Recent Advances in Muconic Acid Extraction Process
Abstract
:1. Introduction
2. Muconic Acid Properties
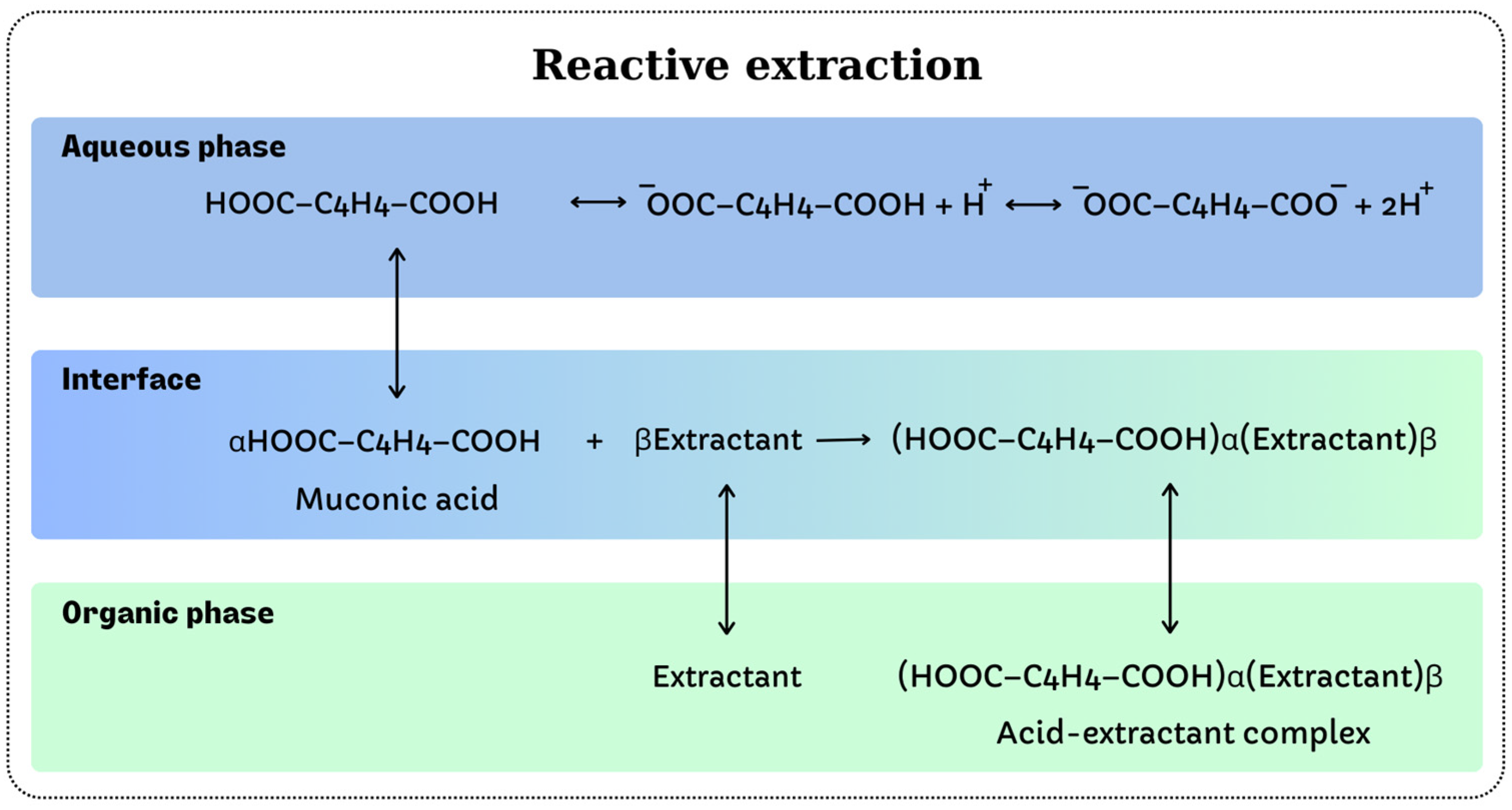
3. Reactive Extraction
- diffusion of the extractant at the separation interface between the two phases;
- diffusion of the solute at the separation interface between the two phases;
- interfacial reaction between solute and extractant;
- diffusion of the acid–extractant complex resulting from the interfacial reaction in the organic phase.
- distribution coefficient;
- separation factor;
- extraction capacity;
- solvent selectivity;
- density;
- surface tension;
- vapor pressure;
- mutual solubility with the mixture to be separated;
- degree of flammability;
- toxicity;
- price.
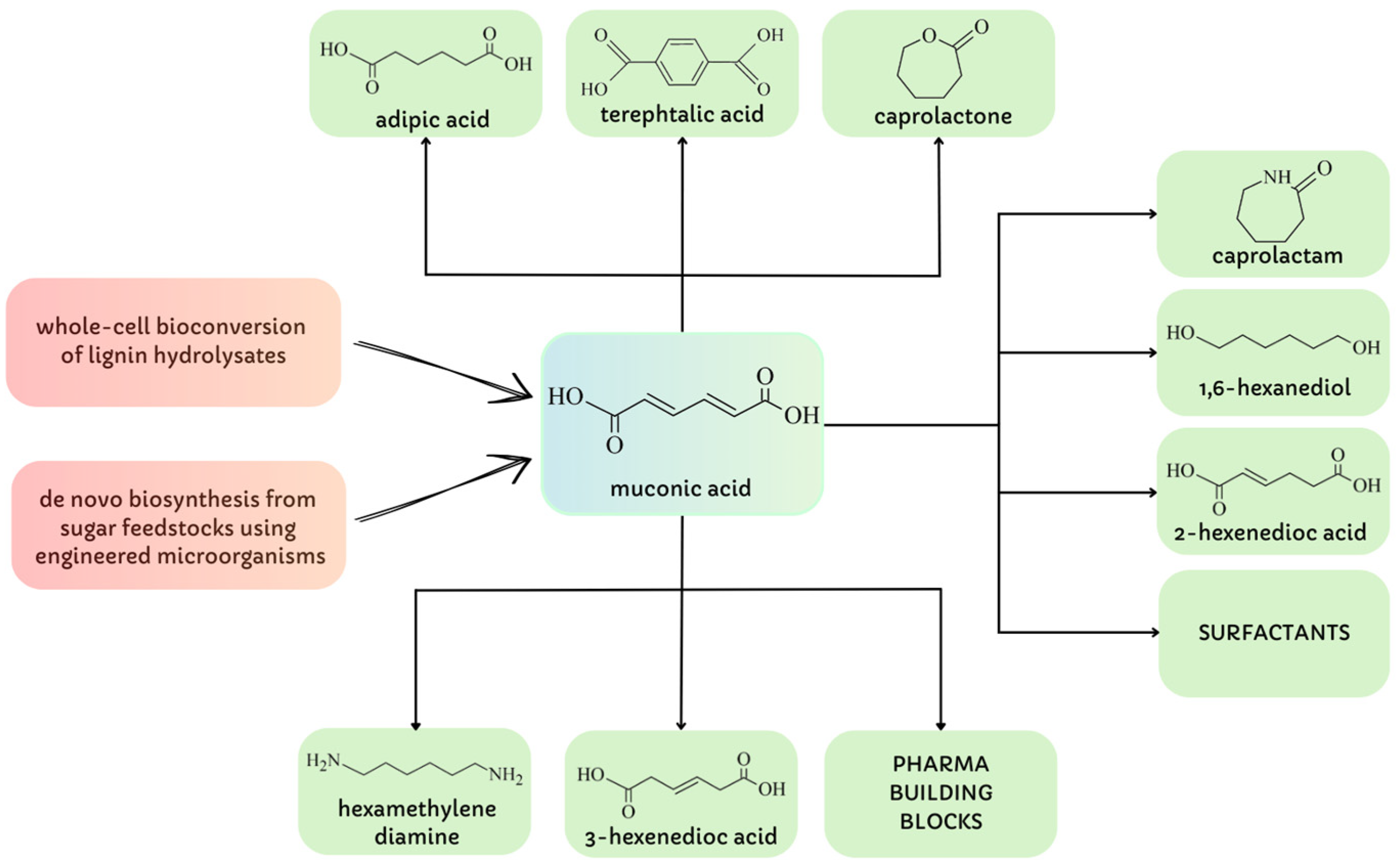
4. Muconic Acid Extraction
- treatment of the fermentation broth with activated carbon to remove color and protocatechuic acid;
- MA precipitation at low pH (2) and low temperature (5 °C);
- spray drying [45].
4.1. Solvent Screening
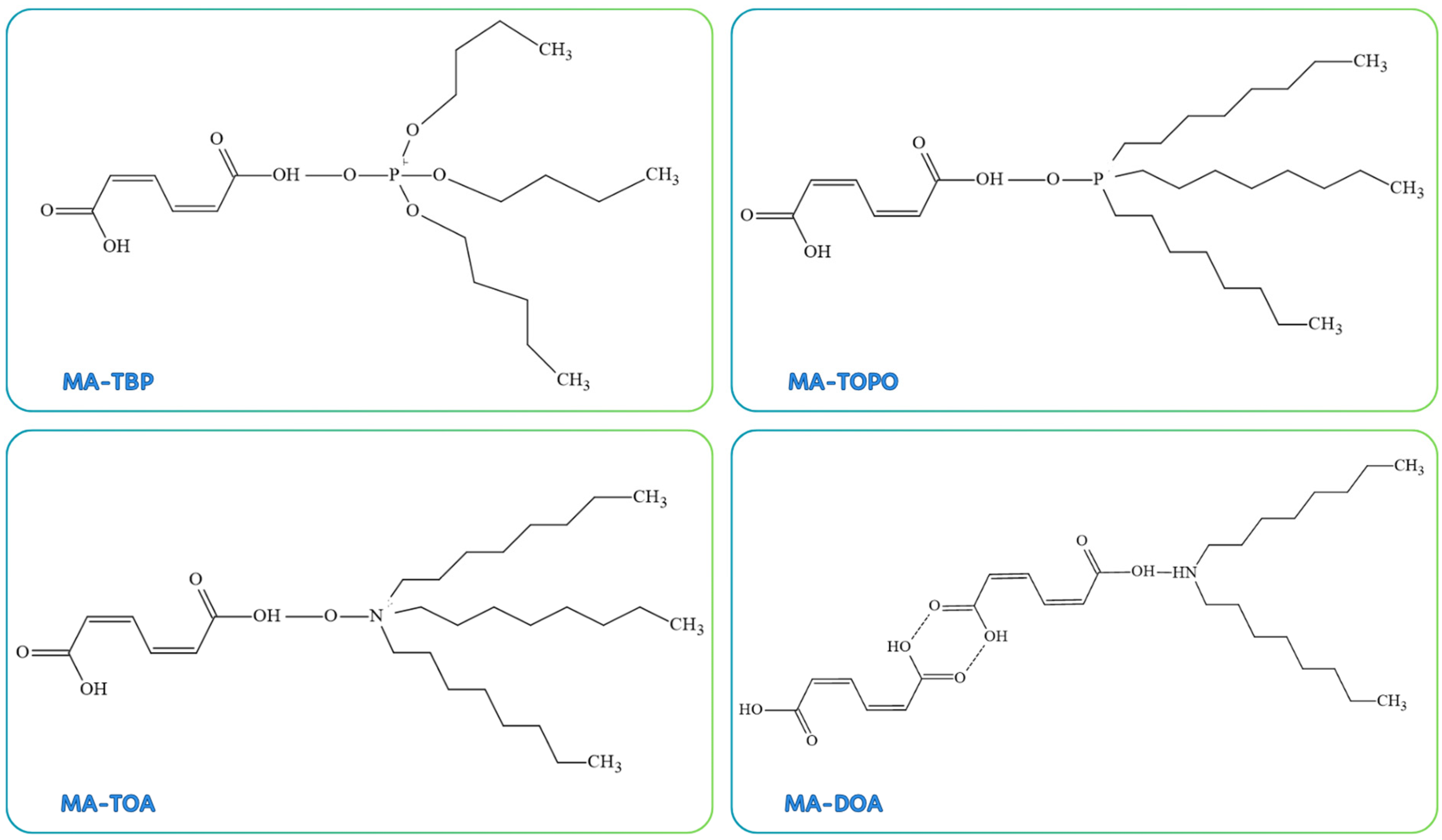
4.2. Reactive Extraction Systems Used for MA Separation
4.2.1. Reactive Extraction Mechanism
- [MA]aq + [E]org→ [MA:E]org,
- [MA]aq + [MA:E]org→ [(MA)2: E]org,
- [MA]aq + [MAn-1:E]org→ [MAn:E]org.
4.2.2. Reactive Extraction Influencing Factors
4.3. Direct Extraction Processes for MA Separation
4.4. Stripping Process for MA Recovery from Organic Phase
- A pH-shift, which implies the use of an aqueous solution (citrate buffer, with a pH interval of 3–7.8) as the re-extraction phase, results in a yield of 99% at a pH higher than 7 due to the dissociated form of MA at pH values higher than its pKa.
- The use of amines that are soluble in water as an extra reactive element (propylamine (M-C3), butylamine (M-C4), hexylamine (M-C6), and tri-n-propylamine (T-C3)). The acid is again extracted into an aqueous phase along with a complex of amines that are water-soluble. The efficiency of different concentrations of amines used for re-extraction was analyzed through the partition coefficient, KD. Using M-C3, after re-extraction, KD yields infinity, indicating the complete recovery of MA from the organic phase at high amine concentration in the organic phase. Additionally, similar results were obtained when using M-C4. These results indicated that the complex stoichiometry is a 2:1 ratio due to the binding of one molecule of M-C3 or M-C4 per MA carboxylic group [59].
- Analyzing these results, the use of water-soluble amines can be considered the best stripping strategy, as it provides 100% re-extraction efficiency.
5. Conclusions and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, J.; Jiang, Y.; Ding, B.; Wang, Y.; Qiu, H.; Dai, S.; Zhao, X.; Hou, Z. Zirconium phosphate supported copper catalyst for selective oxidation of phenol to cis, cis-muconic acid. Appl. Catal. A Gen. 2023, 664, 119351. [Google Scholar] [CrossRef]
- Dehghani, F.; Omidi, F.; Heravizadeh, O.; Yousefinejad, S. Solidified floating organic droplet microextraction coupled with HPLC for rapid determination of trans, trans muconic acid in benzene biomonitoring. Sci. Rep. 2021, 11, 15751. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Liu, P.; Sun, J.; Wu, Y.; Zhang, Y.; Chen, W.; Lin, J.; Wang, Q.; Ma, Y. Engineering catechol 1, 2-dioxygenase by design for improving the performance of thecis, cis-muconic acid synthetic pathway in Escherichia coli. Sci. Rep. 2015, 5, 13435. [Google Scholar] [CrossRef] [PubMed]
- Almqvist, H.; Veras, H.; Li, K.; Garcia Hidalgo, J.; Hulteberg, C.; Gorwa-Grauslund, M.; Skorupa Parachin, N.; Carlquist, M. Muconic acid production using engineered Pseudomonas putida KT2440 and a guaiacol-rich fraction derived from kraft lignin. ACS Sustain. Chem. Eng. 2021, 9, 8097–8106. [Google Scholar] [CrossRef]
- Ling, C.; Peabody, G.L.; Salvachúa, D.; Kim, Y.M.; Kneucker, C.M.; Calvey, C.H.; Monninger, M.A.; Munoz, N.M.; Poirier, B.C.; Ramirez, K.J.; et al. Muconic acid production from glucose and xylose in Pseudomonas putida via evolution and metabolic engineering. Nat. Commun. 2022, 13, 4925. [Google Scholar] [CrossRef]
- Lee, H.N.; Shin, W.S.; Seo, S.Y.; Choi, S.S.; Song, J.S.; Kim, J.Y.; Park, J.H.; Lee, D.; Sang, Y.K.; Lee, S.J.; et al. Corynebacterium Cell Factory Design and Culture Process Optimization for Muconic Acid Biosynthesis. Sci. Rep. 2018, 8, 18041. [Google Scholar] [CrossRef]
- Wang, G.; Øzmerih, S.; Guerreiro, R.; Meireles, A.C.; Carolas, A.; Milne, N.; Jensen, M.K.; Ferreira, B.S.; Borodina, I. Improvement of cis,cis-Muconic Acid Production in Saccharomyces cerevisiae through Biosensor-Aided Genome Engineering. ACS Synth. Biol. 2020, 9, 634–646. [Google Scholar] [CrossRef]
- Kakko, N.; Rantasalo, A.; Koponen, T.; Vidgren, V.; Kannisto, M.; Maiorova, N.; Nygren, H.; Mojzita, D.; Penttilä, M.; Jouhten, P. Inducible Synthetic Growth Regulation Using the ClpXP Proteasome Enhances cis,cis-Muconic Acid and Glycolic Acid Yields in Saccharomyces cerevisiae. ACS Synth. Biol. 2023, 12, 1021–1033. [Google Scholar] [CrossRef]
- Pyne, M.E.; Bagley, J.A.; Narcross, L.; Kevvai, K.; Exley, K.; Davies, M.; Wang, Q.; Whiteway, M.; Martin, V.J.J. Screening non-conventional yeasts for acid tolerance and engineering Pichia occidentalis for production of muconic acid. Nat. Commun. 2023, 14, 5294. [Google Scholar] [CrossRef]
- Choi, S.; Lee, H.N.; Park, E.; Lee, S.J.; Kim, E.S. Recent Advances in Microbial Production of cis,cis-Muconic Acid. Biomolecules 2020, 10, 1238. [Google Scholar] [CrossRef]
- He, S.; Wang, W.; Wang, W.; Hu, H.; Xu, P.; Tang, H. Microbial production of cis,cis-muconic acid from aromatic compounds in engineered Pseudomonas. Syn. Syst. Biotechno. 2023, 8, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Djas, M.; Henczka, M. Reactive extraction of carboxylic acids using organic solvents and supercritical fluids: A review. Sep. Purif. Technol. 2018, 201, 106–119. [Google Scholar] [CrossRef]
- Inyang, V.; Lokhat, D. Reactive Extraction of Malic Acid using Trioctylamine in 1–Decanol: Equilibrium Studies by Response Surface Methodology Using Box Behnken Optimization Technique. Sci. Rep. 2020, 10, 2400. [Google Scholar] [CrossRef]
- Eda, S.; Borra, A.; Parthasarathy, R.; Bankupalli, S.; Bhargava, S.; Thella, P.K. Recovery of levulinic acid by reactive extraction using tri-n-octylamine in methyl isobutyl ketone: Equilibrium and thermodynamic studies and optimization using Taguchi multivariate approach. Sep. Purif. Technol. 2018, 197, 314–324. [Google Scholar] [CrossRef]
- Blaga, A.C.; Dragoi, E.N.; Tucaliuc, A.; Kloetzer, L.; Cascaval, D. Folic Acid Ionic-Liquids-Based Separation: Extraction and Modelling. Molecules 2023, 28, 3339. [Google Scholar] [CrossRef] [PubMed]
- Blaga, A.C.; Dragoi, E.N.; Munteanu, R.E.; Cascaval, D.; Galaction, A.I. Gallic Acid Reactive Extraction with and without 1-Octanol as Phase Modifier: Experimental and Modeling. Fermentation 2022, 8, 633. [Google Scholar] [CrossRef]
- Blaga, A.C.; Tucaliuc, A.; Kloetzer, L. Applications of Ionic Liquids in Carboxylic Acids Separation. Membranes 2022, 12, 771. [Google Scholar] [CrossRef]
- Lazar, R.G.; Blaga, A.C.; Dragoi, E.N.; Galaction, A.I.; Cascaval, D. Mechanism, influencing factors exploration and modelling on the reactive extraction of 2-ketogluconic acid in presence of a phase modifier. Sep. Purif. Technol. 2021, 255, 117740. [Google Scholar] [CrossRef]
- Lazar, R.G.; Blaga, A.C.; Dragoi, E.N.; Galaction, A.I.; Cascaval, D. Application of reactive extraction for the separation of pseudomonic acids: Influencing factors, interfacial mechanism, and process modeling. Can. J. Chem. Eng. 2021, 100, S246–S257. [Google Scholar] [CrossRef]
- Aşçı, Y.S.; Lalikoglu, M. Development of new hydrophobic deep eutectic solvents based on trioctylphosphine oxide for reactive extraction of carboxylic acids. Ind. Eng. Chem. Res. 2021, 60, 1356–1365. [Google Scholar] [CrossRef]
- Kanzaki, R. Deep eutectic solvents for liquid–liquid extraction. Anal. Sci. 2023, 39, 1021–1022. [Google Scholar] [CrossRef]
- Antony, F.M.; Wasewar, K.L. Ionic liquids as green solvents in process industry for reaction and separation: Emphasizing on protocatechuic acid recovery. Chem. Eng. Commun. 2023, 210, 2138–2145. [Google Scholar] [CrossRef]
- Ayan, E.; Baylan, N.; Çehreli, S. Optimization of reactive extraction of propionic acid with ionic liquids using central composite design. Chem. Eng. Res. Des. 2020, 53, 666–676. [Google Scholar] [CrossRef]
- Sprakel, L.M.J.; Schuur, B. Solvent developments for liquid-liquid extraction of carboxylic acids in perspective. Sep. Purif. Technol. 2019, 211, 935–957. [Google Scholar] [CrossRef]
- Esen Marti, M.; Zeidan, H. Using eco-friendly alternatives for the recovery of pyruvic acid by reactive extraction. Sep. Purif. Technol. 2023, 312, 123309. [Google Scholar] [CrossRef]
- Demmelmayer, P.; Steiner, L.; Weber, H.; Kienberger, M. Thymol-menthol-based deep eutectic solvent as a modifier in reactive liquid–liquid extraction of carboxylic acids from pretreated sweet sorghum silage press juice. Sep. Purif. Technol. 2023, 310, 123060. [Google Scholar] [CrossRef]
- Liu, H.; Jin, Y.; Zhang, R.; Ning, Y.; Yu, Y.; Xu, P.; Deng, L.; Wang, F. Recent advances and perspectives on production of value-added organic acids through metabolic engineering. Biotechnol. Adv. 2023, 23, 108076. [Google Scholar] [CrossRef]
- Xie, N.Z.; Liang, H.; Huang, R.B.; Xu, P. Biotechnological production of muconic acid: Current status and future prospects. Biotechnol. Adv. 2014, 32, 615–622. [Google Scholar] [CrossRef]
- Khalil, I.; Quintens, G.; Junker, T.; Dusselier, M. Muconic acid isomers as platform chemicals and monomers in the biobased economy. Green Chem. 2020, 22, 1517–1541. [Google Scholar] [CrossRef]
- Fisher Scientific Company. Cis,Cis-Acid Muconic, MSDS Nr. AC297760000; Fisher Scientific Company: Hampton, NH, USA, 2023. [Google Scholar]
- Dell’Anna, M.N.; Laureano, M.; Bateni, H.; Matthiesen, J.E.; Zaza, L.; Zembrzuski, M.P.; Paskach, T.J.; Tessonnier, J.P. Electrochemical hydrogenation of bioprivileged cis,cis-muconic acid to trans-3-hexenedioic acid: From lab synthesis to bench-scale production and beyond. Green Chem. 2021, 23, 6456–6468. [Google Scholar] [CrossRef]
- Shanks, B.H.; Keelinga, P.L. Bioprivileged molecules: Creating value from biomass. Green Chem. 2017, 19, 3177–3185. [Google Scholar] [CrossRef]
- Liu, P.; Zheng, Y.; Yuan, Y.; Zhang, T.; Li, Q.; Liang, Q.; Su, T.; Qi, Q. Valorization of Polyethylene Terephthalate to Muconic Acid by Engineering Pseudomonas putida. Int. J. Mol. Sci. 2022, 23, 10997. [Google Scholar] [CrossRef] [PubMed]
- Antony, F.M.; Wasewar, K.L. The Sustainable Approach of Process Intensification in Biorefinery Through Reactive Extraction Coupled with Regeneration for Recovery of Protocatechuic Acid. Appl. Biochem. Biotechnol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Qin, H.; Song, Z.; Cheng, H.; Chen, L.; Qi, Z. Toward reactive extraction processes for synthesizing long-chain esters: A general approach by tuning bifunctional deep eutectic solvent. Chem. Eng. J. 2022, 445, 136664. [Google Scholar] [CrossRef]
- Sevindik, Y.E.; Gök, A.; Lalikoglu, M.; Gülgün, S.; Güven, E.Y.; Gürkaş-Aydın, Z.; Yağcı, M.Y.; Turna, Ö.C.; Aydın, M.A.; Aşçı, Y.S. Investigation of the effectiveness of edible oils as solvent in reactive extraction of some hydroxycarboxylic acids and modeling with multiple artificial intelligence models. Biomass Convers. Biorefin. 2023, 13, 13253–13265. [Google Scholar] [CrossRef]
- De, B.S.; Dixit, R.J.; Anand, A.; Dhongde, V.R.; Basu, S. Experimental, equilibrium modelling, and column design for the reactive separation of biomass-derived 2-furoic acid. Can. J. Chem. Eng. 2023, 101, 3167–3179. [Google Scholar] [CrossRef]
- Yıldız, E.; Lalikoglu, M.; Aşçı, Y.S.; Tarım, B.S. Investigation of reactive extraction of monocarboxylic acids with menthol-based hydrophobic deep eutectic solvent by response surface methodology. Sep. Sci. Technol. 2023, 58, 1450–1459. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, L.; Yuan, P.; Xu, X.; Yang, J. Extraction of volatile fatty acids from aqueous solution by in situ formed deep eutectic solvent with methyltrioctylammonium chloride. Biomass Convers. Biorefin. 2023, 15. [Google Scholar] [CrossRef]
- Datta, D.; Kumar, S.; Uslu, H. Status of the Reactive Extraction as a Method of Separation. J. Chem. 2015, 2015, 853789. [Google Scholar] [CrossRef]
- Schügerl, K. Solvent Extraction in Biotechnology: Recovery of Primary and Secondary Metabolites; Springer: Berlin, Germany, 2013. [Google Scholar]
- Bart, H. Reactive Extraction, 1st ed.; Springer: Berlin, Germany, 2001. [Google Scholar]
- Kakku, S.; Gaikwad, S.M.; Gaikwad, S.; Taralkar, S.V.; Billa, S.B.; Chakinala, A.G.; Chakinala, N. Reactive extraction of gluconic acid using trioctylamine in different diluents. Chem. Eng. Technol. 2022, 45, 417–424. [Google Scholar] [CrossRef]
- Yoshikawa, N.; Mizuno, S.; Ohta, K.; Suzuki, M. Microbial production of cis, cis-muconic acid. J. Biotechnol. 1990, 14, 209–210. [Google Scholar] [CrossRef]
- Kohlstedt, M.; Starck, S.; Barton, N.; Stolzenberger, J.; Selzer, M.; Mehlmann, K.; Schneider, R.; Pleissner, D.; Rinkel, J.; Dickschat, J.S.; et al. From lignin to nylon: Cascaded chemical and biochemical conversion using metabolically engineered Pseudomonas putida. Metab. Eng. 2018, 47, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Vardon, D.R.; Franden, M.A.; Johnson, C.W.; Karp, E.M.; Guarnieri, M.T.; Linger, J.G.; Salm, M.J.; Strathmann, T.J.; Beckham, G.T. Adipic acid production from lignin. Energy Environ. Sci. 2015, 8, 617–628. [Google Scholar] [CrossRef]
- Wang, G.; Tavares, A.; Schmitz, S.; França, L.; Almeida, H.; Cavalheiro, J.; Carolas, A.; Øzmerih, S.; Blank, L.M.; Ferreira, B.S.; et al. An integrated yeast-based process for cis,cis-muconic acid production. Biotechnol. Bioeng. 2022, 119, 376–387. [Google Scholar] [CrossRef]
- Traore, M.; Gong, A.J.; Wang, Y.W.; Qiu, L.N.; Bai, Y.Z.; Zhao, W.Y.; Liu, Y.; Chen, Y.; Liu, Y.; Wu, H.L.; et al. Research progress of rare earth separation methods and technologies. J. Rare Earths 2023, 41, 182–189. [Google Scholar] [CrossRef]
- Demir, Ö.; Gök, A.; Uslu, H.; Kırbaşlar, Ş.İ. Reactive extraction of cis,cis-muconic acid from aqueous solution using phosphorus-bonded extractants, tri-n-octylphosphineoxide and tri-n-butyl phosphate: Equilibrium and thermodynamic study. Sep. Purif. Technol. 2021, 272, 118899. [Google Scholar] [CrossRef]
- Nicolaï, T.; Deparis, Q.; Foulquié-Moreno, M.R.; Thevelein, J.M. In-situ muconic acid extraction reveals sugar consumption bottleneck in a xylose-utilizing Saccharomyces cerevisiae strain. Microb. Cell Factories 2021, 20, 114. [Google Scholar] [CrossRef]
- Tönjes, S.; Uitterhaegen, E.; De Brabander, P.; Verhoeven, E.; Delmulle, T.; De Winter, K.; Soetaert, W. In situ product recovery as a powerful tool to improve the fermentative production of muconic acid in Saccharomyces cerevisiae. Biochem. Eng. J. 2023, 190, 108746. [Google Scholar] [CrossRef]
- Gorden, J.; Zeiner, T.; Brandenbusch, C. Reactive extraction of cis,cis-muconic acid. Fluid Phase Equilibria 2015, 393, 78–84. [Google Scholar] [CrossRef]
- Scelfo, S.; Pirone, R.; Russo, N. Thermodynamics of cis,cis-muconic acid solubility in various polar solvents at low temperature range. J. Mol. Liq. 2016, 222, 823–827. [Google Scholar] [CrossRef]
- Eyal, A.M.; Canari, R. Effect of pH on dicarboxylic acids extraction by amine-based extractants. Ind. Eng. Chem. Re.s 1995, 34, 1789–1798. [Google Scholar] [CrossRef]
- Carraher, J.M.; Carter, P.; Rao, R.G.; Forrester, M.J.; Pfennig, T.; Shanks, B.H.; Cochran, E.W.; Tessonnier, J.P. Solvent-driven isomerization of cis,cis-muconic acid for the production of specialty and performance-advantaged cyclic biobased monomers. Green Chem. 2020, 22, 6444–6454. [Google Scholar] [CrossRef]
- Gadekar-Shinde, S.; Kumar, R.B.; Gaikwad, S. Reactive extraction of caproic acid using tri n-octylamine+2 octanol system. Mater. Today Proc. 2023, 72, 260–267. [Google Scholar] [CrossRef]
- Nolte, L.; Nowaczyk, M.; Brandenbusch, C. Monitoring and investigating reactive extraction of (di)carboxylic acids using online FTIR–Part I: Characterization of the complex formed between itaconic acid and tri-n-octylamine. J. Mol. Liq. 2022, 352, 118721. [Google Scholar] [CrossRef]
- Poştaru, M.; Bompa, A.S.; Galaction, A.I.; Blaga, A.C.; Caşcaval, D. Comparative study on pantothenic acid separation by reactive extraction with tri-n-octylamine and di-(2-ethylhexyl) phosphoric acid. Chem. Biochem. Eng. Q. 2016, 30, 81–92. [Google Scholar] [CrossRef]
- Gorden, J.; Zeiner, T.; Sadowski, G.; Brandenbusch, C. Recovery of cis,cis-muconic acid from organic phase after reactive extraction. Sep. Purif. Technol. 2016, 169, 1–8. [Google Scholar] [CrossRef]
- Straathof, A.J.J. The proportion of downstream costs in fermentative production processes. In Comprehensive Biotechnology, 2nd ed.; Moo-Young, M., Ed.; Elsevier: Toronto, ON, Canada, 2011; pp. 811–814. [Google Scholar]
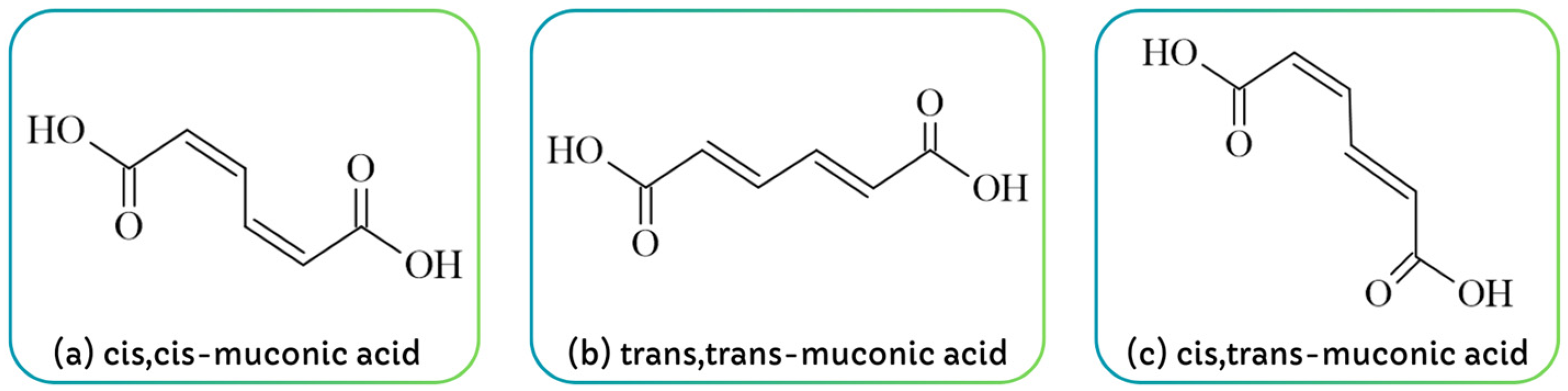

| Property | Details | Ref. |
|---|---|---|
| Molecular mass | 142.11 g/mol | [30] |
| State of aggregation | Solid | [30] |
| Color | Light yellow | [30] |
| Molecular formula | C6H6O4 | [30] |
| Melting point | 194–196 °C (cis,cis-muconic acid) | [30] |
| 191 °C (cis,trans-muconic acid) | [29] | |
| 300 °C (trans, trans-muconic acid) | [29] | |
| Solubility in water | 1 g/L (cis,cis-muconic acid) | [31] |
| 5.2 g/L (cis,trans-muconic acid) | [31] | |
| 0.1 g/L (trans,trans-muconic acid) | [29] | |
| Stability | Stable under normal conditions | [30] |
| Acidity constant, pKA | 2.9 (cis,cis-muconic acid) (pka1) | [29] |
| 3.57 (cis,cis-muconic acid)(pka2) | [29] | |
| 2.9 (cis,trans muconic acid) | [29] | |
| 3,4 (trans,trans-muconic acid) | [29] |
| Organic Phase | Extraction Conditions | K/Yield, % | Ref. |
|---|---|---|---|
| Physical Solvents | |||
| 1-butanol | 5 mL equal volumes at both aqueous and organic phases 120 rpm for two hours in a temperature-controlled water bath shaker initial acid concentration: 0.005–0.007 mol/kg | 6.146 | [49] |
| isoamyl alcohol | 2.846 | [49] | |
| methyl ethyl ketone | 1.180 | [49] | |
| methyl isobutyl ketone | 1.074 | [49] | |
| di-isobutyl ketone | 0.672 | [49] | |
| iso-butanol | 3.948 | [49] | |
| n-hexane | 0.636 | [49] | |
| dimethyl glutarate | 2.951 | [49] | |
| ethyl propionate | 1.941 | [49] | |
| diethyl carbonate | 1.199 | [49] | |
| polypropylene glycol 4000 | aqueous phase pH 4.25 ± 0.05 20% (v/v) solvent shaken vigorously at 30 °C for 30 min | 5.78 | [50] |
| Reactive solvents | |||
| CYTOP 503 | 20% (v/v) solvent shaken at 900 rpm for 30 min at 30 °C aqueous phase pH 4.25 | 37.9 | [51] |
| CYPHOS IL101 | 510 | [51] | |
| CYPHOS IL104 | 399 | [51] | |
| Aliquat 336 | 93.7 | ||
| Tri-octylamine | 3.4, 94.08% | [52] | |
| Tributylphosphate | 6.4 | [51] | |
| Solvent | Extractant | Extraction Conditions | K | Extr. Efficiency, % | Ref. |
|---|---|---|---|---|---|
| 1-butanol | TBP | 5 mL equal volumes at both aqueous and organic phases 120 rpm for two hours in a temperature-controlled water bath shaker Initial acid concentration: 0.0008–0.00215 mol/kg Extractant concentration 0.47–2.06 mol/kg | 6.83 | 87.013 | [49] |
| isoamyl alcohol | TBP | 3.18 | 75.546 | [49] | |
| methyl ethyl ketone | TBP | 2.73 | 70.897 | [49] | |
| methyl isobutyl ketone | TBP | 5.17 | 82.524 | [49] | |
| di-isobutyl ketone | TBP | 3.54 | 74.438 | [49] | |
| Iso-butanol | TBP | 6.49 | 85.979 | [49] | |
| n-hexane | TBP | 5.86 | 83.293 | [49] | |
| dimethyl glutarate | TBP | 6.56 | 86.161 | [49] | |
| ethyl propionate | TBP | 6.32 | 85.214 | [49] | |
| diethyl carbonate | TBP | 5.33 | 83.017 | [49] | |
| 1-butanol | TOPO | 5 mL equal volumes at both aqueous and organic phases 120 rpm for two hours in a temperature-controlled water bath shaker Initial acid concentration: 0.005–0.007 mol/kg Extractant concentration 0.135–0.756 mol/kg | 5.51 | 84.627 | [49] |
| isoamyl alcohol | TOPO | 5.26 | 83.305 | [49] | |
| methyl ethyl ketone | TOPO | 2.82 | 72.887 | [49] | |
| methyl isobutyl ketone | TOPO | 4.83 | 81.929 | [49] | |
| di-isobutyl ketone | TOPO | 4.07 | 78.522 | [49] | |
| iso-butanol | TOPO | 3.93 | 79.629 | [49] | |
| n-hexane | TOPO | 11.26 | 91.351 | [49] | |
| dimethyl glutarate | TOPO | 7.53 | 87.457 | [49] | |
| ethyl propionate | TOPO | 7.78 | 88.126 | [49] | |
| diethyl carbonate | TOPO | 5.49 | 83.795 | [49] | |
| canola oil | CYTOP 503 | 20% (v/v) solvent 900 rpm mixing Time: 30 min T: 30 °C aqueous phase pH: 4.25 Extractant ratio [% v/v]: 25 CYTOP 503; 12.5 CYPHOS IL-101 | 8.70 | - | [51] |
| sunflower FAME | CYTOP 503 | 9.28 | - | [51] | |
| canola oil | CYPHOS IL-101 | 51.33 | - | [51] | |
| sunflower FAME | CYPHOS IL-101 | 27.62 | - | [51] | |
| ethyl oleate | di-n-hexylamine, DHA | Total working volume of 60 mL and a phase ratio of 1:2 aqueous to organic in a 100 mL double-walled glass reactor phase ratio of 1:2 (maq:morg) T = 25 °C 1000 rpm mixing MA concentration 0.035 mol/kg extractant concentration: 0.015 mol/kg | - | 92.02 | [52] |
| ethyl oleate | tri-n-hexylamine, THA | - | 76.07 | [52] | |
| ethyl oleate | tri-n-octylamine, TOA | - | 94.08 | [52] | |
| ethyl oleate | di-n-octylamine, DOA | - | 96.13 | [52] | |
| ethyl oleate + 1-dodecanol | di-n-octylamine, DOA. | phase ratio of 1:2 (maq:morg) T = 25 °C 1000 rpm mixing MA concentration 0.035 mol/kg extractant concentration: 0.1 mol/kg 1-dodecanol weight fraction 0.3 | - | 98.66 | [52] |
| Solvent | Extractant | Extractant Conc., mol/kg | T., °C | K | Extraction Efficiency, % |
|---|---|---|---|---|---|
| Hexane | TOPO | 0.89 | 20 | 14.66 | 93.61 |
| 0.89 | 25 | 16.33 | 94.23 | ||
| 0.71 | 30 | 22.98 | 95.83 | ||
| 0.77 | 35 | 21.39 | 95.53 | ||
| 0.77 | 40 | 18.61 | 94.90 | ||
| Ethyl propionate | TBP | 1.95 | 20 | 2.25 | 88.43 |
| 1.96 | 25 | 3.04 | 89.50 | ||
| 1.95 | 30 | 3.48 | 90.07 | ||
| 2.17 | 35 | 2.89 | 89.73 | ||
| 2.18 | 40 | 2.22 | 89.03 |
| Solvent | Extractant | Extractant Conc., mol/kg | Extraction Efficiency, % | Ref. |
|---|---|---|---|---|
| Ethyl propionate | TOPO | 0.12 | 84.07 | [49] |
| 0.25 | 88.43 | [49] | ||
| 0.39 | 88.98 | [49] | ||
| 0.55 | 91.01 | [49] | ||
| Ethyl propionate | TBP | 0.41 | 79.43 | [49] |
| 0.82 | 82.62 | [49] | ||
| 1.20 | 84.55 | [49] | ||
| 1.57 | 89.08 | [49] | ||
| Ethyl oleate | DOA | 1.98 | 90.37 | [49] |
| 0.01 | 85.14 | [52] | ||
| 0.032 | 96.04 | [52] | ||
| 0.055 | 97.3 | [52] | ||
| Ethyl oleate | TOA | 0.01 | 15.45 | [52] |
| 0.25 | 95.04 | [52] | ||
| 0.5 | 95.81 | [52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blaga, A.C.; Gal, D.G.; Tucaliuc, A. Recent Advances in Muconic Acid Extraction Process. Appl. Sci. 2023, 13, 11691. https://doi.org/10.3390/app132111691
Blaga AC, Gal DG, Tucaliuc A. Recent Advances in Muconic Acid Extraction Process. Applied Sciences. 2023; 13(21):11691. https://doi.org/10.3390/app132111691
Chicago/Turabian StyleBlaga, Alexandra Cristina, Diana Georgiana Gal, and Alexandra Tucaliuc. 2023. "Recent Advances in Muconic Acid Extraction Process" Applied Sciences 13, no. 21: 11691. https://doi.org/10.3390/app132111691
APA StyleBlaga, A. C., Gal, D. G., & Tucaliuc, A. (2023). Recent Advances in Muconic Acid Extraction Process. Applied Sciences, 13(21), 11691. https://doi.org/10.3390/app132111691






