1. Introduction
Dates, or
Phoenix dactylifera L., are widely consumed in northern Africa and the Middle East area in addition to diverse regions including Central and South America and Europe (the southern part) (FAOSTAT, 2022). Due to their high quantities of sugar, dietary fiber, macronutrients, and minerals, dates have long been deemed by Middle Easterners as the ideal meal supplement [
1]. The sweetness and texture of dates are influenced by their adulthood and mellowness stage [
2]. Dates are normally picked and sold in three developmental stages: aged hard (Besser or Khalal), demi-seasoned (Rutab), and seasoned (Tamr). In addition to their nutritional estimate, dates are associated with a number of bioactive effects, i.e., antioxidant, anti-mutagenic, anti-inflammatory, anti-tumor, anti-bacteriological, and immunostimulant [
3,
4]. It is believed that the polyphenols in dates, containing hydroxycinnamates, phenolic acids, flavonoid glycosides, and proanthocyanin oligomers, contribute to their beneficial properties [
5]. Numerous polyphenols contained in date fruits, including pelargonin and derivatives of cinnamic acid, have been demonstrated to enhance cellular resistance recently [
6]. However, these substances are unstable. Microencapsulation techniques in this case might aid in stabilizing polyphenols for use in industrial applications. By protecting bioactive substances from O
2, H
2O, light, and other environmental variables, microencapsulation increases their stability and converts liquid solutions into powders for easier handling [
7]. Dates are popular treats that are sold in a range of cuisines due to their added nutritional value. However, between 12 and 15% of dates contain stones that are often regarded as the fruit’s by-product and are not yet commercially valuable [
2]. Bedouin tribes used to consume roasted date seed infusions, but most societies have not really used them to date, even though date stones contain superior polyphenolic quantities and outstanding antioxidant effects. Abundant research on animals shows biological advantages, such as a decrease in oxidative impairment, progress in memory and conception deficiencies in Alzheimer’s patterns, and anti-diabetic effects [
8]. Given the importance of dates as a dietary component and the emerging benefits of eating raw fruit for health, further in-depth study of date seed biochemical composition is desirable.
On the other hand, one of the most significant issues facing people today is obesity, which is linked to several metabolic disorders like diabetes mellitus and cardiovascular disease. Recently, it has become more common among Eastern children and teenagers [
9]. Recently, dietary fibers extracted from dates which were integrated into biscuit models showed anti-obesity features in animal models by controlling their lipid outline, kidney, blood sugars, and liver activity [
10]. Date palm pits from the Kentichi type had a strong inhibitory impact against key enzymes linked to diabetes and obesity, showing its ability to be used in the agro-food, cosmetic, and pharmaceuticals industries instead of synthetic additives [
11]. The processes behind the anti-obesity benefits are still not completely known. In detail, it has been suggested that the anti-obesity characteristics result from the regulation of the manifestation of lipogenic enzymes in the liver and pale adipose matter [
12] as well as the reticence of α-glucosidase in the starch assimilation in the presence of purple corn’s anthocyanins [
13], which might be occurring in the presence of date seed polyphenols. By accumulating tri-acylglycerols or circulating free fatty acids (FFAs) in response to variations in vitality demands, adipocytes are crucial for retaining lipid homeostasis and strength equilibrium. Since both adipocyte hyperplasia and hypertrophy can result in increased adipose tissue mass, the creation and characteristics of adipocytes have been the emphasis of obesity investigations [
14]. Adipogenesis is a multifaceted process. Firstly, preadipocytes reach contact inhibition, and under induction of the appropriate hormone, the cycle-arrested preadipocytes start the mitotic clonal expansion (MCE) program, and then the cells reenter the growth cycle. After MCE, the preadipocytes initiate a cascade of signals and release multiple adipogenic factors such as PPARγ and C/EBPs, which are two major regulators in controlling adipogenesis. We used an MDI differentiation medium due to its benefits in obtaining better quality differentiation over a short period of time with increased sensitivity to insulin. This medium is also an economical and convenient way to generate adipocyte-like cells for experiments.
Nevertheless, no studies have focused on how date seed polyphenols affect adipocytes, and more research into cellular processes is needed. Our hypothesis was that the date seed polyphenols would have anti-obesity effects by reducing the number of mature adipocytes and lipid levels after being industrially encapsulated in a commercial product. Cell viability was tracked throughout time to investigate the proliferation of preadipocytes. We investigated if they contributed to the drop in lipid content or lipid accumulation during cell differentiation and mature adipocyte lipolysis. We examined the effects of date seed polyphenols that were industrially encapsulated in a commercial product, “pills”, on adipocyte function rather than a single ingredient because it has been shown that the biological effects of a blend of phytonutrients have harmonious influences [
14]. For the convenience and traceability of lipogenic enzymes and the variation in the buildup of mass fat with food, a cell culture method via preadipocyte 3T3-L1 cells was employed. The fate of polyphenols after digestion was also measured. The conclusions of this investigation shed information on the functional behavior of date seed polyphenols after shaping into commercial pills that might be the platform of valorizing agricultural wastes.
2. Materials and Methods
2.1. Chemicals and Reagents
3T3-L1 mouse embryo fibroblasts were donated by ATCC (Manassas, VA, USA). Fetal bovine serum (FBS), penicillin–streptomycin–glutamine, and Dulbecco’s altered Eagle medium (DMEM) were acquired from GIBCO (Invitrogen, NY, USA). PPAR- and polyclonal glyceraldehyde-3-phosphate dehydrogenase (GAPDH)-specific antibodies were purchased from Santa Cruz Bio (Santa Cruz, CA, USA) and Ab Frontier (Seoul, Republic of Korea). Folin–Ciocalteu reagent, 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid (ABTS), gallic acid, and trolox were all acquired from Aladdin Co., Shanghai, China. In March 2022, the Shalli Company sold date seeds of the Siwa variety (Siwa, Marsa Matruh, Egypt). The seeds were transported for 12 h at room temperature (RT) to our faculty and were immediately produced. All the components used in this experiment, including solvents, were of analytical quality.
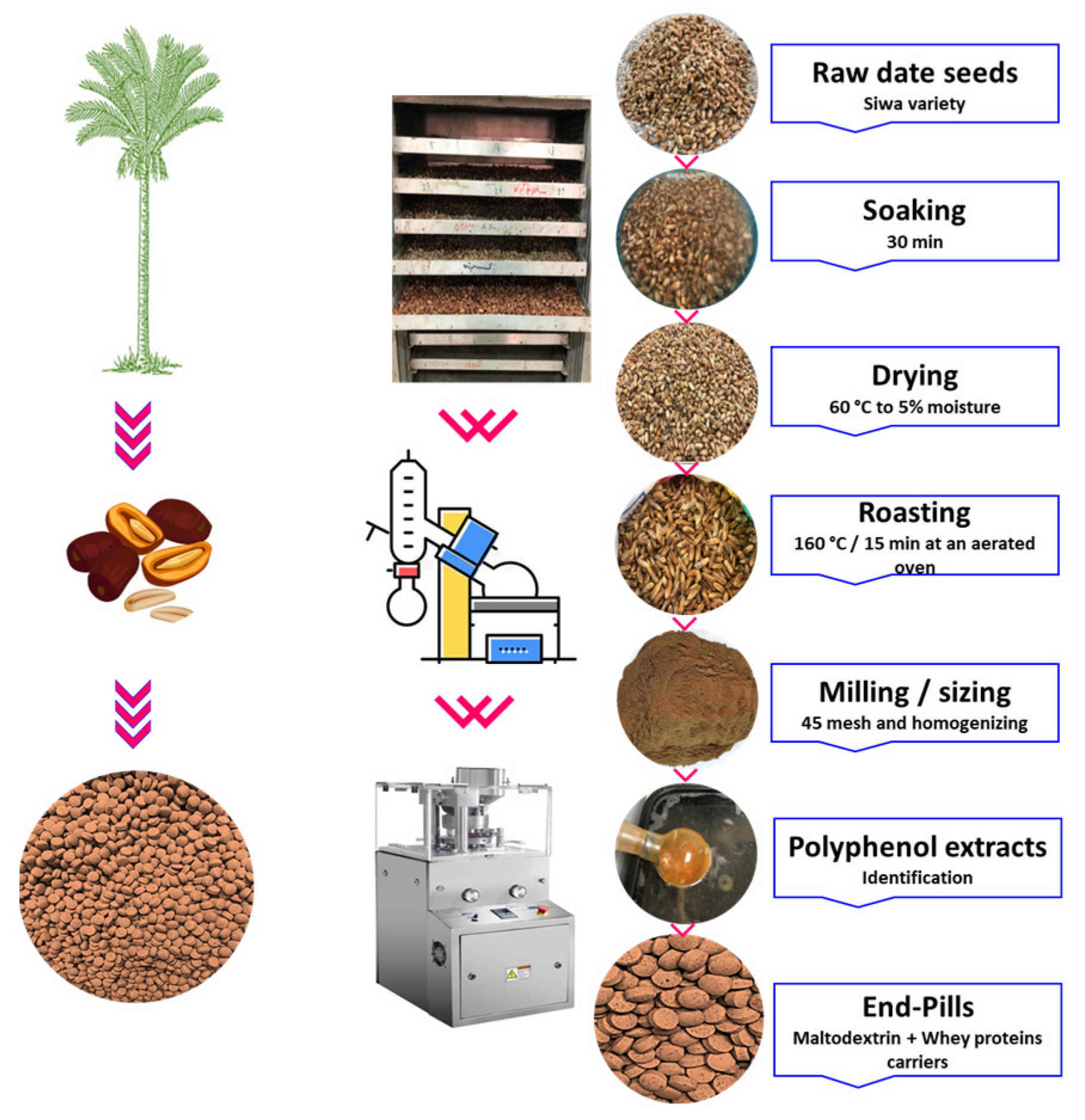
2.2. Commercially Fabricated Date Seed Polyphenol Pills (DSPPs)
The date seeds were first processed by cleaning using a rotary washing and soaking machine from Markoom, Turkey, drying (using an OT-2F from Gilson Co., Inc. in Columbus, OH, USA), roasting (using a Payo Roasting Machine from Dabiige Co. in the Philippines), milling (using a 3 HP Industrial Crusher from Alishan Co., New Delhi, India), sieving to a mesh size of 45 for greater homogeneity and uniformity), and finally shaped using a ZP-15E-19E automatic rotary pill press machine (China) via the processing line of Tadawina Co., (Shubra, Egypt). The polyphenols of date seeds were extracted and purified to eliminate the occurrence of any other substances including proteins, carbohydrates, and lipids. Whey proteins and maltodextrin were utilized to create the pills, and their respective amounts were 400, 500, and 100 mg per gram of each pill for the date’s polyphenols, maltodextrin, and whey proteins, respectively.
Figure 1 provides a summary of the procedures involved in creating the DSPPs produced by pressing and employing a combination of whey proteins and maltodextrin as holders.
2.3. Evaluating DSPP Polyphenols
Polyphenols from DSPPs were isolated by employing our earlier assay [
15]. Briefly, 50 mg of DSPPs was mixed with 1 mL of MeOH and 3 mL of acetone–H
2O–trifluoroacetic acid (TFA, 60:40:0.05,
v/
v/
v), stirred (1 h at RT), centrifuged, separated into two aliquots from the supernatant (400 µL each), and evaporated (35 °C until dryness). The two aliquots were used for chromatography analysis and for measuring the de-polymerized proanthocyanidin (heating at 50 °C/20 min and adding 200 mM sodium acetate to the aliquot dissolved in 200 µL of MeOH-HCl (0.2 mM) containing phloroglucinol (50 g/L) and ascorbic acid (10 g/L). A Waters Acquity UPLC-PDA system (Waters, Milford, CT, USA) and an Acquity BEH C18 column (10 × 1 mm i.d., 1.7 µm) were used for chromatographic analysis. Phases A (H
2O- HCOOH, 99:1,
v/
v) and B (MeOH- HCOOH, 99:1,
v/
v) made up the mobile phase. The column was kept at 35 °C, and the flow rate was 0.08 mL/min. The elution program was as follows: isocratic for 2 min with 30% B, 30–75% B (14–27 min), isocratic for 1 min with 2% B, 2–18% B (1–7 min), isocratic with 18% B (7–9 min), 18–30% B (9–12 min), and isocratic for 2 min with 30% B. The column was then cleaned and reconstituted. The injection volume was 2 µL. An electrospray basis and an ion trap mass analyzer are both parts of the Bruker Daltonics Amazon (Bruker, Hamburg, Germany) mass spectrometer used for the ESI-MS/MS analysis linked to the UPLC-PDA system. The collision energy for fragmentation in the MS
2 tests was set at 1. The positive ion mode (capillary voltage of 2.5 kV; end plate offset of 500 V; temperature of 200 °C; nebulizer gas of 10) and the negative ion mode (capillary voltage of 4.5 kV; end plate offset of 500 V; temperature of 200 °C; nebulizer gas of 10 psi; and dry gas of 5 µL/min) both use ions to vaporize gas. Retention periods, UV-visible spectra, and MS spectra were utilized to distinguish the samples. We assessed our findings to those obtained using more traditional ingredients and found polyphenol-based information in the pertinent literature [
16,
17]. The concentrations of protocatechuic acid, flavan-3-ol monomers and oligomers released via phloroglucolysis were evaluated at 280 nm, hydroxycinnamic acids were evaluated at 320 nm, flavones were evaluated at 250–260 nm, and flavonols were evaluated at 340–360 nm via external standardization curves founded with their comparable substances. Phloroglucinolysis produces expansion units as parallel phloroglucinol derivatives whilst preserving the stereochemistry and possible substituents of proanthocyanin terminal units. The total amount of constitutive units released during phloroglucinolysis was used to calculate the total flavan-3-ol content. Using MarkerLynx software (version SCN781 4.1), the data located under investigation were gathered, united, and regulated. Peaks for metabolite detection were captured using peak distance across at 5% elevation of 1 s, peak-to-peak baseline noise of 1, noise removal of 6, and strength limit of 10,000. The data were aligned using a mass window of 0.05 Da and a retaining time window of 0.2 min.
2.4. Cell Culture and Differentiation
According to an earlier report [
18], 3T3-L1 cells were cultivated in DMEM containing glutamate (292 μg/L), streptomycin (100 μg/mL), and penicillin (100 U/mL). The cells were retained at 37 °C in a moistened atmosphere with 5% CO
2. To induce differentiation, 2-day-old post-confluent 3T3-L1 preadipocytes (D
0) were treated for 2 d with 0.5 μM isobutyl methylxanthine, 1 μM dexamethasone, and 167 nM insulin in DMEM containing 10% FBS (MDI delineation medium) (D
2). After another 4 d of increase in 10% FBS/DMEM (D
8) and 2 d of maintenance in 10% FBS/DMEM with 167 nM insulin, more than 90% of the cells developed into adipocytes with lipid-filled drops (D
4).
2.5. Cell Viability Evaluation
The MTS method (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt, MTS; Celltiter 96 Aqueous One Solution, Promega, Wisconsin) was utilized to accomplish a time course to evaluate the viable cell number in a culture. Cells were seeded at a density of 2500 cells per well in 96-well plates containing preadipocytes that were pre-confluent. After 24 h, either a DMSO vehicle or DSPPs after dissolving in DMSO (25, 50, or 100 μg/mL based on the total polyphenols at pills) was applied. At D
0 for post-confluent preadipocytes, the DSPPs were attached to MDI delineation medium. Cells were cultured for 24, 48, and 72 h in each experiment. Mature adipocytes (D
8) were cultivated with DSPPs for 24 and 48 h. The formazan concentration was determined via a plate reader (ELx808 Bio-Tek Instruments, Winooski, VT, USA) that measures the absorbance at 490 nm and is directly proportional to the number of surviving cells in the culture [
19].
2.6. Oil Red O Staining and Estimation of Lipid Quantity via the Adipo-Red Method
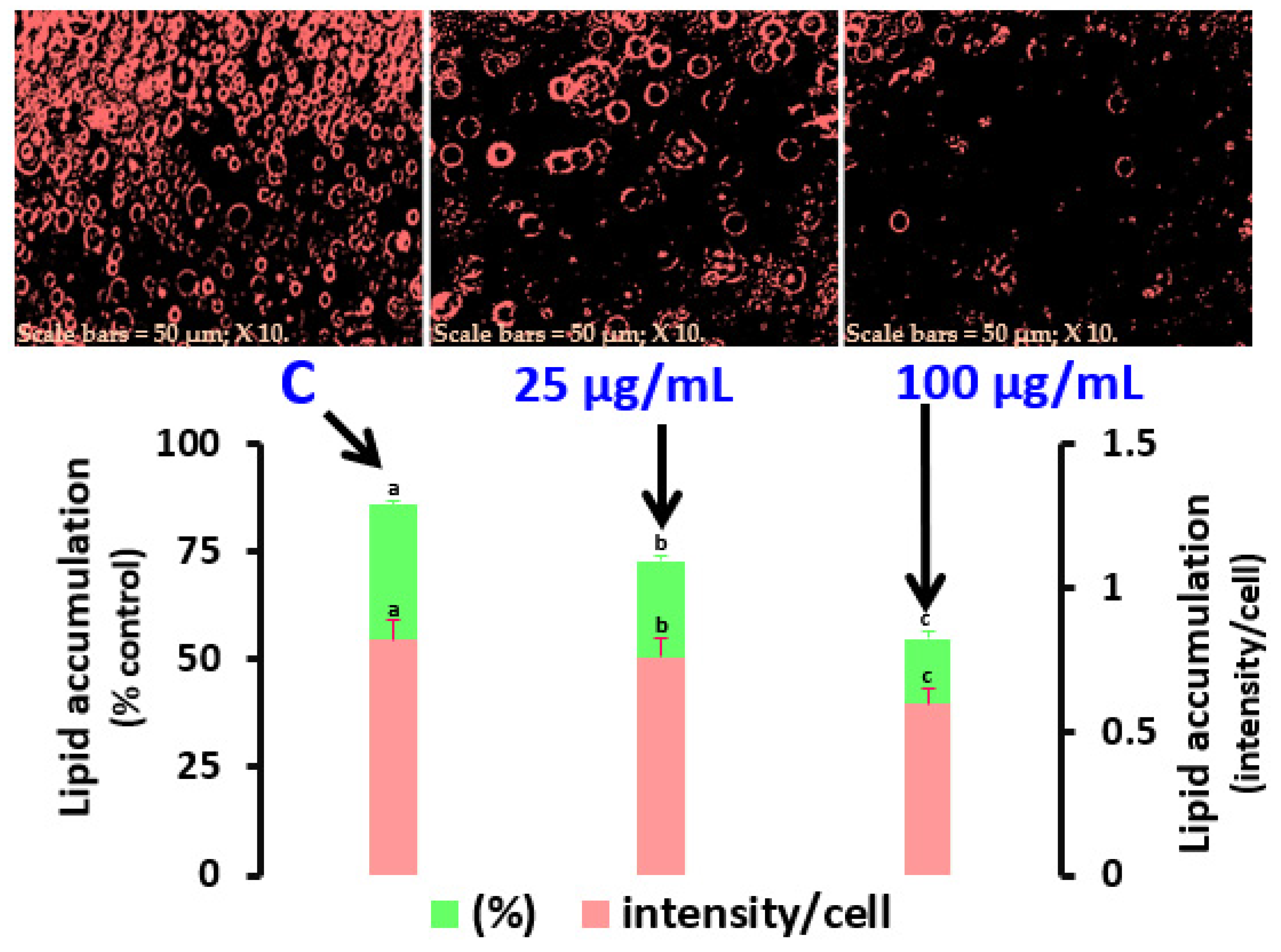
DSPPs (25, 50, and 100 μg/mL) or a DMSO carrier were incubated with the MDI delineation medium at D0 and cultivated for 6 d under treatment (D0–D6). On D6, cells were dyed with Oil Red O after being placed in fresh 10% formalin (pH 7.4, Sigma, St. Louis, MO, USA). After rinsing 3T3-L1 cells on D6 with PBS saline (pH 7.4) and inserting 200 μL of PBS into wells containing 5 μL of the Adipo-Red reagent, the Adipo-Red assay (Lonza Walkersville, Inc., Walkersville, MD, USA) was performed. After 10 min, the amount of lipid accumulation was checked by investigating the absorbance of the Oil Red O solution dissolved in isopropanol at 485 and 572 nm wavelengths for excitation and emission separately.
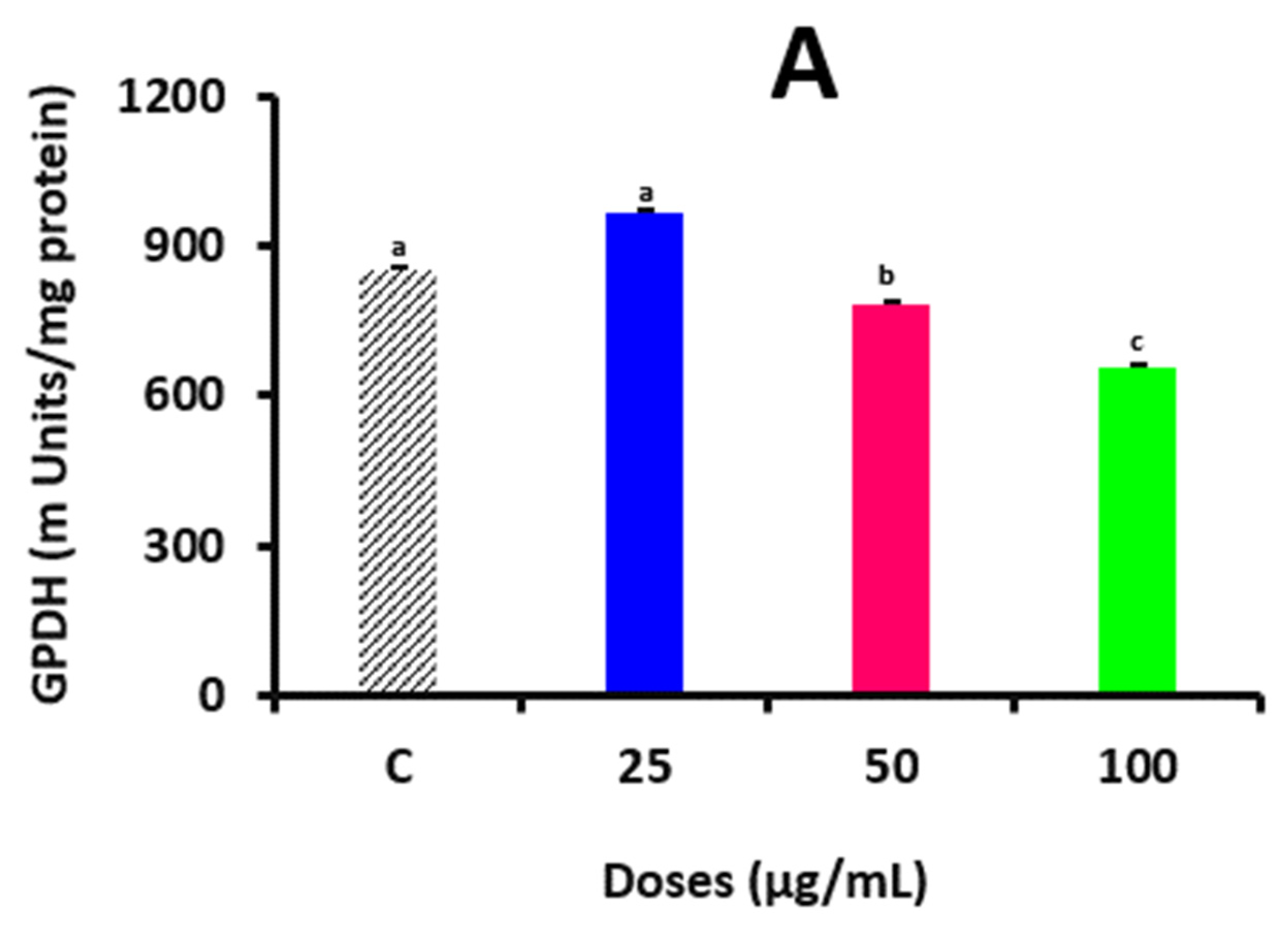
2.7. Evaluating the Activity of Glycerol-3-Phosphate Dehydrogenase Enzyme and Its Binding with Pills’ Polyphenols
A measure of overdue adipocyte development was the glycerol-3-phosphate dehydrogenase (GPDH) test. For 6 d, cells were cultivated in a delineation medium comprising DSPP (D
0–D
6). The cells were then scratched into 0.5 mL of ice-cold buffer that included 0.28 mM sucrose, 5 mM Tris, 1 mM EDTA, and 0.002% β-mercaptoethanol, rinsed 3 times with PBS and kept at 70 °C. The homogenate was centrifugated at 10,000×
g for 10 min at 4 °C after sonicating 3 times for 15 s each. GPDH tests were conducted using the obtained supernatants in accordance with Wise and Green’s techniques [
20]. Briefly, 50 µL TEA solution (0.5 M triethanolamine, 10 mM EDTA, and 10 mM2-mercaptoethanol, pH 7.5) was mixed with 100 µL of 5 mM dihydroxyacetone phosphate, 200 µL of 0.5 mMβ-nicotinamide adenine dinucleotide, and 50 µL of samples in a 98-well order. It was then measured at 340 nm with a spectrophotometer (Multiscan Go, Thermo-Fisher Scientific, Lenexa, KS, USA). The activities were measured in milliunits (mU), where one mU is equivalent to 1 nmol of NADH
2 oxidized in 1 min. The interaction between DSPPs and the GPDH enzyme was also confirmed using a molecular docking approach. As the primary polyphenols in DSPPs, the structures of each unliganded form of the GPDH enzyme (PDB: 3DA1, resolution of 2.16 Å) and epicatechin (PubChem CID-72276) were retrieved from
https://www.rcsb.org/ and
https://pubchem.ncbi.nlm.nih.gov/ (accessed on 2 September 2023) and optimized by combining fractional charges. Then, H
2O was removed, the structure was refined, the energy was reduced, and 3D protonation was performed using MOE software (version 2015) before the GPDH enzyme was employed as a receptor. Epicatechin was used to phenolate the enzyme structure, after which it was docked using the MOE docking tool by locating the strong binding sites using the MOE site discoverer tool, and then, it was engaged by the docking procedure. The procedure was then performed again for the enzyme that phenolates epicatechin, and 10 appropriate docked postures were generated using the gaining function London dG and refined using the algorithm for the force field. Based on root mean square deviation, the MOE-LigX tool was utilized to determine which ligand receptor interaction was most appropriate [
21].
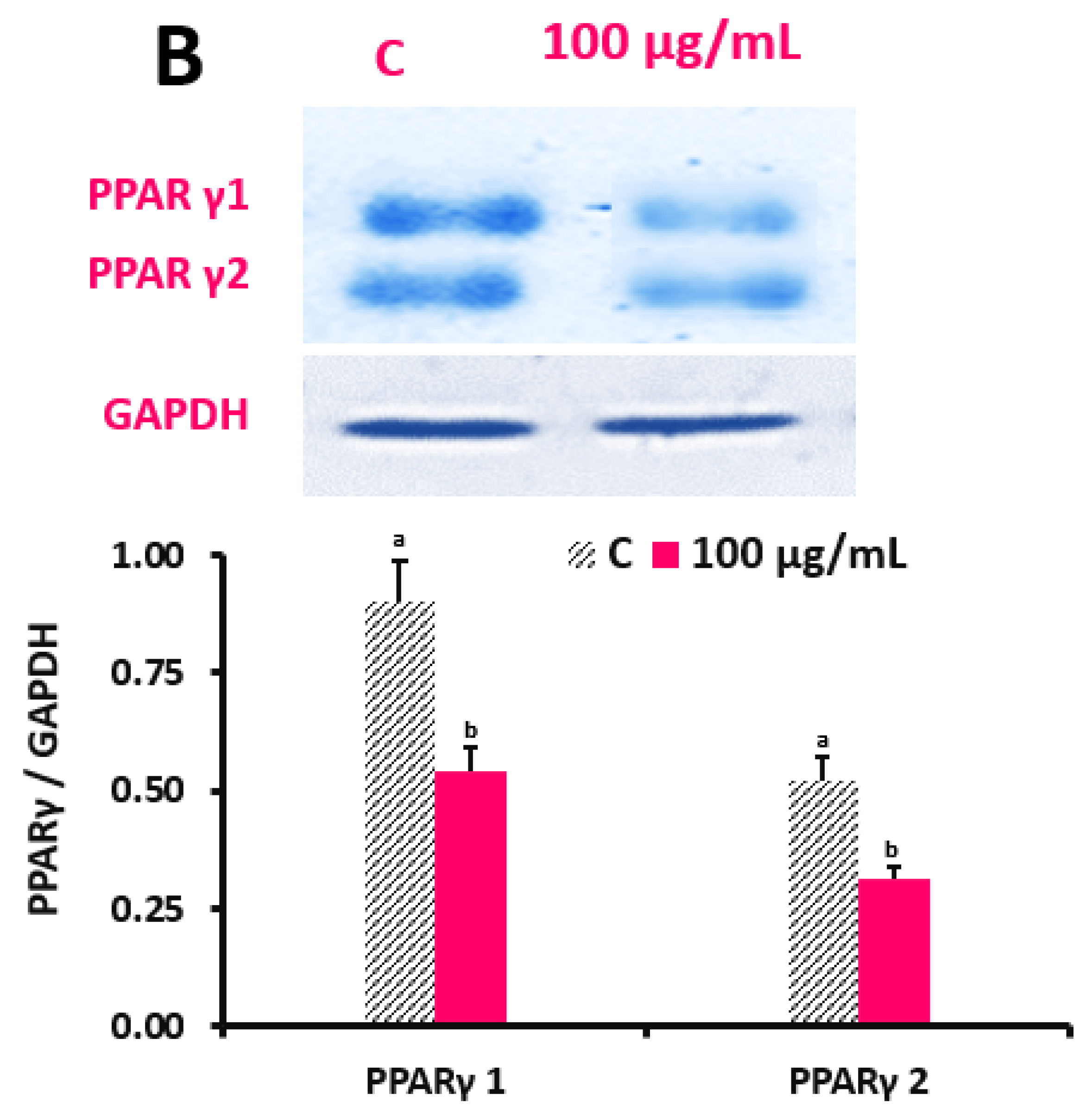
2.8. Western Blot Assessment of PPARγ Expression
PPARγ is a crucial marker of 3T3-L1 cell differentiation. To investigate their impact on the expression of PPARγ, DSPPs (100 μg/mL) were inserted into the differentiation medium through D0-D6. The cold radioimmune precipitation assay buffer (RIPA; pH 7.4) included Tris-HCl, NaCl, Na2EDTA, EGTA, NP-40, Na-deoxycholate, Na-pyrophosphate, Na3VO4, glycerophosphate, and leupeptin by 20 mM, 150 mM, 1 mM, 1 mM, 1% v/v, 1% v/v, 2.5 mM, 1 mM, 1 mM, and 1 μg/mL, separately. The cell lysates were centrifugated at 10,000× g for 10 min at 4 °C. Protein quantity was defined by the Bradford assay (Bio-Rad, CA, USA). Afterwards, 30 μg of protein was transferred to polyvinylidene difluoride membranes after being separated using a 10% sodium dodecyl sulfate–polyacrylamide gel (Bio-Rad). The membranes were then stopped with Tris-buffered saline including 5% nonfat dry milk before being exposed to the primary anti-mouse PPARγ antibody overnight at 4 °C. The layers were washed; then, rabbit anti-mouse immunoglobulin G-coupled with horseradish peroxidase was applied for 1 h at RT (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The ECL method was utilized to produce certain protein bands (Amersham Pharmacia Biotech, Piscataway, NJ, USA). The band concentrations were measured with a FluorChem densitometer and ImageJ software (version 1. 51j8) (National Institute of Health, Bethesda, MD, USA). PPARγ/GAPDH was used to express the results with GAPDH acting as the control.
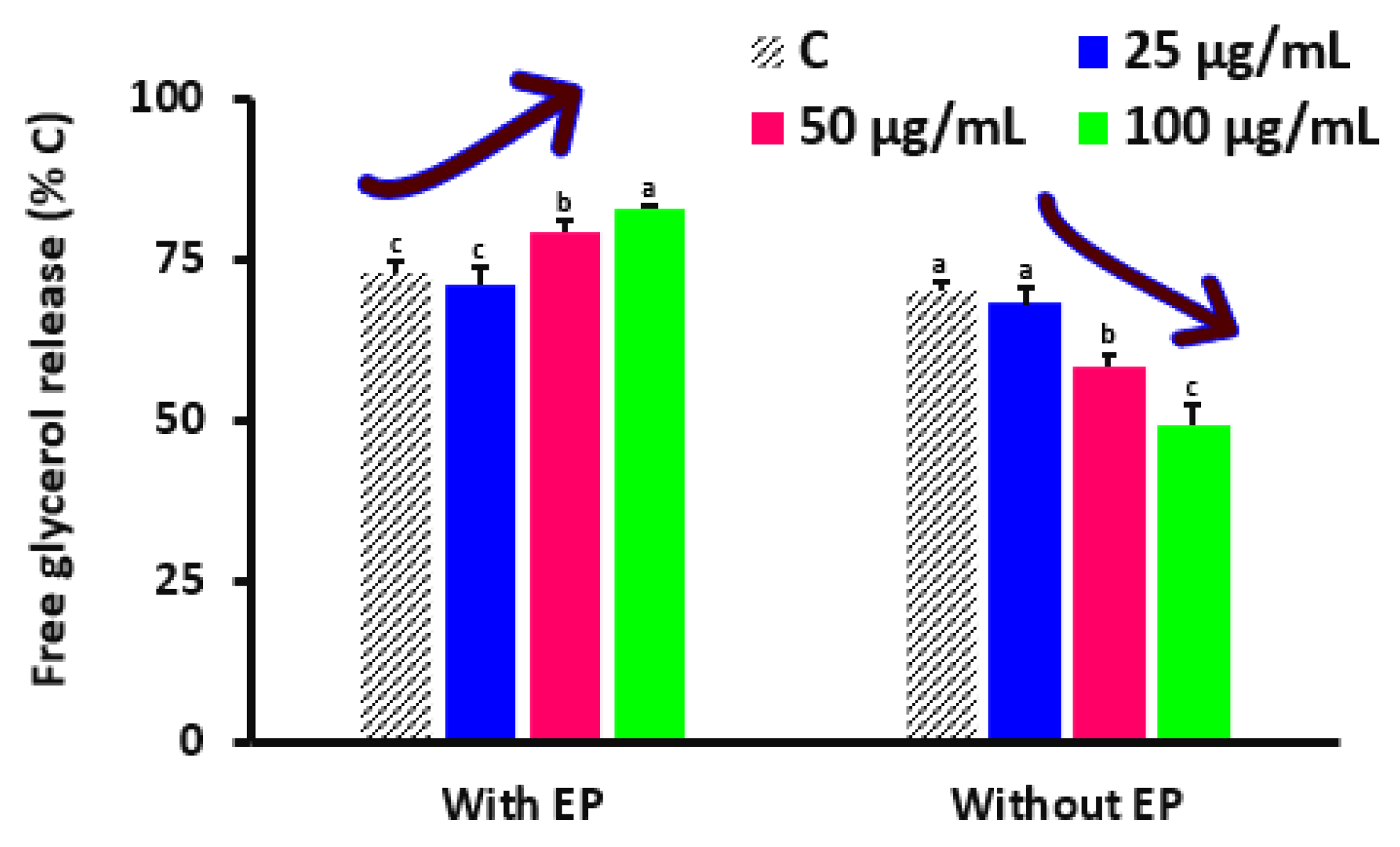
2.9. Lipolysis Assay and Their Interaction Simultaneously
In the presence or deficiency of epinephrine (1 μM), fully developed adipocytes (D
8) were cured for 4 h with DMSO or DSPPs (25, 50, and 100 μg/mL). Each well-conditioned medium was removed and tested for the presence of glycerol. Based on the outcomes of a preliminary test, a time point that was inside the linear scope of glycerol release was selected. The interaction between epicatechin (PubChem CID-72276) and the lipase enzyme (PDB: 1HPL, resolution of 2.3 Å) was studied as explained before. They were optimized; then, the H
2O was removed, the structure was refined, the energy was reduced, 3D protonation was performed, and the docking procedures were ended via the MOE site discoverer means. Afterwards, 10 appropriate docked postures were shaped using the recording function London dG and refined using the algorithm for the force field [
21].
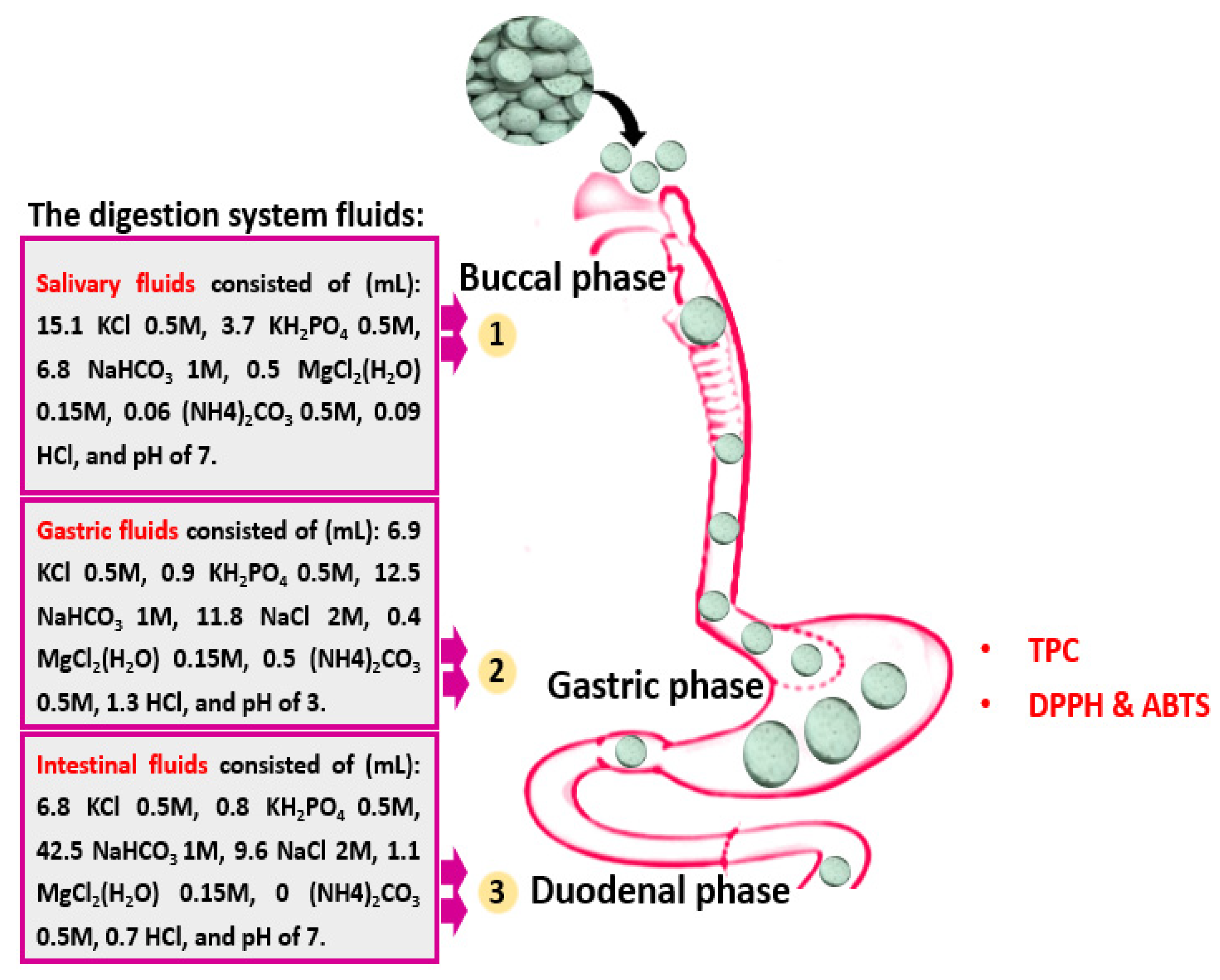
2.10. Total Antioxidative Polyphenols of In Vitro Digested Pills
DSPPs were digested following the protocol described by Khalifa et al. [
22]. Briefly, 25 L of 0.3 M CaCl
2, 0.975 mL of MillQ-H
2O, 7.5 mL of salivary juice, 0.5 mL of α-amylase solution, and 10 mL of DSPP extracts were combined. A stirring incubator (HHIS208 Meena Medical Equipment, Inc., Bedford, TX, USA) was used to incubate the mixture at 37 °C for 2 min at 120 rpm. The remaining liquid was then thoroughly mixed with 6 mL of gastric fluid, 1.6 mL of pepsin solution (2500 U/mL), 4 L of 0.3 M CaCl
2, and 8 mL of MillQ-H
2O before the pH was brought to 3.0 by 1 M of HCl. By adding 1 M NaOH to raise the pH to 7.0 and stop the digestion, the mixture also hatched for 60 min. The outstanding mixture was then combined with duodenal juice (7.7 mL), 3.5 mL pancreatin blend (800 U/mL), 1.75 mL of 160 mM bile salt, 0.3 M CaCl
2, and 14 mL of MillQ-H
2O. The pH was then brought down to 7.0 with NaOH (1 M), and the mixture was incubated for 60 min. The digestion was finished by heating at 95 °C for 5 min after each stage. Following each stage, a 4 mL aliquot of each sample was taken and kept at −80 °C until needed (
Figure 2).
The total polyphenol content (TPC) was determined using Folin’s assay [
7]. A 100 μL aliquot of digested or non-digested DSPPs was blended with 200 μL of Folin–Ciocalteu reagent (1 mL of raw reagent diluted with 9 mL of dH
2O). A total of 800 μL of Na
2CO
3 blend (7.5%,
w/
v) was inserted into the blends within 30 s to 8 min of adding the reagent. The blends were incubated in the dark at RT for 2 h. Their absorbance was then evaluated at 750 nm using a spectrophotometer (Multiscan Go, Thermo-Fisher Scientific, Lenexa, KS, USA). TPC was calculated as an equivalent of gallic acid (mg GAE per g of DSPPs).
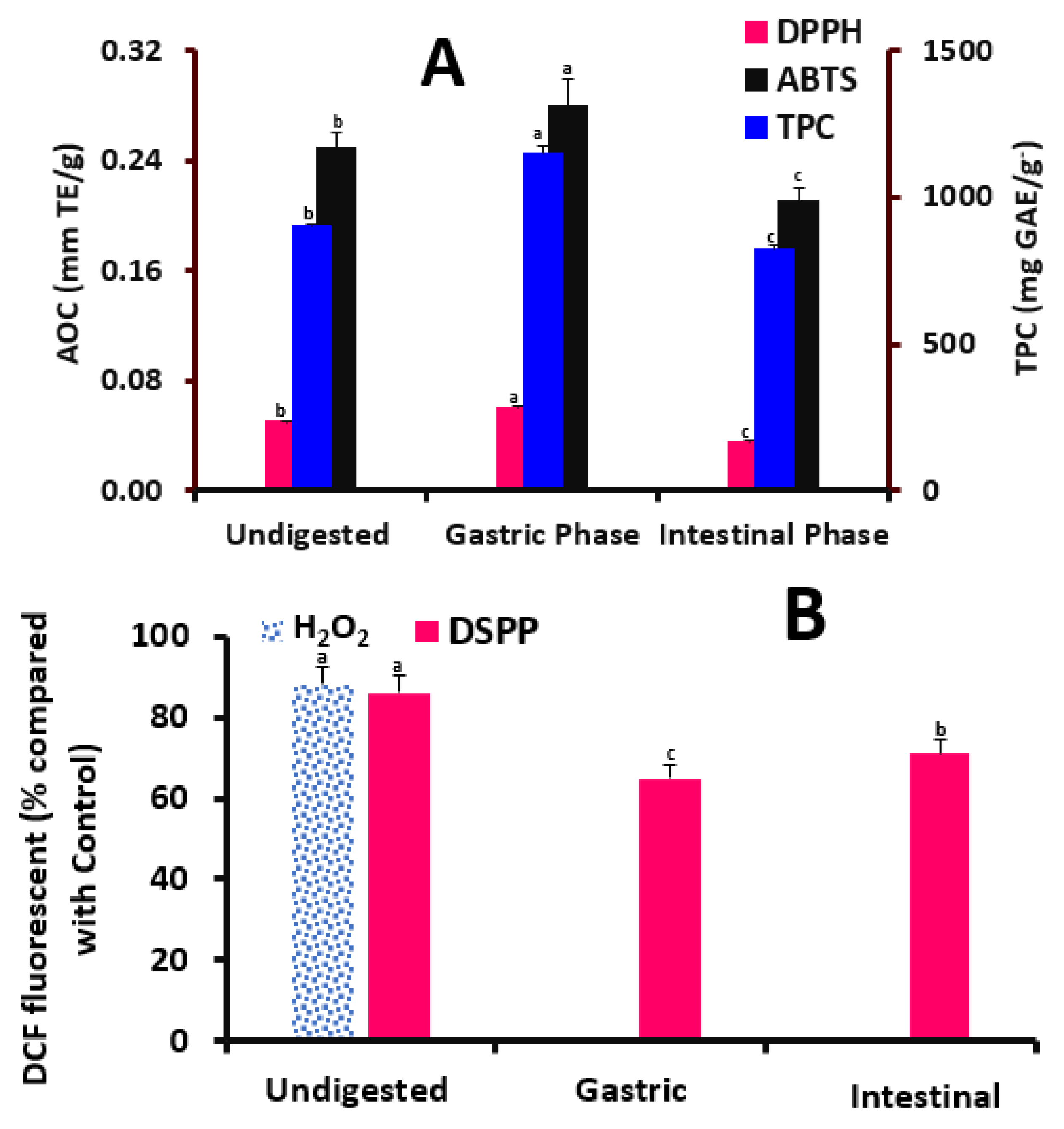
The antioxidant capacity (AOC) of the digested and non-digested DSPPs was measured using DPPH and ABTS radical scavenging capacity assays [
7]. A total of 20 μL of DSPPs was added to 380 μL of SPB (10 mM, pH 7.4) and 400 μL of MeOH solution (DPPH, 0.1 mM). The blend was then dark hatched for 30 min at RT. The absorbance was then scanned at 517 nm using a spectrophotometer. For the ABTS method, 600 μL of ABTS solution prepared in SPB (10 mM, pH 7.4, absorbance of 0.7 at 734) was mixed with 10 μL of DSPPs and measured at a wavelength of 734 after 5 s using a spectrophotometer. The results of both methods were calculated as trolox equivalents (TEs) [
7]. To confirm the results of the AOA, the level of intracellular oxidative status was evaluated by determining 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) oxidation. In brief, cells were cultivated in 96-well black microplates (5 × 10
3 cells per well) for 24 h. The medium was then eradicated and returned by PBS with 10 μM DCFH-DA (Shyuanye, Shanghai, China), and cells were saved in a wetted atmosphere (5% CO
2, 37 °C) for 45 min. Cells were then co-exposed to H
2O
2 (250 μM) and 100 μM DSPPs (non-digested, gastric digested, and intestinal digested extracts based on their total polyphenols calculated as GAE) DSPPs for 1 h. Fluorescence was detected at excitation and emission wavelengths of 492 and 520 nm, respectively.
2.11. Statistical Analysis
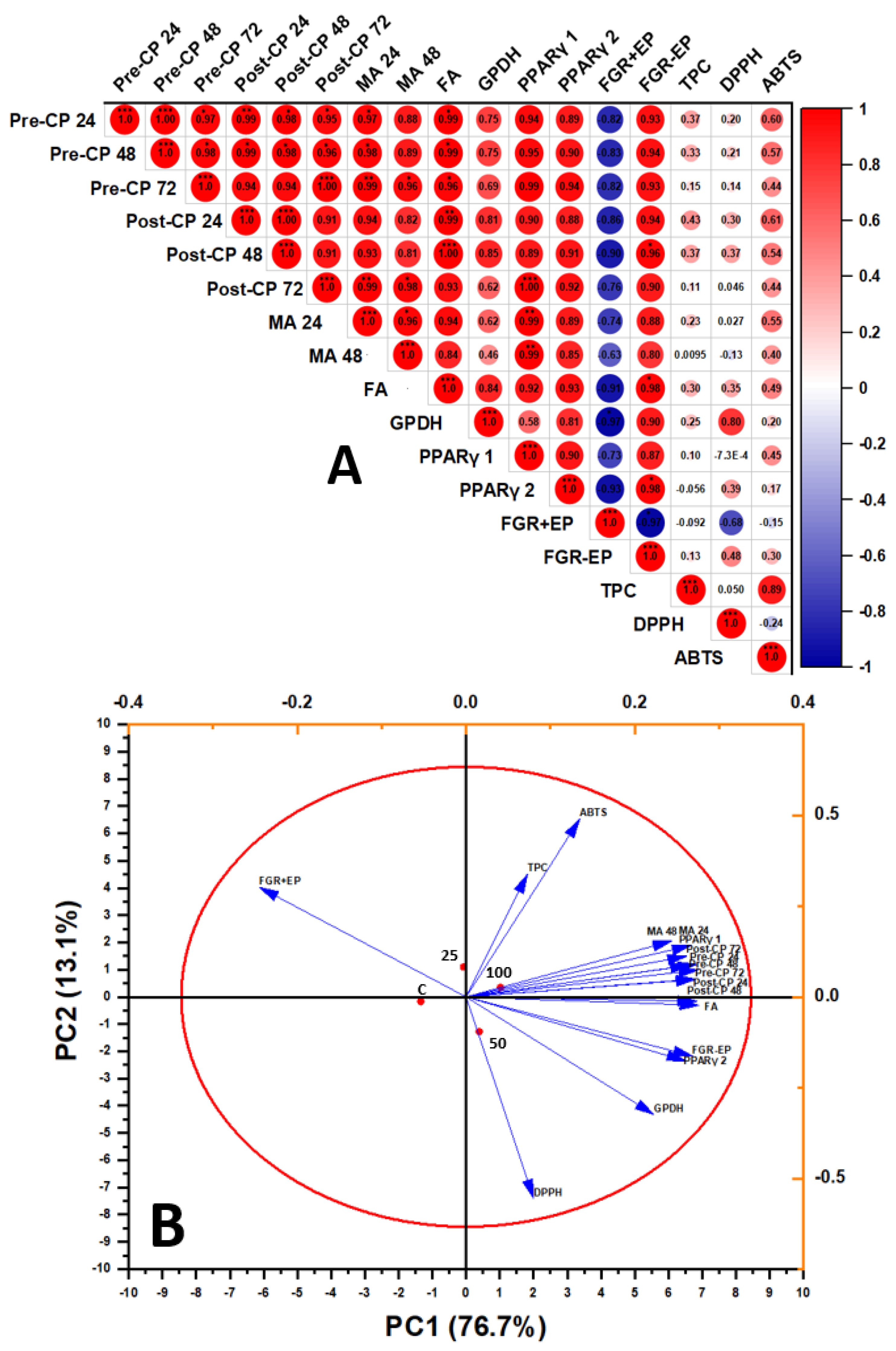
The results are described as the means ± SD. The data were explored via one-way analysis of variance (ANOVA) in SPSS 21.0 Statistics with post hoc Tukey’s tests for multiple variations (SPSS Inc., Chicago, IL, USA). When p < 0.05 was present, statistical outcomes were considered significant. Principal component analysis (PCA) was performed using Minitab, ver. 16 (Minitab, Inc., State College, PA, USA). Bubbles Pearson correlation analysis was performed using OriginPro 2023 (Origin Lab, Co., Northampton, MA, USA).
4. Discussion
Overweightness is characterized as an abnormal buildup of fat in adipose tissue that raises the risk of prolonged diseases involving diabetes, cardiovascular conditions, and some forms of tumors [
24]. In mice given high-fat diets, polyphenols from different plants have been found to reduce mass growth and adipose tissue accumulation [
25,
26]. However, there is no research that shows how DSPPs can reduce adipose tissue bulk. In general, the balance of adipocyte proliferation, lipogenesis, and lipolysis determines the bulk of adipose tissue. To determine whether DSPPs have anti-obesity benefits by preventing the development of mature adipocytes and lowering lipid levels, we looked at the effects of DSPPs on preadipocyte differentiation, proliferation, and lipolysis via 3T3-L1 cells.
We evaluated cell viability at various times throughout cell growth to ascertain the impact of DSPPs on the proliferation of proliferating preadipocytes. The results supported previous research on epicatechin, the major polyphenol in DSPPs, that inhibits the development of 3T3-L1 cells by reducing the quantity of available cells in 3T3-L1 pre-confluent preadipocytes, leading to a decline in cell multiplication [
27]. The fact that lactate dehydrogenase release did not rise suggests that cytotoxicity was not the cause of the reduction in preadipocyte development. In the first few days following stimulus with MDI delineation medium, the following took place: storing lipids by mitotic clonal growth and an irreparable dedication to distinction [
28]. The dosage-related reduction in proliferation in post-confluent distinguishing cells may be a possible strategy for lowering the quantity of adipocytes, given that post-confluent 3T3-L1 preadipocytes suffer many cycles of replication through the first 72 h of distinction. We suggest that DSPPs reduce the amount of adipose tissue in part by preventing preadipocytes’ post-confluent cells from proliferating. After more than 48 h of incubation, 3T3-L1 preadipocytes treated with tea extracts high in epicatechin showed an increase in sub-G1 apoptotic cells and a decrease in G1 cells [
29]. As a result, apoptosis may be the cause of the suppression of preadipocyte growth. Moreover, the accumulation of triacylglycerols plays a major role in determining the cell volume of adipocytes. DSPPs prevented lipid buildup during adipocyte development, according to the findings of lipid blotting and AdipoRed analysis. In the presence of 100 μg/mL of DSPPs, the enzyme activity of GPDH, an indicator of former adipocyte development, was also reduced. The AdipoRed results indicate the number of fat cells and lipid accretion because adipocyte development requires both mitotic clonal growth and lipid storage. Triglyceride biosynthesis involves the conversion of dihydroxyacetone phosphate to glycerol 3-phosphate by the cytoplasmic enzyme GPDH. GPDH activity increases when adipocytes develop and go through final adipogenic differentiation. Meanwhile, GPDH is a crucial enzyme that supplies G3P to produce triacylglycerol in adipose tissue and is thought to be a sign of adipocyte development. One of the primary marker enzymes for differentiation is cellular GPDH. Through the measurement of GPDH activity, the impact of biological variables on lipogenesis and adipogenesis may be approximated. Mammals use the G3P pathway in most of their cell types to synthesize glycerolipids, i.e., triacylglycerol, diacylglycerol, and phospholipids. In obese people, an increased production of triacylglycerol may be attributed to elevated GPDH. As a result, it is possible that the lower lipid content is largely caused by the fewer cells that are present because of the suppression of cell growth. Meanwhile, we are planning to analyze whether DSPPs affect lipogenic processes in adipocytes to elucidate this pathway as well.
The manifestation of transcriptional activators, notably the CCAAT/gravy-attaching protein and PPAR groups, controls adipocyte differentiation [
30]. The ligand-activated transcription factor peroxisome proliferator-stimulated receptor-γ normalizes adipogenesis and is expressed in the initial to mid phases of adipocyte difference. When compared to the control, cells cured with DSPPs exhibited significantly lower levels of PPARγ-1 and 2. These conclusions match those of a prior study [
31] that found polyphenols impede adipocyte development and reduce lipid synthesis while concurrently down-regulating PPARγ and the sterol regulatory element-binding protein (SREBP)1c. A PPARγ ligand may be present in DSPPs. Nevertheless, a luciferase reporter gene experiment has shown that the polyphenols regulate PPARγ and adipocyte-specific genes without increasing PPARγ ligand activity [
32]. AMP-activated protein kinase (AMPK) was shown to be activated by epicatechin in adipocytes and white adipose tissue [
33]. Energy metabolism-related genes that are downstream targets of AMPK activation are phosphorylated and controlled. Recent research has demonstrated that the use of phytochemicals inhibits PPARγ expression and adipogenesis while simultaneously activating AMPK. Alternatively, phytochemicals inhibited adipogenesis by activating the AMPK cascade [
34]. More research must be conducted on evaluations of the representation of additional crucial transcriptional factors linked to adipocyte development. The management of energy homeostasis depends on the failure of triacylglycerols in adipocytes and the discharge of glycerol and fatty acids. The most significant lipase that facilitates lipolysis in response to energy needs is hormone-sensitive lipase. After this, the generated FFAs are transported to the emaciated muscle and other tissues for oxidation and the synthesis of ATP [
35]. The amount of lipolysis induced by epinephrine was somewhat increased by DSPPs. Hence, decreased adiposity might not be primarily due to lipolysis. Remarkably, in completely differentiated adult adipocytes, DSPPs markedly reduced basal lipolysis. In larger mature fat cells, hormone-independent basal lipolysis is visible, and an enhanced amount of disseminating FFAs has been proposed to serve as a facilitator of insulin endurance and cardiovascular disorder linked to overweightness [
36]. Consequently, the observations on the insulin-sensitizing effects of DSPPs may be explained by the antilipolytic impact in the baseline state. Epicatechin may also improve insulin signaling by inhibiting the c-Jun NH2-terminus kinase signaling conduit and decreasing pro-inflammatory cytokines such tumor gangrene factor α with antioxidants in addition to increasing the production of adiponectin and leptin [
37,
38].
Epicatechin from many plants has been found to cause apoptosis, which can reduce the amount of adipocytes in the adipose tissue [
39]. Hence, it is not impossible that DSPPs causes adipocytes to undergo apoptosis. Our findings are consistent with the idea that DSPPs might decrease adipose tissue bulk, which will lead to less mature adipocytes and less lipid buildup. The inhibition of adipocyte development is further shown by the lowering of GPDH action and PPARγ representation. This research is constrained by the sort of preadipocyte growth suppression, and it is yet unclear what molecular processes DSPPs use to prevent adipocyte development, which is under investigation. Recently, Varela et al. [
39] found that the DSPPs significantly declined the viability in MCF-7 and Hep-G2 cells with an inhibition of adipocyte differentiation and lipid gathering during 48 h of polyphenols’ treatments. He also noted that DSPPs preserved antioxidant effects in the digestive milieu and assumes that DSPPs persisted actively in the digestive milieu and showed possible anti-hyperglycemic and anti-adipogenic effects. Meanwhile, the innovative point of our results is that our DSPPs are potentially commercial pills that could be utilized on a large scale. To evaluate the change that occurs during digestion, our current study examined the TPC and AOC during simulated in vitro digestion. Following digestion, the TPC and AOC content in DSPPs rose, showing that they were released from the food matrix during digestion. During digestion, macromolecules obtain a pH that is favorable for hydrolysis and the release of polyphenols attached to them [
40]. As a result, DSPPs that were digested tend to have higher TPC, which supports antioxidant capabilities. This claim is plausible, since polyphenols are what provide the samples’ antioxidant activity; therefore, an increase in the overall phenolic content will correlate to an increase in antioxidant activity. Both the DPPH radical scavenging experiment and ABTS assay findings showed that after digestion, the AOC of DSPPs considerably increased, indicating the release of TPC from the food matrix. The AOC was unaffected by the drop in TPC, as shown by the results of the DPPH and ABTS radical scavenging experiment. The antioxidant capacities measured by DPPH and ABTS methods are based on the occurrence of hydroxylated antioxidative components. Meanwhile, the ABTS radicals are soluble in water and organic solvents, enabling the determination of antioxidant capacity of both hydrophilic and lipophilic compounds/samples. Additionally, the DPPH radicals have higher stability and lower reactivity than ABTS ones. Thus, the results of the ABTS method are higher than that of DPPH in our current study, especially with DSPPs, since it had a group of components rather than only one. DSPPs also protected preadipocytes against H
2O
2-induced detrimental effects, and this property could be ascribed to their direct antioxidant effects versus free radicals in co-exposure conditions. It has been noted that the constitution of polyphenols in terms of hydrophilic and hydrophobic domains could also determine their binding with lipid bilayers. Given that a rudimentary combination of polyphenols is exposed to the digestive process, the results after intestinal digestion are not shocking. It is known that during intestinal digestion, polyphenols suffer irreversible structural changes due to auto-oxidation, isomerization, and conjugation in an alkaline pH, yet certain species are more stable than others, depending on the structure [
41]. A similar finding was noted where Sirisena et al. [
42] showed that DSPPs such as procyanidin B, catechins, and epicatechin decreased with time when digestion moved from the stomach to the intestinal phases. Polyphenol losses between the phases of intestinal and stomach digestion have already been noted with a loss ratio of 44% in wild blueberries [
43].
5. Conclusions
Turning the problem of food processing by-products to products rich in natural polyphenols was recently spotlighted. Therefore, for the first time, we converted date seed polyphenol-rich extracts to healthcare pills produced under large-scale conditions. These are inexpensive sources of polyphenols that have many biological effects, namely anti-obesity activity. After tabulating, the anti-obesity effect of DSPPs was elucidated in 3T3-L1 cells, and their antioxidative polyphenols after digestion were also quantified. The total polyphenols in date seeds were found to be 51.167 g/kg, where epicatechin was a key polyphenol. Results also showed that DSPPs dose-dependently delayed the proliferation of adipocytes in 3T3-L1 cells. DSPPs, especially at a dose of 100 µg/mL, decreased the viable cells quantity, basal lipolysis of adipocytes, and lipid accumulation-related genes. Epicatechin, the key polyphenol of DSPPs, also effectively interacted with both lipase and GPDH enzymes. The pills also showed a high content of polyphenols with antioxidant capacities after digestion, showing the high release of those components after digestion and the importance of the tabulating technique to maintain those active components. In conclusion, the current findings suggested that DSPPs may have anti-obesity effects that could be commercially industrialized as a healthcare tablet.