Abstract
Spent portable batteries belong to the category of hazardous waste, sometimes dumped together with non-hazardous municipal waste in landfills, resulting in various aquatic environments. Their presence in the aquatic environment leads to changes in its quality and its contamination with heavy metals or other toxic elements. This paper highlights the portable battery waste’s influence on the aquatic environment in stagnant conditions. Therefore, three types of batteries and three solutions with different pH values were used to represent the possible media existing in nature: acid (pH = 4.00), rainwater (pH = 5.63), and alkaline (pH = 8.00). After 180 days, the results showed changes in the chromatics and composition of the initial solutions. The analyses showed decreased pH, increased conductivity, and the transfer of several heavy metals into solutions (Cu, Pb, Zn, Ni, and Fe). Thus, there were slight exceedances of the maximum allowed values for water quality class I (Order no. 161/2006) in the case of Cu and Pb and higher exceedances in the case of Zn, Ni, and Fe. Zinc–carbon batteries stand out because of the release of Pb and Fe ions. The same applies to lithium manganese dioxide batteries because of Ni ions as well as zinc–manganese alloy batteries because of Cu and Zn ions. Altogether, the negative influence of spent batteries on the aquatic environment is noticed, and the measures for the implementation of safe disposal and processing are necessary.
1. Introduction
In many countries, especially underdeveloped or developing countries, discarded municipal waste may contain hazardous waste like spent portable batteries. Thus, spent portable batteries can reach landfills or various aquatic environments where, under the influence of temperature, precipitation, and the acidity or alkalinity of the storage environment, they can be corroded, releasing heavy metals and other harmful components into the environment, i.e., the aquatic system [1,2,3,4,5,6]. One of the most important hazards associated with landfilling is the release of leachate containing heavy metals and toxic compounds, which could pose a serious environmental impact and a threat to living species [2,7,8,9,10,11,12,13,14,15].
The amount of disposed lithium-ion batteries is estimated to progressively increase, as demonstrated in the last decade, which saw an increase from 10,700 tons in 2012 to 250,000 tons in 2020 [16,17,18].
According to Ordoñez et al. (2016) [14], 4000 tons of used lithium-ion batteries could generate about 1100 tons of heavy metals and more than 200 tons of toxic electrolytes. Thus, the released toxic elements can reach the groundwater, surface water, or the air, endangering the environment and human health [14].
An alkaline portable battery consists of an anode (negative electrode), a cathode (positive electrode), and an electrolyte (a liquid solution through which ions move) [19,20]. Usually, it uses powdered zinc (55–70%) as a negative electrode, manganese dioxide (MnO: 79–85%) with graphite as a positive electrode, and highly conductive potassium hydroxide (KOH: 35–52%) as an electrolyte [19,20,21]. In addition, a battery may have the structure of paper, metal (steel), plastic, etc. [19].
Based on the chemical nature of the active elements in anodes and cathodes, Almeida et al. (2006) [19] classified batteries into seven categories, as follows: alkaline zinc–manganese dioxide, zinc–carbon, mercury oxide, silver oxide, zinc–air, lithium, and nickel–cadmium.
Batteries could be divided into two categories: primary and secondary [22,23]. The primary ones are not rechargeable, which means that they become waste after the end of their lives, but the secondary ones are rechargeable, which allows more cycles of use.
According to Eurostat (2020) [24], out of the 191,000 tons of portable batteries sold in the EU in 2018, close to 48% were collected for recycling. In some countries, used portable batteries are not collected entirely separately, and there is a risk that they will be thrown into municipal waste dumps. Emphasizing the recycling process would mean reducing waste that could endanger the environment [18].
Wasted batteries discharged into the environment are a major source of heavy metals, especially of lead, lithium, nickel, cadmium, and cobalt but also of electrolytes like lithium perchlorate (LiClO4), lithium tetrafluoroborate (LiBF4), and lithium hexafluorophosphate (LiPF6), all of which contribute to the contamination of water and leachate [3,4,5,25,26,27]. Heavy metals seep into the soil or different watercourses after being released and could lead to acidification that can destroy aquatic ecosystems and damage biodiversity [28,29,30]. Some metals like nickel and cobalt and their compounds might be carcinogens, while others like LiCoO2 should be mutagenic [31,32,33].
The presence of portable lithium-ion batteries in saline aquatic environments leads to the release of heavy metals in the liquid environment as a result of the corrosion process of the metal casing and, thus, to an increase in salt content by the release of electrolyte (rich in salts and organic solvents) [1,34,35]. Some researchers claim that the greatest release of heavy metals from spent batteries occurs in low-pH solutions [30], and other researchers claim that this happens more in concentrated saline solutions [1].
To understand the behavior of portable alkaline batteries in aquatic systems, Xara et al. (2009) [36] performed an experiment using a deionized solution and nitric acid solution in which they introduced both whole and cut batteries [36]. After the experiment, it was found that only Hg was significantly dissolved compared to its content in the batteries. [36]. Knowing the structure and composition of used batteries allows a better understanding of the impact they have on environmental factors and human health [19,37]. The presence of batteries in the body of a municipal waste deposit leads to an increase in the concentration of lead, mercury, manganese, and zinc in the leachate, constituting a danger to the quality of underground water [30,37].
Most of the research carried out by researchers around the world has focused on the recovery and recycling of the useful components of used batteries and less on the negative impact they could have on the environment if they are stored with household waste.
The objective of this research is to highlight the influence of spent portable battery waste on the aquatic environment. For this purpose, three types of batteries and three solutions with different pH values were used and were observed experimentally for a period of 180 days to track the loss of battery mass and the transfer of heavy metals, such as Cu, Pb, Zn, Ni, and Fe, into the immersion solution.
2. Materials and Methods
2.1. Preparation of the Experiment
Through this experiment, the transfer conditions of heavy metals (Cu, Pb, Zn, Ni, Fe, Mn, and Cd) from the contents of portable alkaline batteries in the aquatic environment or in the leachate of a municipal deposit were followed.
The batteries used in the experiment were taken from a used battery collection container, batteries from the primary and Non-rechargeable categories. Nine identical batteries from three categories were selected: 1. zinc–carbon battery R6KG AA (A); 2. lithium manganese dioxide battery (B) and zinc–manganese alloy battery (C). No tests were performed on the materials from which the batteries were made. The presentation of their composition was based on the data provided by the technical data sheets related to these types of batteries (Table 1).

Table 1.
Content of the battery [38,39,40].
The batteries were not destroyed; they were only inserted into the stagnation solutions, and before that, they were carefully checked for leaks or deformations. They were immersed in the liquid medium without any mechanical intervention on them.
The 27 batteries were immersed in three solutions with different pHs: acidic solution (pH = 4.00), a synthetic solution with pH similar to that of rainwater (pH = 5.63), and alkaline solution (pH = 8.00) for 180 days, thus simulating three different aqueous environments. The choice of acidity state of solutions pH 4.00, pH 5.63, and pH 8.00 was based on the intention to simulate an acidic and alkaline aquatic environment that could exist in a natural state.
All solutions were obtained using bidistilled water (with pH = 6.80) to simulate an environment free of possible interferences. The acidic solution was obtained by acidifying with nitric acid (HNO3, 65%) to obtain the solution with a pH of 5.63. It was necessary to acidify using all nitric acid (65%), and the basic solution was obtained by adding potassium hydroxide (KOH). All the used chemicals (nitric acid, potassium hydroxide) were of analytical grade (Merck, Darmstadt, Germany) and were used as received without additional purification. A Cyclon (Fistreem International, Cambridge, UK) water purification system was used to produce double distilled water as extraction media for the samples and prepare the standard solutions.
For the experiment, 3 types of batteries in three replicates each were used. Thus, there were 27 pieces of batteries and 27 containers (with a capacity of 370 mL), each one filled with 300 mL solution. To prevent the solution evaporation, each container was closed with a threaded cap. Each type of battery, together with replicates, was stored in three types of solutions (pH = 4.00, pH = 5.63, and pH = 8.00), resulting in 9 containers for each type of battery and a total of 27 containers for the whole experiment (Table 2).

Table 2.
Symbols of containers with batteries and solutions were used in the experiment.
2.2. Solution Analysis
Initially and at the end of the experiment, measurements were made regarding the initial and final weight of the batteries, using an analytical balance, Adventurer type (Ohaus, Parsippany, NJ, USA). At the end of the experiment, the batteries were rinsed with double-distilled water to remove impurities and then brought to a constant mass in the laboratory environment.
After the passage of the 180 days in which the batteries stagnated in the immersion solutions with different pHs, Equation (1) was used to calculate the mass loss of the batteries.
where Ml—mass loss; Mi—mass initial [g]; Mf—mass final [g].
Weekly, during the 180 days of the experiment, the containers were monitored for color variation, and once every 30 days, the pH and conductivity of the solutions in the containers were determined using a WTW pH-meter, type 730 InoLab and a Thermofischer conductometer, type Orion 3D Star, respectively. At the end of the test period, a visual analysis was carried out regarding the degree of corrosion of the batteries.
2.3. Concentration of Heavy Metals
The element concentrations in the extracts were determined using a Perkin Elmer, type AAnalyst 700 atomic absorption spectrometer (Perkin Elmer, Norwalk, CT, USA), with flame and graphite furnace atomizer, with continuum source background correction and specific hollow cathode lamp for each element. The operation parameters, gas flows, wavelengths, and slit widths were selected according to producer instructions (Table 3).

Table 3.
Instrumental parameters applied to AAS for the heavy metal analysis.
Each metallic element was determined according to the European methods of analysis (ISO 15586:2003, ISO 8288:1986) [41,42] and validated in-house in the specific conditions of the laboratory; consequently, fit for purpose is demonstrated. The accuracy of the analysis was checked by analyzing with each series of samples a certified reference material, CRM (TM-24-4 from Environmental and Climate Change Canada, lot 0916). The limits of quantification of metallic elements (Cu = 3.00 µg L−1; Pb = 3.00 µg L−1; Zn = 0.075 mg L−1; Ni = 3.00 µg L−1; Fe = 0.150 mg L−1; Mn = 0.05 mg L−1; Cd = 0.200 µg L−1) were confirmed on samples with the addition of certified reference material, by applying the “3 sigma” principle in a fidelity test by repeatability, with a degree of recovery of 90–110% being accepted. In the analysis of CRM, recovery degrees between 90.0 and 105.1% and a relative standard deviation (RSD) of reproducibility between 2.9 and 8.5% were obtained for all elements. The atomic absorption spectrometry analytical technique was performed on calibration curves, with correlation factor R2 ≥ 0.9986 obtained by diluting mono-element reference solutions with traceability to NIST (Sigma Aldrich, Darmstadt, Germany). The obtained results were interpreted according to the values of Order 161/2006 presented in Table 4 [43].

Table 4.
Classification of metals in quality classes according to Order no. 161/2006 [43].
3. Results
3.1. Solution Analysis
During the stagnation of the batteries in the solutions, superficial corrosion of the case occurred. No deep damage or destruction of the case was observed, regardless of the type of battery used or the immersion solution.
According to the graph in Figure 1, it can be seen that the batteries immersed in the alkaline medium suffered the greatest mass losses: A3 (2.08%), B3 (1.59%), C3 (1.64%), followed by the acid solution and rainwater. The lowest mass losses were observed for batteries immersed in a pH 5.63 solution (rainwater). Here, the lowest loss was for battery C (0.29%).
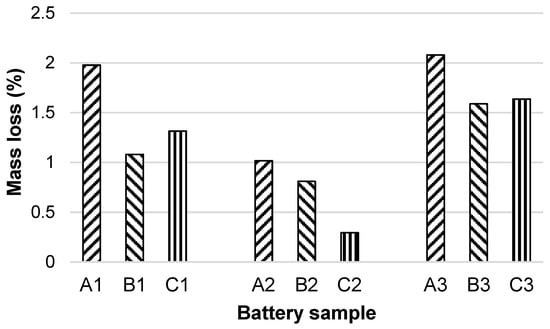
Figure 1.
Percentage mass loss for each battery type after 180 days of solutions contact.
Battery A shows the highest mass loss in the case of all three pHs of the contact solutions. The highest value is recorded for battery A in the basic solution (2.08%), followed by battery A in the acidic solution (1.98%).
3.1.1. Chromatic Changes in Contact Solutions
In the first week of the experiment, a slight yellowish coloration of the solutions was observed, especially in the acidic and alkaline ones. A greater accentuation of this color toward yellow-brown was found in batteries A and B immersed in the solution with pH 4 but also in those in the alkaline solution (pH 8.00), in which case the solution was almost brown. The light brown coloration also occurred for the solutions with pH = 4.00 in the immersed C batteries.
The solutions with pH 4.00 and pH 8.00 started even in the second week to change their aspect into a yellow-light rust color. After 4 weeks, the solution with pH 5.63 started to color, too, into the same chromatic.
In the last week of the experiment, the color of the solutions was more accentuated, especially in the containers with acid and alkaline solutions. Moderate staining was observed for those solutions with a pH of 5.63. The strongest colored solution was the alkaline one with B batteries. Exceptionally, the acidic solutions with immersed B batteries had no more accentuated coloration compared to the first part of the contact period, keeping their brown color.
At the end of the period of battery contact with solutions, the acid and alkaline media were the most reactive with the surface of the immersed batteries, leading to the intense coloration of the solutions. The solution with pH 5.63 was yellow-brown for all three types of batteries, and in the case of C batteries, the color of the solution remained slightly yellowish.
At the end of contact, the batteries showed visible changes in the surface layer, highlighting areas of corrosion, especially on the metal and inscriptions cover.
During the test, this trend intensified the color; thus, at the end of the test, the acidic and alkaline solutions had a rusty color and the solution with pH 5.63 had a light brown aspect. It is possible that this coloring was also influenced by the initial colors on the batteries, which is why it is considered not relevant for the intended purpose.
3.1.2. pH
During the experimentation period, the pH of the solutions was measured every 30 days so that at the end of the 180-day period, the results indicated a slight decrease in pH for all three solutions with the A, B, and C immersed batteries (Figure 2). The most decreased pH was for A batteries immersed in an acid solution.
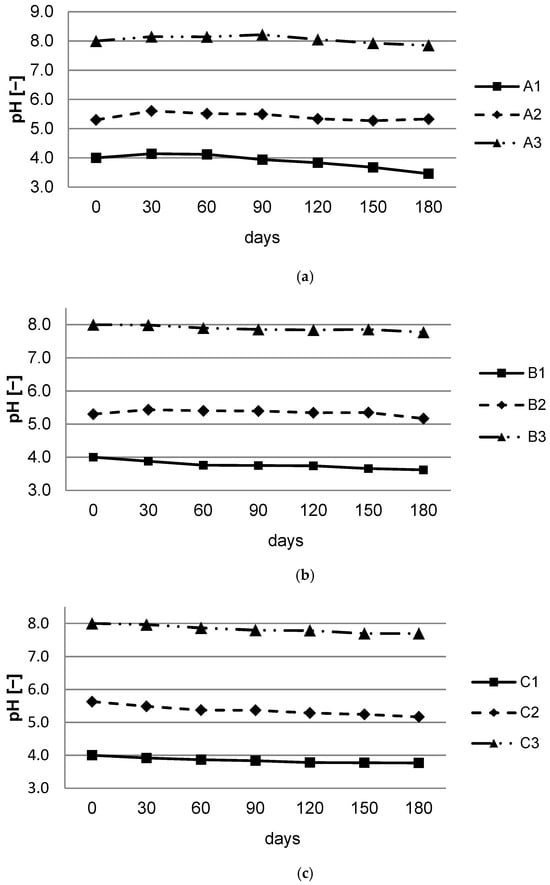
Figure 2.
Average pH dynamics for spent batteries: (a) Battery A; (b) Battery B; (c) Battery C.
During the 180 days, a small variation can be observed in the pH of the immersion solution. In the case of battery A, there is an increase in the first 30 days, followed by a decrease (Figure 2a). At batteries B and C, a decrease in pH could be observed since the beginning of the experiment. This decrease continues until the end of the test period. These pH variations can be observed in all cases, regardless of the type of battery or the solution in which it was immersed.
3.1.3. Conductivity
Figure 3 shows the evolution of conductivity during the 180 days of the experiment, in which an increase can be observed for all nine samples.
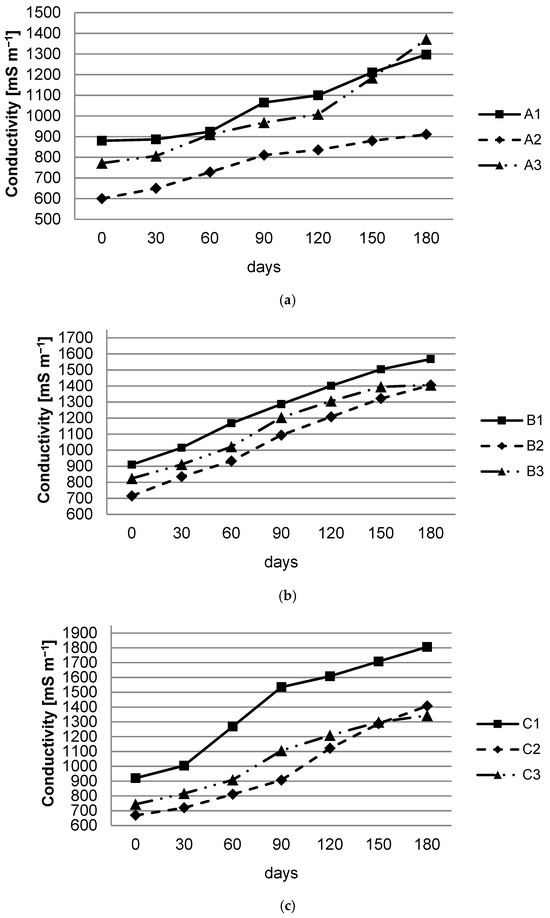
Figure 3.
Conductivity evolution for spent batteries: (a) Battery A; (b) Battery B; (c) Battery C.
The highest increase of conductivity was recorded for battery C1 (from 920 mS m−1 to 1806 mS m−1), followed by battery B1 (from 910 mS m−1 to 1568 mS m−1). The lowest conductivity values were in the case of the A2 battery (from 600 mS m−1 to 911 mS m−1), followed by the C2 battery (from 669 mS m−1 to 1407 mS m−1).
Regardless of the type of battery, the conductivity was higher in the case of the immersion solution with pH 4.00, followed by the solution with pH 8.00, and the lowest value was recorded in the solution with pH 5.63 at the end of the experiment, the lowest conductivity was observed in Type A batteries (911–1371 mS m−1), B batteries having a slightly higher conductivity (1406–1568 mS m−1), while in the case of C batteries, the conductivity increased significantly (1343–1806 mS m−1).
3.2. Concentration of Heavy Metals
In the case of manganese and cadmium ions, their concentrations were below the detection limit of the spectrometer. The exception was in the case of manganese in type A batteries immersed in the solution with pH 5.63 (Mn: 0.31 mg/L) and at pH 8.00 (Mn: 0.69 mg/L).
Therefore, in the case of the solution with pH 5.63 in which the A batteries were immersed, there was an exceeding of the maximum allowed value for the quality class III of surface water (0.3 mg/L) (Order no. 161/2006) [43]. An exceedance was observed for this class and for the solution with pH 8.00 in which batteries A were introduced.
The copper ion concentration values obtained for the three solutions revealed that only for the C batteries, immersed in a solution with pH 8.00, a slight exceeding of the maximum concentration allowed for quality class I (20.00 µg L−1) (Order no. 161/2006) [43] was observed being far below the maximum allowed limit for the other quality classes (Figure 4).
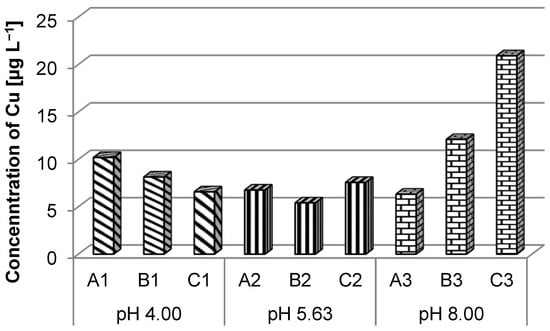
Figure 4.
Copper ions concentration in different pH solutions.
Analyzing the concentration of copper depending on the type of battery and the pH of the solution, it can be observed that the higher the pH of the solution, the lower the concentration of copper, so that at pH = 8.00, the concentration of copper was 6.33 µg L−1, and in the case of battery C, the concentration of Cu decreased with increasing pH.
As seen in Figure 5, battery A recorded high Pb concentrations regardless of the pH value of the solution. The highest concentration was observed in the case of sample A2 (4.09 µg L−1). The concentration of Pb in the solutions in which the type C batteries were immersed decreased with the increase in pH. For batteries A and B, it was not possible to establish whether the pH influences the amount of Pb that was transferred into the solution because there were variations depending on the pH of the immersion solution.
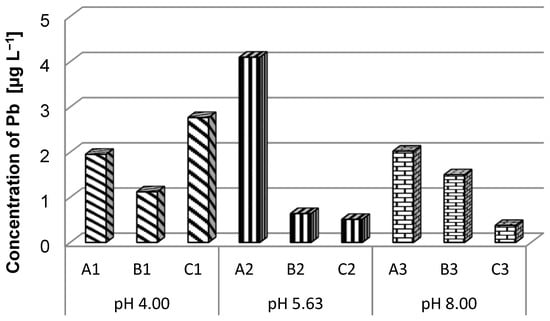
Figure 5.
Lead ion concentration in different pH solutions.
The results of laboratory tests indicated a high concentration of zinc in the case of type C batteries, highlighting a double concentration in the case of solutions with pH = 5.63 compared to solutions with pH = 8 (Figure 6). However, even the highest concentration of 12.05 µg L−1 (sample C2) was significantly lower than the maximum limit provided for class I surface water quality (Order no. 161/2006) [43].
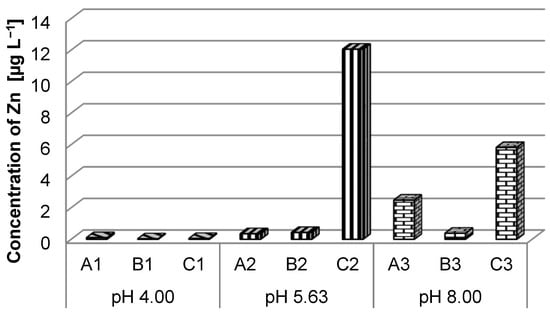
Figure 6.
Zinc ions concentration in different pH solutions.
At pH = 4.00, the concentration of zinc in the solution was insignificant (Figure 6), regardless of the type of battery used in the experiment, but it increased with the pH value, highlighting that battery C has the highest concentrations at pH = 5.63 and pH = 8.00, respectively. A higher concentration is also noticeable in the case of battery A at pH = 8.00 (sample A3 = 2.48 µg L−1) compared to the values obtained at low pH (4.00 and 5.63, respectively). It can be concluded that a higher pH value influences the Zn concentration transferred into the immersion solution.
In the case of nickel ions for the three types of solutions, there were exceedances of the maximum allowed value for the first quality class of surface water (10.00 µg L−1) (Order no. 161/2006) [43]. Thus, slight exceedances were recorded for the solution with pH 8.00 in which batteries A were immersed and the solution with pH 5.63 containing batteries C. There was a higher exceedance in the case of the solution with pH 5.63 in which batteries B were immersed (Figure 7).
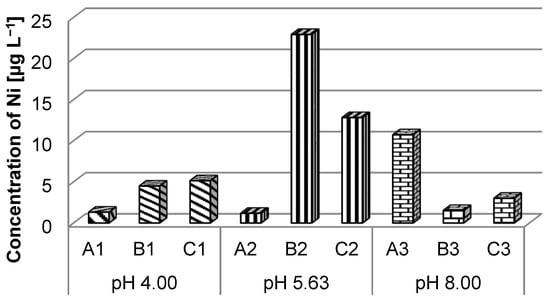
Figure 7.
Nickel ions concentration in different pH solutions.
The highest concentrations of nickel (B2: 22.93 µg L−1 and C2: 12.83 µg L−1) were recorded in the solution with pH = 5.63 (batteries B and C), the exception being battery A (Figure 7), which has higher concentrations elevated at pH = 8.00 (A3: 10.73 µg L−1). Following these results, it can be concluded that at pH = 5.63, the transfer of nickel from the battery to the solution takes place.
The presence of Zn and Ni ions as corrosion products in the lithium-ion battery stagnation solution could possibly be explained by the Zn- and Ni-coated battery steel housing protecting it against external damaging factors [44].
For total iron (Figure 8), the analysis results indicated exceeding the maximum allowed limit (0.3 mg L−1) for quality class I of surface waters for all nine investigated samples. An approximately nine times exceedance of this limit can be observed in the case of sample B2, with a much higher exceedance, over 100 times, in the case of sample A3 (Order no. 161/2006) [43]. Samples B2 and A3 show very high iron concentrations, exceeding the maximum limit allowed for quality class V of surface waters (2 mg L−1). (Order no. 161/2006) [43].
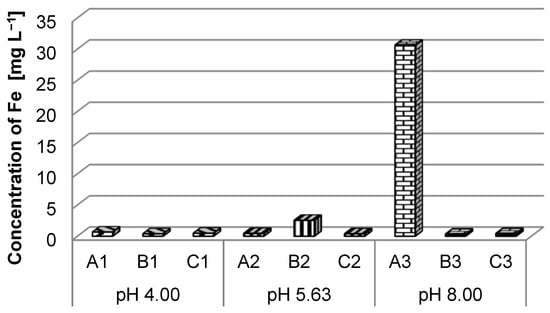
Figure 8.
Iron ion concentration in different pH solutions.
Fe (30.64 mg L−1) exceeded the 5th water quality class (˃2 mg L−1). In the case of iron, there was a huge difference between the maximum concentration and the rest of the iron concentration (most of them were in the second water quality state). Based on observation of the case state at the end of tests and assuming that the case was made of steel according to the technical data sheet (Table 1), there was a hypothesis that the iron ions could be released by superficial corrosion of the battery case.
Comparing the results obtained in this research with the results of another study carried out under the same conditions, for AA batteries that come into contact with various contact/immersion solutions (nitric acid solutions and deionized water, respectively) with a pH = 4.00, the obtained concentration of Pb (1.9 µg L−1) and Cu (10 µg L−1) is considerably higher than the concentration extracted in the study carried out by Xará et al., (2009) [36] (Pb = 0 µg L−1; Cu = 0 µg L−1) and the results obtained by Karnchanawong and Limpiteeprakanb, (2009) (Pb = 0.07 µg L−1) [30], respectively.
In the case of zinc, in the solution with pH = 4.00, values were obtained below the detection limit of the instrument (<0.01 µg L−1), and in the case of using nitric acid, 0.39 µg L−1 and 1.5 µg L−1 were, respectively, obtained when using deionized water [36]. When the batteries were cut/crushed, the zinc concentration increased considerably (560 µg L−1 and 380 µg L−1, respectively [30,36].
Regarding Mn, Cd, and Ni, the results were similar to those obtained by Xará et al. (2009) (Mn = 0 µg L−1 and Cd = 0 µg L−1, Ni = 1.1 µg L−1) [36], and when the batteries were cut/crushed, higher concentrations were obtained in the case of manganese: 53 µg L−1 [30] and 2.1 µg L−1 [36]. This can be explained by the fact that the batteries were cut/crushed; if the stagnation time of the batteries was longer, the metal concentrations would probably have been higher.
The amount of Fe (0.6 µg L−1) obtained in the present study was much lower than that reported by Xará et al. (2009) obtained (2.8 µg L−1 in deionized water and 8 µg L−1 in nitric acid, respectively) [36].
The results of the experiment carried out by Karnchanawong and Limpiteeprakanb (2009) illustrated the fact that at lower pH levels, more metals were leached than at higher pH levels, but the results obtained in this study highlighted the fact that each metal reacted differently [30]. The leaching of metals depends on the pH and the interaction time with the solution. It was observed that the lowest pH continued to decrease, which means that the presence of batteries in the acid environment enhanced acidification [43].
The conductivity trend was to increase in all solutions regardless of the initial pH of the solution. The highest concentration of copper was identified in the alkaline solution for type C batteries, so we can accept that the transfer was closely dependent on the highest copper content available for this type of battery. The lead has been identified in the highest concentration for battery type A in the neutral solubilization medium (of ordinary precipitation), so the ease with which this element solubilizes is caused by the initial concentrations and its chemical bonds; type A batteries probably contain the highest concentrations of lead and combinations thereof.
The zinc has been released mainly in a neutral environment for type C batteries, as well as in a neutral environment, thus proving that from this type of battery, zinc is solubilized from its compounds, in significant proportions, in a neutral environment, but basic.
The nickel was found in the highest concentrations in the neutral and basic medium for type B and C batteries, significantly more for type B compared to type A or C batteries.
The iron concentrations have been identified in the basic solubilization medium for type A batteries.
To express the potential of contamination of the aquatic environment with metals by the three types of batteries, after 180 days of stagnation, the highest and the lowest concentrations of metals released by each type of battery in solution were considered. Thus, the degree of pollution that, under the given conditions, the three categories of batteries can have on the aquatic environment is highlighted. Each type of battery released higher or lower concentrations of metals depending on the pH of the solution in which it stagnated. The highest concentrations of metals released in the aquatic environment belonged to battery A for Pb (4.9 µg L−1, pH 5.63) and Fe (30.64 mg L−1, pH 8.00), battery B for Ni (22.93 µg L−1, pH 5.63), and battery C for Cu (20.92 µg L−1, pH 8.00) and Zn (12.05 µg L−1, pH 5.63).
The stand-out sources of the lowest metal concentrations were as follows: battery B for Cu (5.41 µg L−1, pH 5.63), Zn (0.03 µg L−1, pH 4.00), and Fe (0.34 mg L−1, pH 8.00); battery A for Ni (1.18 µg L−1, pH 5.63); and battery C for Pb (0.38 µg L−1, pH 8.00). Extreme values of the concentrates were noted for identical pHs and the same element but for different types of batteries. Such situations were noted for Fe and Ni, which presented extreme concentrations in an alkaline environment (pH 8.00), and for Ni, which presented extreme concentrations in an environment with pH 5.63. The same is not so for Pb, which showed the highest value in the environment with pH 5.63 and the lowest in the environment with pH 8.00; Zn, which recorded the highest concentration in the environment with pH 5.63 and the lowest in the environment with pH 8.00; and copper, which showed the highest value in the solution with pH 800 and the lowest value at pH 5.63. Therefore, both the pH of the solution and the stagnant battery’s type influence the degree to which metals are released in the solution.
The risks for water quality, aquatic biodiversity, and human health differ depending on the type of battery and the pH of the respective water. Thus, battery A stands out through the release of Pb and Fe ions, battery B through Ni ions, and battery C through Cu and Zn ions.
Considering that certain metals are transferred into the tested solutions, it can be concluded that they can reach the environment and affect the health of organisms and people living in the respective area. In this sense, a selective collection that is as sustainable as possible is necessary, and in the areas where there is already such contamination, remediation of the water and soil affected by this type of pollution is likewise needed.
4. Conclusions
In many countries, and especially in underdeveloped and developing countries, used portable batteries are not rigorously collected selectively. As a result, part of them end up in municipal waste deposits together with residual waste. During their stagnation in these waste deposits, used batteries can come into contact with different liquid environments, either with leachate or with surface waters, where they are subjected to corrosion over time. Under these conditions, these battery wastes’ metals and other dangerous components can be released.
Considering these circumstances, a simulation was made of the conditions in which three types of spent batteries, a zinc–carbon battery (A), lithium manganese dioxide battery (B), and zinc–manganese alloy battery (C), were contacted with synthetic liquid environments of different pHs: an acidic environment (pH 4.00), an environment similar to rainwater (pH 5.63), and an alkaline environment (pH 8.00) These three types of used batteries were immersed in these liquid environments and allowed to stagnate for 180 days. During this time interval, pH and conductivity were measured once every 30 days. During the test period, the pH had a decreasing trend, while the conductivity recorded an upward trend. The pH monitoring showed the possible influence of batteries on the water acidification state over a certain period (180 days).
At the end of the test period (after 180 days), no major damage to the battery case was observed, the corrosion process being superficial for all types of used batteries. The concentrations of heavy metals (Cd, Mn, Cu, Pb, Zn, Ni, and Fe) in the stagnation solution were compared with those regulated for surface waters in Romania by Order 161/2006 to establish the water quality class. Mn and Cd were below the detection limit of the spectrometer, except for battery A in the pH 5.63 solution, where the Mn concentration was 0.31 mg L−1, and the pH 8.00 solution, where the Mn was 0.69 mg/L (quality class IV). To determine the water quality class correlated with each metal, the highest metal concentration was taken into account: Cu: 20.92 µg L−1 (quality class II); Pb: 4.09 µg L−1 (quality class I); Zn: 12.05 µg L−1 (quality class I); Ni: 22.93 µg L−1 (quality class II); and Fe: 30.64 mg L−1 (quality class V). Metal concentrations in aquatic environments are not strictly influenced by the pH of the initial solutions but by the type of used batteries. Battery A stands out through the release of Pb and Fe ions, battery B through Ni ions, and battery C through Cu and Zn ions. Thus, in order to assess the degree of pollution that the presence of used batteries in aquatic environments would have, the highest and lowest concentrations of released metals were taken into account. It was noted that at the same pH, the same element was released into the environment both at the maximum value and at the minimum value, the difference caused by the types of batteries. The maximum and minimum values of the metals were associated with different pHs, with the exception of Fe and Ni, which had maximum and minimum concentrations at the same pH value (Fe in the solution with pH 8.00 and Ni in the solution with pH 5.63).
Technical analyses and tests of the batteries were not carried out so that some results can be interpreted and accepted based on assumptions correlated with technical data for the characterization of the batteries provided in the specific technical sheets. To be able to evaluate the quality of the solutions at the end of the test, the metal concentrations in the solutions were compared with the maximum allowed values provided in the National Normative Act for surface waters (Order no. 161/2006). The maximum concentrations of the elements in the solution gave a certain degree of quality to the respective aquatic environment.
The heavy metals in aquatic environments pose a threat to the quality of environmental factors and human health. As has been demonstrated, used batteries thrown into the environment can be a source of water pollution with heavy metals. In order to prevent such a negative outcome, it is very important to carry out adequate management of battery waste by rigorously collecting it at the source and treating and recycling it.
It was concluded that the pH of the stagnation solution, the type of battery, and the nature of the metal influenced the mechanism of the metal leaching process in the aquatic environment. Considering this, additional research is necessary in the future.
Author Contributions
Conceptualization, I.S., A.M., I.M.S. and V.M.; methodology, I.S. and I.M.S.; investigation, I.S. and A.M.; validation, I.S., A.M., V.M. and I.M.S.; resources, I.S., A.M. and V.M.; data curation, I.S., V.M. and I.M.S.; writing—original draft preparation, I.S. and I.M.S.; writing—review and editing, I.S., A.M., V.M. and I.M.S.; visualization, I.S., I.M.S. and V.M.; supervision, I.S., V.M. and I.M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Xiao, J.; Guo, J.; Zhan, L.; Xu, Z. A cleaner approach to the discharge process of spent lithium ion batteries in different solutions. J. Clean. Prod. 2020, 255, 120064. [Google Scholar] [CrossRef]
- Kanmani, S.; Gandhimathi, R. Assessment of heavy metal contamination in soil due to leachate migration from an open dumping site. Appl. Water Sci. 2013, 3, 193–205. [Google Scholar] [CrossRef]
- Bhat, R.A.; Singh, D.V.; Qadri, H.; Dar, G.H.; Dervash, M.A.; Bhat, S.A.; Unal, B.T.; Ozturk, M.; Hakeem, K.R.; Yousaf, B. Vulnerability of municipal solid waste: An emerging threat to aquatic ecosystems. Chemosphere 2022, 287, 132223. [Google Scholar] [CrossRef] [PubMed]
- Melchor-Martínez, E.M.; Macias-Garbett, R.; Malacara-Becerra, A.; Iqbal, H.M.N.; Sosa-Hernandez, J.E.; Parra-Saldívar, R. Environmental impact of emerging contaminants from battery waste: A mini review. Case Stud. Chem. Environ. Eng. 2021, 3, 100104. [Google Scholar] [CrossRef]
- Sethurajan, M.; Gaydardzhiev, S. Bioprocessing of spent lithium ion batteries for critical metals recovery—A review. Resour. Conserv. Recycl. 2021, 165, 105225. [Google Scholar] [CrossRef]
- Ishak, A.R.; Mohamad, S.; Soo, T.K.; Hamid, F.S. Leachate and Surface Water Characterization and Heavy Metal Health Risk on Cockles in Kuala Selangor. Procedia Soc. Behav. Sci. 2016, 222, 263–271. [Google Scholar] [CrossRef]
- Beinabaj, S.M.H.; Heydariyan, H.; Aleii, H.M.; Hosseinzadeh, A. Concentration of heavy metals in leachate, soil, and plants in Tehran’s landfill: Investigation of the effect of landfill age on the intensity of pollution. Heliyon 2023, 9, e13017. [Google Scholar] [CrossRef]
- Agbeshie, A.A.; Adjei, R.; Anokye, J.; Banunle, A. Municipal waste dumpsite: Impact on soil properties and heavy metal concentrations, Sunyani, Ghana. Sci. Afr. 2020, 8, e00390. [Google Scholar] [CrossRef]
- Guo, X.; Song, Y.; Nan, J. Flow evaluation of the leaching hazardous materials from spent nickel-cadmium batteries discarded in different water surroundings. Environ. Sci. Pollut. Res. 2018, 25, 5514–5520. [Google Scholar] [CrossRef]
- Grover, S.; Sibi, G. Metal Leachate from Alkaline Battery Litters: A threat to Aquatic Organisms. Int. J. Microbiol. Curr. Res. 2019, 1, 26–28. [Google Scholar] [CrossRef]
- Mrozik, W.; Rajaeifar, M.A.; Heidrich, O.; Christensen, P. Environmental impacts, pollution sources and pathways of spent lithium-ion batteries. Energy Environ. Sci. 2021, 14, 6099–6612. [Google Scholar] [CrossRef]
- Rajesh, R.; Kanakadhurga, D.; Prabaharan, N. Electronic waste: A critical assessment on the unimaginable growing pollutant, legislations and environmental impacts. Environ. Chall. 2022, 7, 100507. [Google Scholar] [CrossRef]
- Borjac, L.; El Joumaa, M.; Kawach, R.; Youssef, L.; Blake, D.A. Heavy metals and organic compounds contamination in leachates collected from Deir Kanoun Ras El Ain dump and its adjacent canal in South Lebanon. Heliyon 2019, 5, e02212. [Google Scholar] [CrossRef] [PubMed]
- Ordoñez, J.; Gago, E.J.; Girard, A. Processes and technologies for the recycling and recovery of spent lithium-ion batteries. Renew. Sustain. Energy Rev. 2016, 60, 195–205. [Google Scholar] [CrossRef]
- Lee, J.-C.; Pandey, B.D. Bio-processing of solid wastes and secondary resources for metal extraction—A review. Waste Manag. 2012, 32, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.; Kim, Y. Technologies of lithium recycling from waste lithium ion batteries: A review. Mater. Adv. 2021, 2, 3234–3250. [Google Scholar] [CrossRef]
- Thompson, D.L.; Hartley, J.M.; Lambert, S.M.; Shiref, M.; Harper, G.D.J.; Kendrick, E.; Anderson, P.; Ryder, K.S.; Gaines, L.; Abbott, A.P. The importance of design in lithium ion battery recycling—A critical review. Green Chem. 2020, 22, 7585–7603. [Google Scholar] [CrossRef]
- Dobó, Z.; Dinh, T.; Kulcsár, T. A review on recycling of spent lithium-ion batteries. Energy Rep. 2023, 9, 6362–6395. [Google Scholar] [CrossRef]
- Almeida, M.F.; Xara, S.M.; Delgado, J.; Costa, C.A. Characterization of spent AA household alkaline batteries. Waste Manag. 2006, 26, 466–476. [Google Scholar] [CrossRef]
- He, K.; Zhang, Z.Y.; Alai, L.; Zhang, F.S. A green process for exfoliating electrode materials and simultaneously extracting electrolyte from spent lithium-ion batteries. J. Hazard. Mater. 2019, 375, 43–51. [Google Scholar] [CrossRef]
- Hurd, J.D.; Muchnick, D.M.; Schedler, M.F.; Mele, T. Recycling of Consumer Dry Cell Batteries (Pollution Technology Review), 1st ed.; William Andrew: Norwich, NY, USA, 1993; p. 288. [Google Scholar]
- Kosaraju, S. A Review of the Importance of Recycling Lithium-ion Batteries for Lithium, in View of Impending Electric Vehicle Industry. Industrial Materials Recycling. 2012. SE-412. Available online: https://publications.lib.chalmers.se/records/fulltext/165368.pdf (accessed on 12 August 2023).
- Sethurajan, M.; Van Hullebusch, E.D.; Fontana, D.; Akcil, A.; Deveci, H.; Batinic, B.; Leal, J.P.; Gasche, T.A.; Ali Kucuker, M.; Kuchta, K.; et al. Recent advances on hydrometallurgical recovery of critical and precious elements from end of life electronic wastes—A review. Critic. Rev. Environ. Sci. Technol. 2019, 49, 212–275. [Google Scholar] [CrossRef]
- Eurostat Statistics Explained. Waste Statistics-Recycling of Batteries and Accumulators. 2020. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Waste_statistics_-_recycling_of_batteries_and_accumulators (accessed on 12 August 2023).
- Xu, J.; Thomas, H.R.; Francis, R.W.; Lum, K.R.; Wang, J.; Liang, B. A review of processes and technologies for the recycling of lithium-ion secondary batteries. J. Power Sources 2008, 177, 512–527. [Google Scholar] [CrossRef]
- Kang, D.H.P.; Chen, M.; Ogunseitan, O.A. Potential environmental and human health impacts of rechargeable lithium batteries in electronic waste. Environ. Sci. Technol. 2013, 47, 5495–5503. [Google Scholar] [CrossRef] [PubMed]
- Horeh, N.B.; Mousavi, S.M.; Baniasadi, M. Use of adapted metal tolerant Aspergillus niger to enhance bioleaching efficiency of valuable metals from spent lithium-ion mobile phone batteries. J. Clean. Prod. 2018, 197, 1546–1557. [Google Scholar] [CrossRef]
- Twagirayezu, G.; Irumva, O.; Huang, K.; Xia, H.; Uwimana, A.; Nizeyimana, J.C.; Manzi, H.P.; Nambajemariya, F.; Itangishaka, A.C. Environmental Effects of Electrical and Electronic Waste on Water and Soil: A Review. Pol. J. Environ. Stud. 2022, 31, 2507–2525. [Google Scholar] [CrossRef]
- Kuchhal, P.; Sharma, U.C. Battery waste management. In Environmental Science and Engineering; Vol. 5 Solid Waste Management; Gurjar, B.R., Sharma, U.C., Singh, N., Eds.; Studium Press LLC: Houston, TX, USA, 2017; pp. 141–155. [Google Scholar]
- Karnchanawong, S.; Limpiteeprakanb, P. Evaluation of heavy metal leaching from spent household batteries disposed in municipal solid waste. Waste Manag. 2009, 29, 550–558. [Google Scholar] [CrossRef]
- Shekhar, A.R.; Parekh, M.H.; Pol, V.G. Worldwide ubiquitous utilization of lithium-ion batteries: What we have done, are doing, and could do safely once they are dead? J. Power Sources 2022, 523, 231015. [Google Scholar] [CrossRef]
- Vimmerstedt, L.J.; Ring, S.; Hammel, C.J. Current Status of Environmental, Health, and Safety Issues of Lithium Ion Electric Vehicle Batteries. 1995; NREL/TP-463-7673, UC Category 1501, DE95009295. Available online: https://www.nrel.gov/docs/legosti/old/7673.pdf (accessed on 12 August 2023).
- Casimir, A.; Zhang, H.; Ogoke, O.; Amine, J.C.; Lu, J.; Wu, G. Silicon-based anodes for lithium-ion batteries: Effectiveness of materials synthesis and electrode preparation. Nano Energy 2016, 27, 359–376. [Google Scholar] [CrossRef]
- Zhang, G.; He, Y.; Feng, Y.; Wang, H.; Zhang, T.; Xie, W.; Zhu, X. Enhancement in liberation of electrode materials derived from spent lithium-ion battery by pyrolysis. J. Clean. Prod. 2018, 199, 62–68. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, X.-Y.; Yan, F.-Y. Tribocorrosion behaviour of type S31254 steel in seawater: Identification of corrosion–wear components and effect of potential. Mater. Chem. Phys. 2016, 179, 273–281. [Google Scholar] [CrossRef]
- Xará, S.M.; Delgado, J.N.; Almeida, M.F.; Costa, C.A. Laboratory study on the leaching potential of spent alkaline batteries. Waste Manag. 2009, 29, 2121–2131. [Google Scholar] [CrossRef] [PubMed]
- Maiti, S.K.; De, S.; Hazra, T.; Debsarkar, A.; Dutta, A. Characterization of Leachate and Its Impact on Surface and Groundwater Quality of a Closed Dumpsite—A Case Study at Dhapa, Kolkata, India. Procedia Environ. Sci. 2016, 35, 391–399. [Google Scholar] [CrossRef]
- Toshiba Batteries (TB). Technical Characteristics, Heavy Duty AA BP-4. 2021. Available online: https://www.toshiba-lifestyle.com/jp/living/batteries/files/carbon-zinc_9V_en.pdf (accessed on 12 August 2023).
- Duracell. Product Safety Data Sheet (PSDS). 2012. Available online: http://sds.staples.com/msds/818693.pdf (accessed on 12 August 2023).
- Product Information Sheet (PIS). Panasonic Batteries, Panasonic Industrial Company. 2020. Available online: https://assets.omron.eu/downloads/msds/en/v1/panasonic_cr-series_button_cells_msds_document_en.pdf (accessed on 12 August 2023).
- ISO 15586:2003; Water Quality—Determination of Trace Elements Using Atomic Absorption Spectrometry with Graphite Furnace. International Organization for Standardization: Geneva, Switzerland, 2003.
- ISO 8288:1986; Water Quality—Determination of Cobalt, Nickel, Copper, Zinc, Cadmium and Lead—Flame Atomic Absorption Spectrometric Methods. International Organization for Standardization: Geneva, Switzerland, 1986.
- ORDER no. 161 from October 13, 2016 for the Approval of the Norm Regarding the Classification of Surface Water Quality in Order to Establish the Ecological Status of Water Bodies. Available online: http://www.monitoruljuridic.ro/act/ordin-nr-161-din-13-octombrie-2016-privind-aprobarea-planului-integrat-de-ac-iune-pentru-gestionarea-unei-situa-ii-de-criz-n-domeniul-imigra-iei-emitent-185101.html (accessed on 10 August 2023). (In Romanian).
- Takahashi, T.; Ishizuka, K.; Kawanishi, K. Properties of Nickel-Coated Steel Sheets for Battery Case, Nippon Steel & Sumitomo Metal Technical Report, 2015 No. 108, UDC 669. 14-408. 2:669. 248. 7. Available online: https://www.nipponsteel.com/en/tech/report/nssmc/pdf/108-16.pdf (accessed on 15 October 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).