Stress Corrosion Cracking Analysis of a Hot Blast Stove Shell with an Internal Combustion Chamber
Abstract
:1. Introduction
2. Numerical Modelling
2.1. SCC Growth Model
2.2. Theoretical Corrosion Rate
- In the first part, based on the assumed mixed concentration (300 ppm) of NOx and SOx, the NO2 and SO3, which would cause the formation of HNO3 and H2SO4, were estimated as a function of the flue gas temperature employed by the function by Olsen et al. [30] and Fleig et al. [31]. The initial concentration of sulfur trioxide, nitrogen dioxide, and water in the flue gas for the reference blast temperature is described in Table 2.
- The acid distribution was calculated using the controlled condensation method to determine the SCC propagation rate.
2.2.1. Theoretical Water Condensation Rate
2.2.2. Theoretical Acid Condensation Rate
2.2.3. Numerical Calculation of Condensation Rates
2.3. Calculation of the Phenomenological Relationship of SCC
2.4. Operation Condition of the HBS
3. Results and Discussion
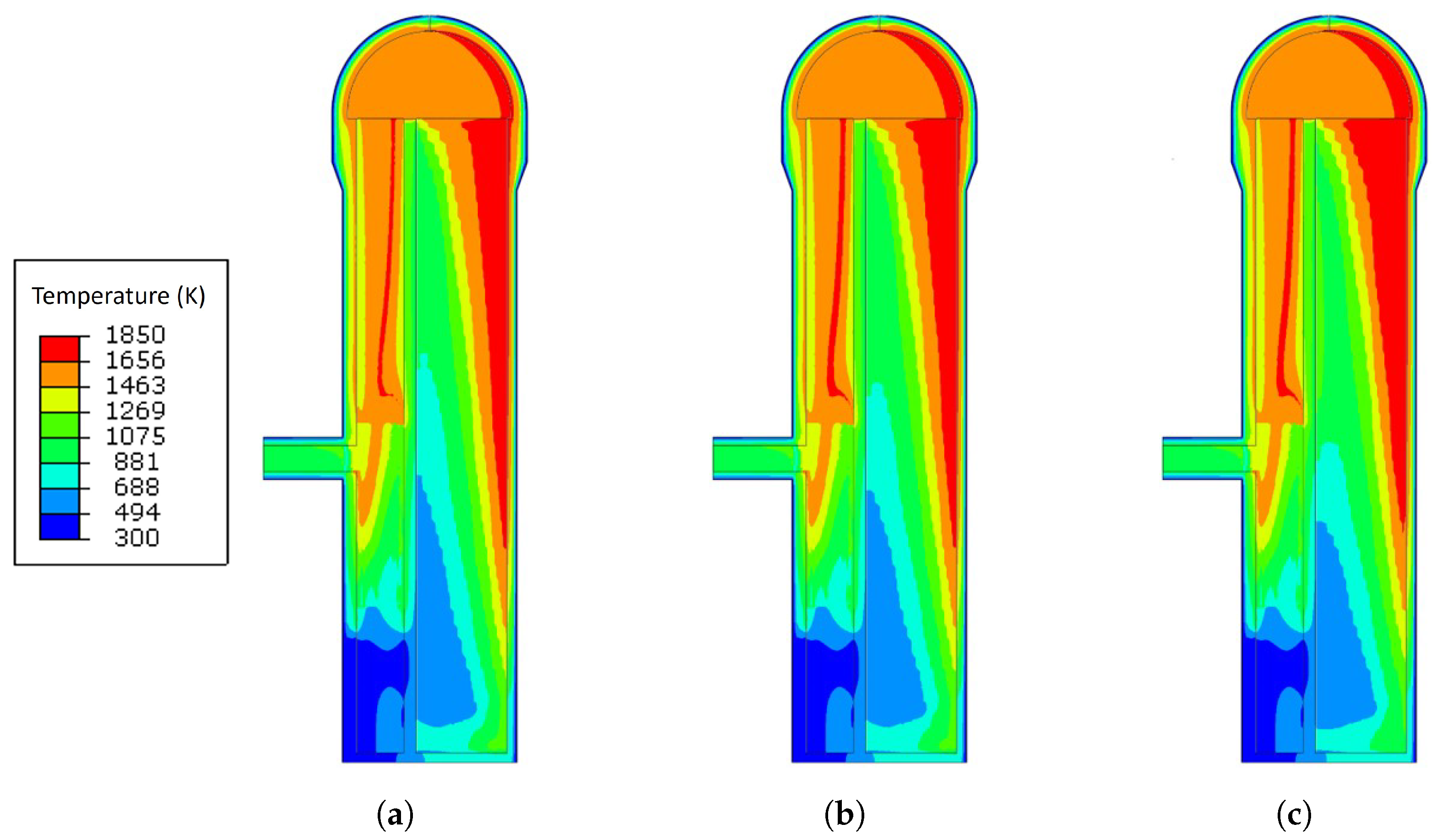
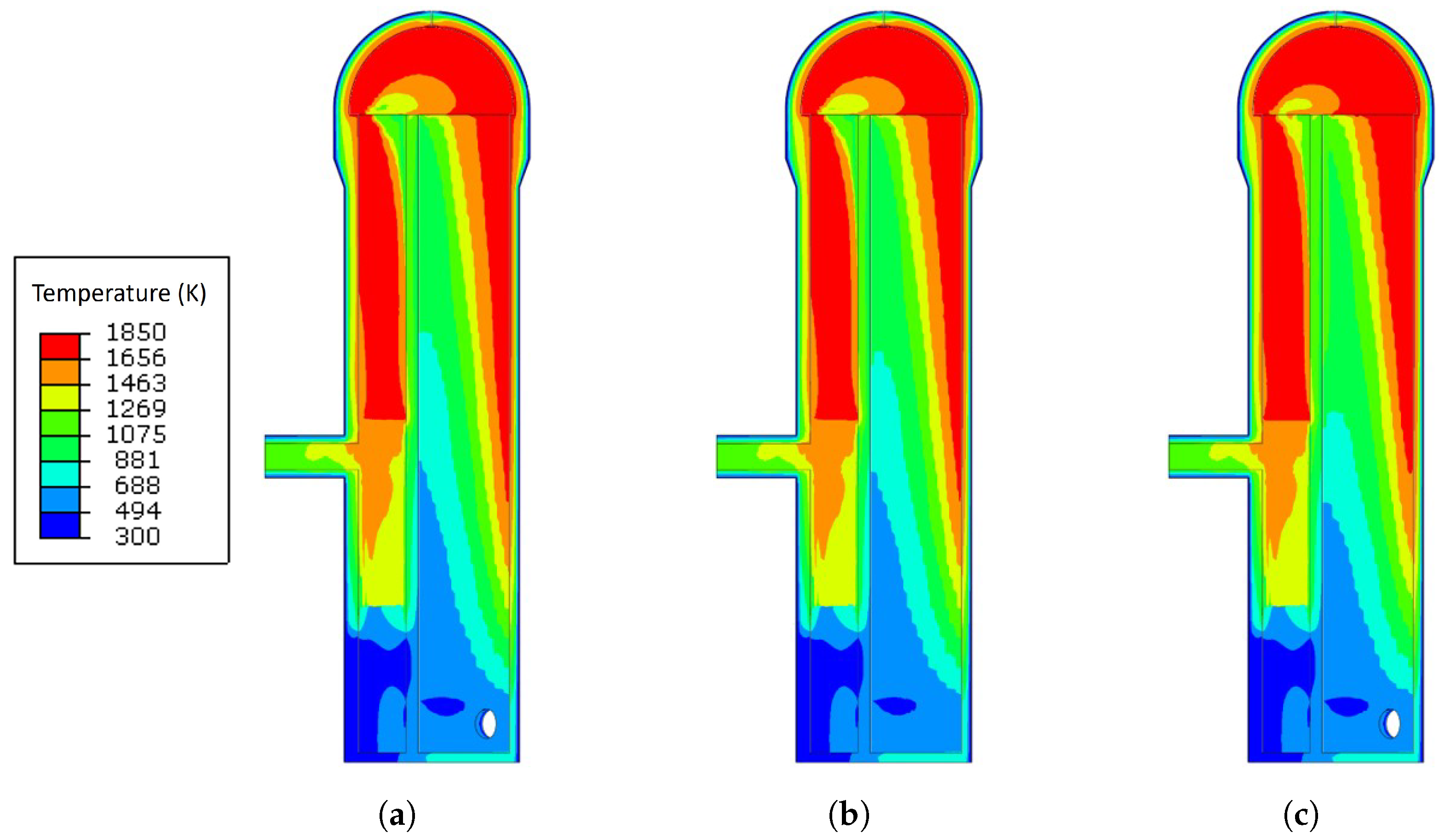
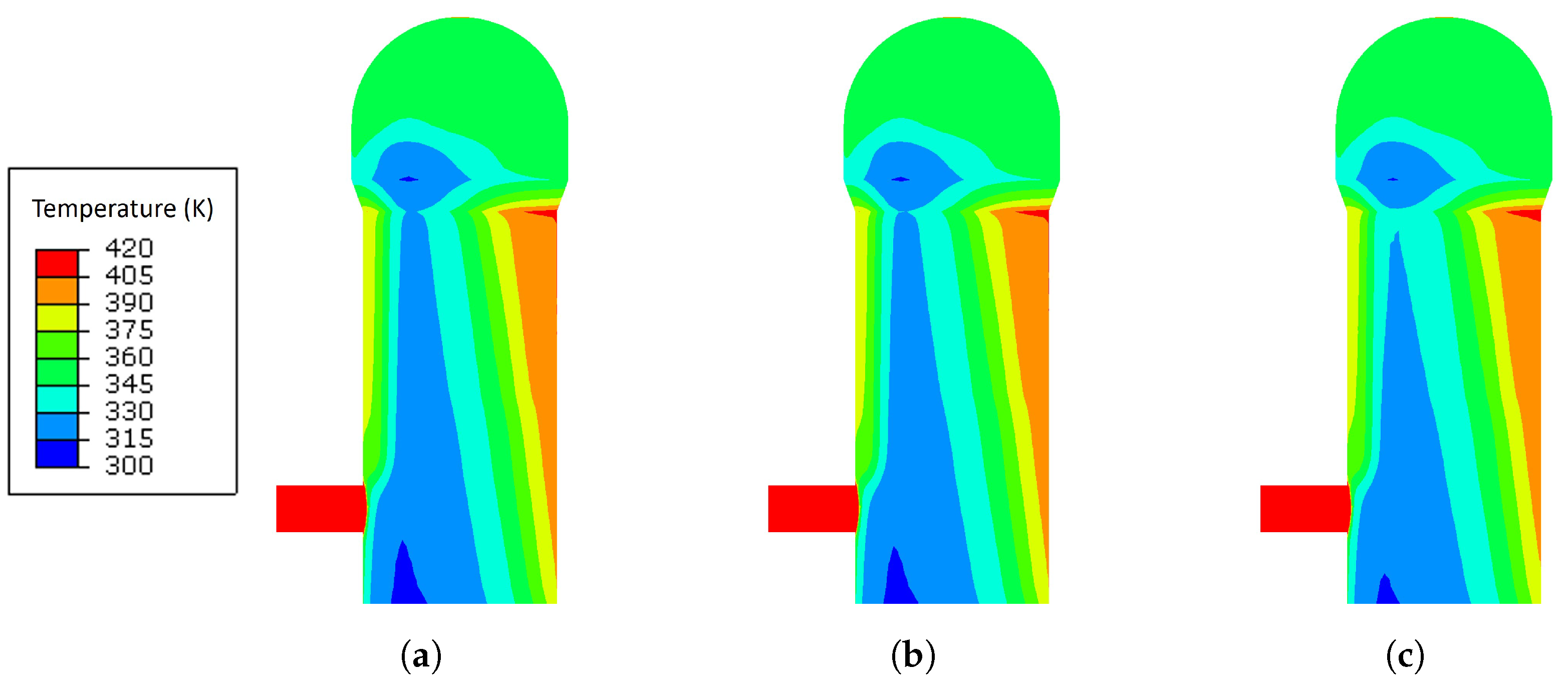
3.1. Temperature Analysis Results with Different Blast Temperatures
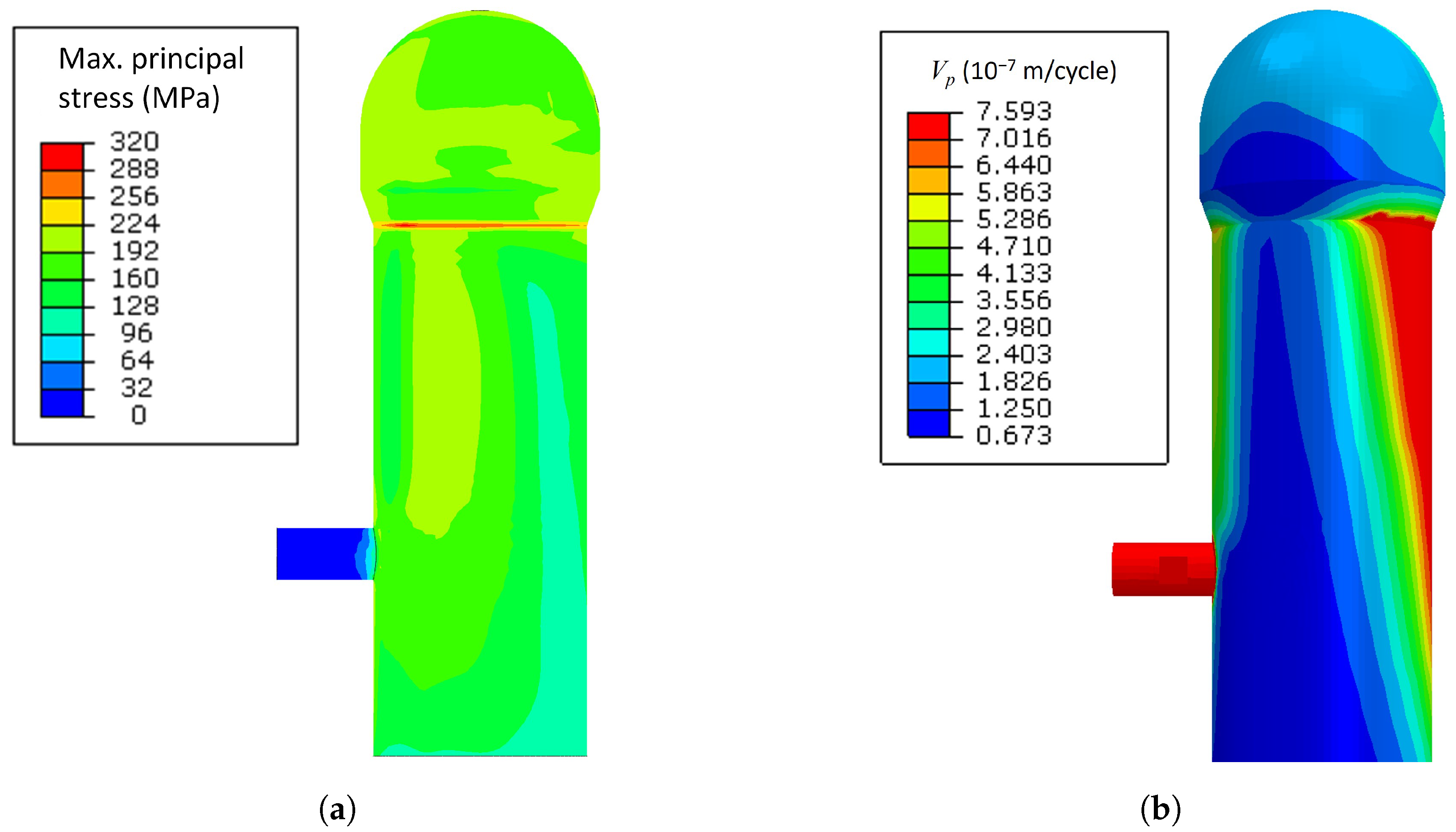
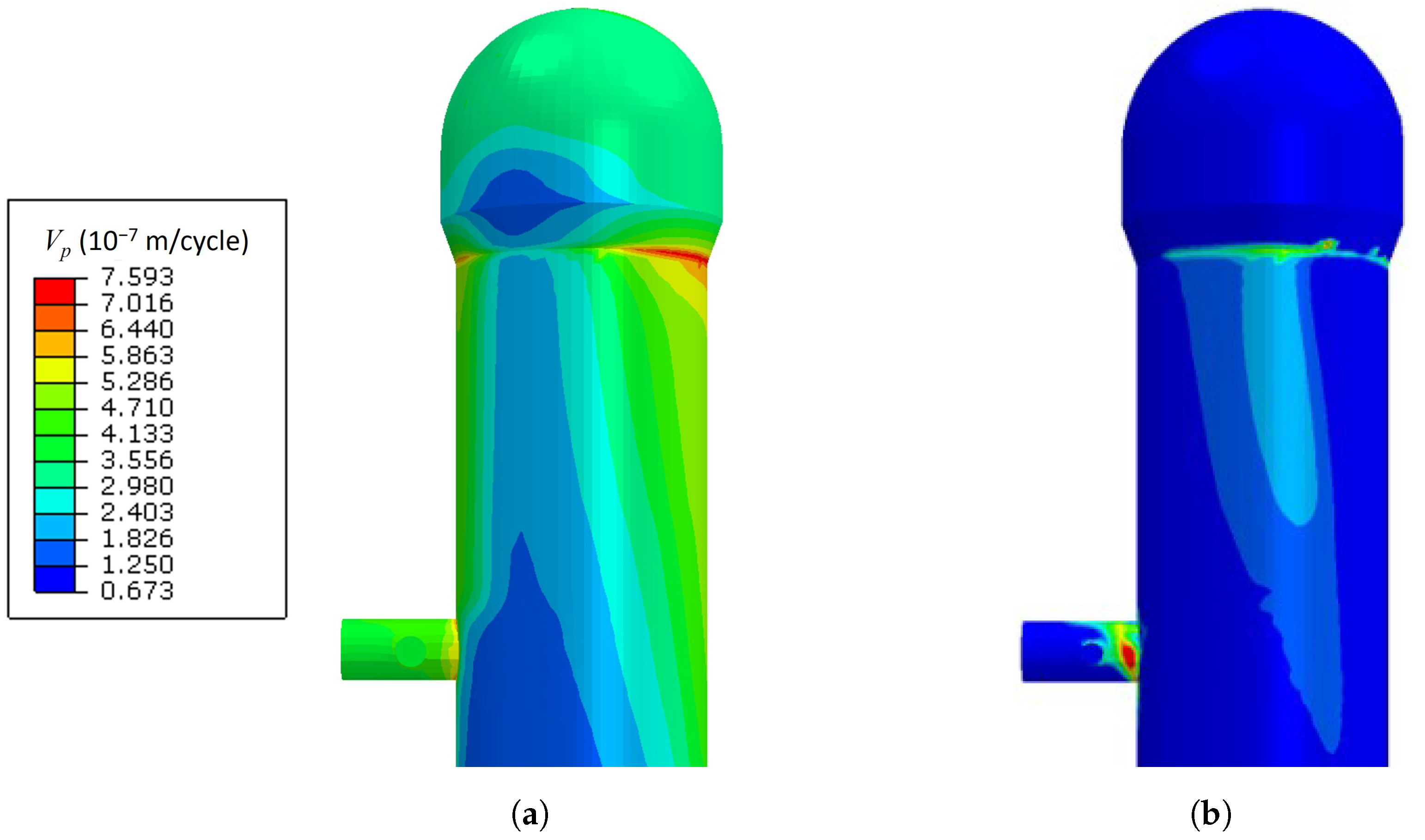
3.2. Crack Propagation Velocity Prediction Results from SCC SMM
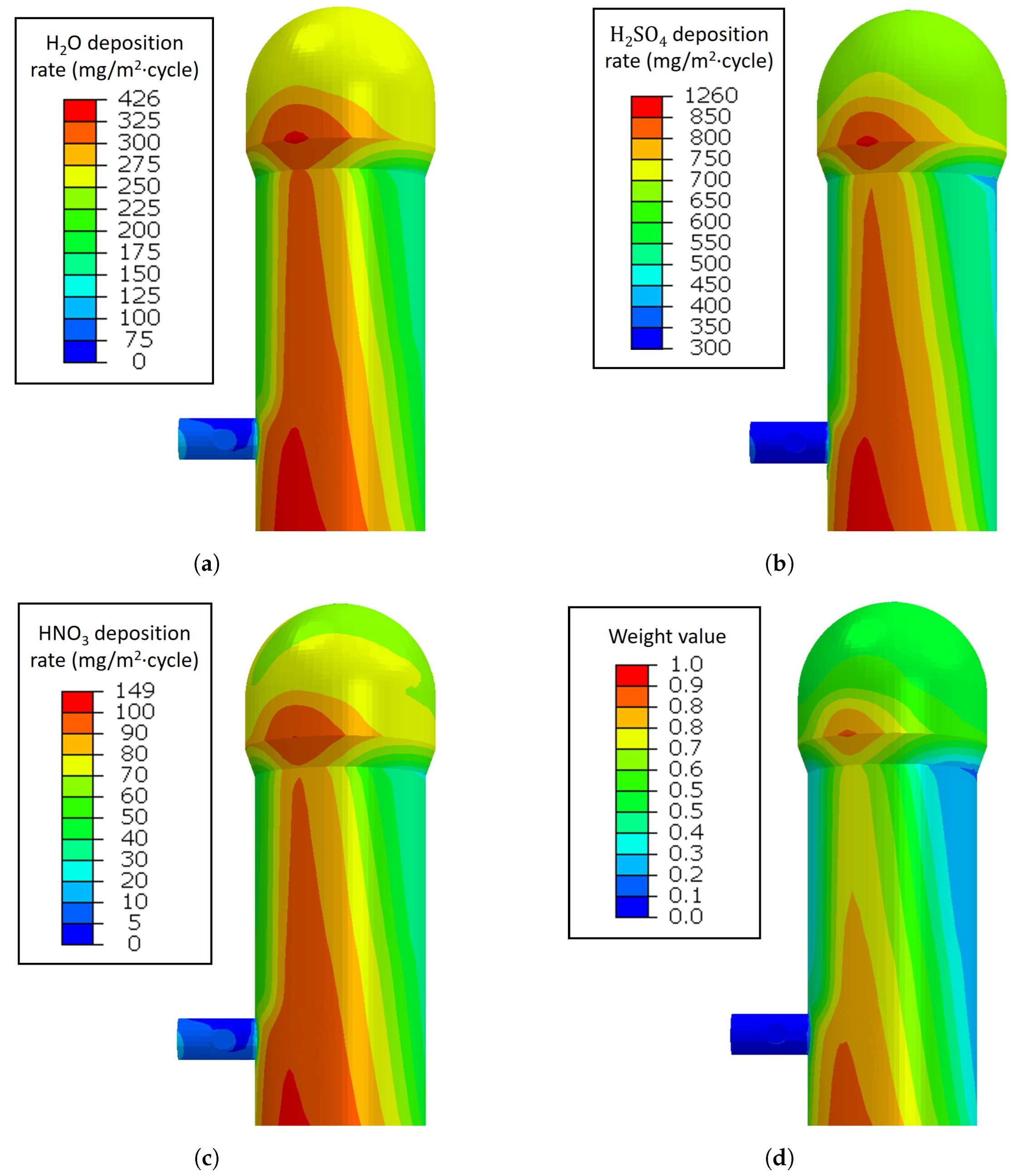
3.3. Prediction Results Combined with Acid Corrosion Rates
3.4. Discussion
4. Conclusions
- Without considering the weight factor, the crack propagation velocity was the largest at the conical region of the upper combustion chamber. The causes of the high crack propagation velocity are higher maximum principal stress and temperature distributions on the region.
- The deposition rates of the water, sulfuric, and nitric acids were found to condense more at the lower part of the HBS. However, at the conical region between the checker and combustion chambers, the deposition rates were higher than in the other part of the conical region.
- The weight factor on the crack propagation rate made it possible to consider the effect of the acid condensation rates on the crack propagation rates. The crack propagation rates in the regions with high temperatures became lower since there was no sufficient condensed acid to be expected.
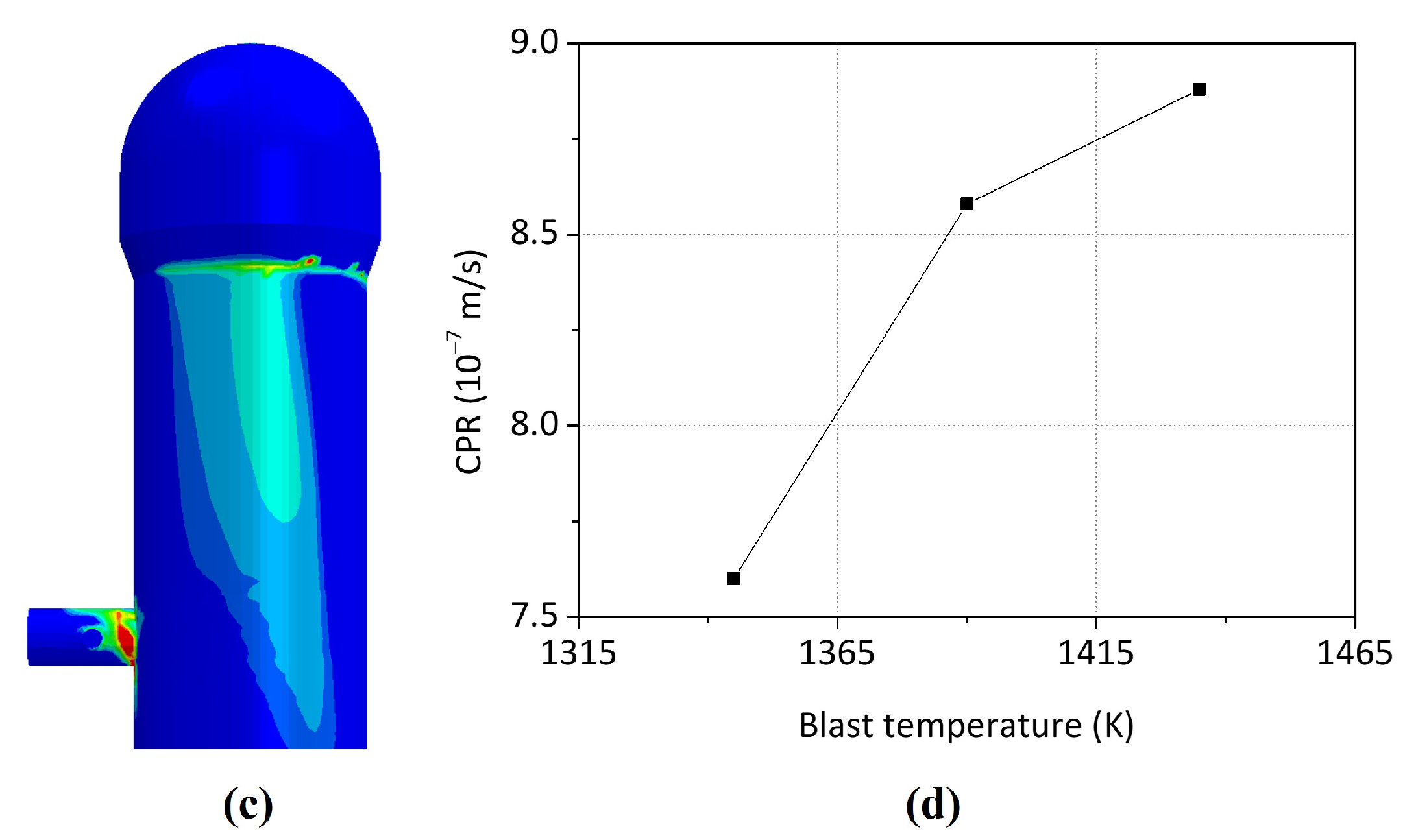
- The increase in the blast temperature had a low effect on the shell temperature. With the increase of 90 K on the blast temperature, the shell temperature only increased by 5.2 K maximum.
- The blast temperature had less effect on the maximum principal stress generated during the operation. However, when the blast temperature was increased by 90 K, the maximum crack propagation rate was increased from m/cycle to m/cycle, which means that there was a 16.7% increase since the stress corrosion cracking is affected by the shell temperature.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADP | acid dew point |
| CFD | computational fluid dynamics |
| CPR | crack propagation rate |
| FEA | finite element analysis |
| HBS | hot blast stove |
| LTC | low-temperature corrosion |
| SCC | stress corrosion cracking |
| SMM | surface mobility mechanism |
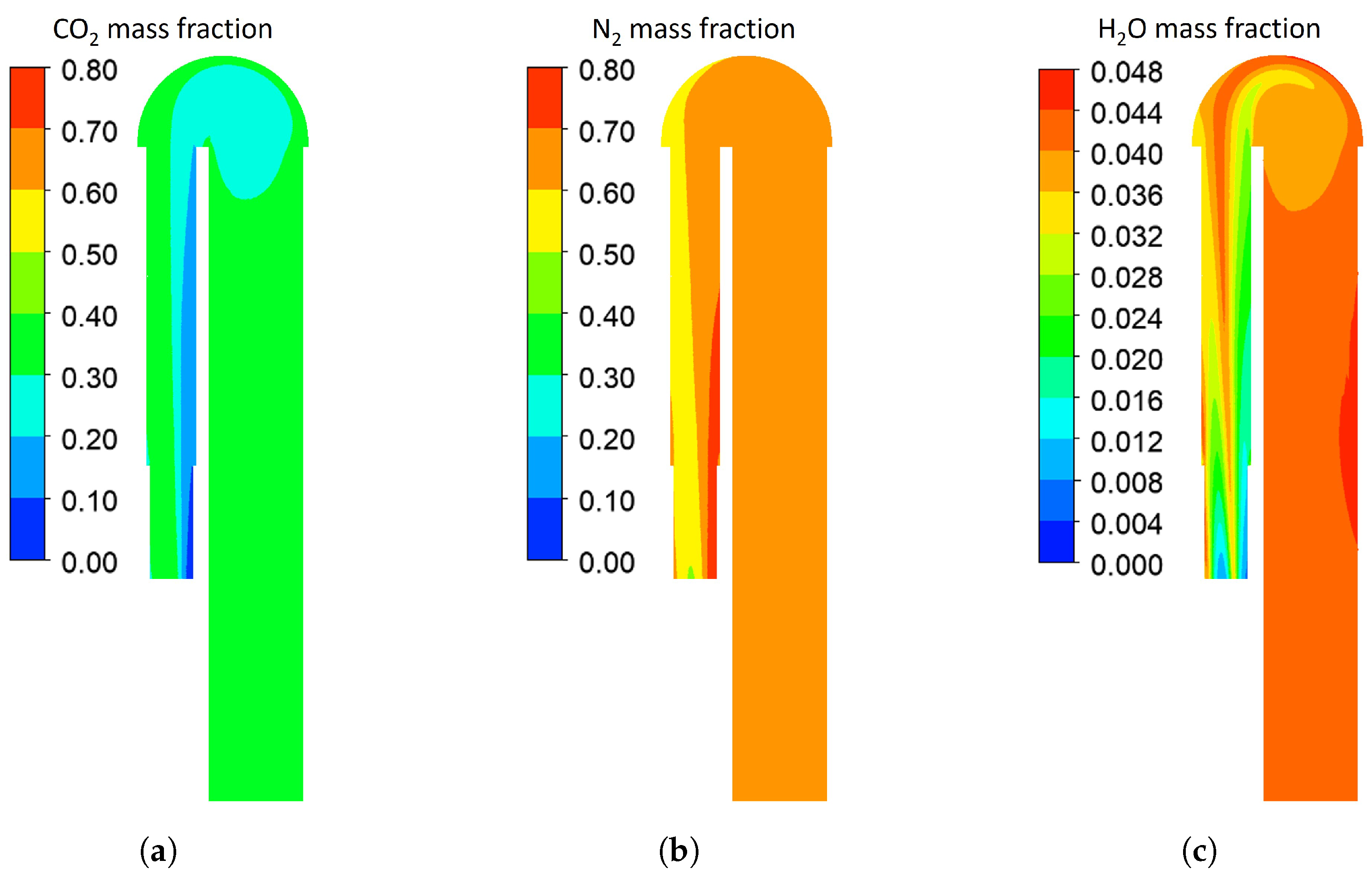
Appendix A. Computational Fluid Dynamics Model of the Hot Blast Stove
References
- Huijbregts, W.; Leferink, R. Latest advances in the understanding of acid dewpoint corrosion: Corrosion and stress corrosion cracking in combustion gas condensates. Anti-Corros. Methods Mater. 2004, 51, 173–188. [Google Scholar] [CrossRef]
- Sanchez, J.; Fullea, J.; Andrade, C.; Alonso, C. Stress corrosion cracking mechanism of prestressing steels in bicarbonate solutions. Corros. Sci. 2007, 49, 4069–4080. [Google Scholar] [CrossRef]
- Cattant, F.; Crusset, D.; Féron, D. Corrosion issues in nuclear industry today. Mater. Today 2008, 11, 32–37. [Google Scholar] [CrossRef]
- Linden, D. Long term operating experience with corrosion control in industrial axial flor compressors. In Proceedings of the 40th Turbomachinery Symposium, Houston, TX, USA, 24–27 September 2011. [Google Scholar]
- Dwivedi, S.K.; Vishwakarma, M.; Soni, A. Advances and researches on non destructive testing: A review. Mater. Today Proc. 2018, 5, 3690–3698. [Google Scholar] [CrossRef]
- Cherniavsky, A. Estimation of the causes of cracking in the casing of a hot blast duct. Eng. Fail. Anal. 2020, 116, 104738. [Google Scholar] [CrossRef]
- Leferink, R.; Huijbregts, W. Nitrate stress corrosion cracking in waste heat recovery boilers. Anti-Corros. Methods Mater. 2002, 49, 118–126. [Google Scholar] [CrossRef]
- Scott, P.M. An Analysis of Primary Water Stress Corrosion Cracking in PWR Steam Generators. In Proceedings of the NEA/CSNI-UNIPEDE Specialist Meeting on Operating Experience with Steam Generators, Brussels, Belgium, 16–20 September 1991. [Google Scholar]
- Jenks, A.R.; White, G.A.; Crooker, P. Crack growth rates for evaluating PWSCC of thick-wall alloy 690 material and alloy 52, 152, and variant welds. In Proceedings of the Pressure Vessels and Piping Conference, Waikoloa, HI, USA, 16–20 July 2017; American Society of Mechanical Engineers: New York, NY, USA, 2017; Volume 58004, p. V06BT06A012. [Google Scholar]
- Woodtli, J.; Kieselbach, R. Damage due to hydrogen embrittlement and stress corrosion cracking. Eng. Fail. Anal. 2000, 7, 427–450. [Google Scholar] [CrossRef]
- Logan, H.L. Film-rupture mechanism of stress corrosion. J. Res. Natl. Bur. Stand. 1952, 48, 99–105. [Google Scholar] [CrossRef]
- Vermilyea, D.A. A theory for the propagation of stress corrosion cracks in metals. J. Electrochem. Soc. 1972, 119, 405. [Google Scholar] [CrossRef]
- Shi, J.; Fekete, B.; Wang, J.; Macdonald, D.D. Customization of the coupled environment fracture model for predicting stress corrosion cracking in Alloy 600 in PWR environment. Corros. Sci. 2018, 139, 58–67. [Google Scholar] [CrossRef]
- Hall, M., Jr. An alternative to the Shoji crack tip strain rate equation. Corros. Sci. 2008, 50, 2902–2905. [Google Scholar] [CrossRef]
- Shoji, T.; Suzuki, S.; Ballinger, R. Theoretical Prediction of SCC Growth Behavior—Threshold and Plateau Growth Rate; Technical Report; NACE International: Houston, TX, USA, 1995. [Google Scholar]
- Speidel, M.O. Stress corrosion cracking of stainless steels in NaCl solutions. Metall. Trans. A 1981, 12, 779–789. [Google Scholar] [CrossRef]
- Fan, T.; Sutton, M.; Zhang, L. Plane stress steady crack growth in a power-law hardening material. Int. J. Fract. 1997, 86, 327–341. [Google Scholar] [CrossRef]
- Koshiishi, M.; Hashimoto, T.; Obata, R. Application of the FRI crack growth model for neutron-irradiated stainless steels in high-temperature water of a boiling water reactor environment. Corros. Sci. 2017, 123, 278–288. [Google Scholar] [CrossRef]
- Galvele, J. A stress corrosion cracking mechanism based on surface mobility. Corros. Sci. 1987, 27, 1–33. [Google Scholar] [CrossRef]
- Galvele, J. Surface mobility mechanism of stress-corrosion cracking. Corros. Sci. 1993, 35, 419–434. [Google Scholar] [CrossRef]
- Galvele, J. Recent developments in the surface-mobility stress-corrosion-cracking mechanism. Electrochim. Acta 2000, 45, 3537–3541. [Google Scholar] [CrossRef]
- Vogt, H.; Speidel, M. Stress corrosion cracking of two aluminium alloys: A comparison between experimental observations and data based on modelling. Corros. Sci. 1998, 40, 251–270. [Google Scholar] [CrossRef]
- Kim, S.; Yu, H.; Nam, K.; Choi, B. Failure analysis and structural improvement for cracked circular finned tube. Eng. Fail. Anal. 2018, 92, 95–106. [Google Scholar] [CrossRef]
- Mou, Y.; Lian, Z.; Yu, H.; Lin, T.; Han, Z.; Zhang, Q. SCC evaluation of composite materials for natural gas absorber based on experimental and numerical methods. Eng. Fail. Anal. 2020, 110, 104450. [Google Scholar] [CrossRef]
- Wang, W.Y.; Tseng, Y.S.; Yeh, T.K. Evaluation of crack growth of chloride-induced stress corrosion cracking in dry storage system under different environmental conditions. Prog. Nucl. Energy 2020, 130, 103534. [Google Scholar] [CrossRef]
- Ramírez, E.I.; Figueroa, C.G.; Romero, J.L.; Ramos, E.; Schouwenaars, R.; Ortiz, A. Stress corrosion cracking of the slip-ring connectors of a 2 MW wind turbine. Eng. Fail. Anal. 2022, 141, 106732. [Google Scholar] [CrossRef]
- Wang, W.Y.; Tseng, Y.S.; Yeh, T.K. Tolerance assessment and crack growth of chloride-induced stress corrosion cracking for Chinshan dry storage system. Prog. Nucl. Energy 2022, 147, 104210. [Google Scholar] [CrossRef]
- Park, D.; Guo, F.; Choi, J.; Park, J.H.; Kim, N. Temperature and Thermal Stress Analysis of a Hot Blast Stove with an Internal Combustion Chamber. Processes 2023, 11, 707. [Google Scholar] [CrossRef]
- Jeong, K.; Levy, E.K. Theoretical prediction of sulfuric acid condensation rates in boiler flue gas. Int. J. Heat Mass Transf. 2012, 55, 8010–8019. [Google Scholar] [CrossRef]
- Olsen, D.B.; Kohls, M.; Arney, G. Impact of oxidation catalysts on exhaust NO2/NOx ratio from lean-burn natural gas engines. J. Air Waste Manag. Assoc. 2010, 60, 867–874. [Google Scholar] [CrossRef]
- Fleig, D.; Andersson, K.; Normann, F.; Johnsson, F. SO3 formation under oxyfuel combustion conditions. Ind. Eng. Chem. Res. 2011, 50, 8505–8514. [Google Scholar] [CrossRef]
- Karamavruc, A. Obtaining bulk flow based heat transfer coefficients for thermal evaluation of turbochargers. In Proceedings of the IMechE Conference, C6231-110, London, UK, 17–18 May 2016. [Google Scholar]
- Antoine, M.C. Nouvelle relation entre les tensions et les temperatures. C. R. Held Seanc. Acad. Sci. Paris 1888, 107, 681–684. [Google Scholar]
- Verhoff, F.; Banchero, J. Predicting Dew Points of Flue Gases. Chem. Eng. Prog. 1974, 78, 71–72. [Google Scholar]
- Kiang, Y.H. Predicting Dew Points of Acid Gases. Chem. Eng. 1981, 88, 127. [Google Scholar]
- Valencia, M.P.P. Condensation of Water Vapor and Acid Mixtures From Exhaust Gases; Institut für Energietechnik: Esslingen, Germany, 2004. [Google Scholar]
- Prando, D.; Brenna, A.; Diamanti, M.V.; Beretta, S.; Bolzoni, F.; Ormellese, M.; Pedeferri, M. Corrosion of titanium: Part 1: Aggressive environments and main forms of degradation. J. Appl. Biomater. Funct. Mater. 2017, 15, e291–e302. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Liu, Z.; Yao, C.; Li, B. Numerical study and structural optimization of a top combustion hot blast stove. Adv. Mech. Eng. 2015, 7, 709675. [Google Scholar] [CrossRef]
- Blekkenhorst, F.; Brandenburg, J.; Stolwijk, C. Stress corrosion cracking in hot blast stoves at Hoogovens Ijmuiden. Iron Steel Eng. 1980, 57, 55–59. [Google Scholar]
- Harp, G.; Klima, R.; Sucker, D. Gastatmosphaere und Kondensat beim Winderhitzerprozess im Hinblick auf die Spannungsrisskorrosion. Stahl Eisen 1985, 105, 99–104. [Google Scholar]
- An, G.H.; Park, T.J. Mechanism of Stress Corrosion Cracking of Hot Stove Shells and Preventive Measures. In Proceedings of the Korean Society of Tribologists and Lubrication Engineers Conference, Korean Tribology Society, Busan, Republic of Korea, 16–17 May 2002; pp. 279–284. [Google Scholar]
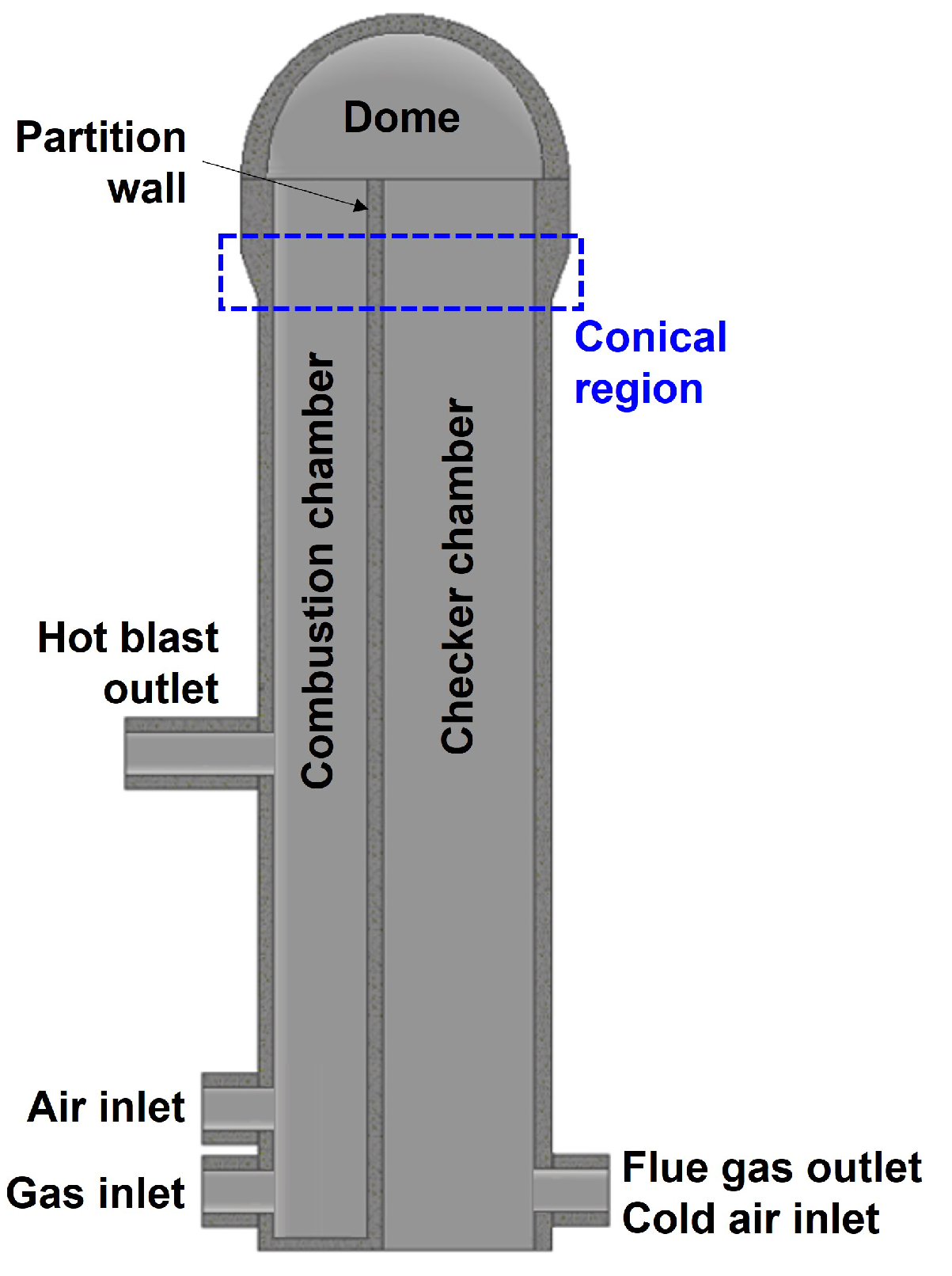


| Literature | Target Component | Analysis Method |
|---|---|---|
| Kim et al. [23] | Circular finned tube | CFD and FEA |
| Mou et al. [24] | Natural gas absorber made of composite materials | FEA |
| Wang et al. [25] | Dry storage system | CFD |
| Ramírez et al. [26] | Slip-ring connectors of wind turbine | FEA |
| Wang et al. [27] | Dry storage system | CFD |
| Species | Initial Concentration |
|---|---|
| SO3 | 780 mg/m |
| NO2 | 564 mg/m |
| H2O | 6% wet |
| Compound | Temperature Range (°C) | Reaction Mechanism |
|---|---|---|
| Sulfuric acid | ||
| Condensation of H2SO4 | ||
| Nitric acid | ||
Condensation of HNO3 |
| Component | CO2 | O2 | CO | H2 | H2O | N2 |
|---|---|---|---|---|---|---|
| Composition (%) | 18.1 | 0.1 | 24.5 | 0.3 | 0.2 | 56.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, D.; Guo, F.; Kim, N. Stress Corrosion Cracking Analysis of a Hot Blast Stove Shell with an Internal Combustion Chamber. Appl. Sci. 2023, 13, 12297. https://doi.org/10.3390/app132212297
Park D, Guo F, Kim N. Stress Corrosion Cracking Analysis of a Hot Blast Stove Shell with an Internal Combustion Chamber. Applied Sciences. 2023; 13(22):12297. https://doi.org/10.3390/app132212297
Chicago/Turabian StylePark, Donghwi, Feng Guo, and Naksoo Kim. 2023. "Stress Corrosion Cracking Analysis of a Hot Blast Stove Shell with an Internal Combustion Chamber" Applied Sciences 13, no. 22: 12297. https://doi.org/10.3390/app132212297
APA StylePark, D., Guo, F., & Kim, N. (2023). Stress Corrosion Cracking Analysis of a Hot Blast Stove Shell with an Internal Combustion Chamber. Applied Sciences, 13(22), 12297. https://doi.org/10.3390/app132212297






