Abstract
Bimetallic nanoparticles (BMNPs) combine unique and synergistic properties of two metals, allowing new specific applications. In this study, bimetallic AuFe nanoparticles and their conjugates with methotrexate (MTX) were obtained with an environmentally safe method of metal-vapor synthesis. The composition and electronic structure of the particles were investigated with X-ray photoelectron spectroscopy (XPS), energy dispersive X-ray (EDX) spectrum and X-ray absorption spectroscopy (XANES and EXAFS). The effects of BMNP-MTX conjugates on human primary cells and tumor cell lines were evaluated with neutral red uptake and MTT in vitro cytotoxicity assays. Bright-field microscopy analyses of tumor spheroid size and evaluations of tumor spheroid vitality based on SFDA AM staining were carried out. In vitro assays for an antibacterial activity evaluation of the generated samples were performed. The influence of BMNP-MTX on cytokine production with normal leukocytes was assessed using ELISA. X-ray analyses of the samples demonstrated that gold was in the ground state Au0 as well as Au+ and Au3+ states are present in small quantities, whereas iron existed as a mixture of non-histometric oxides with states close to Fe2+ and Fe3+. The modification of the AuFe system with MTX is accompanied by a threefold increase in the relative proportion of the Au+ state. BMNP-MTX conjugates demonstrated significant antitumor activity compared to the drug alone, which proves the ability of the generated nanoconjugates to improve the effectiveness of MTX therapy. This was confirmed by a marked reduction in the size and vitality of AuFe-MTX-treated 3D tumor spheroids. In addition to their selective antitumor activity, AuFe-MTX exhibited moderate antibacterial activity and induced sample-specific cytokine production with normal human leukocytes—which points to an immunostimulatory potential. The present findings indicate important and diverse biological properties of BMNP-MTX conjugates and thus highlight perspectives for their biomedical applications and new immune-specific abilities.
1. Introduction
Hybrid nanoparticles composed of two different metal species open up wide opportunities for their application due to synergistic effects [1] and the achievement of specific functional properties (biological, catalytic, optical, thermal, magnetic and other characteristics). Bimetallic nanoparticles (BMNPs) are successfully used in various fields, such as catalysis, wastewater treatment, agriculture and others [2,3,4], and are also being investigated as potential antimicrobial agents and antitumor agents in the field of biomedicine [5,6]. The ability of such nanomaterials to interact with cells (specifically cancer cells and inflamed tissues), their wide availability and good biocompatibility combined with specific surface characteristics and abilities for diverse functionalization makes them promising tools for the creation of new drugs, improvement of standard drug’s effectiveness and application in clinical practice [7]. They could serve as convenient drug carriers and, in addition, could exhibit therapeutic potential depending on their specific composition and properties and thus enhance current therapeutic strategies and contribute to the development of new ones. A number of studies have already demonstrated the ability of BMNPs to serve as drug delivery systems, photothermal ablation and magnetic hyperthermia agents [5]. They also serve as versatile tools for diagnostical bioimaging [6], theranostics [5], immunotherapy [8] and the generation of improved and more sophisticated biosensors [6].
Bimetallic nanosystems containing iron are among the most studied objects whose properties can be regulated by a modification with other metals [9]. Heterometallic nanoparticles containing gold and iron demonstrate improved optical and magnetic properties [10] and hence improve their efficient application in many fields [11], including medicine [5,12]. The introduction of gold into iron oxide nanoparticles does not reduce the magnetic characteristics of the system and allows the use of external magnetic influence to control the AuFe system as a whole [5,13]. Remarkable magnetic properties have been demonstrated for AuFe alloy nanoparticles, which makes them promising candidates for application as contrast agents in computed tomography (CT) and magnetic resonance imaging (MRI) diagnostic techniques [13]. Recent studies have proven the benefits of AuFe BMNPs and their potential use in magnetic guidance and magnetic hyperthermia therapy [14,15]. Another important property of this type of bimetallic nanomaterials is their ability for facilitated clearance from the body. Torresan et al. have shown self-degradation of AuFe BMNPs over time and a significant reduction of their long-term accumulation in vivo compared to monometallic gold and iron oxide NPs, thus proving their utility as safe multifunctional nanomedicines [12]. In addition, iron oxide nanoparticles have been shown to possess intrinsic catalytic activity by mimicking natural innate peroxidases [16]. Such activity contributes and expands their biomedical application and could even supplement the properties and therapeutic application of bimetallic nanosystems that contain them. Furthermore, the facilitated functionalization of AuFe BMNPs was reported by different research groups [15,17]. These findings were recently supplemented with studies showing the use of Au-iron oxide magnetic NPs for the development of self-assembly systems that serve as nanoplatforms for biologically active molecules, such as oxytocin and insulin, that are paving the way for new sustainable green methods for BMNP functionalization by improving their further application as drug carriers [18].
The structure and electronic state of AuFe nanoparticles vary significantly depending on the methods used for their synthesis [19,20], which makes it possible to control the properties of the synthesized systems. This stimulates the development of new methods for the synthesis of AuFe systems and a comprehensive study of their physicochemical properties. A potential candidate for being an efficient method of the production of BMNPs is metal-vapor synthesis (MVS). It enables the formation of bimetallic nanocomplexes “in situ” under a vacuum or an inert atmosphere in the presence of a stabilizing organic solvent, even for highly reactive systems such as iron NPs, to obtain bimetallic materials whose synthesis is impossible or difficult to implement using traditional methods, due to the multistep process and the high cost of metal complex precursors [21]. This method can be carried out in a technologically closed cycle and is environmentally friendly.
Classical chemotherapeutics are associated with detrimental influences on normal tissues, resulting in various side effects. Other common disadvantages limiting the effectiveness of some chemotherapeutic agents are low solubility and a short half-life. A typical example is methotrexate (MTX)—an antitumor and anti-inflammatory drug applied in the treatment of certain tumors, rheumatoid arthritis and other inflammatory disorders. Renal excretion of administered MTX has been shown to account for up to 90% of the drug dose [22]. Therefore, the conjugation of BMNPs with MTX has been suggested to reduce minimum effective dosages, allow specific drug targeting to eliminate adverse effects, improve the drug bioavailability and half-life and avoid the need for repetitive treatment. The efficacy of gold–iron alloy nanoparticles in hyperthermia-controlled MTX release has been demonstrated [23], as well as the potential of gold–iron oxide magnetic nanoparticles for specific targeting of doxorubicin to cancer cells and hyperthermia treatment [24], including the application of theranostics in oral cancer [15].
In this study, for the first time, bimetallic AuFe nanoparticles and their conjugates with MTX were effectively obtained with an environmentally safe metal-vapor synthesis method, and their potential for biomedical applications was proven. Our experiments included detailed physicochemical evaluations of the obtained bimetallic drug nanocarriers and multilateral analyses of BMNP-MTX biological activities encompassing antitumor, antibacterial and immunological properties.
2. Materials and Methods
2.1. Materials
Acetone (>99.5%) was dried and distilled over zeolites in an atmosphere of purified argon. Toluene (99.8%) was dried and distilled over Na in an atmosphere of purified argon. Before synthesis, the solvent was degassed with alternating freeze–thaw cycles. Our materials also included metals—gold foil (99.99%); iron foil (99.9%); tungsten rod (ø 2.0 mm, 99.8%)—and methotrexate (4-Amino-N10-methylpteroyl-L-glutamic acid; Sigma-Aldrich RTC, Laramie, WY, USA).
2.2. Synthesis of Metal Nanoparticles
AuFe nanoparticles were obtained with metal-vapor synthesis (MVS), the scheme of which is shown in Figure 1. The MVS method makes it possible to obtain metal nanoparticles using previously described techniques [25]. Experiments on the production of bimetallic nanomaterials AuFe were performed in situ in a vacuum or an inert atmosphere, since Fe nanoparticles are very active and easily interact with traces of moisture and oxygen in an organic reagent. The nature of the organic reagent used in the MVS affects the size and composition of the synthesized particles, as well as the electronic state of the metal and the biological activity of the system as a whole [26,27,28]. Acetone (Ac) and toluene (Tol) were used as organic reagents in the synthesis of bimetallic nanoparticles.

Figure 1.
Scheme of synthesis of bimetallic nanoparticles modified with methotrexate.
In the experiment, Au and Fe were simultaneously evaporated with a total mass of about 0.3 g and 130–150 mL of organic solvent. Acetone was dried and distilled over zeolites in an atmosphere of purified Ar, and toluene was dried over sodium and then degassed in a vacuum with alternating freeze–thaw cycles. The joint condensation of metal vapors and organic reagent was carried out in a quartz 5 L reactor. Metal vapors were obtained by resistive heating of a tungsten evaporator (rod ø 2 mm) containing Au and Fe foils. The joint condensation of metal vapors with acetone or toluene was carried out in a vacuum of 10−2 Pa on the reactor walls that were cooled with liquid nitrogen. The feed rate of the organic reagent was controlled with a fine-tuning tap. After the synthesis was completed, the cooling was removed, the co-condensate matrix was heated to room temperature and the reactor was filled with argon. The resulting organosol of metals in an organic reagent in situ was siphoned from the reactor into a vacuum flask containing 30 mg of methotrexate (MTX) and 10 mL of the absolute solvent used in the synthesis. The contents of the flask were stirred for 2 h at a temperature of 40 °C, after which the organic reagent was removed in a vacuum of 10−1 Pa.
2.3. Energy Dispersive X-ray Studies
The energy dispersive X-ray spectrum (EDX) characterizes the elemental composition of the samples. EDX studies were carried out using a QUANTAS 75 spectrometer, Bruker (Billerica, MA, USA).
2.4. Small-Angle X-ray Scattering and X-ray Photoelectron Spectroscopy
Small-angle X-ray scattering (SAXS) is an effective method for obtaining information about the size distribution of nanoparticles. X-ray photoelectron spectroscopy (XPS) can determine the characteristics of different chemical states of elements on the surface of a material. SAXS and XPS measurements and processing of experimental curves were carried out according to the method described in the paper by [29].
2.5. Cell Types and In Vitro Culture Conditions
Four human cell lines were used to assess the cytotoxic and antitumor properties of BMNPs and BMNP-MTX samples in vitro—lung adenocarcinoma A549 (ATCC CCL-185™), HeLa derived from cervical adenocarcinoma (ATCC CCL-2™), HT-29 colorectal adenocarcinoma (ATCC HTB-38) and normal fetal foreskin fibroblast cells denoted as HFFC (CLS Cell Lines Service GmbH, Eppelheim, Germany). Adenocarcinoma cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM), supplemented with 10% fetal bovine serum (FBS), 100 µg/mL streptomycin and 100 IU penicillin (all from Merck KGaA, Darmstadt, Germany) and designated as complete DMEM. HFFC cells were cultured in DMEM containing 15% FBS and the same amount of antibiotics. Prior to the experiments, all cell types were grown in 75 cm2 culture dishes (TPP, Trasadingen, Switzerland) until reaching 80% confluency. The cultures were maintained under standard conditions (37 °C, high humidity and a mixture of 5% CO2/95% atmospheric air in the gas phase).
2.6. Evaluation of Cytotoxicity and Antitumor Activity
2.6.1. MTT and Neutral Red Assays
The MTT assay was used to assess the metabolic activity of cells treated with BMNPs or BMNP-MTX conjugates based on the reduction of 3-(4,5-dimethylthiazol-2-yl)-2,4-diphenyl tetrazolium bromide (MTT) in a cellular mitochondria [30]. The neutral red uptake (NRU) assay was performed to determine the extent of cell viability with the measurement of the amount of neutral red (NR) accumulated in the lysosomes of the living functional cells [31]. Prior to both assay types, cell suspensions with a concentration of 1 × 105 cells/mL were seeded on 96-well plates (TPP, Trasadingen, Switzerland) (100 μL/well) and cultured under standard conditions for 24 h. The cells were then treated with different concentrations of nanoparticles and BMNP-MTX conjugates—10, 50, 100 and 200 μg/mL) for 24, 48, 72 and 96 h. For this aim, 5 mg/mL nanomaterials were suspended in Dulbecco’s phosphate-buffered saline (DPBS) and sonicated for 300 s. To achieve the test-concentrations, BMNPs stock suspensions were diluted in a cell culture medium. MTX served as a positive control for all in vitro cytotoxicity tests. The compound was assayed in the same concentrations as the nanoparticles. In addition, a control of untreated cells cultured for the same time period in a standard growth medium was analyzed in all experiments. All samples were analyzed in triplicates.
At the end of every test period (24, 48, 72, 96 h), MTT (Merck KGaA, Darmstadt, Germany) or NR (Merck KGaA, Darmstadt, Germany) solution was added to the cell culture medium to reach a final concentration of 0.5 mg/mL for MTT and 0.005 mg/mL for the NRU assays. The plates were incubated for 2 h in the dark at 37 °C, 5% CO2 and high humidity. After that, the culture medium was removed, and the cells were washed with DPBS. Formazan accumulated in MTT-treated cells was solubilized using 100 μL/well dimethyl sulfoxide (DMSO, Merck KGaA, Darmstadt, Germany). The plates were incubated at room temperature under continuous mild shaking for 15 min. Absorbance was then measured at 570 nm using a SpectraMax i3x spectrophotometer (Molecular Devices, San Jose, CA, USA). For the NR assays, after washing with DPBS 100 μL/well, aqueous solutions of ethanol and glacial acetic acid (50%:1%) were pipetted to the plates in order to extract the dye accumulated in vital cells. The culture vessels were incubated for 15 min at room temperature with continuous gentle shaking, which was followed by a measurement of absorbance at 540 nm using a SpectraMax i3x spectrophotometer (Molecular Devices, San Jose, CA, USA). The obtained data were presented as percent inhibition of cell viability and/or metabolic activity, which was determined based on absorbance units from treated cells and cells cultured under standard conditions without the addition of an extract. The concentration of the test sample inhibiting the viability/metabolic activity of 50% of the cells (IC50) was determined at 72 h.
2.6.2. Experiments with Tumor Spheroids
Tumor spheroids were formed with HT-29 cells. A total of 200 μL culture medium containing 5000 cells/well were seeded in a 96-well round-bottom plate (Corning Inc., Glendale, AZ, USA) treated with a polymer for ultra-low cell attachment. In 24 h after the formation of 3D spheroids, 100 μg/mL BMNPs or BMNP-MTX samples were added to the culture wells and the multicellular aggregates were treated for a total period of 96 h. Spheroids cultured for the same test period in complete DMEM served as an untreated control. Mean spheroid diameter was defined at 24 and 96 h of culture after the addition of the test sample. Measurements of ten spheroids were performed for each sample using an Inverso microscope (Medline Scientific, Chalgrove, Oxon, UK), Si-3000 high-resolution digital camera and XLiCap software (Medline Scientific, Chalgrove, Oxon, UK).
To evaluate the viability of spheroid-forming HT-29 cells, staining with 5-carboxyfluorescein diacetate, acetoxymethyl ester (5-CFDA AM) (Invitrogen, Molecular probesTM, Eugene, OR, USA) was performed. This reagent penetrates the cell membrane, and active esterases of viable cells convert it to a fluorescent probe (5-carboxyfluorescein) that is retained in the cytoplasm. 5-CFDA AM was added to the culture medium of the control spheroids and spheroids treated for 96 h with BMNPs and BMNP-MTX to a final concentration of 2.7 μg/mL. The samples were incubated at 37 °C in the dark for 30 min. Then, the culture medium containing 5-CFDA AM was replaced with DPBS and the spheroid staining was analyzed with fluorescence microscopy (ZEISS Axiovert 5, Carl Zeiss Microscopy GmbH, Oberkochen, Germany).
2.7. Antibacterial Activity Assays
To analyze the antibacterial activity of AuFe and AuFe-MTX samples, experiments with one Gram-negative/Escherichia coli (ATCC 25922)/and one Gram-positive species/Bacillus cereus (ATCC 11778)/were carried out. The bacteria were cultured in a Mueller–Hinton medium (agar or broth) (Merck KGaA, Darmstadt, Germany) at 37 °C.
2.7.1. Agar Diffusion Tests
To perform agar diffusion assays, 50 μL of bacterial suspension (1 × 106 colony-forming units (cfu)/mL) were inoculated onto nutrient agar. Then, 20 µL containing 200 μg of sample (AuFe NPs, AuFe-MTX) was pipetted on sterile paper discs (6 mm diameter) and placed in different areas of the inoculated culture vessel. A 20 µL buffer solution containing 20 IU penicillin and 20 µg streptomycin was used as a positive control for antibacterial activity. The culture dishes inoculated with E. coli or B. cereus were incubated with test samples for 24 h at 37 °C. After that, the diameter of the areas with inhibited bacterial growth was measured. During these assays, all samples were analyzed in duplicates.
2.7.2. Determination of Minimal Inhibitory and Bactericidal Concentrations
The minimal inhibitory concentration (MIC) of the nanomaterials was determined with the plate microdilution method [32]. Twofold serial dilutions were prepared from 10 mg/mL stock solutions of all samples. Ceftriaxone (MIP Pharma GmbH, Blieskastel-Niederwurzbach, Germany) was assayed in the same concentrations as the nanomaterials served as an antibiotic control. Furthermore, 100 μL of the resulting diluted samples were pipetted on a 96-well plate. Then, 100 μL of bacterial suspension was added in a final concentration of 1 × 106 cfu/mL. Hence, the highest assayed concentration of the test sample was 5 mg/mL. The following controls were used in the assays: 200 μL broth containing only 100 μL test sample and 100 μL culture medium; 200 μL broth containing 100 μL culture medium and 100 μL bacteria. The cultures were incubated for 24 h at 37 °C, and then a 20 μL/well 5 mg/mL MTT solution was added to the plates. Metabolically active cells are able to reduce the yellow tetrazolium salt MTT to a purple-colored formazan product. Therefore, addition of MTT to the samples allows to determine cellular viability and metabolic activity. The culture plates were incubated for 1 h at 37 °C, after which absorbance was measured at 570 nm using a SpectraMax i3x spectrophotometer (Molecular Devices, San Jose, CA, USA).
To determine the minimum bactericidal concentration (MBC), 50 μL of the samples, determined as the minimal inhibitory concentration, was inoculated into two separate Petri dishes containing antibiotic-free nutrient agar. Similarly, one or two samples corresponding to concentrations higher than the MIC and one concentration lower than the MIC were also tested. The culture dishes were incubated for 24 h at 37 °C. MBC was defined as the concentration of the test sample at which no bacterial colonies were observed.
2.7.3. Longitudinal Assessment of Bacterial Viability
In addition, the longitudinal effects of the nanomaterials on the bacterial cultures’ viability were estimated at three different time points during culture—12, 24 and 36 h. Then, 3 mL bacterial cultures with a concentration of 1 × 106 cfu/mL were seeded and incubated at 37 °C for 36 h. For each bacterial species (E. coli or B. cereus), four samples were established. These were untreated control in a standard liquid growth medium, a culture treated with 200 μg/mL AuFe nanoparticles, bacteria cultured in the presence of 200 μg/mL AuFe(Ac)-MTX conjugates (nanomaterials produced in acetone as the organic dispersion medium) and a sample treated with 200 μg/mL AuFe(Tol)-MTX conjugates (nanomaterials produced in toluene as the organic dispersion medium). All samples were assayed in triplicates. During the culture period, 100 μL samples were collected at 12 and 24 h, as well as at the end of the experiment (36 h), in order to assess the relative level of viable bacteria. The samples were pipetted onto a 96-well culture plate (Costar, Corning Inc., New York, NY, USA). MTT solution (Merck KgaA, Darmstadt, Germany) was added to each sample well reaching a final concentration of 0.5 mg/mL. The plates were incubated for 1 h at 37 °C, and then absorbance at 570 nm was measured using a SpectraMax i3x spectrophotometer (Molecular Devices, San Jose, CA, USA).
2.8. Ex Vivo Culture of Leukocytes and Measurement of Cytokine Production
Leukocytes were isolated from venous blood collected in BD Vacutainer® K2EDTA tubes (Becton, Dickinson and Company (BD), Franklin Lakes, NJ, USA) from 3 healthy volunteers (2 men and 1 woman, age range: 30–49 years). Standard hematological parameters were analyzed and confirmed to be in the reference range. The blood samples were centrifuged at 1000× g for 15 min, and then the plasma was discarded. The erythrocytes were lysed with a 0.84% NH4Cl buffer for 8 to 10 min, and the samples were washed twice with DPBS. The isolated leukocytes were pooled and centrifuged at 1000 rpm for 10 min. The resulting cell pellet was resuspended in complete DMEM. The cell suspension was divided into three aliquots for a triplicate experiment. The cells were seeded on a 12-well culture plate (TPP, Trasadingen, Switzerland) at 1 × 106 cells/mL and treated for 24 h with 100 μg/mL nanoparticles or BMNP-MTX conjugates. A triplicate control cultured in complete DMEM without a test sample was also set. At the end of the experiment, 100 μL culture media were collected from all samples. The concentrations of interleukin-2 (IL-2) and interferon-γ (IFN-γ) were determined in the collected samples with an enzyme-linked immunosorbent assay (ELISA). For this aim, human IL-2 and IFN-γ ELISA kits (eBiosciencesTM, Thermo Fisher Scientific Inc., Waltham, MA, USA) were used, and the assays were performed according to the manufacturer’s instructions.
2.9. Statistics
StatView software (version 5.0) (SAS Institute, Cary, NC, USA) was used to apply analysis of variance (ANOVA). Statistically significant differences between the analyzed samples were determined using Fisher’s PLSD test. p values lower than 0.05 were regarded as statistically significant.
3. Results
3.1. Antitumor Activity of AuFe-MTX Conjugates
The results from the biological activity analyses include AuFe-MTX obtained with MVS using an acetone dispersion medium (denoted in the figures as AuFe(Ac)-MTX), AuFe-MTX conjugate produced using a toluene dispersion medium (marked in the figures as AuFe(Tol)-MTX) and AuFe nanoparticles (NPs) generated in a toluene organic dispersion medium. Only one BMNP sample was included in the assays and served as a representative unconjugated control. Our previous studies have indicated that NPs obtained with MVS using an acetone or toluene dispersion medium do not significantly differ in their biological activity [33].
Standard in vitro cytotoxicity assays based on the cellular ability for reducing the MTT or NR uptake were performed with three adenocarcinoma cell lines and normal human fibroblasts. Their purpose was to assess the antitumor potential of BMNP conjugates. All cell types tested showed time-dependent and concentration-dependent responses against the treatment with AuFe-MTX conjugates (Figure 2, Table 1). Normal fibroblasts were the least affected by the addition of BMNP-MTX to the culture media, which highlights an antitumor potential of the tested samples. The level of inhibition detected in HFFC cultures did not exceed 25% at the highest tested concentrations of the samples. Furthermore, these negative effects were measured in the early test periods—24 h and 48 h—which indicate the ability of the cells to overcome the cytotoxic influence of BMNP-MTX and highlight the good biocompatibility potential of the samples (Figure 2g,h). The opposite trend is clear from the results obtained with adenocarcinoma cells. More than 50% inhibition of A549 and HeLa tumor cell metabolic activity was detected after 72 h of treatment with nanoconjugates (Table 1). The calculated IC50 concentrations were higher than MTX applied alone, which could be attributed to the potential gradual mode of MTX release by the conjugates. An important activity of BMNP-MTX samples detected with both MTT and NR assays was their higher inhibitory potential when they were applied at low concentrations (Figure 2). Interestingly, a BMNP-MTX concentration of 10 μg/mL induced a significantly higher inhibition of tumor cell metabolic activity and vitality compared to MTX (Figure 2a–f). These effects gradually increased in magnitude during the experiment, reaching a maximum at the 72 or 96 h mark. The HT-29 cell line showed the lowest sensitivity towards the BMNP-MTX treatment among the tested tumor cell lines. In general, AuFe NPs did not show negative effects at low concentrations, except for the longest test period (96 h) with A549 cells (Figure 2a). Thus, the inhibition induced with AuFe-MTX conjugates could be attributed to the effective targeting of the drug and its possible synergistic effects.
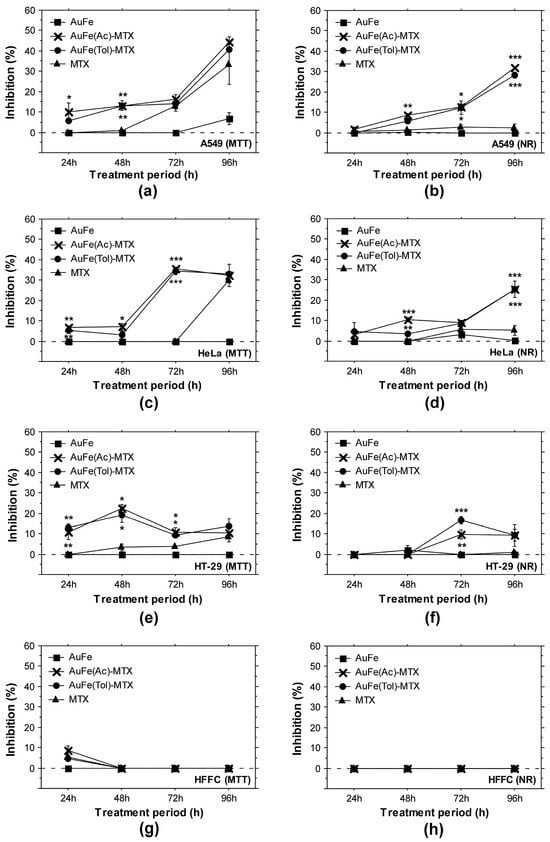
Figure 2.
Longitudinal in vitro cytotoxicity and antitumor potential of BMNPs and BMNP-MTX samples. Data represent ± standard error of the mean (±SEM). All graphs display results for A549 (a,b), HeLa (c,d), HT-29 (e,f) and fibroblast (g,h) cells treated with 10 μg/mL AuFe-MTX conjugates, AuFe NPs or methotrexate (MTX). (a,c,e,g) MTT assay results; (b,d,f,h) NRU assay data. The asterisk sign indicates statistically significant difference between the test sample and MTX control. *, p < 0.05; **, p < 0.01; ***, p < 0.001.

Table 1.
Inhibiting concentration 50% (IC50) measured with MTT assays after 72 h treatment with BMNP-MTX.
MTT and NRU assay analyses were followed by experiments with tumor spheroids formed with HT-29 cells. The aim of these studies was to confirm the detected antitumor potential and prove the efficacy of BMNP-MTX on the level of multicellular tumor structure. HT-29 cells were seeded on polymer-treated ultra-low attachment culture plates. After 24 h, the formed spheroids were treated with 100 μg/mL AuFe and AuFe-MTX conjugates for 96 h. The diameter of the tumor cell aggregates was measured after treatment at two time points—24 h and 96 h. In addition, at the end of the experiment, cellular viability was examined using 5-carboxyfluorescein diacetate, acetoxymethyl ester (5-CFDA AM)—a membrane-permeable reagent converted by cellular esterases to a fluorescent probe (5-carboxyfluorescein) which is retained only in viable cells [34]. The obtained results confirmed the inhibitory effects determined by the studies with monolayer cultures but also emphasized a stronger antitumor potential for AuFe(Ac)-MTX (Figure 3). This sample significantly reduced the spheroid size and vitality of HT-29 in comparison to the untreated spheroids and even when compared to AuFe(Tol)-MTX-treated multicellular tumor structures (Figure 3a–c). The AuFe(Tol)-MTX sample showed to be effective in monolayer tumor cell cultures but was unable to inhibit the development and vitality of the preformed 3D tumor aggregates. This may be due to the limited ability of AuFe(Tol)-MTX to penetrate multicellular tumor structures; however, additional experiments are needed to prove this hypothesis.

Figure 3.
BMNP-MTX treatment effects on tumor spheroids. Mean spheroid diameter after treatment with 100 μg/mL BMNP-MTX for 24 h (a) and 96 h (b). The results represent ±SEM for 10 spheroids per test sample. Picture (c) shows spheroid treated for 96 h with AuFe(Ac)-MTX; (d) spheroid cultured for 96 h in medium containing AuFe(Tol)-MTX; (e) spheroid treated for 96 h with AuFe NPs; (f) untreated spheroid (control). The black bar indicates 100 μm. Asterisks mark statistically significant differences between the test sample and untreated control spheroids—*, p < 0.05.
3.2. Antibacterial Activity of BMNP-MTX
Agar diffusion and microdilution assays indicated moderate inhibitory activity of BMNP-MTX against Gram-negative and Gram-positive bacteria which was higher compared to AuFe NPs (Table 2). The mean size of the measured inhibition zones indicated that B. cereus cultures were more sensitive to the BMNP-MTX treatment. Again, AuFe(Ac)-MTX demonstrated superior properties and the highest activity among the tested samples.

Table 2.
Minimal inhibiting concentrations (mg/mL), minimal bactericidal concentrations (mg/mL) and inhibition zone (mm) determined after 24 h treatment of bacterial cultures.
To longitudinally characterize the ability of the AuFe-MTX samples to influence bacterial growth and vitality, we performed suspension culture experiments and analyzed cells treated with 200 μg/mL nanomaterials at different time points (12, 24 and 36 h). Bacterial culture viability was analyzed based on the samples’ ability to metabolize tetrazoulim salt MTT. The obtained results demonstrated the potential to slightly reduce the viability of both bacterial types (Gram-positive and Gram-negative) for 12 h and 24 h cultures (Figure 4). The higher inhibition effect against B. cereus was confirmed by these experiments, and a significant reduction in cellular vitality was observed after a 12 h and 24 h treatment with AuFe(Ac)-MTX.
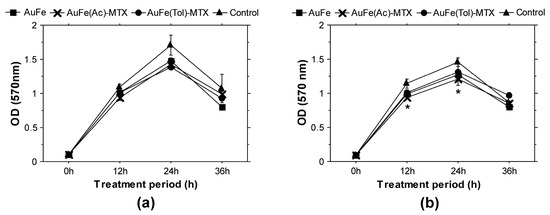
Figure 4.
Longitudinal evaluations of the effects of BMNP-MTX on bacterial growth and viability. (a) Gram-negative bacteria (E. coli); (b) Gram-positive bacteria (B. cereus). All samples were analyzed in triplicates. The graphs display mean results (±SEM). *, p < 0.05.
3.3. BMNP-MTX Conjugates Affect Cytokine Production by Peripheral Blood Leukocytes
To investigate the potential influence of BMNP-MTX on cytokine production, isolated normal white blood cells were treated ex vivo with 100 μg/mL AuFe-MTX conjugates for 24 h. After that, the levels of secretion in the culture media interleukin-2 and interferon-γ were analyzed with ELISA. The results from these experiments are shown in Figure 5. Interestingly, a 24 h treatment of leukocytes with AuFe(Ac)-MTX resulted in a significantly increased production of IL-2 (Figure 5a). In addition, all samples—the two nanoconjugates and AuFe—activated the release of IFN-γ via ex vivo-treated white blood cells (Figure 5b). The treatment with AuFe(Tol)-MTX led to more than a threefold increase in IFN-γ levels in the culture media. These data clearly indicate the immune-specific activity of BMNPs and BMNP-MTX conjugates. Future experiments will elucidate the role of these effects in detail.

Figure 5.
IL-2 (a) and IFN-γ (b) production with peripheral blood leukocytes treated ex vivo with 100 μg/mL BMNP-MTX. Asterisks indicate statistically significant differences between the test sample and the untreated control. ***, p < 0.001.
3.4. Physicochemical Analyses
Biological evaluations highlighted the AuFe-MTX sample generated in the acetone organic dispersion medium as a nanocarrier with better properties compared to AuFe(Tol)-MTX. Therefore, the physicochemical evaluations were concentrated on this sample. Figure 6 shows the EDX spectra of bimetallic AuFe nanoparticles obtained in the reaction with acetone and their conjugates with MTX. Along with the presence of metals in the AuFe system, a significant amount of carbon material has been registered, which may indicate the chemisorption of acetone or its fragments on the metal surface.
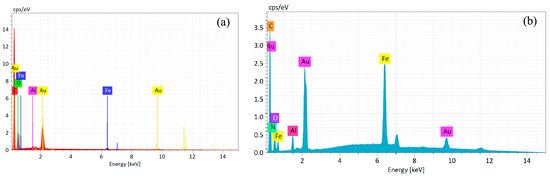
Figure 6.
Energy dispersive X-ray spectra of AuFe (a) and AuFe-MTX (b).
The composition of the surface plays a key role in the biological activity, catalysis and other properties of metal nanoparticles. AuFe nanoparticles and their conjugates with MTX were studied with X-ray photoelectron spectroscopy, which is the leading analytical method used for the characterization of various chemical/physical forms of elements on the surface of materials. The relative atomic concentrations of the elements were determined from the survey spectra of the samples (see Supplementary Materials, Figure S1) (Table 3).

Table 3.
X-ray photoelectron spectroscopy (XPS) quantification data (at. %) of samples comprising MTX, AuFe and AuFe-MTX.
The Au 4f photoelectron spectra of the AuFe (1) and AuFe-MTX (2) samples, shown in Figure 7, are described by three spin-orbit doublets 4f7/2-4f5/2 preserving the 4f7/2/4f5/2 branching ratio of 4/3. The characteristics of the photoelectronic peaks and their interpretations are presented in Table 4.
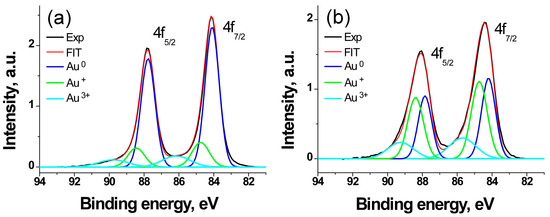
Figure 7.
The Au 4f photoelectron spectra of samples AuFe (a) and AuFe-MTX (b).

Table 4.
Characteristics of photoelectronic spectra Au 4f (Eb—binding energy; W—peak width; Irel—relative intensity).
The binding energy of the Au 4f7/2 peak in the spectrum of AuFe nanoparticles equal to 84.1 eV is 0.1 eV higher than the value for pure Au and can be attributed to the formation of a Au-Fe bond. The value of 84.8 eV corresponds to the Au+ state. As for the binding energy of 86.2 eV, it corresponds to the interaction of gold atoms with hydroxyl groups of the shell with the formation of the Au3+ state [35].
The binding energies corresponding to the Au0 and Au+ states in the Au 4f spectrum of the bimetal sample with MTX are 0.1 eV higher than in the AuFe, whereas it is 0.5 eV higher for the Au3+ state, which (by analogy with the work by Brown et al. [35]) should be attributed to the interaction of gold atoms with the carboxyl groups of metatrexate. This leads both to an increase in the relative fraction of this state and to an increase in the binding energy of the corresponding doublet. The interaction of MTX with AuFe is accompanied by a threefold increase in the relative proportion of the Au+ state. In other words, MTX acts as an oxidizer of gold.
The Fe 2p spectra of the AuFe and AuFe-MTX samples (Figure 8) include Fe 2p3/2-Fe 2p1/2 doublets with binding energies of 710.8–724.3 and 710.5–724.1 eV, respectively, and a satellite (sat) at ~718 eV. The latter is a fingerprint of the Fe3+ state. The given energies also correspond to the Fe3+ state. However, the difference in the binding energies of Fe 2p3/2 and Fe 2p3/2-Fe 2p1/2 spin-orbit splitting indicate a change in the electron density on the iron atoms after their interaction with MTX, which is also accompanied by a decrease in FWHM from 4.65 to 4.15 eV.
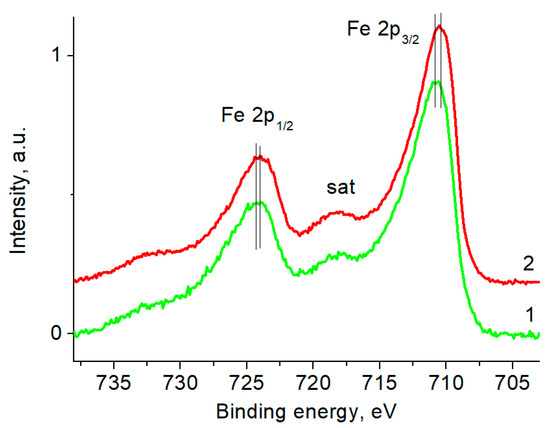
Figure 8.
The Fe 2p photoelectron spectra of samples AuFe (1) and AuFe-MTX (2).
The photoelectron spectra of Au 4f and Fe 2p indicate that the introduction of metatrexate into the AuFe changes the charge states of the Au atoms to a greater extent than those of the Fe atoms. In the Au 4f spectrum, a significant increase in the proportion of Au+ and Au3+ states is observed, while there is a slight decrease in the signal in the Fe 2p spectrum from the satellite peak and a slight shift of the Fe 2p peak to a low-energy region, indicating an increase in the proportion of the Fe2+ state. This is manifested in the change in the Fe 2p/Au 4f signal intensity ratio, which indicates the segregation of gold atoms to the surface of the conjugate. Since the sampling depth of the Fe 2p photoelectrons is less than that of the Au 4f photoelectrons, this leads to a noticeable decrease in the Fe 2p’s signal intensity. Nevertheless, the bulk content remains unchanged.
Figure 9 shows the C 1s spectra of MTX, AuFe and AuFe-MTX samples. An important fact is that it reflects the practical disappearance of the signal at about 283.5 eV, which is characteristic of the metal-carbon bond or low-molar-mass C-C/C-H fragments [36], after the interaction of MTX with AuFe.
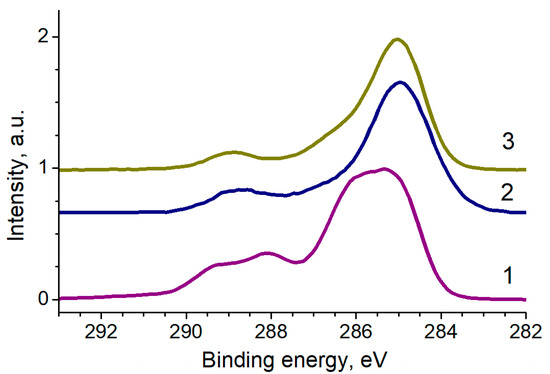
Figure 9.
The C 1s photoelectron spectra of samples MTX (1), AuFe (2) and AuFe-MTX (3), normalized by the intensity of the main peak.
Figure 10 shows the N 1s spectra approximated by three Gaussian peaks, the characteristics of which are provided in Table 5.
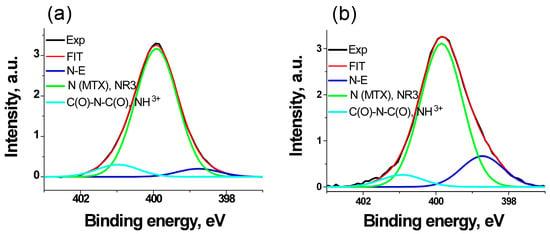
Figure 10.
The N 1s photoelectron spectra of samples MTX (a) and AuFe-MTX (b).

Table 5.
Characteristics of photoelectronic spectra N 1s (Eb—binding energy; W—peak width; Irel—relative intensity).
The peak at 399.9 eV is a characteristic of MTX. The binding energy of 398.8 eV is close to that of pyridine and may also be attributed to the metal–nitrogen bond. However, due to the absence of such bonds in MTX, it can be attributed to intermolecular interactions. At the same time, when interacting with active iron black, the relative intensity of this peak increased approximately three times, and only 1.5 times with less active gold black [29]. In the AuFe-MTX sample, the relative intensity of this peak is the same as in the spectrum of the Fe-MTX sample [29]; therefore, it may be preferentially assigned to the Fe-N bond. The peak at ~401 eV occupies an intermediate position between the values of 400.6 and 401.46 eV, which is characteristic of the C(O)-N-C(O) and NH3+ groups [37] and, apparently, can be attributed to the intermolecular interactions with the formation of hydrogen bonds between the oxygen atoms of the C(O)N groups and the hydrogen atoms of the neighboring molecule.
The size distribution of the AuFe nanoparticles and their conjugates with MTX was analyzed using small-angle X-ray scattering. The experimental scattering profiles from the bimetallic nanoparticles, their conjugates with MTX and their distribution functions DV(R) of the scattering particles (obtained with volume dimensions in the acetone medium) are presented in Figure 11.
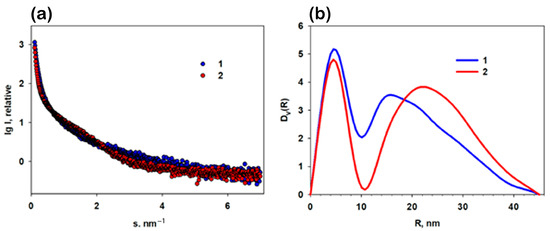
Figure 11.
Small-angle X-ray scattering (SAXS) patterns from the AuFe nanoparticles obtained in acetone medium along with those from their conjugates AuFe-MVX: (a) experimental SAXS curves from AuFe (1) and AuFe-MTX (2). Panel of (b) the volume size distribution functions DV(R) calculated from AuFe (1) and AuFe-MTX (2).
The analysis of the experimental SAXS curves showed that they correspond to poly-disperse systems. The initial sections of the SAXS patterns show an upward trend, which indicates the presence of large aggregates in the samples. In the experimental curves, there is practically no scattering from the spherical shape of nanoparticles (form factor). However, the SAXS curves from AuFe show a weak presence of a form factor, possibly from individual particles and/or their small aggregates, in contrast to the curve describing AuFe-MTX (Figure 11a, curve 2).
The distribution functions DV(R) calculated with the method from [38] are shown in Figure 11b. The functions are bimodal. It was found that the bimetallic samples are characterized by the presence of highly homogeneous populations of individual NPs along with a wide fraction of large aggregates. Fe metal particles contain both small particles with an average radius of about 4.6 nm and aggregates with radii up to 45 nm. In the presence of MTX, the number of aggregates increases, and the proportion of small conjugate nanoparticles becomes more pronounced. Apparently, the introduction of MTX leads to an increased interaction between the nanoparticles and, as a consequence, to the formation of a larger number of large aggregates in conjugates.
Additional information about the electronic state and local environment of gold and iron atoms in composites was obtained using XANES (X-ray absorption near the edge of the structure) and EXAFS (extended X-ray absorption of a fine structure), absorption at the K-edge of iron absorption and at the L3-edge of gold absorption (see Supplementary Materials, Methods).
Comprehensive studies have shown that the environment of iron atoms is similar to the structure of Fe3O4, and the spectra of gold in the sample did not show a significant difference from metallic gold. The Fourier transformant of the Au spectrum detected a complex structure with additional maxima at short distances, but EXAFS did not confirm the formation of AuFe alloy particles. The powder diffraction pattern of the AuFe sample indicates an amorphous structure with Bragg peaks being consistent with bulk gold and, possibly, magnetite.
4. Discussion
Nowadays, the development of nanomaterials for specialized biomedical applications is a leading research branch that opens numerous perspectives. Nanoparticles exhibit superior properties compared to the bulk material, providing them with unique abilities and numerous beneficial applications [39]. Bimetallic nanoparticles, in particular, contain two nano-sized metals, which leads to structural changes introduced by an additional degree of freedom [5]. Synergistic effects induced by the combination of different metals could enhance the physical, chemical and biological properties of the nanomaterial, resulting in improved optical, magnetic, catalytic, thermal, antibacterial, antitumor, anti-inflammatory or immunomodulatory properties [5,10,39], as well as new specific activities.
In our previous studies, we have proven that biologically active gold and iron nanoparticles can be generated by the environmentally safe method of metal-vapor synthesis [33]. Both types of metal nanoparticles have been shown to selectively accumulate in tumor and inflamed tissues [39]. Their further modification with drugs could allow them to achieve a better therapeutic efficacy, a reduction in negative side effects and prolonged circulation time in blood. Improvement of solubility, tissue availability and the penetration profile together with the diminution of adverse effects is particularly necessary for MTX—a well-known antitumor and anti-inflammatory agent used for the treatment of certain tumors and inflammatory conditions (rheumatoid arthritis, psoriasis). The benefits of MTX conjugation with nanoparticles, particularly metal nanoparticles, have already been reported [40,41]. Our previous studies also contribute to this field, demonstrating antitumor properties of Au and Fe monometallic MTX conjugates produced with MVS [29,42]. Interestingly, Au NPs-MTX and Fe NPs-MTX generated using the MVS method showed highest inhibitory potential against HT-29 rectal adenocarcinoma cells in our previous study [29], while the data in this study showed a better efficacy against lung A549 and cervical HeLa adenocarcinoma cells. This difference could be due to the use of bimetallic nanoparticles and their specific physicochemical properties manifested in combination with the modification using MTX. Another interesting finding in this study is the high effectiveness of the BMNP-MTX nanomaterial at low concentrations. Treatment with the same concentration of MTX or AuFe NPs did not induce significant inhibition of tumor cell metabolic activity and viability. On the other hand, we could not detect stronger than MTX-induced inhibition during the treatment with higher concentrations of BMNP-MTX. A possible reason for this could be the saturation effect of the sample’s concentration and the inactive state of BMNPs-bound MTX. Li et al. have proven that MTX exerts its activity only after its release from the conjugate with AuFe alloy nanoparticles [23]. In the present research scenario, we suggest that AuFe-MTX samples used in higher concentrations (100–200 μg/mL) could prevent effective cellular uptake and drug release, while lower concentrations of the conjugate favor synergistic effects together with efficient interactions with cells and drug release. Further experiments need to be performed to confirm this hypothesis.
Our results on the antitumor activity of AuFe-MTX nanocarriers are exclusively based on in vitro experiments, which could be considered as a partial limitation of our study. However, an important step in the process of the validation of a certain substance or nanomaterial as an antitumor agent includes in vitro analyses. Moreover, our experiments with 3D spheroids demonstrate the efficacy of BMNP-MTX on the level of multicellular tumor structures. The results precede and motivate future in vivo evaluations of AuFe-MTX, for which effective concentration ranges of the test agent as well as tumor cell type specific effects were defined. Another perspective for our further investigations includes the analyses of the potential of MVS-produced AuFe-MTX samples to serve as hyperthermia or radiosensitizing agents providing stringent site-specific drug release under the influence of external magnetic or X-ray signals. Recent findings demonstrate such ability of drug-modified AuFe NPs. Li et al. have shown a controlled drug release via hyperthermia mediated using MTX-conjugated iron–gold alloy nanoparticles [23]. Nosrati et al. have reported efficient tumor chemoradiation therapy in a murine model of breast cancer using curcumin and folic acid-loaded Fe3O4-Au bimetallic heteronanostructures [43].
An important biological activity to investigate is antibacterial potential and, eventually, activity against antibiotic-resistant species. The reason to investigate the bactericidal effects of BMNP-MTX is that both components of this conjugate have been shown to possess such abilities. The antibacterial properties of MTX have been described [44]. The present data indicate moderate antibacterial activity for the studied AuFe NPs. Taken together, in a joint agent, MVS-generated AuFe NPs and MTX could exhibit a stronger antibacterial activity. However, our data do not confirm such enhanced properties.
The emerging role of nanomaterials concerns their immunomodulatory potential. Gold, iron and iron oxide nanoparticles have demonstrated intrinsic immune-regulatory and anti-inflammatory properties [39]. In this study, we have analyzed the levels of two main cytokines—Il-2 and IFN-γ. Although they are generally considered as pro-inflammatory, IL-2 and IFN-γ play complex roles to support functional immune responses. IFN-γ is necessary for macrophage activation to increase phagocytosis and enhance antitumor properties, and is used for the destruction of intracellular pathogens [45]. IL-2 is essential for the maintenance of a peripheral tolerance, T-lymphocyte growth and differentiation [46]. Therefore, the increase caused by the AuFe-MTX treatment IL-2 and IFN-γ production and secretion should not be solely attributed to the exacerbation of pro-inflammatory responses. In fact, it should be considered as a complex immuno-stimulatory effect with a prospective potential that will be further analyzed in our future studies.
In conclusion, this study describes the generation and characterization of biologically active bimetallic nanoparticles and their conjugation with MTX based on the environmentally safe method of metal-vapor synthesis, which enables an effective production of bimetallic drug nanocarriers, and is a simple approach for drug conjugation that does not involve additional organic reactions, improving the sustainability of the process and possible future manufacture. The obtained modified nanomaterials were subjected to physicochemical analyses and multilateral biological activity evaluations. BMNP-MTX demonstrated prominent antitumor activity even after the treatment of tumor cells with low sample concentrations and inhibition of multicellular 3D tumor aggregates’ growth and vitality. This indicates that they could serve as efficient drug carriers of antitumor agents and could act synergistically. In addition, AuFe-MTX demonstrated an ability to stimulate cytokine production with peripheral blood leukocytes, proving to have complex biological effects and a vast potential for biomedical applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app132312894/s1, Figure S1: Survey of XPS spectra of the AuFe and AuFe-MTX sample; Methods [47,48,49].
Author Contributions
Conceptualization, T.B. and A.V. (Alexander Vasil’kov); methodology, T.B., A.V. (Alexander Vasil’kov) and B.D.; formal analysis, T.B., A.V. (Alexander Vasil’kov), A.V. (Anastasiia Voronova), A.N., D.M., I.T. and B.D.; biological evaluations, T.B., D.M., I.T. and B.D.; synthesis of nanomaterials, A.V. (Alexander Vasil’kov) and A.V. (Anastasiia Voronova); characterization of nanomaterials, A.N., A.V. (Alexander Vasil’kov) and A.V. (Anastasiia Voronova); writing—original draft preparation, T.B., A.V. (Alexander Vasil’kov), A.V. (Anastasiia Voronova), A.N., D.M., I.T. and B.D.; writing—review and editing, T.B. and A.V. (Alexander Vasil’kov); supervision, T.B. and A.V. (Alexander Vasil’kov); project administration, T.B. and A.V. (Anastasiia Voronova); funding acquisition, T.B. and A.V. (Alexander Vasil’kov). All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Science Foundation of Bulgaria (NSFB), project no. KP-06-RUSSIA/14, and the Ministry of Science and Higher Education of the Russian Federation, contract no. 075-03-2023-642. During this work, the equipment of the INEOS RAS Molecular Composition Research Center was used.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Local Ethical Committee at the University of Plovdiv “Paisii Hilendarski”, Bulgaria (protocol no. 5 from 10 June 2020).
Informed Consent Statement
The blood sampling procedure was performed in accordance with the Declaration of Helsinki for the experiments involving humans. The three healthy volunteers that participated in this study signed written informed consent forms prior to the initiation of this study.
Data Availability Statement
Data are contained within this article and the Supplementary Material.
Acknowledgments
The authors are grateful to E. Shtykova and V. Volkov for SAXS research and to Y. Zubavichus for XANES/EXAFS research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Guardia, P.; Nitti, S.; Materia, M.E.; Pugliese, G.; Yaacoub, N.; Greneche, J.M.; Lefevre, C.; Manna, L.; Pellegrino, T. Gold-iron oxide dimers for magnetic hyperthermia: The key role of chloride ions in the synthesis to boost the heating efficiency. J. Mater. Chem. B 2017, 5, 4587–4594. [Google Scholar] [CrossRef] [PubMed]
- Seeburg, D.P.; Liu, D.; Radnik, J.; Atia, H.; Pohl, M.-M.; Schneider, M.; Martin, A.; Wohlrab, S. Structural Changes of Highly Active Pd/MeOx (Me = Fe, Co, Ni) during Catalytic Methane Combustion. Catalysts 2018, 8, 42. [Google Scholar] [CrossRef]
- Scaria, J.; Nidheesh, P.V.; Kumar, M.S. Synthesis and applications of various bimetallic nanomaterials in water and wastewater treatment. J. Environ. Manag. 2020, 259, 110011. [Google Scholar] [CrossRef] [PubMed]
- Basavegowda, N.; Mandal, T.K.; Baek, K.H. Bimetallic and trimetallic nanoparticles for active food packaging applications: A review. Food Bioprocess Technol. 2020, 13, 30–44. [Google Scholar] [CrossRef]
- Makada, H.; Habib, S.; Singh, M. Bimetallic nanoparticles as suitable nanocarriers in cancer therapy. Sci. Afr. 2023, 20, e01700. [Google Scholar] [CrossRef]
- Xia, C.; Jin, X.; Garalleh, H.A.; Garaleh, M.; Wu, Y.; Hill, J.M.; Pugazhendhi, A. Optimistic and possible contribution of nanomaterial on biomedical applications: A review. Environ. Res. 2023, 218, 114921. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ahmad, H.; Parveen, T.; Ahmad, A.; Oves, M.; Ismail, I.M.I.; Qari, H.A.; Umar, K.; Mohamad Ibrahim, M.N. Recent Advances in Metal Decorated Nanomaterials and Their Various Biological Applications: A Review. Front. Chem. 2020, 8, 341. [Google Scholar] [CrossRef]
- Park, W.; Heo, Y.J.; Han, D.K. New opportunities for nanoparticles in cancer immunotherapy. Biomater. Res. 2018, 22, 24. [Google Scholar] [CrossRef]
- Tomitaka, A.; Ota, S.; Nishimoto, K.; Arami, H.; Takemura, Y.; Nair, M. Dynamic magnetic characterization and magnetic particle imaging enhancement of magnetic-gold core-shell nanoparticles. Nanoscale 2019, 11, 6489–6496. [Google Scholar] [CrossRef]
- Srinoi, P.; Chen, Y.-T.; Vittur, V.; Marquez, M.D.; Lee, T.R. Bimetallic nanoparticles: Enhanced magnetic and optical properties for emerging biological applications. Appl. Sci. 2018, 8, 1106. [Google Scholar] [CrossRef]
- Amsarajan, S.; Jagirdar, B. Air-Stable Carbon-Fe Based Magnetic Nanostructures. Eur. J. Inorg. Chem. 2019, 2019, 1374–1383. [Google Scholar] [CrossRef]
- Torresan, V.; Forrer, D.; Guadagnini, A.; Badocco, D.; Pastore, P.; Casarin, M.; Selloni, A.; Coral, D.; Ceolin, M.; Fernandez van Raap, M.B.; et al. 4D Multimodal Nanomedicines Made of Nonequilibrium Au-Fe Alloy Nanoparticles. ACS Nano 2020, 14, 12840–12853. [Google Scholar] [CrossRef] [PubMed]
- Amendola, V.; Scaramuzza, S.; Litti, L.; Meneghetti, M.; Zuccolotto, G.; Rosato, A.; Nicolato, E.; Marzola, P.; Fracasso, G.; Anselmi, C.; et al. Magneto-plasmonic Au-Fe alloy nanoparticles designed for multimodal SERS-MRI-CT imaging. Small 2014, 10, 2476–2486. [Google Scholar] [CrossRef] [PubMed]
- Sanad, M.F.; Meneses-Brassea, B.P.; Blazer, D.S.; Pourmiri, S.; Hadjipanayis, G.C.; El-Gendy, A.A. Superparamagnetic Fe/Au Nanoparticles and Their Feasibility for Magnetic Hyperthermia. Appl. Sci. 2021, 11, 6637. [Google Scholar] [CrossRef]
- Tsai, M.T.; Sun, Y.S.; Keerthi, M.; Panda, A.K.; Dhawan, U.; Chang, Y.H.; Lai, C.F.; Hsiao, M.; Wang, H.Y.; Chung, R.J. Oral Cancer Theranostic Application of FeAu Bimetallic Nanoparticles Conjugated with MMP-1 Antibody. Nanomaterials 2021, 12, 61. [Google Scholar] [CrossRef] [PubMed]
- Munir, S.; Shah, A.A.; Rahman, H.; Bilal, M.; Rajoka, M.S.R.; Khan, A.A.; Khurshid, M. Nanozymes for medical biotechnology and its potential applications in biosensing and nanotherapeutics. Biotechnol. Lett. 2020, 42, 357–373. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Wang, Y.; Qin, Y.X. Promoting neuroregeneration by applying dynamic magnetic fields to a novel nanomedicine: Superparamagnetic iron oxide (SPIO)-gold nanoparticles bounded with nerve growth factor (NGF). Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1337–1347. [Google Scholar] [CrossRef]
- Lau, E.; Ahlen, M.; Cheung, O.; Ganin, A.Y.; Smith, D.G.E.; Yiu, H.H.P. Gold-iron oxide (Au/Fe3O4) magnetic nanoparticles as the nanoplatform for binding of bioactive molecules through self-assembly. Front. Mol. Biosci. 2023, 10, 1143190. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Du, X.W.; Singh, S.C.; Kulinich, S.A.; Yang, S.; He, J.; Cai, W. Nanomaterials via laser ablation/irradiation in liq-uid: A review. Adv. Funct. Mater. 2012, 22, 1333–1353. [Google Scholar] [CrossRef]
- Lyon, J.L.; Fleming, D.A.; Stone, M.B.; Schiffer, P.; Williams, M.E. Synthesis of Fe oxide core/Au shell nanoparticles by itera-tive hydroxylamine seeding. Nano Lett. 2004, 4, 719–723. [Google Scholar] [CrossRef]
- Pitzalis, E.; Psaro, R.; Evangelisti, C. From metal vapor to supported single atoms, clusters and nanoparticles: Recent advances to heterogeneous catalysts. Inorg. Chim. Acta 2022, 533, 120782. [Google Scholar] [CrossRef]
- Seideman, P.; Beck, O.; Eksborg, S.; Wennberg, M. The pharmacokinetics of methotrexate and its 7-hydroxy metabolite in patients with rheumatoid arthritis. Br. J. Clin. Pharmacol. 1993, 35, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Xu, M.; Dhawan, U.; Liu, W.C.; Wu, K.T.; Liu, X.R.; Lin, C.; Zhao, G.; Wu, Y.C.; Chung, R.J. Iron-gold alloy nanoparticles serve as a cornerstone in hyperthermia-mediated controlled drug release for cancer therapy. Int. J. Nanomed. 2018, 13, 5499–5509. [Google Scholar] [CrossRef]
- Desai, M.; Paiva-Santos, A.; Nimbalkar, M.; Sonawane, K.; Patil, P.; Pawar, K. Iron tolerant Bacillus badius mediated bimetallic magnetic iron oxide and gold nanoparticles as Doxorubicin carrier and for hyperthermia treatment. J. Drug Deliv. Sci. Technol. 2023, 81, 104214. [Google Scholar] [CrossRef]
- Olenin, A.; Leenson, I.; Lisichkin, G. Cryochemical Co-condensation of Metal Vapors and Organic Compounds. In Direct Synthesis of Metal Complexes; Kharisov, B., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 143–179. [Google Scholar] [CrossRef]
- Vasil’kov, A.; Rubina, M.; Naumkin, A.; Buzin, M.; Dorovatovskii, P.; Peters, G.; Zubavichus, Y. Cellulose-Based Hydrogels and Aerogels Embedded with Silver Nanoparticles: Preparation and Characterization. Gels 2021, 7, 82. [Google Scholar] [CrossRef] [PubMed]
- Vasil’kov, A.; Batsalova, T.; Dzhambazov, B.; Naumkin, A. XPS study of silver and copper nanoparticles demonstrated selective anticancer, proapoptotic, and antibacterial properties. Surf. Interface Anal. 2022, 54, 189–202. [Google Scholar] [CrossRef]
- Barbaro, D.; Di Bari, L.; Gandin, V.; Marzano, C.; Ciaramella, A.; Malventi, M.; Evangelisti, C. Glucose-coated superparamagnetic iron oxide nanoparticles prepared by metal vapor synthesis can target GLUT1 overexpressing tumors: In vitro tests and in vivo preliminary assessment. PLoS ONE 2022, 17, e0269603. [Google Scholar] [CrossRef]
- Vasil’kov, A.; Voronova, A.; Batsalova, T.; Moten, D.; Naumkin, A.; Shtykova, E.; Volkov, V.; Teneva, I.; Dzhambazov, B. Evolution of Gold and Iron Oxide Nanoparticles in Conjugates with Methotrexate: Synthesis and Anticancer Effects. Materials 2023, 16, 3238. [Google Scholar] [CrossRef]
- Mladenova, T.; Batsalova, T.; Dzhambazov, B.; Mladenov, R.; Teneva, I.; Stoyanov, P.; Bivolarska, A. Antitumor and Immunomodulatory Properties of the Bulgarian Endemic Plant Betonica bulgarica Degen et Neic. (Lamiaceae). Plants 2022, 11, 1689. [Google Scholar] [CrossRef]
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef]
- Moyo, B.; Mukanganyama, S. Antibacterial Effects of Cissus welwitschii and Triumfetta welwitschii Extracts against Escherichia coli and Bacillus cereus. Int. J. Bacteriol. 2015, 2015, 162028. [Google Scholar] [CrossRef] [PubMed]
- Batsalova, T.; Moten, D.; Butenko, I.; Dzhambazov, B.; Vasil’kov, A. Biological and physicochemical properties of gold and iron nanoparticles produced by green synthesis method. In Proceedings of the 22nd SGEM International Multidisciplinary Scientific GeoConference 2022, Albena, Bulgaria, 4–10 July 2022; pp. 11–22. [Google Scholar]
- Rodrigues da Cunha, M.J.; Souza Freitas, B.L.; Nasser Fava, N.M.; Sabogal-Paz, L.P. CFDA-AM staining to assess the metabolic activity of Giardia duodenalis cysts inactivated by chlorine, boiling and ultraviolet irradiation. J. Water Health 2022, 20, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.A.; Fujimori, Y.; Ringleb, F.; Shao, X.; Stavale, F.; Nilius, N.; Sterrer, M.; Freund, H.J. Oxidation of Au by surface OH: Nucleation and electronic structure of gold on hydroxylated MgO(001). J. Am. Chem. Soc. 2011, 133, 10668–10676. [Google Scholar] [CrossRef] [PubMed]
- Klabunde, K.; Davis, S.; Hattori, H.; Tanaka, Y. Clustering of metal atoms in organic media: V. Production of tailored selectivity in nickel catalysts: Comparisons with Raney nickel. J. Catal. 1978, 54, 254–268. [Google Scholar] [CrossRef]
- Beamson, G.; Briggs, D. High Resolution XPS of Organic Polymers: The Scienta ESCA300 Database; Wiley: Hoboken, NJ, USA, 1992. [Google Scholar]
- Svergun, D.I. Determination of the regularization parameter in indirect-transform methods using perceptual criteria. J. Appl. Cryst. 1992, 25, 495–503. [Google Scholar] [CrossRef]
- Agarwal, H.; Nakara, A.; Shanmugam, V.K. Anti-inflammatory mechanism of various metal and metal oxide nanoparticles synthesized using plant extracts: A review. Biomed. Pharmacother. 2019, 109, 2561–2572. [Google Scholar] [CrossRef]
- Álvarez-González, B.; Rozalen, M.; Fernández-Perales, M.; Álvarez, M.A.; Sánchez-Polo, M. Methotrexate gold nanocarriers: Loading and release study: Its activity in colon and lung cancer cells. Molecules 2020, 25, 6049. [Google Scholar] [CrossRef]
- Kim, J.H.; Umemura, M.; Eguchi, H.; Ishikawa, Y. Methotrexate-Transferrin-Functionalized Fe(Salen)-Polypyrrole Nano-composites for Targeted Photo-/Magneto-Thermal Cancer treatments. J. Compos. Sci. 2022, 6, 136. [Google Scholar] [CrossRef]
- Batsalova, T.; Moten, D.; Voronova, A.; Dzhambazov, B.; Vasil’kov, A. Anticancer and antibacterial potential of methotrexate loaded iron nanoparticles produced by green synthesis method. In Proceedings of the 23rd International Multidisciplinary Scientific Geoconference—SGEM23, Albena, Bulgaria, 1–10 July 2023; pp. 3–16. [Google Scholar]
- Nosrati, H.; Baghdadchi, Y.; Abbasi, R.; Barsbay, M.; Ghaffarlou, M.; Abhari, F.; Mohammadi, A.; Kavetskyy, T.; Bochani, S.; Rezaeejam, H.; et al. Iron oxide and gold bimetallic radiosensitizers for synchronous tumor chemoradiation therapy in 4T1 breast cancer murine model. J. Mater. Chem. B 2021, 9, 4510–4522. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, Z.P.; Lin, Z.; Wei, G.; Wen, X.; Li, S.; Yang, X.; Zhang, Q.; Jing, C.; Dai, Y.; et al. Drug Repurposing by Siderophore Conjugation: Synthesis and Biological Evaluation of Siderophore-Methotrexate Conjugates as Antibiotics. Angew. Chem. 2022, 61, e202204139. [Google Scholar] [CrossRef]
- Tau, G.; Rothman, P. Biologic functions of the IFN-gamma receptors. Allergy 1999, 54, 1233–1251. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, M.F.; Oxenius, A. Interleukin 2: From immunostimulation to immunoregulation and back again. EMBO Rep. 2007, 8, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Chernyshov, A.; Veligzhanin, A.; Zubavichus, Y. Structural Materials Science End-Station at the Kurchatov Synchrotron Radiation Source: Recent Instrumentation Upgrades and Experimental Results. Nucl. Instrum. Methods Phys. Res. A. 2009, 603, 95–98. [Google Scholar] [CrossRef]
- Newville, M. EXAFS analysis using FEFF and FEFFIT. J. Synchrotron Radiat. 2001, 8, 96–100. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).