Featured Application
Application of femtosecond laser pulses in assisted reproduction technologies is a novel field of biology and medicine. No attempts to arrange experimental data of laser ablation of embryo zona pellucida have been made yet. The results obtained are of practical importance for implementation in microsurgery procedures, including laser-assisted hatching and embryo tagging.
Abstract
We applied femtosecond laser pulses for microsurgery of the zona pellucida (ZP) of mouse embryos in terms of assisted reproductive technologies. The embryos were subjected to a series of laser pulses (wavelength of 514 nm, pulse duration of 280 fs, repetition rate of 2.5 kHz). Optical microscopy was used to study the dependence of the ZP cut width on the pulse energy E and velocity v of the laser beam. It is shown that the same value of the cut width can be obtained for different combinations of these parameters. The boundaries of admissible values were found to be E = (19–52) nJ, v = (0.001–0.03) mm/s; recommendations on their proper choice are given. An analytical expression of the cut width of the ZP for a given combination of laser-pulse energy, beam velocity, and pulse repetition rate is proposed. This simple and easy-to-use equation allows for quick prediction of ZP cut width in embryo microsurgery procedures of laser-assisted hatching and embryo laser tagging using femtosecond laser pulses.
1. Introduction
Laser sources were first used in assisted reproductive technologies (ART) three decades ago [1] to study sperm motility. Since then, the range of procedures has been significantly expanded and includes sperm immobilization, laser-assisted hatching (LAH), laser-assisted biopsy of embryonic cells, etc. Different types of lasers have been used in ART, including neodymium:yttrium–aluminum–garnet (Nd: YAG) at a radiation wavelength of 1064 nm for sperm capture in 1989 [1,2] and zona pellucida microsurgery at 532 nm [3] in 1991. In 1992, a UV excimer laser at a wavelength of 248 nm was proposed for ZP dissection [4]. However, it was not widely used because of the potentially high cytotoxic and mutagenic effects. Later, in 1992 and 1995, several studies were conducted using infrared lasers—erbium:yttrium–aluminum–garnet (Er:YAG) [5] and holmium:yttrium–scandian–gallium–garnet (Ho:YSGG) [6], generating radiation at wavelengths of 2900 and 2100 nm, respectively. However, a real breakthrough in ART occurred with the use of a semiconductor InGaAsP laser (1480 nm), which is still applied in clinical practice for dissection of the zona pellucida (ZP) [7,8]. Laser pulses 10–20 ms long with a power of 60–70 mW focused into a spot 2–3 μm in diameter were initially applied to drill the ZP and to form an opening 5–7 μm wide [7].
The main danger of using millisecond laser dissectors for embryo microsurgery is the thermal effect on the cells adjacent to the zona pellucida. This issue was raised in a study by Tucker and Ball [9], who constructed isotherms for two types of laser sources: Zylos-tk (0.5 ms, 300 mW) [10] and Fertilase (20 ms, 47 mW) [11]. Laser absorption models and thermal conductivity of the medium [12,13] were taken into account. The size of the area heated to 60 °C/80 °C was shown to be 16 μm/13 μm and 37 μm/15 μm for Zylos-tk and Fertilase lasers, respectively, much broader than the beam focus diameter. So, the choice was made in favor of a system with a shorter pulse duration. Since then, clinical guidelines for ZP microsurgery of the embryo using millisecond laser dissectors have been developed. The optimal parameters for laser-assisted hatching have been estimated to be 300 mW and 0.5–1 ms to reduce the thermal effect [13]. Local thinning of the ZP or opening formation, performed typically on embryo day three (postfertilization, ), are the possible approaches implemented for LAH (see the figures in e.g., Ref. [14]). A comprehensive review of current protocols applied for embryo-assisted hatching can be found elsewhere [15], while a detailed description of all procedures performed in fertility clinics is presented in [16,17].
Another procedure widely applied in fertility clinics is the trophectoderm (TE) biopsy. It is considered to be a sensitive and reliable tool for preimplantation genetic screening (PGS) to identify aneuploid embryos. The latter have little potential to result in a viable pregnancy and cannot be distinguished from normal embryos using standard morphological evaluations [18]. Some significant results in embryo quality estimations were, however, demonstrated with special image-processing protocols using an artificial neural network [19]. For TE biopsies, the blastocyst is held with the holding pipette, and TE cells are drawn carefully into the biopsy pipette by applying gentle suction. Subsequently, the TE cells are gently pulled away from the blastocyst, while laser pulses are applied. The detachment of the TE cells are normally achieved with less than five laser pulses [20]. Special care should be taken when selecting laser-pulse energy to avoid thermal damage of blastocyst cells and carbonization of the biopsy sample.
Femtosecond lasers have been widely applied in developmental biology for several decades; their high potential in the ART has also been recently demonstrated [21]. These lasers represent a promising alternative to millisecond dissectors. The difference in the fundamental processes of laser–matter interaction makes it possible to eliminate the above disadvantages attributed to high pulse duration and temperature concerns. Nonlinear mechanisms of absorption of femtosecond laser radiation in an aqueous medium [22,23] result in the localization of the laser effect within the point of the focused beam, significantly increasing the accuracy of microsurgical procedures. This, in turn, improves the safety of laser microsurgery of embryos and expands the scope of femtosecond lasers in the ART.
Controlled laser-assisted hatching (CLAH) is a novel technology developed using femtosecond laser pulses [24]. It consists of the dissection of the zona pellucida and, in contrast to millisecond pulses, can be performed at a late stage of embryo development. At the blastocyst stage, embryo cells have already differentiated into the trophectoderm cells and the intracellular mass (ICM). So, CLAH enables the embryologist to choose the hatching site (“ICM-forward” or “TE-forward”) for subsequent cell biopsy. Moreover, femtosecond CLAH procedures do not impair embryo development and increase the hatching rate.
Femtosecond laser pulses have also been implemented in noncontact laser-tagging technology to tag preimplantation embryos to identify them. It could help to prevent reported medical accidents relating to embryo mix-ups [25]. This brings prolonged emotional distress to parents and the loss of reputation to the clinic. The technology consists of applying alphanumeric designations to embryo ZPs using laser engraving. The cuts, which are 1–1.5 μm wide, form a set of arbitrary alphanumeric characters that can be recognized by the embryologist. The width of the cut is crucial since it affects the readability of the code.
Despite the minimal risk of embryo thermal damage, parameters for femtosecond laser exposure should be chosen carefully. Microsurgery of the ZP is performed with a series of femtosecond laser pulses, and a cut of a given width can be obtained with a different combination of parameters, including the energy and the laser beam velocity. To the best of our knowledge, the issue of the influence of laser-radiation parameters on the formed cut of the zona pellucida has not been considered previously. The purpose of this research was to study the effect of laser parameters on the cut width, as well as to provide recommendations for choosing the optimal values for ZP microsurgery in the framework of assisted reproductive technologies.
2. Materials and Methods
2.1. Experimental Setup
Embryo microsurgery was performed using the custom-built “Femtosecond laser scalpel” system shown in Figure 1. A femtosecond ytterbium laser (TETA, Avesta LLC, Moscow, Russia) generated ultrashort pulses with the following parameters: duration fs, energy μJ, radiation wavelength nm, pulse repetition rate kHz. Femtosecond laser pulses were further frequency-doubled in a DKDP crystal (radiation wavelength nm). Laser radiation was then directed to the right-side port of an Olympus IX-71 inverted microscope and was focused using a 20× UPlanFL microlens (Olympus, Japan) with a numerical aperture NA into a spot ∼2 μm in diameter. An attenuation unit and a telescope installed along the beam pathway between the laser and the microscope were used to adjust the energy of the pulses and to match the beam diameter with the microlens aperture. A small portion of the laser beam was reflected from a thin glass plate (Fresnel reflection of about 8%) and directed to a photodiode (DET36A2, Thorlabs Inc., Newton, NJ, USA) to control the energy of the laser pulses. The signal of the photodiode was calibrated through linear regression of its amplitude and the energy of the laser pulses at the output of the microlens registered by laser sensor (S120VC, Thorlabs Inc., Newton, NJ, USA).
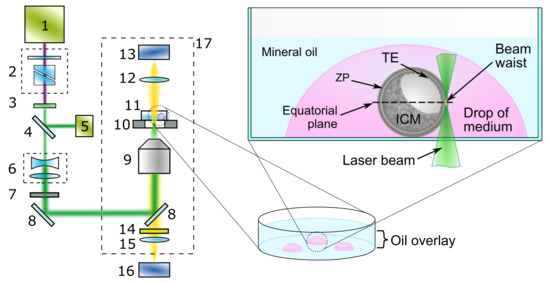
Figure 1.
Schematic setup of the system for femtosecond laser-assisted ZP microsurgery: 1—femtosecond ytterbium laser; 2—beam attenuator; 3—second-harmonic generator; 4—thin glass plate; 5—photodiode; 6—telescope; 7—electromechanical shutter; 8—dichroic mirrors; 9—microlens; 10—X–Y motorized stage; 11—Petri dish with embryo; 12—condenser lens; 13—microscope lamp; 14—notch filter; 15—tube lens; 16—CMOS camera; 17—inverted microscope. Schematic presentation of glass-bottom Petri dish containing a blastocyst (in a fresh drop of embryo manipulation medium) during the process of laser-assisted ZP cut.
A special Petri dish with a glass bottom was used for embryo microsurgery. The dish was mounted on the motorized stage (Märzhäuser Wetzlar, Wetzlar, Germany) of the microscope to move the embryo relative to the stationary laser beam. Embryo images were recorded with a CMOS camera (DFK 72AUC02, The Imaging Source, Bremen, Germany). The image magnification factor μm/pixel was preliminarily estimated using a standard Ronchi slide (100 lp/mm, #57-888, Edmund Optics, Barrington, NJ, USA) installed on the microscope stage.
To automate the microsurgery procedure, a custom-built software was used. It allowed the operator to visualize the embryo; to control laser parameters such as the energy, the pulse repetition rate, and the laser beam divergence; as well as to set the laser beam’s trajectory. To do this, a primitive element (a line or a curve) was drawn right above the live embryo image from the camera to set the form of a cut. Each drawn element was converted to a sequence of commands to the motorized stage. Position of the motorized stage during ZP cutting and synchronous laser shutter opening/closing were controlled automatically. When focusing, the laser beam’s diameter decreases along the beam axis, resulting in growth of the laser intensity. The latter reaches its maximum in the beam waist. Assuming a Gaussian shape of femtosecond laser pulse in space and time and a relation for peak power , pulse peak intensity is determined using equation [26]:
where μm is the radius of the focused laser beam at level, and fs is the pulse duration full width at half maximum. Laser-assisted ZP cutting occurs as soon as the incoming laser intensity in the beam waist exceeds the threshold. For 280 fs laser pulses at 514 nm with the energy of 19 nJ per pulse, which is high enough for ZP microdissection, the estimated pulse intensity is about 1.7 TW cm−2.
2.2. Embryo Collection, Culture, and Monitoring
In this paper we focus on analysis of the experimental data obtained in our previous study [25]. C57BL/6J mice were used for embryo collection. Zygotes were collected from fresh oviducts according to standard protocol [27]. After retrieval from mice, embryos were cultured in the CO2 incubator until the experiment. Details on embryo collection, culture, and monitoring can be found elsewhere [24,25]. All manipulations with animals were performed according to the European Convention for the Protection of Vertebrate Animals, Strasbourg, 18.III.1986, Directive 2010/63/EU, 22 September 2010 (annexes III, IV), the Order No. 755 of the USSR Ministry of Health, 12 August 1977, and the Laboratory Practice Rules in the RF and were approved by the Bioethics Committee of the Moscow State University [25].
2.3. Embryo Microsurgery
Embryos (typically on day 3 postcoitum, hereafter ), were transferred into embryo fresh drops of human tubal fluid medium in Petri dishes under mineral oil balanced with the medium. A special Petri dish with a glass bottom 170 μm thick (#100350, SPL Lifesciences, Pocheon, Republic of Korea) was used. The dish was placed on a motorized microscope stage. After finding the embryo in the dish, the laser beam was focused in the equatorial plane, i.e., the plane of the maximum cross-section diameter of an embryo (Figure 1).
The dissection of the ZP was performed with a sequence of laser pulses, with the embryo moving linearly relative to the laser beam at a given velocity v in the radial direction from the outer border of the ZP to the inner one. For simplicity, below, we refer to the “laser beam velocity” v, implying the movement of the embryo.
2.4. Measuring the Cut Width
To determine the width of the cut, a section profile of the image was built perpendicular to the cut. The section profile is the dependence of the image brightness on the spatial coordinate. An example of laser cuts and a cut cross section is shown in Figure 2A,B. A cut in a photo may have a white border, primarily on one side. We suppose that this is caused by a combination of optical effects, including refraction and total internal reflection. When the cut is illuminated by a microscope lamp, the rays of light travel within the bulk of the ZP and between the walls of the cut. The white border appears when the ray of light being reflected from a wall of the cut is directed right to the microscope camera. The opposite wall stays in shadow and the dark area indicates the opposite boundary of the cut. For thin cuts, the amount of light scattered by the wall decreases, and the white border may disappear. A mean value of and shown in Figure 2B was taken as a cut width. The difference between and determined the inaccuracy in width estimation. Averaging was carried out over 50 sections. The measurements were carried out three times for different embryos. The average value of the cut width was finally obtained, as well as , characterizing the minimum–maximum cut width spread in the series. It should be noted that the term “cut” here stands for laser-affected area of the ZP, including complete protein dissociation or density changes in the ZP causing alternations in its index of refraction.
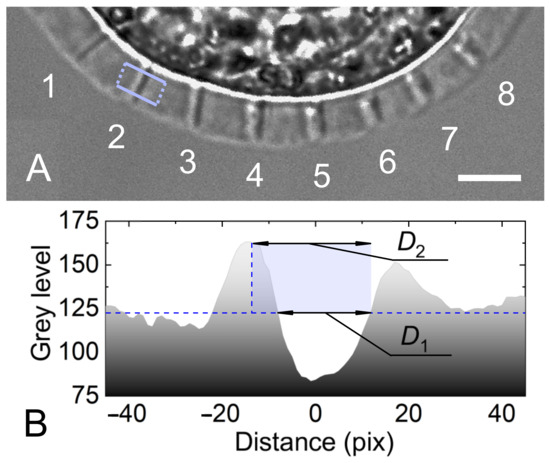
Figure 2.
(A) An example of radial cuts of the ZP performed at different energies of laser pulses E for velocity mm/s and pulse repetition rate kHz. Pulse energies for cuts 1—19 nJ; 2—24 nJ; 3—31 nJ; 4—38.6 nJ; 5—43 nJ; 6—46 nJ; 7—48 nJ; 8—51 nJ. Scale bar is 10 μm. Rectangle shows the area of multiple cross sections. (B) Cross section of cut 2.
3. Results and Discussion
Dissection of the embryo zona pellucida consists of its local destruction as a result of the absorption of energy of the laser pulses following a given repetition rate. The ZP of a mouse embryo consists of three glycoproteins: ZP1, ZP2, and ZP3. Molecules of glycoproteins ZP2 and ZP3 are connected alternately and form chains that link to each other through “bridges” of ZP1 molecules, resulting in a network-like structure [28].
Following an approach by Vogel et al. [23] for cell nanosurgery, biological objects are considered as water. Such an approach is to some extent fair for the ZP, since the mesh of proteins forming it is permeable to water molecules that can absorb laser photons. Interaction of a femtosecond laser pulse with water then leads to the formation of “free” electrons in the conduction band as a result of multiphoton ionization processes and the Zener tunneling effect [23,29]. Due to the nonlinearity of the absorption process, the electrons are strongly localized in the focal spot of the laser beam. One of the possible mechanisms for the destruction of proteins that form the ZP is a photochemical effect, leading either to the dissociation of water molecules and the creation of reactive oxygen species that destroy proteins, or to the direct rupture of bonds (the mechanisms of femtosecond nanosurgery of cells are described in detail elsewhere [23,30]). Another distinctive feature of all the aforementioned effects is their high localization within the size of the laser beam’s waist, which makes it possible to perform a cut of the zona pellucida narrower than the laser beam’s diameter, i.e., .
In this paper, the cuts of the zona pellucida were made for a series of energies E of femtosecond laser pulses (see Figure 2A) following with a repetition rate kHz and velocity mm/s. The blue circles in Figure 3 demonstrate the obtained dependence of the average cut width on energy E. The light-blue area indicates the limits (Min–Max) of the values obtained and characterizes the error in determining the width of the cut.
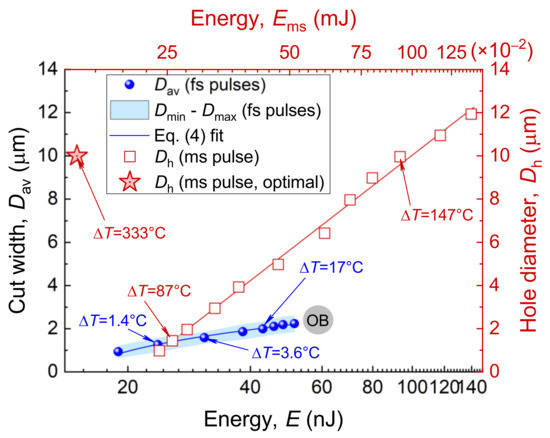
Figure 3.
ZP microsurgery with femtosecond and millisecond laser pulses. Circles demonstrate average cut width as a function of laser-pulse energy E for femtosecond laser pulses; velocity mm/s and pulse repetition rate kHz. Open boxes denote hole diameter in the ZP drilled using a millisecond laser pulse with power mW. Data for () are adopted from the open-archive source, Ref [8]. A star stands for parameters considered optimal for the LAH procedure [13]. OB—optical breakdown.
In case of millisecond pulse duration, a single pulse is enough to perform a cut. Let us consider the differences in the effects induced by millisecond and femtosecond pulses. When a pulse from a millisecond laser dissector is directed at the ZP, denaturation of the proteins occurs since the temperature in the laser focal spot can reach several hundred degrees. Tadir et al. [13] have shown that for laser-pulse parameters considered to be close to optimal (pulse duration ms and power mW for LAH procedure), the temperature at the beam’s center reaches 370 °C. This forms a region of highly superheated water, which is an excellent solvent. Thus, it is not surprising that the region of the ZP nearest to the waist of the laser beam rapidly disappears [12]. The temperature, however, decreases in a radial direction and reaches about 130 °C at a distance of 5 μm, corresponding to the radius of a hole drilled in the ZP using the laser-pulse parameters given above [13]. Such a large hole of 10 μm in size (red star in Figure 3) obtained for a focused beam diameter of ∼3 μm is due to the high thermal conductivity of the aqueous medium surrounding the embryo and to the heat transfer to the adjacent area. It is worth mentioning that a perforation of about 1 μm in diameter can, however, be formed using a millisecond laser pulse. The diameter of the drilled hole as a function of duration of the laser pulse for a given power mW has been previously studied [8]. The data are presented in Figure 3 (red squares), wherein the energy of a millisecond laser pulse is used for the x–axis. However, such a low power is not in line with recommendations for laser-assisted ZP microsurgery due to the high energy deposited.
The model presented earlier in Ref. [13] was used to estimate the temperature at the center point of a millisecond pulse. It is based on a solution to the heat-conduction equation with the power of the pulse P, its duration , and the radius of the beam used as input parameters. We have estimated temperature growth for both millisecond exposure modes—the “high” power ( ms, mW) and “low” power ( ms, mW), resulting in the same ZP opening size μm. The beam geometry in the calculations was the same; values were taken from Ref. [13]. Although the temperature increase °C for the “low” power exposure regime is lower than that for the “high” power ( °C), a longer pulse duration leads to greater heating at a distance of 10–15 μm from beam center, increasing the risk of cell damage in the region adjacent to the laser focus.
In the case of femtosecond pulse duration, ZP dissection is performed using a sequence of laser pulses with energy that is four orders of magnitude lower than that used for millisecond pulses. To make a cut in the ZP that is ∼1 μm wide, femtosecond laser pulses with energies of about 23 nJ and following with a repetition rate of 2.5 kHz are required, with a beam velocity of mm/s. The temperature increase in the area of interaction due to absorption of a single laser pulse reaches °C and falls down to zero for tens of microseconds. To make this estimation, we used the model described in detail in Ref. [31] and an approach proposed by Vogel et al. [23]. Calculations were made for a drop of water absorbing the pulse. First, calculation of concentration dynamics of “free” electrons due to absorption of the femtosecond laser pulse of a given intensity and beam geometry was conducted. The energy is released when recombination of the electrons transferred to the conduction band occurs. This gives us the spatial and temporal power profiles of the heating source for subsequent solving of the heat-conduction equation.
An increase in the energy of the laser pulses finally results in the optical breakdown of water (OB in Figure 3). The energies close to the OB are not suitable for ZP dissection due to the high risk of cavitation bubbles forming, which can cause displacement of an embryo lying freely on the bottom of the dish, and due to high temperatures. Analysis of the obtained spatial profile of the temperature showed that even for energies close to the OB, temperature changes are highly localized and occur within an area with a radius of . Our calculations for a series of femtosecond pulses have shown that heat accumulation in the interaction area is negligible for pulses with repetition rates in the kHz range.
Millisecond pulse exposure used to perform ZP drilling 1 μm in diameter results in a much higher heating of °C compared to femtosecond exposure. Direct comparison of laser effects in cases of femtosecond and millisecond laser pulses is, of course, flawed due to multi- and single-pulse regimes. Figure 3, however, makes it possible to compare the energies of both pulse durations required for ZP microdissections, as well as the temperatures reached.
The cut width D is determined not only by the pulse energy E, but by a set of parameters. They include the velocity of the laser beam v, its radius , and the repetition rate f, which altogether determine the spatial overlap of laser pulses when the laser beam moves along the trajectory: . To find the optimal parameters of laser exposure for given and f, a series of experiments were carried out for different velocities v and pulse energies E, followed by a cut-width estimation.
Three laser-pulse energies were considered: nJ (slightly above the threshold, at which laser cut becomes visible), nJ, and nJ. Increasing the pulse energy made it possible to raise the velocity relative to the nominal one ( mm/s) reported earlier (see dependence in Figure 3). The embryos with laser cuts performed at , , and , are shown in Figure 4A, B, and C, respectively. The maximum velocity, for which the cut is still visible, increases as the energy of the laser pulses grows. Some additional measurements were made at nJ for very low velocities of 0.25 and 0.5 μm/s in Figure 4C. The pulse repetition rate kHz was kept constant for all the series of experiments.
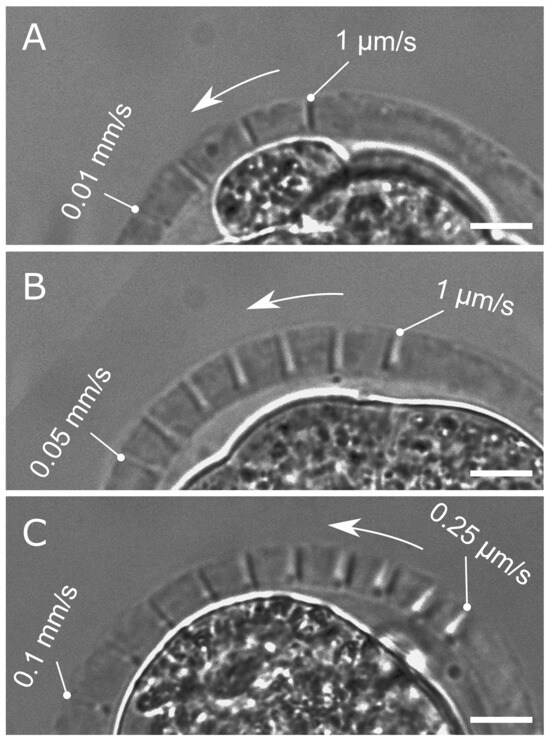
Figure 4.
Laser cuts of the zona pellucida for different velocities v. (A) nJ, mm/s; (B) nJ, mm/s; (C) nJ, mm/s. Scale bar is 10 μm.
Markers (•) in Figure 5 demonstrate the measured values obtained for the set of energies , , and shown in Figure 4. The maximal error bars for each series are shown. The lines represent linear regression . Coefficients and are summarized in Table 1.
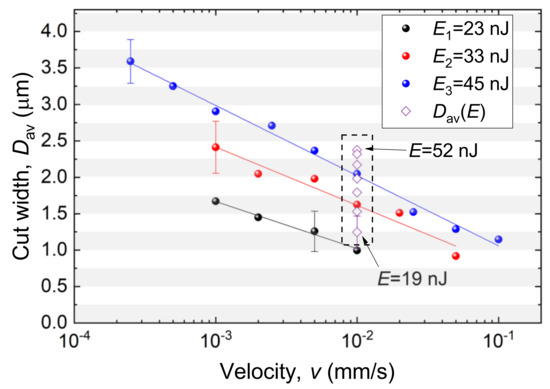
Figure 5.
Cut width as a function of velocity v for a set of laser-pulse energies. Pulse repetition rate kHz; beam radius μm. represents the data from Figure 3. The whiskers represent the averaged over the number of measurements in the series.

Table 1.
Linear regression coefficients.
The data in Figure 5 demonstrate good agreement with the values obtained earlier (see Figure 3) for the velocity mm/s (denoted here by ◇). It can be seen that the desired value of the cut width D can be obtained for various combinations of laser-pulse energy and velocity. For example, μm is achievable by combining nJ and mm/s; nJ and mm/s; nJ and mm/s (for pulse repetition rate kHz and beam waist radius μm). These values can be scaled to another pulse repetition rate f by appropriately changing the velocity v, e.g., for kHz, halved v values should be considered (Figure 5).
For a beam with radius of μm moving with a velocity of mm/s, it takes 224 μs to travel a distance equal to its diameter. Five tens of pulses affect nearly the same place. The damage area begins increasing in size, starting from the center of the beam where the intensity is maximal. Each pulse makes the damage area wider. The slower the velocity, the greater the number of pulses affecting the same area. For mm/s, the number of pulses reaches five thousands, and the width of the cut is much greater than that of 0.1 mm/s. Further velocity decreases would result in approaching a diameter of a stationary hole formed in the ZP. Wang et al. [32] performed similar investigations of femtosecond laser ablation in agarose and glass samples using a series of laser pulses and a stationary laser beam.
Laser energy is deposited into the media when the laser intensity in the focal region exceeds the intensity threshold for nonlinear absorption. Radial intensity distribution is given by the equation
For a given laser energy E, the intensity meets the threshold at a defined transverse radius (the sample is damaged within the ):
Using the relation for average power , Wang et al. [32] derive an equation for the radius of damaged area (shown as a blue curve in Figure 3):
Finding the analytical expression for the cut width D as a function of two variables (energy E and the velocity v) is of practical interest. Using linear regression for according to Figure 5 and a square root function in Equation (4) for energy E, the functional of the following form can be written as
Adjustment of the parameters was performed in the numeric computing environment Matlab R2021b (MathWorks, Natick, MA, USA) using the fittype function. For the energy values E (in nJ) and the velocity of the laser beam v (in mm/s), the coefficients were found to be , , , and . The approximation error of the experimental data by the constructed surface (Figure 6) did not exceed 0.15 μm, and the coefficient of determination . The function presented is valid for energies between 19 and 52 nJ and for velocities in the range of 0.00025 and 0.1 mm/s.
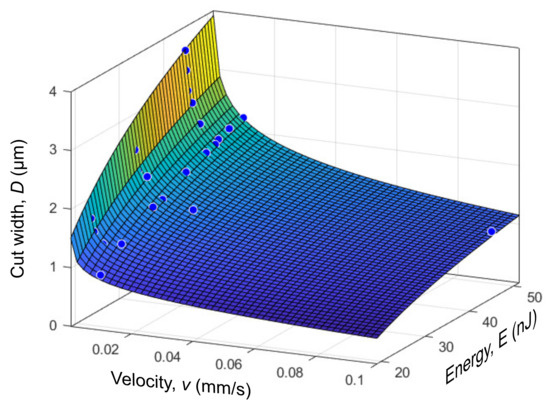
Figure 6.
Dependence of the cut width D on the energy of laser pulses E and velocity v. The approximation error does not exceed 0.15 μm.
When choosing the optimal combination of laser-exposure parameters, the following considerations should be followed. In configuration with a stationary laser beam and a moving microscope stage with an embryo in a Petri dish, velocities mm/s are unsuitable due to the high acceleration experienced by the embryo at the beginning of the movement and the high risk of displacement from its initial position (an embryo lies freely at the bottom of the dish and is not anchored). On the other hand, velocities mm/s significantly increase the dissection time, as well as the total time the embryos stay outside the incubator. Thus, the choice of parameters should begin with setting the desired width of the zona pellucida cut. To minimize the thermal impact, the lowest energy values should be chosen for the specified cut width D, taking into account the above restrictions on the allowable velocities in the range mm/s in accordance with the dependencies shown in Figure 4 and Figure 5.
The popularity of femtosecond ytterbium lasers has grown substantially over the past decade. They provide pulses 250–500 fs long, with energies of up to several tens of microjoules. The fibre format of such a laser makes it compact compared to conventional femtosecond ones and provides low maintenance. So, selecting ytterbium lasers for microsurgery procedures seems to be a good choice for technological applications in the future. We have applied a second harmonic of laser radiation at a wavelength of 514 nm. It falls into a window of minimum water absorption, enabling the laser pulse to reach the embryo equatorial plane with minimal losses. When applying laser radiation at the fundamental frequency in the near-infrared spectral region, linear absorption would become stronger, making the absorption process less sensitive to laser-pulse energy fluctuations. Direct comparison of cut-width dependencies obtained at nm with the results for near-infrared pulses seems to be interesting from both fundamental and practical points of view. The issue of wavelength dependence for spatial resolution and microsurgery precision is the subject of our future investigations.
4. Conclusions
In this paper, femtosecond laser pulses were utilized for the microdissections of the zona pellucida of mouse embryos. Experimental studies on the effects of laser-exposure parameters on ZP cut width were carried out. The dependence of the latter both on the energy of laser pulses at a given laser beam velocity and on the velocity at different energies of laser pulses was obtained. It is shown that the specified value of the cut width can be achieved with different combinations of laser exposure parameters. The analysis of the range of their admissible values resulted in the optimal range for velocities v = (0.001–0.03) mm/s and the selection of minimal laser-pulse energy for the desired cut width. Analytical expression, enabling one to quickly estimate the cut width for a chosen laser-pulse energy E, laser beam velocity v, and pulse repetition rate f, is presented. The results obtained are of practical importance for the implementation in embryo microsurgery procedures of laser-assisted hatching and embryo tagging.
Author Contributions
Conceptualization, D.S.S.; validation, M.A.F.; formal analysis, I.V.I.; investigation, D.S.S., M.A.F. and I.V.I.; writing—original draft preparation, D.S.S.; writing—review and editing, D.S.S., M.A.F. and I.V.I.; visualization, I.V.I.; supervision, D.S.S.; project administration, D.S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Higher Education of the Russian Federation (State Assignment No. 075-01129-23-00).
Institutional Review Board Statement
The animal study protocol was approved by the Bioethics Committee of the Moscow State University (protocol code 72-j; 26 March 26 2018).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The experiments were conducted using the Unique Facility “Terawatt Femtosecond Laser Complex” at the Center for Collective Usage “Femtosecond Laser Complex” of the Joint Institute for High Temperatures of the Russian Academy of Sciences (JIHT RAS).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ART | assisted reproductive technologies |
| CLAH | controlled laser-assisted hatching |
| ICM | intracellular mass |
| LAH | laser-assisted hatching |
| PGS | preimplantation genetic screening |
| TE | trophectoderm |
| UV | ultraviolet |
| ZP | zona pellucida |
References
- Tadir, Y.; Wright, W.H.; Vafa, O.; Ord, T.; Asch, R.H.; Berns, M.W. Micromanipulation of sperm by a laser generated optical trap. Fertil. Steril. 1989, 52, 870–873. [Google Scholar] [CrossRef] [PubMed]
- Tadir, Y.; Wright, W.H.; Vafa, O.; Ord, T.; Asch, R.H.; Berns, M.W. Force generated by human sperm correlated to velocity and determined using a laser generated optical trap. Fertil. Steril. 1990, 53, 944–947. [Google Scholar] [CrossRef] [PubMed]
- Tadir, Y.; Wright, W.; Vafa, O.; Liaw, L.; Asch, R.; Berns, M. Micromanipulation of gametes using laser microbeams. Hum. Reprod. 1991, 6, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Blanchet, G.B.; Russell, J.B.; Fincher, C.R.; Portmann, M. Laser micromanipulation in the mouse embryo: A novel approach to zona drilling. Fertil. Steril. 1992, 57, 1337–1341. [Google Scholar] [CrossRef] [PubMed]
- Feichtinger, W.; Strohmer, H.; Fuhrberg, P.; Radivojevic, K.; Antinori, S.; Pepe, G.; Versaci, C. Photoablation of oocyte zona pellucida by erbium-yag laser for in-vitro fertilisation in severe male infertility. Lancet 1992, 339, 811. [Google Scholar] [CrossRef] [PubMed]
- Neev, J.; Schiewe, M.C.; Sung, V.W.; Kang, D.; Hezeleger, N.; Berns, M.W.; Tadir, Y. Assisted hatching in mouse embryos using a noncontact Ho:YSGG laser system. J. Assist. Reprod. Genet. 1995, 12, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Rink, K.; Delacretaz, G.P.; Salathe, R.P.; Senn, A.; Nocera, D.; Germond, M.; Fakan, S. 1.48-um diode laser microdissection of the zona pellucida of mouse zygotes. In Proceedings of the Laser-Tissue Interact V and Ultraviolet Radiation Hazards; Jacques, S.L., Sliney, D.H., Belkin, M., Eds.; SPIE: Bellingham, WA, USA, 1994; Volume 2134, pp. 412–422. [Google Scholar] [CrossRef]
- Germond, M.; Nocera, D.; Senn, A.; Rink, K.; Delacrétaz, G.; Fakan, S. Microdissection of mouse and human zona pellucida using a 1.48 µm diode laser beam: Efficacy and safety of the procedure. Fertil. Steril. 1995, 64, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Tucker, M.; Ball, G. Assisted hatching as a technique for use in human in vitro fertilization and embryo transfer is long overdue for careful and appropriate study. J. Clin. Embryol. 2009, 12, 9–14. [Google Scholar]
- Sagoskin, A.W.; Levy, M.J.; Tucker, M.J.; Richter, K.S.; Widra, E.A. Laser assisted hatching in good prognosis patients undergoing in vitro fertilization-embryo transfer: A randomized controlled trial. Fertil. Steril. 2007, 87, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Venkat, G.; Thornhill, A.; Wensvoort, S.; Craft, I. Does laser-assisted hatching using partial zona thinning (LAH) improve outcome in frozen embryo transfer (FET) cycles? J. Clin. Embryol. 2008, 11, 17–30. [Google Scholar]
- Douglas-Hamilton, D.H.; Conia, J. Thermal effects in laser-assisted pre-embryo zona drilling. J. Biomed. Opt. 2001, 6, 205. [Google Scholar] [CrossRef] [PubMed]
- Tadir, Y.; Douglas-Hamilton, D.H. Laser Effects in the Manipulation of Human Eggs and Embryos for In Vitro Fertilization. Methods Cell Biol. 2007, 82, 409–431. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Cha, J.H.; Shin, S.H.; Kim, Y.J.; Lee, S.K.; Park, C.K.; Pak, K.A.; Yoon, J.S.; Park, S.Y. Effects of laser-assisted thinning versus opening on clinical outcomes according to maternal age in patients with repeated implantation failure. Lasers Med. Sci. 2019, 34, 1889–1895. [Google Scholar] [CrossRef] [PubMed]
- Alteri, A.; Viganò, P.; Maizar, A.A.; Jovine, L.; Giacomini, E.; Rubino, P. Revisiting embryo assisted hatching approaches: A systematic review of the current protocols. J. Assist. Reprod. Genet. 2018, 35, 367–391. [Google Scholar] [CrossRef] [PubMed]
- Davidson, L.M.; Liu, Y.; Griffiths, T.; Jones, C.; Coward, K. Laser technology in the ART laboratory: A narrative review. Reprod. Biomed. Online 2019, 38, 725–739. [Google Scholar] [CrossRef]
- Rosen, M.; Yang, X.; Marsh, P.; Runge, A.; Olivera, G.; Ribeiro, S.; Simbulan, R.; Quinn, M. Gamete and Embryo Manipulation, 8th ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 823–856.e14. [Google Scholar] [CrossRef]
- Verlinsky, Y.; Ginsberg, N.; Lifchez, A.; Valle, J.; Moise, J.; Strom, C.M. Analysis of the first polar body: Preconception genetic diagnosis. Hum. Reprod. 1990, 5, 826–829. [Google Scholar] [CrossRef]
- Chéles, D.S.; Ferreira, A.S.; de Jesus, I.S.; Fernandez, E.I.; Pinheiro, G.M.; Dal Molin, E.A.; Alves, W.; de Souza, R.C.M.; Bori, L.; Meseguer, M.; et al. An Image Processing Protocol to Extract Variables Predictive of Human Embryo Fitness for Assisted Reproduction. Appl. Sci. 2022, 12, 3531. [Google Scholar] [CrossRef]
- Costa-Borges, N.; Mestres, E.; Garcia, M.; Vanrell, I.; Rink, K.; Levtonov, M.; Calderón, G. Trophectoderm biopsy of blastocysts using the assisted by a laser system. Appl. Note 2016, 1–8. [Google Scholar]
- Ilina, I.; Sitnikov, D. From Zygote to Blastocyst: Application of Ultrashort Lasers in the Field of Assisted Reproduction and Developmental Biology. Diagnostics 2021, 11, 1897. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Venugopalan, V. Mechanisms of Pulsed Laser Ablation of Biological Tissues. Chem. Rev. 2003, 103, 577–644. [Google Scholar] [CrossRef]
- Vogel, A.; Noack, J.; Hüttman, G.; Paltauf, G. Mechanisms of femtosecond laser nanosurgery of cells and tissues. Appl. Phys. B 2005, 81, 1015–1047. [Google Scholar] [CrossRef]
- Ilina, I.V.; Khramova, Y.V.; Ivanova, A.D.; Filatov, M.A.; Silaeva, Y.Y.; Deykin, A.V.; Sitnikov, D.S. Controlled hatching at the prescribed site using femtosecond laser for zona pellucida drilling at the early blastocyst stage. J. Assist. Reprod. Genet. 2021, 38, 517–529. [Google Scholar] [CrossRef]
- Ilina, I.V.; Khramova, Y.V.; Filatov, M.A.; Sitnikov, D.S. Application of femtosecond laser microsurgery in assisted reproductive technologies for preimplantation embryo tagging. Biomed. Opt. Express 2019, 10, 2985–2995. [Google Scholar] [CrossRef]
- Shchatsinin, I. Free Clusters and Free Molecules in Strong, Shaped Laser Fields. Ph.D. Thesis, Freie Universität, Berlin, Germany, 2009. [Google Scholar] [CrossRef]
- Hogan, B.; Beddington, R.; Costantini, F.; Lacy, E. Manipulating the Mouse Embryo: A Laboratory Manual; Cold Spring Harbor Lab.: New York, NY, USA, 2014; p. 814. [Google Scholar]
- Wassarman, P.M. Zona pellucida glycoproteins. Annu. Rev. Biochem. 1988, 57, 415–442. [Google Scholar] [CrossRef]
- Joglekar, A.P.; Liu, H.H.; Meyhofer, E.; Mourou, G.; Hunt, A.J. Optics at critical intensity: Applications to nanomorphing. Proc. Natl. Acad. Sci. USA 2004, 101, 5856–5861. [Google Scholar] [CrossRef] [PubMed]
- Hoy, C.L.; Ferhanoglu, O.; Yildirim, M.; Kim, K.H.; Karajanagi, S.S.; Chan, K.M.C.; Kobler, J.B.; Zeitels, S.M.; Ben-Yakar, A. Clinical Ultrafast Laser Surgery: Recent Advances and Future Directions. IEEE J. Sel. Top. Quantum Electron. 2014, 20, 242–255. [Google Scholar] [CrossRef]
- Sitnikov, D.; Ilina, I.; Pronkin, A. Assessment of the thermal effect of femtosecond and millisecond laser pulses in microsurgery of mammalian embryos. Quantum Electron. 2022, 52, 482. [Google Scholar] [CrossRef]
- Wang, Y.L.; Grooms, N.W.F.; Chung, S.H. Transverse and axial resolution of femtosecond laser ablation. J. Biophotonics 2022, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).