Low Relative Handgrip Strength Is Associated with a High Risk of Non-Alcoholic Fatty Liver Disease in Italian Adults: A Retrospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Anthropometric Measurements
2.3. HGS Measurement
2.4. Liver Transient Elastography (TE)
2.5. Biochemical Evaluation
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, S.H.; Gong, H.S. Measurement and interpretation of handgrip strength for research on sarcopenia and osteoporosis. J. Bone Metab. 2020, 27, 85. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A. Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Auyeung, T.W.; Lee, J.; Leung, J.; Kwok, T.; Woo, J. The selection of a screening test for frailty identification in community-dwelling older adults. J. Nutr. Health Aging 2014, 18, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Fielding, R.A.; Vellas, B.; Evans, W.J.; Bhasin, S.; Morley, J.E.; Newman, A.B.; van Kan, G.A.; Andrieu, S.; Bauer, J.; Breuille, D. Sarcopenia: An undiagnosed condition in older adults. Current consensus definition: Prevalence, etiology, and consequences. International working group on sarcopenia. J. Am. Med. Dir. Assoc. 2011, 12, 249–256. [Google Scholar] [CrossRef]
- Alexandre, T.; Duarte, Y.; Santos, J.; Wong, R.; Lebrao, M. Sarcopenia according to the European Working Group on Sarcopenia in Older People (EWGSOP) versus dynapenia as a risk factor for mortality in the elderly. J. Nutr. Health Aging 2014, 18, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Valentini, L.; Schaper, L.; Buning, C.; Hengstermann, S.; Koernicke, T.; Tillinger, W.; Guglielmi, F.W.; Norman, K.; Buhner, S.; Ockenga, J. Malnutrition and impaired muscle strength in patients with Crohn’s disease and ulcerative colitis in remission. Nutrition 2008, 24, 694–702. [Google Scholar] [CrossRef]
- Tak, Y.J.; Lee, J.G.; Yi, Y.H.; Kim, Y.J.; Lee, S.; Cho, B.M.; Cho, Y.H. Association of handgrip strength with dietary intake in the Korean population: Findings based on the seventh Korea National Health and Nutrition Examination Survey (KNHANES VII-1), 2016. Nutrients 2018, 10, 1180. [Google Scholar] [CrossRef]
- Bohannon, R.W. Dynamometer measurements of hand-grip strength predict multiple outcomes. Percept. Mot. Ski. 2001, 93, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Leong, D.P.; Teo, K.K.; Rangarajan, S.; Lopez-Jaramillo, P.; Avezum Jr, A.; Orlandini, A.; Seron, P.; Ahmed, S.H.; Rosengren, A.; Kelishadi, R. Prognostic value of grip strength: Findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet 2015, 386, 266–273. [Google Scholar] [CrossRef]
- Kim, K.; Ho, J.-H. Handgrip Strength and Mortality in Elderly Koreans: Evidence from the Korea Longitudinal Study of Ageing. Asia Pac. J. Public. Health 2020, 32, 302–309. [Google Scholar] [CrossRef]
- Bae, E.-J.; Park, N.-J.; Sohn, H.-S.; Kim, Y.-H. Handgrip strength and all-cause mortality in middle-aged and older Koreans. Int. J. Environ. Res. Public. Health 2019, 16, 740. [Google Scholar] [CrossRef]
- Schaap, L.A.; Van Schoor, N.M.; Lips, P.; Visser, M. Associations of sarcopenia definitions, and their components, with the incidence of recurrent falling and fractures: The longitudinal aging study Amsterdam. J. Gerontol. Ser. A 2018, 73, 1199–1204. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Sayiner, M.; Koenig, A.; Henry, L.; Younossi, Z.M. Epidemiology of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis in the United States and the Rest of the World. Clin. Liver Dis. 2016, 20, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M. Non-alcoholic fatty liver disease–A global public health perspective. J. Hepatol. 2019, 70, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Farrell, A.; Ryan, M.; Howell, J. Epidemiology of non-alcoholic fatty liver disease-related hepatocellular carcinoma: A western perspective. Hepatoma Res. 2020, 6, 18. [Google Scholar] [CrossRef]
- Dunn, W.; Xu, R.; Wingard, D.L.; Rogers, C.; Angulo, P.; Younossi, Z.M.; Schwimmer, J.B. Suspected nonalcoholic fatty liver disease and mortality risk in a population-based cohort study. Am. J. Gastroenterol. 2008, 103, 2263. [Google Scholar] [CrossRef]
- Lee, J.I.; Kim, M.C.; Moon, B.S.; Song, Y.S.; Han, E.N.; Lee, H.S.; Son, Y.; Kim, J.; Han, E.J.; Park, H.-J. The relationship between 10-year cardiovascular risk calculated using the pooled cohort equation and the severity of non-alcoholic fatty liver disease. Endocrinol. Metab. 2016, 31, 86–92. [Google Scholar] [CrossRef]
- Dulai, P.S.; Singh, S.; Patel, J.; Soni, M.; Prokop, L.J.; Younossi, Z.; Sebastiani, G.; Ekstedt, M.; Hagstrom, H.; Nasr, P. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology 2017, 65, 1557–1565. [Google Scholar] [CrossRef]
- Guillet, C.; Boirie, Y. Insulin resistance: A contributing factor to age-related muscle mass loss? Diabetes Metab. 2005, 31, S20–S25. [Google Scholar] [CrossRef] [PubMed]
- Beyer, I.; Mets, T.; Bautmans, I. Chronic low-grade inflammation and age-related sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Merli, M.; Dasarathy, S. Sarcopenia in non-alcoholic fatty liver disease: Targeting the real culprit? J. Hepatol. 2015, 63, 309–311. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.S.; Li, G. Skeletal muscle myostatin gene expression and sarcopenia in overweight and obese middle-aged and older adults. JCSM Clin. Rep. 2021, 6, 137–142. [Google Scholar] [CrossRef]
- Wilkes, J.J.; Lloyd, D.J.; Gekakis, N. Loss-of-function mutation in myostatin reduces tumor necrosis factor alpha production and protects liver against obesity-induced insulin resistance. Diabetes 2009, 58, 1133–1143. [Google Scholar] [CrossRef]
- Zhang, C.; McFarlane, C.; Lokireddy, S.; Bonala, S.; Ge, X.; Masuda, S.; Gluckman, P.D.; Sharma, M.; Kambadur, R. Myostatin-deficient mice exhibit reduced insulin resistance through activating the AMP-activated protein kinase signalling pathway. Diabetologia 2011, 54, 1491–1501. [Google Scholar] [CrossRef]
- Yao, Y.; Miao, X.; Zhu, D.; Li, D.; Zhang, Y.; Song, C.; Liu, K. Insulin-like growth factor-1 and non-alcoholic fatty liver disease: A systemic review and meta-analysis. Endocrine 2019, 65, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Bian, A.; Ma, Y.; Zhou, X.; Guo, Y.; Wang, W.; Zhang, Y.; Wang, X. Association between sarcopenia and levels of growth hormone and insulin-like growth factor-1 in the elderly. BMC Musculoskelet. Disord. 2020, 21, 214. [Google Scholar] [CrossRef]
- Ding, R.B.; Bao, J.; Deng, C.X. Emerging roles of SIRT1 in fatty liver diseases. Int. J. Biol. Sci. 2017, 13, 852–867. [Google Scholar] [CrossRef]
- Lee, D.; Goldberg, A.L. SIRT1 protein, by blocking the activities of transcription factors FoxO1 and FoxO3, inhibits muscle atrophy and promotes muscle growth. J. Biol. Chem. 2013, 288, 30515–30526. [Google Scholar] [CrossRef]
- Liu, C.-W.; Huang, C.-C.; Hsu, C.-F.; Li, T.-H.; Tsai, Y.-L.; Lin, M.-W.; Tsai, H.-C.; Huang, S.-F.; Yang, Y.-Y.; Hsieh, Y.-C.; et al. SIRT1-dependent mechanisms and effects of resveratrol for amelioration of muscle wasting in NASH mice. BMJ Open Gastroenterol. 2020, 7, e000381. [Google Scholar] [CrossRef]
- Buscemi, C.; Ferro, Y.; Pujia, R.; Mazza, E.; Boragina, G.; Sciacqua, A.; Piro, S.; Pujia, A.; Sesti, G.; Buscemi, S. Sarcopenia and Appendicular Muscle Mass as Predictors of Impaired Fasting Glucose/Type 2 Diabetes in Elderly Women. Nutrients 2021, 13, 1909. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Kim, D.J.; Plank, L.D. Association of grip strength with non-alcoholic fatty liver disease: Investigation of the roles of insulin resistance and inflammation as mediators. Eur. J. Clin. Nutr. 2020, 74, 1401–1409. [Google Scholar] [CrossRef] [PubMed]
- Meng, G.; Wu, H.; Fang, L.; Li, C.; Yu, F.; Zhang, Q.; Liu, L.; Du, H.; Shi, H.; Xia, Y. Relationship between grip strength and newly diagnosed nonalcoholic fatty liver disease in a large-scale adult population. Sci. Rep. 2016, 6, 33255. [Google Scholar] [CrossRef] [PubMed]
- Lee, K. Relationship between handgrip strength and nonalcoholic fatty liver disease: Nationwide surveys. Metab. Syndr. Relat. Disord. 2018, 16, 497–503. [Google Scholar] [CrossRef]
- Kim, B.-J.; Ahn, S.H.; Lee, S.H.; Hong, S.; Hamrick, M.W.; Isales, C.M.; Koh, J.-M. Lower hand grip strength in older adults with non-alcoholic fatty liver disease: A nationwide population-based study. Aging 2019, 11, 4547. [Google Scholar] [CrossRef]
- Kang, S.; Moon, M.K.; Kim, W.; Koo, B.K. Association between muscle strength and advanced fibrosis in non-alcoholic fatty liver disease: A Korean nationwide survey. J. Cachexia Sarcopenia Muscle 2020, 11, 1232–1241. [Google Scholar] [CrossRef]
- Cho, J.; Lee, I.; Park, D.-H.; Kwak, H.-B.; Min, K. Relationships between Socioeconomic Status, Handgrip Strength, and Non-Alcoholic Fatty Liver Disease in Middle-Aged Adults. Int. J. Environ. Res. Public. Health 2021, 18, 1892. [Google Scholar] [CrossRef]
- Lee, S.B.; Kwon, Y.J.; Jung, D.H.; Kim, J.K. Association of Muscle Strength with Non-Alcoholic Fatty Liver Disease in Korean Adults. Int. J. Env. Res. Public. Health 2022, 19, 1675. [Google Scholar] [CrossRef]
- Gan, D.; Wang, L.; Jia, M.; Ru, Y.; Ma, Y.; Zheng, W.; Zhao, X.; Yang, F.; Wang, T.; Mu, Y.; et al. Low muscle mass and low muscle strength associate with nonalcoholic fatty liver disease. Clin. Nutr. 2020, 39, 1124–1130. [Google Scholar] [CrossRef]
- Wijarnpreecha, K.; Kim, D.; Raymond, P.; Scribani, M.; Ahmed, A. Associations between sarcopenia and nonalcoholic fatty liver disease and advanced fibrosis in the USA. Eur. J. Gastroenterol. Hepatol. 2019, 31, 1121–1128. [Google Scholar] [CrossRef]
- Petermann-Rocha, F.; Gray, S.R.; Forrest, E.; Welsh, P.; Sattar, N.; Celis-Morales, C.; Ho, F.K.; Pell, J.P. Associations of muscle mass and grip strength with severe NAFLD: A prospective study of 333,295 UK Biobank participants. J. Hepatol. 2022, 76, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-H.; Cai, J.-J.; She, Z.-G.; Li, H.-L. Noninvasive evaluation of nonalcoholic fatty liver disease: Current evidence and practice. World J. Gastroenterol. 2019, 25, 1307. [Google Scholar] [CrossRef]
- Eddowes, P.J.; Sasso, M.; Allison, M.; Tsochatzis, E.; Anstee, Q.M.; Sheridan, D.; Guha, I.N.; Cobbold, J.F.; Deeks, J.J.; Paradis, V. Accuracy of FibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology 2019, 156, 1717–1730. [Google Scholar] [CrossRef]
- Mikolasevic, I.; Orlic, L.; Franjic, N.; Hauser, G.; Stimac, D.; Milic, S. Transient elastography (FibroScan®) with controlled attenuation parameter in the assessment of liver steatosis and fibrosis in patients with nonalcoholic fatty liver disease-Where do we stand? World J. Gastroenterol. 2016, 22, 7236. [Google Scholar] [CrossRef] [PubMed]
- Bonder, A.; Afdhal, N. Utilization of FibroScan in clinical practice. Curr. Gastroenterol. Rep. 2014, 16, 372. [Google Scholar] [CrossRef] [PubMed]
- Pujia, A.; Mazza, E.; Ferro, Y.; Gazzaruso, C.; Coppola, A.; Doldo, P.; Grembiale, R.D.; Pujia, R.; Romeo, S.; Montalcini, T. Lipid oxidation assessed by indirect calorimetry predicts metabolic syndrome and type 2 diabetes. Front. Endocrinol. 2019, 9, 806. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation, Geneva, 8-11 December 2008; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Montalcini, T.; Ferro, Y.; Salvati, M.A.; Romeo, S.; Miniero, R.; Pujia, A. Gender difference in handgrip strength of Italian children aged 9 to 10 years. Ital. J. Pediatr. 2016, 42, 16. [Google Scholar] [CrossRef]
- Hardy, R.; Cooper, R.; Aihie Sayer, A.; Ben-Shlomo, Y.; Cooper, C.; Deary, I.J.; Demakakos, P.; Gallacher, J.; Martin, R.M.; McNeill, G. Body mass index, muscle strength and physical performance in older adults from eight cohort studies: The HALCyon programme. PLoS ONE 2013, 8, e56483. [Google Scholar] [CrossRef] [PubMed]
- Karlas, T.; Petroff, D.; Sasso, M.; Fan, J.-G.; Mi, Y.-Q.; de Lédinghen, V.; Kumar, M.; Lupsor-Platon, M.; Han, K.-H.; Cardoso, A.C. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J. Hepatol. 2017, 66, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Montalcini, T.; Pujia, A.; Donini, L.M.; Frittitta, L.; Galvano, F.; Natali, A.; Pironi, L.; Porrini, M.; Riso, P.; Rivellese, A.A.; et al. A Call to Action: Now Is the Time to Screen Elderly and Treat Osteosarcopenia, a Position Paper of the Italian College of Academic Nutritionists MED/49 (ICAN-49). Nutrients 2020, 12, 2662. [Google Scholar] [CrossRef]
- Miele, L.e.a. General Practitioner databaSe NAFLD (GPS-NAFLD) study: Italian regional variability of the NAFLD prevalence rate. Dig. Liver Dis. 2021, 53, S30–S31. [Google Scholar] [CrossRef]
- Petta, S.; Di Marco, V.; Pipitone, R.M.; Grimaudo, S.; Buscemi, C.; Craxì, A.; Buscemi, S. Prevalence and severity of nonalcoholic fatty liver disease by transient elastography: Genetic and metabolic risk factors in a general population. Liver Int. Off. J. Int. Assoc. Study Liver 2018, 38, 2060–2068. [Google Scholar] [CrossRef]
- Colicchio, P.; Tarantino, G.; del Genio, F.; Sorrentino, P.; Saldalamacchia, G.; Finelli, C.; Conca, P.; Contaldo, F.; Pasanisi, F. Non-alcoholic fatty liver disease in young adult severely obese non-diabetic patients in South Italy. Ann. Nutr. Metab. 2005, 49, 289–295. [Google Scholar] [CrossRef]
- Bellentani, S.; Saccoccio, G.; Masutti, F.; Crocè, L.S.; Brandi, G.; Sasso, F.; Cristanini, G.; Tiribelli, C. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann. Intern. Med. 2000, 132, 112–117. [Google Scholar] [CrossRef]
- Estes, C.; Anstee, Q.M.; Arias-Loste, M.T.; Bantel, H.; Bellentani, S.; Caballeria, J.; Colombo, M.; Craxi, A.; Crespo, J.; Day, C.P.; et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J. Hepatol. 2018, 69, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Fu, S.; Li, J.; Liu, D.; Tan, Y. Association between grip strength and non-alcoholic fatty liver disease: A systematic review and meta-analysis. Front. Med. 2022, 9, 988566. [Google Scholar] [CrossRef]
- Xu, L.; Lu, W.; Li, P.; Shen, F.; Mi, Y.-Q.; Fan, J.-G. A comparison of hepatic steatosis index, controlled attenuation parameter and ultrasound as noninvasive diagnostic tools for steatosis in chronic hepatitis B. Dig. Liver Dis. 2017, 49, 910–917. [Google Scholar] [CrossRef]
- Pang, Q.; Zhang, J.-Y.; Song, S.-D.; Qu, K.; Xu, X.-S.; Liu, S.-S.; Liu, C. Central obesity and nonalcoholic fatty liver disease risk after adjusting for body mass index. World J. Gastroenterol. WJG 2015, 21, 1650. [Google Scholar] [CrossRef] [PubMed]
- Linge, J.; Ekstedt, M.; Leinhard, O.D. Adverse muscle composition is linked to poor functional performance and metabolic comorbidities in NAFLD. JHEP Rep. 2021, 3, 100197. [Google Scholar] [CrossRef] [PubMed]
- Oshida, N.; Shida, T.; Oh, S.; Kim, T.; Isobe, T.; Okamoto, Y.; Kamimaki, T.; Okada, K.; Suzuki, H.; Ariizumi, S.-i.; et al. Urinary levels of titin-N fragment, a skeletal muscle damage marker, are increased in subjects with nonalcoholic fatty liver disease. Sci. Rep. 2019, 9, 19498. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Park, S.W.; Harris, T.B.; Kritchevsky, S.B.; Nevitt, M.; Schwartz, A.V.; Simonsick, E.M.; Tylavsky, F.A.; Visser, M.; Newman, A.B. The loss of skeletal muscle strength, mass, and quality in older adults: The health, aging and body composition study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2006, 61, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.B.; Kupelian, V.; Visser, M.; Simonsick, E.M.; Goodpaster, B.H.; Kritchevsky, S.B.; Tylavsky, F.A.; Rubin, S.M.; Harris, T.B. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2006, 61, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Enomoto, H.; Yoh, K.; Iwata, Y.; Sakai, Y.; Kishino, K.; Ikeda, N.; Takashima, T.; Aizawa, N.; Takata, R. Health-related quality of life in chronic liver diseases: A strong impact of hand grip strength. J. Clin. Med. 2018, 7, 553. [Google Scholar] [CrossRef] [PubMed]
- Yoh, K.; Nishikawa, H.; Enomoto, H.; Iwata, Y.; Ikeda, N.; Aizawa, N.; Nishimura, T.; Iijima, H.; Nishiguchi, S. Grip strength: A useful marker for composite hepatic events in patients with chronic liver diseases. Diagnostics 2020, 10, 238. [Google Scholar] [CrossRef] [PubMed]
- Hanai, T.; Shiraki, M.; Imai, K.; Suetsugu, A.; Takai, K.; Moriwaki, H.; Shimizu, M. Reduced handgrip strength is predictive of poor survival among patients with liver cirrhosis: A sex-stratified analysis. Hepatol. Res. 2019, 49, 1414–1426. [Google Scholar] [CrossRef] [PubMed]
- Kotoh, Y.; Saeki, I.; Yamasaki, T.; Sasaki, R.; Tanabe, N.; Oono, T.; Matsuda, T.; Hisanaga, T.; Matsumoto, T.; Hidaka, I. Effect of Handgrip Strength on Clinical Outcomes of Patients with Hepatocellular Carcinoma Treated with Lenvatinib. Appl. Sci. 2020, 10, 5403. [Google Scholar] [CrossRef]
- Tapper, E.B.; Konerman, M.; Murphy, S.; Sonnenday, C.J. Hepatic encephalopathy impacts the predictive value of the Fried Frailty Index. Am. J. Transplant. 2018, 18, 2566–2570. [Google Scholar] [CrossRef]
- Wang, C.W.; Feng, S.; Covinsky, K.E.; Hayssen, H.; Zhou, L.-Q.; Yeh, B.M.; Lai, J.C. A comparison of muscle function, mass, and quality in liver transplant candidates: Results from the functional assessment in liver transplantation (FrAILT) study. Transplantation 2016, 100, 1692. [Google Scholar] [CrossRef]
- Sultan, P.; Hamilton, M.A.; Ackland, G.L. Preoperative muscle weakness as defined by handgrip strength and postoperative outcomes: A systematic review. BMC Anesthesiol. 2012, 12, 1. [Google Scholar] [CrossRef]
- Srikanthan, P.; Hevener, A.L.; Karlamangla, A.S. Sarcopenia exacerbates obesity-associated insulin resistance and dysglycemia: Findings from the National Health and Nutrition Examination Survey III. PLoS ONE 2010, 5, e10805. [Google Scholar] [CrossRef]
- Serra, C.; Bhasin, S.; Tangherlini, F.; Barton, E.R.; Ganno, M.; Zhang, A.; Shansky, J.; Vandenburgh, H.H.; Travison, T.G.; Jasuja, R. The role of GH and IGF-I in mediating anabolic effects of testosterone on androgen-responsive muscle. Endocrinology 2011, 152, 193–206. [Google Scholar] [CrossRef]
- Elkina, Y.; Von Haehling, S.; Anker, S.D.; Springer, J. The role of myostatin in muscle wasting: An overview. J. Cachexia Sarcopenia Muscle 2011, 2, 143. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.J. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology 2002, 123, 1705–1725. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Clinical. National Institute for Health and Clinical. National Institute for Health and Care Excellence: Guidelines. In Non-Alcoholic Fatty Liver Disease: Assessment and Management; National Institute for Health and Care Excellence (NICE): London, UK, 2003. [Google Scholar]
- Rinella, M.E.; Neuschwander-Tetri, B.A.; Siddiqui, M.S.; Abdelmalek, M.F.; Caldwell, S.; Barb, D.; Kleiner, D.E.; Loomba, R. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology 2023, 77, 1797–1835. [Google Scholar] [CrossRef]
- Lauschke, V.M. Practice guidance documents for the diagnosis and management of non-alcoholic fatty liver disease-recent updates and open questions. Hepatobiliary Surg. Nutr. 2023, 12, 780–784. [Google Scholar] [CrossRef] [PubMed]
- Neuschwander-Tetri, B.A.; Caldwell, S.H. Nonalcoholic steatohepatitis: Summary of an AASLD Single Topic Conference. Hepatology 2003, 37, 1202–1219. [Google Scholar] [CrossRef]
- Sanyal, D.; Mukherjee, P.; Raychaudhuri, M.; Ghosh, S.; Mukherjee, S.; Chowdhury, S. Profile of liver enzymes in non-alcoholic fatty liver disease in patients with impaired glucose tolerance and newly detected untreated type 2 diabetes. Indian. J. Endocrinol. Metab. 2015, 19, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.; Petta, S.; Hiriart, J.B.; Cammà, C.; Wong, G.L.; Marra, F.; Vergniol, J.; Chan, A.W.; Tuttolomondo, A.; Merrouche, W.; et al. Validity criteria for the diagnosis of fatty liver by M probe-based controlled attenuation parameter. J. Hepatol. 2017, 67, 577–584. [Google Scholar] [CrossRef]
- Zhang, X.; Wong, G.L.; Wong, V.W. Application of transient elastography in nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 2020, 26, 128–141. [Google Scholar] [CrossRef]
- Shen, F.; Zheng, R.D.; Shi, J.P.; Mi, Y.Q.; Chen, G.F.; Hu, X.; Liu, Y.G.; Wang, X.Y.; Pan, Q.; Chen, G.Y.; et al. Impact of skin capsular distance on the performance of controlled attenuation parameter in patients with chronic liver disease. Liver Int. Off. J. Int. Assoc. Study Liver 2015, 35, 2392–2400. [Google Scholar] [CrossRef]
- Kimura, S.; Tanaka, K.; Oeda, S.; Inoue, K.; Inadomi, C.; Kubotsu, Y.; Yoshioka, W.; Okada, M.; Isoda, H.; Kuwashiro, T.; et al. Effect of skin-capsular distance on controlled attenuation parameter for diagnosing liver steatosis in patients with nonalcoholic fatty liver disease. Sci. Rep. 2021, 11, 15641. [Google Scholar] [CrossRef] [PubMed]
- Oeda, S.; Tanaka, K.; Oshima, A.; Matsumoto, Y.; Sueoka, E.; Takahashi, H. Diagnostic Accuracy of FibroScan and Factors Affecting Measurements. Diagnostics 2020, 10, 940. [Google Scholar] [CrossRef] [PubMed]
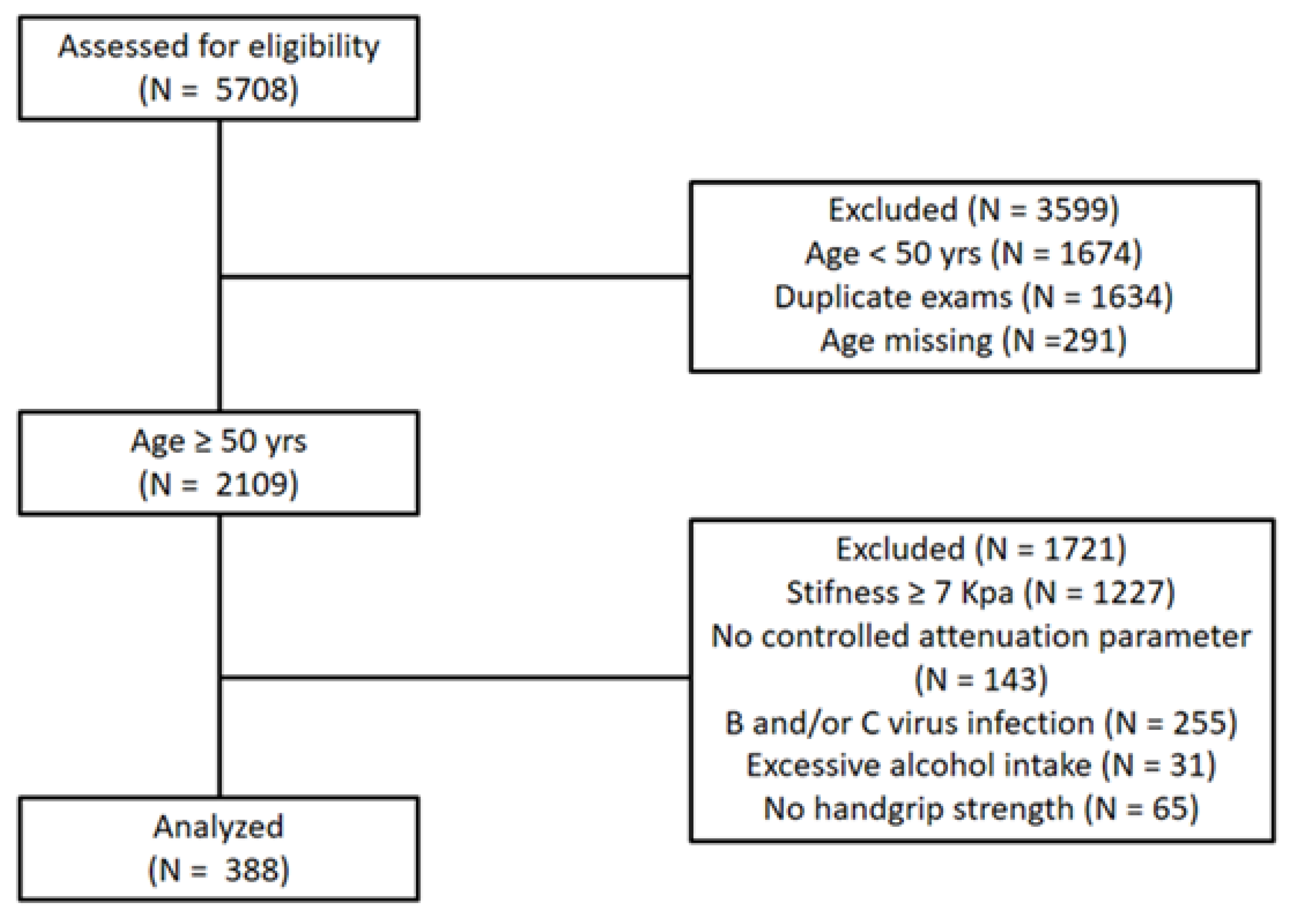
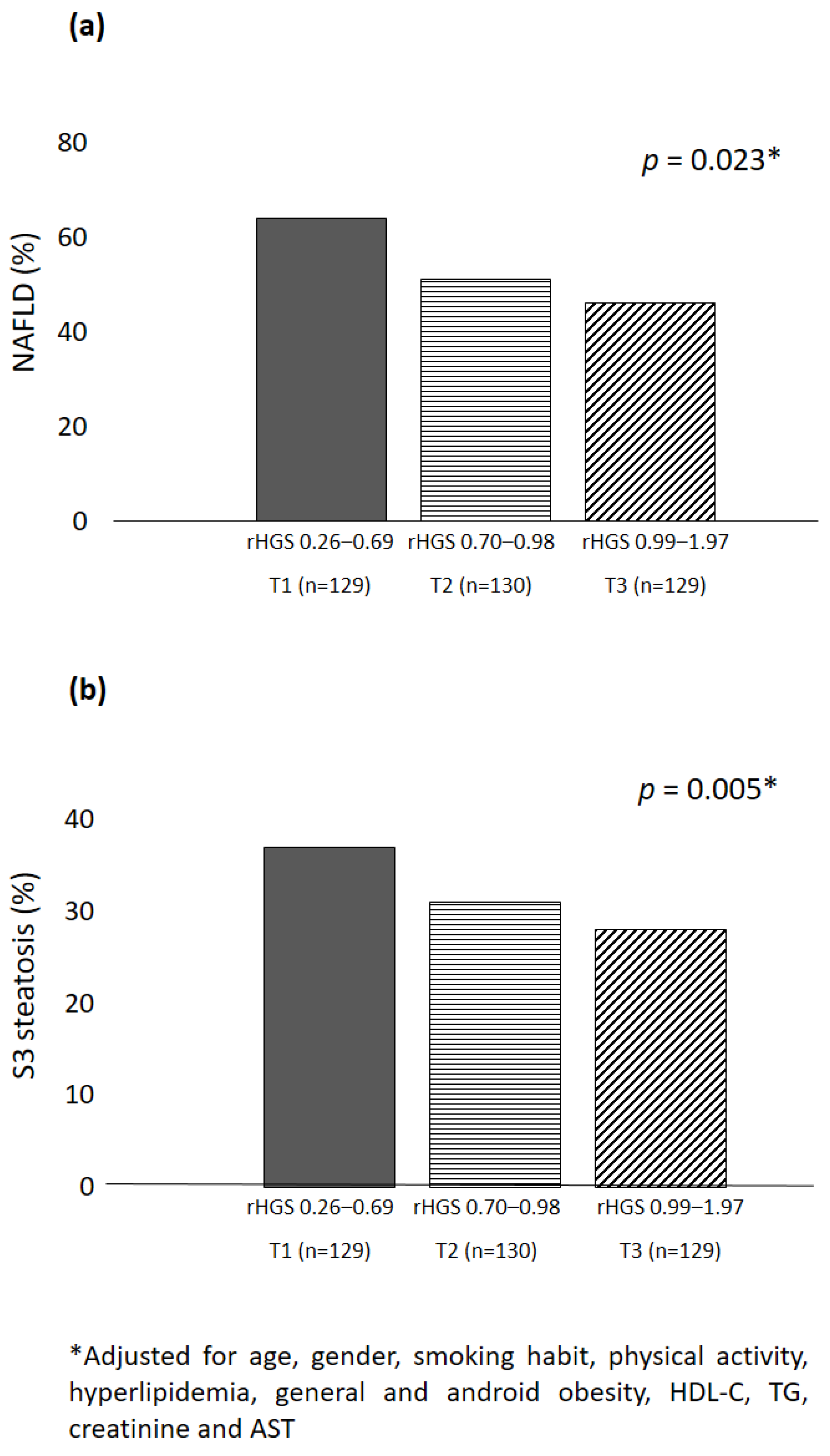
| Variables | Total (n = 388) | T1 (n = 129) | T2 (n = 130) | T3 (n = 129) | p for Trend | p for Post Hoc Analysis |
|---|---|---|---|---|---|---|
| rHGS (Range) | 0.26–1.97 | 0.26–0.69 | 0.70–0.98 | 0.99–1.97 | ||
| Age (years) | 60.8 ± 7.1 | 62.4 ± 7 | 61.8 ± 7 | 58.1 ± 6 | <0.001 | T1 vs. T3 < 0.001 T2 vs. T3 < 0.001 |
| BMI (kg/m2) | 28.7 ± 4.4 | 30.8 ± 5 | 27.9 ± 4 | 27.3 ± 4 | <0.001 | T1 vs. T2 < 0.001 T1 vs. T3 < 0.001 |
| HGS (kg) | 25.5 ± 9.3 | 17.9 ± 3 | 22.4 ± 4 | 36.1 ± 7 | <0.001 | T1 vs. T2 < 0.001 T1 vs. T3 < 0.001 T2 vs. T3 < 0.001 |
| WHR | 0.91 ± 0.08 | 0.89 ± 0.07 | 0.89 ± 0.09 | 0.94 ± 0.09 | <0.001 | T1 vs. T3 < 0.001 T2 vs. T3 < 0.001 |
| SBP (mmHg) | 125 ± 16 | 125 ± 16 | 126 ± 18 | 125 ± 13 | 0.85 | / |
| DBP (mmHg) | 76 ± 10 | 76 ± 11 | 76 ± 9 | 77 ± 9 | 0.56 | / |
| Glucose (mg/dL) | 99 ± 21 | 99 ± 16 | 98 ± 24 | 99 ± 22 | 0.92 | / |
| TC (mg/dL) | 208 ± 42 | 210 ± 39 | 208 ± 42 | 206 ± 46 | 0.68 | / |
| HDL-C (mg/dL) | 57 ± 16 | 61 ± 17 | 59 ± 16 | 52 ± 14 | <0.001 | T1 vs. T3 < 0.001 T2 vs. T3 0.001 |
| TG (mg/dL) | 129 ± 70 | 123 ± 62 | 119 ± 62 | 144 ± 81 | 0.010 | T2 vs. T3 0.019 |
| LDL-C (mg/dL) | 125 ± 38 | 125 ± 36 | 124 ± 37 | 125 ± 40 | 0.99 | / |
| Creatinine (mg/dL) | 0.81 ± 0.17 | 0.77 ± 0.2 | 0.75 ± 0.1 | 0.91 ± 0.2 | <0.001 | T1 vs. T3 < 0.001 T2 vs. T3 < 0.001 |
| AST (IU/L) | 22 ± 7 | 21 ± 7 | 21 ± 7 | 23 ± 8 | 0.018 | T1 vs. T3 0.039 |
| ALT (IU/L) | 23 ± 14 | 21 ± 12 | 23 ± 17 | 25 ± 13 | 0.09 | / |
| γGT (IU/L) | 29 ± 26 | 36 ± 41 | 23 ± 15 | 29 ± 24 | 0.15 | / |
| CAP (Db/m) | 256 ± 48 | 266 ± 48 | 252 ± 49 | 249 ± 47 | 0.012 | T1 vs. T3 0.021 |
| IQR (%) | 20 ± 6 | 20 ± 6 | 21 ± 6 | 20 ± 6 | 0.90 | |
| aCAP § (Db/m) | / | 269 ± 5 | 253 ± 4 | 237 ± 6 | 0.001 | T1 vs. T2 0.023 T1 vs. T3 0.001 |
| Stiffness (KPa) | 4.8 ± 1 | 4.9 ± 1 | 4.7 ± 1 | 4.7 ± 1 | 0.49 | / |
| Prevalence (%) | ||||||
| Gender, female | 70 | 98 | 85 | 26 | <0.001 | / |
| Smokers | 27 | 19 | 25 | 38 | 0.001 | / |
| Physical activity | 35 | 18 | 37 | 50 | <0.001 | / |
| T2DM | 9 | 12 | 9 | 7 | 0.22 | / |
| Dyslipidaemia | 69 | 65 | 62 | 81 | 0.007 | / |
| Hypertension | 47 | 54 | 45 | 42 | 0.06 | / |
| General Obesity | 34 | 50 | 32 | 20 | <0.001 | / |
| Android Obesity | 73 | 72 | 66 | 80 | 0.034 | / |
| OR (95% CI) | p | |
|---|---|---|
| Model 1 | ||
| High rHGS | 1 (ref) | |
| Mild rHGS | 2.48 (1.26–4.87) | 0.008 |
| Low rHGS | 4.85 (2.30–10.20) | <0.001 |
| Gender | 2.70 (1.40–5.21) | 0.003 |
| Model 2 | ||
| High rHGS | 1 (ref) | |
| Mild rHGS | 2.02 (0.91–4.48) | 0.08 |
| Low rHGS | 3.63 (1.49–8.82) | 0.004 |
| General Obesity | 2.44 (1.41–4.28) | 0.002 |
| Model 3 | ||
| High rHGS | 1 (ref) | |
| Mild rHGS | 2.56 (1.17–5.59) | 0.018 |
| Low rHGS | 5.30 (2.24–12.57) | <0.001 |
| Android Obesity | 2.39 (1.36–4.18) | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maurotti, S.; Pujia, R.; Mazza, E.; Pileggi, M.F.; Arturi, F.; Tarsitano, M.G.; Montalcini, T.; Pujia, A.; Ferro, Y. Low Relative Handgrip Strength Is Associated with a High Risk of Non-Alcoholic Fatty Liver Disease in Italian Adults: A Retrospective Cohort Study. Appl. Sci. 2023, 13, 12489. https://doi.org/10.3390/app132212489
Maurotti S, Pujia R, Mazza E, Pileggi MF, Arturi F, Tarsitano MG, Montalcini T, Pujia A, Ferro Y. Low Relative Handgrip Strength Is Associated with a High Risk of Non-Alcoholic Fatty Liver Disease in Italian Adults: A Retrospective Cohort Study. Applied Sciences. 2023; 13(22):12489. https://doi.org/10.3390/app132212489
Chicago/Turabian StyleMaurotti, Samantha, Roberta Pujia, Elisa Mazza, Maria Francesca Pileggi, Franco Arturi, Maria Grazia Tarsitano, Tiziana Montalcini, Arturo Pujia, and Yvelise Ferro. 2023. "Low Relative Handgrip Strength Is Associated with a High Risk of Non-Alcoholic Fatty Liver Disease in Italian Adults: A Retrospective Cohort Study" Applied Sciences 13, no. 22: 12489. https://doi.org/10.3390/app132212489
APA StyleMaurotti, S., Pujia, R., Mazza, E., Pileggi, M. F., Arturi, F., Tarsitano, M. G., Montalcini, T., Pujia, A., & Ferro, Y. (2023). Low Relative Handgrip Strength Is Associated with a High Risk of Non-Alcoholic Fatty Liver Disease in Italian Adults: A Retrospective Cohort Study. Applied Sciences, 13(22), 12489. https://doi.org/10.3390/app132212489








