Abstract
Improving methods for landless production of bioproducts is considered an important stage in the development of the modern bioeconomy. In this context, microalgal biomass is one of the most promising sources of valuable substances due to its rich biochemical composition. Despite the high adaptability of microalgae to various environmental factors, the effectiveness of cultivation systems depends on precisely selected parameters. Both the light conditions and the supply of inorganic carbon sources are key in determining the efficiency of photoautotrophic cultivation. In this work, the effect of a high daily photosynthetic photon flux density (PPFD) ranging from 37.44 to 112.32 mol m−2 day−1 on the growth and productivity of a novel Scenedesmaceae alga, strain EZ-B1, was assessed. The next stage of cultivation consisted of selecting the optimal CO2 concentration. Improved performance of microalga during cultivation in a photobioreactor was achieved at 112.32 mol m−2 day−1 (24 h photoperiod) and by supplying 2% CO2, as evidenced by the high biomass productivity (0.69 g L−1 day−1), total biomass yield (5.23 g L−1), and ammonium nitrogen consumption rate. The data obtained suggest that a higher level of PPFD led to the highest growth rate of the novel strain and the highest biomass productivity, which, in practice, will increase production capacity.
1. Introduction
The modern biotechnology of microalgae is developing along with the concept of integrated production systems, the interconnection of which ensures the efficient use of resources and the reduction of anthropogenic pressure on the environment [1]. The results of many studies prove that various microalgae successfully purify both wastewater and flue gases while simultaneously producing valuable metabolic products [2,3]. Although the environmental aspects of microalgae cultivation deservedly occupy a place among promising “green” technologies [4], the scalability of algal biomass production models is limited by technical and economic factors. The most important tasks in developing cost-effective cultivation models are the improvement of the designs of cultivation systems and the optimization of growth parameters in accordance with the physiological characteristics of different strains [5].
The main obstacles to growing microalgae are associated with a low biomass density, which increases cultivation costs, increases the risk of contamination, and leads to low yields of the targeted metabolic products. For microalgae as photosynthetic microorganisms, the intensity and duration of light, as well as the CO2 concentration, are the main environmental parameters that significantly affect the cultures’ growth [6]. It has been noted that the optimization of both parameters should be considered in combination [7,8]. Microalgae species adapt to different light conditions; however, to ensure optimal growth, it is important to establish several basic light parameters, including intensity, frequency, and emitted spectrum [9]. The intensity and duration of light influence the rate of photosynthesis, as well as the specific growth rate of an algal culture [10], the synthesis of commercially important products [11], and the removal of nutrients from the medium [12]. At the same time, providing the algal cells with light of sufficient intensity depends on the surface-to-volume ratio of a photobioreactor and the efficiency of the mixing devices [13]. Equipping reactors with artificial light sources increases production costs, but at the same time stabilizes biomass production, making it independent of external factors [14]. Under natural conditions, the duration of light is determined by seasonal and daily fluctuations, while application of artificial light sources makes it possible to control the light and dark cycles or to cultivate microalgae under continuous light [12].
In a liquid medium, gaseous inorganic carbon passes into a dissolved form, available to algal cells in the form of CO2(aq) or HCO3− [15]. The degree of dissolution, and hence the efficiency of CO2 fixation by algal cells, depends on the size of the gas bubbles and the residence time in the liquid. The degree of CO2 biofixation is determined by the difference between the concentration of gas entering and exiting a photobioreactor, by the amount of carbon in the biomass, and by other methods using sophisticated analytical equipment [16,17]. Parameters such as light intensity, temperature, and pH have a significant impact on photosynthetic carbon fixation degree [18]. Studies on the ability to utilize CO2 and the effect of the CO2 concentration on biomass production have been studied for several microalgae of the genera Chlorella [19,20], Scenedesmus [20,21,22], Chlorococcum [23], and other microalgae, which are characterized by rapid growth and high tolerance of stress factors. However, most studies are conducted to find evidence for the use of microalgae from different geographic regions for CO2 sequestration, and the optimal light requirements of the microalgae are often not considered. Species with high CO2 tolerance have great potential in carbon dioxide sequestration technologies [24,25].
Our research work aimed to identify the combined effects of light regimens and CO2 concentrations on the growth of newly isolated indigenous microalga within the family Scenedesmaceae. Additionally, the biochemical composition of the cells and nutrient removal by cells from the synthetic nutrient medium were studied. It has previously been shown that a variety of microalgae of the family Scenedesmaceae can be used as agents for removing inorganic nutrients from contaminated waters [26], promising sources of valuable products, CO2 fixation agents, and as feed substitutes [27,28]. The selection of optimal growth conditions for algae of the family Scenedesmaceae has been the focus of many research groups [20,21,22,27,28,29]. However, many studies have been conducted using a narrow range of conditions or have optimized their growth using only one parameter. Thus, in the work of Patil and Kaliwala [29], the cultivation of an indigenous species, Tetradesmus bajacalifornicus, was carried out at different CO2 concentrations but at a constant light intensity (180 μmol m−2 s−1). The authors noted a significant effect of CO2 concentration on the growth and biochemical composition of the cells. In another work [20], Tetradesmus obliquus, along with representatives of the genus Chlorella, were cultured in a glass bubble column photobioreactor at 74 μmol m−2 s−1 and at various CO2 concentrations. Among the tested microalgae, T. obliquus had high growth parameters and lipid production levels, and the authors noted that T. obliquus showed high adaptation to elevated levels of CO2. Among the studies devoted to the study of the influence of light on microalgae of the family Scenedesmaceae, there are few studies where high levels of PPFD were tested. In many studies, the PPFD values rarely exceed 150 µmol m−2 s−1 [20,29,30,31,32], which does not allow to fully evaluate the capabilities of the algae.
A previous study demonstrated that the novel Scenedesmaceae microalga, strain EZ-B1, accumulated a high protein level when grown in diluted anaerobic digester effluent, a residue of anaerobic digesters [33]. In the present study, we aimed to study the effect of high PPFD on the metabolism of this microalga, considering that high PPFD is a metabolic shift strategy to increase the synthesis of certain products, including pigments [24,34]. Considering that the high tested light intensities can cause photoinhibition of microalgae [35], in this work, cultivation was carried out both under continuous and periodic light. It is also known that the use of different photoperiods makes it possible to change the biochemical composition of microalgal cells [9,36]. A series of experiments were carried out to analyze the effects of daily PPFD (37.44–112.32 mol m−2 day−1), photoperiods (16 h and 24 h), and various CO2 concentrations (1–4%) on algal growth, biomass productivity, nutrient removal, and accumulation of selected metabolic products in a well-controlled Labfors 4 Lux photobioreactor.
2. Materials and Methods
2.1. Microalgal Strain and Culture Media
Scenedesmaceae alga (Chlorophyta), strain EZ-B1, was isolated by us recently, and the growth was tested in culture media prepared from anaerobic digester effluent [33]. This strain demonstrated a maximum biomass productivity of 0.35 ± 0.02 g L−1 day−1 in such a medium. The growth of the pure algal culture was maintained on a solidified standard Bold’s basal medium (BBM) [37] supplemented with antibiotics [33]. The inoculum was prepared, and cells were cultured in 500 mL Erlenmeyer flasks with a standard BBM medium containing sodium nitrate (41 mg NO3−–N L−1) as a main nitrogen source. All treatments in the photobioreactor were carried out with BBM, but the nitrogen source was changed to ammonium chloride (~165 mg NH4+–N L−1). At non-toxic concentrations, ammonium is often reported to provide higher growth rates compared to nitrate and urea for a wide range of microalgae species [38,39]. Nutrient media were autoclaved and added to Petri dishes, flasks, or the photobioreactor under aseptic conditions.
2.2. Photoautotrophic Cultivation
Cells for inoculation were obtained by subculturing algal cells from agar medium into 500 mL Erlenmeyer flasks containing standard BBM. The flasks were placed in a shaker–incubator, providing a stable temperature (28 °C), stirring (120 rpm), and continuous light (200 µmol photons m−2 s−1). After 5 days, the cells were collected from the standard medium by centrifugation, washed with a sterile buffer solution (0.05 M Na2HPO4 + KH2PO4; pH 7.0), and added into the photobioreactor. Finally, an initial optical density of 0.05 (measured at 750 nm) was obtained.
The experiments were divided into two main blocks. In the first block, PPFD in the range of 650 µmol m−2 s−1 to 1300 µmol m−2 s−1 at a concentration of 2% CO2 was tested under a 16 h photoperiod (periodic light, PL). In the second block, under a 24 h photoperiod (continuous light, CL), 650 µmol m−2 s−1 (at a concentration of 2% CO2) and 1300 µmol m−2 s−1 (at different CO2 concentrations, 1–4%) were tested. Considering the photoperiod values, the tested values of instantaneous PPFD were converted into daily PPFD expressed in mol m−2 day−1. Thus, 1 µmol m−2 s−1 = 0.0576 mol m−2 day−1 with periodic light (16 h photoperiod), whereas 1 µmol m−2 s−1 = 0.0864 mol m−2 day−1 with continuous light (24 h photoperiod) (Table 1).

Table 1.
Experimental design of photoautotrophic cultivation of Scenedesmaceae strain EZ-B1.
Photoautotrophic cultivation was carried out in the autoclavable 3.6 L Labfors 4 Lux photobioreactor (Infors HT, Bottmingen, Switzerland) with a working volume of 2.6 L and 16 Gro-Lux fluorescent tubes (1 tube = 120 lm). The PPFD was controlled by changing the number of operating tubes. Additionally, the photosynthetically active radiation was measured using a PAR meter (Apogee Instruments, Logan, UT, USA). Carbon dioxide mixed with air was supplied to the photobioreactor through a 0.20 µm Midisart filter (Sartorius Stedim Biotech, Göttingen, Germany). The aeration rate was 0.7 L min−1, and the O2 and CO2 concentrations were measured using a gas analyzer (Infors HT, Bottmingen, Switzerland). A constant temperature level (28 °C) was maintained by recirculating deionized water through an A600 circulating cooler (Scientific Equipment, NV-Lab, Tomsk, Russia). The stability of the pH level (7.0 ± 0.02) was ensured by a system automatically supplying 2 M NaOH to the culture medium, which was measured in real time using an EasyFerm Plus PHI K8 425 electrode (Hamilton, OH, USA). Foam control was also performed automatically by supplying a sterile 2% antifoam solution (Antifoam B, Sigma-Aldrich, St. Louis, MO, USA).
2.3. Harvesting and General Characterization of Biomass
The algal dry weight was determined periodically. For each weight measurement, 2 mL of the suspension was taken into microcentrifuge tubes, and the cells were collected by centrifugation at 5000× g for 5 min. The cells were washed with distilled water, after which the centrifugation was repeated. The tubes were left in a thermostat at 80 °C for 24 h. The dried biomass was weighed using an analytical balance, and the concentration of biomass in 1 L of algal suspension was calculated. For the final weight measurements, the entire biomass was collected. The algal suspension was transferred into 50 mL Falcon tubes, and the cells were collected by centrifugation at 5000× g for 5 min. The obtained biomass was washed twice with distilled water. One part of the algal biomass was dried in a thermostat at 60 °C for 24 h and then used for protein measurements, while the other part was placed in crucibles and dried at 105 °C in a dry oven for 16 h (to determine the total solids or final dry matter). The weight of the algal biomass was expressed in g L−1. To determine the volatile solids in the biomass, it was placed in crucibles in a muffle furnace at 550 °C for 2 h. After cooling, the crucibles were weighed, and the volatile solids were then calculated.
2.4. Assessment of Algal Culture Growth, Nutrient Removal, and Biochemical Composition of Biomass
The optical density of the culture was determined using a Lambda 35 spectrophotometer (Perkin Elmer, Singapore) at 750 nm. The number of cells was counted using a counting chamber.
The specific growth rate and biomass productivity were calculated using the equations reported by Pedersen et al. [40]. The specific growth rate of microalga (day−1) was calculated during the exponential phase of the growth. Biomass productivity (g L−1 day−1) was calculated by dividing the final biomass yield (dry matter) by the total number of days within the experiment required to reach the stationary phase.
The content of chlorophylls (a, b) and carotenoids (mg L−1) was determined by the method using dimethyl sulfoxide as a solvent, as described previously [33,38]. Briefly, 1 mL of microalgal culture was centrifuged at 10,000× g for 5 min, after which the supernatant was discarded. After that, 1 mL of preheated dimethyl sulfoxide was added to the precipitate, resuspended, incubated for 5 min in a thermoshaker TS-100C (BioSan, Riga, Latvia) at 60 °C, and then centrifuged at 10,000× g for 5 min. Absorption was measured at 480, 649, and 665 nm using a Lambda 35 spectrophotometer (Perkin Elmer, Singapore). The chlorophyll and carotenoid concentrations were calculated using the equation reported by Welburn [41].
A commercial Bio-Rad protein assay kit (Munich, Germany) was used to determine the content of proteins as described previously [38]. Briefly, approximately 20 mg of dried algal biomass was combined with 1 mL of 0.5 M NaOH in a 2 mL metal lysing tube (MP Biomedicals, Illkrich, France) with two types of beads (0.6 g of 0.1 mm zirconium/silica beads and 0.4 g of 1.0 mm glass beads) and then homogenized for two 30 s pulses at speed level 6.0 m s−1 with a 5 min cooling period between pulses. The extracted samples were incubated at 500 rpm in a thermoshaker TS-100C (BioSan, Riga, Latvia) at 60 °C. The mixtures were then transferred to new, clean tubes, and after centrifugation at 2000× g for 5 min, the supernatants were used for protein measurement.
The degree of ammonium nitrogen removal was assessed by the colorimetric method using Nessler’s reagent. Prior to analysis, cells were pelleted by centrifugation at 10,000× g for 3 min. Then, 100 µL of the supernatant was diluted with deionized water, mixed on a vortex, and 100 µL of Nessler’s reagent was added. After 5 min of incubation, the absorbance was measured using a Lambda 35 spectrophotometer at 425 nm. The NH4+–N content was expressed in mg L−1.
The concentration of phosphate in the growth media was evaluated at the end of the cultivation using ion chromatography (Dionex ICS-900 Ion Chromatography System, Thermo Fisher Scientific, Wilmington, DE, USA) [16].
2.5. Statistical Analysis
Each treatment was conducted in duplicate, and all analyses were measured in triplicate. The mean values are presented together with standard deviations. The Tukey method and 95% confidence interval were used to compare differences (Minitab software version 20.2.0, State College, PA, USA).
3. Results and Discussion
3.1. Algal Culture Growth Rates and Biomass Yield under Various Growth Conditions
In this work, photoautotrophic cultivation of Scenedesmaceae alga, strain EZ-B1, was carried out in modified BBM. Parameters such as temperature, pH, aeration, and mixing rates were maintained at the same level in all experiments by using an automatic photobioreactor control system. To assess the effect of light conditions on the growth of the algal culture, two modes were selected. During the first mode, with a 16 h photoperiod, a wide range of daily PPFD was tested, from a minimum of 37.44 mol m−2 day−1 to a maximum of 74.88 mol m−2 day−1. During the second mode of the study, a 24 h photoperiod with 56.16 mol photons m−2 day−1 and 112.32 mol photons m−2 day−1 was evaluated. At this stage, in addition to the different PPFD values, the influence of various CO2 concentrations ranging from 1.0% to 4.0% (aeration rate: 0.7 L min−1) was further assessed. The experimental design is presented in Table 1.
The growth dynamics of the algal culture Scenedesmaceae strain EZ-B1 are presented based on the average optical density values measured at a wavelength of 750 nm (Figure 1A,B). As the photosynthetic photon flux density increased (a 16 h photoperiod; 2% CO2), the exponential growth duration of the microalga decreased from approximately 256 h at 37.44 mol m−2 day−1 (PL1) to approximately 208 h at 74.88 mol m−2 day−1 (PL5). In the range of 37.44–56.16 mol photons m−2 day−1, the final values of the OD750 did not differ, while at 65.55 mol photons m−2 day−1 and 74.88 mol photons m−2 day−1, higher OD750 values were reached. The maximum OD750 value in experiments with periodic light was 8.80 ± 0.15 obtained at 74.88 mol photons m−2 day−1 (PL5) at 232 h of cultivation (Figure 1A). During the dark period, the optical density of the algal culture did not change substantially (not shown in the figure).
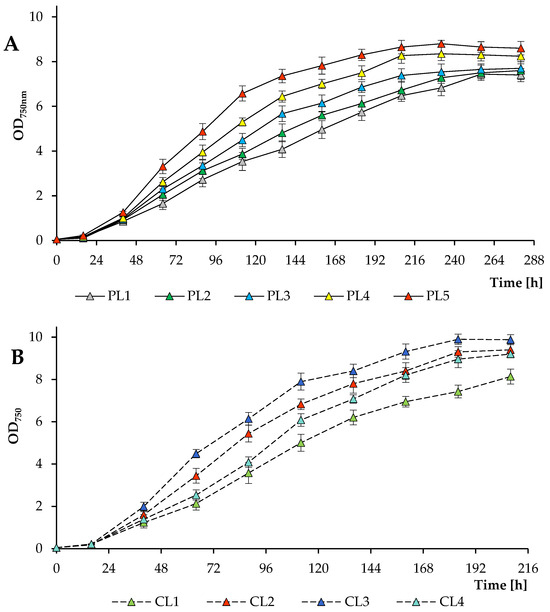
Figure 1.
Change of OD750 during growth of Scenedesmaceae alga, strain EZ-B1, under periodic (A) and continuous (B) light with various CO2 concentrations.
Similar growth patterns of the strain EZ-B1 were observed with a 24 h photoperiod, but higher PPFD values resulted in higher biomass yields (when the CL1 and CL3 treatments were compared). Thus, the exponential phase at 112.32 mol photons m−2 day−1 ended after about 184 h, whereas the growth of the culture at 56.16 mol photons m−2 day−1 was delayed. Furthermore, different CO2 concentrations were tested at the maximum PPFD (when the CL2, CL3, and CL4 treatments were compared). The final OD750 values were comparable over the CO2 concentration range studied, but 2% CO2 was found to be optimal in terms of biomass production (Figure 1B). It is worth noting that in the experiment with a high daily PPFD, no photoinhibition effect was observed, which, in addition to the resistance of the strain to higher light intensity, is also associated with the presence of a shadow effect on microalgae when cultivated in a photobioreactor, which was also observed in another study [12].
The optimal growth of microalga at high PPFD values was also indicated by the specific growth rate (Figure 2). The minimum and maximum values of the specific growth rate with a 16 h photoperiod were 0.47 ± 0.01 day−1 (at 37.44 mol photons m−2 day−1) and 0.61 ± 0.01 day−1 (at 74.88 mol photons m−2 day−1). With a 24 h photoperiod, the specific growth rates obtained at 112.32 mol photons m−2 day−1 and 2% CO2 were higher than the values obtained at 56.16 mol photons m−2 day−1 at the same CO2 level and amounted to 0.72 ± 0.01 day−1. A decrease or an increase in CO2 concentrations to 1% and 4%, respectively, resulted in a decrease in the specific growth rates. The benefits of continuous lighting were additionally confirmed by the biomass productivity (Figure 3), final dry weight values, volatile solids, and cell concentration (Table 2).
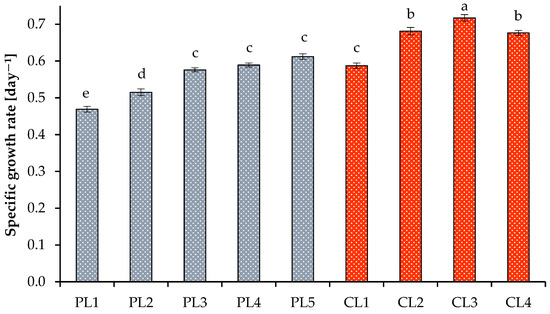
Figure 2.
Specific growth rate of Scenedesmaceae alga, strain EZ-B1, under periodic light (PL) and continuous light (CL). Means that do not share a letter are significantly different (ANOVA, Tukey method, α = 0.05).
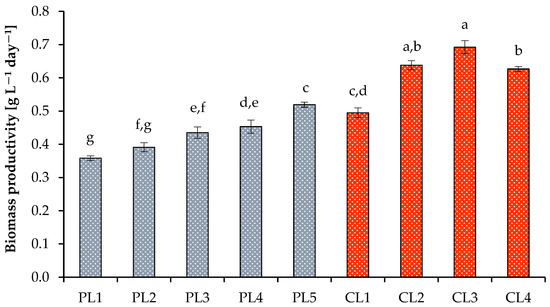
Figure 3.
Biomass productivity of Scenedesmaceae alga, strain EZ-B1, under periodic light (PL) and continuous light (CL). Means that do not share a letter are significantly different (ANOVA, Tukey method, α = 0.05).

Table 2.
Scenedesmaceae strain EZ-B1 characteristics.
Thus, the highest biomass productivity was observed at daily PPFD = 112.32 mol m−2 day−1 and 2.0% CO2 and reached 0.69 ± 0.02 g L−1 day−1. Finally, a daily PPFD of 74.88 mol m−2 day−1 and 2% CO2 (16 h photoperiod) yielded 4.55 ± 0.11 g L−1 of dry weight with a high content of organic matter (4.11 ± 0.10 g L−1), while the maximum values of dry weight and volatile substances of algal biomass under continuous light were obtained when cultivating alga at 112.32 mol photons m−2 day−1 and 2% CO2 (5.23 ± 0.18 g L−1 and 4.68 ± 0.16 g L−1, respectively).
These data show that the light conditions and the concentration of inorganic carbon have a significant effect on the production of algal biomass and are considered species-specific. As many researchers have noted, optimizing the CO2 concentration when cultivating various algae will both increase the content of metabolic products and optimize cell growth [42,43,44]. Vanags et al. [45] demonstrated that both the light intensity and an additional CO2 supply stimulated the growth of Desmodesmus communis. The optimal values of these parameters were 300 μmol m−2 s−1, which was the maximum tested light intensity, and a supply of 4% CO2. In a study performed by Nzayisenga et al. [46], it was noted that a higher light intensity increased the biomass yield of Chlorella vulgaris and Tetradesmus obliquus, but since the optimal growth of cultures was observed in the range of 150–300 µE m−2 s−1, it was more appropriate to culture at 150 μE m−2 s−1. The authors also showed that for another culture of the genus Desmodesmus, light with an intensity of 300 µE m−2 s−1 was optimal since it significantly increased the biomass yield. In another study, the maximum biomass productivity of C. vulgaris was obtained at a light intensity of 10,000 lux and 2% CO2 concentration [47]. Maximum biomass yield values during the cultivation of Scenedesmus dimorphus under various parameters were achieved at 15,000 lux and a 12 h photoperiod [48]. Chunzhuk et al. [49] evaluated the effect of various CO2 concentrations ranging from 0.04 to 9% on the growth of several strains of microalgae and cyanobacteria. The growth rate of three strains (C. vulgaris, Chlorella ellipsoidea, and Elliptochloris subsphaerica) had a positive correlation with the CO2 concentration, while high CO2 had a negative effect on the growth of other strains (Gloeotila pulchra and Arthrospira platensis), which is due to their adaptive mechanisms or features of carbon assimilation. Cultivation of T. obliquus at a 2.5% CO2 concentration increased the specific growth rate, but at both 2.5% CO2 and 5.0% CO2, the maximum biomass concentrations were at the same level [20]. According to a study performed by Montoya-Vallejo et al. [50], at higher PPFD values, both the specific growth rate and the biomass yield of Chlorella sorokiniana increased. This is consistent with the results obtained in this study, but for another algal species. Perin et al. [51] revealed a clear positive correlation between light intensity and biomass concentration when cultivating Nannochromis gaditana in an open system. Since microalgae species have different light saturation limits, which are determined by their acquired adaptation mechanisms to light conditions, excess light has an inhibitory effect on culture growth. Difusa et al. [30] identified light conditions for the optimal growth of two strains of the genus Scenedesmus, under which growth kinetics were significantly improved. In the range of 27 µmol photons m−2 s−1 to 81 µmol photons m−2 s−1, the specific growth rate and biomass productivity of both strains increased, but the maximum light intensity studied (94.5 µmol photons m−2 s−1) had an inhibitory effect on the growth of algal cultures when grown in flasks. Our research demonstrates that high PPFD values stimulate the more effective growth kinetics of Scenedesmaceae microalga, strain EZ-B1, in the photobioreactor under controlled conditions. Table 3 demonstrates additional comparisons with several other Scenedesmaceae strains.

Table 3.
Comparison of biomass yield, biomass productivity, and specific growth rates of various Scenedesmaceae strains under different optimal growth conditions (batch treatments).
3.2. Production of Metabolites under Various Growth Conditions
Proteins, carbohydrates, lipids, and pigments synthesized by microalgae are biomolecules with high added value. The use of these products makes the cultivation of algal strains commercially viable [59]. It is known that both qualitative and quantitative characteristics of light affect the metabolic composition of cells; therefore, an optimal light regimen not only increases the productivity of biomass but also increases its value [60]. In turn, the light regimen affects the efficiency of assimilation of inorganic carbon, which is converted into various compounds during photosynthesis [61,62].
Microalgae synthesize various types of pigments, which are widely used in the food industry, pharmacology, and cosmetology, competing with synthetic ones [63]. In this study, the concentrations of pigments (chlorophyll a, chlorophyll b, and total carotenoids) were estimated during the cultivation of Scenedesmaceae strain EZ-B1 in the photobioreactor to estimate the photosynthetic potential of the culture (Table 2; Figure 4). Under different photoautotrophic growth conditions, the total chlorophyll and total carotenoid content of the cultures varied from 83.2 ± 3 mg L−1 to 102.3 ± 4 mg L−1 and from 16.0 ± 0.6 mg L−1 to 18.2 ± 0.8 mg L−1, respectively (at the end of cultivation). The maximum total pigment concentrations were achieved at 56.16 mol m−2 day−1 with a 16 h photoperiod.
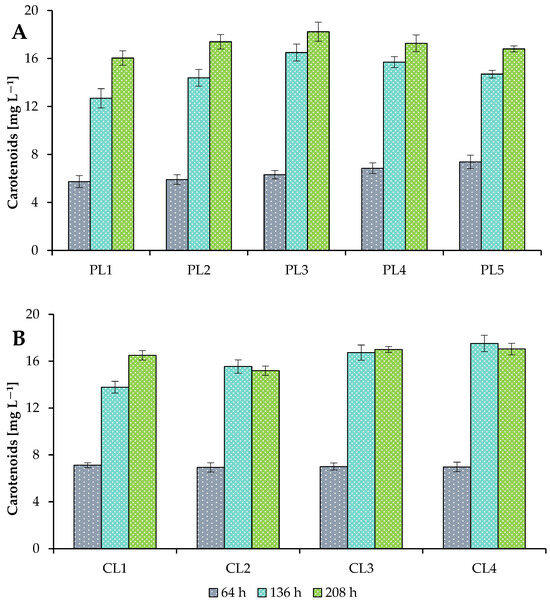
Figure 4.
Concentrations of total carotenoids of Scenedesmaceae alga, strain EZ-B1, at 64, 136, and 208 h of cultivation under periodic light (PL; A) and continuous light (CL; B).
Bialevich et al. [64] noted that with increasing PPFD (from 100 µmol m−2 s−1 to 500 µmol m−2 s−1), the concentration of total pigments in the cells of Parachlorella kessleri decreased, but for two other algal species, an inverse relationship was observed. Thus, when Chlamydomonas reinhardtii and Desmodesmus quadricauda were cultivated at the maximum tested PPFD value, the concentrations of chlorophyll a, chlorophyll b, and total carotenoids were significantly higher. Increasing light intensity has been shown to be important for the accumulation of total carotenoids by Dunaliella tertiolecta under all conditions tested and to have an uncertain effect on chlorophyll synthesis [34]. The maximum chlorophyll content of Scenedesmus acutus in the study performed by Kaymaz and Cetin [31] was recorded for a 24 h photoperiod, but the chlorophyll content was reached more quickly under 18 h and 12 h photoperiods. Yang et al. [65] studied the effect of CO2 concentration (0.05–100%) on the synthesis of pigments and found that increasing the CO2 concentration to a certain level promoted the accumulation of pigments in T. obliquus culture. Also, the authors demonstrated that more than 30% CO2 had an inhibitory effect on the photosynthesis of microalgae and significantly reduced the concentration of all pigments.
Proteins obtained from microalgae are a promising alternative to animal and plant proteins [66]. The protein content varies sharply among different species of microalgae, ranges from 12 to 70% of dry weight, and also depends on the cultivation conditions [67]. In this study, the protein content in cells of Scenedesmaceae strain EZ-B1 increased with increasing light intensity with a 16 h photoperiod, but under continuous light conditions (112.32 mol m−2 day−1), the protein content was lower (Figure 5). The maximum protein content in the biomass of the strain was 30.5 ± 3.4% of dry weight with a 16 h photoperiod and 112.32 mol m−2 day−1. Additionally, it was found that, regardless of the carbon dioxide concentration, at the same high light intensity, the protein content was at the same level (17–18% of dry weight). It is worth considering that the protein content in the dry biomass of algal cells was determined at the end of cultivation; therefore, differences may be associated not only with the tested growth conditions but also with the time when the culture entered the stationary phase of growth (a change in the cultivation period can cause a change in the ratio of cellular metabolites).
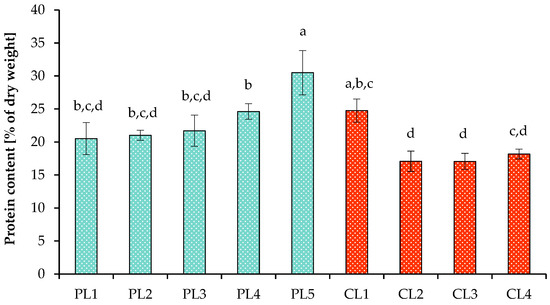
Figure 5.
Protein content of the biomass of Scenedesmaceae alga, strain EZ-B1, under periodic light (PL) and continuous light (CL). Means that do not share a letter are significantly different (ANOVA, Tukey method, α = 0.05).
Changing light intensity in the range of 130–520 µmol m−2 s−1 did not affect the protein content in cells of C. vulgaris when grown in closed bioreactor, as was shown in a study performed by Metsoviti et al. [68]. However, in another study, when C. vulgaris was cultivated at the maximum light intensity tested (300 µE m−2 s−1), the protein content was 1.46× higher compared to the minimum intensity [69]. When studying the effect of photoperiod on the synthesis of various metabolites in S. acutus [31], it was revealed that under 24 h and 18 h photoperiods, the maximum protein content was reached on day 10, while with a 12 h photoperiod, the maximum concentration was achieved on day 8. However, the maximum protein content did not differ significantly between different photoperiods. This is probably due to the light intensity, which was only 55 µmol photons m−2 s−1. In the work presented here, the final biomass protein content decreased under a 24 h photoperiod at the maximum average daily PPFD. In another study [70], the final protein content in culture of C. vulgaris was higher with a 16 h photoperiod and significantly decreased with a 20 h photoperiod. In terms of CO2 concentration, the maximum protein content in cells of Scenedesmus bajacalifornicus was recorded at 15% CO2 by Patil and Kaliwal [29].
3.3. Carbon and Nitrogen Nutrition
To determine the efficiency of the assimilation of carbon dioxide supplied to the photobioreactor (CL2–CL4 treatments), the CO2 output was measured (Figure 6). Since it was previously established that the concentration of CO2, as well as the assimilation of ammonium by microalgae with the simultaneous release of hydrogen ions (protons), destabilizes the pH level and leads to its decrease [71], the pH level was maintained throughout all treatments in the automatic mode by supplying 2 M NaOH and kept at 7.0 ± 0.02.
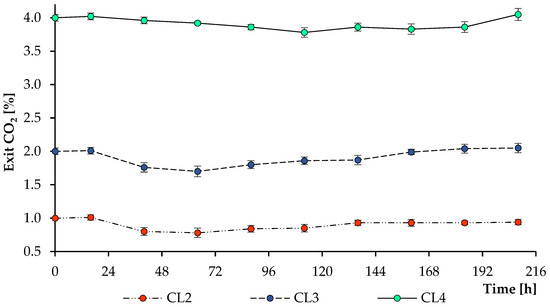
Figure 6.
Changes in the released CO2 level observed during the growth of Scenedesmaceae alga, strain EZ-B1, under continuous light (CL) at 112.32 mol m−2 day−1 and different initial CO2 concentrations (1%, 2%, and 4%).
Figure 1 and Figure 6 illustrate that the period of intensive growth of the culture was accompanied by a decrease in the carbon dioxide output and, consequently, by effective carbon assimilation. With an increase in cell concentration and complete utilization of ammonium from the growth medium, an increase in the CO2 concentration in the output was observed, which can be explained by the simultaneous assimilation of CO2 and its active release by cells. In our recent work [38], cultivation of Chlorella sorokiniana in a growth medium in the presence of ammonium ions stimulated cells to more efficiently uptake carbon dioxide than during cultivation in the presence of nitrate ions or in effluent from biogas reactors. Effluent from biogas reactors is a by-product of the anaerobic digestion process that must be disposed of to prevent harmful effects on the environment [72,73]. In turn, an additional CO2 supply affects the removal of nutrients from the growth medium, as shown by Molino et al. [27], where nitrate removal was increased from 5.0 to 87.2% when the CO2 concentration was increased from 0.0 to 3.0%. It is worth noting that the additional supply of carbon dioxide during the cultivation of microalgae not only optimizes the growth of photoautotrophic microorganisms but also has an advantage in the environmental aspect.
In all experiments, the initial total ammonium nitrogen concentration was about 165 mg L−1 (Figure 7). The dynamics of ammonium nitrogen removal from the nutrient medium were influenced by both the PPFD level and the concentration of supplied CO2. At 37.44–56.16 mol photons m−2 day−1 (PL1–PL3), almost all nitrogen was removed during 112 h of cultivation. With a further increase in PPFD level (PL4 and PL5), complete removal of nitrogen was observed after 88 h of cultivation. Cultivation under continuous light (112.32 mol m−2 day−1) with a supply of 1 and 2% CO2 (CL2 and CL3) shifted the point of almost complete nitrogen removal to 64 h. In the case of phosphate utilization by algal cells, 90–98% of phosphates were removed from the culture media by the end of cultivation.
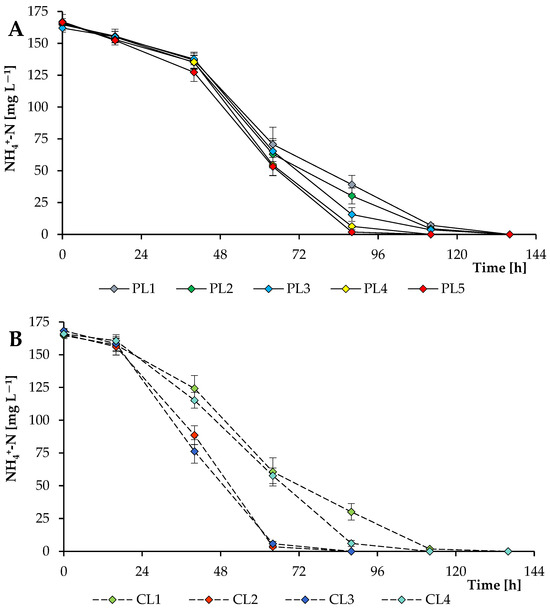
Figure 7.
Ammonium nitrogen utilization by Scenedesmaceae alga, strain EZ-B1, under periodic light (Pl; A) and continuous light (CL; B).
Nitrogen can be supplied in various forms to support the growth of green algae, but different species prefer specific forms and concentrations of nitrogen [38,39,74,75]. One previous study investigated the effect of different forms of nitrogen (NaNO3, CO(NH2)2, and NH4Cl) in different concentrations on the growth of Chlorella sp. GN1 in flat photobioreactors [74]. The authors demonstrated that Chlorella sp. GN1 grew significantly better on media containing CO(NH2)2 and NaNO3, while algal cell growth was inhibited by increasing the NH4Cl concentration. In the following work [75], CO(NH2)2, NaNO3, KNO3, NH4NO3, NH4Cl, NH4CH3CO2, (NH4)2SO4, and NH4HCO3 were tested for the growth of Chlorella sp. HS2. The authors observed a very low biomass productivity when cultivated in a medium enriched with NH4Cl, NH4NO3, and (NH4)2SO4. However, these works did not apply pH control in the medium, which could affect the results. The use of high concentrations of NH4+ as a nitrogen source appears to release H+, decrease the pH, and inhibit algal growth. pH control is a very important parameter because it influences the physiological state of the cells and the growth kinetics of microalgae. For example, the optimal growth of S. dimorphus was found at pH 7.0, and its growth was almost completely inhibited at pH values of 4.0, 5.0, 10.0, and 11.0 [48]. Worth mentioning is a study on another green alga, Chlorella, for which the authors successfully optimized cultivation to produce important secondary metabolites such as lipids. The researchers optimized the growth medium, urea, phosphate supply, and the stepwise light intensity to increase lipid accumulation [44]. Because the preferred nitrogen source varies among microalgae species, research is necessary to find the optimal nitrogen source and concentration.
The emphasis that researchers are currently placing on reducing the time and costs of growing microalgae of biotechnological importance is certainly justified and includes a search for fast-growing strains, the selection of media, including cheap ones, and the control of metabolism through basic cultivation factors. The selection of important parameters, such as sources of constructive and energy metabolism, is the starting point for expanding the commercialization of algal biotechnologies. Although many microalgae are cultivated heterotrophically or mixotrophically, a photoautotrophic regimen has many advantages (including the capture of carbon dioxide), but despite the existing knowledge, this regimen requires additional, enormous research attention. Thus, the optimal level of light flux with the corresponding optimal concentration of inorganic carbon is critical for growth and serves as a cornerstone for the development of efficient, large-scale technologies to produce algal biomass or their metabolites.
This work confirms that algal growth kinetics and productivity are closely related to the basic parameters of controlled photoautotrophic cultivation and provides a theoretical basis that is important for controlling algal metabolism depending on the cultivation goals. All optimization models showed quite high biomass yields and major metabolic products, but it was the high PPFD regimens that resulted in the highest biomass yield and nutrient removal. Thus, the results obtained from batch cultivation of promising alga within Scenedesmaceae ensure that optimized light conditions will stimulate the production of valuable algal biomass while reducing the cultivation time.
4. Conclusions
The results of this study demonstrated that optimal light conditions significantly increased the productivity of a microalgal cultivation system in photoautotrophic mode. High daily PPFD values stimulated the growth of the microalga Scenedesmaceae strain EZ-B1 and shortened the cultivation time. The combined influence of several parameters is important to consider when choosing cultivation conditions. Carbon dioxide concentrations also affected the growth kinetics and productivity of this algal culture. Thus, by optimizing the cultivation parameters of Scenedesmaceae strain EZ-B1 in modified Bold’s basal medium, its biomass productivity and specific growth rate were increased. Thus, the maximum average values of the specific growth rate, biomass productivity, and total biomass yield were established at 112.32 mol photons m−2 day−1 and 2% CO2 and were 0.72 day−1, 0.69 g L−1 day−1, and 5.23 g L−1, respectively. In addition, the strain EZ-B1 has been shown to be a producer of valuable metabolites such as proteins, carotenoids, and chlorophylls.
Author Contributions
Conceptualization, E.E.Z. and A.M.Z.; methodology, E.E.Z. and A.M.Z.; software, E.E.Z., S.S.B. and A.M.Z.; validation, E.E.Z., S.S.B. and A.M.Z.; formal analysis, E.E.Z., S.S.B. and A.M.Z.; investigation, E.E.Z., S.S.B., K.A.Y. and A.M.Z.; resources, E.E.Z.; data curation, E.E.Z. and A.M.Z.; writing—original draft preparation, E.E.Z.; writing—review and editing, S.S.B. and A.M.Z.; visualization, E.E.Z. and S.S.B.; supervision, A.M.Z.; project administration, A.M.Z.; funding acquisition, E.E.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation and the Cabinet of Ministers of the Republic of Tatarstan within the framework of the scientific project No. 22-24-20044.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
This paper has been supported by the Kazan Federal University Strategic Academic Leadership Program (PRIORITY-2030).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ahmad, A.; Ashraf, S.S. Sustainable food and feed sources from microalgae: Food security and the circular bioeconomy. Algal Res. 2023, 74, 103185. [Google Scholar] [CrossRef]
- Lizzul, A.; Hellier, P.; Purton, S.; Baganz, F.; Ladommatos, N.; Campos, L. Combined remediation and lipid production using Chlorella sorokiniana grown on wastewater and exhaust gases. Bioresour. Technol. 2014, 151, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Goswami, R.K.; Agrawal, K.; Verma, P. Microalgal-based remediation of wastewater: A step towards environment protection and management. Environ. Qual. Manag. 2022, 32, 105–112. [Google Scholar] [CrossRef]
- Yap, J.K.; Sankaran, R.; Chew, K.W.; Munawaroh, H.S.H.; Ho, S.H.; Rajesh, B.J.; Show, P.L. Advancement of green technologies: A comprehensive review on the potential application of microalgae biomass. Chemosphere 2021, 281, 130886. [Google Scholar] [CrossRef] [PubMed]
- Leong, W.H.; Rawindran, H.; Ameen, F.; Alam, M.M.; Chai, Y.H.; Ho, Y.C.; Lam, M.K.; Lim, J.W.; Tong, W.Y.; Bashir, M.J.K.; et al. Advancements of microalgal upstream technologies: Bioengineering and application aspects in the paradigm of circular bioeconomy. Chemosphere 2023, 339, 139699. [Google Scholar] [CrossRef]
- Masojídek, J.; Ranglová, K.; Lakatos, G.E.; Silva Benavides, A.M.; Torzillo, G. Variables governing photosynthesis and growth in microalgae mass cultures. Processes 2021, 9, 820. [Google Scholar] [CrossRef]
- Schultze, L.K.P.; Simon, M.-V.; Li, T.; Langenbach, D.; Podola, B.; Melkonian, M. High light and carbon dioxide optimize surface productivity in a Twin-Layer biofilm photobioreactor. Algal Res. 2015, 8, 37–44. [Google Scholar] [CrossRef]
- Kim, S.; Moon, M.; Kwak, M.; Lee, B.; Chang, Y.K. Statistical optimization of light intensity and CO2 concentration for lipid production derived from attached cultivation of green microalga Ettlia sp. Sci. Rep. 2018, 8, 15390. [Google Scholar] [CrossRef]
- Maltsev, Y.; Maltseva, K.; Kulikovskiy, M.; Maltseva, S. Influence of light conditions on microalgae growth and content of lipids, carotenoids, and fatty acid composition. Biology 2021, 10, 1060. [Google Scholar] [CrossRef]
- Chowdury, K.H.; Nahar, N.; Deb, U.K. The growth factors involved in microalgae cultivation for biofuel production: A Review. Comput. Water Eng. Environ. Eng. 2020, 9, 185–215. [Google Scholar] [CrossRef]
- Diaz-MacAdoo, D.; Mata, M.T.; Riquelme, C. Influence of irradiance and wavelength on the antioxidant activity and carotenoids accumulation in Muriellopsis sp. isolated from the Antofagasta coastal desert. Molecules 2022, 27, 2412. [Google Scholar] [CrossRef]
- González-Camejo, J.; Viruela, A.; Ruano, M.V.; Barat, R.; Seco, A.; Ferrer, J. Effect of light intensity, light duration and photoperiods in the performance of an outdoor photobioreactor for urban wastewater treatment. Algal Res. 2019, 40, 101511. [Google Scholar] [CrossRef]
- Benner, P.; Meier, L.; Pfeffer, A.; Krüger, K.; Oropeza Vargas, J.E.; Weuster-Botz, D. Lab-scale photobioreactor systems: Principles, applications, and scalability. Bioprocess Biosyst. Eng. 2022, 45, 791–813. [Google Scholar] [CrossRef] [PubMed]
- Kratky, L.; Jirout, T.; Belohlav, V. Economic feasibility study for artificial lighting of microalgal flat-panel photobioreactors. Int. J. Environ. Sci. Technol. 2023, 20, 12089–12100. [Google Scholar] [CrossRef]
- Le Gouic, B.; Marec, H.; Pruvost, J.; Cornet, J.F. Investigation of growth limitation by CO2 mass transfer and inorganic carbon source for the microalga Chlorella vulgaris in a dedicated photobioreactor. Chem. Eng. Sci. 2021, 233, 116388. [Google Scholar] [CrossRef]
- Ziganshina, E.E.; Bulynina, S.S.; Ziganshin, A.M. Comparison of the photoautotrophic growth regimes of Chlorella sorokiniana AM-02 in a photobioreactor for enhanced biomass productivity. Biology 2020, 9, 169. [Google Scholar] [CrossRef]
- Lim, Y.A.; Chong, M.N.; Foo, S.C.; Ilankoon, I.M.S.K. Analysis of direct and indirect quantification methods of CO2 fixation via microalgae cultivation in photobioreactors: A critical review. Renew. Sustain. Energy Rev. 2021, 137, 110579. [Google Scholar] [CrossRef]
- Xu, P.; Li, J.; Qian, J.; Wang, B.; Liu, J.; Xu, R.; Chen, P.; Zhou, W. Recent advances in CO2 fixation by microalgae and its potential contribution to carbon neutrality. Chemosphere 2023, 319, 137987. [Google Scholar] [CrossRef]
- Adamczyk, M.; Lasek, J.; Skawińska, A. CO2 biofixation and growth kinetics of Chlorella vulgaris and Nannochloropsis gaditana. Appl. Biochem. Biotechnol. 2016, 179, 1248–1261. [Google Scholar] [CrossRef]
- Assunção, J.; Batista, A.P.; Manoel, J.; da Silva, T.L.; Marques, P.; Reis, A.; Gouveia, L. CO2 utilization in the production of biomass and biocompounds by three different microalgae. Eng. Life Sci. 2017, 17, 1126–1135. [Google Scholar] [CrossRef]
- Samarpita, B.; Abhijit Sarma, R.; Kaustubha, M.; Aloke, K.G. Enhanced CO2 sequestration by a novel microalga: Scenedesmus obliquus SA1 isolated from bio-diversity hotspot region of Assam, India. Bioresour. Technol. 2013, 143, 369–377. [Google Scholar]
- Maryshamya, A.; Rajasekar, T.; Rengasamy, R. Carbon sequestration potential of Scenedesmus quadricauda (Turpin) and evaluation on Zebra fish (Danio rerio). Aquac. Rep. 2019, 13, 100178. [Google Scholar] [CrossRef]
- Ota, M.; Kato, Y.; Watanabe, H.; Watanabe, M.; Sato, Y.; Smith, R.L.; Inomata, H. Fatty acid production from a highly CO2 tolerant alga, Chlorocuccum littorale, in the presence of inorganic carbon and nitrate. Bioresour. Technol. 2009, 100, 5237–15242. [Google Scholar] [CrossRef]
- Ahmad, S.; Iqbal, K.; Kothari, R.; Singh, H.M.; Sari, A.; Tyagi, V.V. A critical overview of upstream cultivation and downstream processing of algae-based biofuels: Opportunity, technological barriers and future perspective. J. Biotechnol. 2022, 351, 74–98. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, D.; Chang, H.; Li, S.; Ho, S.H. Recent progress on converting CO2 into microalgal biomass using suspended photobioreactors. Bioresour. Technol. 2022, 363, 127991. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, X.; Wan, C.; Chen, B.; Bai, F. Efficient biosorption of cadmium by the self-flocculating microalga Scenedesmus obliquus AS-6-1. Algal Res. 2016, 16, 427–433. [Google Scholar] [CrossRef]
- Molino, A.; Mehariya, S.; Karatza, D.; Chianese, S.; Iovine, A.; Casella, P.; Marino, T.; Musmarra, D. Bench-scale cultivation of microalgae Scenedesmus almeriensis for CO2 capture and lutein production. Energies 2019, 12, 2806. [Google Scholar] [CrossRef]
- Toledo-Cervantes, A.; Morales, M.; Novelo, E.; Revah, S. Carbon dioxide fixation and lipid storage by Scenedesmus obtusiusculus. Bioresour. Technol. 2013, 130, 652–658. [Google Scholar] [CrossRef]
- Patil, L.; Kaliwal, B. Effect of CO2 Concentration on growth and biochemical composition of newly isolated indigenous microalga Scenedesmus bajacalifornicus BBKLP-07. Appl. Biochem. Biotechnol. 2016, 182, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Difusa, A.; Talukdar, J.; Kalita, M.C.; Mohanty, K.; Goud, V.V. Effect of light intensity and pH condition on the growth, biomass and lipid content of microalgae Scenedesmus species. Biofuels 2015, 6, 37–44. [Google Scholar] [CrossRef]
- Kaymaz, N.; Cetin, A. The effect of photoperiod on growth, and protein, lipid and chlorophyll content in Scenedesmus acutus. Turk. J. Sci. Technol. 2020, 15, 79–84. [Google Scholar]
- Xie, Y.; Zhao, X.; Chen, J.; Yang, X.; Ho, S.-H.; Wang, B.; Chang, J.-S.; Shen, Y. Enhancing cell growth and lutein productivity of Desmodesmus sp. F51 by optimal utilization of inorganic carbon sources and ammonium salt. Bioresour. Technol. 2017, 244, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Ziganshina, E.E.; Bulynina, S.S.; Yureva, K.A.; Ziganshin, A.M. Growth parameters of various green microalgae species in effluent from biogas reactors: The importance of effluent concentration. Plants 2022, 11, 3583. [Google Scholar] [CrossRef]
- Minhas, A.K.; Gaur, S.; Adholeya, A. Influence of light intensity and photoperiod on the pigment and, lipid production of Dunaliella tertiolecta and Nannochloropsis oculata under three different culture medium. Heliyon 2023, 9, 12801. [Google Scholar] [CrossRef]
- Ramanna, L.; Rawat, I.; Bux, F. Light enhancement strategies improve microalgal biomass productivity. Renew. Sustain. Energy Rev. 2017, 80, 765–773. [Google Scholar] [CrossRef]
- Sartori, R.B.; Vendruscolo, R.G.; Ribeiro, S.R.; Furlan, V.J.M.; Wagner, R.; Zepka, L.Q.; Jacob-Lopes, E. The role of photo-cycles in the modulation of growth and biochemical profile of microalgae: Part I-Food interest compounds. Life 2022, 12, 462. [Google Scholar] [CrossRef] [PubMed]
- Nichols, H.W.; Bold, H.C. Trichosarcina polymorpha Gen. et Sp. Nov. J. Phycol. 1965, 1, 34–38. [Google Scholar] [CrossRef]
- Ziganshina, E.E.; Bulynina, S.S.; Ziganshin, A.M. Growth characteristics of Chlorella sorokiniana in a photobioreactor during the utilization of different forms of nitrogen at various temperatures. Plants 2022, 11, 1086. [Google Scholar] [CrossRef]
- Collos, Y.; Harrison, P.J. Acclimation and toxicity of high ammonium concentrations to unicellular algae. Mar. Pollut. Bull. 2014, 80, 8–23. [Google Scholar] [CrossRef]
- Pedersen, T.C.; Gardner, R.D.; Gerlach, R.; Peyton, B.M. Assessment of Nannochloropsis gaditana growth and lipid accumulation with increased inorganic carbon delivery. J. Appl. Phycol. 2018, 30, 2155–2166. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Xie, S.; Lin, F.; Zhao, X.; Gao, G. Enhanced lipid productivity coupled with carbon and nitrogen removal of the diatom Skeletonema costatum cultured in the high CO2 level. Algal Res. 2022, 61, 102589. [Google Scholar] [CrossRef]
- Wu, M.; Gao, G.; Jian, Y.; Xu, J. High CO2 increases lipid and polyunsaturated fatty acid productivity of the marine diatom Skeletonema costatum in a two-stage model. J. Appl. Phycol. 2022, 34, 43–50. [Google Scholar] [CrossRef]
- Muthuraj, M.; Chandra, N.; Palabhanvi, B.; Kumar, V.; Das, D. Process Engineering for high-cell-density cultivation of lipid rich microalgal biomass of Chlorella sp. FC2 IITG. Bioenergy Res. 2015, 8, 726–739. [Google Scholar] [CrossRef]
- Vanags, J.; Kunga, L.; Dubencovs, K.; Galvanauskas, V.; Balode, M.; Grigs, O. The effect of shaking, CO2 concentration and light intensity on biomass growth of green microalgae Desmodesmus communis. Environ. Res. Eng. Manag. 2015, 70, 73–79. [Google Scholar] [CrossRef][Green Version]
- Nzayisenga, J.C.; Farge, X.; Groll, S.L.; Sellstedt, A. Effects of light intensity on growth and lipid production in microalgae grown in wastewater. Biotechnol. Biofuels 2020, 13, 4. [Google Scholar] [CrossRef]
- Kusuma, T.C.; Pratiwi, R.A.; Septiandre, A.; Zulaikah, S. Effect of light intensity, CO2 gas concentration, culturing period and walne nutrient concentrations on biomass and lipid productivity of Chlorella vulgaris in sea water media. MATEC Web Conf. 2018, 156, 03024. [Google Scholar] [CrossRef][Green Version]
- Li, L.; Chi, K.A. Comparative study of Scenedesmus dimorphus cultured with synthetic and actual wastewater. Water 2021, 13, 3060. [Google Scholar] [CrossRef]
- Chunzhuk, E.A.; Grigorenko, A.V.; Kiseleva, S.V.; Chernova, N.I.; Ryndin, K.G.; Kumar, V.; Vlaskin, M.S. The influence of elevated CO2 concentrations on the growth of various microalgae strains. Plants 2023, 12, 2470. [Google Scholar] [CrossRef]
- Montoya-Vallejo, C.; Guzmán Duque, F.L.; Quintero Díaz, J.C. Biomass and lipid production by the native green microalgae Chlorella sorokiniana in response to nutrients, light intensity, and carbon dioxide: Experimental and modeling approach. Front. Bioeng. Biotechnol. 2023, 11, 1149762. [Google Scholar] [CrossRef]
- Perin, G.; Gambaro, F.; Morosinotto, T. Knowledge of regulation of photosynthesis in outdoor microalgae cultures is essential for the optimization of biomass productivity. Front. Plant Sci. 2022, 13, 846496. [Google Scholar] [CrossRef] [PubMed]
- Rodas-Zuluaga, L.I.; Castañeda-Hernández, L.; Castillo-Vacas, E.I.; Gradiz-Menjivar, A.; López-Pacheco, I.Y.; Castillo-Zacarías, C.; Boully, L.; Iqbal, H.M.N.; Parra-Saldívar, R. Bio-capture and influence of CO2 on the growth rate and biomass composition of the microalgae Botryococcus braunii and Scenedesmus sp. J. CO2 Util. 2021, 43, 101371. [Google Scholar] [CrossRef]
- Chandra, T.S.; Deepak, R.S.; Kumar, M.M.; Mukherji, S.; Chauhan, V.S.; Sarada, R.; Mudliar, S.N. Evaluation of indigenous fresh water microalga Scenedesmus obtusus for feed and fuel applications: Effect of carbon dioxide, light and nutrient sources on growth and biochemical characteristics. Bioresour. Technol. 2016, 207, 430–439. [Google Scholar] [CrossRef]
- Huang, B.; Shan, Y.; Yi, T.; Tang, T.; Wei, W.; Quinn, N.W.T. Study on high-CO2 tolerant Scenedesmus sp. and its mechanism via comparative transcriptomic analysis. J. CO2 Util. 2020, 42, 101331. [Google Scholar] [CrossRef]
- Eze, C.N.; Ogbonna, I.O.; Aoyagi, H.; Ogbonna, J.C. Comparison of growth, protein and carotenoid contents of some freshwater microalgae and the effects of urea and cultivation in a photobioreactor with reflective broth circulation guide on Desmodesmus subspicatus LC172266. Braz. J. Chem. Eng. 2022, 39, 23–33. [Google Scholar] [CrossRef]
- Luu, T.N.; Alsafra, Z.; Corato, A.; Corsaro, D.; Le, H.A.; Eppe, G.; Remacle, C. Isolation and characterization of two microalgal isolates from Vietnam with potential for food, feed, and biodiesel production. Energies 2020, 13, 898. [Google Scholar]
- Soares, J.; Kriiger Loterio, R.; Rosa, R.M.; Santos, M.O.; Nascimento, A.G.; Santos, N.T.; Williams, T.C.R.; Nunes-Nesi, A.; Arêdes Martins, M. Scenedesmus sp. cultivation using commercial-grade ammonium sources. Ann. Microbiol. 2017, 68, 35–45. [Google Scholar] [CrossRef]
- Ho, S.-H.; Chan, M.-C.; Liu, C.-C.; Chen, C.-Y.; Lee, W.-L.; Lee, D.-J.; Chang, J.-S. Enhancing lutein productivity of an indigenous microalga Scenedesmus obliquus FSP-3 using light-related strategies. Bioresour. Technol. 2014, 152, 275–282. [Google Scholar] [CrossRef]
- Levasseur, W.; Perré, P.; Pozzobon, V. A review of high value-added molecules production by microalgae in light of the classification. Biotechnol. Adv. 2020, 41, 107545. [Google Scholar] [CrossRef]
- Parveen, A.; Bhatnagar, P.; Gautam, P.; Bisht, B.; Nanda, M.; Kumar, S.; Vlaskin, M.S.; Kumar, V. Enhancing the bio-prospective of microalgae by different light systems and photoperiods. Photochem. Photobiol. Sci. 2023, 22, 2687–2698. [Google Scholar] [CrossRef]
- Liran, O.; Shemesh, E.; Tchernov, D. Investigation into the CO2 concentrating step rates within the carbon concentrating mechanism of Synechocystis sp. PCC6803 at various pH and light intensities reveal novel mechanistic properties. Algal Res. 2018, 33, 419–429. [Google Scholar] [CrossRef]
- Naira, V.R.; Das, D.; Maiti, S.K. Real time light intensity based carbon dioxide feeding for high cell-density microalgae cultivation and biodiesel production in a bubble column photobioreactor under outdoor natural sunlight. Bioresour. Technol. 2019, 284, 43–55. [Google Scholar] [CrossRef]
- Cao, K.; Cui, Y.; Sun, F.; Zhang, H.; Fan, J.; Ge, B.; Cao, Y.; Wang, X.; Zhu, X.; Wei, Z.; et al. Metabolic engineering and synthetic biology strategies for producing high-value natural pigments in microalgae. Biotechnol. Adv. 2023, 68, 108236. [Google Scholar] [CrossRef]
- Bialevich, V.; Zachleder, V.; Bišová, K. The effect of variable light source and light intensity on the growth of three algal species. Cells 2022, 11, 1293. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, J.; Song, M.; Yu, J.; Yu, X.; Ding, B.; Chen, X. Analysis of photosynthetic pigments pathway produced by CO2-toxicity-induced Scenedesmus obliquus. Sci. Total Environ. 2023, 867, 161309. [Google Scholar] [CrossRef]
- Colgrave, M.L.; Dominik, S.; Tobin, A.B.; Stockmann, R.; Simon, C.; Howitt, C.A.; Belobrajdic, D.P.; Paull, C.; Vanhercke, T. Perspectives on future protein production. J. Agric. Food Chem. 2021, 69, 15076–15083. [Google Scholar] [CrossRef] [PubMed]
- Lucakova, S.; Branyikova, I.; Hayes, M. Microalgal proteins and bioactives for food, feed, and other applications. Appl. Sci. 2022, 12, 4402. [Google Scholar] [CrossRef]
- Metsoviti, M.N.; Papapolymerou, G.; Karapanagiotidis, I.T.; Katsoulas, N. Effect of light intensity and quality on growth rate and composition of Chlorella vulgaris. Plants 2019, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Salman, J.; Baiee, M. Effect of phosphorus concentration and light intensity on protein content of microalga Chlorella vulgaris. Mesop. Environ. J. 2016, 2, 75–86. [Google Scholar]
- Kendirlioglu, G.; Agirman, N.; Cetin, A.K. The effects of photoperiod on the growth, protein amount and pigment content of Chlorella vulgaris. Turk. J. Sci. Technol. 2015, 10, 7–10. [Google Scholar]
- Ziganshina, E.E.; Bulynina, S.S.; Ziganshin, A.M. Assessment of Chlorella sorokiniana growth in anaerobic digester effluent. Plants 2021, 10, 478. [Google Scholar] [CrossRef]
- Ziganshina, E.E.; Belostotskiy, D.E.; Bulynina, S.S.; Ziganshin, A.M. Influence of granular activated carbon on anaerobic co-digestion of sugar beet pulp and distillers grains with solubles. Processes 2020, 8, 1226. [Google Scholar] [CrossRef]
- Ziganshina, E.E.; Bulynina, S.S.; Ziganshin, A.M. Impact of granular activated carbon on anaerobic process and microbial community structure during mesophilic and thermophilic anaerobic digestion of chicken manure. Sustainability 2022, 14, 447. [Google Scholar] [CrossRef]
- Feng, P.; Xu, Z.; Qin, L.; Alam, A.; Wang, Z.; Zhu, S. Effects of different nitrogen sources and light paths of flat plate photobioreactors on the growth and lipid accumulation of Chlorella sp. GN1 outdoors. Bioresour. Technol. 2020, 301, 122762. [Google Scholar] [CrossRef] [PubMed]
- Nayak, M.; Suh, W.I.; Chang, Y.K.; Lee, B. Exploration of two-stage cultivation strategies using nitrogen starvation to maximize the lipid productivity in Chlorella sp. HS2. Bioresour. Technol. 2019, 276, 110–118. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).