The Effects of Angelica ternata Extract from Kyrgyzstan on the Formation of Candida albicans ATCC 10231 Biofilms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Collection
2.2. Angelica Ternata Extract Preparation
2.3. Quantitative Determination of the Amount of Flavonoids in ATE
2.4. Microbial Strain and Culture Medias
2.5. Biofilm Formation Ability
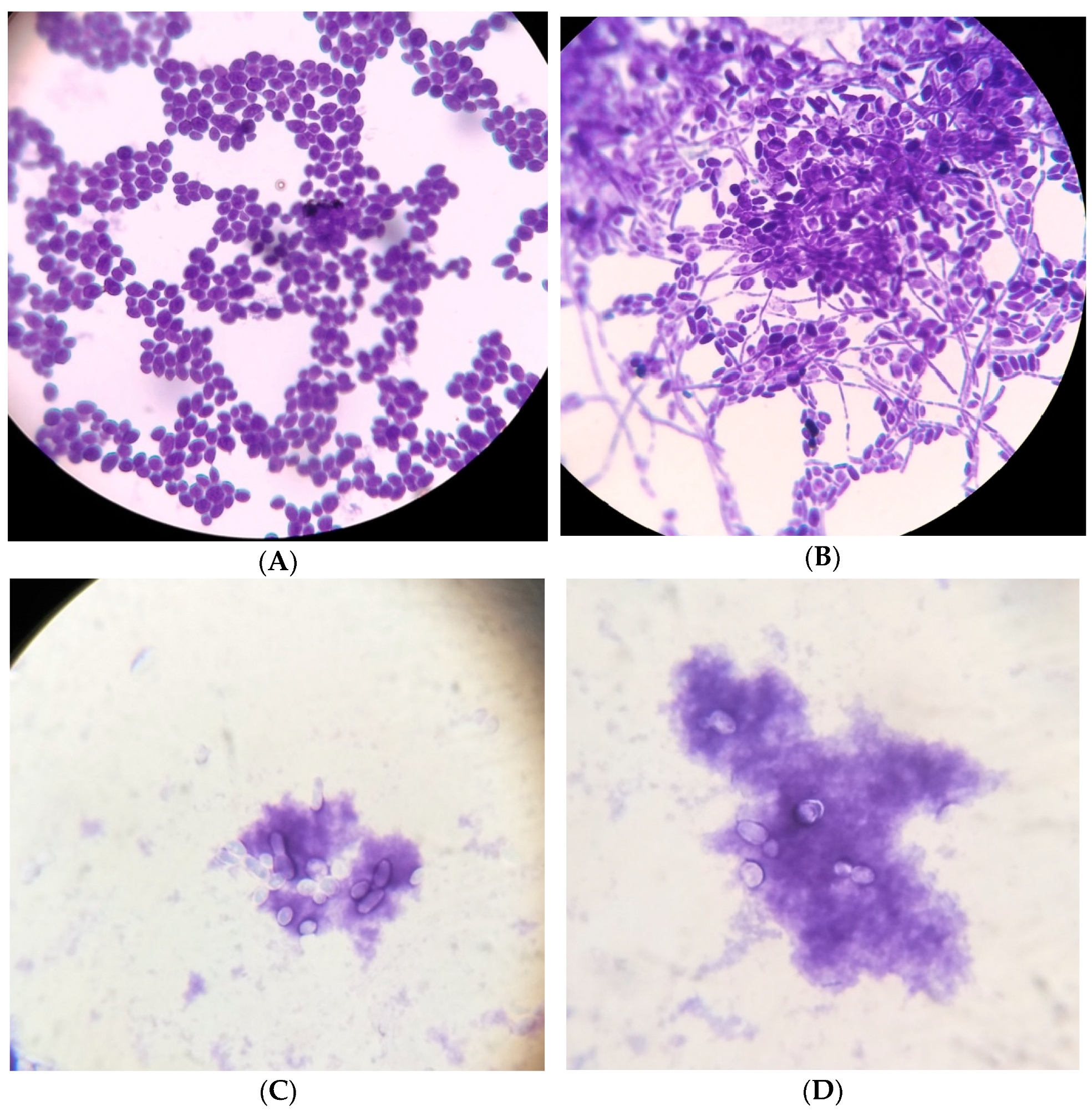
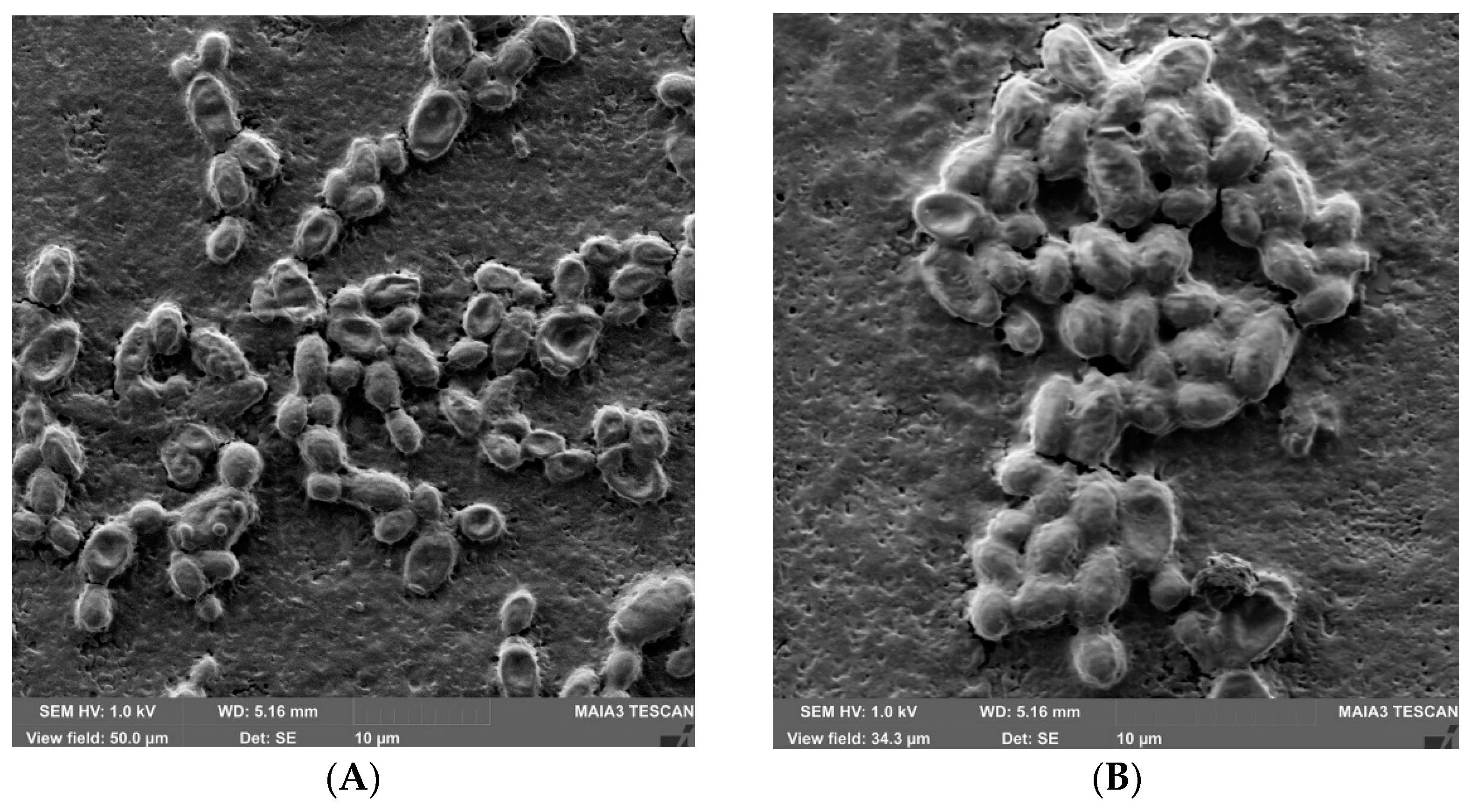
2.6. Morphological Characteristics of Candida Biofilms
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ponde, N.O.; Lortal, L.; Ramage, G.; Naglik, J.R.; Richardson, J.P. Candida albicans biofilms and polymicrobial interactions. Crit. Rev. Microbiol. 2021, 47, 91–111. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Cerdeira, C.; Gregorio, M.C.; Molares-Vila, A.; López-Barcenas, A.; Fabbrocini, G.; Bardhi, B.; Sinani, A.; Sánchez-Blanco, E.; Arenas-Guzmán, R.; Hernandez-Castro, R. Biofilms and vulvovaginal candidiasis. Colloids Surf. B Biointerfaces 2019, 174, 110–125. [Google Scholar] [CrossRef] [PubMed]
- Sachivkina, N.P.; Lenchenko, E.M.; Mannapova, R.T.; Strizhakov, A.A.; Romanova, E.V.; Lukina, D.M. Candida Biofilm Modeling: Past and Present. Farmatsiya 2019, 68, 18–22. [Google Scholar] [CrossRef]
- Hereidia, M.; Andes, D. Production and Isolation of the Candida Species Biofilm Extracellular Matrix. Methods Mol. Biol. 2022, 2542, 257–268. [Google Scholar] [CrossRef] [PubMed]
- do Rosário Esteves Guimarães, C.; de Freitas, H.F.; Barros, T.F. Candida albicans antibiofilm molecules: Analysis based on inhibition and eradication studies. Braz. J. Microbiol. 2023, 54, 37–52. [Google Scholar] [CrossRef]
- Montoya, C.; Kurylec, J.; Ossa, A.; Orrego, S. Cyclic strain of poly (methyl methacrylate) surfaces triggered the pathogenicity of Candida albicans. Acta Biomater. 2023, 170, 415–426. [Google Scholar] [CrossRef]
- Sachivkina, N.; Podoprigora, I.; Bokov, D. Morphological characteristics of Candida albicans, Candida krusei, Candida guilliermondii, and Candida glabrata biofilms, and response to Farnesol. Vet. World 2021, 14, 1608–1614. [Google Scholar] [CrossRef]
- Kernien, J.F.; Johnson, C.J.; Bayless, M.L.; Chovanec, J.F.; Nett, J.E. Neutrophils from Patients with Invasive Candidiasis Are Inhibited by Candida albicans Biofilms. Front. Immunol. 2020, 11, 587956. [Google Scholar] [CrossRef]
- Bezerra, N.V.F.; Brito, A.C.M.; de Medeiros, M.M.D.; Leite, K.L.d.F.; Bezerra, I.M.; de Almeida, L.F.D.; Aires, C.P.; Cavalcanti, Y.W. Glucose supplementation effect on the acidogenicity, viability, and extracellular matrix of Candida single- and dual-species biofilms. J. Investig. Clin. Dent. 2019, 10, e12412. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Tabassum, N.; Jeong, G.-J.; Jung, W.-K.; Kim, Y.-M. Inhibition of Mixed Biofilms of Candida albicans and Staphylococcus aureus by β-Caryophyllene-Gold Nanoparticles. Antibiotics 2023, 12, 726. [Google Scholar] [CrossRef]
- Ruiz-Duran, J.; Torres, R.; Stashenko, E.E.; Ortiz, C. Antifungal and Antibiofilm Activity of Colombian Essential Oils against Different Candida Strains. Antibiotics 2023, 12, 668. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, T.M.; Ożarowski, M.; Seremak-Mrozikiewicz, A.; Wolski, H.; Adamczak, A. Plant Preparations and Compounds with Activities against Biofilms Formed by Candida spp. J. Fungi 2021, 7, 360. [Google Scholar] [CrossRef] [PubMed]
- Smolarz, M.; Zawrotniak, M.; Satala, D.; Rapala-Kozik, M. Extracellular Nucleic Acids Present in the Candida albicans Biofilm Trigger the Release of Neutrophil Extracellular Traps. Front. Cell Infect. Microbiol. 2021, 11, 681030. [Google Scholar] [CrossRef]
- Kulig, K.; Karnas, E.; Woznicka, O.; Kuleta, P.; Zuba-Surma, E.; Pyza, E.; Osyczka, A.; Kozik, A.; Rapala-Kozik, M.; Karkowska-Kuleta, J. Insight Into the Properties and Immunoregulatory Effect of Extracellular Vesicles Produced by Candida glabrata, Candida parapsilosis, and Candida tropicalis Biofilms. Front. Cell Infect. Microbiol. 2022, 12, 879237. [Google Scholar] [CrossRef] [PubMed]
- Dunn, M.J.; Fillinger, R.J.; Anderson, L.M.; Anderson, M.Z. Automated quantification of Candida albicans biofilm-related phenotypes reveals additive contributions to biofilm production. NPJ Biofilms Microbiomes 2020, 6, 36. [Google Scholar] [CrossRef]
- Pereira, R.; Dos Santos Fontenelle, R.O.; de Brito, E.H.S.; de Morais, S.M. Biofilm of Candida albicans: Formation, regulation and resistance. J. Appl. Microbiol. 2021, 131, 11–22. [Google Scholar] [CrossRef]
- Staniszewska, M. Virulence Factors in Candida species. Curr. Protein Pept. Sci. 2020, 21, 313–323. [Google Scholar] [CrossRef]
- Kaur, J.; Nobile, C.J. Antifungal drug-resistance mechanisms in Candida biofilms. Curr. Opin. Microbiol. 2023, 71, 102237. [Google Scholar] [CrossRef] [PubMed]
- Tulasidas, S.; Rao, P.; Bhat, S.; Manipura, R. A study on biofilm production and antifungal drug resistance among Candida species from vulvovaginal and bloodstream infections. Infect. Drug Resist. 2018, 11, 2443–2448. [Google Scholar] [CrossRef]
- Balaji, T.; Manushankar, C.M.; Al-Ghanim, K.A.; Kamaraj, C.; Thirumurugan, D.; Thanigaivel, S.; Nicoletti, M.; Sachivkina, N.; Govindarajan, M. Padina boergesenii-Mediated Copper Oxide Nanoparticles Synthesis, with Their Antibacterial and Anticancer Potential. Biomedicines 2023, 11, 2285. [Google Scholar] [CrossRef]
- Jafri, H.; Ahmad, I. Thymus vulgaris essential oil and thymol inhibit biofilms and interact synergistically with antifungal drugs against drug resistant strains of Candida albicans and Candida tropicalis. J. Mycol. Med. 2020, 30, 100911. [Google Scholar] [CrossRef] [PubMed]
- Demin, K.A.; Refeld, A.G.; Bogdanova, A.A.; Prazdnova, E.V.; Popov, I.V.; Kutsevalova, O.Y.; Ermakov, A.M.; Bren, A.B.; Rudoy, D.V.; Chistyakov, V.A.; et al. Mechanisms of Candida Resistance to Antimycotics and Promising Ways to Overcome It: The Role of Probiotics. Probiotics Antimicrob. Proteins 2021, 13, 926–948. [Google Scholar] [CrossRef]
- Shafaroudi, A.M.; Gorji, N.E.; Nasiri, P.; Javidnia, J.; Saravi, M.E. Antifungal Properties of Zataria multiflora on Candida species: A Systematic Review. J. Evid. Based Integr. Med. 2022, 27, 2515690X221132272. [Google Scholar] [CrossRef] [PubMed]
- Afthab, J.; Khatoon, N.; Zhou, L.; Yao, T.; Shi, S. Hepatoprotective Angelica sinensis silver nanoformulation against multidrug resistant bacteria and the integration of a multicomponent logic gate system. Nanoscale 2020, 12, 19149–19158. [Google Scholar] [CrossRef] [PubMed]
- Olabode, I.R.; Sachivkina, N.; Karamyan, A.; Mannapova, R.; Kuznetsova, O.; Bobunova, A.; Zhabo, N.; Avdonina, M.; Gurina, R. In Vitro Activity of Farnesol against Malassezia pachydermatis Isolates from Otitis Externa Cases in Dogs. Animals 2023, 13, 1259. [Google Scholar] [CrossRef]
- Ramasubbu, K.; Padmanabhan, S.; Al-Ghanim, K.A.; Nicoletti, M.; Govindarajan, M.; Sachivkina, N.; Rajeswari, V.D. Green Synthesis of Copper Oxide Nanoparticles Using Sesbania grandiflora Leaf Extract and Their Evaluation of Anti-Diabetic, Cytotoxic, Anti-Microbial, and Anti-Inflammatory Properties in an In-Vitro Approach. Fermentation 2023, 9, 332. [Google Scholar] [CrossRef]
- Gucwa, K.; Milewski, S.; Dymerski, T.; Szweda, P. Investigation of the Antifungal Activity and Mode of Action of Thymus vulgaris, Citrus limonum, Pelargonium graveolens, Cinnamomum cassia, Ocimum basilicum, and Eugenia caryophyllus Essential Oils. Molecules 2018, 23, 1116. [Google Scholar] [CrossRef] [PubMed]
- Oves, M.; Rauf, M.A.; Aslam, M.; Qari, H.A.; Sonbol, H.; Ahmad, I.; Zaman, G.S.; Saeed, M. Green synthesis of silver nanoparticles by Conocarpus lancifolius plant extract and their antimicrobial and anticancer activities. Saudi J. Biol. Sci. 2022, 29, 460–471. [Google Scholar] [CrossRef] [PubMed]
- Liaqat, N.; Jahan, N.; Anwar, T.; Qureshi, H. Green synthesized silver nanoparticles: Optimization, characterization, antimicrobial activity, and cytotoxicity study by hemolysis assay. Front. Chem. 2022, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.J.; Kim, O.S.; Yoo, S.R.; Seo, C.S.; Kim, Y.; Shin, H.K. Anti-inflammatory and antioxidant activity of the traditional herbal formula Gwakhyangjeonggi-san via enhancement of heme oxygenase-1 expression in RAW264.7 macrophages. Mol. Med. Rep. 2016, 13, 4365–4371. [Google Scholar] [CrossRef]
- Lee, S.K.; Cho, H.K.; Cho, S.H.; Kim, S.S.; Nahm, D.H.; Park, H.S. Occupational asthma and rhinitis caused by multiple herbal agents in a pharmacist. Ann. Allergy Asthma Immunol. 2001, 86, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Nai, J.; Zhang, C.; Shao, H.; Li, B.; Li, H.; Gao, L.; Dai, M.; Zhu, L.; Sheng, H. Extraction, structure, pharmacological activities and drug carrier applications of Angelica sinensis polysaccharide. Int. J. Biol. Macromol. 2021, 183, 2337–2353. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.F.; Li, C.Y.; Fu, Y.P.; Jiang, Q.X.; Peng, X.; Li, L.X.; Song, X.; Zhao, X.H.; Li, Y.P.; Chen, X.F.; et al. The comparison of preliminary structure and intestinal anti-inflammatory and anti-oxidative activities of polysaccharides from different root parts of Angelica sinensis (Oliv.) Diels. J. Ethnopharmacol. 2022, 295, 115446. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.L.; Zeng, R.; Gu, C.M.; Qu, Y.; Huang, L.F. Angelica sinensis in China-A review of botanical profile, ethnopharmacology, phytochemistry and chemical analysis. J. Ethnopharmacol. 2016, 190, 116–141. [Google Scholar] [CrossRef] [PubMed]
- Zeng, P.; Li, J.; Chen, Y.; Zhang, L. The structures and biological functions of polysaccharides from traditional Chinese herbs. Prog. Mol. Biol. Transl. Sci. 2019, 163, 423–444. [Google Scholar] [CrossRef] [PubMed]
- Lü, J.; Jiang, C.; Schell, T.D.; Joshi, M.; Raman, J.D.; Xing, C. Angelica gigas: Signature Compounds, In Vivo Anticancer, Analgesic, Neuroprotective and Other Activities, and the Clinical Translation Challenges. Am. J. Chin. Med. 2022, 50, 1475–1527. [Google Scholar] [CrossRef]
- Ryu, S.; Nam, S.H.; Baek, J.S. Green Synthesis of Silver Nanoparticles (AgNPs) of Angelica Gigas Fabricated by Hot-Melt Extrusion Technology for Enhanced Antifungal Effects. Materials 2022, 15, 7231. [Google Scholar] [CrossRef]
- Oh, S.T.; Lee, S.; Hua, C.; Koo, B.-S.; Pak, S.C.; Kim, D.-I.; Jeon, S.; Shin, B.A. Decursin induces apoptosis in glioblastoma cells, but not in glial cells via a mitochondria-related caspase pathway. Korean J. Physiol. Pharmacol. 2019, 23, 29–35. [Google Scholar] [CrossRef]
- Zhao, H.; Feng, Y.L.; Wang, M.; Wang, J.J.; Liu, T.; Yu, J. The Angelica dahurica: A Review of Traditional Uses, Phytochemistry and Pharmacology. Front. Pharmacol. 2022, 13, 896637. [Google Scholar] [CrossRef]
- Li, C.; Cai, Q.; Wu, X.; Tan, Z.; Yao, L.; Huang, S.; Zhang, W.; Hong, Z.; Chen, Z.; Zhang, L. Anti-inflammatory Study on the Constituents of Angelica sinensis (Oliv.) Diels, Angelica dahurica (Hoffm.) Benth. & Hook.f. ex Franch. & Sav., Angelica pubescence Maxim and Foeniculum vulgare Mill. Essential Oils. J. Oleo Sci. 2022, 71, 1207–1219. [Google Scholar] [CrossRef]
- Yang, W.T.; Ke, C.Y.; Wu, W.T.; Lee, R.P.; Tseng, Y.H. Effective Treatment of Bovine Mastitis with Intramammary Infusion of Angelica dahurica and Rheum officinale Extracts. Evid. Based Complement. Altern. Med. 2019, 2019, 7242705. [Google Scholar] [CrossRef]
- Abdulkarim, A.; Gileva, A.A.; Blinova, O.L.; Belonogova, V.D.; Turyshev, A.Y. Development of a procedure for quantification of the total flavonoid content in the ground burnut (Tribulus terrestris) herb. Farmatsiya 2020, 69, 17–22. [Google Scholar] [CrossRef]
- Sachivkina, N.; Vasilieva, E.; Lenchenko, E.; Kuznetsova, O.; Karamyan, A.; Ibragimova, A.; Zhabo, N.; Molchanova, M. Reduction in Pathogenicity in Yeast-like Fungi by Farnesol in Quail Model. Animals 2022, 12, 489. [Google Scholar] [CrossRef] [PubMed]
- Senyagin, A.; Sachivkina, N.; Das, M.; Semenova, V.; Kuznetsova, O.; Ibragimova, A. The Effects of L-Lysine-α-oxidase Enzyme and Trichoderma harzianum Rifai Culture Liquid on the Formation of Biofilms by Uropathogenic Multiresistant E. coli. Fermentation 2023, 9, 710. [Google Scholar] [CrossRef]
- Alves-Silva, J.; Zuzarte, M.; Cavaleiro, C.; Salgueiro, L. Antibiofilm Effect of Lavandula multifida Essential Oil: A New Approach for Chronic Infections. Pharmaceutics 2023, 15, 2142. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Pruthi, V.; Poluri, K.M. Mechanistic insights into Candida biofilm eradication potential of eucalyptol. J. Appl. Microbiol. 2021, 131, 105–123. [Google Scholar] [CrossRef]


| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Action 1 | Hbb 100 µL | Hbb 100 µL | Hbb 100 µL | Hbb 100 µL | Hbb 100 µL | Hbb 100 µL | Hbb 100 µL | Hbb 100 µL | Hbb 100 µL | Hbb 100 µL | Hbb 100 µL | |
| Action 2 | +ATE 200 µL | |||||||||||
| Action 3 | Not titrated | Transfer 100 µL | Transfer 100 µL | Transfer 100 µL | Transfer 100 µL | Transfer 100 µL | Transfer 100 µL | Transfer 100 µL | Transfer 100 µL | Transfer 100 µL | Transfer 100 µL | Transfer 100 µL |
| Titer of ATE | Control – no ATE | 1/1 | 1/2 | 1/4 | 1/8 | 1/16 | 1/32 | 1/64 | 1/128 | 1/256 | 1/512 | 1/1024 |
| Action 4 | +100 µL of culture | +100 µL of culture | +100 µL of culture | +100 µL of culture | +100 µL of culture | +100 µL of culture | +100 µL of culture | +100 µL of culture | +100 µL of culture | +100 µL of culture | +100 µL of culture | +100 µL of culture |
| Action 5 | Wait for 48 h | |||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ATE concentration according to rutine | Control no ATE | 1.896 | 0.948 | 0.474 | 0.237 | 0.119 | 0.06 | 0.03 | 0.015 | 0.008 | 0.004 | 0.002 |
| Average OD of 3 repeating the experiment | 0.525 ± 0.022 | 0.123 ± 0.018 ** | 0.142 ± 0.024 ** | 0.160 ± 0.021 ** | 0.154 ± 0.017 ** | 0.185 ± 0.019 ** | 0.194 ± 0.015 ** | 0.202 ± 0.023 ** | 0.211 ± 0.018 ** | 0.369 ± 0.031 * | 0.383 ± 0.024 * | 0.498 ± 0.018 |
| Average decrease OD, % | 0 | 76.6 | 73.0 | 69.5 | 70.7 | 64.8 | 63.0 | 61.5 | 59.8 | 29.7 | 27.0 | 5.1 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Action 1 | C. albicans in Hbb 100 µL | C. albicans in Hbb 100 µL | C. albicans in Hbb 100 µL | C. albicans in Hbb 100 µL | C. albicans in Hbb 100 µL | C. albicans in Hbb 100 µL | C. albicans in Hbb 100 µL | C. albicans in Hbb 100 µL | C. albicans in Hbb 100 µL | C. albicans in Hbb 100 µL | C. albicans in Hbb 100 µL | C. albicans in Hbb 100 µL |
| Action 2 | Wait 48 h | |||||||||||
| Action 3 | +100 µL Hbb | + Not titrated ATE | + titrated ATE | + titrated ATE | + titrated ATE | + titrated ATE | + titrated ATE | + titrated ATE | + titrated ATE | + titrated ATE | + titrated ATE | + titrated ATE |
| Titer of ATE | Control – no ATE | 1/1 | 1/2 | 1/4 | 1/8 | 1/16 | 1/32 | 1/64 | 1/128 | 1/256 | 1/512 | 1/1024 |
| ATE concentration according to rutine | Control no ATE | 1.896 | 0.948 | 0.474 | 0.237 | 0.119 | 0.06 | 0.03 | 0.015 | 0.008 | 0.004 | 0.002 |
| Action 4 | Wait for 6 h | |||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ATE concentration according to rutine | Control no ATE | 1.896 | 0.948 | 0.474 | 0.237 | 0.119 | 0.06 | 0.03 | 0.015 | 0.008 | 0.004 | 0.002 |
| Average OD of 3 repeating the experiment | 0.653 ± 0.025 | 0.323 ± 0.028 ** | 0.449 ± 0.020 * | 0.461 ± 0.019 * | 0.454 ± 0.021 * | 0.485 ± 0.019 * | 0.504 ± 0.025 * | 0.502 ± 0.026 * | 0.522 ± 0.018 * | 0.559 ± 0.023 * | 0.584 ± 0.025 * | 0.668 ± 0.028 |
| Average decrease OD, % | 0 | 50.5 | 31.2 | 29.4 | 30.5 | 25.7 | 22.8 | 23.1 | 20.1 | 14.4 | 10.6 | −2.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sachivkina, N.; Karamyan, A.; Semenova, V.; Ignatiev, A.; Abdurasulov, A.; Muratova, R.; Emilbekova, D.; Ermatova, V.; Risvanli, A.; Salykov, R.; et al. The Effects of Angelica ternata Extract from Kyrgyzstan on the Formation of Candida albicans ATCC 10231 Biofilms. Appl. Sci. 2023, 13, 12042. https://doi.org/10.3390/app132112042
Sachivkina N, Karamyan A, Semenova V, Ignatiev A, Abdurasulov A, Muratova R, Emilbekova D, Ermatova V, Risvanli A, Salykov R, et al. The Effects of Angelica ternata Extract from Kyrgyzstan on the Formation of Candida albicans ATCC 10231 Biofilms. Applied Sciences. 2023; 13(21):12042. https://doi.org/10.3390/app132112042
Chicago/Turabian StyleSachivkina, Nadezhda, Arfenya Karamyan, Valentina Semenova, Aleksej Ignatiev, Abdugani Abdurasulov, Rakhima Muratova, Dinara Emilbekova, Venera Ermatova, Ali Risvanli, Ruslan Salykov, and et al. 2023. "The Effects of Angelica ternata Extract from Kyrgyzstan on the Formation of Candida albicans ATCC 10231 Biofilms" Applied Sciences 13, no. 21: 12042. https://doi.org/10.3390/app132112042
APA StyleSachivkina, N., Karamyan, A., Semenova, V., Ignatiev, A., Abdurasulov, A., Muratova, R., Emilbekova, D., Ermatova, V., Risvanli, A., Salykov, R., Ibragimova, A., & Neborak, E. (2023). The Effects of Angelica ternata Extract from Kyrgyzstan on the Formation of Candida albicans ATCC 10231 Biofilms. Applied Sciences, 13(21), 12042. https://doi.org/10.3390/app132112042








