Supplementation of Nutraceuticals from Dwarf Kiwi and Apple Improves Lipid Profile in Overweight Adults
Abstract
1. Introduction
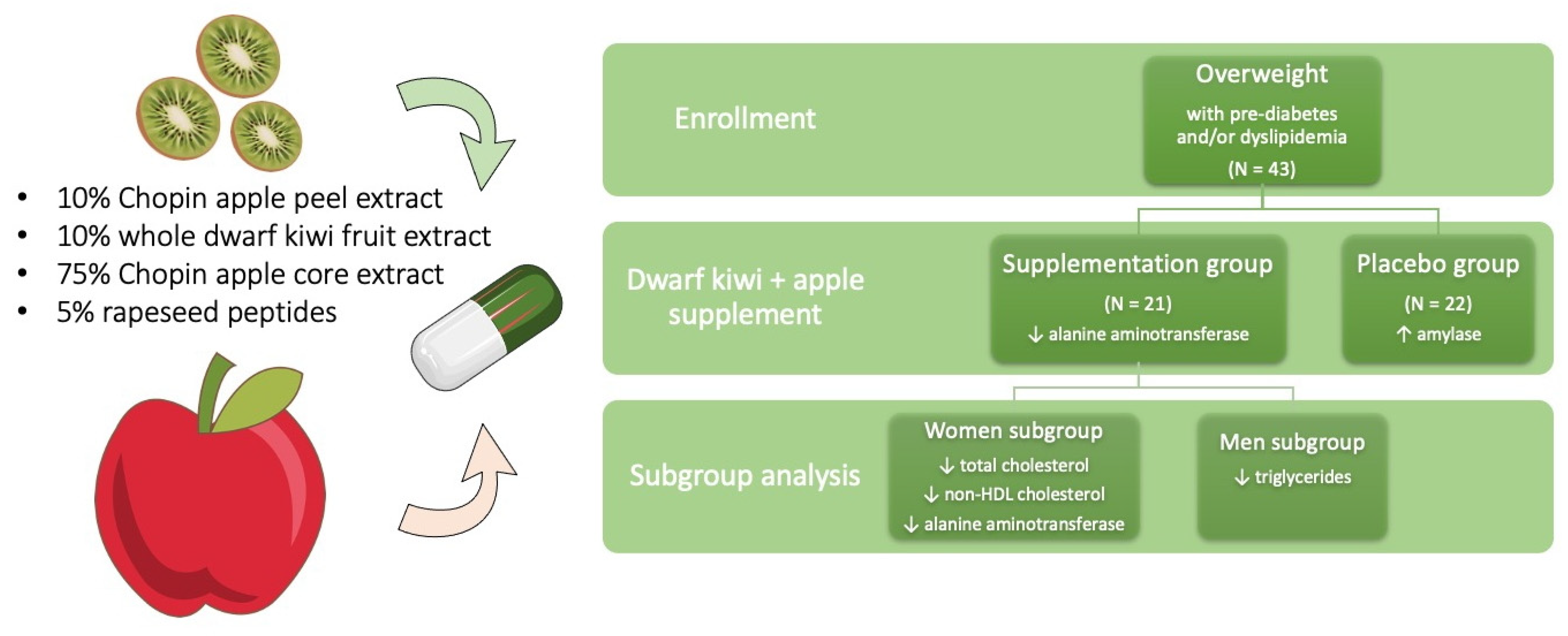
2. Materials and Methods
2.1. Ethical Aspects
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Limitations of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef]
- Alshammary, A.F.; Alharbi, K.K.; Alshehri, N.J.; Vennu, V.; Khan, I.A. Metabolic syndrome and coronary artery disease risk: A meta-analysis of observational studies. Int. J. Environ. Res. Public Health 2021, 18, 1773. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Obesity: Preventing and Managing the Global Epidemic; Report of a WHO Consultation; Technical Report Series; World Health Organization: Geneva, Switzerland, 2000; Volume 894.
- International Diabetes Federation. The IDF consensus worldwide definition of the metabolic syndrome. Obes. Metab. 2005, 2, 47–49. [Google Scholar] [CrossRef]
- Monserrat-Mesquida, M.; Quetglas-Llabrés, M.; Capó, X.; Bouzas, C.; Mateos, D.; Pons, A.; Tur, J.A.; Sureda, A. Metabolic Syndrome Is Associated with Oxidative Stress and Proinflammatory State. Antioxidants 2020, 9, 236. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.Y.; Kim, J.Y.; Kang, H.T.; Han, K.H.; Shim, J.Y. Effect of fruits and vegetables on metabolic syndrome: A systematic review and meta-analysis of randomized controlled trials. Int. J. Food Sci. Nutr. 2015, 66, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Tsao, R.; Yang, R.; Young, J.C.; Zhu, H. Polyphenolic Profiles in Eight Apple Cultivars Using High-Performance Liquid Chromatography (HPLC). J. Agric. Food Chem. 2003, 51, 6347–6353. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, K.; Wu, X.; Liu, R.H. Antioxidant activity of apple peels. J. Agric. Food Chem. 2003, 51, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Wu, X.; Zhang, M.; Zhou, Z.; Liu, Y. Comparative study on the effects of apple peel polyphenols and apple flesh polyphenols on cardiovascular risk factors in mice. Clin. Exp. Hypertens. 2018, 40, 65–72. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The health benefits of dietary fibre. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef]
- Chen, J.P.; Chen, G.C.; Wang, X.P.; Qin, L.; Bai, Y. Dietary fiber and metabolic syndrome: A meta-analysis and review of related mechanisms. Nutrients 2018, 10, 24. [Google Scholar] [CrossRef]
- Shinozaki, K.; Okuda, M.; Sasaki, S.; Kunitsugu, I.; Shigeta, M. Dietary fiber consumption decreases the risks of overweight and hypercholesterolemia in Japanese children. Ann. Nutr. Metab. 2015, 67, 58–64. [Google Scholar] [CrossRef]
- Zhou, Q.; Wu, J.; Tang, J.; Wang, J.J.; Lu, C.H.; Wang, P.X. Beneficial effect of higher dietary fiber intake on plasma HDL-C and TC/HDL-C ratio among Chinese rural-to-urban migrant workers. Int. J. Environ. Res. Public Health 2015, 12, 4726–4738. [Google Scholar] [CrossRef]
- Zhu, X.; Sun, X.; Wang, M.; Zhang, C.; Cao, Y.; Mo, G.; Liang, J.; Zhu, S. Quantitative assessment of the effects of beta-glucan consumption on serum lipid profile and glucose level in hypercholesterolemic subjects. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 714–723. [Google Scholar] [CrossRef]
- Hollænder, P.L.B.; Ross, A.B.; Kristensen, M. Whole-grain and blood lipid changes in apparently healthy adults: A systematic review and meta-analysis of randomized controlled studies1-3. Am. J. Clin. Nutr. 2015, 102, 556–572. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.L.; Otto, B.; Reich, S.-C.; Weickert, M.O.; Steiniger, J.; Machowetz, A.; Rudovich, N.N.; Möhlig, M.; Katz, N.; Speth, M.; et al. Arabinoxylan consumption decreases postprandial serum glucose, serum insulin and plasma total ghrelin response in subjects with impaired glucose tolerance. Eur. J. Clin. Nutr. 2007, 61, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Kalinowska, M.; Gryko, K.; Wróblewska, A.M.; Jabłońska-Trypuć, A.; Karpowicz, D. Phenolic content, chemical composition and anti-/pro-oxidant activity of Gold Milenium and Papierowka apple peel extracts. Sci. Rep. 2020, 10, 14951. [Google Scholar] [CrossRef] [PubMed]
- Popiolek-Kalisz, J.; Blaszczak, P.; Fornal, E. Dietary Isorhamnetin Intake Is Associated with Lower Blood Pressure in Coronary Artery Disease Patients. Nutrients 2022, 14, 4586. [Google Scholar] [CrossRef]
- Popiolek-Kalisz, J.; Fornal, E. Dietary Isorhamnetin Intake Is Inversely Associated with Coronary Artery Disease Occurrence in Polish Adults. Int. J. Environ. Res. Public Health 2022, 19, 12546. [Google Scholar] [CrossRef]
- Lee, K.-H.; Park, E.; Lee, H.-J.; Kim, M.-O.; Cha, Y.-J.; Kim, J.-M.; Lee, H.; Shin, M.-J. Effects of daily quercetin-rich supplementation on cardiometabolic risks in male smokers. Nutr. Res. Pract. 2011, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Egert, S.; Bosy-Westphal, A.; Seiberl, J.; Kürbitz, C.; Settler, U.; Plachta-Danielzik, S.; Wagner, A.E.; Frank, J.; Schrezenmeir, J.; Rimbach, G.; et al. Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: A double-blinded, placebo-controlled cross-over study. Br. J. Nutr. 2009, 102, 1065–1074. [Google Scholar] [CrossRef]
- Lee, Y.M.; Yoon, Y.; Yoon, H.; Park, H.M.; Song, S.; Yeum, K.J. Dietary anthocyanins against obesity and inflammation. Nutrients 2017, 9, 1089. [Google Scholar] [CrossRef] [PubMed]
- Vugic, L.; Colson, N.; Nikbakht, E.; Gaiz, A.; Holland, O.J.; Kundur, A.R.; Singh, I. Anthocyanin supplementation inhibits secretion of pro-inflammatory cytokines in overweight and obese individuals. J. Funct. Foods 2020, 64, 103596. [Google Scholar] [CrossRef]
- Azzini, E.; Venneria, E.; Ciarapica, D.; Foddai, M.S.; Intorre, F.; Zaccaria, M.; Maiani, F.; Palomba, L.; Barnaba, L.; Tubili, C.; et al. Effect of Red Orange Juice Consumption on Body Composition and Nutritional Status in Overweight/Obese Female: A Pilot Study. Oxidative Med. Cell. Longev. 2017, 2017, 1672567. [Google Scholar] [CrossRef] [PubMed]
- Bhaswant, M.; Brown, L.; Mathai, M.L. Queen Garnet plum juice and raspberry cordial in mildly hypertensive obese or overweight subjects: A randomized, double-blind study. J. Funct. Foods 2019, 56, 119–126. [Google Scholar] [CrossRef]
- Garcia-Herrera, P.; Maieves, H.A.; Vega, E.N.; Perez-Rodriguez, M.L.; Fernandez-Ruiz, V.; Iriondo-DeHond, A.; del Castillo, M.D.; Sanchez-Mata, M.C. Dwarf Kiwi (Actinidia arguta Miq.), a Source of Antioxidants for a Healthy and Sustainable Diet. Molecules 2022, 27, 5495. [Google Scholar] [CrossRef]
- Ferguson, A.R.; Ferguson, L.R. Are kiwifruit really good for you? Acta Hortic. 2003, 610, 131–138. [Google Scholar] [CrossRef]
- Debersaques, F.; Mekers, O.; Decorte, J.; Van Labeke, M.C.; Schoedl-Hummel, K.; Latocha, P. Challenges faced by commercial kiwiberry (Actinidia argute planch.) Production. Acta Hortic. 2015, 1096, 435–442. [Google Scholar] [CrossRef]
- Williams, M.H.; Boyd, L.M.; McNeilage, M.A.; MacRae, E.A.; Beatson, R.A.; Martin, P.J. Development and Commercialization of ‘Baby Kiwi’ (Actinidia arguta planch.); International Society for Horticultural Science: Leuven, Belgium, 2003. [Google Scholar]
- Drummond, L. Chapter Three—The Composition and Nutritional Value of Kiwifruit. In Advances in Food and Nutrition Research; Boland, M., Moughan, P.J., Eds.; Academic Press: Cambridge, MA, USA, 2013; Volume 68, pp. 33–57. [Google Scholar] [CrossRef]
- Latocha, P.; Krupa, T.; Wołosiak, R.; Worobiej, E.; Wilczak, J. Antioxidant activity and chemical difference in fruit of different Actinidia sp. Int. J. Food Sci. Nutr. 2010, 61, 381–394. [Google Scholar] [CrossRef]
- Latocha, P.P. The Nutritional and Health Benefits of Kiwiberry (Actinidia arguta)—A Review. Plant Foods Hum. Nutr. 2017, 72, 325–334. [Google Scholar] [CrossRef]
- Kim, H.; Bae, M.; Lim, S.; Lee, W.; Kim, S. A Water-Soluble Extract from Actinidia arguta Ameliorates Psoriasis-Like Skin Inflammation in Mice by Inhibition of Neutrophil Infiltration. Nutrients 2018, 10, 1399. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.-J.; Lim, S.; Lee, D.S.; Ko, K.R.; Lee, W.; Kim, S. Water soluble extracts from Actinidia arguta, PG102, attenuates house dust mite-induced murine atopic dermatitis by inhibiting the mTOR pathway with Treg generation. J. Ethnopharmacol. 2016, 193, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Lian, L.; Zhang, S.; Yu, Z.; Ge, H.; Qi, S.; Zhang, X.; Long, L.; Xiong, X.; Chu, D.; Ma, X.; et al. The dietary freeze-dried fruit powder of Actinidia arguta ameliorates dextran sulphate sodium-induced ulcerative colitis in mice by inhibiting the activation of MAPKs. Food Funct. 2019, 10, 5768–5778. [Google Scholar] [CrossRef]
- Leontowicz, M.; Leontowicz, H.; Jesion, I.; Bielecki, W.; Najman, K.; Latocha, P.; Park, Y.-S.; Gorinstein, S. Actinidia arguta supplementation protects aorta and liver in rats with induced hypercholesterolemia. Nutr. Res. 2016, 36, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Kim, S.; Lee, S.-H.; Park, H.-W.; Chang, Y.-S.; Min, K.-U.; Cho, S.-H. The effects of PG102, a water-soluble extract from Actinidia arguta, on serum total IgE levels: A double-blind, randomized, placebo-controlled exploratory clinical study. Eur. J. Nutr. 2011, 50, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Bąk-Sosnowska, M.; Białkowska, M.; Bogdanski, P.; Chomiuk, T.; Gałązka-Sobotka, M.; Holecki, M.; Jarosińska, A.; Jezierska-Kazberuk, M.; Kamiński, P.; Kłoda, K.; et al. Zalecenia Kliniczne Dotyczące Postępowania u Chorych na Otyłość 2022. Stanowisko Polskiego Towarzystwa Leczenia Otyłości; Medycyna Praktyczna: Krakow, Poland, 2022. [Google Scholar]
- Iłowiecka, K.; Glibowski, P.; Skrzypek, M.; Styk, W. The Long-Term Dietitian and Psychological Support of Obese Patients Who Have Reduced Their Weight Allows Them to Maintain the Effects. Nutrients 2021, 13, 2020. [Google Scholar] [CrossRef]
- Henríquez, C.; Speisky, H.; Chiffelle, I.; Valenzuela, T.; Araya, M.; Simpson, R.; Almonacid, S. Development of an ingredient containing apple peel, as a source of polyphenols and dietary fiber. J. Food Sci. 2010, 75, H172–H181. [Google Scholar] [CrossRef]
- Popiolek-Kalisz, J.; Glibowski, P. Apple Peel Supplementation Potential in Metabolic Syndrome Prevention. Life 2023, 13, 753. [Google Scholar] [CrossRef]
| Overall (n = 43) | Supplementation Group (n = 21) | Placebo Group (n = 22) | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD [±] | Mean | SD [±] | Mean | SD [±] | p | |
| Anthropometric parameters | |||||||
| FM [kg] | 33.85 | 11.67 | 36.11 | 13.37 | 31.69 | 9.60 | 0.47 |
| FM% | 35.67 | 6.80 | 36.26 | 7.44 | 35.11 | 6.25 | 0.45 |
| Body mass [kg] | 93.57 | 21.05 | 97.72 | 22.53 | 89.61 | 19.21 | 0.37 |
| BMI [kg/m2] | 31.66 | 5.86 | 32.75 | 6.66 | 30.62 | 4.92 | 0.47 |
| Biochemical parameters | |||||||
| Renal function | |||||||
| Creatinine [mg/dL] | 0.74 | 0.20 | 0.68 | 0.11 | 0.79 | 0.24 | 0.36 |
| GFR [mL/min/1.73 m2] | 107.43 | 22.26 | 113.69 | 21.59 | 101.45 | 21.69 | 0.15 |
| Uric acid [mg/dL] | 5.36 | 1.15 | 5.40 | 1.03 | 5.32 | 1.27 | 0.42 |
| Liver function | |||||||
| GGTP [UI/L] | 33.02 | 31.91 | 32.52 | 19.79 | 33.50 | 40.79 | 0.19 |
| AST [UI/L] | 20.65 | 8.43 | 21.66 | 9.49 | 19.70 | 7.38 | 0.54 |
| ALT [UI/L] | 26.85 | 17.66 | 29.65 | 19.02 | 24.17 | 16.25 | 0.16 |
| Albumin [g/L] | 45.91 | 2.60 | 45.89 | 2.96 | 45.94 | 2.28 | 0.77 |
| Glucose metabolism and pancreas function | |||||||
| Glucose [mg/dL] | 102.35 | 11.04 | 102.81 | 9.13 | 101.91 | 12.81 | 0.53 |
| HbA1C [%] | 5.33 | 0.35 | 5.33 | 0.37 | 5.32 | 0.34 | 0.73 |
| Insulin [mU/mL] | 15.69 | 10.23 | 16.74 | 9.37 | 14.70 | 11.12 | 0.26 |
| HOMA-IR | 0.15 | 0.09 | 0.16 | 0.10 | 0.14 | 0.08 | 0.47 |
| Amylase [UI/L] | 53.76 | 21.71 | 54.11 | 26.09 | 53.42 | 17.15 | 0.72 |
| Lipid profile | |||||||
| Total cholesterol [mg/dL] | 201.07 | 40.36 | 206.35 | 45.67 | 196.27 | 35.25 | 0.30 |
| HDL [mg/dL] | 50.71 | 20.30 | 53.20 | 26.88 | 48.45 | 11.82 | 0.87 |
| LDL [mg/dl] | 117.82 | 35.24 | 120.50 | 41.12 | 115.52 | 30.18 | 0.69 |
| TG [mg/dL] | 160.28 | 108.40 | 165.49 | 94.97 | 155.55 | 121.37 | 0.47 |
| Non-HDL [mg/dL] | 150.37 | 41.14 | 153.18 | 45.29 | 147.81 | 37.87 | 0.40 |
| Products [Portions/Week] | Overall [Mean ± SD] | Supplementation Group [Mean ± SD] | Placebo Group [Mean ± SD] | p |
|---|---|---|---|---|
| Fruits | 6.29 ± 3.07 | 6.21 ± 1.82 | 6.36 ± 3.96 | 0.89 |
| Vegetables | 6.58 ± 1.78 | 6.71 ± 0.96 | 6.45 ± 2.32 | 0.28 |
| Legumes | 0.72 ± 1.07 | 0.98 ± 1.32 | 0.48 ± 0.72 | 0.15 |
| Fish | 0.86 ± 0.81 | 0.83 ± 0.91 | 0.89 ± 0.72 | 0.64 |
| Red meat | 1.24 ± 1.38 | 1.17 ± 1.29 | 1.32 ± 1.48 | 0.87 |
| Poultry | 2.55 ± 1.65 | 2.52 ± 1.74 | 2.57 ± 1.59 | 0.99 |
| Processed meat | 3.48 ± 2.82 | 3.86 ± 2.83 | 3.11 ± 2.83 | 0.31 |
| Eggs | 3.21 ± 1.83 | 3.10 ± 1.79 | 3.32 ± 1.90 | 0.86 |
| Milk | 3.03 ± 3.07 | 2.83 ± 3.03 | 3.23 ± 3.16 | 0.80 |
| Other dairy | 4.83 ± 2.82 | 5.07 ± 2.15 | 4.59 ± 3.37 | 0.37 |
| White bread | 2.72 ± 2.81 | 2.81 ± 2.69 | 2.64 ± 2.98 | 0.61 |
| Wholegrain bread | 3.52 ± 2.74 | 3.71 ± 2.61 | 3.34 ± 2.90 | 0.55 |
| Groats | 3.42 ± 2.42 | 3.74 ± 2.21 | 3.11 ± 2.61 | 0.35 |
| Potatoes | 2.15 ± 1.85 | 2.05 ± 1.77 | 2.25 ± 1.95 | 0.87 |
| Sweets | 3.86 ± 3.08 | 4.48 ± 3.61 | 3.27 ± 2.39 | 0.36 |
| Kiwi | 0 | 0 | 0 | 1.00 |
| Apples | 0.53 ± 0.50 | 0.57 ± 0.51 | 0.50 ± 0.51 | 0.64 |
| Supplementation Group | Placebo Group | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 60 | p | Day 0 | Day 60 | p | |||||
| Mean | SD [±] | Mean | SD [±] | Mean | SD [±] | Mean | SD [±] | |||
| FM [kg] | 36.11 | 13.37 | 37.05 | 13.25 | 0.02 | 31.69 | 9.60 | 31.97 | 9.19 | 1.00 |
| FM% | 36.26 | 7.44 | 36.96 | 6.95 | 0.02 | 35.11 | 6.25 | 35.55 | 5.35 | 1.00 |
| Creatinine [mg/dL] | 0.68 | 0.11 | 0.68 | 0.10 | 0.69 | 0.79 | 0.24 | 0.75 | 0.22 | 0.047 |
| GFR [mL/min/1.73 m2] | 113.69 | 21.59 | 114.81 | 22.17 | 0.56 | 101.45 | 21.69 | 106.37 | 22.24 | 0.10 |
| Uric acid [mg/dL] | 5.40 | 1.03 | 5.37 | 0.91 | 0.84 | 5.32 | 1.27 | 5.11 | 1.31 | 0.20 |
| GGTP [UI/L] | 32.52 | 19.79 | 33.53 | 18.47 | 0.77 | 33.50 | 40.79 | 28.32 | 25.47 | 0.58 |
| Amylase [UI/L] | 54.11 | 26.09 | 57.04 | 28.70 | 0.05 | 53.42 | 17.15 | 55.62 | 15.03 | 0.02 |
| Total cholesterol [mg/dL] | 206.35 | 45.67 | 198.43 | 49.29 | 0.10 | 196.27 | 35.25 | 188.00 | 29.85 | 0.08 |
| HDL [mg/dL] | 53.20 | 26.88 | 56.38 | 26.34 | 0.13 | 48.45 | 11.82 | 52.14 | 13.71 | 0.02 |
| LDL [mg/dl] | 120.50 | 41.12 | 112.95 | 42.60 | 0.01 | 115.52 | 30.18 | 107.52 | 25.64 | 0.02 |
| TG [mg/dL] | 165.49 | 94.97 | 144.70 | 71.50 | 0.37 | 155.55 | 121.37 | 153.48 | 155.70 | 0.59 |
| Non-HDL [mg/dL] | 153.18 | 45.29 | 141.93 | 49.26 | 0.07 | 147.81 | 37.87 | 135.83 | 33.15 | 0.01 |
| AST [UI/L] | 21.66 | 9.49 | 18.85 | 5.17 | 0.11 | 19.70 | 7.38 | 21.45 | 11.30 | 0.92 |
| ALT [UI/L] | 29.65 | 19.02 | 23.80 | 13.76 | 0.01 | 24.17 | 16.25 | 20.64 | 13.37 | 0.17 |
| Glucose [mg/dL] | 102.81 | 9.13 | 101.48 | 11.88 | 0.24 | 101.91 | 12.81 | 98.82 | 9.56 | 0.08 |
| HbA1C [%] | 5.33 | 0.37 | 5.22 | 0.32 | 0.02 | 5.32 | 0.34 | 5.20 | 0.27 | 0.01 |
| Insulin [mU/mL] | 16.74 | 9.37 | 15.32 | 7.93 | 0.30 | 14.70 | 11.12 | 12.23 | 6.15 | 0.37 |
| HOMA-IR | 0.16 | 0.10 | 0.15 | 0.09 | 0.57 | 0.14 | 0.08 | 0.14 | 0.06 | 0.36 |
| Albumin [g/L] | 45.89 | 2.96 | 45.14 | 2.27 | 0.27 | 45.94 | 2.28 | 45.47 | 1.79 | 0.26 |
| Body mass [kg] | 97.72 | 22.53 | 98.43 | 22.50 | 0.33 | 89.61 | 19.21 | 89.23 | 19.20 | 0.59 |
| BMI [kg/m2] | 32.75 | 6.66 | 32.98 | 6.65 | 0.36 | 30.62 | 4.92 | 30.50 | 4.89 | 0.68 |
| Supplementation Group | Placebo Group | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 60 | p | Day 0 | Day 60 | p | |||||
| Mean | SD [±] | Mean | SD [±] | Mean | SD [±] | Mean | SD [±] | |||
| FM [kg] | 37.85 | 12.10 | 39.46 | 12.48 | 0.07 | 31.51 | 8.90 | 31.31 | 8.87 | 0.80 |
| FM% | 39.73 | 4.69 | 40.01 | 4.71 | 0.06 | 37.76 | 4.51 | 37.71 | 4.29 | 0.81 |
| Creatinine [mg/dL] | 0.64 | 0.10 | 0.64 | 0.09 | 0.91 | 0.71 | 0.26 | 0.69 | 0.24 | 0.22 |
| GFR [mL/min/1.73 m2] | 111.84 | 25.12 | 111.26 | 24.95 | 0.97 | 101.70 | 20.84 | 106.36 | 24.00 | 0.26 |
| Uric acid [mg/dL] | 5.15 | 1.06 | 5.25 | 0.99 | 0.55 | 4.91 | 1.02 | 4.66 | 1.03 | 0.08 |
| GGTP [UI/L] | 29.57 | 20.29 | 29.51 | 17.21 | 0.88 | 30.00 | 48.50 | 22.93 | 26.84 | 0.71 |
| Amylase [UI/L] | 54.41 | 31.06 | 57.11 | 33.78 | 0.24 | 57.38 | 18.76 | 57.56 | 14.80 | 0.19 |
| Total cholesterol [mg/dL] | 220.15 | 36.69 | 208.43 | 37.09 | 0.04 | 187.57 | 20.30 | 181.93 | 26.49 | 0.28 |
| HDL [mg/dL] | 59.00 | 31.68 | 62.64 | 30.43 | 0.17 | 53.21 | 10.37 | 56.57 | 12.88 | 0.08 |
| LDL [mg/dl] | 133.23 | 38.42 | 117.86 | 38.56 | 0.02 | 111.57 | 16.70 | 103.29 | 19.68 | 0.03 |
| TG [mg/dL] | 140.04 | 64.75 | 139.24 | 69.06 | 0.34 | 114.13 | 41.52 | 110.15 | 53.20 | 0.63 |
| Non-HDL [mg/dL] | 161.17 | 41.00 | 145.69 | 41.75 | 0.02 | 134.34 | 18.37 | 125.26 | 26.60 | 0.09 |
| AST [UI/L] | 20.51 | 6.96 | 17.93 | 3.41 | 0.12 | 15.90 | 3.62 | 18.59 | 11.09 | 1.00 |
| ALT [UI/L] | 25.41 | 12.05 | 19.07 | 6.13 | 0.01 | 15.86 | 6.03 | 14.22 | 4.76 | 0.75 |
| Glucose [mg/dL] | 102.29 | 9.35 | 101.21 | 10.56 | 0.43 | 97.93 | 6.64 | 96.86 | 5.60 | 0.62 |
| HbA1C [%] | 5.33 | 0.38 | 5.22 | 0.30 | 0.05 | 5.16 | 0.17 | 5.09 | 0.15 | 0.18 |
| Insulin [mU/mL] | 17.01 | 8.86 | 15.01 | 7.26 | 0.29 | 9.56 | 3.03 | 10.00 | 4.30 | 0.71 |
| HOMA-IR | 0.16 | 0.09 | 0.14 | 0.09 | 0.50 | 0.10 | 0.04 | 0.12 | 0.04 | 0.15 |
| Albumin [g/L] | 45.41 | 2.98 | 44.71 | 2.11 | 0.38 | 46.28 | 2.54 | 45.18 | 1.88 | 0.03 |
| Body mass [kg] | 93.56 | 20.04 | 94.41 | 21.06 | 0.35 | 82.19 | 15.54 | 81.89 | 15.61 | 0.89 |
| BMI [kg/m2] | 33.17 | 6.03 | 33.37 | 6.29 | 0.38 | 29.85 | 4.54 | 29.76 | 4.52 | 1.00 |
| Supplementation Group | Placebo Group | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 60 | p | Day 0 | Day 60 | p | |||||
| Mean | SD [±] | Mean | SD [±] | Mean | SD [±] | Mean | SD [±] | |||
| FM [kg] | 32.63 | 16.05 | 34.21 | 15.29 | 0.11 | 32.01 | 11.37 | 33.13 | 10.25 | 0.74 |
| FM% | 29.31 | 7.22 | 30.85 | 6.89 | 0.11 | 30.49 | 6.40 | 31.77 | 5.08 | 0.74 |
| Creatinine [mg/dL] | 0.78 | 0.06 | 0.75 | 0.06 | 0.18 | 0.92 | 0.16 | 0.87 | 0.12 | 0.15 |
| GFR [mL/min/1.73 m2] | 117.39 | 12.75 | 121.91 | 14.18 | 0.22 | 101.00 | 24.60 | 106.38 | 20.34 | 0.31 |
| Uric acid [mg/dL] | 5.89 | 0.81 | 5.60 | 0.72 | 0.34 | 6.04 | 1.41 | 5.91 | 1.43 | 0.95 |
| GGTP [UI/L] | 38.43 | 18.76 | 41.57 | 19.55 | 0.40 | 39.63 | 23.57 | 37.75 | 21.16 | 0.89 |
| Amylase [UI/L] | 53.53 | 13.31 | 56.90 | 16.52 | 0.11 | 46.49 | 11.92 | 52.23 | 15.80 | 0.04 |
| Total cholesterol [mg/dL] | 180.71 | 52.28 | 178.43 | 66.48 | 0.94 | 211.50 | 50.47 | 198.63 | 34.17 | 0.25 |
| HDL [mg/dL] | 42.43 | 8.54 | 43.86 | 5.73 | 0.55 | 40.13 | 9.73 | 44.38 | 12.14 | 0.13 |
| LDL [mg/dL] | 87.40 | 29.34 | 103.14 | 51.58 | 0.31 | 123.43 | 48.19 | 116.00 | 35.03 | 0.30 |
| TG [mg/dL] | 212.74 | 127.15 | 155.63 | 80.61 | 0.047 | 228.05 | 177.63 | 229.31 | 239.05 | 0.74 |
| Non-HDL [mg/dL] | 138.33 | 52.32 | 134.40 | 64.90 | 0.94 | 171.39 | 51.75 | 154.33 | 37.00 | 0.11 |
| AST [UI/L] | 23.94 | 13.65 | 20.69 | 7.61 | 0.58 | 26.34 | 7.74 | 26.45 | 10.47 | 0.74 |
| ALT [UI/L] | 38.13 | 27.66 | 33.27 | 19.81 | 0.30 | 38.71 | 18.58 | 31.88 | 16.36 | 0.40 |
| Glucose [mg/dL] | 103.86 | 9.30 | 102.00 | 15.11 | 0.47 | 108.88 | 17.97 | 102.35 | 13.96 | 0.07 |
| HbA1C [%] | 5.33 | 0.37 | 5.23 | 0.37 | 0.17 | 5.61 | 0.37 | 5.41 | 0.31 | 0.02 |
| Insulin [mU/mL] | 16.20 | 11.04 | 15.93 | 9.73 | 0.94 | 23.68 | 14.46 | 16.13 | 7.21 | 0.08 |
| HOMA-IR | 0.16 | 0.11 | 0.17 | 0.10 | 1.00 | 0.20 | 0.11 | 0.18 | 0.07 | 0.67 |
| Albumin [g/L] | 46.86 | 2.88 | 46.00 | 2.51 | 0.58 | 45.34 | 1.71 | 45.96 | 1.65 | 0.38 |
| Body mass [kg] | 106.04 | 26.46 | 106.46 | 24.77 | 0.73 | 102.60 | 18.85 | 102.08 | 18.90 | 0.46 |
| BMI [kg/m2] | 31.90 | 8.22 | 32.00 | 7.75 | 0.80 | 31.98 | 5.57 | 31.81 | 5.54 | 0.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popiolek-Kalisz, J.; Glibowski, P.; Solarska, E. Supplementation of Nutraceuticals from Dwarf Kiwi and Apple Improves Lipid Profile in Overweight Adults. Appl. Sci. 2024, 14, 1324. https://doi.org/10.3390/app14041324
Popiolek-Kalisz J, Glibowski P, Solarska E. Supplementation of Nutraceuticals from Dwarf Kiwi and Apple Improves Lipid Profile in Overweight Adults. Applied Sciences. 2024; 14(4):1324. https://doi.org/10.3390/app14041324
Chicago/Turabian StylePopiolek-Kalisz, Joanna, Paweł Glibowski, and Ewa Solarska. 2024. "Supplementation of Nutraceuticals from Dwarf Kiwi and Apple Improves Lipid Profile in Overweight Adults" Applied Sciences 14, no. 4: 1324. https://doi.org/10.3390/app14041324
APA StylePopiolek-Kalisz, J., Glibowski, P., & Solarska, E. (2024). Supplementation of Nutraceuticals from Dwarf Kiwi and Apple Improves Lipid Profile in Overweight Adults. Applied Sciences, 14(4), 1324. https://doi.org/10.3390/app14041324







