1. Introduction
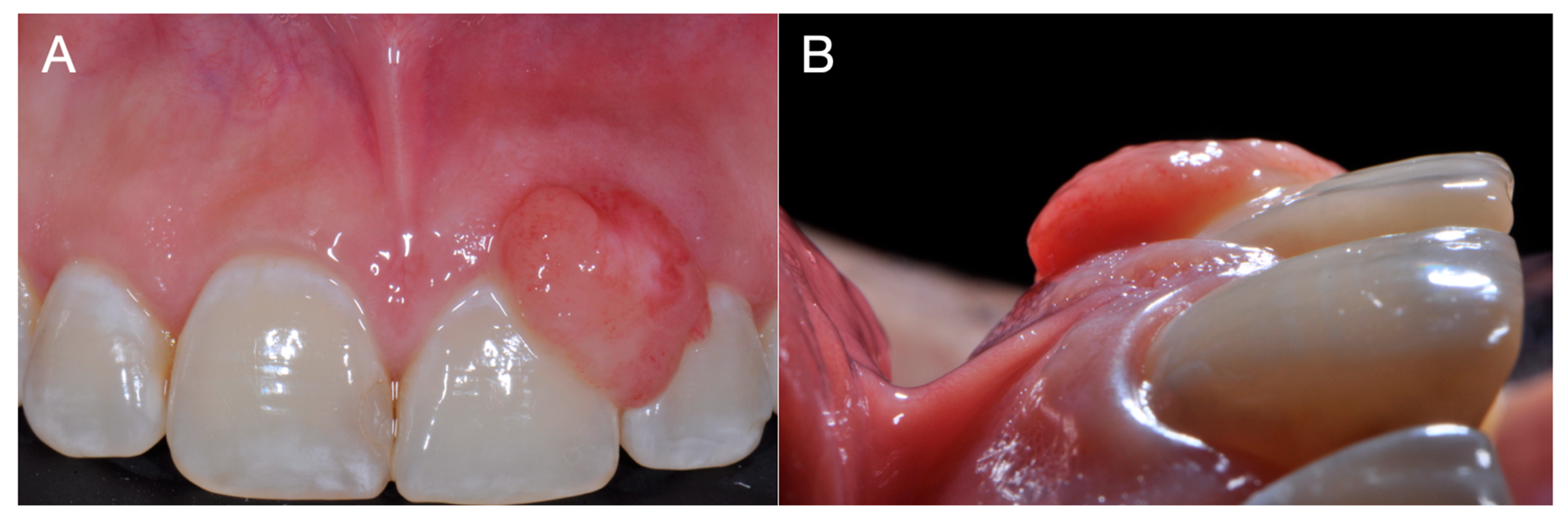
Peripheral giant cell granuloma (PGCG) is the most common oral giant cell lesion. It appears as an extraosseous soft tissue nodule that originates from the connective tissue of the periosteum or the periodontal ligament membrane, and is localized within the soft tissues [
1,
2,
3,
4]. This pathology is identified by other names, such as giant cell reparative granuloma, osteoclastoma, giant cell epulis, and giant cell hyperplasia of the oral mucosa [
5].
The lesion has a predilection for the buccal gingiva rather than the lingual or palatal. It occurs more frequently in the lower jaw rather than the upper jaw, especially in the incisors and canines [
3,
6]. It is often located in the interdental papilla, edentulous alveolar ridge, or marginal gingiva in completely or partially edentulous patients, resulting in gingival contour changes that can cause tooth mobility and displacement [
3,
4,
7]. It is more frequent in women than in men, with a 2:1 incidence ratio, and the incidence is higher in the age group of 30–50 years [
3,
5,
6,
8]. While rare, it may occur in pediatric patients as well [
3,
5]. In most cases, PGCG is asymptomatic; however, the presence of the lesion can be bothersome when it interferes with occlusion or is associated with an infectious or ulcerative process [
3,
4,
5]. The size of the lesion may vary from a small papule to a massive enlargement which does not typically exceed a diameter of 5 cm [
3]. In certain cases, progressive growth may lead to significant tumors that compromise speech, chewing, or swallowing, with evolution time ranging from three months to four years [
3,
4].
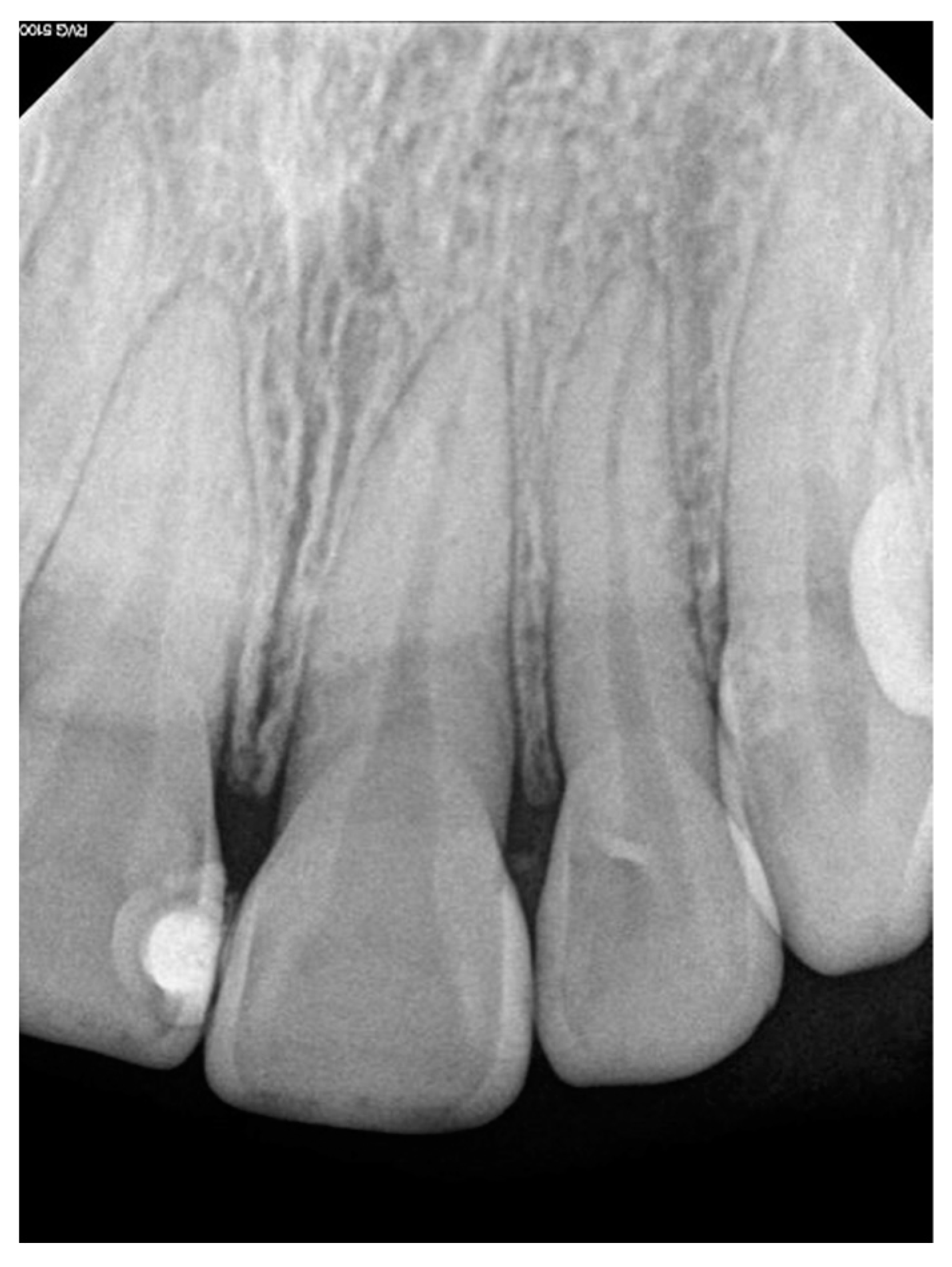
PGCG develops extraosseously; however, in edentulous areas, a cup-shaped superficial erosion of the underlying bone may be noted, termed bone flattening or “saucerization”. However, in dentate areas widening of the periodontal ligament space, destruction of the alveolar crest, and tooth displacement may be noted [
3,
4].
Although the etiology of PGCG remains uncertain, it may be related to various factors, including trauma, inflammatory foci, nutritional impact, maladjusted restorations, overextended fillings, occlusal trauma, genetic predisposition, dental calculus, and the use of orthodontic appliances [
3,
5,
9]. Additionally, hormonal changes during pregnancy have been considered a possible contributing factor. However, due to the limited number of clinical cases described in the literature, the association between PGCG and pregnancy remains controversial [
10,
11,
12]. The objective of this study was to describe the surgical management of a clinical case of upper jaw PGCG associated with pregnancy.
3. Discussion
Peripheral giant cell granuloma (PGCG) is an exophytic, extra-osseous, and non-neoplastic pathology that originates in the periosteum or periodontal ligament [
1]. The etiology of PGCG is unknown; however, hormones have been considered as contributing factors, although this association is controversial. Few clinical cases of pregnancy-associated PGCG have been described [
10,
11,
12]. This study presents a case of pregnancy-associated PGCG in the upper jaw.
The complexity of the etiology of GPCG involves several hypotheses of reactive processes, the nature of multinucleated giant cells, and the influence of cytokines and differentiation factors [
1,
4,
13,
14,
15]. GPCG is generally considered a reactive lesion, which means that it is probably triggered by external factors such as the presence of local irritants such as dental biofilm, dental calculus, overflowing restorations, indiscriminate use of toothpicks, chronic infections, food, and tooth impaction [
1,
4,
13,
14,
15]. One theory of the nature of the multinucleated giant cells in GPCG proposes that they are osteoclasts, possibly residual from physiological tooth resorption or in reaction to injury to the periosteum; evidence supporting this includes the presence of receptors for calcitonin in these cells and their ability to excavate bone in vitro [
4]. On the other hand, studies suggest that the giant cells in the GPCG could represent a reactive component derived from bone marrow mononuclear cells in response to an unknown stimulus from the stromal tissue [
4]. Recently, research has shown that stromal cells in GPCG secrete various cytokines and differentiation factors. These factors are believed to stimulate the migration of monocytes into the tumor tissue and promote the fusion of these cells into multinucleated osteoclast-like giant cells [
4,
13]. The imbalance in the Receptor Activator of Nuclear Factor κB Ligand (RANKL)/Receptor Activator of Nuclear Factor κB (RANK)/osteoprotegerin (OPG) system could be a contributing factor in the development of GPCG. Alteration in the balance between RANKL and OPG, either by an increase in RANKL or a decrease in OPG, could lead to increased differentiation and activity of osteoclasts and potentially to the formation of multinucleated giant cells [
4]. However, it is important to recognize that the etiology of GPCG is multifactorial, and this last mechanism is only one of the possible factors involved.
The roles played by sex hormones in pregnancy in relation to the initiation, development, and progression of PGCG are controversial. During pregnancy, hormonal changes, particularly those involving estrogen and progesterone, promote alterations in the oral cavity. These alterations encompass physiological, vascular, microbiological, and cellular changes, creating a favorable environment for the initiation and development of various pathologies [
16,
17,
18,
19,
20,
21,
22,
23]. Among the physiological changes that significantly contribute to the prevalence of oral lesions, especially in gingival tissue, are an approximate 1500 mL increase in blood volume (normal volume in women being 4 to 4.5 L), establishing a framework clinical diagnosis for physiological anemia, which can stimulate gingival bleeding and the possible increase in blood glucose levels due to the decrease in sensitivity to insulin by pregnancy hormones, potentially generating a decrease in the elasticity and increase in the permeability of blood capillaries [
16,
22]. Alterations in the microcirculatory system during pregnancy involve swelling of endothelial cells, increased adhesion of platelets and leukocytes to vessel walls, microthrombi formation, changes in perivascular mast cells, increased vascular permeability, and vascular proliferation [
16,
22]. Estrogen and progesterone, in conjunction with inflammatory mediators, can induce changes in vascular responses and connective tissue turnover in the periodontium, leading to a higher prevalence of inflammatory processes during hormonal fluctuations [
22]. Hormones during pregnancy can induce an increase in Vascular Endothelial Growth Factor (VEGF) and basic Fibroblast Growth Factor (bFGF), which are activators of angiogenesis, altering the vascular response. VEGF is particularly important, as it acts as a mitogenic factor for endothelial cells, triggering neovascularization within granulomas, and thereby contributing to their appearance and growth. Additionally, other vascular factors such as connective tissue growth factor, angiopoietin-1, angiopoietin-2, Tie-2, ephrinB2, ephrinB4, and decorin have been reported to be involved in profound inflammation and angiogenesis in granulomas. Therefore, the set of these factors, stimulated or modulated by pregnancy hormones, could facilitate the formation of peripheral giant cell granulomas by promoting a proinflammatory and proangiogenic environment in the oral cavity. The oral microbiota undergo changes during pregnancy, in particular an increase in populations of aerobic and anaerobic bacteria such as
Porphyromonas gingivalis, Bacteroides melaninogenicus, and
Prevotella intermedia [
16,
19]. These bacteria affect target cells such as keratinocytes and fibroblasts. Cellular changes in the gingival epithelium are characterized by decreased keratinization, an increase in glycogen content, proliferation of fibroblasts, blockage of collagen degradation, and an increase in the rate of oral mucosa desquamation, favoring bacterial proliferation and resulting in oral lesions [
19]. These changes lead to alterations in the epithelial barrier, resulting in heightened responses to irritants, especially in the dental biofilm [
18]. Additionally, there is a decrease in the antimicrobial activity of peripheral neutrophils, which are essential components of the innate immune defenses of periodontal tissues. Furthermore, in the oral cavity there is a decrease in pH and a consequent reduction in salivary buffer capacity during pregnancy. Combined with modifications or alterations in eating habits and oral care practices, this increases the risk of microorganism growth and the development of oral lesions [
16]. These conditions generated by hormonal changes during pregnancy favor the prevalence and/or severity of certain conditions in the oral cavity [
22]. This effect is particularly noticeable after the second month, as it is precisely at this point that there are increases in plasma levels of estrogen and progesterone [
16,
21]. In their clinical case description of PGCG associated with pregnancy, Caillouette and Mattar (1978) reported that high levels of estrogens and gonadotropic hormones increase the deposition of glycogen in the epithelium and exert a positive trophic effect on the buccal mucosa [
10]. These changes lead to hyperplasia of the papillae and blood vessels, resulting in increased vascularity, which in turn favors the development of PGCG. The surgical management of this clinical case involved the extraction of three teeth from the lower jaw to facilitate tumor removal. Additionally, the lesion extended to the mandibular canal, necessitating the sacrifice of the inferior alveolar nerve. In contrast, in the present case no dental organ or nerve sacrifice was necessary for PGCG removal. Subsequently, Csillag et al. (1997) described a clinical case of GCG removal from the mandible which required the extraction of the second molar associated with the lesion. The authors suggested that hormonal and hemodynamic changes during pregnancy may act as stimulating factors for GCG. On the other hand, Patil et al. (2018) reported a clinical case involving the surgical removal of a PGCG in a patient who manifested the lesion during the last trimester of pregnancy. The patient presented generalized gingival inflammation, bleeding on probing, and calculus during clinical examination. Additionally, radiographic examination identified bone resorption in the molar region. In the present case report, no alterations were observed in the soft or hard tissues of the oral cavity. In a descriptive study involving 26 patients with PGCG, Günhan (1998) identified the presence of estrogen and progesterone receptors using the immuno-peroxidase technique [
23]. Estrogen receptor positivity was found in stromal cells in fourteen patients, and in ten of these patients the osteoclast-like giant cells exhibited estrogen receptor immunostaining. Based on these results, it was concluded that the cells forming the peripheral giant cell granuloma might be potential targets for estrogens and that the lesion might be influenced by hormonal conditions. In contrast, Shirani (2008) carried out a descriptive study in 20 patients to determine the relationship between PGCG and circulating levels of sex hormones (testosterone, estrogen, and progesterone) [
24]. However, no significant association was found between PGCG and sex hormones, and it was concluded that PGCG does not directly depend on testosterone, estrogen, and progesterone studies [
25]. Therefore, it was suggested that the role of sex hormones in the development of PGCG might be secondary to local factors such as trauma and poor oral hygiene. In the clinical case described here, we believe that the changes in the oral cavity caused by hormones during pregnancy may be considered as factors that contribute to the onset and progression of PGCG, as no other local triggering conditions for this lesion were present. However, further studies are needed to clarify this association.
Generally, PGCGs occur in soft tissue without affecting the bone, as was observed in the present study, which is consistent with findings from other studies [
13,
26]. However, giant cells may be activated as an inflammatory response, making them participate as osteoclasts and leading to the resorption of alveolar bone [
3]. Osteoclastic activity may be associated with exuberant stromal cell proliferation and bone remodeling [
21]. Resorptive functions may be modulated by estrogen receptors on osteoclasts. On the other hand, the proliferation of osteoclasts in the pathology may be related to other factors.
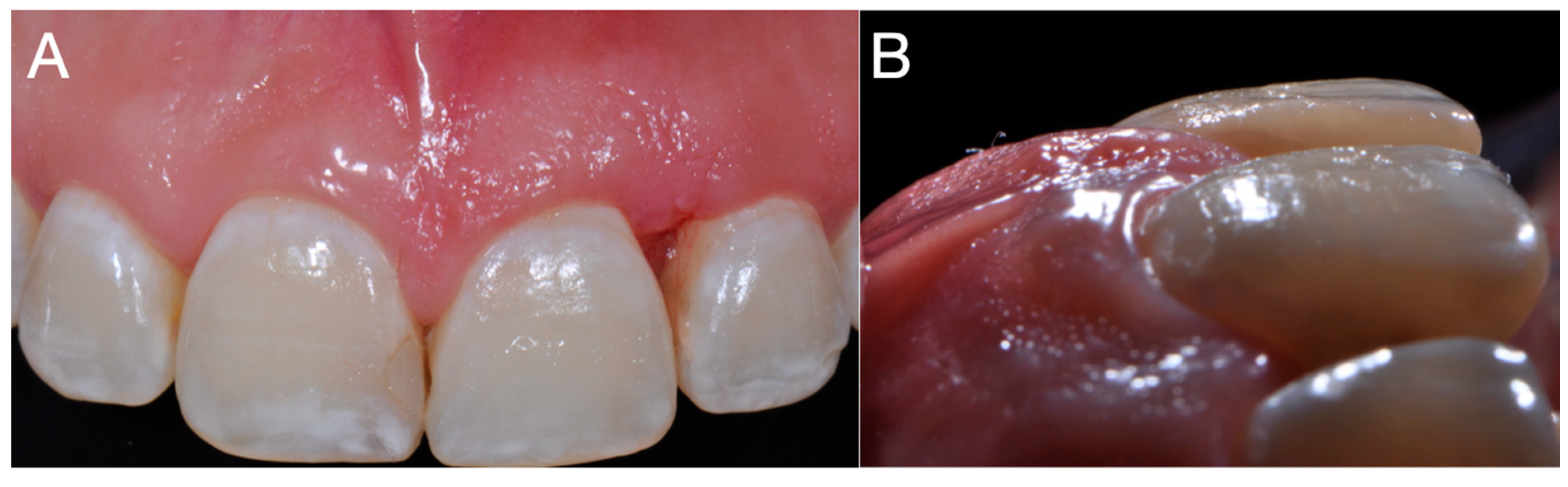
The treatment of choice for PGCG is surgical excision, as implemented in the present clinical case and in previous studies [
1,
7,
14,
27]. This surgical removal method involves excising the lesion along with extensive curettage of the periosteum at the base of the lesion. The associated teeth do not need to be extracted if they are healthy. In fact, it has even been suggested that extraction is contraindicated [
2,
27]. In addition, it is advisable to remove local irritants. Various treatment modalities have been suggested for PGCG, including intralesional steroids, subcutaneous or nasal calcitonin, subcutaneous injections of alpha-interferon, and the protein tyrosine kinase inhibitor imatinib. However, there is insufficient evidence to support the efficacy of these methods [
27]. Using a CO
2 laser or laser resection is another viable treatment option for granuloma removal [
27]. The CO
2 laser provides advantages, including reduced bleeding risk and superior coagulation properties. However, it may not be suitable when the lesion affects adjacent bone, in which case thorough surgical curettage becomes necessary [
27]. In a study by Chaparro (2005), CGCG excision and biopsy was performed via CO
2 laser in an operation involving two cases and with a cold scalpel in three cases [
15]. Laser resection is a technique that allows wound sterilization, causes less intraoperative bleeding, does not require sutures, and provides greater postoperative comfort. However, if the resection is superficial, the condition may reoccur [
2]. The surgical modality followed by curettage was implemented in the present case because the lesion involved adjacent bone and because adding curettage reduces the risk of recurrence compared to excision alone.
The recurrence rate of PGCG varies widely [
28,
29]. Chrcanovic (2018) analyzed 2824 reported cases of PGCG lesions in the literature and found that the overall recurrence rate after treatment was 9.5% [
6]. The analysis revealed that when PGCG was treated solely with surgical excision, the recurrence rate was 16%. However, when surgical excision was followed with curettage, the chance of recurrence was reduced by 85% relative to excision alone. Interestingly, other factors such as age, lesion size, duration of follow-up, gender, location of lesion, clinical symptoms, and bone erosion did not appear to influence the probability of recurrence. Additionally, recurrence is associated with not eliminating local irritating factors adjacent to the site where the injury developed, such as hard deposits, food impact, ill-fitting dentures, overhanging restorations, etc. [
3,
12].
Healthcare professionals need to be aware of the potential impact of pregnancy-associated hormones on the oral cavity. Dentists should be vigilant in monitoring oral lesions during prenatal care. Managing PGCG in any trimester of pregnancy requires a multidisciplinary approach involving dental professionals, obstetricians, and other healthcare providers. Collaboration among specialists ensures the formulation of a safe and effective treatment plan that considers the unique challenges posed by pregnancy [
28]. Having a patient-centered approach that addresses her concerns is essential for optimizing treatment outcomes and overall patient satisfaction. Providing reassurance and addressing any anxiety or concerns regarding dental treatment during pregnancy lead to better patient compliance and outcomes [
29]. Additionally, it is important to inform pregnant patients that the use of local anesthesia during surgery is safe and does not pose a significant risk to the fetus [
30]. Regarding surgical management in pregnant women with PGCG, there are no special considerations; however, it is suggested that in this patient group the use of 2% lidocaine with epinephrine 1:200,000 is the anesthetic agent that best balances safety and effectiveness [
22]. Semisupine positioning, blood pressure monitoring, and reassurance are recommended for high-risk mothers in order to reduce the risk of transient changes in blood pressure, hypoxemia, and hypoglycemia [
22]. In patients with underlying conditions, such as eclampsia, hypertension, hypotension, and gestational diabetes, dentists should use epinephrine with caution and monitor the anesthetic dose [
19]. Implementing oral care recommendations for pregnant women is essential for aiding the prevention and early detection of developing oral lesions. These recommendations include promoting good oral hygiene practices [
30,
31,
32,
33], scheduling regular dental check-ups [
33], maintaining a balanced diet [
34], and implementing monitoring and follow-up when lesions are observed [
35].
Finally, future research should aim to evaluate the association between circulating levels of estrogen and progesterone in various trimesters of pregnancy and the presence of PGCG. This research would help to clarify the controversial link between hormonal changes during pregnancy and this condition. Additionally, further studies are needed to investigate the benefits of the various treatment modalities employed for PGCG.