Abstract
This study aimed to assess functional substances and antioxidant effects with respect to planting intervals and collection timing, with the goal of developing efficient planting methods for the mass harvesting of Rosa davurica and enhancing the availability of the plant’s edible parts in Korea. High-Performance Liquid Chromatography (HPLC) was employed to analyze the ethyl gallate content, a recognized functional component present in R. davurica, while the antioxidant effect was verified using DPPH, ABTS, and SOD assays. The findings revealed an increased yield of buds and leaves in R. davurica when the planting interval was reduced to 120 × 50 cm compared to the conventional 120 × 100 cm spacing. Specifically, the content of ethyl gallate remained consistent across different collection periods, and this result was associated with the varying planting intervals. While there were no significant changes in total polyphenol and flavonoid content, the collection from September demonstrated higher levels. Furthermore, the study established that leaf antioxidant activity, determined through IC50 values of DPPH and ABTS, surpassed that of the buds, with no significant difference in SOD activity being observed. Overall, no substantial differences were observed in the content of functional components between the buds and leaves of R. davurica, regardless of planting interval and collection timing. These results contribute valuable insights for optimizing planting methods for the large-scale collection of R. davurica leaves.
1. Introduction
Reactive oxygen species (ROS) are generated during the bioenergetic processes inherent in human respiration or induced by environmental stimuli [1]. It is recognized for its participation in cellular growth and differentiation; however, without effective in vivo elimination, the prolonged presence of free radicals leads to oxidative stress [2]. Upon the manifestation of an oxidative stress milieu within the human body, accelerated aging, genetic damage, skin pigmentation, and the initiation of chronic inflammation ensue, thereby precipitating a spectrum of diseases, including but not limited to hypertension, diabetes, arteriosclerosis, and stroke [3]. The mitigation of oxidative stress necessitates a crucial emphasis on providing antioxidants to the body.
The presently employed synthetic antioxidants, namely butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT), have been documented to evoke significant adverse effects upon consumption at specified or elevated levels [4]. Hence, the significance of antioxidant materials derived from natural sources becomes paramount. Natural antioxidants, predominantly abundant in vegetable raw materials as opposed to animal counterparts, encompass compounds capable of actively neutralizing reactive oxygen species without undergoing conversion into other oxidized radicals, such as flavonoids and polyphenols [5].
The Rosaceae family exhibits a global distribution, being particularly prevalent in Europe, North America, and Asia [6]. In Korea, this botanical family comprises 207 species spanning 35 genera across 4 subfamilies, including notable representatives such as Fragaria (strawberry), Rubus (berry), Prunus (apricot and peach), Malus (apple), Pyrus (pear), and Chaenomeles (quince). Among these, R. davurica (saengyeolgui) stands out as a perennial plant within the Rosaceae family, manifesting as a deciduous broad-leaved shrub primarily found in Korea, notably in Gangwon-do, situated at elevations ranging from 200 to 1200 m above sea level.
R. davurica finds utility in the private sector as a source of sustenance, with its roots and flowers primarily being employed for addressing ailments by strengthening the stomach; making menstruation even; alleviating indigestion; and stopping diarrhea, stomach pain, and dysmenorrhea. Furthermore, the plant’s flowers contribute to the formulation of perfumes and cosmetic products. Notably, the leaves and fruits of R. davurica boast a richness in ascorbic acid, commonly recognized as vitamin C, endowing them with notable properties, such as antioxidant, anticancer, and anti-aging effects [7,8]. A recent study revealed that a 30% ethanol extract from R. davurica inhibited UVB-induced photoaging [9]. Also, a similar extract inhibited the release of cytokines and histamine in activated DNP-IgE mast cells, thereby exhibiting potential in managing allergic diseases [10].
R. davurica, thriving in acidic soil, can be cultivated across diverse Korean regions. The cultivation methods include seed collection and sowing (August to September), shovel cultivation using root shovels, and tissue cultivation involving transplantation into a growth medium for differentiation. Conventionally, optimal spacing for planting R. davurica involves a recess width of 120 cm and an inter-row distance of 100 cm. Proximity beyond recommended measures is reported to impede work efficiency due to overlapping branches. Planting interval, representing the quantity of plants per unit area, holds significant relevance in farm management. Specifically, farm productivity and space optimization can be enhanced through the judicious control of the planting interval. Notably, the traditional method of planting R. davurica, designed primarily for fruit collection purposes, may not align with the objectives of cultivating R. davurica for food-grade net extraction.
Presently, R. davurica is utilized for food consumption, primarily in bud, petal, and fruit forms. Although dictionaries distinguish between the buds and the leaves, the R. davurica fruit and total production calculation table at the Jeongseon saengyeolgui Farming Association Corporation represents the leaves as “bud (leaf)”. This proves that discerning between buds and leaves with the naked eye appears challenging. Consequently, if there is no discernible difference in the composition of buds and leaves, opting for leaves as a food source may prove more productive. Thus, expanding the utilization scope to include leaves becomes imperative.
In this study, 30% ethanol extract of R. davurica was generated and employed in experimentation by harvesting both buds and leaves in accordance with the planting intervals. The objective was to enhance the bud yield suitable for use as a food source from R. davurica. Variations in the surface substances, total flavonoids, and polyphenols of the produced extract were scrutinized, and antioxidant efficacy was assessed through 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azino-bis-3-ethylbenzo-thiazoline-6-sulfonic acid (ABTS) radical scavenging activity and superoxide dismutase (SOD) activity evaluations.
2. Materials and Methods
2.1. Chemicals
DPPH, ABTS, 2,2′-azobis(2-amidinopropane) dihydrochloride (AAPH), AlCl3, quercetin, gallic acid, and Folin–Ciocalteau’s phenol reagent were obtained from Sigma Chemical (St. Louis, MO, USA). EtOH, CH3COOK, MeOH, and formic acid were bought from Daejung chemicals & metals Co., Ltd. (Siheung-si, Gyeonggi-do, Republic of Korea) and Junsei chemical Co., Ltd. (Saitama, Tokyo, Japan), respectively. All other materials used were purchased from commercial sources in the highest grades available and were used as received without any further purification.
2.2. Sample Preparations
This study aimed to investigate the content of beneficial components in R. davurica leaves according to planting intervals. For this purpose, a trial plot was established in the spatial domain of Wonsam-myeon, Cheoin-gu, Yongin-si, Gyeonggi-do, with standard fertilization (N 5.5-P2O5 6.3-K2O 15.6-manure 3000 kg/10a) applied uniformly. The seedlings of R. davurica were subjected to a factorial experiment with planting intervals of 120 × 50 cm and 120 × 100 cm, and the harvest dates were differentiated into two periods in May and September. The experiment was conducted in triplicate using a randomized block design. Harvesting was conducted on R. davurica within a 1-are (1a) area at two different planting intervals (120 × 50 cm and 120 × 100 cm). Following the harvest, a total of 4 samples, each representing three replicates per experimental plot, were collected for analysis. The method of Oh S et al. was used to extract R. davurica exactly as it was [10].
2.3. High-Performance Liquid Chromatography (HPLC) Analysis
At a concentration of 10 mg/mL, the sample was produced in 50% methanol. In methanol, serial dilutions (2.5–1000 µg/mL) of the standard chemical (ethyl gallate) were produced. HPLC was conducted on a Dionex Chromelon TM chromatography data system with P580 and UVD100 detectors (Thermo Fisher Scientific Inc., Waltham, MA, USA). Chromatographic separation was conducted on an Inno C-18 column (5 µm, 4.6 × 250 nm; Young Jin). The mobile phase was made up of (A) water and (B) acetonitrile, both of which contained 0.1% formic acid, and the gradient was as follows: 95% A/5% B at 0 min; 95% A/5% B at 3 min; 85% A/15% B at 8 min; 78% A/22% B at 20 min; 76% A/24% B at 24 min; 60% A/40% B at 28 min; 5% A/95% B at 31 min; 5% A/95% B at 35 min; 95% A/5% B at 35.1 min; and 95% A/5% B at 38 min. The temperature of the column was 25 °C, the flow rate was 1.0 mL/min, and the injected volume was 10 µL.
2.4. Total Flavonoid Content Measurement
The total flavonoid content (TFC) was determined following the method of Moreno et al. [11]. Briefly, 0.5 mL of the R. davurica extract was mixed with 4.3 mL of 95% EtOH, 10% AlCl3 0.1 mL, and 1M CH3COOK 0.1 mL. The mixture was thoroughly stirred and left at room temperature for 40 min. Subsequently, absorbance was measured at 450 nm using the FilterMax F5 microplate reader (Molecular Devices, San Francisco, CA, USA). The total flavonoid content in the extract was calculated from the standard calibration curve obtained using quercetin as the reference substance in a concentration range spanning from 31.25 to 1000 µg/mL.
2.5. Total Polyphenol Content Measurement
The total polyphenol content (TPC) was measured using a modified Folin–Denis method [12]. The R. davurica extract was dissolved in dimethyl sulfoxide (DMSO) to a specific concentration. Subsequently, 0.5 mL of the solution was placed in a test tube, and 7 mL of distilled water, 0.5 mL of Folin–Ciocalteau’s phenol reagent, and 1 mL of saturated Na2CO3 solution were successively added. The mixture was stirred for 1 min and left at room temperature for 1 h before measuring absorbance at 625 nm using the FilterMax F5 microplate reader (Molecular Devices, San Francisco, CA, USA). The total phenol content in the extract was calculated from the standard calibration curve obtained using gallic acid as the reference substance in a concentration range spanning from 6.25 to 100 µg/mL.
2.6. DPPH Radical Scavenging Activity
The DPPH radical scavenging activity was measured with modifications to the Blois M.S. method [13]. Specifically, the extract was dissolved in methanol, and 40 µL of the extract, at various concentrations (1, 10, 50, 100, and 250 μg/mL), was added to a 96-well plate, followed by the addition of 160 µL of 2 × 10−4 M DPPH solution. After mixing for 10 s, the plate was shaded, and the reaction was allowed to proceed in an incubator for 30 min. Absorbance was measured at 595 nm using the FilterMax F5 microplate reader (Molecular Devices, San Francisco, CA, USA). The DPPH radical scavenging activity was expressed as the percentage difference using the following formula:
DPPH radical inhibition (%) = (OD0 − ODX)/OD0 × 100
In this formula, OD0 represents the negative control, and ODX represents the sample.
2.7. ABTS Radical Scavenging Activity
The antioxidant activity of the sample against ABTS was measured following the method of Re et al. [14]. ABTS was dissolved in distilled water to a concentration of 7 mM, and then 2.4 mM potassium persulfate was added to generate ABTS radicals. The solution was left at room temperature for 12–16 h before use. The generated ABTS solution was diluted with 99% ethanol to adjust the absorbance to 0.70 ± 0.02 at 620 nm. The scavenging activity was assessed by mixing 100 µL of the ABTS solution with 100 µL of the extract (1, 10, 50, 100, and 250 μg/mL), and after a 15 min reaction, absorbance was measured at 620 nm using the FilterMax F5 microplate reader (Molecular Devices, San Francisco, CA, USA). The inhibition rate was calculated using the following formula:
ABTS+ radical inhibition (%) = (OD0 − ODX)/OD0 × 100
In this formula, OD0 represents the negative control, while ODX represents the sample.
2.8. SOD Activity
SOD activity was measured using EZ-SOD assay kits (DoGenBio, Seoul, Republic of Korea). The superoxide radicals generated by xanthine oxidase (XOD) react with water-soluble tetrazolium salt (WST) in the reaction mixture to form WST–formazan. Consequently, in the presence of high SOD activity, the oxygen radicals produced by XOD are scavenged by SOD, resulting in a relative reduction in formazan formation. The formed formazan was then measured for absorbance at 450 nm using the FilterMax F5 microplate reader (Molecular Devices, San Francisco, CA, USA).
2.9. Data Analysis and Statistical Processing
Sigma plot 12.0 (San Jose, CA, USA) and GraphPad Prism 8 were used for data analysis, and statistical significance was assessed using a one-way analysis of variance (ANOVA) with subsequent post hoc testing utilizing the Student–Newman–Keuls test. Statistical significance was considered when the p-value was equal to or below 0.05.
3. Results
3.1. Yield Comparison According to Planting Interval and Collecting Period for R. davurica
The influence of planting interval and collecting period on the yield of R. davurica buds was depicted in a three-dimensional profile using Sigma plot 12.0 (San Jose, CA, USA). The quantities of bud and leaf harvest according to the planting interval (120 × 50 cm, 120 × 100 cm) and harvest date (May, Sep) for R. davurica were investigated (Table 1). The results of this study showed that bud and leaf quantities varied with different planting intervals and harvesting dates. At 120 × 50 cm, compared to 120 × 100 cm spacing, bud yield increased by 145.8% and 141.9% in May and September, respectively. Similarly, leaf yield increased by 116.14% and 131.6% in May and September, respectively. The 3D profile indicated that reducing inter-row distance significantly influenced bud and leaf production in R. davurica (Figure 1).

Table 1.
Effects of planting interval and collecting time on R. davurica bud and leaf yield.
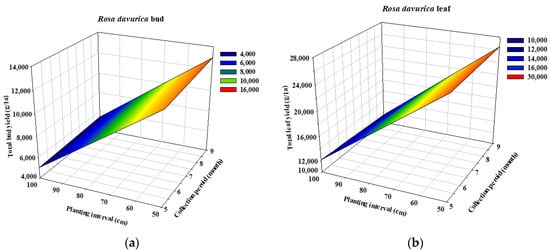
Figure 1.
Three-dimensional mesh plot representing the effects of planting interval and collecting time on R. davurica yield. (a) R. davurica bud; (b) R. davurica leaf.
3.2. Analysis of Indicator Components of R. davurica
According to a study by Fang et al., indicator substances such as ethyl gallate and ellagic acid are known to exist in R. davurica leaves [9]. Ethyl gallate was selected as the indicator in this study, and its levels were analyzed in buds and leaves harvested in May and September under planting intervals of 120 × 50 cm and 120 × 100 cm. The amounts of ethyl gallate (RT 20 min) were measured to be 0.78 mg/g, 0.81 mg/g, 0.83 mg/g, and 0.82 mg/g in the buds and 8.02 mg/g, 8.14 mg/g, 8.22 mg/g, and 8.39 mg/g in the leaves, respectively (Figure 2). These findings indicate that the composition of R. davurica is not significantly affected by planting interval and harvest date.
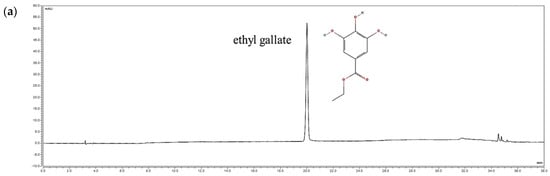
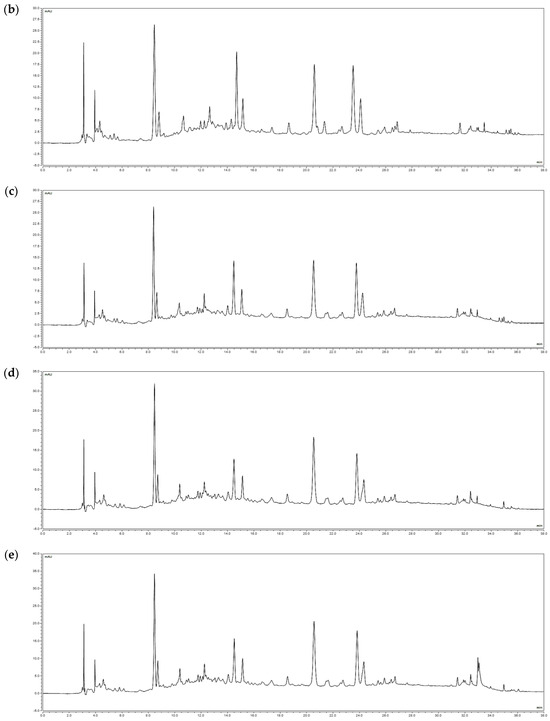
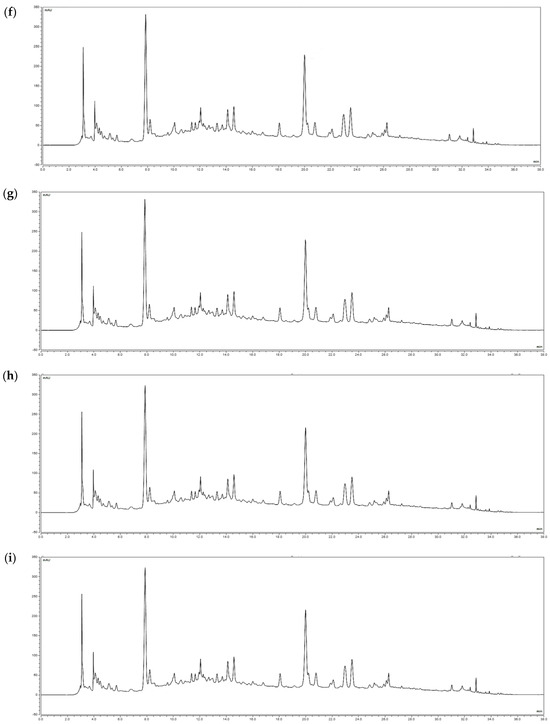
Figure 2.
HPLC chromatograms of R. davurica buds and leaves collected in May and September with different planting intervals. (a) Ethyl gallate standard; (b) 120 × 50 cm in May (buds); (c) 120 × 100 cm in May (buds); (d) 120 × 50 cm in September (buds); (e) 120 × 100 cm in September (buds); (f) 120 × 50 cm in May (leaves); (g) 120 × 100 cm in May (leaves); (h) 120 × 50 cm in September (leaves); (i) 120 × 100 cm in September (leaves).
3.3. Total Flavonoid and Polyphenol Contents
An experiment was conducted to examine the differences in the TFCs and TPCs of R. davurica buds and leaves based on planting interval and harvest date (Table 2). For both the buds and leaves harvested in May and September, the variations in TFC and TPC were minimal, ranging from 2 to 3 mg QE and GAE/g extract. Additionally, the differences in content between the harvest date did not exceed 30.

Table 2.
Contents of flavonoids and polyphenols for different R. davurica bud and leaf planting intervals and collecting times. All data are shown as the mean ± SD.
3.4. Radical Scavenging Activities
DPPH and ABTS experiments were conducted to evaluate the antioxidant capacity of the R. davurica buds and leaves concerning harvest date and planting interval. In both the DPPH and ABTS assays, the R. davurica buds and leaves demonstrated a concentration-dependent increase in antioxidant activity across all conditions (Figure 3 and Figure 4). The half-maximal inhibitory concentration (IC50) values were compared. The September harvest under the 120 × 100 cm condition yielded the lowest IC50 value for both the buds and leaves in the DPPH assay (Table 3). The differences in the bud and leaf IC50 values were generally minimal (within a range of 3 µg/mL), except for the buds harvested in May under the 120 × 50 cm condition. Similarly, in the ABTS experiment, all samples showed great radical scavenging activity, and the corresponding IC50 values were calculated and are shown in Table 4). Among the buds, those harvested in May under the 120 × 100 cm condition exhibited the highest antioxidant activity, while the leaves harvested in September under the 120 × 50 cm condition showed the lowest IC50 values. Through comparing the DPPH and ABTS results, it was observed that the leaves consistently showed lower IC50 values, indicating that they had higher antioxidant capabilities compared to the buds in all experiments.
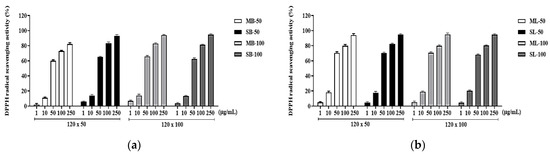
Figure 3.
Inhibitory effects of DPPH radicals of R. davurica buds (a) and leaves (b) according to planting intervals and collecting times. MB, May bud; SB, September bud; ML, May leaf; SL, September leaf. MB/ML/SB/SL-50 and MB/ML/SB/SL-100 refer to the planting intervals. All data are shown as the mean ± SD.
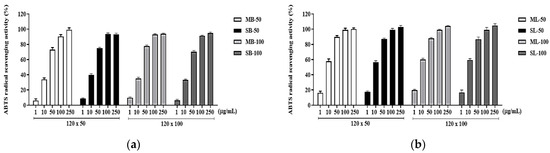
Figure 4.
Inhibitory effects of ABTS radicals of R. davurica buds (a) and leaves (b) according to planting interval and collecting time. MB, May bud; SB, September bud; ML, May leaf; SL, September leaf. MB/ML/SB/SL-50 and MB/ML/SB/SL-100 refer to planting interval. All data are shown as the mean ± SD.

Table 3.
IC50 values of DPPH radicals of R. davurica buds and leaves according to planting interval and collecting time.

Table 4.
IC50 values of the ABTS radicals R. davurica buds and leaves according to planting interval and collecting time.
3.5. SOD Activity
To assess the antioxidant capacity of R. davurica buds and leaves in facilitating the conversion of superoxide anions, a SOD assay was conducted. No significant differences were observed in SOD activity among the bud and leaf extracts harvested in May and September at planting intervals of 120 × 50 cm and 120 × 100 cm (Figure 5). Regardless of planting interval, both the May and September harvests exhibited SOD activity in the 40% range for the buds and the 55–60% range for the leaves.
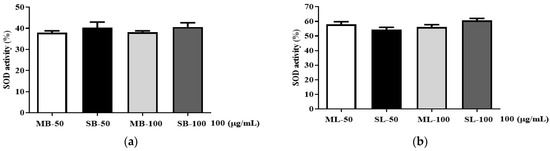
Figure 5.
Effects of planting interval and collecting time on SOD relative activity of Rosa davurica buds (a) and leaves (b). MB, May bud; SB, September bud; ML, May leaf; SL, September leaf. MB/ML/SB/SL-50 and MB/ML/SB/SL-100 refer to planting interval. All data are shown as the mean ± SD.
4. Discussion
R. davurica can be planted all over South Korea, but it thrives on rich, loamy soil. The best planting interval is indicated to be 120 cm row spacing and 100 cm inter-row spacing. According to the South Korean Ministry of Food and Drug Safety, R. davurica can only be ingested in the form of fruit, buds, and petals. Shin et al. discovered that the vitamin content of R. davurica varied based on the portion of the plant taken, with the leaves having the highest concentration [15]. This means that to ensure its best utilization as a food source, the large-scale harvesting of components like buds and leaves is required. However, when R. davurica is planted at the recommended density, bud size reduces dramatically. As a result, this study was carried out to identify whether the production of R. davurica buds and leaves improved depending on the reproductive density (120 × 50 cm, 120 × 100 cm) and harvest date (May and September).
The verification revealed considerable variations in the amounts of buds and leaves at various planting intervals and harvest dates. Regardless of harvest date, decreasing the planting interval from 100 cm to 50 cm boosted bud and leaf yields by more than 100% (Table 1). When harvest date was removed, September showed a considerable increase in yield for both buds and leaves, although statistical significance was not reached. As a result, it is claimed that planting interval influences yield more than harvest date. In order to enhance the cultivation efficiency of R. davurica, a 50 cm planting interval is suggested as a possibly better option than the standard 100 cm spacing. Furthermore, when the ethyl gallate content of R. davurica buds and leaves was evaluated using HPLC, there were no variations in component levels based on planting interval or harvest date (Figure 2). This means that the two previously mentioned parameters have no effect on the composition of R. davurica buds and leaves.
Numerous recent studies have indicated that flavonoid molecules, which are widely distributed in plants, play a variety of physiological roles and have important properties, including antioxidant activity [16]. Furthermore, the phenolic hydroxyl (OH) groups found in phenolic compounds are known to have physiological features such as antioxidant, anticancer, and antibacterial actions via binding to proteins and other materials [17,18]. In general, studies have indicated that as total polyphenol concentration increases, so do physiological activities such as antioxidant properties [19]. The purpose of this study was to determine the changes in the overall flavonoid and polyphenol contents of R. davurica buds and leaves based on planting interval and harvest date (Table 2). According to our comparison of the experimental results, the flavonoid contents of R. davurica bud and leaf extracts had no significant differences based on planting interval and harvest date. Kim et al.’s study observed that the flavonoid content of Turnera diffusa leaves was 108.8 mg QE/g extract [20]. Furthermore, Quyen et al. observed that the polyphenol content of Centella asiatica ranged between 2.14 and 2.82 mg GAE/g extract [21]. In this experiment, the flavonoid and polyphenol contents of R. davurica bud and leaf extracts were greater. These results indicate that, depending on plant interval and harvest date, the R. davurica bud and leaf extracts found in this study contain a rather high quantity of polyphenols. Furthermore, while planting interval had no effect on overall flavonoid and polyphenol concentrations, harvesting in September proved to be more successful overall.
DPPH is exceptionally stable in organic solvents such as ethanol due to its mechanism of proton radical scavenging, making it ideal for measuring antioxidant activity [22]. The extracts prepared from the R. davurica buds and leaves revealed considerably greater activity levels for DPPH free radical scavenging at varied concentrations depending on planting interval and harvesting date (Table 3). The DPPH scavenging activity assay findings revealed very little variation between the leaf and bud extracts obtained in September and May at planting intervals of 120 × 100 cm and 120 × 50 cm, respectively (Figure 3). However, for the samples collected in September, a minor but noticeable enhancement in radical scavenging ability was seen, similar to previous results for total flavonoid and polyphenol levels. Because ABTS oxidizes to the cationic radical form when exposed to oxidants or peroxyl radicals, the antioxidant’s ability to scavenge cationic ABTS was evaluated. The IC50 values for ABTS radical activity revealed only minor changes in scavenging ability between the extracts of leaves and buds taken in September and May, respectively, at planting intervals of 120 × 100 cm and 120 × 50 cm (Table 4, Figure 4). These findings confirm the general conclusion that planting intervals have no effect on R. davurica bud compositional dynamics. The free radical scavenging capacity of the R. davurica bud and leaf extracts appears to vary slightly depending on when the plants are seeded and harvested.
SOD, an important antioxidant enzyme, catalyzes the conversion of superoxide anions, which are constantly formed throughout a variety of cellular metabolic activities, into oxygen and hydrogen peroxide [23]. SOD is present in both plants and mammals. Depending on the metal to which it is connected, it can take several forms, including Cu/Zn SOD, Mn SOD, and Fe SOD [24]. The SOD activity values were identical in every scenario and showed no discernible fluctuations, which was consistent with the prior activity experiment results. As a result, an economic study is required to determine the optimal planting interval and harvest date, as the bioactive components in R. davurica buds and leaves appear unaffected by planting intervals and harvest date.
5. Conclusions
We conducted research on the antioxidant characteristics of R. davurica buds and leaves in order to optimize the planting intervals and harvest dates to boost the output of these plant parts, given their high potential value as food. The acquired results are expected to provide useful insights for optimizing cultivation practices, particularly for the large-scale harvesting of R. davurica buds and leaves. The following are the important findings: When planted at a closer interval of 120 × 50 cm compared to the conventional 120 × 100 cm planting interval, both the R. davurica buds and leaves showed an upward trend in yield. Planting interval and harvest date had no effect on the level of the indicator component, ethyl gallate, in the R. davurica buds and leaves. Our analysis of total polyphenol and flavonoid contents in the R. davurica buds and leaves revealed minor changes in the content of these beneficial chemicals regardless of planting interval and harvest date. The antioxidant capacity study revealed that both the R. davurica buds and leaves had exceptional antioxidant qualities regardless of planting interval or harvest date.
Author Contributions
Conceptualization, S.O.; methodology, S.O.; software, S.Z. and M.K.; validation, S.Z., M.K. and S.O.; formal analysis, S.Z., M.K. and S.O.; investigation, S.O.; resources, S.O.; data curation, S.O.; writing—original draft preparation, M.K.; writing—review and editing, S.O.; visualization, S.Z.; supervision, S.O.; project administration, S.O.; funding acquisition, S.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data relevant to this study are available in this paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ježek, J.; Cooper, K.F.; Strich, R. Reactive Oxygen Species and Mitochondrial Dynamics: The Yin and Yang of Mitochondrial Dysfunction and Cancer Progression. Antioxidants 2018, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Izzo, C.; Vitillo, P.; Di Pietro, P.; Visco, V.; Strianese, A.; Virtuoso, N.; Ciccarelli, M.; Galasso, G.; Carrizzo, A.; Vecchione, C. The Role of Oxidative Stress in Cardiovascular Aging and Cardiovascular Diseases. Life 2021, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Mizobuchi, M.; Ishidoh, K.; Kamemura, N. A comparison of cell death mechanisms of antioxidants, butylated hydroxyanisole and butylated hydroxytoluene. Drug Chem. Toxicol. 2022, 45, 1899–1906. [Google Scholar] [CrossRef] [PubMed]
- Unsal, V.; Dalkıran, T.; Çiçek, M.; Kölükçü, E. The Role of Natural Antioxidants Against Reactive Oxygen Species Produced by Cadmium Toxicity: A Review. Adv. Pharm. Bull. 2020, 10, 184–202. [Google Scholar] [CrossRef] [PubMed]
- Soundararajan, P.; Won, S.Y.; Kim, J.S. Insight on Rosaceae Family with Genome Sequencing and Functional Genomics Perspective. Biomed. Res. Int. 2019, 2019, 7519687. [Google Scholar] [CrossRef]
- Osada, A.; Horikawa, K.; Wakita, Y.; Nakamura, H.; Ukai, M.; Shimura, H.; Jitsuyama, Y.; Suzuki, T. Rosa davurica Pall., a useful Rosa species for functional rose hip production with high content of antioxidants and multiple antioxidant activities in hydrophilic extract. Sci. Hortic. 2022, 291, 110528. [Google Scholar] [CrossRef]
- Razgonova, M.P.; Bazhenova, B.A.; Zabalueva, Y.Y.; Burkhanova, A.G.; Zakharenko, A.M.; Kupriyanov, A.N.; Sabitov, A.S.; Ercisli, S.; Golokhvast, K.S. Rosa davurica Pall., Rosa rugosa Thumb., and Rosa acicularis Lindl. Originating from Far Eastern Russia: Screening of 146 Chemical Constituents in Three Species of the Genus Rosa. Appl. Sci. 2022, 12, 9401. [Google Scholar] [CrossRef]
- Fang, M.; Lee, H.M.; Oh, S.; Zheng, S.; Bellere, A.D.; Kim, M.; Choi, J.; Kim, M.; Yu, D.; Yi, T.H. Rosa davurica inhibits skin photoaging via regulating MAPK/AP-1, NF-κB, and Nrf2/HO-1 signaling in UVB-irradiated HaCaTs. Photochem. Photobiol. Sci. 2022, 21, 2217–2230. [Google Scholar] [CrossRef]
- Lim, S.; Oh, S.; Nguyen, Q.T.N.; Kim, M.; Zheng, S.; Fang, M.; Yi, T.H. Rosa davurica Inhibited Allergic Mediators by Regulating Calcium and Histamine Signaling Pathways. Plants 2023, 12, 1572. [Google Scholar] [CrossRef]
- Moreno, M.I.; Isla, M.I.; Sampietro, A.R.; Vattuone, M.A. Comparison of the free radical-scavenging activity of propolis from several regions of Argentina. J. Ethnopharmacol. 2000, 71, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Folin, O.; Denis, W. On phosphotungstic-phosphomolybdic compounds as color reagents. J. Appl. Biol. Chem. 1912, 12, 239–243. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use or a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Park, Y.A.; Lee, E.J.; Shin, T.Y. Inhibition of immediate-type allergic reaction by Rosa davurica Pall. in a murine model. J. Ethnopharmacol. 1999, 67, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Yeo, J. Bioactivities of Phenolics by Focusing on Suppression of Chronic Diseases: A Review. Int. J. Mol. Sci. 2018, 19, 1573. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.; et al. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2021, 27, 233. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Kim, M.; Ha, L.K.; Oh, S.; Fang, M.; Zheng, S.; Bellere, A.D.; Jeong, J.; Yi, T.H. Antiphotoaging Effects of Damiana (Turnera diffusa) Leaves Extract via Regulation AP-1 and Nrf2/ARE Signaling Pathways. Plants 2022, 11, 1486. [Google Scholar] [CrossRef]
- Quyen, N.T.C.; Quyen, N.T.N.; Quy, N.N.; Quan, P.M. Evaluation of total polyphenol content, total flavonoid content, and antioxidant activity of Centella asiatica. IOP Conf. Ser. Mater. Sci. Eng. 2020, 991, 012020. [Google Scholar] [CrossRef]
- Morteza, J.; Azam, J. Antioxidant potential and DPPH radical scavenging kinetics of water-insoluble flavonoid naringenin in aqueous solution of micelles. Colloids Surf. A Physicochem. Eng. Asp. 2016, 489, 392–399. [Google Scholar]
- Zheng, M.; Liu, Y.; Zhang, G.; Yang, Z.; Xu, W.; Chen, Q. The Applications and Mechanisms of Superoxide Dismutase in Medicine, Food, and Cosmetics. Antioxidants 2023, 12, 1675. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).