Numerical Evaluation Using the Finite Element Method on Frontal Craniocervical Impact Directed at Intervertebral Disc Wear
Abstract
1. Introduction
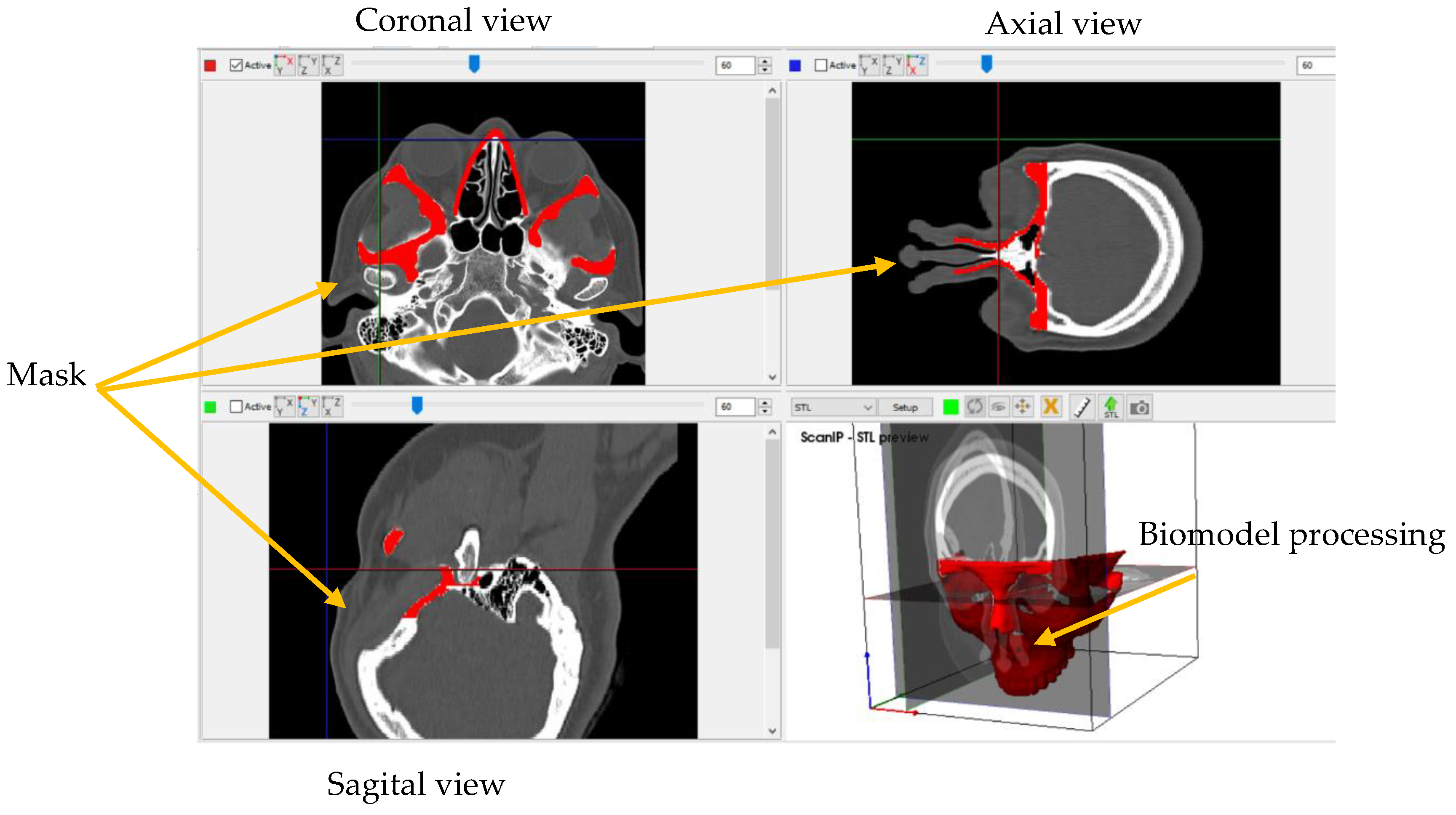
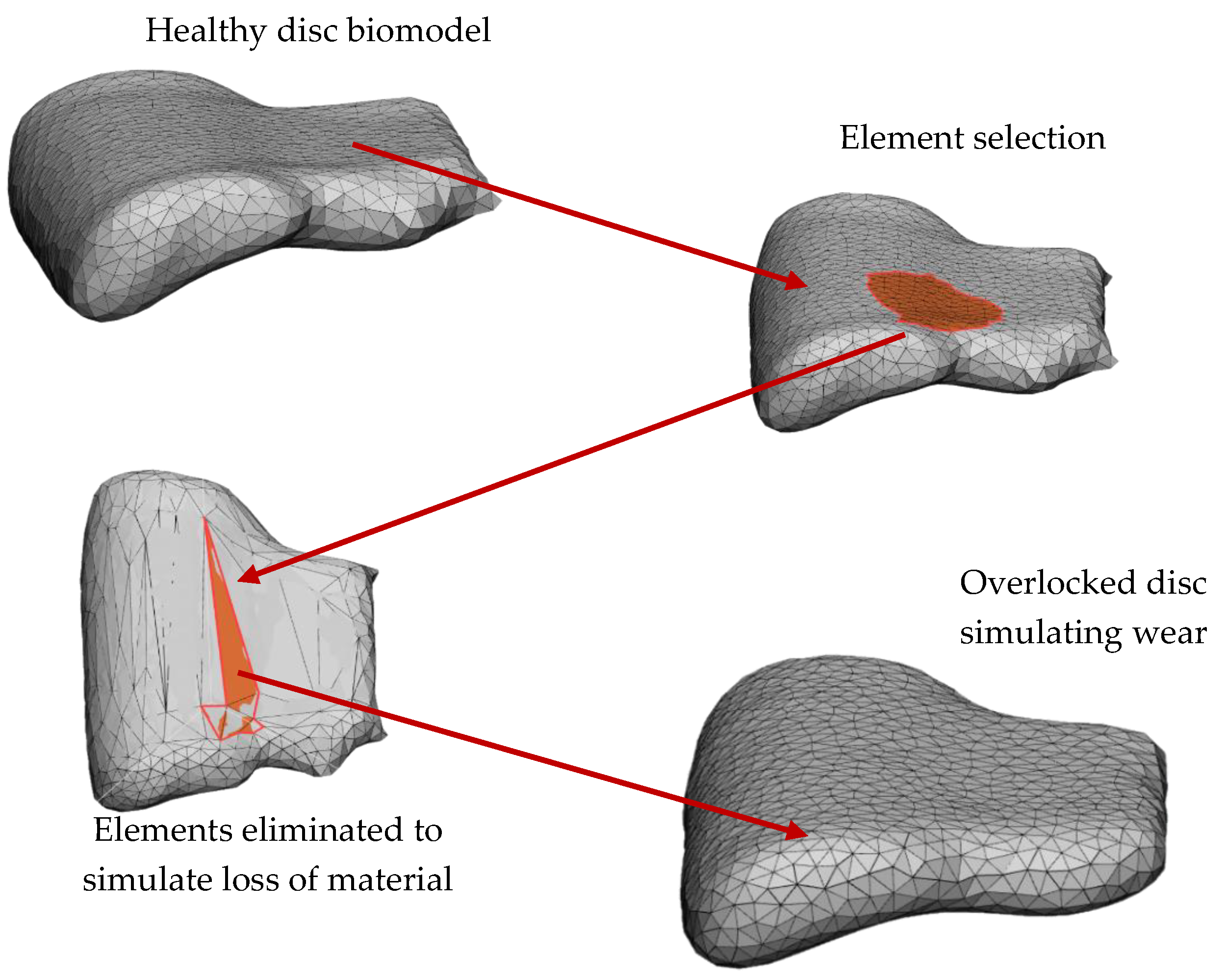
2. Methods
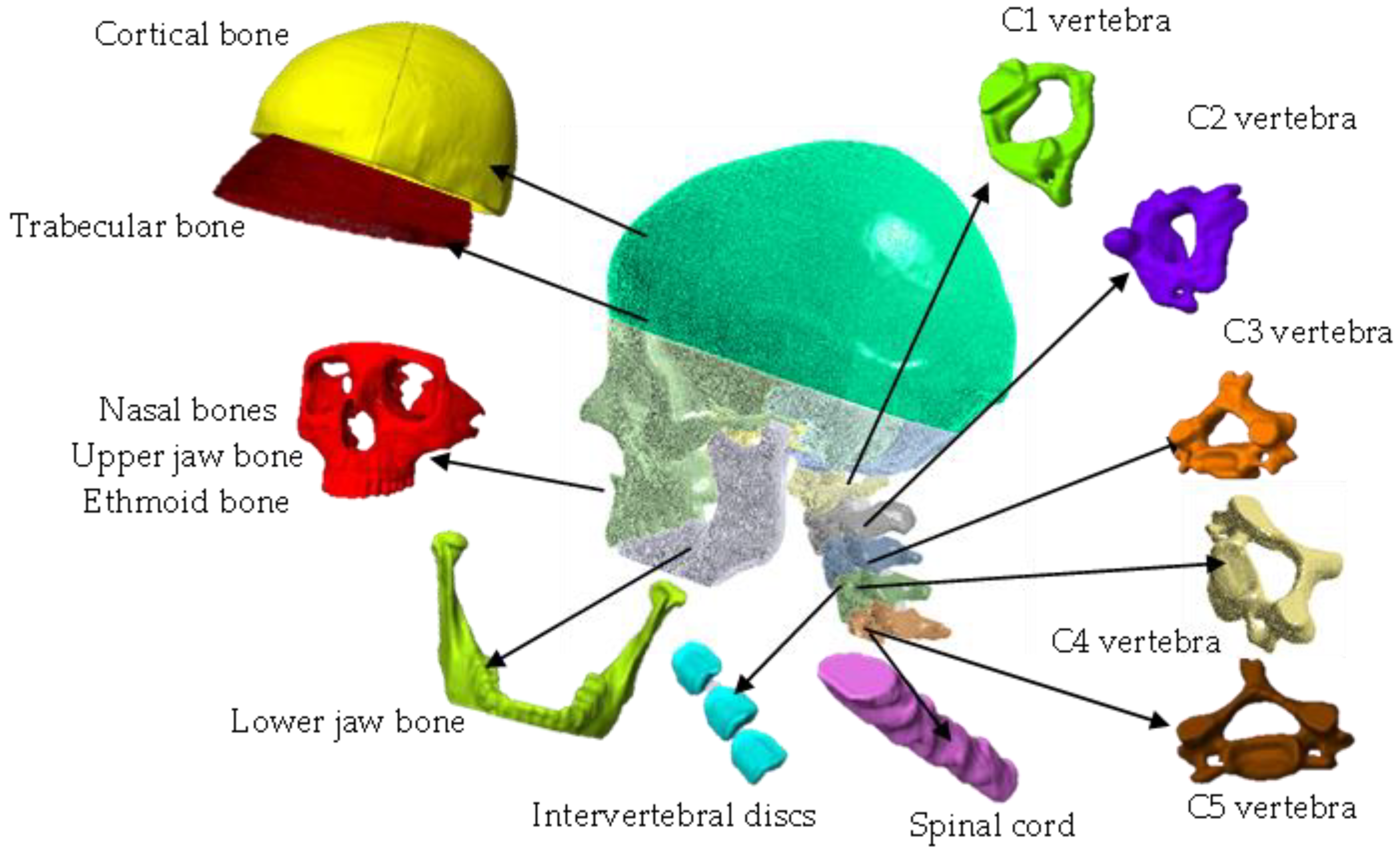
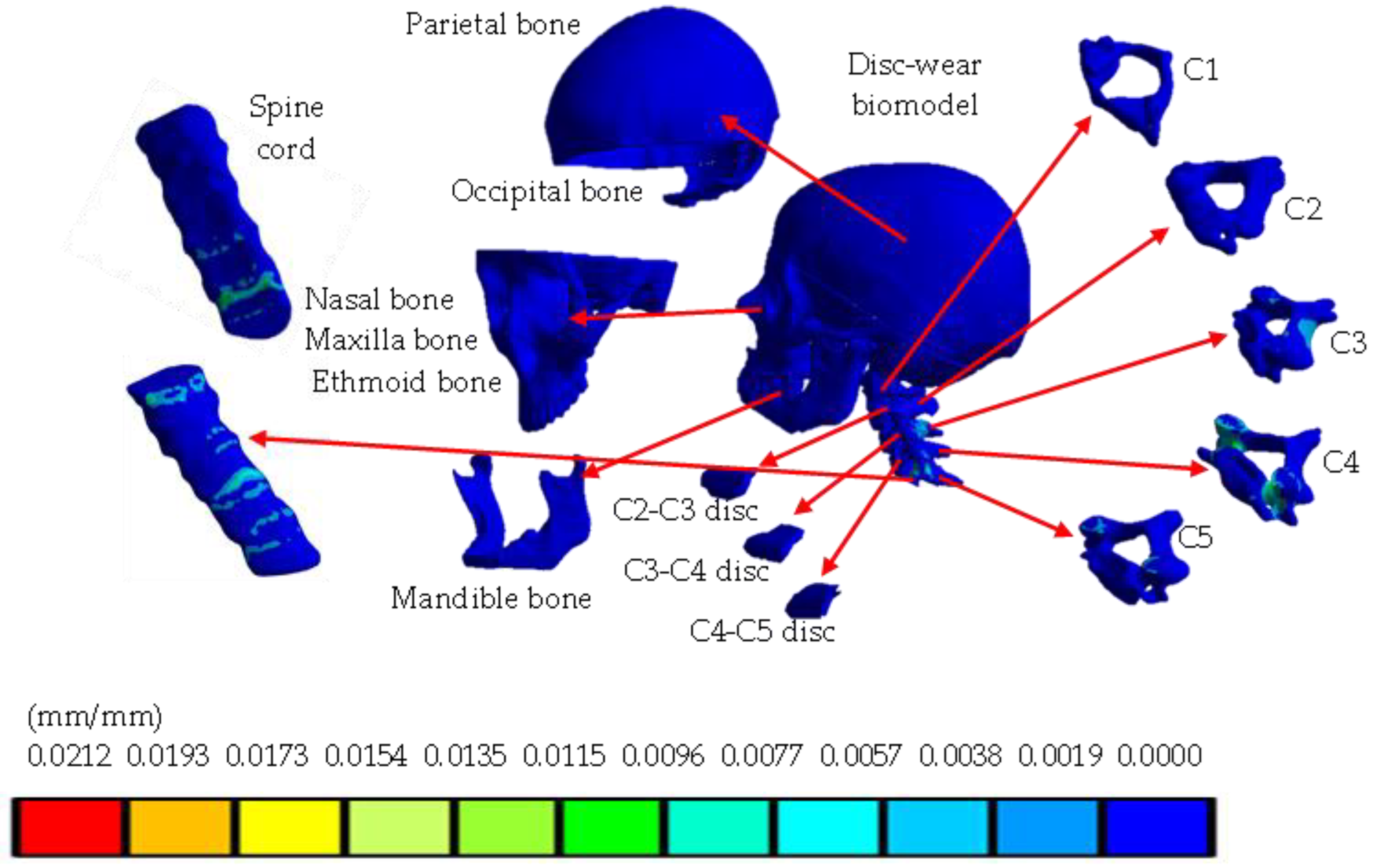
Materials
- Skull.
- Spinal cord.
- Cervical region: C1, C2, C3, C4, C5.
- Intervertebral disc.
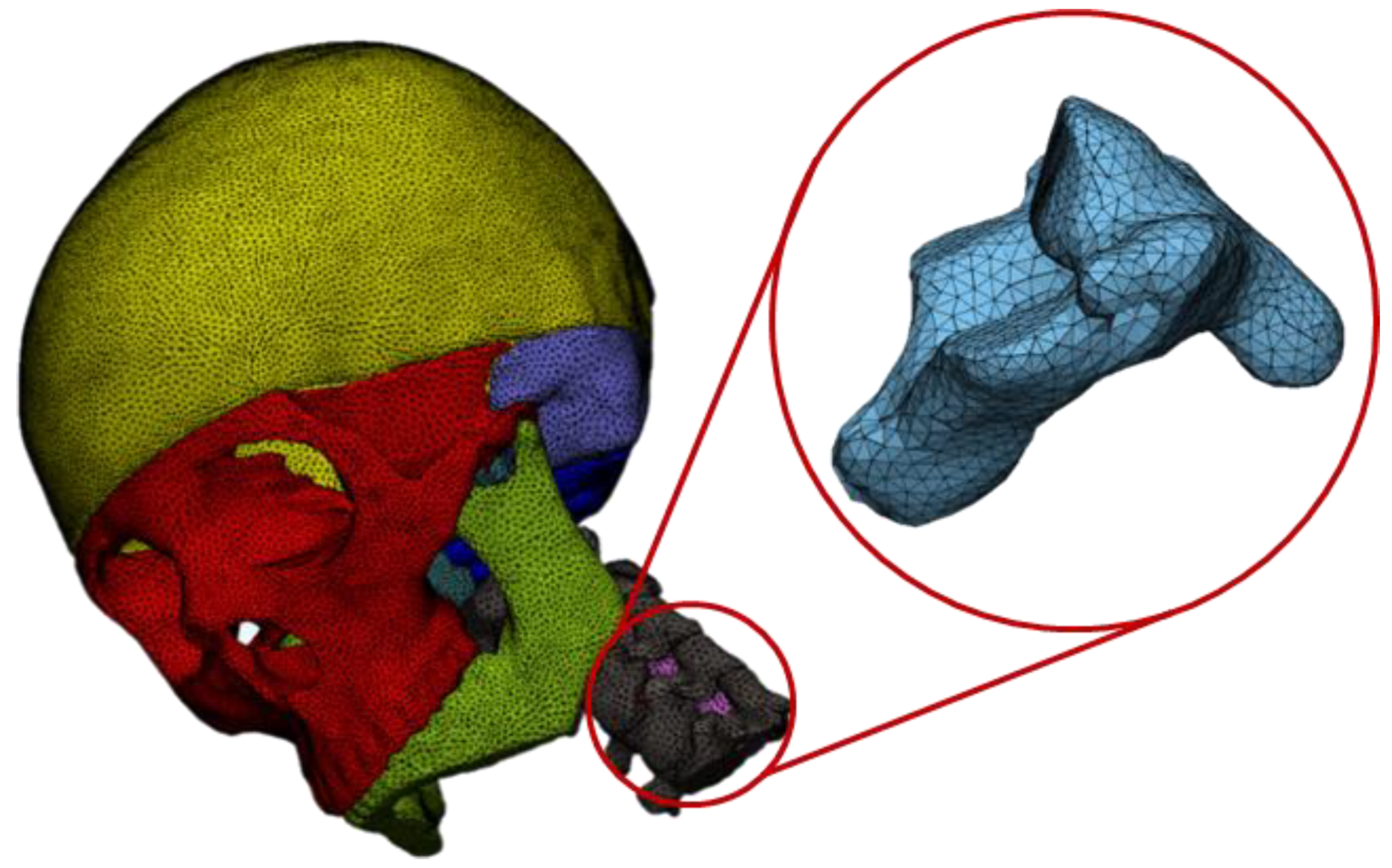
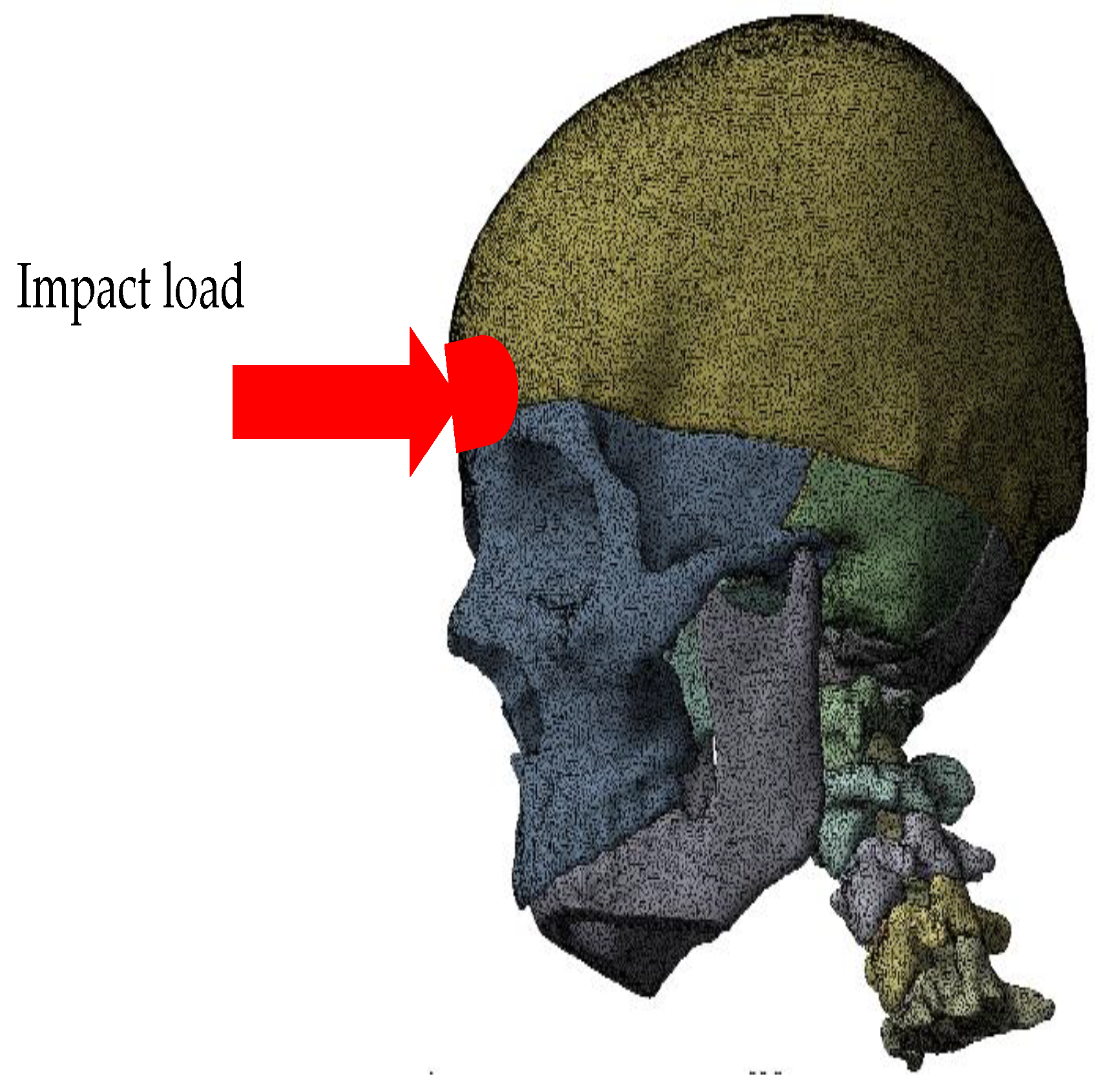
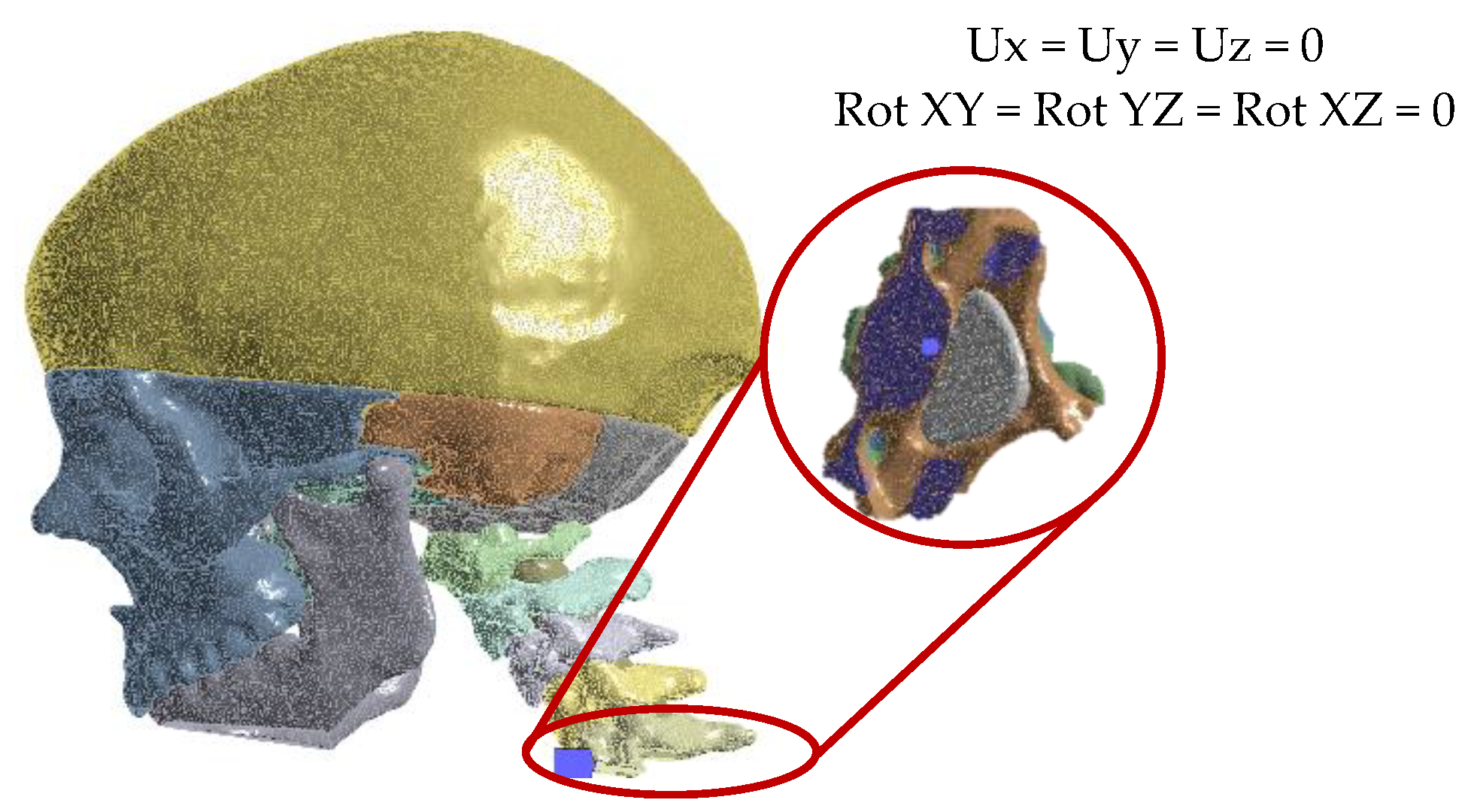
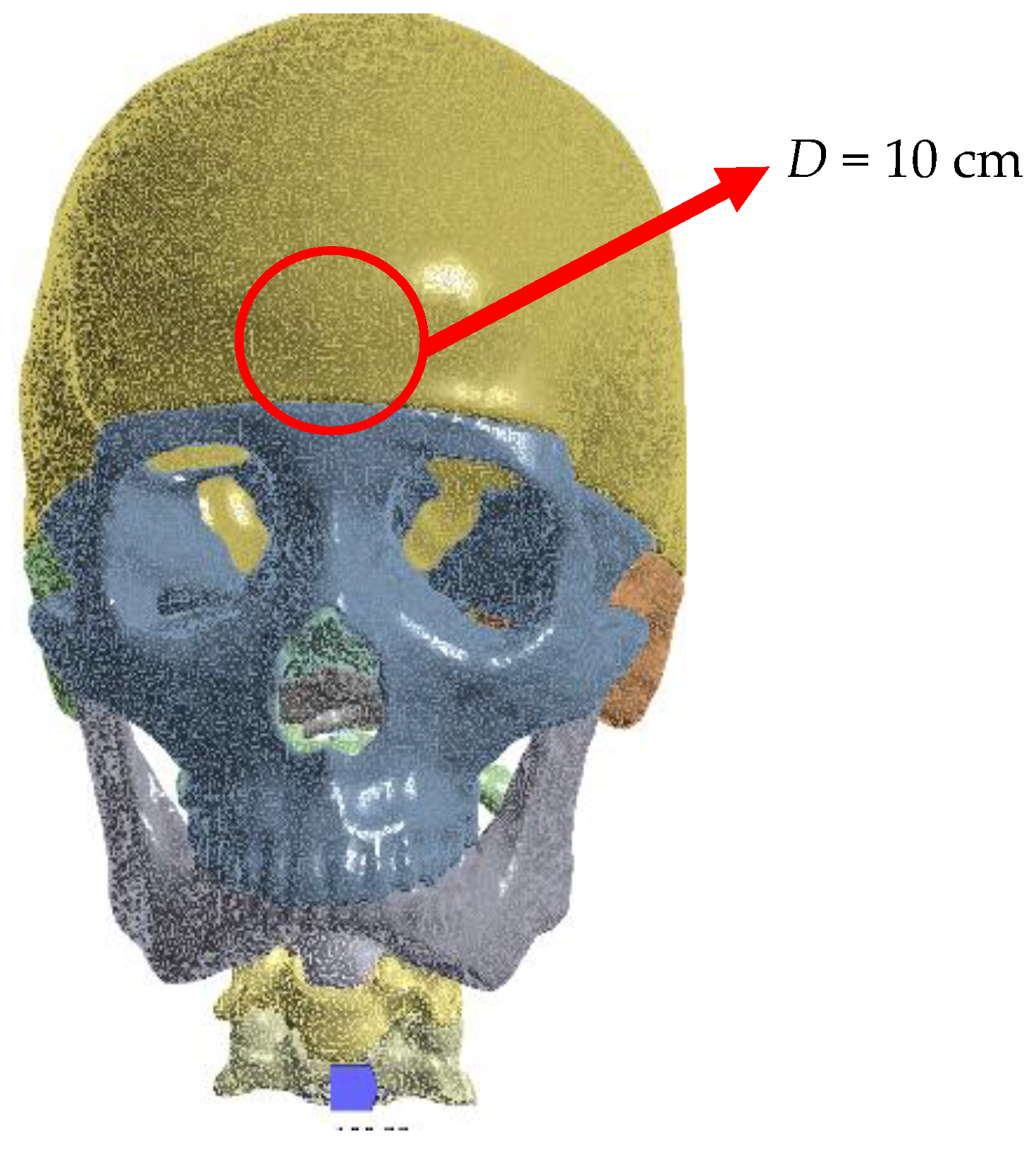
3. Numerical Analysis
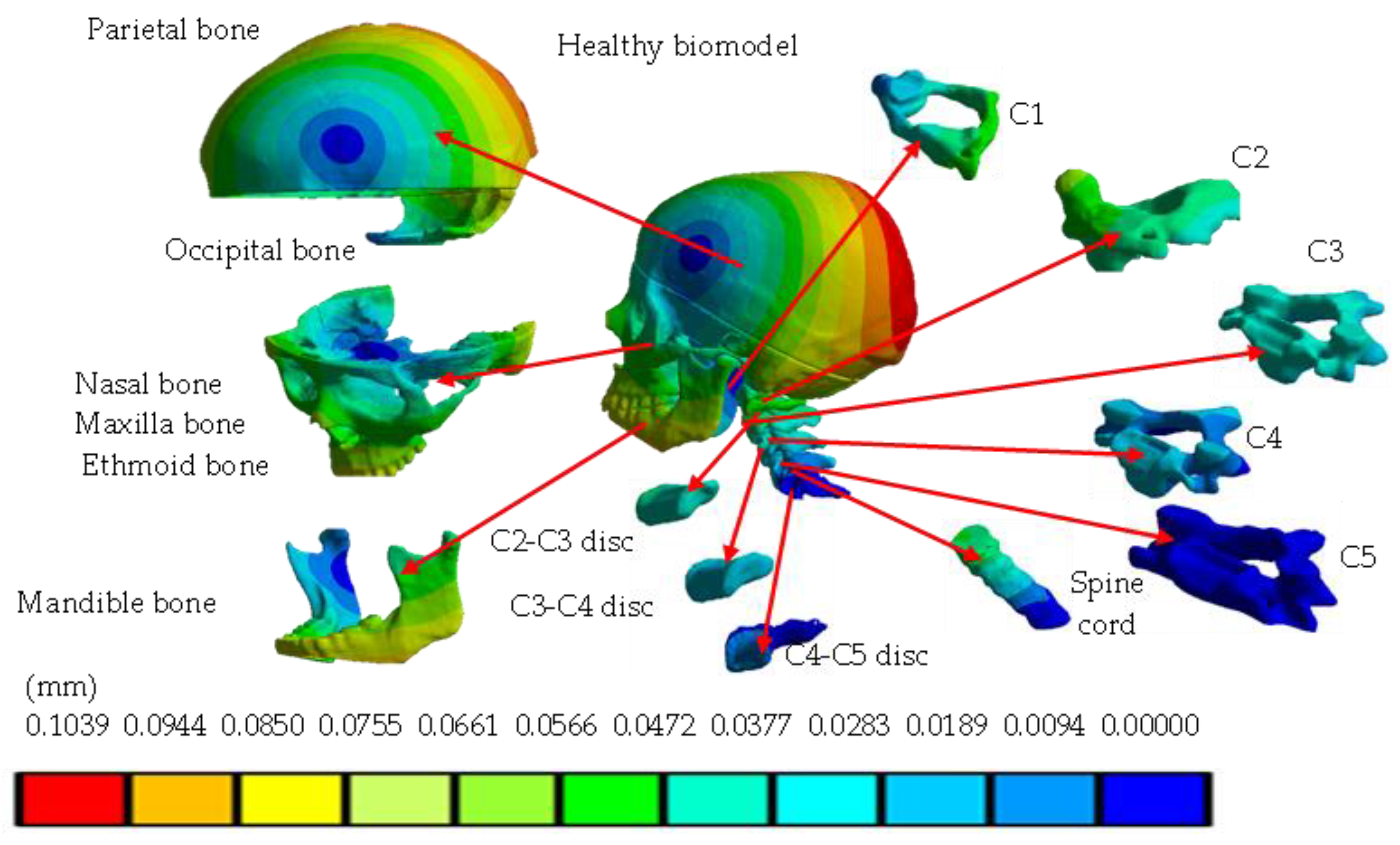
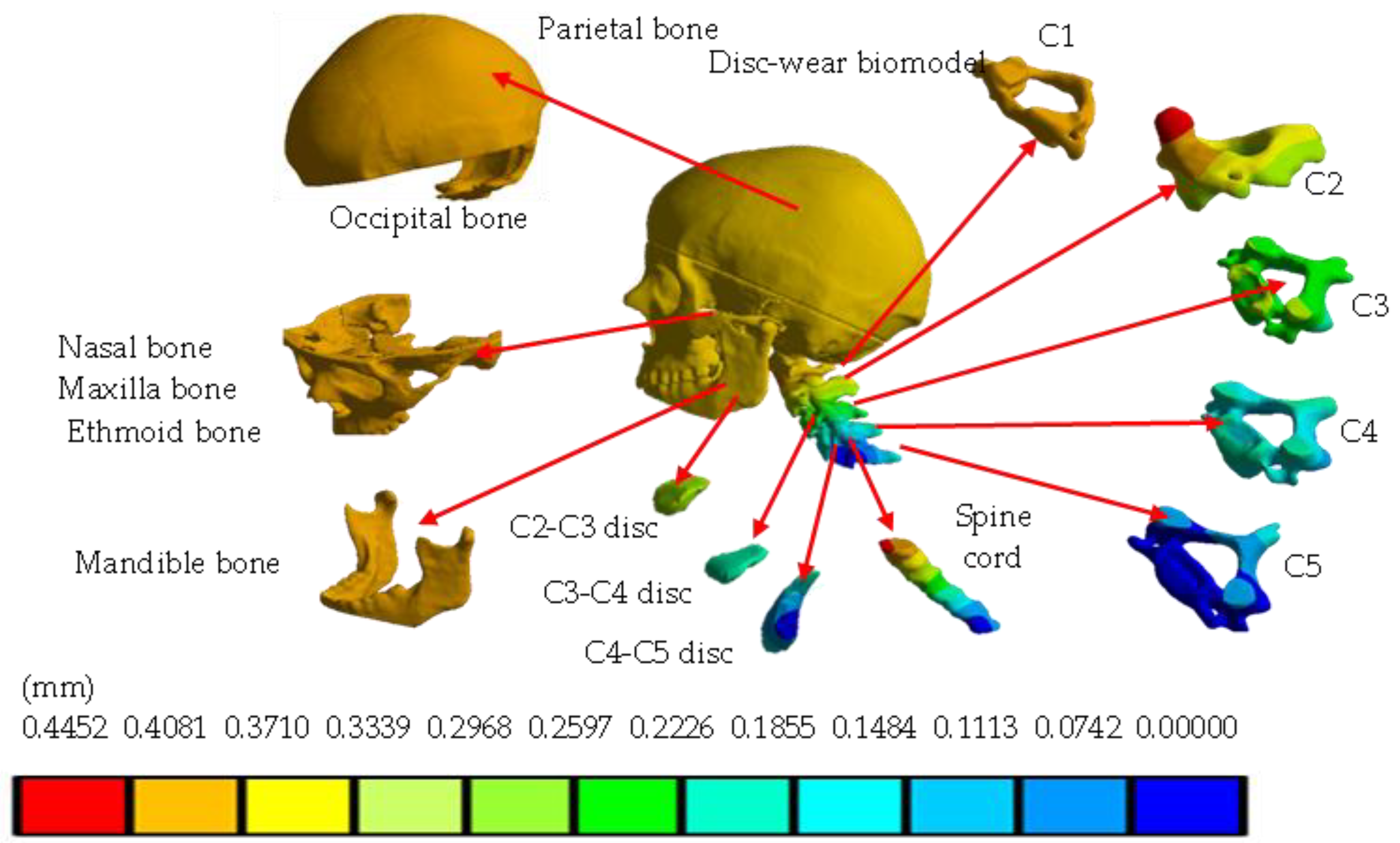
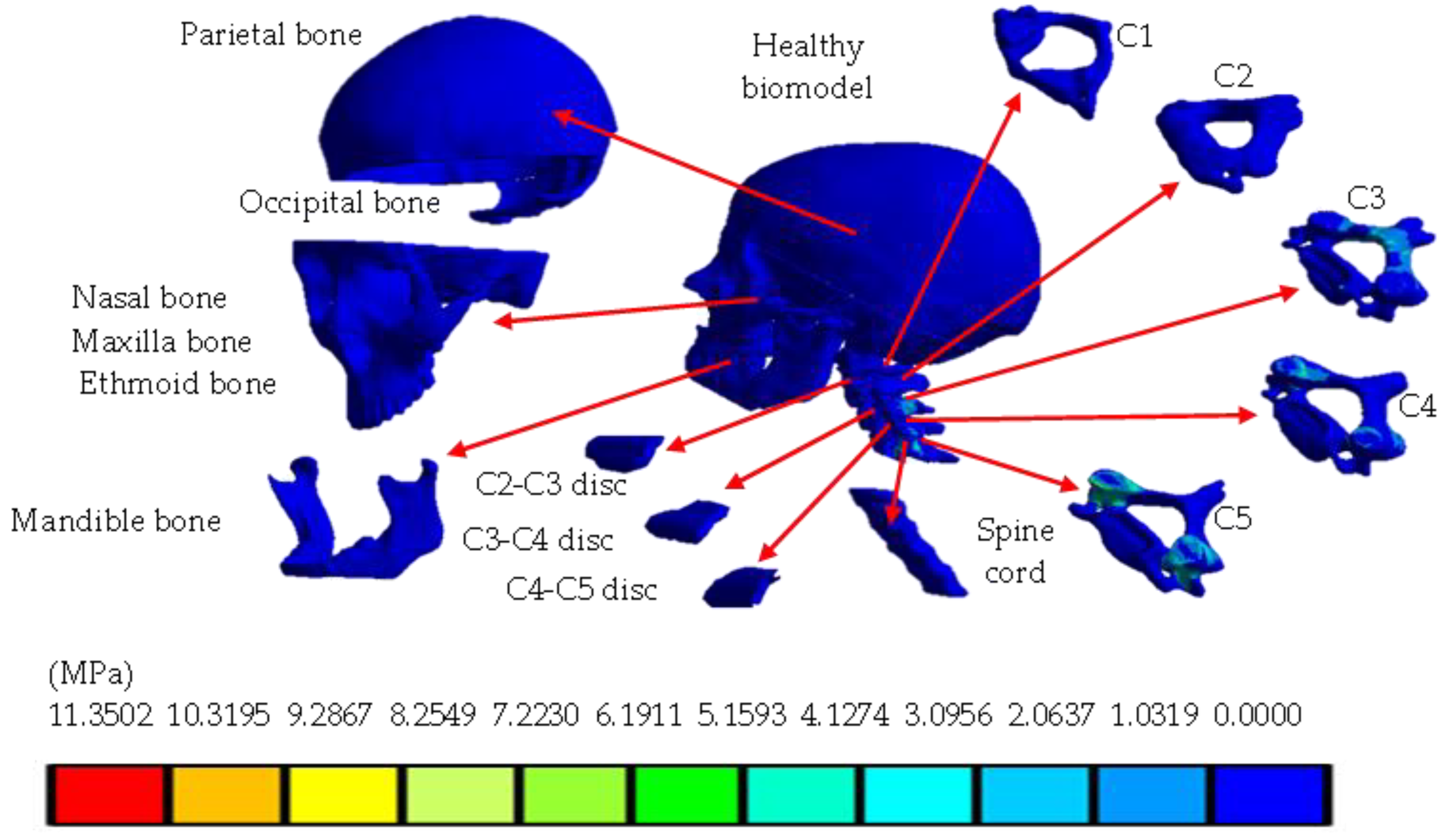
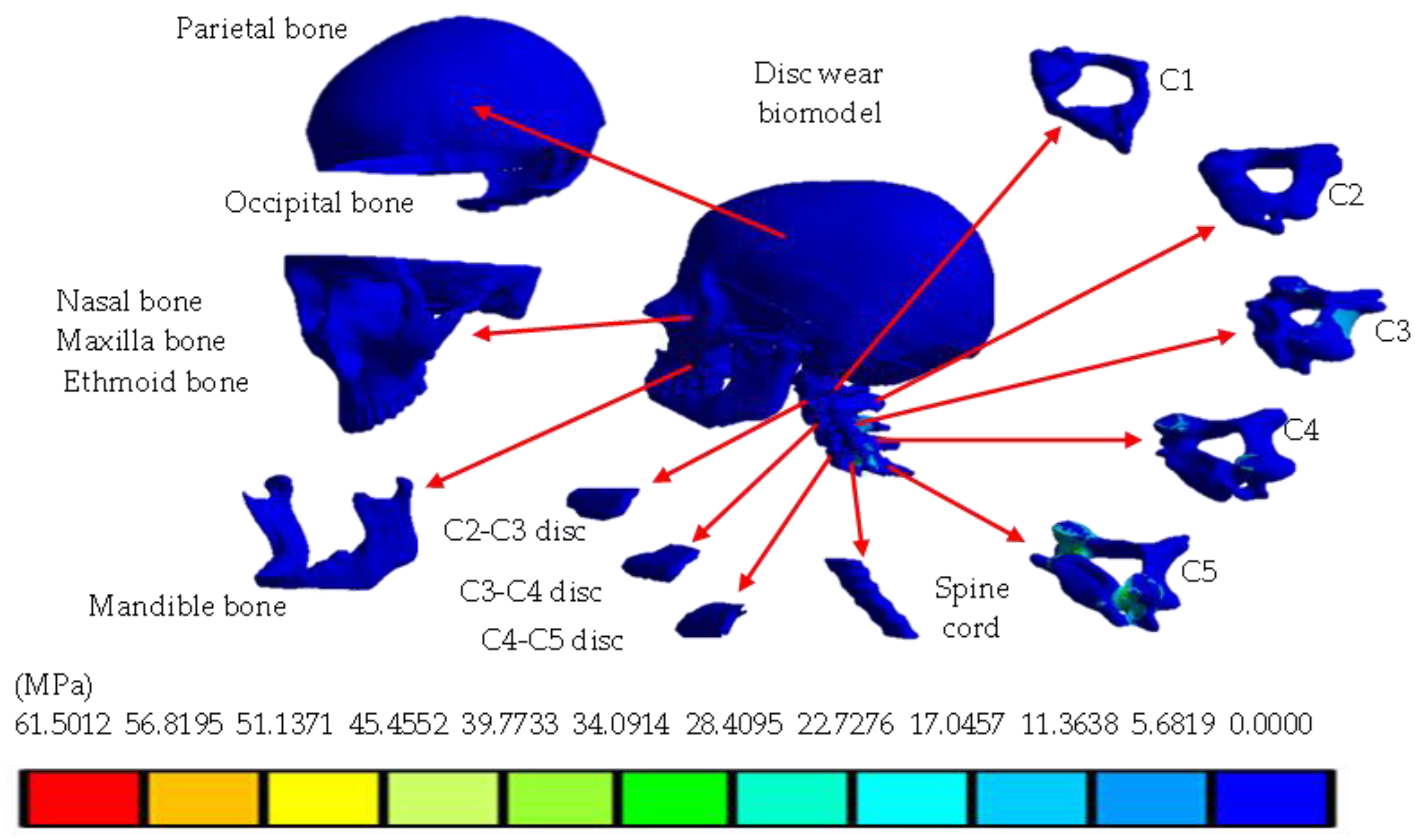
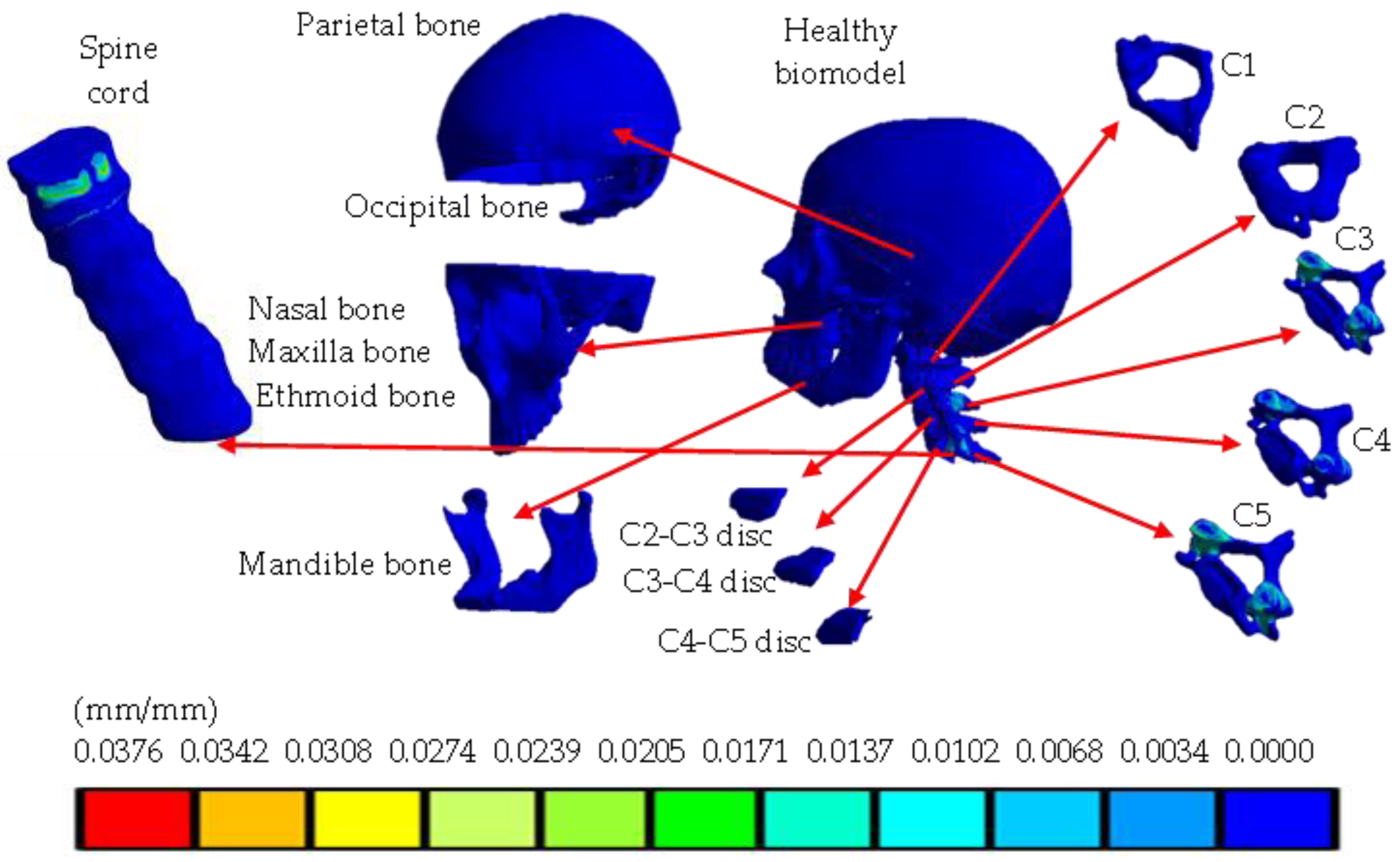
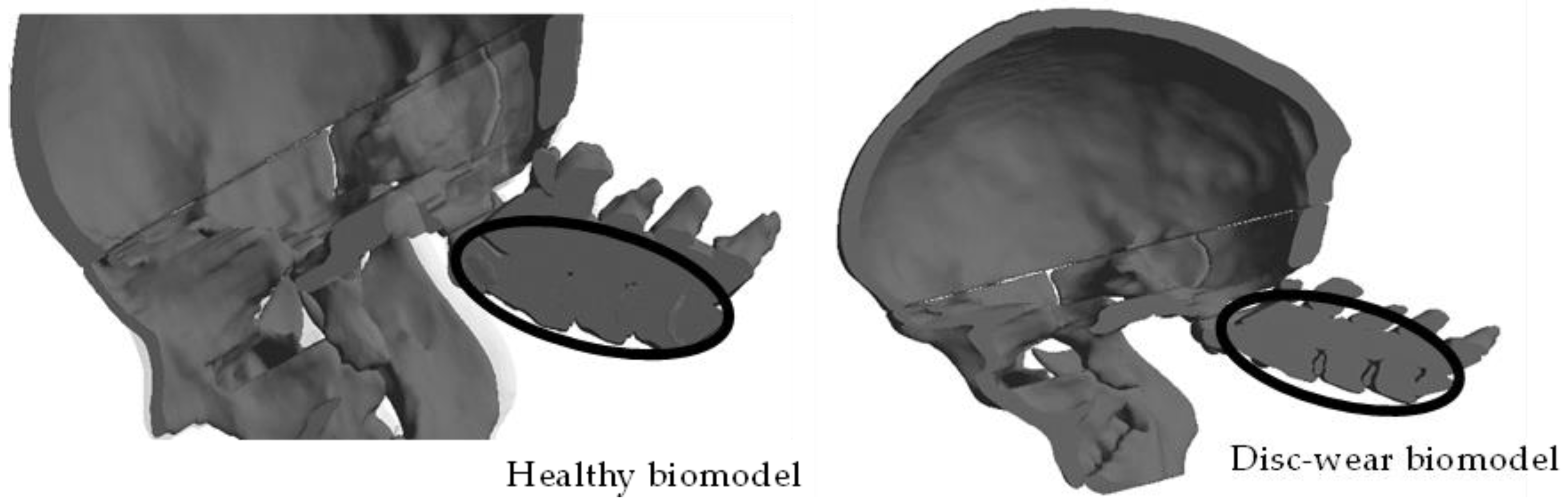
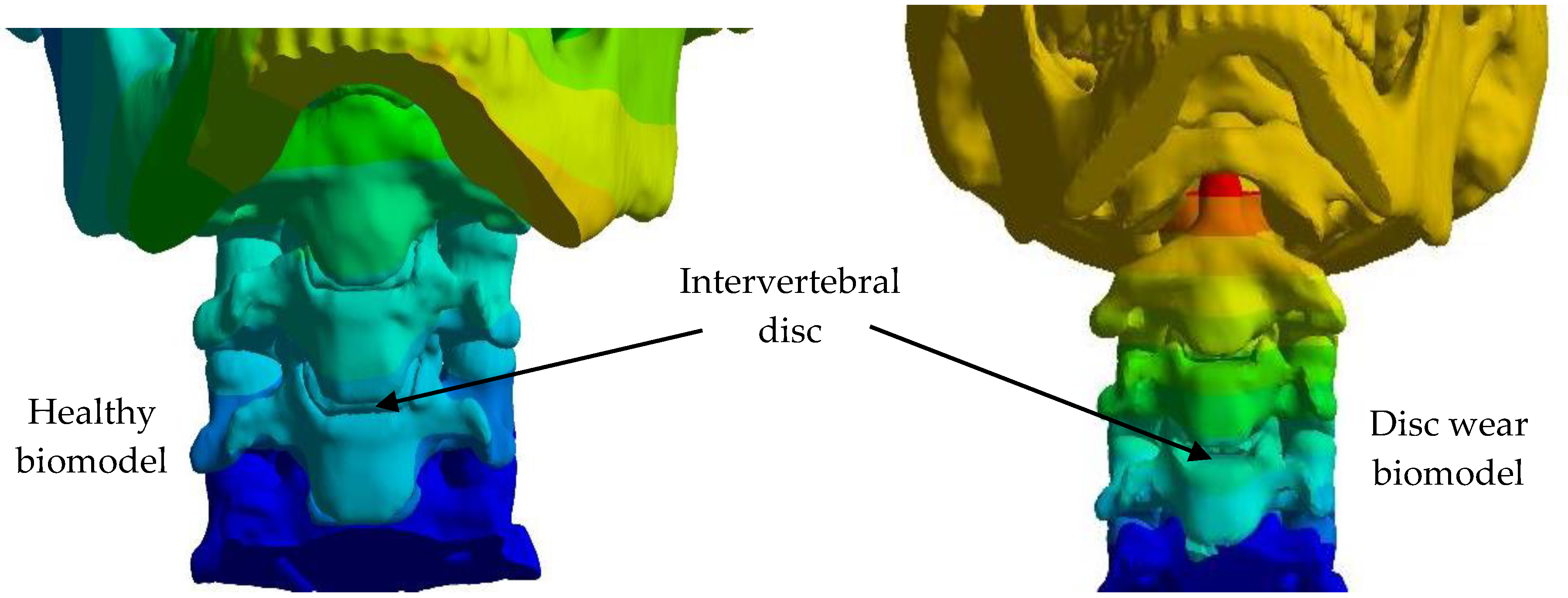
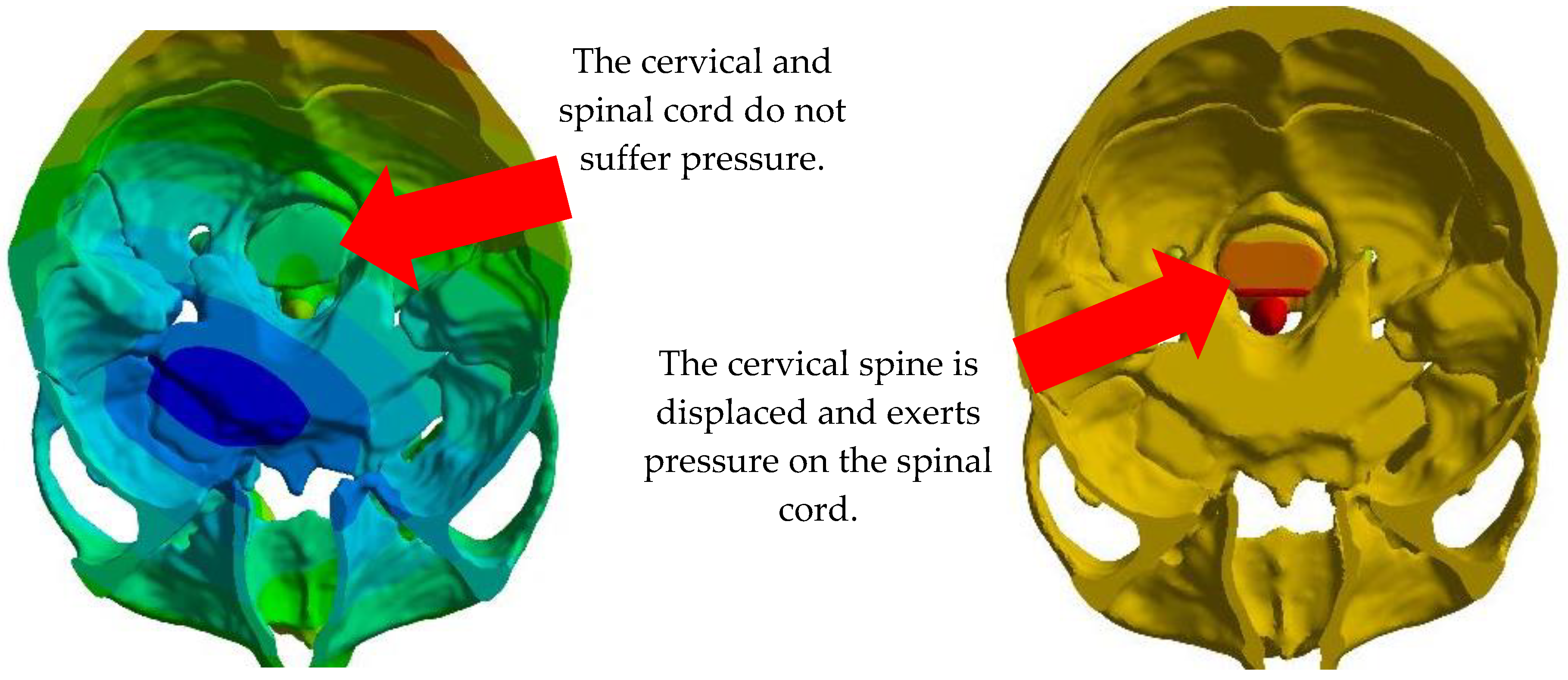
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Concept | Healthy Condition | Disc-Wear Condition | ||
|---|---|---|---|---|
| Minimal | Maximum | Minimal | Maximum | |
| Total displacement (mm) | 0 | 0.1039 | 0 | 0.4452 |
| Displacement X axis (mm) | −0.0444 | 0.0653 | −0.02043 | 0.00941 |
| Displacement Y axis (mm) | −0.0724 | 0.0882 | −0.3297 | 0.0911 |
| Displacement Z axis (mm) | −0.0727 | 0.0531 | −0.3163 | 0.1465 |
| Total strain (mm/mm) | 0 | 0.0376 | 0 | 0.0212 |
| Strain X axis (mm/mm) | −0.0174 | 0.0082 | −0.0042 | 0.0031 |
| Strain Y axis (mm/mm) | −0.0204 | 0.0133 | −0.0123 | 0.0044 |
| Strain Z axis (mm/mm) | −0.0215 | 0.0108 | −0.0117 | 0.0070 |
| Von Mises stress (MPa) | 0 | 11.3502 | 0 | 62.501 |
| Maximum principal stress (MPa) | −4.7549 | 12.6048 | −14.507 | 59.984 |
| Minimum principal stress (MPa) | −15.5285 | 3.4748 | −68.268 | 6.4836 |
| Maximum shear stress (MPa) | 0 | 5.8328 | 0 | 34.632 |
| Nominal stress X axis (MPa) | −8.0763 | 5.8252 | −24.959 | 22.713 |
| Nominal stress Y axis (MPa) | −11.9718 | 7.1022 | −31.995 | 24.682 |
| Nominal stress Z axis (MPa) | −14.9241 | 11.2437 | −65.264 | 55.777 |
| Shear stress XY plane (MPa) | −2.0796 | 2.3934 | −11.901 | 12.005 |
| Shear stress YZ plane (MPa) | −4.1420 | 5.1737 | −25.828 | 25.919 |
| Shear plane XZ plane (MPa) | −3.8517 | 3.3848 | −11.612 | 21.842 |
References
- Frank, R.M.; Beaulieu-Jones, B.; Sánchez, G.; Vopat, B.; Logan, C.; Price, M.D.; Provencher, M.T. Epidemiology of Injuries Identified at the NFL Scouting Combine and Their Impact on Performance in the National Football League: Evaluation of 2203 Athletes From 2009 to 2015. Arthroscopy 2017, 33, e101–e102. [Google Scholar] [CrossRef][Green Version]
- Craig, A.; Tran, Y.; Wijesuriya, N.; Middleton, J. Fatigue and tiredness in people with spinal cord injury. J. Psychosom. Res. 2012, 73, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Mez, J.; Daneshvar, D.H.; Kiernan, P.T.; Abdolmohammadi, B.; Alvarez, V.E.; Huber, B.R.; McKee, A.C. Clinico–pathological evaluation of chronic traumatic encephalopathy in players of American football. JAMA 2017, 318, 360–370. [Google Scholar] [CrossRef]
- Gause, P.R.; Godinsky, R.J.; Burns, K.S.; Dohring, E.J. Lumbar disk herniations and radiculopathy in athletes. Clin. Sports Med. 2021, 40, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Mead, L.B.; Millhouse, P.W.; Krystal, J.; Vaccaro, A.R. C1 fractures: A review of diagnoses, management options, and outcomes. Curr. Rev. Musculoskelet. Med. 2016, 9, 255–262. [Google Scholar] [CrossRef]
- Kumar, V.; Gaurav, A.; Dhatt, S.S.; Neradi, D.; Kumar, S.; Shetty, A. Traumatic cervical disc protruding postero-laterally mimicking lateral flexion type injury of cervical spine: A case report. SN Compr. Clin. Med. 2021, 3, 2060–2063. [Google Scholar] [CrossRef]
- Benzakour, T.; Igoumenou, V.; Mavrogenis, A.F.; Benzakour, A. Current concepts for lumbar disc herniation. Int. Orthop. 2019, 43, 841–851. [Google Scholar] [CrossRef]
- Khalid, M.; Tufail, S.; Aslam, Z.; Butt, A. Osteoarthritis: From complications to cure. Int. J. Clin. Rheumatol. 2017, 12, 160–167. [Google Scholar] [CrossRef]
- Patel, P.D.; Canseco, J.A.; Houlihan, N.; Gabay, A.; Grasso, G.; Vaccaro, A.R. Overview of minimally invasive spine surgery. World Neurosurg. 2020, 142, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.C.; Kuo, C.; Loza, J.; Kurt, M.; Laksari, K.; Yanez, L.Z.; Camarillo, D.B. Detection of American football head impacts using biomechanical features and support vector machine classification. Sci. Rep. 2017, 8, 855. [Google Scholar] [CrossRef] [PubMed]
- Jakanani, G.; Kenningham, R.; Bolia, A. Active retropharyngeal hemorrhage from an acute thyrocervical artery injury: A rare complication of hyperextension cervical spine injury. J. Emerg. Med. 2012, 43, e39–e41. [Google Scholar] [CrossRef]
- Mendoza-Puente, M.; Oliva-Pascual-Vaca, Á.; Rodriguez-Blanco, C.; Heredia-Rizo, A.M.; Torres-Lagares, D.; Ordoñez, F.J. Risk of headache, temporomandibular dysfunction, and local sensitization in male professional boxers: A case-control study. Arch. Phys. Med. Rehabil. 2014, 95, 1977–1983. [Google Scholar] [CrossRef] [PubMed]
- Cejudo, A.; Centenera-Centenera, J.M.; Santonja-Medina, F. The Potential Role of Hamstring Extensibility on Sagittal Pelvic Tilt, Sagittal Spinal Curves and Recurrent Low Back Pain in Team Sports Players: A Gender Perspective Analysis. Int. J. Environ. Res. Public Health 2021, 18, 8654. [Google Scholar] [CrossRef]
- Alizadeh, M.; Knapik, G.G.; Mageswaran, P.; Mendel, E.; Bourekas, E.; Marras, W.S. Biomechanical musculoskeletal models of the cervical spine: A systematic literature review. Clin. Biomech. 2020, 71, 115–124. [Google Scholar] [CrossRef]
- Benítez, J.M.; Montáns, F.J. The mechanical behavior of skin: Structures and models for the finite element analysis. Comput. Struct. 2017, 190, 75–107. [Google Scholar] [CrossRef]
- Zhu, J.B.; Zhou, T.; Liao, Z.Y.; Sun, L.; Li, X.B.; Chen, R. Replication of internal defects and investigation of mechanical and fracture behavior of rock using 3D printing and 3D numerical methods in combination with X-ray computerized tomography. Int. J. Rock Mech. Min. Sci. 2018, 106, 198–212. [Google Scholar] [CrossRef]
- Urits, I.; Burshtein, A.; Sharma, M.; Testa, L.; Gold, P.A.; Orhurhu, V.; Kaye, A.D. Low back pain, a comprehensive review: Pathophysiology, diagnosis, and treatment. Curr. Pain Headache Rep. 2019, 23, 23. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Liu, N.S.; Li, H.G.; Zhang, B.; Tian, S.W.; Tan, M.G.; Sandoz, B. A review of neck injury and protection in vehicle accidents. Transp. Saf. Environ. 2019, 1, 89–105. [Google Scholar] [CrossRef]
- Chi, Q.; Liu, P.; Liang, H. Biomechanics Assist Measurement, Modeling, Engineering Applications, and Clinical Decision Making in Medicine. Bioengineering 2022, 10, 20. [Google Scholar] [CrossRef]
- Zhang, J.K.; Alimadadi, A.; ReVeal, M.; Del Valle, A.J.; Patel, M.; O’Malley, D.S.; Mattei, T.A. Litigation involving sport-related spinal injuries: A comprehensive review of reported legal claims in the United States. Spine J. 2022, 23, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Rebatú, A.G.; Cárdenas, A.A.; Falfan, R.R.; Fernández, J.A.B. Análisis mecánico-estructural del injerto óseo en el segmento C3-C5 de la columna cervical como tratamiento de las fracturas con método de elementos finitos. Rev. Espec. Médico-Quirúrgicas 2013, 18, 195–199. [Google Scholar]
- Carrasco Hernandez, F. Análisis Numérico de Cargas de Impacto Sobre Cráneo Humano. Ph.D. Thesis, Instituto Politécnico Nacional, Mexico City, Mexico, 2020; pp. 57–59. [Google Scholar]
- Hernández-Vázquez, R.A.; Urriolagoitia-Sosa, G.; Marquet-Rivera, R.A.; Romero-Ángeles, B.; Mastache-Miranda, O.A.; Vázquez-Feijoo, J.A.; Urriolagoitia-Calderón, G.M. High-biofidelity biomodel generated from three-dimensional im-aging (cone-beam computed tomography): A methodological proposal. Comput. Math. Methods Med. 2020, 2020, 4292501. [Google Scholar] [CrossRef]
- Hernández-Vázquez, R.A.; Romero-Ángeles, B.; Urriolagoitia-Sosa, G.; Vázquez-Feijoo, J.A.; Vázquez-López, Á.; Urriolagoitia-Calderón, G. Numerical analysis of masticatory forces on a lower first molar, considering the contact between dental tissues. Appl. Bionics Biomech. 2018, 2018, 4196343. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Prasad, R.B. Stress analysis of cortical bone of the human femur. Mater. Today Proc. 2021, 44, 2054–2060. [Google Scholar] [CrossRef]
- Correa-Corona, M.I.; Urriolagoitia-Sosa, G.; Romero-Ángeles, B.; Urriolagoitia-Luna, A.; Maya-Anaya, D.; Suarez-Hernández, M.d.l.L.; Trejo-Enríquez, A.; Urriolagoitia-Calderón, G.M. Application of the finite element model using 3D modeling of a human bone system with osteoporosis for biomedical and mechanical analysis. MedCrave Online J. Appl. Bionics Biomech. 2023, 7, 14–15. [Google Scholar]
- Wang, J.L.; Parnianpour, M.; Shirazi-Adl, A.; Engin, A.E.; Li, S.; Patwardhan, A. Development and validation of a viscoelastic finite element model of an L2/L3 motion segment. Theor. Appl. Fract. Mech. 1997, 28, 81–93. [Google Scholar] [CrossRef]
- Zambrano, L.; Lammardo, A.; Mller-Karger, C. Modelo numérico del anillo fibroso: Revisión del estado del arte. Rev. Fac. Ing. Univ. Cent. Venez. 2013, 28, 117–130. [Google Scholar]
- Nieto, J.; Minor, A.; Alvarez, J. Determinación de esfuerzos en el cráneo humano por medio del método del elemento finito. In Simposio de Ingeniería de Sistemas y Automática en Bioingeniería, Primer Congreso Español de Informática; Thomson: Washington, DC, USA, 2005. [Google Scholar]
- Breedlove, E.L.; Breedlove, K.M.; Bowman, T.G.; Lininger, M.R.; Nauman, E.A. Impact attenuation capabilities of new and used football helmets. Smart Mater. Struct. 2023, 32, 064004. [Google Scholar] [CrossRef]
- Trejo Enriquez, A. Design of a Helmet to Dissipate Energy by Means of Finite Element Failure. Master’s Thesis, Instituto Politécnico Nacional, Mexico City, Mexico, 2020; pp. 57–59. [Google Scholar]
- Jacobson, B.H.; Conchola, E.G.; Glass, R.G.; Thompson, B.J. Longitudinal morphological and performance profiles for American, NCAA Division I football players. J. Strength Cond. Res. 2013, 27, 2347–2354. [Google Scholar] [CrossRef]


| Properties | Cortical Bone | Trabecular Bone |
|---|---|---|
| Young´s modulus | 12,000 MPa | 100 MPa |
| Density | 1700 kg/m3 | 0.14 g/cm3 |
| Poisson ratio | 0.35 | 0.20 |
| Properties | Nucleus Pulposus | Annulus Fibrosus |
|---|---|---|
| Young´s modulus | 1 MPa | 8.4 MPa |
| Density | 997 kg/m3 | 433 kg/m3 |
| Poisson ratio | 0.40 | 0.35 |
| Properties | Cortical Bone | Trabecular Bone |
|---|---|---|
| Young´s modulus | 15,000 MPa | 200 MPa |
| Density | 1900 kg/m3 | 430 g/cm3 |
| Poisson ratio | 0.30 | 0.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trejo-Enriquez, A.; Urriolagoitia-Sosa, G.; Romero-Ángeles, B.; García-Laguna, M.Á.; Guzmán-Baeza, M.; Martínez-Reyes, J.; Rojas-Castrejon, Y.Y.; Gallegos-Funes, F.J.; Patiño-Ortiz, J.; Urriolagoitia-Calderón, G.M. Numerical Evaluation Using the Finite Element Method on Frontal Craniocervical Impact Directed at Intervertebral Disc Wear. Appl. Sci. 2023, 13, 11989. https://doi.org/10.3390/app132111989
Trejo-Enriquez A, Urriolagoitia-Sosa G, Romero-Ángeles B, García-Laguna MÁ, Guzmán-Baeza M, Martínez-Reyes J, Rojas-Castrejon YY, Gallegos-Funes FJ, Patiño-Ortiz J, Urriolagoitia-Calderón GM. Numerical Evaluation Using the Finite Element Method on Frontal Craniocervical Impact Directed at Intervertebral Disc Wear. Applied Sciences. 2023; 13(21):11989. https://doi.org/10.3390/app132111989
Chicago/Turabian StyleTrejo-Enriquez, Alfonso, Guillermo Urriolagoitia-Sosa, Beatriz Romero-Ángeles, Miguel Ángel García-Laguna, Martín Guzmán-Baeza, Jacobo Martínez-Reyes, Yonatan Yael Rojas-Castrejon, Francisco Javier Gallegos-Funes, Julián Patiño-Ortiz, and Guillermo Manuel Urriolagoitia-Calderón. 2023. "Numerical Evaluation Using the Finite Element Method on Frontal Craniocervical Impact Directed at Intervertebral Disc Wear" Applied Sciences 13, no. 21: 11989. https://doi.org/10.3390/app132111989
APA StyleTrejo-Enriquez, A., Urriolagoitia-Sosa, G., Romero-Ángeles, B., García-Laguna, M. Á., Guzmán-Baeza, M., Martínez-Reyes, J., Rojas-Castrejon, Y. Y., Gallegos-Funes, F. J., Patiño-Ortiz, J., & Urriolagoitia-Calderón, G. M. (2023). Numerical Evaluation Using the Finite Element Method on Frontal Craniocervical Impact Directed at Intervertebral Disc Wear. Applied Sciences, 13(21), 11989. https://doi.org/10.3390/app132111989






