Formulation by Design of an Innovative Tea Tree Oil Nanoemulgel Incorporating Mupirocin for Enhanced Wound Healing Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Designing the Experiment
2.3. The Formulation of Nanoemulsions
2.4. The Characterization of Mupirocin-Loaded NE Formulations
2.4.1. Particle Characterization
2.4.2. In Vitro Drug Release Study
2.5. The Formulation of Nanoemulgel
2.6. The Characterization of NE Formulation
2.6.1. Visual Inspection
2.6.2. pH Determination
2.6.3. Viscosity Measurement
2.6.4. Spreadability Test
2.6.5. Drug Content
2.7. The In Vitro Drug Release Study of NEG Formulation
2.8. Drug-Excipient Compatibility Studies (Fourier-Transform Infrared Spectroscopy (FTIR) Studies)
2.9. Scanning Electron Microscopy (SEM)
2.10. Stability Study
2.11. The Antibacterial Activity of Mupirocin-Loaded NEG against Different Bacterial Strains
2.12. Animals and Statement of Ethical Approval
2.13. Studies on Skin Irritation
2.14. The In Vivo Evaluation of Wound Healing Efficiency
2.14.1. Design of the Experiment
- Group I: rats were kept to heal without treatment (control group);
- Group II: rats were given commercial ointment (50 mg);
- Group III: rats were given blank NEG (50 mg);
- Group IV: rats were given mupirocin-loaded NEG (50 mg of 2% NEG) [38].
2.14.2. Excision Wound Establishment
2.14.3. Wound Area Measurement
2.15. Statistical Analysis
3. Results
3.1. Designing the Experiment
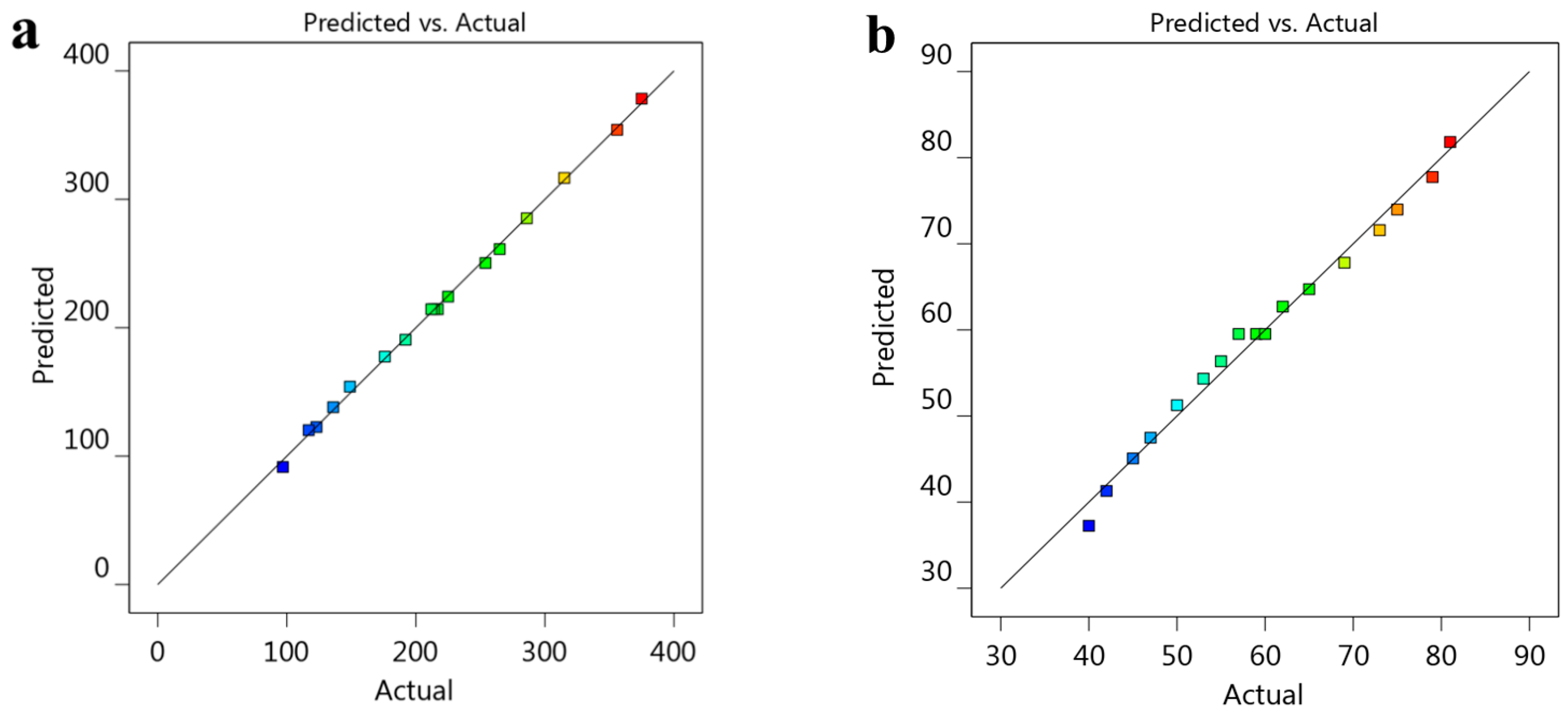
Fitting Models and Analyzing Statistical Data
3.2. The Characterization of Mupirocin-Loaded NE Formulations
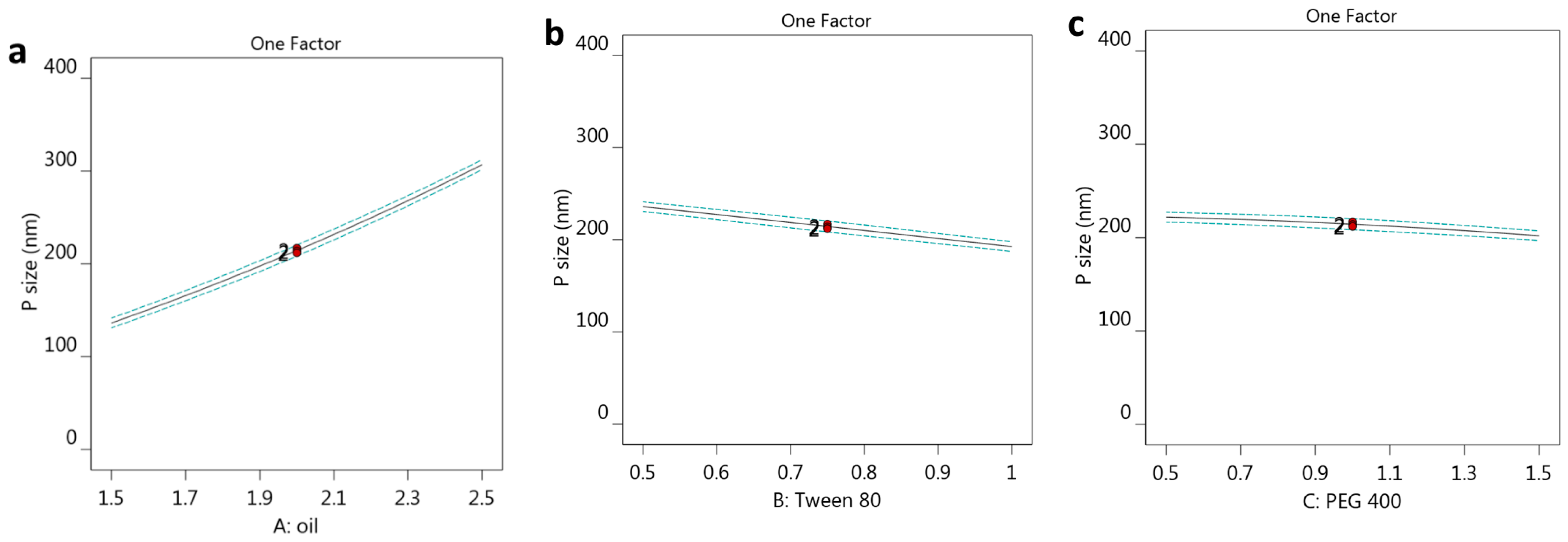
3.2.1. The Effect of Selected Factors on Y1
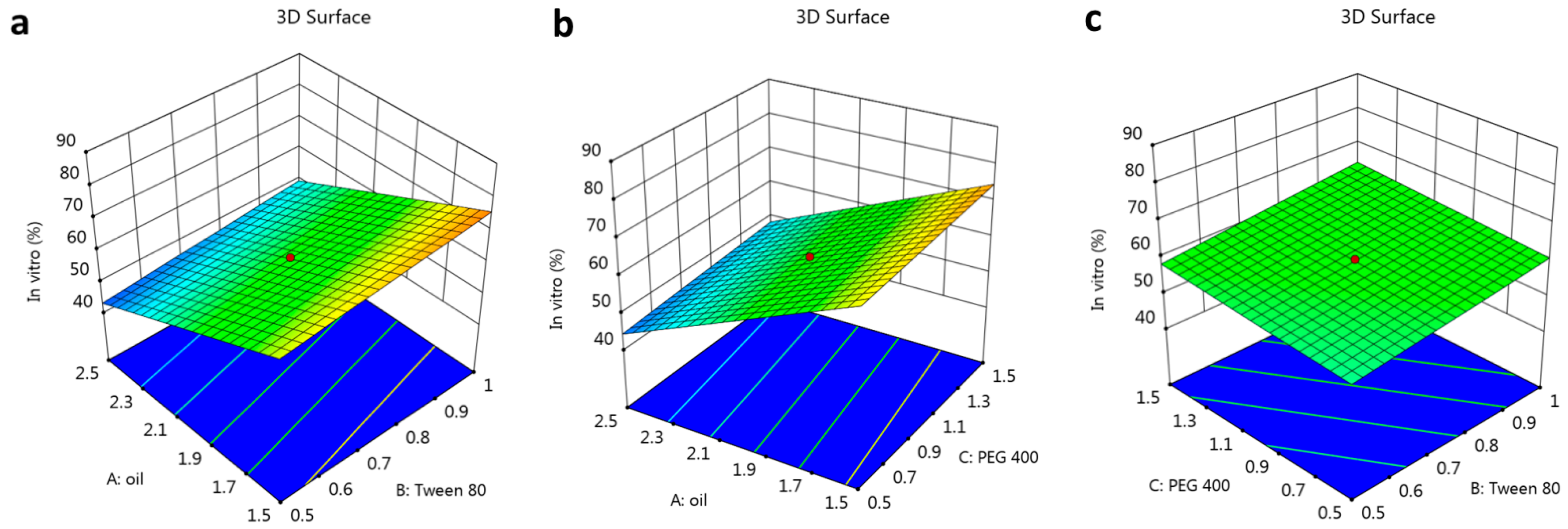
3.2.2. The Impact of Selected Factors on Y2
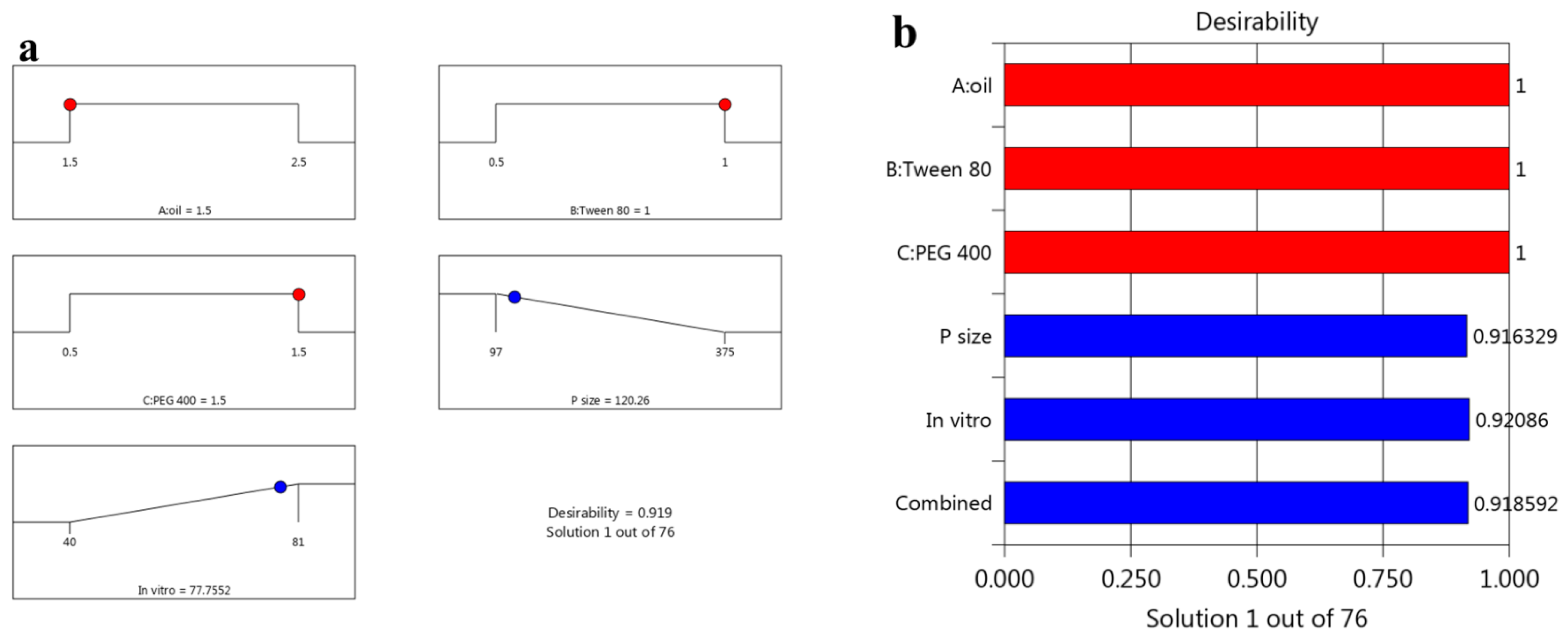
3.3. Optimizing the Data
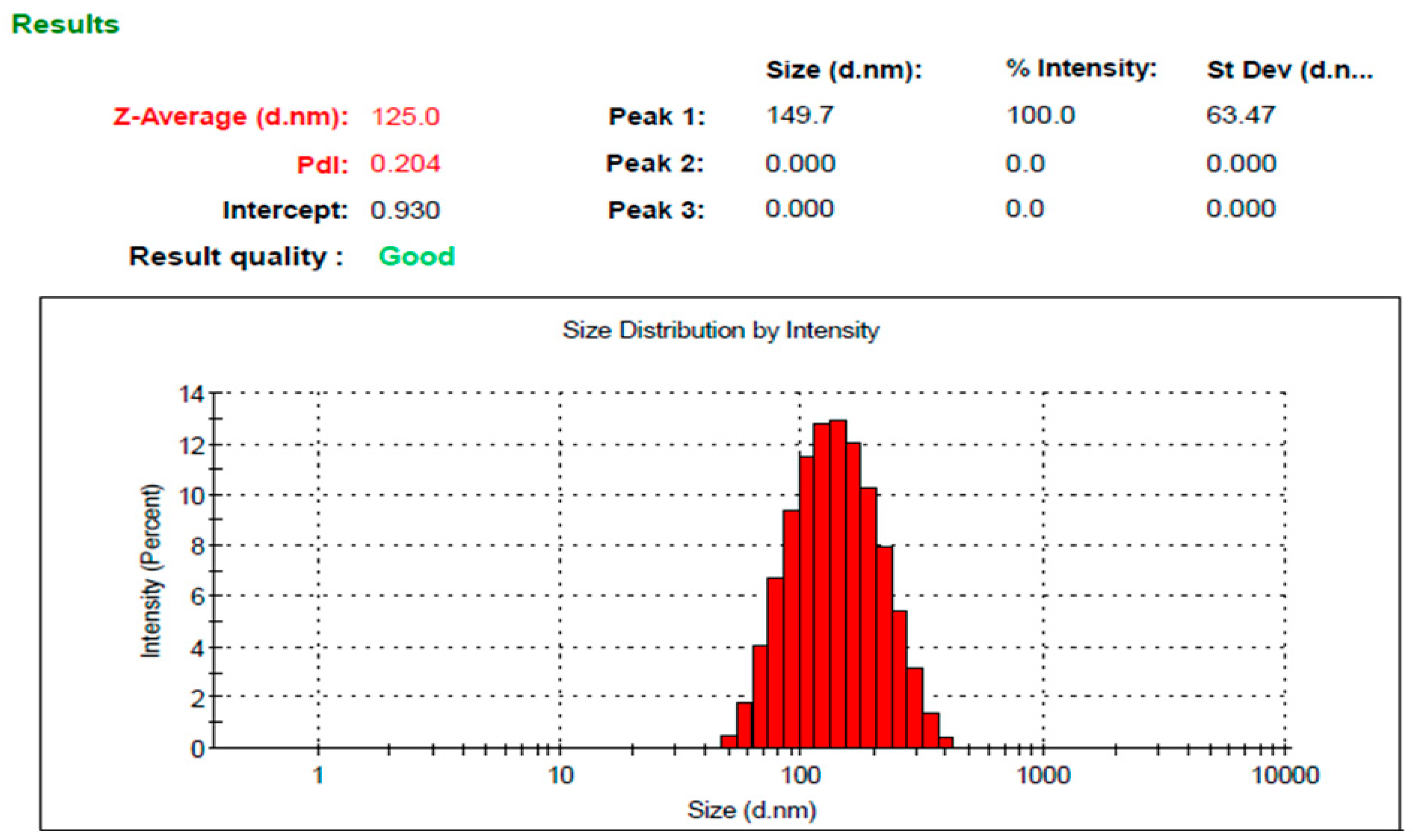
3.4. The Characterization of the Optimized Mupirocin NE
3.4.1. Visual Inspection
3.4.2. pH Determination
3.4.3. Viscosity Measurement
3.4.4. Spreadability Test
3.4.5. Drug Content
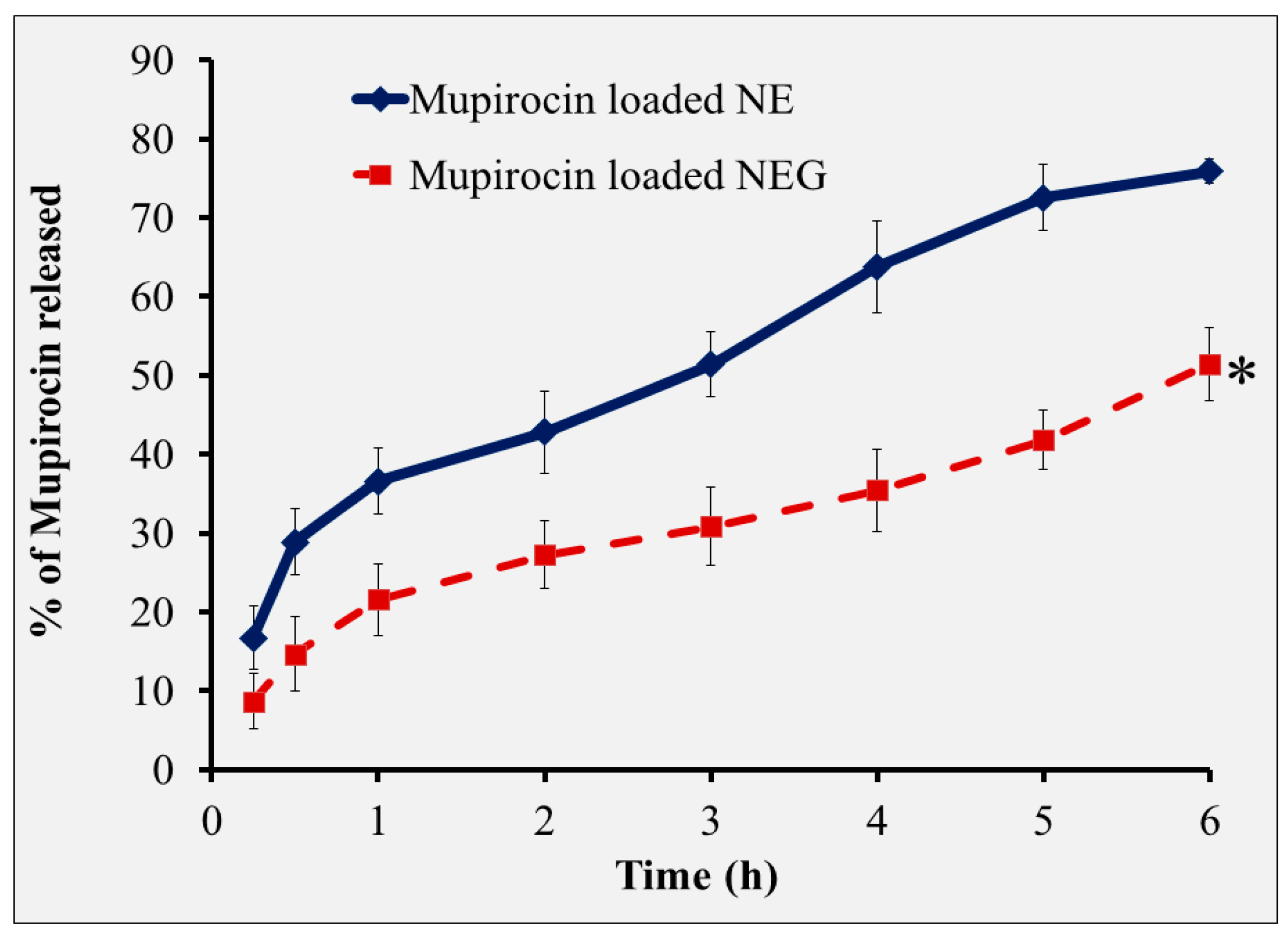
3.5. The In Vitro Drug Release Study of NEG Formulation
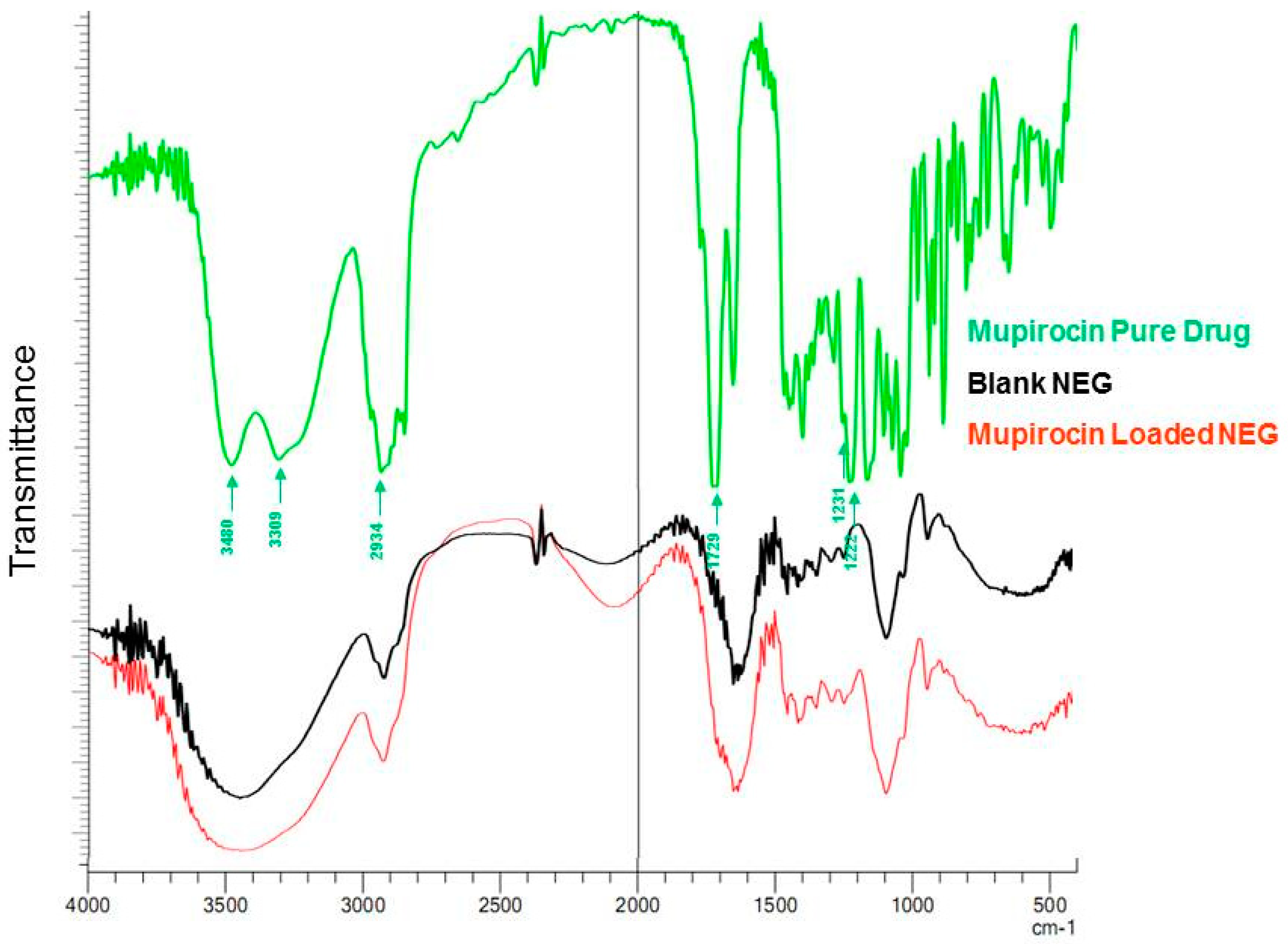
3.6. FTIR (Fourier-Transform Infrared Spectroscopy) Study
3.7. Scanning Electron Microscopy (SEM)
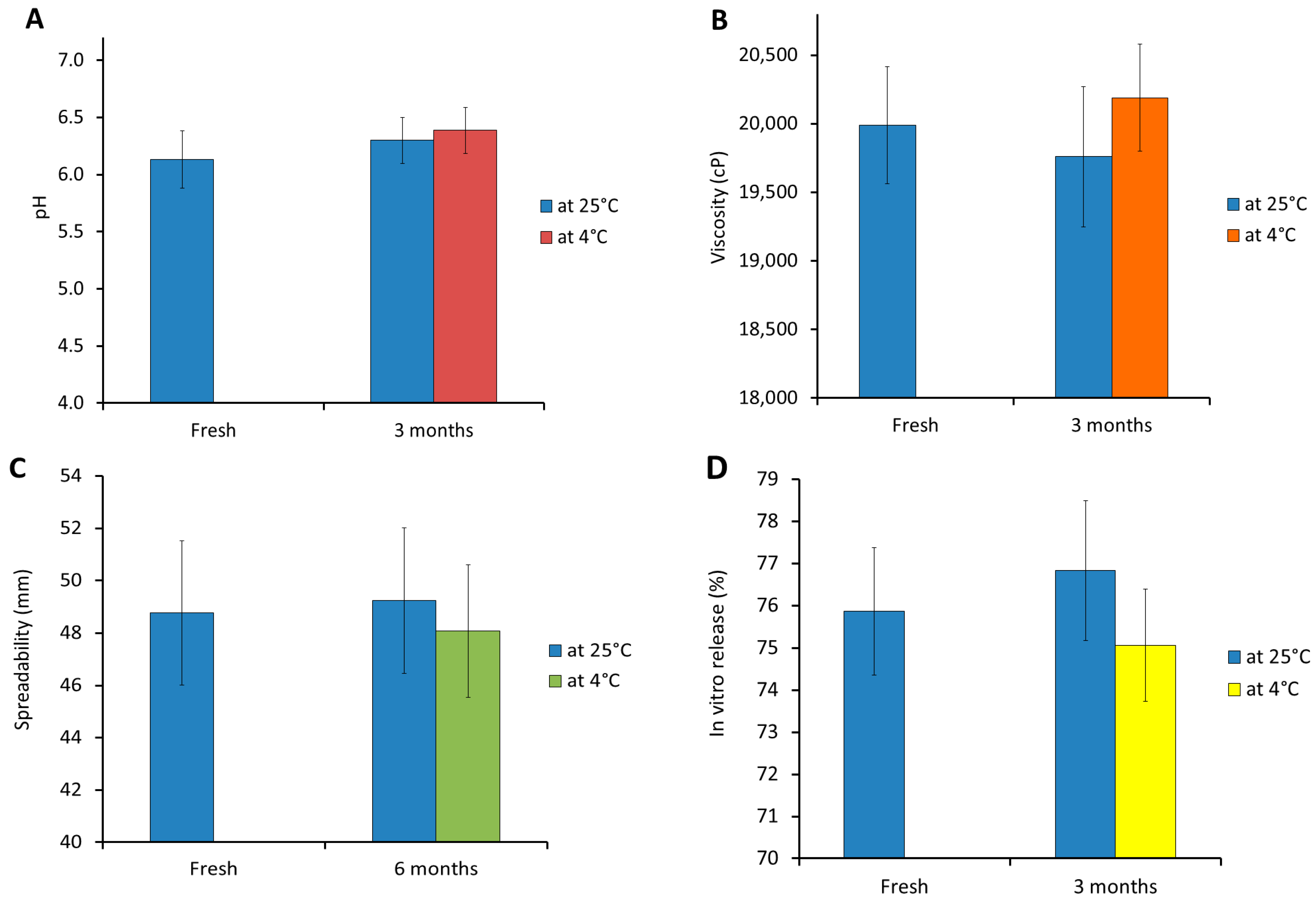
3.8. Stability Study
3.9. The Antibacterial Activity of Mupirocin-Loaded NEG against MRSA Bacterial Strain
3.10. Studies on Skin Irritation
3.11. The In Vivo Evaluation of Wound Healing Efficiency
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Connolly, J.A.; Wilson, A.; Macioszek, M.; Song, Z.; Wang, L.; Mohammad, H.H.; Yadav, M.; di Martino, M.; Miller, C.E.; Hothersall, J. Defining the genes for the final steps in biosynthesis of the complex polyketide antibiotic mupirocin by Pseudomonas fluorescens NCIMB10586. Sci. Rep. 2019, 9, 1542. [Google Scholar] [CrossRef] [PubMed]
- David, S.R.; Malek, N.; Mahadi, A.H.; Chakravarthi, S.; Rajabalaya, R. Development of controlled release silicone adhesive-based mupirocin patch demonstrates antibacterial activity on live rat skin against Staphylococcus aureus. Drug Des. Dev. Ther. 2018, 12, 481–494. [Google Scholar] [CrossRef]
- Twilley, D.; Reva, O.; Meyer, D.; Lall, N. Mupirocin Promotes Wound Healing by Stimulating Growth Factor Production and Proliferation of Human Keratinocytes. Front. Pharmacol. 2022, 13, 862112. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Kaushik, D. Mupirocin Mounted copper nanoparticle offered augmented drug delivery against resistant bacteria. Indian J. Pharm. Educ. Res. 2020, 54, 637–646. [Google Scholar] [CrossRef]
- Shehata, T.M.; Elsewedy, H.S. Paclitaxel and myrrh oil combination therapy for enhancement of cytotoxicity against breast cancer; QbD approach. Processes 2022, 10, 907. [Google Scholar] [CrossRef]
- Sharmeen, J.B.; Mahomoodally, F.M.; Zengin, G.; Maggi, F. Essential Oils as Natural Sources of Fragrance Compounds for Cosmetics and Cosmeceuticals. Molecules 2021, 26, 666. [Google Scholar] [CrossRef] [PubMed]
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca alternifolia (Tea Tree) oil: A review of antimicrobial and other medicinal properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef]
- Corona-Gómez, L.; Hernández-Andrade, L.; Mendoza-Elvira, S.; Suazo, F.M.; Ricardo-González, D.I.; Quintanar-Guerrero, D. In vitro antimicrobial effect of essential tea tree oil(Melaleuca alternifolia), thymol, and carvacrol on microorganisms isolated from cases of bovine clinical mastitis. Int. J. Vet. Sci. Med. 2022, 10, 72–79. [Google Scholar] [CrossRef]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of antibacterial action of three monoterpenes. Antimicrob. Agents Chemother. 2005, 49, 2474–2478. [Google Scholar] [CrossRef]
- Yadav, E.; Kumar, S.; Mahant, S.; Khatkar, S.; Rao, R. Tea tree oil: A promising essential oil. J. Essent. Oil Res. 2017, 29, 201–213. [Google Scholar] [CrossRef]
- Tan, C.; McClements, D.J. Application of advanced emulsion technology in the food industry: A review and critical evaluation. Foods 2021, 10, 812. [Google Scholar] [CrossRef] [PubMed]
- Nasr, A.M.; Aboelenin, S.M.; Alfaifi, M.Y.; Shati, A.A.; Elbehairi, S.E.I.; Elshaarawy, R.F.; Elwahab, N.H.A. Quaternized Chitosan Thiol Hydrogel-Thickened Nanoemulsion: A Multifunctional Platform for Upgrading the Topical Applications of Virgin Olive Oil. Pharmaceutics 2022, 14, 1319. [Google Scholar] [CrossRef] [PubMed]
- Shehabeldine, A.M.; Doghish, A.S.; El-Dakroury, W.A.; Hassanin, M.M.H.; Al-Askar, A.A.; AbdElgawad, H.; Hashem, A.H. Antimicrobial, Antibiofilm, and Anticancer Activities of Syzygium aromaticum Essential Oil Nanoemulsion. Molecules 2023, 28, 5812. [Google Scholar] [CrossRef] [PubMed]
- Marwa, A.; Iskandarsyah; Jufri, M. Nanoemulsion curcumin injection showed significant anti-inflammatory activities on carrageenan-induced paw edema in Sprague-Dawley rats. Heliyon 2023, 9, e15457. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhang, J.; Liu, X.; Lu, Y.; Shi, Y.; Li, X.; Wang, S.; Huang, J.; Liu, H.; Zhou, H.; et al. Antioxidant nanoemulsion loaded with latanoprost enables highly effective glaucoma treatment. J. Control. Release 2023, 361, 534–546. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.C.; Chen, Y.C.; Wu, M.T.; Wang, C.C.; Wu, Y.T. Nanoemulsion as a strategy for improving the oral bioavailability and anti-inflammatory activity of andrographolide. Int. J. Nanomed. 2018, 13, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Monge, C.; Stoppa, I.; Ferraris, C.; Bozza, A.; Battaglia, L.; Cangemi, L.; Miglio, G.; Pizzimenti, S.; Clemente, N.; Gigliotti, C.L. Parenteral Nanoemulsions Loaded with Combined Immuno-and Chemo-Therapy for Melanoma Treatment. Nanomaterials 2022, 12, 4233. [Google Scholar] [CrossRef]
- Rai, V.K.; Mishra, N.; Yadav, K.S.; Yadav, N.P. Nanoemulsion as pharmaceutical carrier for dermal and transdermal drug delivery: Formulation development, stability issues, basic considerations and applications. J. Control. Release 2018, 270, 203–225. [Google Scholar] [CrossRef]
- Krishnan, V.; Mitragotri, S. Nanoparticles for topical drug delivery: Potential for skin cancer treatment. Adv. Drug Deliv. Rev. 2020, 153, 87–108. [Google Scholar] [CrossRef]
- Tapfumaneyi, P.; Imran, M.; Mohammed, Y.; Roberts, M.S. Recent advances and future prospective of topical and transdermal delivery systems. Front. Drug Deliv. 2022, 2, 957732. [Google Scholar] [CrossRef]
- Makvandi, P.; Caccavale, C.; Della Sala, F.; Zeppetelli, S.; Veneziano, R.; Borzacchiello, A. Natural Formulations Provide Antioxidant Complement to Hyaluronic Acid-Based Topical Applications Used in Wound Healing. Polymers 2020, 12, 1847. [Google Scholar] [CrossRef] [PubMed]
- Donthi, M.R.; Munnangi, S.R.; Krishna, K.V.; Saha, R.N.; Singhvi, G.; Dubey, S.K. Nanoemulgel: A Novel Nano Carrier as a Tool for Topical Drug Delivery. Pharmaceutics 2023, 15, 164. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, H.; Gorain, B.; Pandey, M.; Chatterjee, L.A.; Sengupta, P.; Das, A.; Molugulu, N.; Kesharwani, P. Recent Update on Nanoemulgel as Topical Drug Delivery System. J. Pharm. Sci. 2017, 106, 1736–1751. [Google Scholar] [CrossRef]
- Debnath, S.; Aishwarya, M.; Babu, M.N. Formulation by design: An approach to designing better drug delivery systems. Pharma Times 2018, 50, 9–14. [Google Scholar]
- Nainggolan, E.A.; Banout, J.; Urbanova, K. Application of Central Composite Design and Superimposition Approach for Optimization of Drying Parameters of Pretreated Cassava Flour. Foods 2023, 12, 2101. [Google Scholar] [CrossRef]
- Elsewedy, H.S.; Shehata, T.M.; Soliman, W.E. Tea Tree Oil Nanoemulsion-Based Hydrogel Vehicle for Enhancing Topical Delivery of Neomycin. Life 2022, 12, 1011. [Google Scholar] [CrossRef] [PubMed]
- Shehata, T.M.; Almostafa, M.M.; Elsewedy, H.S. Development and Optimization of Nigella sativa Nanoemulsion Loaded with Pioglitazone for Hypoglycemic Effect. Polymers 2022, 14, 3021. [Google Scholar] [CrossRef]
- Elsewedy, H.S.; Younis, N.S.; Shehata, T.M.; Mohamed, M.E.; Soliman, W.E. Enhancement of anti-inflammatory activity of optimized niosomal colchicine loaded into jojoba oil-based emulgel using response surface methodology. Gels 2021, 8, 16. [Google Scholar] [CrossRef]
- Donthi, M.R.; Saha, R.N.; Singhvi, G.; Dubey, S.K. Dasatinib-Loaded Topical Nano-Emulgel for Rheumatoid Arthritis: Formulation Design and Optimization by QbD, In Vitro, Ex Vivo, and In Vivo Evaluation. Pharmaceutics 2023, 15, 736. [Google Scholar] [CrossRef]
- Razzaq, F.A.; Asif, M.; Asghar, S.; Iqbal, M.S.; Khan, I.U.; Khan, S.-U.-D.; Irfan, M.; Syed, H.K.; Khames, A.; Mahmood, H. Glimepiride-loaded nanoemulgel; development, in vitro characterization, ex vivo permeation and in vivo antidiabetic evaluation. Cells 2021, 10, 2404. [Google Scholar] [CrossRef]
- Zaki, N.M.; Awad, G.A.; Mortada, N.D.; Abd ElHady, S.S. Enhanced bioavailability of metoclopramide HCl by intranasal administration of a mucoadhesive in situ gel with modulated rheological and mucociliary transport properties. Eur. J. Pharm. Sci. 2007, 32, 296–307. [Google Scholar] [CrossRef]
- Elsewedy, H.S.; Shehata, T.M.; Soliman, W.E. Shea Butter Potentiates the Anti-Bacterial Activity of Fusidic Acid Incorporated into Solid Lipid Nanoparticle. Polymers 2022, 14, 2436. [Google Scholar] [CrossRef] [PubMed]
- Salamanca, C.H.; Barrera-Ocampo, A.; Lasso, J.C.; Camacho, N.; Yarce, C.J. Franz diffusion cell approach for pre-formulation characterisation of ketoprofen semi-solid dosage forms. Pharmaceutics 2018, 10, 148. [Google Scholar] [CrossRef] [PubMed]
- Ojo, O.E.; Ilomuanya, M.O.; Sekunowo, O.I.; Gbenebor, O.P.; Adeosun, S.O. Development and characterization of mupirocin encapsulated in animal bone-derived hydroxyapatite for management of chronic wounds. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 82. [Google Scholar] [CrossRef]
- Elsewedy, H.S.; Shehata, T.M.; Almostafa, M.M.; Soliman, W.E. Hypolipidemic activity of olive oil-based nanostructured lipid carrier containing atorvastatin. Nanomaterials 2022, 12, 2160. [Google Scholar] [CrossRef] [PubMed]
- Nathan, P.; Law, E.J.; Murphy, D.F.; MacMillan, B.G.J.B. A laboratory method for selection of topical antimicrobial agents to treat infected burn wounds. Burns 1978, 4, 177–187. [Google Scholar] [CrossRef]
- Si, S.; Swain, S.; Kanungo, S.; Gupta, R. Preparation and evaluation of gels from gum of Moringa oleifera. Indian J. Pharm. Sci. 2006, 68, 777. [Google Scholar]
- Kamlungmak, S.; Nakpheng, T.; Kaewpaiboon, S.; Mudhar Bintang, M.A.K.; Prom-In, S.; Chunhachaichana, C.; Suwandecha, T.; Srichana, T. Safety and Biocompatibility of Mupirocin Nanoparticle-Loaded Hydrogel on Burn Wound in Rat Model. Biol. Pharm. Bull. 2021, 44, 1707–1716. [Google Scholar] [CrossRef]
- Chollet, J.L.; Jozwiakowski, M.J.; Phares, K.R.; Reiter, M.J.; Roddy, P.J.; Schultz, H.J.; Ta, Q.V.; Tomai, M.A. Development of a topically active imiquimod formulation. Pharm. Dev. Technol. 1999, 4, 35–43. [Google Scholar] [CrossRef]
- Quazi, A.; Patwekar, M.; Patwekar, F.; Mezni, A.; Ahmad, I.; Islam, F. Evaluation of Wound Healing Activity (Excision Wound Model) of Ointment Prepared from Infusion Extract of Polyherbal Tea Bag Formulation in Diabetes-Induced Rats. Evid. -Based Complement. Altern. Med. ECAM 2022, 2022, 1372199. [Google Scholar] [CrossRef]
- Wróblewska, M.; Szymańska, E.; Winnicka, K. The Influence of Tea Tree Oil on Antifungal Activity and Pharmaceutical Characteristics of Pluronic(®) F-127 Gel Formulations with Ketoconazole. Int. J. Mol. Sci. 2021, 22, 11326. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.H.; Hasni, D.; Rohaya, S.; Antasari, M.; Winarti, C. The role of breadfruit OSA starch and surfactant in stabilizing high-oil-load emulsions using high-pressure homogenization and low-frequency ultrasonication. Heliyon 2020, 6, e04341. [Google Scholar] [CrossRef] [PubMed]
- Lemaalem, M.; Ahfir, R.; Derouiche, A.; Filali, M. Static and dynamic properties of decane/water microemulsions stabilized by cetylpyridinium chloride cationic surfactant and octanol cosurfactant. RSC Adv. 2020, 10, 36155–36163. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, P.; Aggarwal, G.; Harikumar, S.L.; Singh, K. Development of self-microemulsifying drug delivery system and solid-self-microemulsifying drug delivery system of telmisartan. Int. J. Pharm. Investig. 2014, 4, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Lukić, M.; Pantelić, I.; Savić, S.D. Towards optimal ph of the skin and topical formulations: From the current state of the art to tailored products. Cosmetics 2021, 8, 69. [Google Scholar] [CrossRef]
- Hassan, D.S.; Hasary, H.J. The impact of viscosity on the dissolution of naproxen immediate-release tablets. J. Taibah Univ. Med. Sci. 2023, 18, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Djiobie Tchienou, G.E.; Tsatsop Tsague, R.K.; Mbam Pega, T.F.; Bama, V.; Bamseck, A.; Dongmo Sokeng, S.; Ngassoum, M.B. Multi-response optimization in the formulation of a topical cream from natural ingredients. Cosmetics 2018, 5, 7. [Google Scholar] [CrossRef]
- Md, S.; Alhakamy, N.A.; Aldawsari, H.M.; Kotta, S.; Ahmad, J.; Akhter, S.; Shoaib Alam, M.; Khan, M.A.; Awan, Z.; Sivakumar, P.M. Improved Analgesic and Anti-Inflammatory Effect of Diclofenac Sodium by Topical Nanoemulgel: Formulation Development—In Vitro and In Vivo Studies. J. Chem. 2020, 2020, 4071818. [Google Scholar] [CrossRef]
- Fong Yen, W.; Basri, M.; Ahmad, M.; Ismail, M. Formulation and evaluation of galantamine gel as drug reservoir in transdermal patch delivery system. Sci. World J. 2015, 2015, 495271. [Google Scholar] [CrossRef]
- Arora, R.; Aggarwal, G.; Harikumar, S.L.; Kaur, K. Nanoemulsion Based Hydrogel for Enhanced Transdermal Delivery of Ketoprofen. Adv. Pharm. 2014, 2014, 468456. [Google Scholar] [CrossRef]
- Alhasso, B.; Ghori, M.U.; Conway, B.R. Development of Nanoemulsions for Topical Application of Mupirocin. Pharmaceutics 2023, 15, 378. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.D.; Mann, C.M.; Markham, J.L.; Gustafson, J.E.; Warmington, J.R.; Wyllie, S.G. Determining the antimicrobial actions of tea tree oil. Molecules 2001, 6, 87–91. [Google Scholar] [CrossRef]
- Abreu, A.C.; Serra, S.C.; Borges, A.; Saavedra, M.J.; Mcbain, A.J.; Salgado, A.J.; Simoes, M. Combinatorial activity of flavonoids with antibiotics against drug-resistant Staphylococcus aureus. Microb. Drug Resist. 2015, 21, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, P.; Singh, A.K.; Singh, A.K.; Shukla, S.; Agarwal, L. Prevalence of Mupirocin Resistant Staphylococcus aureus Isolates Among Patients Admitted to a Tertiary Care Hospital. N. Am. J. Med. Sci. 2014, 6, 403–407. [Google Scholar] [CrossRef]
- Gangwar, A.; Kumar, P.; Singh, R.; Kush, P. Recent advances in mupirocin delivery strategies for the treatment of bacterial skin and soft tissue infection. Future Pharmacol. 2021, 1, 80–103. [Google Scholar] [CrossRef]


| Formula | Independent Variables | Dependent Variables | ||||
|---|---|---|---|---|---|---|
| A (%) | B (%) | C (%) | Particle Size (nm) | In Vitro Release (%) | PDI | |
| F1 | 2.5 | 1 | 1.5 | 265 ± 3.2 | 50 ± 3.0 | 0.467 ± 0.061 |
| F2 | 1.5 | 1 | 0.5 | 123 ± 2.0 | 75 ± 1.8 | 0.348 ± 0.034 |
| F3 | 1.5 | 0.5 | 1.5 | 136 ± 2.5 | 73 ± 2.8 | 0.350 ± 0.049 |
| F4 | 2 | 0.32 | 1 | 254 ± 3.4 | 53 ± 2.6 | 0.573 ± 0.048 |
| F5 | 2 | 0.75 | 0.15 | 225 ± 1.9 | 55 ± 2.2 | 0.276 ± 0.013 |
| F6 | 2 | 0.75 | 1 | 214 ± 3.0 | 59 ± 2.3 | 0.341 ± 0.032 |
| F7 | 2 | 1.17 | 1 | 176 ± 2.9 | 65 ± 3.2 | 0.333 ± 0.031 |
| F8 | 2.5 | 0.5 | 0.5 | 356 ± 4.5 | 42 ± 3.0 | 0.521 ± 0.024 |
| F9 | 1.5 | 0.5 | 0.5 | 149 ± 2.5 | 69 ± 2.7 | 0.319 ± 0.019 |
| F10 | 1.15 | 0.75 | 1 | 97 ± 2.0 | 81 ± 2.6 | 0.323 ± 0.035 |
| F11 | 2 | 0.75 | 1 | 212 ± 3.2 | 60 ± 2.8 | 0.275 ± 0.010 |
| F12 | 2 | 0.75 | 1.8 | 192 ± 1.7 | 62 ± 2.5 | 0.226 ± 0.007 |
| F13 | 2.5 | 1 | 0.5 | 286 ± 3.6 | 47 ± 2.4 | 0.439 ± 0.018 |
| F14 | 2 | 0.75 | 1 | 217 ± 3.5 | 57 ± 3.5 | 0.326 ± 0.012 |
| F15 | 1.5 | 1 | 1.5 | 117 ± 2.1 | 79 ± 3.1 | 0.307 ± 0.021 |
| F16 | 2.5 | 0.5 | 1.5 | 315 ± 4.7 | 45 ± 3.0 | 0.489 ± 0.018 |
| F17 | 2.8 | 0.75 | 1 | 375 ± 3.4 | 40 ± 2.0 | 0.495 ± 0.010 |
| Source | Y1 | Y2 | ||
|---|---|---|---|---|
| F-Value | p-Value | F-Value | p-Value | |
| Model | 639.74 | <0.0001 * | 403.40 | <0.0001 * |
| A | 5245.74 | <0.0001 * | 1126.16 | <0.0001 * |
| B | 339.32 | <0.0001 * | 61.19 | <0.0001 * |
| C | 72.07 | <0.0001 * | 22.84 | 0.0004 * |
| AB | 37.14 | 0.0005 * | ||
| AC | 12.21 | 0.0101 * | ||
| BC | 4.81 | 0.0643 | ||
| A² | 31.41 | 0.0008 * | ||
| B² | 0.0157 | 0.9040 | ||
| C2 | 3.60 | 0.0994 | ||
| Lack of fit | 3.78 | 0.2221 | 0.8965 | 0.6378 |
| R2 analysis | ||||
| R² | 0.9988 | 0.9894 | ||
| Adjusted R² | 0.9972 | 0.9869 | ||
| Predicted R² | 0.9911 | 0.9820 | ||
| Adequate precision | 85.9518 | 62.9684 | ||
| Model | Quadratic | Linear | ||
| Remark | Suggested | Suggested | ||
| Selected Factor | Constraint | |
|---|---|---|
| Tea tree oil concentration (g) | In range | |
| Tween 80 concentration (g) | In range | |
| PEG 400 concentration (g) | In range | |
| Response | Predicted values | Observed values |
| Particle size (nm) | 120.26 ± 4.3 | 125.0 ± 3.6 |
| In vitro release (%) | 77.75 ± 1.45 | 75.86 ± 1.5 |
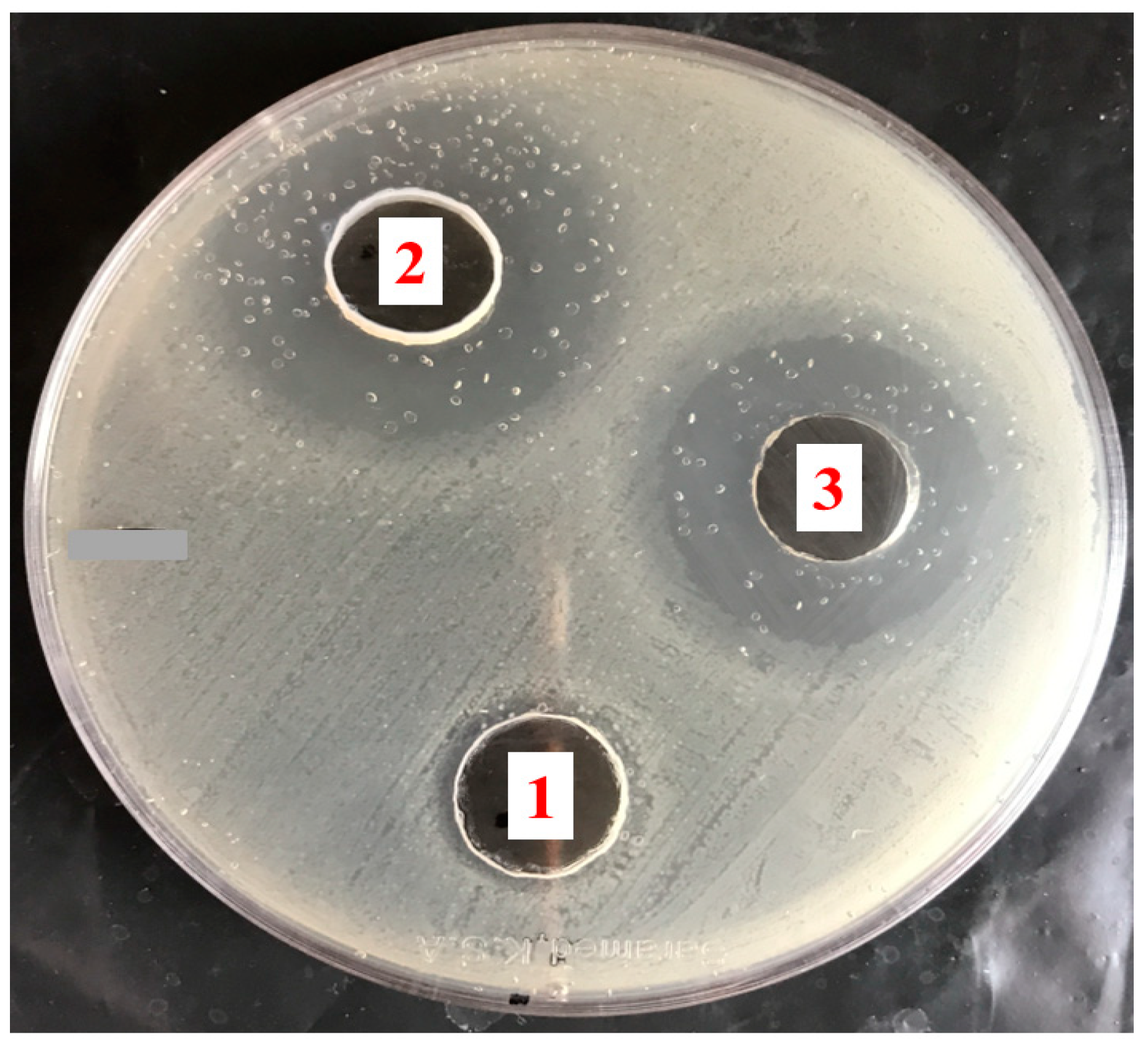
| Inhibition Zone Diameter (cm) | Bacterial Strain |
|---|---|
| MRSA | |
| Blank NEG | 1.8 ± 0.10 |
| Bactroban® ointment | 3.0 ± 0.15 * |
| Mupirocin-loaded NEG | 3.4 ± 0.15 * ° |
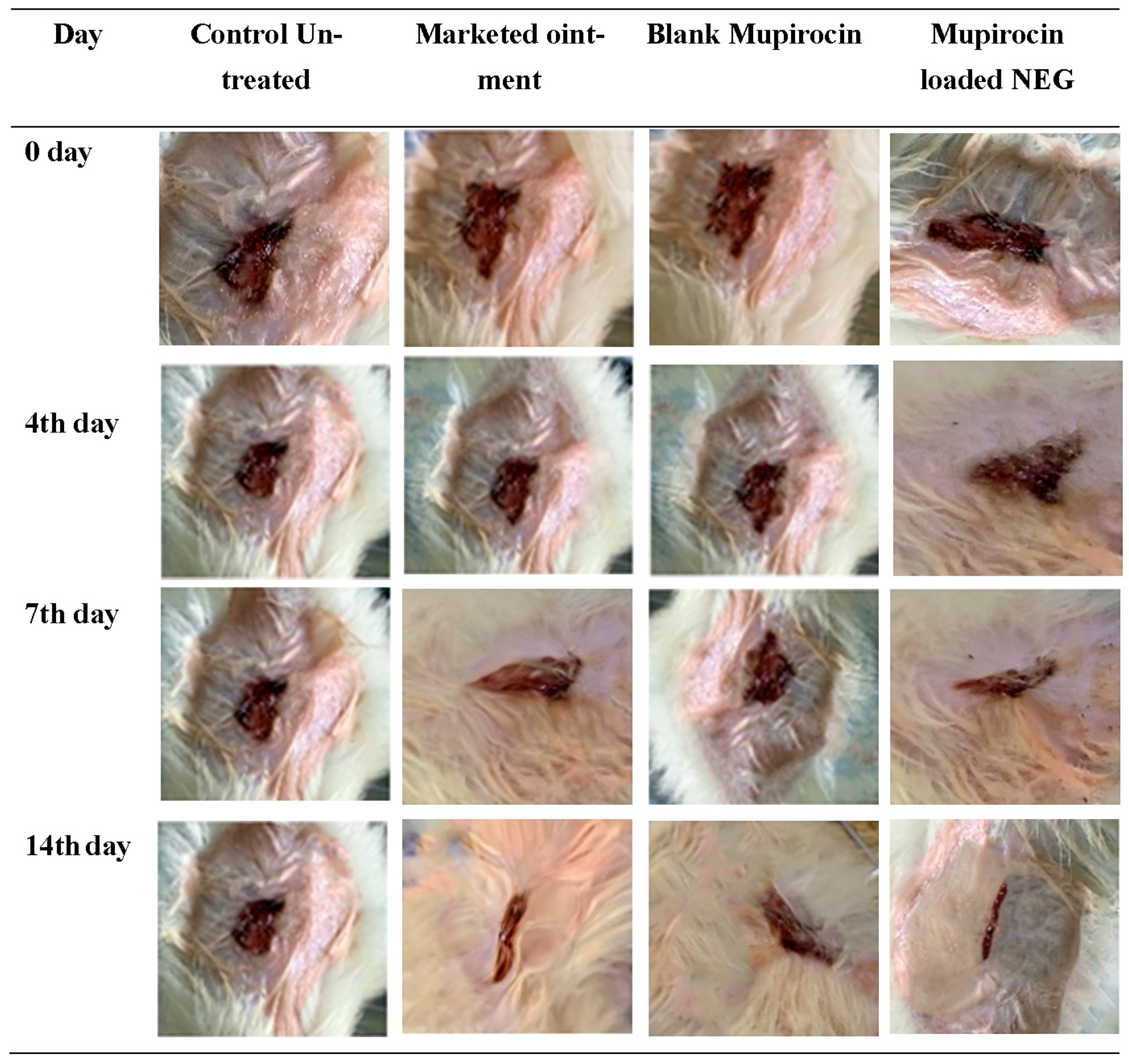
| Days | % of Unhealed Wound Lesions | |||
|---|---|---|---|---|
| Control Untreated | Marketed Ointment | Blank NEG | Mupirocin-Loaded NEG | |
| 0 | 102.8 ± 4.7 | 101 ± 5.3 | 102.8 ± 5.7 | 102.8 ± 5.7 |
| 4 | 97.3 ± 5.1 | 73.3 ± 1.4 * ° | 80 ± 4.0 * $ | 61.63 ± 3.2 * ° $ |
| 7 | 94.0 ± 4.5 | 54.3 ± 3.4 * ° | 72.53 ± 1.53 * $ | 31.67 ± 5.1 * ° $ |
| 14 | 90.3 ± 4.5 | 36.67 ± 3.0 * ° | 50.0 ± 1.0 * $ | 10.27 ± 2.0 * ° $ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bujubarah, M.M.; Elsewedy, H.S.; Shehata, T.M.; Soliman, W.E. Formulation by Design of an Innovative Tea Tree Oil Nanoemulgel Incorporating Mupirocin for Enhanced Wound Healing Activity. Appl. Sci. 2023, 13, 13244. https://doi.org/10.3390/app132413244
Bujubarah MM, Elsewedy HS, Shehata TM, Soliman WE. Formulation by Design of an Innovative Tea Tree Oil Nanoemulgel Incorporating Mupirocin for Enhanced Wound Healing Activity. Applied Sciences. 2023; 13(24):13244. https://doi.org/10.3390/app132413244
Chicago/Turabian StyleBujubarah, Mahdi M., Heba S. Elsewedy, Tamer M. Shehata, and Wafaa E. Soliman. 2023. "Formulation by Design of an Innovative Tea Tree Oil Nanoemulgel Incorporating Mupirocin for Enhanced Wound Healing Activity" Applied Sciences 13, no. 24: 13244. https://doi.org/10.3390/app132413244
APA StyleBujubarah, M. M., Elsewedy, H. S., Shehata, T. M., & Soliman, W. E. (2023). Formulation by Design of an Innovative Tea Tree Oil Nanoemulgel Incorporating Mupirocin for Enhanced Wound Healing Activity. Applied Sciences, 13(24), 13244. https://doi.org/10.3390/app132413244







