Lead Tolerance and Enrichment Characteristics of Three Hydroponically Grown Ornamental Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Material
2.2. Material Cultivation and Experimental Design
2.3. Experimental Methods
2.4. Measurement Items and Methods
2.5. Statistical Analysis
3. Results and Discussion
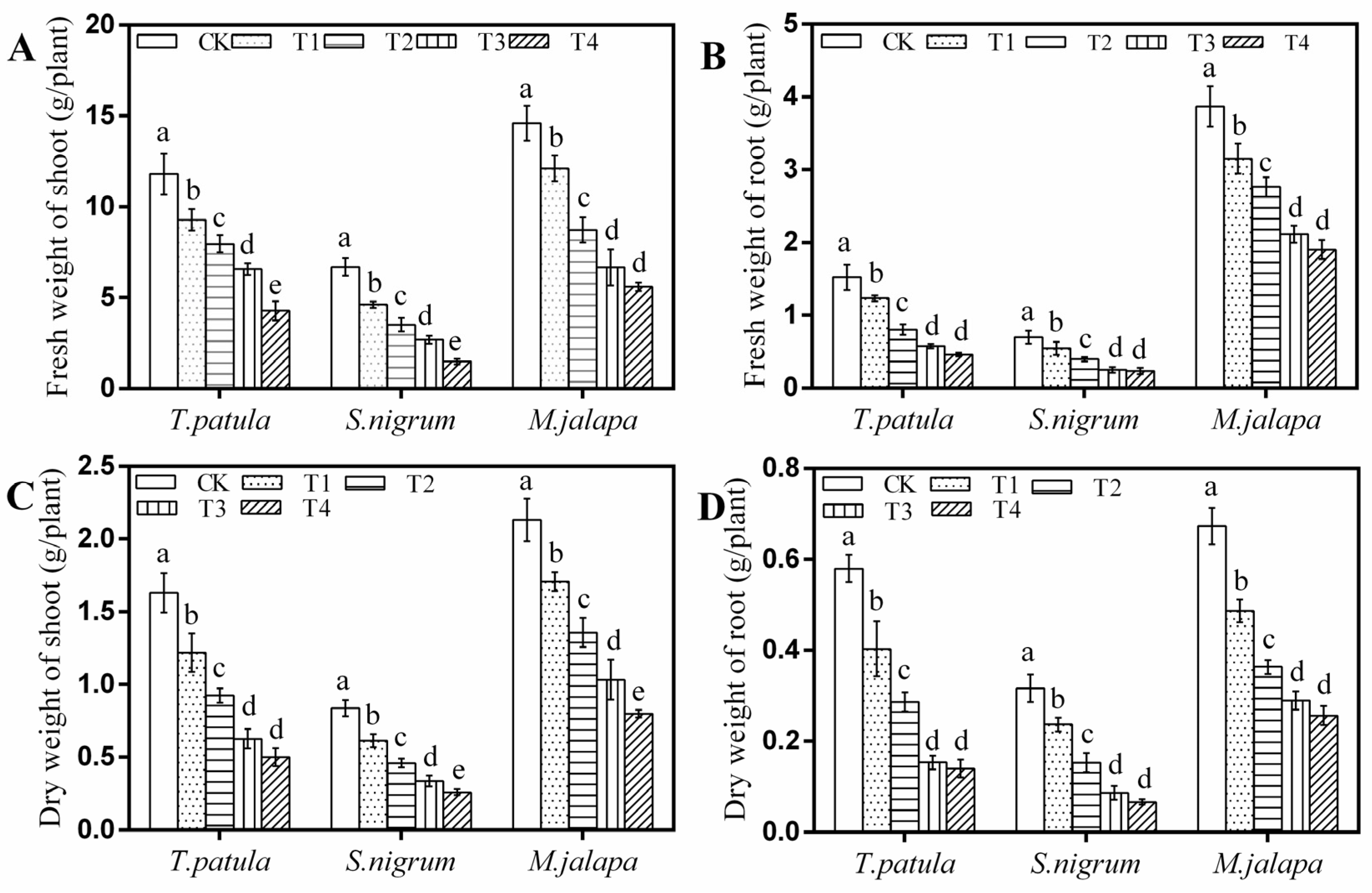
3.1. Effects of Pb Stress on the Biomass of Ornamental Plants
3.2. Effects of Pb Stress on the Tolerance Index of Ornamental Plants
3.3. Effects of Pb Stress on the Pb Content of Ornamental Plants
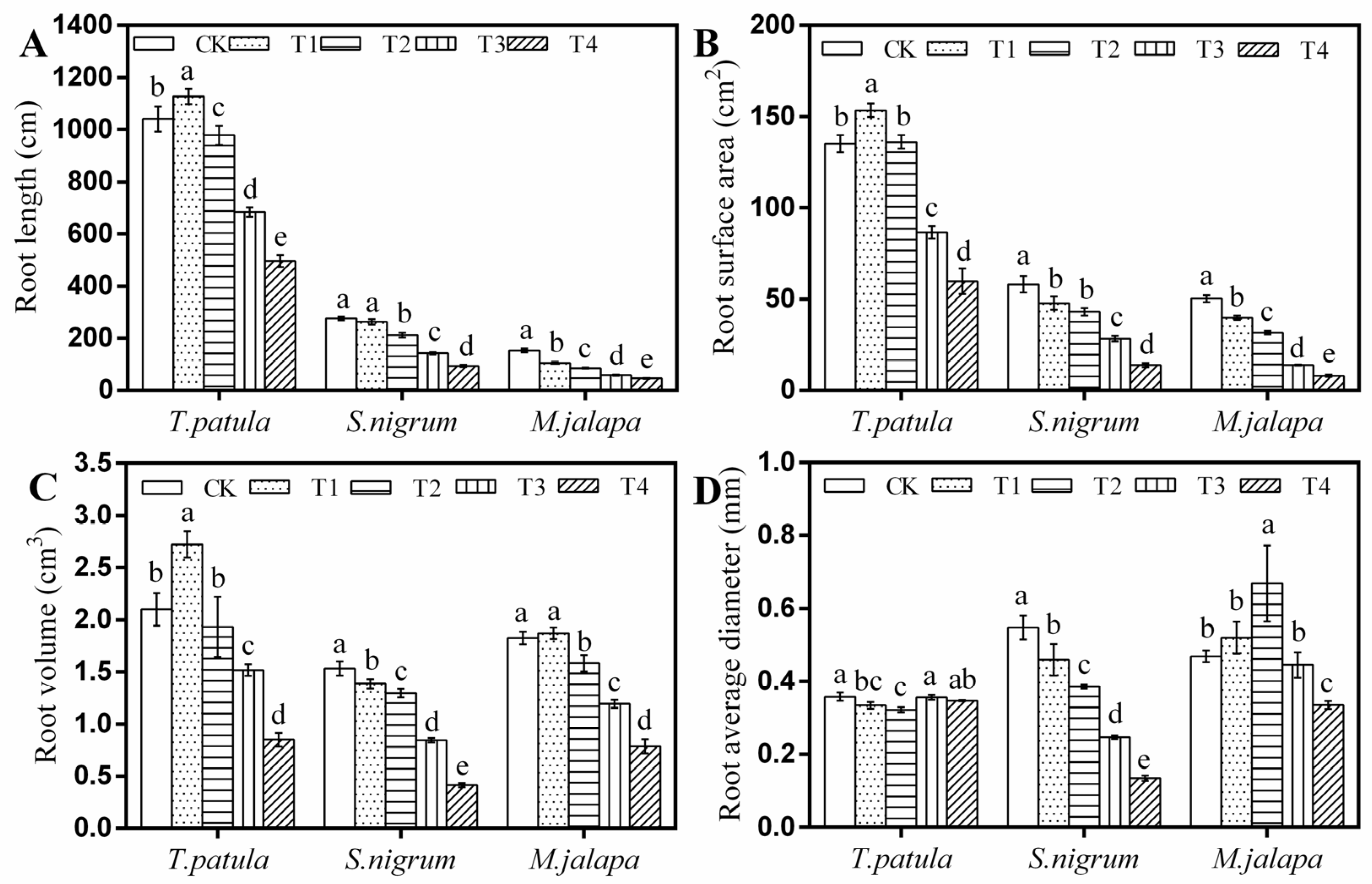
3.4. Effects of Pb Stress on the Root Architecture of Ornamental Plants
3.5. Effects of Pb Stress on the Enrichment Coefficient and Transfer Factor of Ornamental Plants
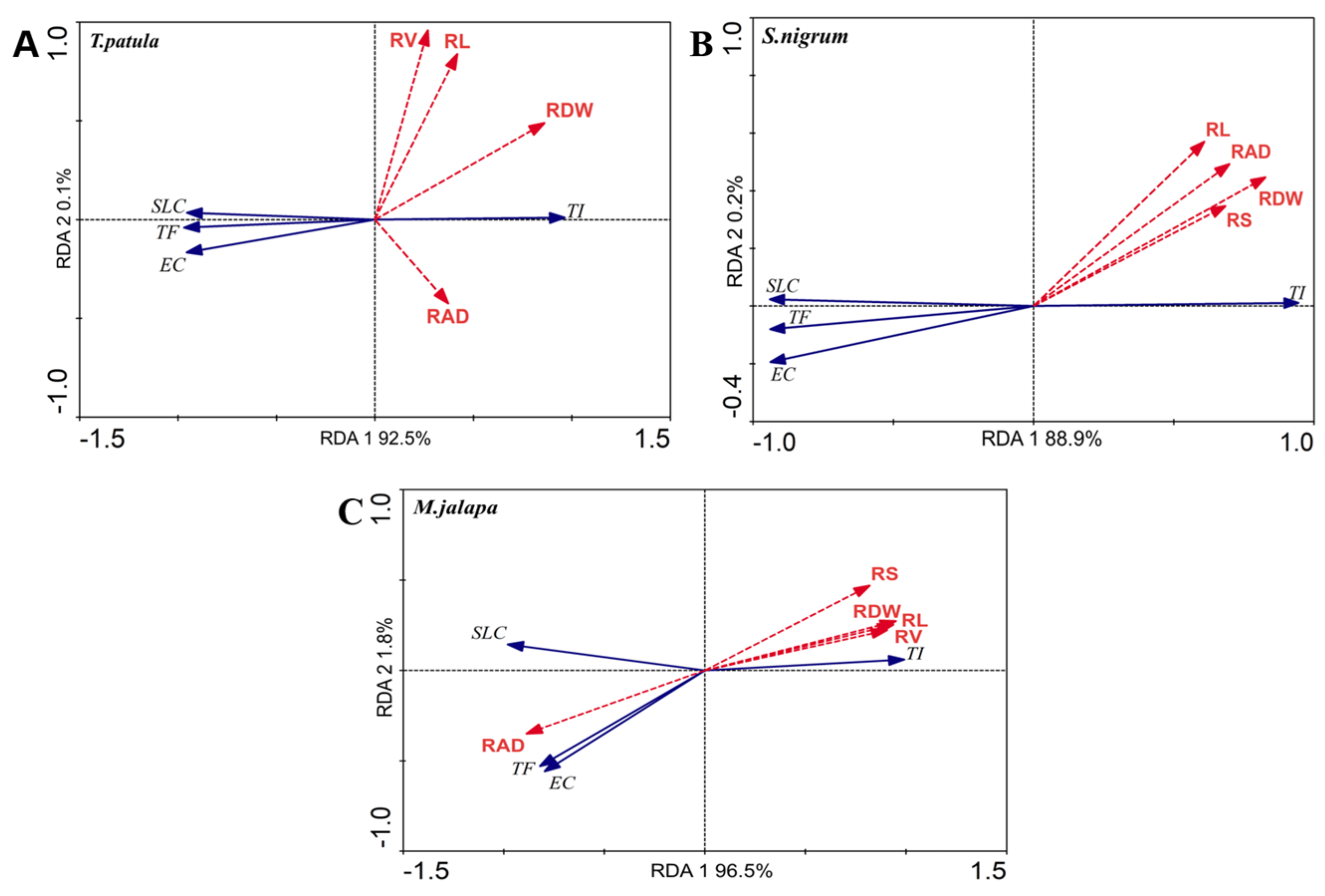
3.6. Relationship between Root Morphological Indices and Tolerance, Enrichment, and Transfer Coefficients of Tested Ornamental Plants
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X.; Wang, Y.; Mahmood, Q.; Islam, E.; Jin, X.; Li, T.; Yang, X.; Liu, D. The effect of EDDS addition on the phytoextraction efficiency from Pb contaminated soil by Sedum alfredii Hance. J. Hazard. Mater. 2009, 168, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Mosa, K.A.; Saadoun, I.; Kumar, K.; Helmy, M.; Dhankher, O.P. Potential Biotechnological Strategies for the Cleanup of Heavy Metals and Metalloids. Front. Plant Sci. 2016, 7, 303. [Google Scholar] [CrossRef]
- Aghelan, N.; Sobhanardakani, S.; Cheraghi, M.; Lorestani, B.; Merrikhpour, H. Evaluation of some chelating agents on phytore mediation efficiency of Amaranthus caudatus L. and Tagetes patula L. in soils contaminated with lead. J. Environ. Ment. Health Sci. Eng. 2021, 19, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Mahar, A.; Wang, P.; Ali, A.; Awasthi, M.K.; Lahori, A.H.; Wang, Q.; Li, R.H.; Zhang, Z.Q. Challenges and opportunities in the phytoremediation of heavy metals contaminated soils: A review. Ecotoxicol. Environ. Saf. 2016, 126, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.Q.; Lu, W.L.; Nasar, J.; Zhang, J.; Yan, L. Growth responses and accumulation characteristics of three ornamentals under copper and lead contamination in a hydroponic-culture experiment. Bull. Environ. Contam. Toxicol. 2019, 103, 854–859. [Google Scholar] [CrossRef]
- Asgari, L.B.; Khadem, M.N.; Maghsoodi, M.R.; Ghorbanpour, M.; Kariman, K. Phytoextraction of heavy metals from contaminated soil, water and atmosphere using ornamental plants: Mechanisms and efficiency improvement strategies. Environ. Sci. Pollut. Res. 2019, 26, 8468–8484. [Google Scholar] [CrossRef]
- Saxena, G.; Purchase, D.; Mulla, S.I.; Saratale, G.D.; Bharagava, R.N. Phytoremediation of heavy metal-contaminated sites: Eco-environmental concerns, field studies, sustainability issues, and future prospects. Rev. Environ. Contam. Toxicol. 2019, 249, 71–131. [Google Scholar]
- Anten, N.P.R.; Chen, B.J.W. Detect thy family: Mechanisms, ecology and agricultural aspects of kin recognition in plants. Plant Cell Environ. 2021, 44, 1059–1071. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, C.; Ji, X.; Yun, C.; Wang, M.; Luo, X. The knowledge domain and emerging trends in phytoremediation: A scientometric analysis with CiteSpace. Environ. Sci. Pollut. Res. 2020, 27, 15515–15536. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.M.; Whipps, J.M. Substrate flow in the rhizosphere. Plant Soil 1990, 129, 1–10. [Google Scholar] [CrossRef]
- Fahr, M.; Laplaze, L.; Bendaou, N.; Hocher, V.; Mzibri, M.E.; Bogusz, D.; Smouni, A. Effect of lead on root growth. Front. Plant Sci. 2013, 4, 175. [Google Scholar] [CrossRef]
- Fasani, E.; Manara, A.; Martini, F.; Furini, A.; DalCorso, G. The potential of genetic engineering of plants for the remediation of soils contaminated with heavy metals. Plant Cell Environ. 2018, 41, 1201–1232. [Google Scholar] [CrossRef] [PubMed]
- Rocha, C.S.; Rocha, D.C.; Kochi, L.Y.; Carneiro, D.N.M.; dos Reis, M.V.; Gomes, M.P. Phytoremediation by ornamental plants: A beautiful and ecological alternative. Environ. Sci. Pollut. Res. 2022, 29, 3336–3354. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.L.; Li, Z.R.; Shao, Z.Q.; Zheng, C.C.; Zou, H.J.; Zhang, J.J. Lead tolerance and enrichment characteristics of several ornamentals under hydroponic culture. Bull. Environ. Contam. Toxicol. 2020, 105, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Ebbs, S.D.; Kochian, L.V. Toxicity of zinc and copper to Brassica species: Implications for phytoremediation. J. Environ. Qual. 1997, 26, 776–781. [Google Scholar] [CrossRef]
- Liu, J.; Xin, X.; Zhou, Q. Phytoremediation of contaminated soils using ornamental plants. Environ. Rev. 2018, 26, 43–54. [Google Scholar] [CrossRef]
- Yan, L.; Li, C.; Zhang, J.; Moodley, O.; Liu, S.; Lan, C.; Gao, Q.; Zhang, W. Enhanced Phytoextraction of Lead from Artificially Contaminated Soil by Mirabilis jalapa with Chelating Agents. Bull. Environ. Contam. Toxicol. 2017, 99, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Cao, X.; Li, X.; Li, T.; Zhang, H.; Cui, X.; Cui, Z. Ecological toxicity of Cd, Pb, Zn, Hg and regulation mechanism in Solanum nigrum L. Chemosphere 2023, 313, 137447. [Google Scholar] [CrossRef] [PubMed]
- Auguy, F.; Fahr, M.; Moulin, P.; Brugel, A.; Laplaze, L.; Mzibri, M.E.; Filali-Maltouf, A.; Doumas, P.; Smouni, A. Lead tolerance and accumulation in Hirschfeldia incana, a Mediterranean Brassicaceae from metalliferous mine spoils. PLoS ONE 2013, 8, e61932. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, M.; Gul, I.; Silvestre, J.; Kallerhoff, J.; Arshad, M. Screening of indigenous ornamental species from different plant families for Pb accumu lation potential exposed to metal gradient in spiked soils. Soil Sediment Contam. Int. J. 2018, 27, 439–453. [Google Scholar] [CrossRef]
- Shao, Z.Q.; Zheng, C.C.; Apostma, J.; Lu, W.L.; Gao, Q.; Gao, Y.Z.; Zhang, J.J. Nitrogen acquisition, fixation and transfer in maize/al falfa intercrops are increased through root contact and morphological responses to interspecies competition. J. Integr. Agric. 2021, 20, 2240–2254. [Google Scholar] [CrossRef]
- Ter Braak, C.J.F.; Smilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination (Version 4.5). 2002. Available online: www.canoco.com (accessed on 11 September 2023).
- Duh, P.D.; Yen, W.J.; Du, P.C.; Yen, G.C. Antioxidant activity of mung bean hulls. J. Am. Oil Chem. Soc. 1997, 74, 1059–1063. [Google Scholar] [CrossRef]
- Cui, S.; Zhang, T.G.; Zhao, S.L.; Li, P.; Zhou, Q.X.; Zhang, Q.R.; Han, Q. Evaluation of three ornamental plants for phytoremedia tion of Pb-contamined soil. Int. J. Phytoremediat. 2013, 15, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Mao, F.; Nan, G.; Cao, M.; Gao, Y.; Guo, L.; Meng, X.; Yang, G. The metal distribution and the change of physiological and biochemical process in soybean and mung bean plants under heavy metal stress. Int. J. Phytoremediat. 2018, 20, 1113–1120. [Google Scholar] [CrossRef]
- Sharma, P.; Dubey, R.S. Lead toxicity in plants. Braz. J. Plant Physiol. 2005, 17, 35–52. [Google Scholar] [CrossRef]
- Bassegio, C.; Campagnolo, M.A.; Schwantes, D.; Gonçalves Junior, A.C.; Manfrin, J.; Schiller, A.D.P.; Bassegio, D. Growth and accumulation of Pb by roots and shoots of Brassica juncea L. Int. J. Phytoremediat. 2020, 22, 134–139. [Google Scholar] [CrossRef]
- Salazar, M.J.; Rodriguez, J.H.; Cid, C.V.; Pignata, M.L. Auxin effects on Pb phytoextraction from polluted soils by Tegetes minuta L. and Bidens pilosa L.: Extractive power of their root exudates. J. Hazard. Mater. 2016, 311, 63–69. [Google Scholar] [CrossRef]
- Rascio, N.; Vecchia, F.D.; Rocca, L.N.; Barbato, R.; Pagliano, C.; Raviolo, M.; Gonnelli, C.; Gabbrielli, R. Metal accumulation and damage in rice (cv. Vialone nano) seedlings exposed to cadmium. Environ. Exp. Bot. 2008, 62, 267–278. [Google Scholar] [CrossRef]
- Eun, S.O.; Shik, Y.H.; Lee, Y. Lead disturbs microtubule organization in the root meristem of Zea mays. Physiol. Plant. 2000, 110, 357–365. [Google Scholar] [CrossRef]
- Verma, S.; Dubey, R.S. Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci. 2003, 164, 645–655. [Google Scholar] [CrossRef]
- Mazumder, M.K.; Moulick, D.; Choudhury, S. Iron (Fe3+)-mediated redox responses and amelioration of oxidative stress in cadmium (Cd2+) stressed mung bean seedlings: A biochemical and computational analysis. J. Plant Biochem. Biotechnol. 2022, 31, 49–60. [Google Scholar] [CrossRef]
- Peng, J.S.; Guan, Y.H.; Lin, X.J.; Xu, X.J.; Xiao, L.; Wang, H.H.; Meng, S. Comparativeunderstanding of metal hyperaccumulation in plants: A mini-review. Environ. Geochem. Health 2021, 43, 1599–1607. [Google Scholar] [CrossRef]

| Ornamental Variety | Treatments | Pb Content (mg/kg) | Enrichment Coefficient | Translocation Factor | ||
|---|---|---|---|---|---|---|
| Shoot | Root | Shoot | Root | |||
| T. patula | CK | 43.2 ± 6.91 e | 55.1 ± 6.99 e | - | - | - |
| T1 | 359.4 ± 28.1 d | 2432.2 ± 178.8 d | 3.59 | 24.3 | 0.15 | |
| T2 | 574.1 ± 47.2 c | 5284.3 ± 517.7 c | 2.87 | 26.4 | 0.11 | |
| T3 | 862.1 ± 47.8 b | 8370.7 ± 466.5 b | 1.72 | 16.7 | 0.10 | |
| T4 | 1074.1 ± 111.8 a | 11,698.4 ± 672.6 a | 1.07 | 11.7 | 0.09 | |
| S. nigrum | CK | 32.1 ± 3.40 e | 54.5 ± 7.29 e | - | - | - |
| T1 | 503.8 ± 34.0 d | 2197.6 ± 213.8 d | 5.04 | 22.0 | 0.23 | |
| T2 | 656.7 ± 41.2 c | 4073.0 ± 205.7 c | 3.28 | 20.4 | 0.16 | |
| T3 | 838.6 ± 42.9 b | 6671.4 ± 244.3 b | 1.68 | 13.3 | 0.13 | |
| T4 | 958.7 ± 40.7 a | 9835.4 ± 180.7 a | 0.96 | 9.84 | 0.10 | |
| M. jalapa | CK | 38.5 ± 4.58 e | 47.1 ± 5.72 e | - | - | - |
| T1 | 150.8 ± 14.2 d | 1472.4 ± 29.2 d | 1.51 | 14.7 | 0.10 | |
| T2 | 339.4 ± 25.5 c | 2603.9 ± 19.9 c | 1.70 | 13.0 | 0.13 | |
| T3 | 642.3 ± 41.8 b | 4900.2 ± 93.4 b | 1.28 | 9.80 | 0.13 | |
| T4 | 975.3 ± 26.0 a | 8139.0 ± 207.2 a | 0.98 | 8.14 | 0.12 | |
| Ornamentals Variety | Root Morphology Index | Explains (%) | F Value | p Value |
|---|---|---|---|---|
| T. patula | RDW | 68.4 | 28.193 | 0.002 |
| RL | 16.3 | 2.523 | 0.152 | |
| RS | 12.8 | 1.913 | 0.182 | |
| RV | 11.1 | 1.631 | 0.246 | |
| RAD | 6.8 | 0.945 | 0.332 | |
| S. nigrum | RDW | 60.7 | 20.101 | 0.002 |
| RL | 33.0 | 6.402 | 0.024 | |
| RS | 41.5 | 9.236 | 0.006 | |
| RV | 29.5 | 5.449 | 0.026 | |
| RAD | 43.4 | 9.951 | 0.006 | |
| M. jalapa | RDW | 87.1 | 88.122 | 0.002 |
| RL | 84.8 | 72.766 | 0.002 | |
| RS | 65.2 | 24.315 | 0.002 | |
| RV | 79.2 | 49.537 | 0.002 | |
| RAD | 76.2 | 41.516 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, Z.; Li, M.; Zheng, J.; Zhang, J.; Lu, W. Lead Tolerance and Enrichment Characteristics of Three Hydroponically Grown Ornamental Plants. Appl. Sci. 2023, 13, 11208. https://doi.org/10.3390/app132011208
Shao Z, Li M, Zheng J, Zhang J, Lu W. Lead Tolerance and Enrichment Characteristics of Three Hydroponically Grown Ornamental Plants. Applied Sciences. 2023; 13(20):11208. https://doi.org/10.3390/app132011208
Chicago/Turabian StyleShao, Zeqiang, Mei Li, Juan Zheng, Jinjing Zhang, and Wenlong Lu. 2023. "Lead Tolerance and Enrichment Characteristics of Three Hydroponically Grown Ornamental Plants" Applied Sciences 13, no. 20: 11208. https://doi.org/10.3390/app132011208
APA StyleShao, Z., Li, M., Zheng, J., Zhang, J., & Lu, W. (2023). Lead Tolerance and Enrichment Characteristics of Three Hydroponically Grown Ornamental Plants. Applied Sciences, 13(20), 11208. https://doi.org/10.3390/app132011208





