Abstract
Transcranial magnetic stimulation (TMS) is a flexible, non-invasive technique that involves the production of a brief magnetic field to stimulate the conductive structures of the brain. When TMS is specifically employed as a single- or paired-pulse over the motor cortex, the function and integrity of the descending motor pathways can be assessed through the generation of a motor evoked potential (MEP). Important MEP-derived measures include the motor threshold, MEP amplitude and latency, central motor conduction time, silent period, intracortical inhibition, and intracortical facilitation. These functional measures may have use in individuals with multiple sclerosis (MS), a common chronic autoimmune disorder of the central nervous system, due to their useful diagnostic and prognostic implications. As a representation of excitability and conductivity, TMS measures may have the potential to serve as objective markers of corticospinal tract integrity, which is a major aspect of clinical disability in MS. Additionally, TMS may be employed to help monitor and provide insight on the effects of therapies for patients with MS over a longitudinal timeframe. In this review, we focus on the application of TMS in the context of MS, with an emphasis on the relationship between TMS measures and widely used clinical assessment measures used for patients with MS.
1. Introduction
Transcranial magnetic stimulation (TMS) is a non-invasive technique that uses a magnetic coil placed over the scalp to electromagnetically induce a current over the adjacent brain. It is a well-tolerated and relatively simple way of directly inducing action potentials within conductive structures. TMS is a flexible technique that has the potential to be used for diagnostic, prognostic, and therapeutic purposes in a variety of diseases.
One disease of particular relevance is multiple sclerosis (MS), a complex autoimmune disease that can have a debilitating effect on the central nervous system. MS typically has a mean onset at 20 to 30 years of age and is more common in females than males, at a ratio of approximately 3:1 [1]. The onset of disease in younger individuals consequently leads to a disproportionate impact on personal and professional development. Depending on the severity and progression, individuals with MS may experience a diminished quality of life stemming from a loss in physical and cognitive function, poorer emotional well-being, and an inability to work or participate in activities [2]. With the prevalence of MS growing on a yearly basis [3], there is a growing need for tools such as TMS that can be used for management and monitoring over the course of the disease. This paper will explore the basic principles of TMS, what measures can be derived from TMS, and the current body of literature relating to the use of TMS in patients with MS.
2. Basic Principles of Transcranial Magnetic Stimulation (TMS)
TMS involves the generation of a magnetic field through an external wire coil to non-invasively stimulate the conductive structures of the adjacent brain. A large pulse of current is passed through a wire coil located on the scalp which results in a strong, brief magnetic field being generated. This resulting field excites neurons and triggers an action potential in a specific area of the brain. TMS can be applied with a variety of frequencies to achieve different purposes. Single- or paired-pulse TMS is often used to explore brain and conductive pathway function, while repetitive TMS (rTMS) can be employed to modulate brain activity for therapeutic purposes in neurological conditions such as depression and Parkinson’s disease [4,5]. In the case of MS, single- and pulse-paired TMS are predominantly employed as useful tools for the purposes of diagnosis, prognostication, and monitoring [6].
Safety Profile
TMS is a generally well-tolerated and safe technique. The majority of side effects arising from TMS, especially single- and paired-pulse studies, are typically mild and transient, with headaches, scalp pain, neck pain, and small changes in hearing sensitivity being the most common [7,8]. In studies employing rTMS, other side effects such as transient acute hypomania induction have been reported in very rare cases. Other side effects such as histotoxicity and structural brain changes have inconsistent data associated with their frequency [8]. By far the most severe adverse event associated with TMS is the induction of seizures. However, the reported frequency is extremely low, with a survey by Lerner et al. suggesting a rate at less than 1 event per 60,000 sessions [9]. An important risk factor for seizures associated with TMS applications includes having a neuropsychiatric disease, such as epilepsy. Other general factors may include sleep deprivation, stress, anxiety, and medical conditions that can lower one’s seizure threshold [10]. According to 2020 expert guidelines from Rossi et al., a history of epilepsy or ferromagnetic metal implants within the head typically serve as exclusionary criteria for TMS application, but this is not necessarily an absolute contraindication [10]. In these cases, it is important for clinicians and researchers to consider the necessity of TMS, as well as the particular protocol employed.
In the case of TMS being used in conjunction with MRI, most cases involve TMS being conducted before or after imaging. However, for concurrent use, it is important that dedicated, MRI-approved TMS coils that do not contain ferromagnetic materials are employed. Manufacturer guidelines may also require these coils to be maintained regularly [8,10].
3. TMS Measures
When TMS is used to stimulate part of the motor cortex, a contraction of the contralateral muscle is produced. Coupled with electromyography (EMG), the elicited motor evoked potential (MEP) can be measured as a functional correlate of conduction through the central and peripheral motor pathways, including the motor cortex, descending corticospinal tract, peripheral nerve, and target muscle. On the basis of abnormalities in MEPs, the resulting measures may provide insight on the presence or absence of dysfunction in the motor pathways [5]. In patients with MS, the targets used to obtain MEPs in the upper limb are typically the abductor pollicis brevis and first dorsal interosseous muscles, as illustrated in Figure 1. In the lower limb, the abductor hallucis and tibialis anterior muscles are the frequently assessed targets [11]. Important measures derived from single- and pulse-paired TMS include the following: motor threshold, MEP amplitude and latency, central motor conduction time, silent period, and short-interval intracortical inhibition and facilitation [7].
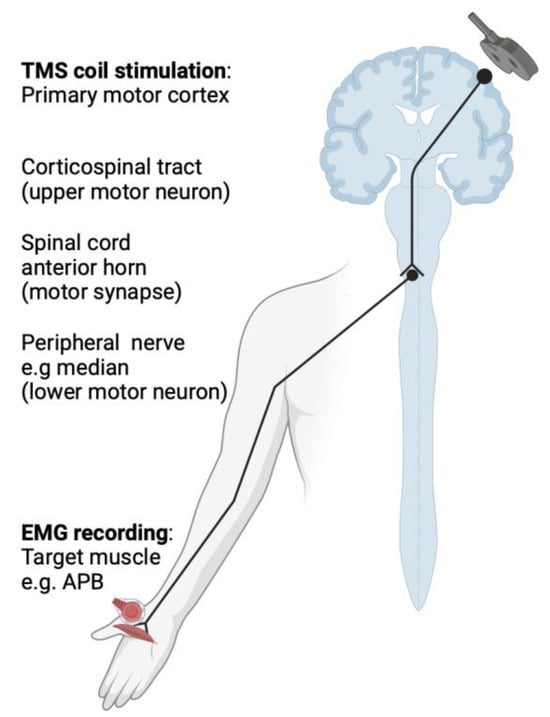
Figure 1.
The basic principles of transcranial magnetic stimulation, depicting the conduction pathway along the corticospinal tract to the target contralateral abductor pollicis brevis.
3.1. Motor Threshold
The motor threshold (MT) is defined as the minimum stimulus intensity capable of evoking MEPs with a given peak-to-peak response in at least 50% of trials [7]. In the clinical use of TMS, the MT must be determined as a foundational step, given that the stimulus intensity must be adjusted to the MT for both the individual patient and session to elicit reliable MEPs for analysis [12,13]. Thresholds for both resting and active muscles can be determined using single-pulse TMS in resting and contracted (at approximately 20% of maximal muscle strength) muscles, respectively, with contracted muscles demanding a lower threshold. Additionally, the intensity of stimulus required to reach the MT will also depend on the location of the target muscle, as lower thresholds are reported for upper limb muscles and higher thresholds are reported for lower limb muscles [13]. In the literature, the MT has been described as a representation for the excitability of the corticospinal pathway [12,14,15], and previous studies have reported abnormal MTs in patients with MS [16]. However, the clinical utility of the MT on its own may be somewhat limited due to wide variation between and even within individuals. Factors such as age, wakefulness, technical setup, pharmacological influence, and posture can all affect the MT [7,12].
3.2. MEP Latency and Amplitude
After the MT for an individual is determined, TMS pulses can be delivered at a suprathreshold intensity to produce MEP recordings. Subsequently, various parameters can be derived, as seen in Figure 2. Of particular importance is the latency of MEP onset and amplitude, which is measured as the peak-to-peak (maximal positive to maximal negative) difference in voltage [12]. If TMS is employed while the target muscle is under contraction, the MEP latency is shorter, and the amplitude is greater. This phenomenon is thought to be the result of voluntary contraction increasing the excitability of motor neurons and decreasing the threshold required to fire [17]. While the MEP amplitude is commonly reported in studies involving TMS, it is also subject to the same factors that influence the MT, leading to high variability between trials [12,18]. Several experimental studies in healthy patients have shown that 10–30 trials may be required for reliable intra- and inter-session reliability of MEP amplitude and latency [19,20,21]. An additional paper by Ammann et al. [18] suggested that the number of trials needed for single-subject reliability largely depends on the error considered to be acceptable by the experimenter, while the number of subjects is more important for hypothesis testing. This is of relevance to the potential utility of MEP amplitude as a measurement of corticospinal excitability.
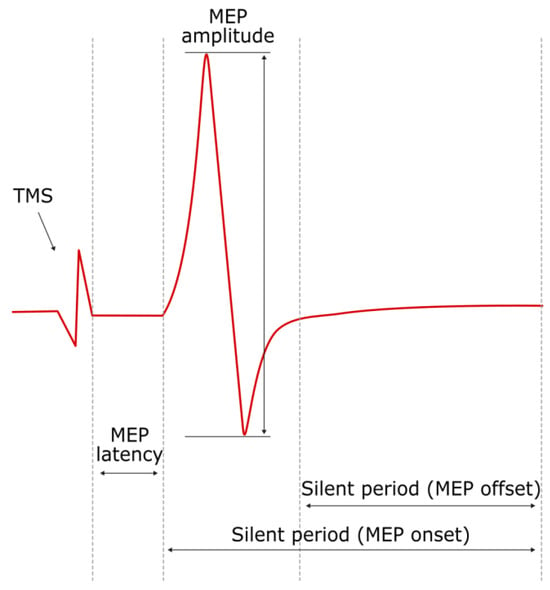
Figure 2.
Example of motor evoked potential produced via TMS to stimulate the motor cortex to stimulate an EMG motor response by the target muscle, showing (i) MEP amplitude (peak-to-peak); (ii) MEP latency; and (iii and iv) silent period from MEP onset and offset.
3.3. Central Motor Conduction Time
To calculate the central motor conduction time (CMCT), both cortical and peripheral measures of latency are required. The peripheral motor latency can be determined by delivering foraminal magnetic stimulation at the level of the exiting motor nerve roots in the appropriate cervical or lumbar vertebral foramen to stimulate the nerve roots. Notably, the intensity of foraminal magnetic stimulation is recommended to be just above the suprathreshold to prevent stimulation of the nerve at a distal location. Subtracting the peripheral latency from the latency of the MEP evoked through TMS will produce the CMCT [12]. This removes the contribution of the peripheral nervous system from the overall latency; therefore, the CMCT is said to be a representation of the conduction time between the primary motor cortex and motor neurons in the spinal cord. It is important to consider the potential effects of physiological confounders such as age, height, and sex on CMCT. A paper by Rossini et al. [13] reported that most studies have found no/weak correlation between age and sex with CMCT. Regarding height, there appears to be no correlation between height and CMCT for the upper limbs; however, there exists a strong correlation between height and CMCT for the lower limbs [22,23,24]. Abnormal CMCT indicates potential dysfunction within the conducting pathway.
3.4. Silent Period
The contralateral silent period (SP) can be defined as the interval in which EMG activity is interrupted and silenced following an MEP upon delivery of a single TMS pulse to a contralateral muscle in a voluntarily state of tonic contraction (typically at 10–20% of the maximal contraction) [25]. Upon the EMG response, the SP immediately follows an MEP and can be defined from either the MEP onset or offset (Figure 2). There is some debate over the mechanisms that contribute to the silent period [26,27]. Previously, the SP has been thought of as wholly reflecting the effects of intracortical inhibition, owing to the activity of GABAergic neurons, and subsequently has been defined as the “cortical” silent period. However, it has been suggested that there is some degree of inhibition that occurs at the level of the spinal cord which contributes to the silent period, particularly in the early portion (around the first 50 ms) of the silent period [27]. Similarly, the ipsilateral silent period (iSP) can also be observed in the ipsilateral homologous muscle upon delivery of TMS using the previously specified parameters. The iSP is thought to be mediated by transcallosal inhibitory neurons from the stimulated to the non-stimulated motor cortex, thereby suggesting that the iSP could reflect the functional integrity of the corpus callosum [6,28].
3.5. Short-Interval Intracortical Inhibition and Facilitation
Using pulse-paired TMS, intracortical inhibition and intracortical facilitation can be examined at varying interstimulus intervals (ISIs). In these assessments, a conditioning stimulus (CS) is first delivered, followed by a test stimulus (TS) at varying interstimulus intervals (ISIs) using a target muscle that is typically at rest. The MEP amplitude from pulse-paired assessments is then compared to that of single-pulse assessments. To assess short-interval intracortical inhibition (ICI), first a subthreshold conditioning stimulus (CS, typically 80% of the RMT) is delivered, followed by a suprathreshold stimulus (typically 120% of the RMT), which is delivered with ISIs of 1–5 ms. This results in an inhibited test response that elicits a lower MEP amplitude. At longer ISIs (7–30 ms), intracortical facilitation (ICF) occurs instead, and the MEP amplitude of the test response increases [7]. ICI is thought to arise from the activation of a low-threshold inhibitory system, leading to a hyperpolarizing inhibitory postsynaptic potential [29]. On the other hand, ICF is thought to arise from the summation of excitatory postsynaptic potentials [29]. ICI and ICF measures have been found to be abnormal in some MS patients [30,31,32], as well as those with other neurodegenerative conditions such as Parkinson’s disease [33]. This is thought to reflect changes in cortical excitability [30]. However, previous studies have reported mixed results regarding the potential association between disability, ICI, and ICF measures [31,34,35].
4. Overview of Multiple Sclerosis
Multiple sclerosis is a chronic autoimmune disease of the central nervous system pathologically characterized by focal and CNS compartmentalized inflammation, demyelination, and axonal loss. It is estimated that 2.8 million people live with MS across the world, with a global increase in prevalence year after year [3]. Clinically, patients with MS often develop prominent motor impairment through their disease including weakness, spasticity, and gait dysfunction, reflective of focal demyelinating lesions involving central motor pathways of the brain and spinal cord [1]. While the pathophysiology of MS involves a heterogeneous spectrum of both focal and diffuse inflammatory and degenerative processes which are present at all disease timepoints, it is classically categorized into discrete clinical phenotypes which have diagnostic, prognostic, and therapeutic implications. These subtypes will be summarized below in Table 1. Only when biomarkers (such as TMS) are established as adequate surrogates of the underlying mechanisms of injury and repair will the field be able to move beyond these crude categorical clinical descriptors.

Table 1.
A summary of clinical phenotypes of MS and their characteristics.
The Use of TMS Measures in MS
In this paper, several studies that have examined TMS measures in patients with MS have been compiled. Where applicable, any MRI measures were also included. The relationship between the TMS measures, clinical assessment measures, and/or MRI measures reported by each study has been summarized below in Table 2. Assessments of conduction both to the upper limbs and lower limbs using TMS have been reported within these studies. However, it is important to note that sensorimotor impairments of the lower limb are often prominent in patients with MS, and so they are the primary focus of many studies [37]. One potential limitation to the generalizability of studies assessing TMS and MS associations is that patients with various subtypes of MS are often grouped together, or limited to certain subtypes of MS.

Table 2.
TMS parameters and clinical assessment measures utilized in studies of patients with MS.
5. TMS Measures in the Diagnosis and Evaluation of MS
In individuals with MS, measures of CMCT are commonly abnormal due to dysfunction involving the corticospinal tract. Numerous studies [31,38,39,40,41,42,43,44,46,48,49,50,51,53,58] have consistently demonstrated markedly prolonged CMCT values in groups of MS patients when compared to control groups. The correlation between CMCT and disability level in MS patients has also been extensively reported, potentially lending credence to CMCT as a marker that could reflect the integrity of the corticospinal pathway. The Expanded Disability Status Scale (EDSS) is the most commonly reported clinical measure, and many studies report a positive association between CMCT and EDSS [31,38,39,41,44,45,50,51]. One criticism of CMCT and EDSS correlations is that while CMCT can be derived from both upper and lower limb TMS measures, EDSS scores, especially within EDSS 4–6, are more greatly affected by lower limb function [60,61]. CMCT has also been reported to have an association with other measures of disability or impairment such as the Scripps Neurologic Rating Scale (SNRS), Ambulation Index (AI), and Multiple Sclerosis Functional Composite Measure (MSFC), as well as those involving strength [38,39,50,53]. However, one study that utilized the Multiple Sclerosis Impact Scale (MSIS, a patient reported score of the physical impacts of MS) did not find any association with CMCT in MS patients [48]. CMCT represents a useful means of objectively quantifying motor dysfunction in MS, and may have utility in both diagnosis and prognostication, secondary to current clinical standards.
As shown in Table 2, abnormal measures of CMCT in patients with MS are frequently reported. However, a number of studies have also found decreased MEP amplitudes, prolonged MEP latencies, and increased MT in patients with MS [16,31,40,42,43,44,46,48,49,51,54,55,56]. The utility of the MT lies in its ability to serve as potential reflection of cortical excitability, which is often decreased in MS patients. In addition, a few studies have specifically examined MEP amplitude and latency in the context of fatigue and MS patients. A study by Petajan et al. [42] involving 16 MS patients with weakness and 16 with normal motor function examined differences in cortical excitability resulting from facilitation via muscle activation of the non-exercised extremity. The study reported that following activation of the homologous non-exercised extremity after fatiguing exercise, MEP amplitude is increased in control and MS patients with normal motor function, but not MS patients with weakness [42]. It is hypothesized that the corpus callosum may play a role in the facilitation of motor responses by inhibiting interneurons that are GABAergic to cortical motor neurons. Thus, the absence of a significant MEP increase following facilitation in MS patients with weakness may suggest a loss in callosal integrity [42]. Another study involving fatigue in MS patients by Coates et al. [56] involved 13 MS patients who reported high fatigability and 13 with low perceived fatigability. Following an exhaustion task, they reported that the highly fatigued group had decreased MEP amplitudes and prolonged MEP latencies, as well as a greater decline in maximal voluntary contraction force. The same paper reported that fatigue severity on the FSS was related to prolonged MEP latencies. However, this was also the case for peripheral muscle fatigability and depressive symptoms, suggesting an interplay between changes in the corticospinal tract and other factors related to perceived fatigue [56]. It appears that other TMS measures such as MEP amplitude and latency could also have a role alongside CMCT in diagnosing and evaluating the clinical features of MS patients, especially in the context of fatigue.
Fewer studies have reported TMS measures relating to the silent period (SP), including duration and onset timing, as well as SICI duration. However, the results seem to be somewhat ambiguous. Tataroglu et al. [44] reported SP abnormalities in over half of the tested MS patients, with the most common abnormality being prolongation of the SP duration. In those with cerebellar dysfunction, the duration of the SP was extremely prolonged [44]. Vucic et al. [31] found a reduced SP duration in SPMS patients as compared to RRMS patients; however, this was not statistically significant. Llufriu et al. reported iSP onset latency to be longer in patients with MS, and also found a correlation with EDSS and MSFC. Neva et al. [16] did not find that the duration of the SP for MS patients was different compared to the control, but did find that the onset of the SP was delayed. Regarding SICI, the same study by Vucic et al. [31] found reduced SICI in SPMS patients, and reported a correlation between SICI and other TMS measures with EDSS. They hypothesized that the observed SICI reduction could point to dysfunction within GABAergic inhibitory interneurons within SPMS patients. However, further validation is required.
5.1. TMS Measures and the Association with MRI Markers
MRI imaging sequences are routinely used to assess the presence and distribution of lesions that characterize MS [1,62]. Highlighting the importance of these measures in research and clinically, T2-weighted sequences and T1-weighted sequences post gadolinium contrast administration form a central part of current diagnostic criteria to establish dissemination in space and time. Given the importance of MRI as a Gold Standard surrogate of MS lesion structural pathology, it is important to explore and understand the association of MRI measures with TMS measures (a putative functional correlate) to clearly establish structure–function relationships which implicitly underpin the promise of TMS measures as useful biomarkers. Kidd et al. [41] monitored a group of 19 MS patients (original n = 20, minus 1 withdrawal) over 12 months, with CMCT measured at the beginning and end of the study, and clinical and MRI examinations conducted at monthly intervals. They reported an increase in CMCT to upper limb muscles correlated with MRI lesion load and the presence of atrophy in the cervical cord. Additionally, while clinical deterioration measured via worsened EDSS scores was observed in 15 of 19 patients, only 4 saw an increase in motor response latencies, all of whom had developed new cord lesions. A more recent study by Kalkers et al. [50] examined a cohort of 52 MS patients and found that T1- and T2-weighted brain lesion volume and the number of spinal cord lesions had moderate positive correlations with CMCT to all limbs (upper/lower, right/left) and a correlation with EDSS results. The paper noted that the number of lesions did not differ significantly between the MS subtype groups. A study by Vucic et al. [31] also found that CMCT was correlated with the T2 brain lesion load. However, a study by Conte et al. [30] examined 30 MS patients and reported that EDSS scores were correlated significantly with TMS measures (CMCT, MEP amplitude, MT, ICI) but not with MRI lesion load. Considering all of this, the association between TMS measures and MRI measures needs clarification: some studies report an association between CMCT and lesion load, while others do not. Kidd et al. [41], in their longitudinal assessment, reported deterioration that was both associated with and independent of changes in CMCT/MRI measures. Furthermore, the spatial distribution of MRI lesions, which focally involve the corticospinal tracts as they course through the frontal lobes, deep white matter tracts, brainstem, and spinal cord, is an important consideration that has not been extensively evaluated. Larger scale, longitudinal studies that collect both TMS and MRI data at varying intervals are necessary to clarify the association between these variables and their clinical utility when combined.
Beyond ‘standard’ MRI measures, a host of new MRI biomarkers have been recently developed in the context of MS. Myelin water fraction (MWF) and the magnetization transfer ratio (MTR) have been proposed as possible structural measures of the myelination state in MS, which may plausibly be associated with TMS measures as functional correlates. In one such study, corpus callosum MWF was correlated with TMS-derived transcallosal inhibition (TCI; a brief suppression of voluntary activity in the primary motor cortex elicited by contralateral stimulation via the corpus callosum), which may suggest an association between structure and function in the context of MS demyelination [63]. Additional novel MRI measures of interest include the central vein sign, a specific feature of MS lesions that may have a diagnostic role, as well as slowly expanding lesions (SELs) and paramagnetic rim lesions (PRLs), which are thought to be evidence of chronic ongoing inflammation at the rim of previously enhancing lesions via MRI as a substrate of progressive injury. Currently, the association of these measures with TMS measures as structural correlates has not been extensively explored.
5.2. TMS Measures in Longitudinal Assessment of MS
Previous work has demonstrated the potential for TMS to be used as a tool in the longitudinal monitoring of disease progression and efficacy of treatments and therapies. Indeed, TMS has been put forward as an important outcome measure in therapeutic trials of remyelinating therapies, a key unmet need in MS. An early study by Kandler et al. [64] utilized TMS to monitor a group of 27 MS patients over a 3 month period. Patients who had experienced a relapse were administered steroids, and the study found a significant correlation between shortened CMCT and those who had a decrease in EDSS score. A similar study by Salle et al. [65] monitored 23 MS patients who were treated with methylprednisolone, examining them at baseline, after treatment, and 2 months later. They reported a significant association between CMCT and EDSS at each examination interval. A study by Sahota et al. [46] also examined a group of 30 MS patients, finding that patients after 3 months who improved clinically on the EDSS by one or more points from baseline had a statistically significant improvement in CMCT. Additionally, a recent study by Tremblay et al. [59] followed a group of 20 MS patients over 48 weeks, where 9 of which were in the treatment group using mesenchymal stem cell (MSC) therapy as a putative treatment to suppress MS inflammation and promote remyelination. While MSC therapy failed to improve neurophysiological and functional outcomes in patients, it was found that MEP latency and CMCT had a strong, positive correlation with impaired dexterity as measured using the 9-Hole Peg Test, although only in the left hand. Overall, the longitudinal association between TMS measures (in particular, CMCT and MEP latency) and changes in disability or dexterity level in MS patients demonstrates the potential of TMS as an objective and dynamic functional correlate. As an increasing number of potential remyelinating treatments are investigated, TMS may form important endpoints in clinical trials and perhaps in clinical practice.
5.3. rTMS Applications in MS
This manuscript has focused on the use of single- and pulse-paired TMS as a potential biomarker in MS, but there have also been significant developments in the application of repetitive TMS (rTMS) as a possible therapy. According to a 2021 review by Aloizou et al., many studies have focused on using rTMS to reduce spasticity, a common symptom of many MS patients [66]. A recent recommendation was made for “probable efficacy of intermittent theta burst stimulation (iTBS) of the leg area of M1 contralateral to the most affected limb (or both M1) in lower limb spasticity”, as found in the evidence-based guidelines for the therapeutic use of rTMS, established by Lefaucheur et al. [67]. In addition to this, the same review by Aloizou et al. expands on other recent studies that have been conducted on the potential of rTMS in addressing cerebellar dysfunction and gait issues, urinary tract dysfunction, cognitive dysfunction, fatigue, and depression. However, sample sizes and differences in protocols continue to be challenges in this field [66].
6. Conclusions
TMS measures have the potential to provide objective information regarding corticospinal tract damage, a major aspect of clinical disability in MS, which may have diagnostic and prognostic implications. Although TMS is a safe and well-tolerated technique, it is currently not a widely employed tool in the clinical context of multiple sclerosis. As the search for therapies to promote remyelinating and repair intensifies, interest in TMS measures as a functional correlate has grown, with TMS measures included as important endpoints in a field where objective biomarkers are sorely lacking. Unlike several MRI measures which reflect established injury (and are therefore not modifiable via interventions), TMS measures are dynamic and therefore have potential utility to monitor patients longitudinally in clinical trials and perhaps one day clinical practice. Moreover, recent developments involving rTMS have demonstrated the potential for TMS to be able to treat symptoms such as spasticity, common in MS patients. Large-scale and longitudinal trials and studies involving TMS are needed to provide further insight on the clinical utility of TMS as an independent or complementary tool for MS and other diseases and to identify the ideal and standardized testing parameters to promote more widespread TMS utilization.
Author Contributions
Conceptualization, A.M.A. and A.S.; investigation, A.S.; writing—original draft preparation, A.S.; writing—review and editing, A.M.A., S.T. and R.I.A.; supervision, A.M.A.; project administration, A.M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- McGinley, M.P.; Goldschmidt, C.H.; Rae-Grant, A.D. Diagnosis and Treatment of Multiple Sclerosis: A Review. JAMA 2021, 325, 765–779. [Google Scholar] [CrossRef]
- Howard, L.; Zwibel, M.D.; Jennifer Smrtka, M.S.N. Improving Quality of Life in Multiple Sclerosis: An Unmet Need. Am. J. Manag. Care 2011, 17, S139. [Google Scholar]
- Walton, C.; King, R.; Rechtman, L.; Kaye, W.; Leray, E.; Marrie, R.A.; Robertson, N.; La Rocca, N.; Uitdehaag, B.; van der Mei, I.; et al. Rising Prevalence of Multiple Sclerosis Worldwide: Insights from the Atlas of MS, Third Edition. Mult. Scler. J. 2020, 26, 1816–1821. [Google Scholar] [CrossRef] [PubMed]
- Klomjai, W.; Katz, R.; Lackmy-Vallée, A. Basic Principles of Transcranial Magnetic Stimulation (TMS) and Repetitive TMS (rTMS). Ann. Phys. Rehabil. Med. 2015, 58, 208–213. [Google Scholar] [CrossRef]
- Rothwell, J. Transcranial Brain Stimulation: Past and Future. Brain Neurosci. Adv. 2018, 2. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, J.-P. Chapter 37—Transcranial Magnetic Stimulation. In Handbook of Clinical Neurology; Levin, K.H., Chauvel, P., Eds.; Clinical Neurophysiology: Basis and Technical Aspects; Elsevier: Amsterdam, The Netherlands, 2019; Volume 160, pp. 559–580. [Google Scholar]
- Simpson, M.; Macdonell, R. The Use of Transcranial Magnetic Stimulation in Diagnosis, Prognostication and Treatment Evaluation in Multiple Sclerosis. Mult. Scler. Relat. Disord. 2015, 4, 430–436. [Google Scholar] [CrossRef]
- Rossi, S.; Hallett, M.; Rossini, P.M.; Pascual-Leone, A. Safety, Ethical Considerations, and Application Guidelines for the Use of Transcranial Magnetic Stimulation in Clinical Practice and Research. Clin. Neurophysiol. 2009, 120, 2008–2039. [Google Scholar] [CrossRef]
- Lerner, A.J.; Wassermann, E.M.; Tamir, D.I. Seizures from Transcranial Magnetic Stimulation 2012–2016: Results of a Survey of Active Laboratories and Clinics. Clin. Neurophysiol. 2019, 130, 1409–1416. [Google Scholar] [CrossRef]
- Rossi, S.; Antal, A.; Bestmann, S.; Bikson, M.; Brewer, C.; Brockmöller, J.; Carpenter, L.L.; Cincotta, M.; Chen, R.; Daskalakis, J.D.; et al. Safety and Recommendations for TMS Use in Healthy Subjects and Patient Populations, with Updates on Training, Ethical and Regulatory Issues: Expert Guidelines. Clin. Neurophysiol. 2021, 132, 269–306. [Google Scholar] [CrossRef]
- Fernández, V. The Use of Motor-Evoked Potentials in Clinical Trials in Multiple Sclerosis. J. Clin. Neurophysiol. 2021, 38, 166–170. [Google Scholar] [CrossRef]
- Groppa, S.; Oliviero, A.; Eisen, A.; Quartarone, A.; Cohen, L.G.; Mall, V.; Kaelin-Lang, A.; Mima, T.; Rossi, S.; Thickbroom, G.W.; et al. A Practical Guide to Diagnostic Transcranial Magnetic Stimulation: Report of an IFCN Committee. Clin. Neurophysiol. 2012, 123, 858–882. [Google Scholar] [CrossRef]
- Rossini, P.M.; Burke, D.; Chen, R.; Cohen, L.G.; Daskalakis, Z.; Di Iorio, R.; Di Lazzaro, V.; Ferreri, F.; Fitzgerald, P.B.; George, M.S.; et al. Non-Invasive Electrical and Magnetic Stimulation of the Brain, Spinal Cord, Roots and Peripheral Nerves: Basic Principles and Procedures for Routine Clinical and Research Application. An Updated Report from an I.F.C.N. Committee. Clin. Neurophysiol. 2015, 126, 1071–1107. [Google Scholar] [CrossRef] [PubMed]
- Stampanoni Bassi, M.; Buttari, F.; Gilio, L.; De Paolis, N.; Fresegna, D.; Centonze, D.; Iezzi, E. Inflammation and Corticospinal Functioning in Multiple Sclerosis: A TMS Perspective. Front. Neurol. 2020, 11, 566. [Google Scholar] [CrossRef] [PubMed]
- Ziemann, U.; Reis, J.; Schwenkreis, P.; Rosanova, M.; Strafella, A.; Badawy, R.; Müller-Dahlhaus, F. TMS and Drugs Revisited 2014. Clin. Neurophysiol. 2015, 126, 1847–1868. [Google Scholar] [CrossRef] [PubMed]
- Neva, J.L.; Lakhani, B.; Brown, K.E.; Wadden, K.P.; Mang, C.S.; Ledwell, N.H.M.; Borich, M.R.; Vavasour, I.M.; Laule, C.; Traboulsee, A.L.; et al. Multiple Measures of Corticospinal Excitability Are Associated with Clinical Features of Multiple Sclerosis. Behav. Brain Res. 2016, 297, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Brum, M.; Cabib, C.; Valls-Solé, J. Clinical Value of the Assessment of Changes in MEP Duration with Voluntary Contraction. Front. Neurosci. 2016, 9, 505. [Google Scholar] [CrossRef]
- Ammann, C.; Guida, P.; Caballero-Insaurriaga, J.; Pineda-Pardo, J.A.; Oliviero, A.; Foffani, G. A Framework to Assess the Impact of Number of Trials on the Amplitude of Motor Evoked Potentials. Sci. Rep. 2020, 10, 21422. [Google Scholar] [CrossRef] [PubMed]
- Bastani, A.; Jaberzadeh, S. A Higher Number of TMS-Elicited MEP from a Combined Hotspot Improves Intra- and Inter-Session Reliability of the Upper Limb Muscles in Healthy Individuals. PLoS ONE 2012, 7, e47582. [Google Scholar] [CrossRef] [PubMed]
- Cuypers, K.; Thijs, H.; Meesen, R.L.J. Optimization of the Transcranial Magnetic Stimulation Protocol by Defining a Reliable Estimate for Corticospinal Excitability. PLoS ONE 2014, 9, e86380. [Google Scholar] [CrossRef]
- Goldsworthy, M.R.; Hordacre, B.; Ridding, M.C. Minimum Number of Trials Required for within- and between-Session Reliability of TMS Measures of Corticospinal Excitability. Neuroscience 2016, 320, 205–209. [Google Scholar] [CrossRef]
- Jaiser, S.R.; Barnes, J.D.; Baker, S.N.; Baker, M.R. A Multiple Regression Model of Normal Central and Peripheral Motor Conduction Times. Muscle Nerve 2015, 51, 706–712. [Google Scholar] [CrossRef]
- Imajo, Y.; Kanchiku, T.; Suzuki, H.; Yoshida, Y.; Funaba, M.; Nishida, N.; Fujimoto, K.; Taguchi, T. Effects of Differences in Age and Body Height on Normal Values of Central Motor Conduction Time Determined by F-Waves. J. Spinal Cord Med. 2017, 40, 181–187. [Google Scholar] [CrossRef]
- Claus, D. Central Motor Conduction: Method and Normal Results. Muscle Nerve 1990, 13, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Fernández, V.; Valls-Sole, J.; Relova, J.L.; Raguer, N.; Miralles, F.; Dinca, L.; Taramundi, S.; Costa-Frossard, L.; Ferrandiz, M.; Ramió-Torrentà, L.; et al. Recommendations for the Clinical Use of Motor Evoked Potentials in Multiple Sclerosis. Neurología 2013, 28, 408–416. [Google Scholar] [CrossRef]
- Škarabot, J.; Mesquita, R.N.O.; Brownstein, C.G.; Ansdell, P. Myths and Methodologies: How Loud Is the Story Told by the Transcranial Magnetic Stimulation-Evoked Silent Period? Exp. Physiol. 2019, 104, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Zeugin, D.; Ionta, S. Anatomo-Functional Origins of the Cortical Silent Period: Spotlight on the Basal Ganglia. Brain Sci. 2021, 11, 705. [Google Scholar] [CrossRef] [PubMed]
- Hübers, A.; Kassubek, J.; Müller, H.-P.; Broc, N.; Dreyhaupt, J.; Ludolph, A.C. The Ipsilateral Silent Period: An Early Diagnostic Marker of Callosal Disconnection in ALS. Ther. Adv. Chronic Dis. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Cash, R.F.H.; Noda, Y.; Zomorrodi, R.; Radhu, N.; Farzan, F.; Rajji, T.K.; Fitzgerald, P.B.; Chen, R.; Daskalakis, Z.J.; Blumberger, D.M. Characterization of Glutamatergic and GABAA-Mediated Neurotransmission in Motor and Dorsolateral Prefrontal Cortex Using Paired-Pulse TMS–EEG. Neuropsychopharmacology 2017, 42, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Conte, A.; Lenzi, D.; Frasca, V.; Gilio, F.; Giacomelli, E.; Gabriele, M.; Marini Bettolo, C.; Iacovelli, E.; Pantano, P.; Pozzilli, C.; et al. Intracortical Excitability in Patients with Relapsing–Remitting and Secondary Progressive Multiple Sclerosis. J. Neurol. 2009, 256, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Vucic, S.; Burke, T.; Lenton, K.; Ramanathan, S.; Gomes, L.; Yannikas, C.; Kiernan, M.C. Cortical Dysfunction Underlies Disability in Multiple Sclerosis. Mult. Scler. J. 2012, 18, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Liepert, J.; Mingers, D.; Heesen, C.; Bäumer, T.; Weiller, C. Motor Cortex Excitability and Fatigue in Multiple Sclerosis: A Transcranial Magnetic Stimulation Study. Mult. Scler. 2005, 11, 316–321. [Google Scholar] [CrossRef]
- Ni, Z.; Bahl, N.; Gunraj, C.A.; Mazzella, F.; Chen, R. Increased Motor Cortical Facilitation and Decreased Inhibition in Parkinson Disease. Neurology 2013, 80, 1746–1753. [Google Scholar] [CrossRef]
- Mori, F.; Kusayanagi, H.; Monteleone, F.; Moscatelli, A.; Nicoletti, C.G.; Bernardi, G.; Centonze, D. Short Interval Intracortical Facilitation Correlates with the Degree of Disability in Multiple Sclerosis. Brain Stimul. 2013, 6, 67–71. [Google Scholar] [CrossRef]
- Nantes, J.C.; Zhong, J.; Holmes, S.A.; Narayanan, S.; Lapierre, Y.; Koski, L. Cortical Damage and Disability in Multiple Sclerosis: Relation to Intracortical Inhibition and Facilitation. Brain Stimul. 2016, 9, 566–573. [Google Scholar] [CrossRef]
- Howard, J.; Trevick, S.; Younger, D.S. Epidemiology of Multiple Sclerosis. Neurol. Clin. 2016, 34, 919–939. [Google Scholar] [CrossRef]
- Pellegrino, L.; Coscia, M.; Muller, M.; Solaro, C.; Casadio, M. Evaluating Upper Limb Impairments in Multiple Sclerosis by Exposure to Different Mechanical Environments. Sci. Rep. 2018, 8, 2110. [Google Scholar] [CrossRef] [PubMed]
- Ingram, D.A.; Thompson, A.J.; Swash, M. Central Motor Conduction in Multiple Sclerosis: Evaluation of Abnormalities Revealed by Transcutaneous Magnetic Stimulation of the Brain. J. Neurol. Neurosurg. Psychiatry 1988, 51, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.M.; Streletz, L.J.; Raab, V.E.; Knobler, R.L.; Lublin, F.D. Lower Extremity Motor Evoked Potentials in Multiple Sclerosis. Arch. Neurol. 1991, 48, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Sue, C.M.; Yiannikas, C.; Clouston, P.D.; Lim, C.L.; Graham, S. Transcranial Cortical Stimulation in Disorders of the Central Motor Pathways. J. Clin. Neurosci. 1997, 4, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Kidd, D.; Thompson, P.D.; Day, B.L.; Rothwell, J.C.; Kendall, B.E.; Thompson, A.J.; Marsden, C.D.; McDonald, W.I. Central Motor Conduction Time in Progressive Multiple Sclerosis. Correlations with MRI and Disease Activity. Brain 1998, 121, 1109–1116. [Google Scholar] [CrossRef]
- Petajan, J.H.; White, A.T. Motor-Evoked Potentials in Response to Fatiguing Grip Exercise in Multiple Sclerosis Patients. Clin. Neurophysiol. 2000, 111, 2188–2195. [Google Scholar] [CrossRef] [PubMed]
- Schmierer, K.; Niehaus, L.; Roricht, S.; Meyer, B. Conduction Deficits of Callosal Fibres in Early Multiple Sclerosis. J. Neurol. Neurosurg. Psychiatry 2000, 68, 633–638. [Google Scholar] [CrossRef][Green Version]
- Tataroglu, C.; Genc, A.; Idiman, E.; Cakmur, R.; Idiman, F. Cortical Silent Period and Motor Evoked Potentials in Patients with Multiple Sclerosis. Clin. Neurol. Neurosurg. 2003, 105, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Tataroglu, C.; Genc, A.; Idiman, E.; Cakmur, R.; Idiman, F. Cortical Relay Time for Long Latency Reflexes in Patients with Definite Multiple Sclerosis. Can. J. Neurol. Sci. 2004, 31, 229–234. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sahota, P.; Prabhakar, S.; Lal, V.; Khurana, D.; Das, C.P.; Singh, P. Transcranial Magnetic Stimulation: Role in the Evaluation of Disability in Multiple Sclerosis. Neurol. India 2005, 53, 197. [Google Scholar] [CrossRef] [PubMed]
- Thickbroom, G.W.; Byrnes, M.L.; Archer, S.A.; Kermode, A.G.; Mastaglia, F.L. Corticomotor Organisation and Motor Function in Multiple Sclerosis. J. Neurol. 2005, 252, 765–771. [Google Scholar] [CrossRef]
- Jørgensen, L.M.; Nielsen, J.E.; Ravnborg, M. MEP Recruitment Curves in Multiple Sclerosis and Hereditary Spastic Paraplegia. J. Neurol. Sci. 2005, 237, 25–29. [Google Scholar] [CrossRef]
- Gagliardo, A.; Galli, F.; Grippo, A.; Amantini, A.; Martinelli, C.; Amato, M.P.; Borsini, W. Motor Evoked Potentials in Multiple Sclerosis Patients without Walking Limitation: Amplitude vs. Conduction Time Abnormalities. J. Neurol. 2007, 254, 220–227. [Google Scholar] [CrossRef]
- Kalkers, N.F.; Strijers, R.L.M.; Jasperse, M.M.S.; Neacsu, V.; Geurts, J.J.G.; Barkhof, F.; Polman, C.H.; Stam, C.J. Motor Evoked Potential: A Reliable and Objective Measure to Document the Functional Consequences of Multiple Sclerosis? Relation to Disability and MRI. Clin. Neurophysiol. 2007, 118, 1332–1340. [Google Scholar] [CrossRef]
- Kale, N.; Agaoglu, J.; Onder, G.; Tanik, O. Correlation between Disability and Transcranial Magnetic Stimulation Abnormalities in Patients with Multiple Sclerosis. J. Clin. Neurosci. 2009, 16, 1439–1442. [Google Scholar] [CrossRef]
- Llufriu, S.; Blanco, Y.; Martinez-Heras, E.; Casanova-Molla, J.; Gabilondo, I.; Sepulveda, M.; Falcon, C.; Berenguer, J.; Bargallo, N.; Villoslada, P.; et al. Influence of Corpus Callosum Damage on Cognition and Physical Disability in Multiple Sclerosis: A Multimodal Study. PLoS ONE 2012, 7, e37167. [Google Scholar] [CrossRef] [PubMed]
- Di Sapio, A.; Bertolotto, A.; Melillo, F.; Sperli, F.; Malucchi, S.; Troni, W. A New Neurophysiological Approach to Assess Central Motor Conduction Damage to Proximal and Distal Muscles of Lower Limbs. Clin. Neurophysiol. 2014, 125, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Manogaran, P.; Vavasour, I.; Borich, M.; Kolind, S.H.; Lange, A.P.; Rauscher, A.; Boyd, L.; Li, D.K.; Traboulsee, A. Corticospinal Tract Integrity Measured Using Transcranial Magnetic Stimulation and Magnetic Resonance Imaging in Neuromyelitis Optica and Multiple Sclerosis. Mult. Scler. J. 2016, 22, 43–50. [Google Scholar] [CrossRef]
- Ayache, S.S.; Créange, A.; Farhat, W.H.; Zouari, H.G.; Lesage, C.; Palm, U.; Abdellaoui, M.; Lefaucheur, J.-P. Cortical Excitability Changes over Time in Progressive Multiple Sclerosis. Funct. Neurol. 2016, 30, 257–263. [Google Scholar] [CrossRef]
- Coates, K.D.; Aboodarda, S.J.; Krüger, R.L.; Martin, T.; Metz, L.M.; Jarvis, S.E.; Millet, G.Y. Multiple Sclerosis-Related Fatigue: The Role of Impaired Corticospinal Responses and Heightened Exercise Fatigability. J. Neurophysiol. 2020, 124, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Pisa, M.; Chieffo, R.; Congiu, M.; Dalla Costa, G.; Esposito, F.; Romeo, M.; Comola, M.; Comi, G.; Leocani, L. Intracortical Motor Conduction Is Associated with Hand Dexterity in Progressive Multiple Sclerosis. Mult. Scler. J. 2021, 27, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Alusi, S.H.; Macerollo, A.; MacKinnon, C.D.; Rothwell, J.C.; Bain, P.G. Tremor and Dysmetria in Multiple Sclerosis: A Neurophysiological Study. Tremor Other Hyperkinetic Mov. 2021, 11, 30. [Google Scholar] [CrossRef]
- Tremblay, F.; Ansari, Y.; Remaud, A.; Freedman, M.S. Neurophysiological Outcomes Following Mesenchymal Stem Cell Therapy in Multiple Sclerosis. Clin. Neurophysiol. 2022, 136, 69–81. [Google Scholar] [CrossRef]
- Meyer-Moock, S.; Feng, Y.-S.; Maeurer, M.; Dippel, F.-W.; Kohlmann, T. Systematic Literature Review and Validity Evaluation of the Expanded Disability Status Scale (EDSS) and the Multiple Sclerosis Functional Composite (MSFC) in Patients with Multiple Sclerosis. BMC Neurol. 2014, 14, 58. [Google Scholar] [CrossRef]
- van Munster, C.E.P.; Uitdehaag, B.M.J. Outcome Measures in Clinical Trials for Multiple Sclerosis. CNS Drugs 2017, 31, 217–236. [Google Scholar] [CrossRef]
- Ömerhoca, S.; Akkaş, S.Y.; İçen, N.K. Multiple Sclerosis: Diagnosis and Differential Diagnosis. Arch. Neuropsychiatry 2018, 55, S1–S9. [Google Scholar] [CrossRef]
- Zhao, E.Y.; Vavasour, I.M.; Zakeri, M.; Borich, M.R.; Laule, C.; Rauscher, A.; Traboulsee, A.; Li, D.K.B.; Boyd, L.A.; MacKay, A.L. Myelin Water Imaging and Transcranial Magnetic Stimulation Suggest Structure-Function Relationships in Multiple Sclerosis. Front. Phys. 2019, 7, 141. [Google Scholar] [CrossRef]
- Kandler, R.H.; Jarratt, J.A.; Davies-Jones, G.A.B.; Gumpert, E.J.W.; Venables, G.S.; Sagar, H.J.; Zeman, A. The Role of Magnetic Stimulation as a Quantifier of Motor Disability in Patients with Multiple Sclerosis. J. Neurol. Sci. 1991, 106, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Salle, J.Y.; Hugon, J.; Tabaraud, F.; Boulesteix, J.M.; Vallat, J.M.; Dumas, M.; Poser, C.M. Improvement in Motor Evoked Potentials and Clinical Course Post-Steroid Therapy in Multiple Sclerosis. J. Neurol. Sci. 1992, 108, 184–188. [Google Scholar] [CrossRef]
- Aloizou, A.-M.; Pateraki, G.; Anargyros, K.; Siokas, V.; Bakirtzis, C.; Liampas, I.; Nousia, A.; Nasios, G.; Sgantzos, M.; Peristeri, E.; et al. Transcranial Magnetic Stimulation (TMS) and Repetitive TMS in Multiple Sclerosis. Rev. Neurosci. 2021, 32, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, J.-P.; Aleman, A.; Baeken, C.; Benninger, D.H.; Brunelin, J.; Di Lazzaro, V.; Filipović, S.R.; Grefkes, C.; Hasan, A.; Hummel, F.C.; et al. Evidence-Based Guidelines on the Therapeutic Use of Repetitive Transcranial Magnetic Stimulation (rTMS): An Update (2014–2018). Clin. Neurophysiol. 2020, 131, 474–528. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).