, M. Cadmium toxicity affects chlorophyll a and b content, antioxidant enzyme activities and mineral nutrient accumulation in strawberry. Biol. Res. 2015, 48, 1–7. [Google Scholar] [CrossRef] [PubMed]
- El-Shintinawy, F.; El-Ansary, A. Differential Effect of Cd2+ and Ni2+ on Amino Acid Metabolism in Soybean Seedlings. Biol. Plant. 2000, 43, 79–84. [Google Scholar] [CrossRef]
- Lycoskoufis, I.H.; Savvas, D.; Mavrogianopoulos, G. Growth, gas exchange, and nutrient status in pepper (Capsicum annuum L.) grown in recirculating nutrient solution as affected by salinity imposed to half of the root system. Sci. Hortic. 2005, 106, 147–161. [Google Scholar] [CrossRef]
- Zhang, T.; Gong, H.; Wen, X.; Lu, C. Salt stress induces a decrease in oxidation energy transfer from phycobilisomes to photosystem II but an increase to photosystem I in the cyanobacterium Spirulina platensis. J. Plant Physiol. 2010, 12, 20. [Google Scholar]
- Shahid, M.A.; Sarkhosh, A.; Khan, N.; Balal, R.M.; Ali, S.; Rossi, R.; Gómez, C.; Mattson, N.; Nasim, W.; Garcia-Sanchez, F. Insights into the Physiological and Biochemical Impacts of Salt Stress on Plant Growth and Development. Agronomy 2020, 10, 938. [Google Scholar] [CrossRef]
- Bless, A.E.; Colin, F.; Crabit, A.; Devaux, N.; Philippon, O.; Follain, S. Landscape evolution and agricultural land salinization in coastal area: A conceptual model. Sci. Total Environ. 2018, 625, 647–656. [Google Scholar] [CrossRef]
- United Nation University Institute for Water. Environment and Health (UNU-INWEH). Annual Report; UNU-INWEH Editions: South Hamilton, Canada, 2014; p. 17. Available online: http://inweh.unu.edu (accessed on 12 October 2022).
- Koukoulakis, P.; Papadopoulos, A. The Problematic Soils and Their Improvement; Stamoulis, A., Ed.; Stamoulis: Athens, Greece, 2007; pp. 23–92. (In Greek). [Google Scholar]
- Maas, E.V. Salt tolerance in plants. Appl. Plant Sci. 1986, 1, 12–26. [Google Scholar]
- Yin, R.; Bai, T.; Ma, F.; Wang, X.; Li, Y.; Yue, Z. Physiological responses and relative tolerance by Chinese apple rootstocks to NaCl stress. Sci. Hortic. 2010, 126, 247–252. [Google Scholar] [CrossRef]
- Fu, M.; Li, C.; Ma, F. Physiological responses and tolerance to NaCl stress in different biotypes of Malus prunifolia. Euphytica 2013, 189, 101–109. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 30, 3–14. [Google Scholar] [CrossRef]
- Koh, R.H.; Song, H.G. Effects of application of Rhodopseudomonas 2 on seed germination and growth of tomato under axenic conditions. J. Microbiol. Biotechnol. 2007, 17, 1805–1810. [Google Scholar] [PubMed]
- Su, P.; Tan, X.; Li, C.; Zhang, D.; Cheng, J.; Zhang, S.; Zhou, X.; Yan, Q.; Peng, J.; Zhang, Z.; et al. Photosynthetic bacterium Rhodopseudomona spalustris GJ-22 induces systemic resistance against viruses. Microb. Biotechnol. 2017, 10, 612–624. [Google Scholar] [CrossRef] [PubMed]
- Welbaum, G.; Sturz, A.V.; Dong, Z.; Nowak, J. Fertilizing soil microorganisms to improve productivity of agroecosystems. Crit. Rev. Plant Sci. 2004, 23, 175–193. [Google Scholar] [CrossRef]
- Compant, S.; Clément, C.; Sessitsch, A. Plant growth-promoting bacteria in the rhizo- and endosphere of plants: Their role, colonization, mechanisms involved and prospects for utilization. Soil Biol. Biochem. 2010, 42, 669–678. [Google Scholar] [CrossRef]
- Lamont, J.R.; Wilkins, O.; Bywater-Ekegärd, M.; Smith, D.L. From yogurt to yield: Potential applications of lactic acid bacteria in plant production. Soil Biol. Biochem. 2017, 111, 1–9. [Google Scholar]
- Zodape, S.T.; Kawarkhe, V.J.; Patolia, J.S.; Warade, A.D. Effect of liquid seaweed fertilizer on yield and quality of okra (Abelmoschus esculentus L.). J. Sci. Ind. Res. 2008, 67, 1115–1117. [Google Scholar]
- Chatzissavvidis, C.; Therios, I. Role of algae in agriculture. In Seaweeds: Agricultural Uses, Biological and Antioxidant Agents; Pomin, V.H., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2014; pp. 1–37. [Google Scholar]
- Trivedi, K.; Anand, K.G.V.; Kubavat, D.; Patidar, R.; Ghosh, A. Drought alleviatory potential of Kappaphycus seaweed extract and the role of the quaternary ammonium compounds as its constituents towards imparting drought tolerance in Zea mays L. J. Appl. Phycol. 2017, 30, 2001–2015. [Google Scholar] [CrossRef]
- Beckett, R.P.; van Staden, J. The effect of seaweed concentrate on the growth and yield of potassium stressed wheat. Plant Soil 1989, 116, 29–36. [Google Scholar] [CrossRef]
- Stanturf, J.A.; Callaham, M.A.; Madsen, P. Soils are fundamental to landscape restoration. In Soils and Landscape Restoration; Stanturf, J.A., Callaham, M.A., Eds.; Academic Press, Elsevier Inc.: Amsterdam, The Netherlands, 2021; pp. 1–37. [Google Scholar] [CrossRef]
- Mumpton, F.A. Uses of natural zeolites in agriculture and industry. Proc. Natl. Acad. Sci. USA 1999, 96, 3463–3470. [Google Scholar] [CrossRef] [PubMed]
- Elliot, M.A.; Edwards, H.M. Comparison of the effects of the synthetic and natural zeolite on laying hen and broiler chicken performance. Poult. Sci. 1991, 70, 2115–2130. [Google Scholar] [CrossRef]
- Polat, E.; Karaca, M.; Demir, H.; Onus, A.N. Use of natural zeolite (clinoptilolite) in agriculture. J. Fruit Ornam. Plant. Res. Spec. 2004, 12, 183–189. [Google Scholar]
- Ramesh, K.; Reddy, D.D. Zeolites and their potential uses in agriculture. Adv. Agron. 2011, 113, 219–240. [Google Scholar]
- Kralova, M.; Hrozinkova, A.; Ruzek, P.; Kovanda, F.; Kolousek, D. Synthetic and natural zeolites affecting the physical-chemical soil properties. Rostl. Vyrob. 1994, 40, 131–138. [Google Scholar]
- Jakkula, V.S.; Wani, S.P. Zeolites: Potential soil amendments for improving nutrient and water use efficiency and agriculture productivity. Sci. Rev. Chem. Commun. 2018, 8, 119–126. [Google Scholar]
- Rehakova, M.; Cuvanova, S.; Dzivak, M.; Rimar, J.; Gavalova, Z. Agricultural and agrochemical uses of natural zeolite of the clinoptilolite type. Curr. Opin. Solid State Mater. Sci. 2004, 8, 397–404. [Google Scholar] [CrossRef]
- Xiubin, H.; Zhanbin, H. Zeolite applications for enhancing water infiltration and retention in loess soil. Resour. Conserv. Recycl. 2001, 34, 45–52. [Google Scholar] [CrossRef]
- Azough, A.; Marashi, S.K.; Babaeinejad, T. Growth characteristics and response of wheat to cadmium, nickel and magnesium sorption affected by zeolite in soil polluted with armaments. J. Adv. Environ. Health Res. 2017, 5, 163–171. [Google Scholar]
- Tahervand, S.; Jalali, M. Sorption and desorption of potentially toxic metals (Cd, Cu, Ni and Zn) by soil amended with bentonite, calcite and zeolite as a function of pH. J. Geochem. Explor. 2017, 181, 148–159. [Google Scholar] [CrossRef]
- Mohd, H.B.; Asima, J.; Arifin, A.; Hazandy, A.H.; Mohd, A.K. Elevation and variability of acidic sandy soil pH: Amended with conditioner, activator, organic and inorganic fertilizers. Afr. J. Agric. Res. 2013, 8, 4020–4024. [Google Scholar]
- Ahmed, O.H.; Sumalatha, G.; Majid, N.M.A. Use of zeolite in maize Zea mays. cultivation on nitrogen, potassium, and phosphorus uptake and use efficiency. Int. J. Phys. Sci. 2010, 5, 2393–2401. [Google Scholar]
- Gholamhoseini, M.; Ghalavand, A.; Khodaei-Joghan, A.; Dolatabadian, A.; Zakikhani, H.; Farmanbar, E. Zeolite-amended cattle manure effects on sunflower yield, seed quality, water use efficiency and nutrient leaching. Soil Tillage Res. 2013, 126, 193–202. [Google Scholar] [CrossRef]
- Szatanik-Kloc, A.; Szerement, J.; Adamczuk, A.; Józefaciuk, G. Effect of Low Zeolite Doses on Plants and Soil Physicochemical Properties. Materials 2021, 14, 2617. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.D.C.; Nicolau, M.C.M.; Checchio, M.V.; Junior, G.D.S.S.; Oliveira, F.D.A.D.; Prado, R.M.; Gratão, P.L. Salt stress alleviation by seed priming with silicon in lettuce seedlings: An approach based on enhancing antioxidant responses. Bragantia 2020, 79, 19–29. [Google Scholar] [CrossRef]
- Sivanesan, I.; Son, M.S.; Lim, C.S.; Jeong, B.R. Effect of soaking of seeds in potassium silicate and uniconazole on germination and seedling growth of tomato cultivars, Seogeon and Seokwang. Afr. J. Biotechnol. 2011, 10, 6743–6749. [Google Scholar]
- Imtiaz, M.; Rizwan, M.S.; Mushtaq, M.A.; Ashraf, M.; Shahzad, S.M.; Yousaf, B.; Saeed, D.A.; Nawaz, M.A.; Mehmood, S.; Tu, S. Silicon occurrence, uptake, transport and mechanisms of heavy metals, minerals and salinity enhanced tolerance in plants with future prospects: A review. J. Environ. Manag. 2016, 183, 521–529. [Google Scholar] [CrossRef]
- Tilman, D.; Fargione, J.; Wolff, B.; D’Antonio, C.; Dobson, A.; Howarth, R.; Swackhamer, D. Forecasting agriculturally driven global environmental change. Science 2001, 292, 281–284. [Google Scholar] [CrossRef]
- Liebman, M.; Schulte, L.A. Enhancing agroecosystem performance and resilience through increased diversification of landscapes and cropping systems. Elementa 2015, 3, 000041. [Google Scholar] [CrossRef]
- Steffen, W.; Richardson, K.; Rockström, J.; Cornell, S.E.; Fetzer, I.; Bennett, E.M.; Sörlin, S. Planetary Boundaries: Guiding Human Development on a Changing Planet. Science. 2015, p. 1259855. Available online: http://www.sciencemag.org/content/347/6223/1259855 (accessed on 15 October 2022).
- DeLonge, M.S.; Miles, A.; Carlisle, L. Investing in the transition to sustainable agriculture. Environ. Sci. Policy 2016, 55, 266–273. [Google Scholar] [CrossRef]
- Mathur, S.; Tomar, R.S.; Jajoo, A. Arbuscular mycorrhizal fungi (AMF) protect photosynthetic apparatus of wheat under drought stress. Photosyn. Res. 2019, 139, 227–238. [Google Scholar] [CrossRef]
- Chatzissavvidis, C.; Therios, I.; Antonopoulou, C. Effect of nitrogen source on olives growing in soils with high boron content. Aust. J. Exp. Agric. 2007, 47, 1491–1497. [Google Scholar] [CrossRef]
- Fales, F.W. The assimilation and degradation of carbohydrates of yeast cells. J. Biol. Chem. 1951, 193, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Scalbert, A.; Monties, B.; Janin, E. Tannins in wood: Comparison of different estimation methods. J. Agric. Food Chem. 1989, 37, 1324–1329. [Google Scholar] [CrossRef]
- Scalbert, A. Plant Polyphenols; Hemingway, R.W., Laks, P.E., Eds.; Plenum Press: New York, NY, USA, 1992; p. 259. [Google Scholar]
- Zhishen, J.; Mengchen, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a measure of ‘‘Antioxidant Power’’: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Yeo, A.R.; Lee, K.S.; Izard, P.; Boursier, P.J.; Flowers, T.J. Short- and long-term effects of salinity on leaf growth in rice (Oryza sativa L.). J. Exp. Bot. 1991, 42, 881–889. [Google Scholar] [CrossRef]
- Cramer, G.R. Response of abscisic acid mutants of Arabidopsis to salinity. Funct. Plant Biol. 2002, 29, 561–567. [Google Scholar] [CrossRef]
- Fricke, W.; Peters, W.S. The biophysics of leaf growth in salt-stressed barley. A study at the cell level. Plant Physiol. 2002, 129, 374–388. [Google Scholar] [CrossRef]
- James, R.A.; Rivelli, A.R.; Munns, R.; von Caemmerer, S. Factors affecting CO2 assimilation, leaf injury and growth in salt-stressed durum wheat. Funct. Plant Biol. 2002, 29, 1393–1403. [Google Scholar] [CrossRef] [PubMed]
- Petridis, A.; Therios, I.; Samouris, G.; Koundouras, S.; Giannakoula, A. Effect of water deficit on leaf phenolic composition, gas exchange, oxidative damage and antioxidant activity of four Greek olive (Olea europaea L.) cultivars. Plant Physiol. Biochem. 2012, 60, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pezeshki, S.R.; DeLaune, R.D.; Patrick, W.H., Jr. Effect of salinity on leaf ionic content and photosynthesis of Taxodium distichum. Am. Midl. Nat. 1988, 119, 185–192. [Google Scholar] [CrossRef]
- Flowers, T.J.; Troke, P.F.; Yeo, A.R. The mechanism of salt tolerance in halophytes. Annu. Rev. Plant Physiol. 1977, 28, 89–121. [Google Scholar] [CrossRef]
- Wyn Jones, R.G.; Storey, R.; Leigh, R.A.; Ahmad, N.; Pollard, A. A hypothesis on cytoplasmic osmoregulation. In Regulation of Cell Membrane Activities in Plants; Marré, E., Cifferi, O., Eds.; Elsevier: Amsterdam, The Netherlands, 1977; pp. 121–136. [Google Scholar]
- Hasegawa, P.M.; Bressan, R.A.; Zhu., J.-K.; Bohnert, H.J. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 463–499. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, M.; Unal, B.T.; Garcia-Caparros, P.; Khursheed, A.; Gul, A.; Hasanuzzaman, M. Osmoregulation and its actions during the droughtstress in plants. Physiol. Plant. 2021, 172, 1321–1335. [Google Scholar] [CrossRef] [PubMed]
- AlKahtani, M.D.F.; Hafez, Y.M.; Attia, K.; Rashwan, E.; Husnain, L.A.; AlGwaiz, H.I.M.; Abdelaal, K.A.A. Evaluation of silicon and proline application on theoxidative machinery in drought-stressed sugar beet. Antioxidants 2021, 10, 398. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, D.; Nadolska-Orczyk, A.; Rich, P.J. Salinity, osmolytes and compatible solutes. In Salinity: Environment—Plants—Molecules; Läuchli, A., Lüttge, U., Eds.; Kluwer: Dordrecht, The Netherlands, 2002; pp. 181–204. [Google Scholar]
- Casas, A.M.; Bressan, R.A.; Hasegawa, P.M. Cell growth and water relations of the halophyte Atriplex nummularia L., in response to NaCl. Plant Cell Rep. 1991, 10, 81–84. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Logan, B.A. Reactive oxygen species and photosynthesis. In Antioxidants and Reactive Oxygen Species in Plants; Smirnoff, N., Ed.; Blackwell: Oxford, UK, 2005; pp. 250–267. [Google Scholar]
- Smirnoff, N. The role of active oxygen in the response to water deficit and desiccation. New Phytol. 1993, 125, 27–58. [Google Scholar] [CrossRef]
- Mittova, V.; Tal, M.; Volokita, M.; Guy, M. Upregulation of the leaf mitochondrial and peroxisomal antioxidative systems in response to salt-induced oxidative stress in the wild salt-tolerant tomato species, Lycopersicon pennellii. Plant Cell. Environ. 2003, 26, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Grace, S.C. Phenolics as antioxidants. In Antioxidants and Reactive Oxygen Species in Plants; Smirnoff, N., Ed.; Blackwell: Oxford, UK, 2005; pp. 141–168. [Google Scholar]
- Reuber, S.; Bornman, J.F.; Weissenböck, G. Phenylpropanoids compounds in primary leaf tissues of rye (Secale cereale). Light response of their metabolism and the possible role in UV-B protection. Physiol. Plant. 1996, 97, 160–168. [Google Scholar] [CrossRef]
- Shirley, B.W. Flavonoid biosynthesis: “new” functions for an “old” pathway. Trends Plant Sci. 1996, 1, 377–382. [Google Scholar]
- Larson, R.A. The antioxidants of higher plants. Phytochemistry 1988, 27, 969–978. [Google Scholar] [CrossRef]
- Noori, M.; Zendehdel, M.; Ahmadi, A. Using natural zeolite for the improvement of soil salinity and crop yield. Toxicol. Environ. Chem. 2006, 88, 77–84. [Google Scholar] [CrossRef]
- Koulympoudi, L.; Orfanoudakis, M.; Sinapidou, E. Improving performance of Phaseolus vulgaris plants by addition of microorganisms and zeolite in soil. In Proceedings of the 27th Scientific Conference of the Greek Society of Horticultural Science, Volos, Greece, 28–29 September 2015. [Google Scholar]
- Gül, A.; Eroğul, D.; Ongun, A.R. Comparison of the use of zeolite and perlite as substrate for crisp-head lettuce. Sci. Hortic. 2005, 106, 464–471. [Google Scholar] [CrossRef]
- Aboul-Magd, M.; Elzopy, K.A.; Zangana, Z.R.M. Effect of zeolite and urea fertilizer on maize grown under saline conditions. Middle East J. Appl. Sci. 2020, 10, 18–25. [Google Scholar]
- Al-Busaidi, A.; Yamamoto, T.; Inoue, M.; Eneji, A.E.; Mori YIrshad, M. Effects of zeolite on soil nutrients and growth of barley following irrigation with saline water. J. Plant Nutr. 2008, 31, 1159–1173. [Google Scholar] [CrossRef]
- Bybordi, A.; Saadat, S.; Zargaripour, P. The effect of zeolite, selenium and silicon on qualitative and quantitative traits of onion grown under salinity conditions. Arch. Agron. Soil Sci. 2018, 64, 520–530. [Google Scholar] [CrossRef]
- Kong, L.; Wang, M.; Bi, D. Selenium modulates the activities of antioxidant enzymes, osmotic homeostasis and promotes the growth of sorrel seedlings under salt stress. Plant Growth Regul. 2005, 45, 155–163. [Google Scholar] [CrossRef]
- Mahmoud, A.W.M.; Abdeldaym, E.A.; Abdelaziz, S.M.; El-Sawy, M.B.I.; Mottaleb, S.A. Synergetic effects of Zinc, Boron, Silicon, and Zeolite nanoparticles on confer Tolerance in potato plants subjected to salinity. Agronomy 2019, 10, 19. [Google Scholar] [CrossRef]
- Durán, P.; Acuña, J.J.; Armada, E.; López-Castillo, O.M.; Cornejo, P.; Mora, M.L.; Azcón, R. Inoculation with selenobacteria and arbuscular mycorrhizal fungi to enhance selenium content in lettuce plants and improve tolerance against drought stress. J. Soil Sci. Plant Nutr. 2016, 16, 201–225. [Google Scholar] [CrossRef]
- Olle, M.; Ngouajio, M.; Siomos, A. Vegetable quality and productivity as influenced by growing medium: A review. Zemdirb. Agric. 2012, 99, 399–408. [Google Scholar]
- Lee, S.-K.; Lur, H.-S.; Liu, C.-T. From Lab to Farm: Elucidating the beneficial roles of photosynthetic bacteria in sustainable agriculture. Microorganisms 2021, 9, 2453. [Google Scholar] [CrossRef]
- Holguin, G.; Vazquez, P.; Bashan, Y. The role of sediment microorganisms in the productivity, conservation, and rehabilitation ofmangrove ecosystems: An overview. Biol. Fertil. Soils 2001, 33, 265–278. [Google Scholar] [CrossRef]
- Franche, C.; Lindström, K.; Elmerich, C. Nitrogen-fixing bacteria associated with leguminous and non-leguminous plants. Plant Soil 2009, 321, 35–59. [Google Scholar] [CrossRef]
- Olivares, J.; Bedmar, E.J.; Sanjuán, J. Biological nitrogen fixation in the context of global change. Mol. Plant-Microbe Interact. 2013, 26, 486–494. [Google Scholar] [CrossRef]
- Hsu, S.H.; Shen, M.W.; Chen, J.C.; Lur, H.S.; Liu, C.T. The photosynthetic bacterium Rhodopseudomonas palustris strain PS3 exerts plant growth-promoting effects by stimulating nitrogen uptake and elevating auxin levels in expanding leaves. Front. Plant Sci. 2021, 12, 573–634. [Google Scholar] [CrossRef]
- Rouphael, Y.; Kyriacou, M.C.; Petropoulos, S.A.; De Pascale, S.; Colla, G. Improving vegetable quality in controlled environments. Sci. Hortic. 2018, 234, 275–289. [Google Scholar] [CrossRef]
- Sakpirom, J.; Kantachote, D.; Nunkaew, T.; Khan, E. Characterizations of purple non-sulfur bacteria isolated from paddyfields, and identification of strains with potential for plant growth-promotion, greenhouse gas mitigation and heavy metalbioremediation. Res. Microbiol. 2017, 168, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Nunkaew, T.; Kantachote, D.; Kanzaki, H.; Nitoda, T.; Ritchie, R.J. Effects of 5-aminolevulinic acid (ALA)-containing supernatants from selected Rhodopseudomonas palustris strains on rice growth under NaCl stress, with mediating effects on chlorophyll, photosynthetic electron transport and antioxidative enzymes. Electron. J. Biotechnol. 2014, 17, 4. [Google Scholar] [CrossRef]
- Saikeur, A.; Choorit, W.; Prasertsan, P.; Kantachote, D.; Sasaki, K. Influence of precursors and inhibitor on the production of extracellular 5-aminolevulinic acid and biomass by Rhodopseudomonas palustris KG31. Biosci. Biotechnol. Biochem. 2009, 73, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Jin, X.; Liao, W.; Hu, L.; Dawuda, M.M.; Zhao, X.; Tang, Z.; Gong, T.; Yu, J. 5-Aminolevulinic Acid (ALA) alleviated salinity stress in cucumber seedlings by enhancing chlorophyll synthesis pathway. Front. Plant Sci. 2018, 9, 635. [Google Scholar] [CrossRef] [PubMed]
- Barka, E.A.; Nowak, J.; Clément, C. Enhancement of chilling resistance of inoculated grapevine plantlets with a plant growth-promoting rhizobacterium, Burkholderia phytofirmans strain Ps JN. Appl. Env. Microb. 2006, 70, 7246–7252. [Google Scholar] [CrossRef]
- Sziderics, A.H.; Rasche, F.; Trognitz, F.; Wilhelm, E.; Sessitsch, A. Bacterial endophytes contribute to abiotic stress adaptation in pepper plants (Capsicum annuum L.). Can. J. Microb. 2007, 53, 1195–1202. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Lugtenberg, B. Use of Plant Growth-Promoting Rhizobacteria to Alleviate Salinity Stress in Plants. In Use of Microbes for the Alleviation of Soil Stresses; Miransari, M., Ed.; Springer Science+Business Media: New York, NY, USA, 2014; Volume 1. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C. Proline accumulation in plants: A review. Amino Acids 2008, 35, 753–759. [Google Scholar] [CrossRef]
- Sathya, B.; Indu, H.; Seenivasan, R.; Geetha, S. Influence of seaweed liquid fertilizer on the growth and biochemical composition of legume crop. Cajanus cajan (L.) Mill sp. J. Phytol. 2010, 2, 50–63. [Google Scholar]
- Cluzet, S.; Torregrosa, C.; Jacquet, C.; Lafitte, C.; Fournier, J.; Mercier, L.; Salamagne, S.; Briand, X.; Esquerré-Tugayé, M.T.; Dumas, B. Gene expression profiling and protection of Medicago truncatula against a fungal infection in response to an elicitor from the green alga Ulva spp. Plant Cell Environ. 2004, 27, 917–928. [Google Scholar] [CrossRef]
- Ramkissoon, A.; Ramsubhag, A.; Jayaraman, J. Phytoelicitor activity of three Caribbean seaweed species on suppression of pathogenic infections in tomato plants. J. Appl. Phycol. 2017, 29, 3235–3244. [Google Scholar] [CrossRef]
- Kasim, W.A.; Hamada, E.A.M.; Shams El-Din, N.G.; Eskander, S.K. Influence of seaweed extracts on the growth, some metabolic activities and yield of wheat grown under drought stress. Int. J. Agri. Agri. R. 2015, 7, 173–189. [Google Scholar]
- Epstein, E.; Bloom, A.J. Mineral Nutrition of Plants: Principles and Perspectives, 2nd ed.; Sinauer Associates Inc.: Sunderland, UK, 2005. [Google Scholar]
- Guerriero, G.; Hausman, J.F.; Legay, S. Silicon and the plant extracellular matrix. Front. Plant Sci. 2016, 7, 463. [Google Scholar] [CrossRef]
- Luyckx, M.; Hausman, J.-F.; Lutts, S.; Guerriero, G. Silicon and Plants: Current knowledge and technological perspectives. Front. Plant Sci. 2017, 8, 411. [Google Scholar] [CrossRef] [PubMed]
- Fauteux, F.; Remus-Borel, W.; Menzies, J.G.; Belanger, R.R. Silicon and plant disease resistance against pathogenic fungi. FEMS Microbiol. Lett. 2005, 249, 1–6. [Google Scholar] [CrossRef]
- Goto, M.; Ehara, H.; Karita, S.; Takabe, K.; Ogawa, N.; Yamada, Y.; Ogawa, S.; Yahaya, M.S.; Morita, O. Protective effect of silicon on phenolic biosynthesis and ultraviolet spectral stress in rice crop. Plant Sci. 2003, 164, 349–356. [Google Scholar] [CrossRef]
- Shen, X.; Zhou, Y.; Duan, L.; Li, Z.; Eneji, A.E.; Li, J. Silicon effects on photosynthesis and antioxidant parameters of soybean seedlings under drought and ultraviolet-B radiation. J. Plant Physiol. 2010, 167, 1248–1252. [Google Scholar] [CrossRef]
- Gong, H.J.; Chen, K.M.; Chen, G.C.; Wang, S.M.; Zhang, C.L. Effects of silicon on growth of wheat under drought. J. Plant Nutr. 2003, 26, 1055–1063. [Google Scholar] [CrossRef]
- Adrees, M.; Ali, S.; Rizwan, M.; Zia-Ur-Rehman, M.; Ibrahim, M.; Abbas, F.; Farid, M.; Qayyum, M.F.; Irshad, M.K. Mechanisms of silicon-mediated alleviation of heavy metal toxicity in plants: A review. Ecotoxicol. Environ. Saf. 2015, 119, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Nwugo, C.C.; Huerta, A.J. Silicon-induced cadmium resistance in rice (Oryza sativa). J. Plant Nutr. Soil Sci. 2008, 171, 841–848. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Singh, V.P.; Prasad, S.M.; Chauhan, D.K.; Dubey, N.K.; Rai, A.K. Silicon-mediated alleviation of Cr (VI) toxicity in wheat seedlings as evidenced by chlorophyll florescence, laser induced breakdown spectroscopy and anatomical changes. Ecotoxicol. Environ. Saf. 2015, 113, 133–144. [Google Scholar] [CrossRef]
- Wani, S.H.; Kumar, V.; Shriram, V.; Sah, S.K. Phytohormones and their metabolic engineering for abiotic stress tolerance in cropplants. Crop. J. 2016, 4, 162–176. [Google Scholar] [CrossRef]
- Tsavkelova, E.A.; Klimova, S.Y.; Cherdyntseva, T.A.; Netrusov, A.I. Microbial producers of plant growth stimulators and theirpractical use: A review. Appl. Biochem. Microbiol. 2006, 42, 117–126. [Google Scholar] [CrossRef]
- Miao, J.-S.; Liu, J.; Liu, Y.-L.; Wu, J.-F. Effect of photosynthetic bacterial on photosynthesis and antioxidant enzyme system ofwatermelon seedlings in early spring. North. Hortic. 2014, 21, 42–44. [Google Scholar]
- Xu, J.; Feng, Y.; Wang, Y.; Lin, X. Effect of rhizobacterium Rhodopseudomonas palustris inoculation on Stevia rebaudiana plant growth and soil microbial community. Pedosphere 2018, 28, 793–803. [Google Scholar] [CrossRef]
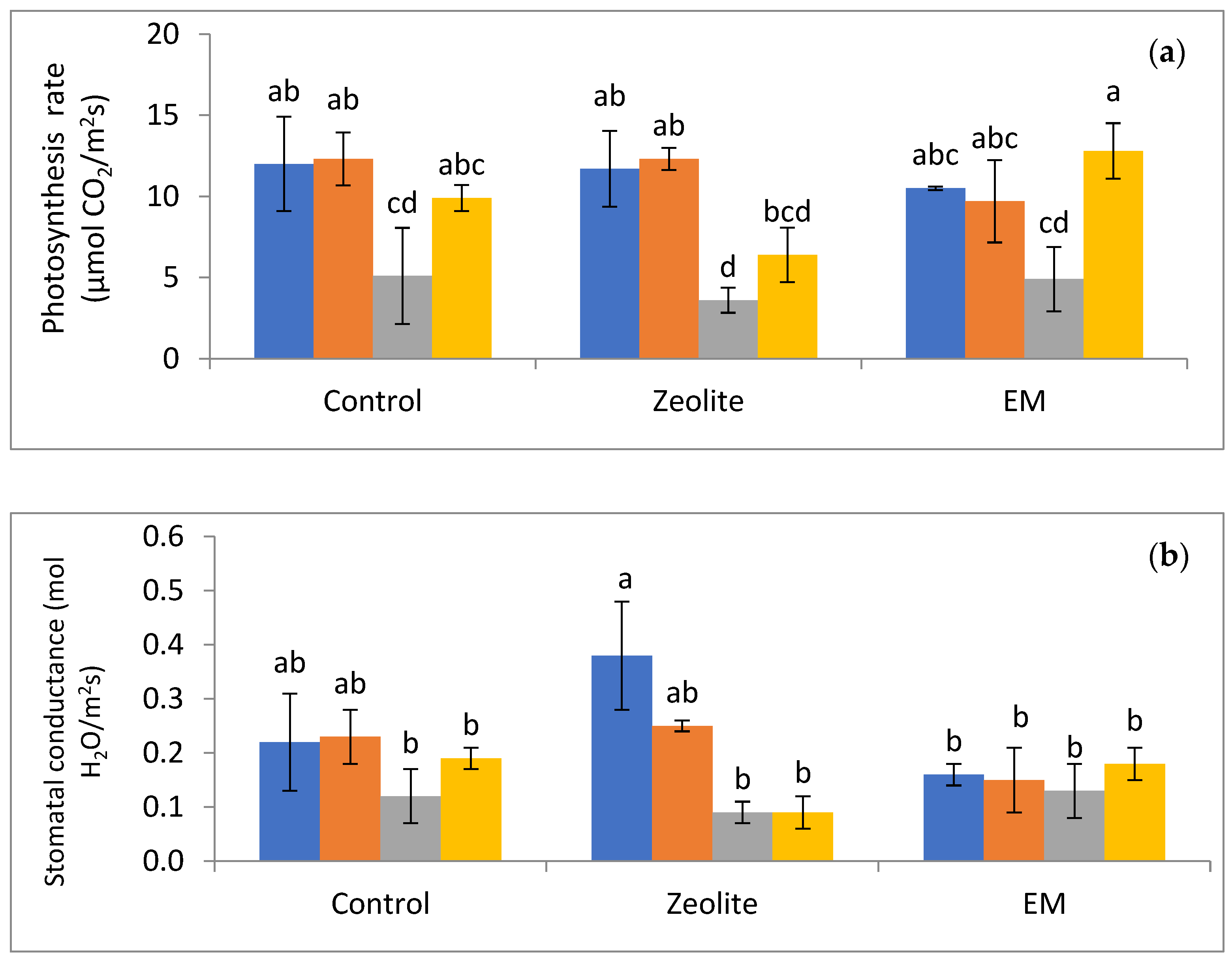


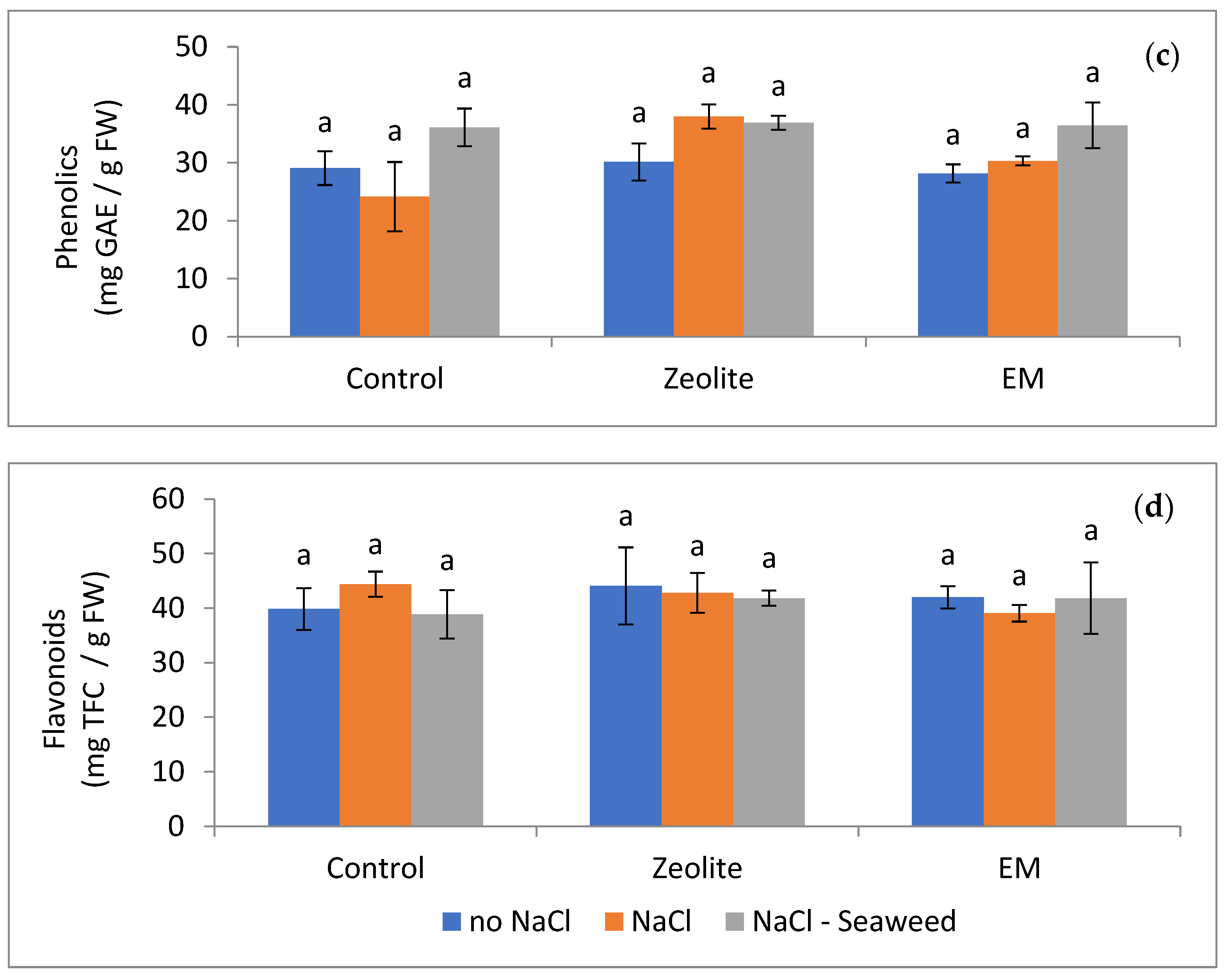
| Treatments–Substrate Additives | Sub- Treatments | Leaf DW (g) | Stem DW (g) | Root DW (g) | Relative Growth Rate (Height–mm mm−1 d−1) |
|---|---|---|---|---|---|
| Control | I | 4.37 ± 0.36 ab | 14.93 ± 3.01 ab | 11.23 ± 1.66 ab | 0.0063 ± 0.002 a |
| II | 2.34 ± 0.16 d | 16.02 ± 2.47 ab | 13.19 ± 2.22 ab | 0.0033 ± 0.002 a | |
| III | 3.51 ± 0.29 bc | 13.64 ± 2.90 ab | 11.00 ± 1.76 ab | 0.0063 ± 0.001 a | |
| IV | 3.61 ± 0.24 bc | 15.59 ± 2.46 ab | 12.57 ± 2.85 ab | 0.0042 ± 0.001 a | |
| Zeolite | I | 5.07 ± 0.55 a | 19.92 ± 3.19 a | 18.86 ± 4.32 a | 0.0055 ± 0.002 a |
| II | 4.14 ± 0.23 abc | 11.97 ± 1.39 b | 10.96 ± 2.14 ab | 0.0079 ± 0.001 a | |
| III | 4.28 ± 0.03 ab | 13.25 ± 0.74 ab | 14.53 ± 1.50 ab | 0.0058 ± 0.002 a | |
| IV | 4.57 ± 0.33 ab | 16.47 ± 1.59 ab | 11.77 ± 1.22 ab | 0.0064 ± 0.001 a | |
| EM | I | 4.68 ± 0.42 ab | 17.26 ± 0.95 ab | 18.32 ± 4.19 a | 0.0058 ± 0.002 a |
| II | 3.02 ± 0.60 cd | 15.14 ± 1.44 ab | 13.61 ± 0.96 ab | 0.0031 ± 0.001 a | |
| III | 4.42 ± 0.44 ab | 12.28 ± 1.31 b | 9.56 ± 1.28 b | 0.0078 ± 0.001 a | |
| IV | 3.99 ± 0.18 abc | 13.10 ± 1.30 ab | 11.73 ± 1.02 ab | 0.0051 ± 0.002 a |
| Treatments–Substrate Additives | Sub-Treatments | Proline (μmol/g FW) | Carbohydrates (μmol/g FW) | Carotenoids (mg/g FW) |
|---|---|---|---|---|
| Control | I | 2.09 ± 0.82 bc | 59.97 ± 12.2 a | 564.13 ± 78.32 bcd |
| II | 0.75 ± 0.27 c | 69.20 ± 7.54 a | 687.04 ± 84.71 a–d | |
| III | 1.07 ± 0.70 c | 43.56 ± 10.74 a | 687.78 ± 21.53 a–d | |
| IV | 1.65 ± 0.55 bc | 57.66 ± 12.26 a | 823.88 ± 31.00 ab | |
| Zeolite | I | 1.72 ± 0.45 bc | 71.97 ± 10.70 a | 847.60 ± 100.35 a |
| II | 2.44 ± 0.86 bc | 56.69 ± 12.73 a | 543.32 ± 79.53 cd | |
| III | 1.33 ± 0.42 c | 39.45 ± 6.04 a | 524.13 ± 24.62 cd | |
| IV | 1.09 ± 0.20 c | 59.21 ± 6.12 a | 736.79 ± 6.67 abc | |
| EM | I | 1.10 ± 0.39 c | 64.88 ± 2.96 a | 673.78 ± 47.16 a–d |
| II | 5.63 ± 0.54 a | 65.88 ± 12.76 a | 681.11 ± 18.51 a–d | |
| III | 4.16 ± 0.63 ab | 48.34 ± 2.34 a | 430.80 ± 24.74 d | |
| IV | 1.76 ± 0.30 bc | 55.48 ± 11.83 a | 602.32 ± 3.56 a–d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koulympoudi, L.; Chatzissavvidis, C.; Giannakoula, A.E. Physiological and Biochemical Responses of Apple (Malus domestica Borkh.) to Biostimulants Application and Substrate Additives under Salinity Stress. Appl. Sci. 2023, 13, 1290. https://doi.org/10.3390/app13031290
Koulympoudi L, Chatzissavvidis C, Giannakoula AE. Physiological and Biochemical Responses of Apple (Malus domestica Borkh.) to Biostimulants Application and Substrate Additives under Salinity Stress. Applied Sciences. 2023; 13(3):1290. https://doi.org/10.3390/app13031290
Chicago/Turabian StyleKoulympoudi, Louloudia, Christos Chatzissavvidis, and Anastasia Evripidis Giannakoula. 2023. "Physiological and Biochemical Responses of Apple (Malus domestica Borkh.) to Biostimulants Application and Substrate Additives under Salinity Stress" Applied Sciences 13, no. 3: 1290. https://doi.org/10.3390/app13031290
APA StyleKoulympoudi, L., Chatzissavvidis, C., & Giannakoula, A. E. (2023). Physiological and Biochemical Responses of Apple (Malus domestica Borkh.) to Biostimulants Application and Substrate Additives under Salinity Stress. Applied Sciences, 13(3), 1290. https://doi.org/10.3390/app13031290







