Ultrasound-Assisted Extraction of Specific Phenolic Compounds from Petroselinum crispum Leaves Using Response Surface Methodology and HPLC-PDA and Q-TOF-MS/MS Identification
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of the Samples
2.3. Ultrasound-Assisted Extraction (UAE)
2.4. Experimental Design
2.5. Verification of the Statistical Model
2.6. Determination of Phenolic Compounds with HPLC-PDA
2.7. Identification of Phenolic Compounds with HPLC-QTOF-MS
2.8. Evaluation of Antioxidant Activity
2.9. Determination of Phenolic Compounds
2.10. Statistical Analysis
3. Results and Discussion
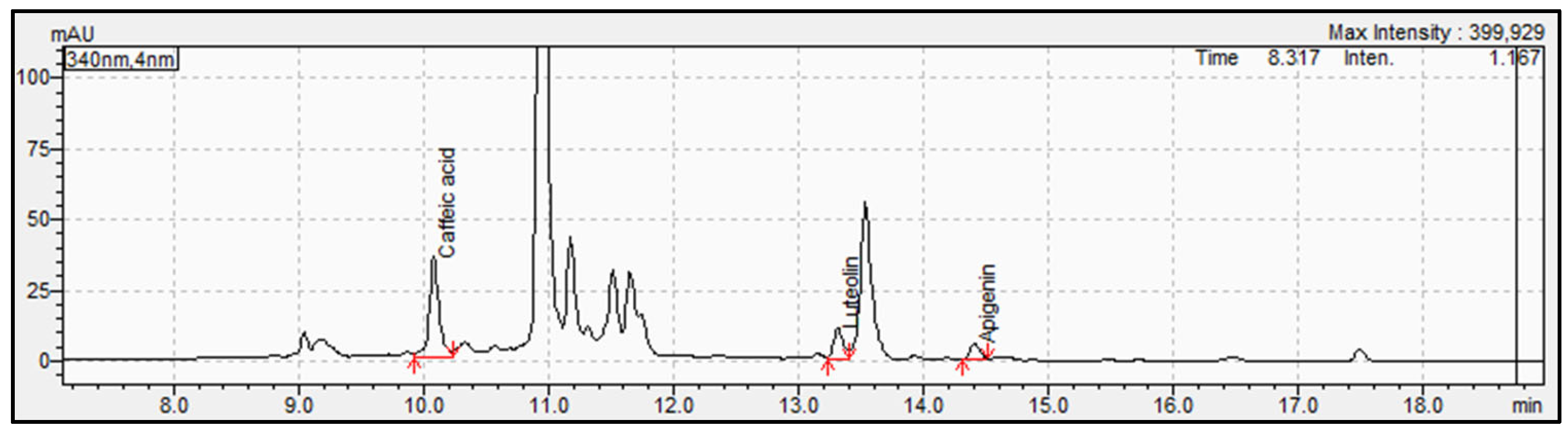
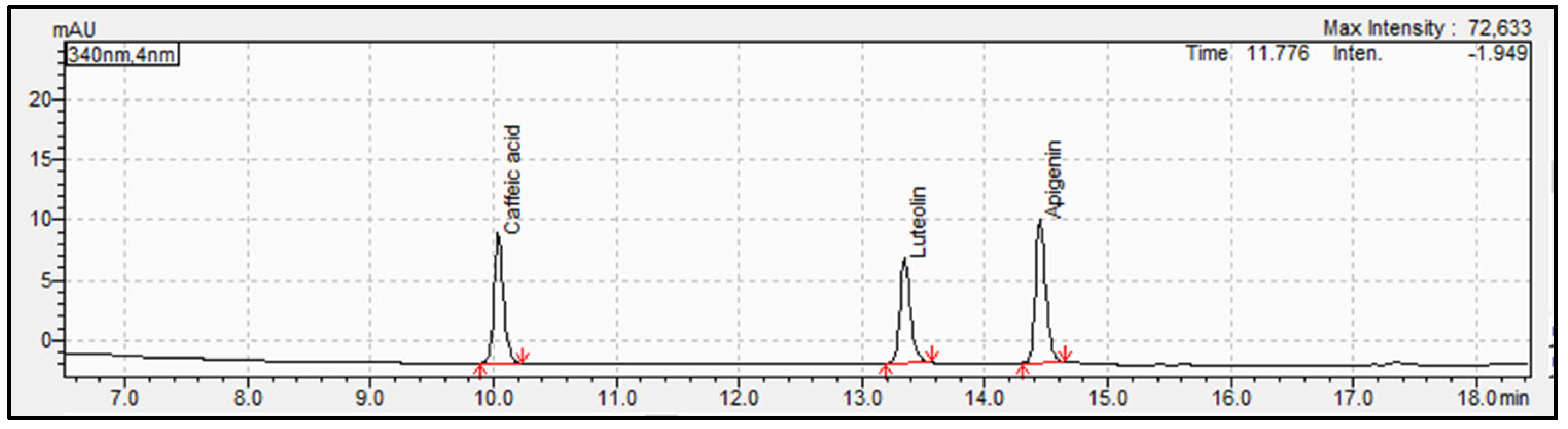
3.1. Determination and Identification of Phenolic Compounds in Parsley Samples by HPLC-PDA and UHPLC-QTOF-MS
3.2. Model Fitting
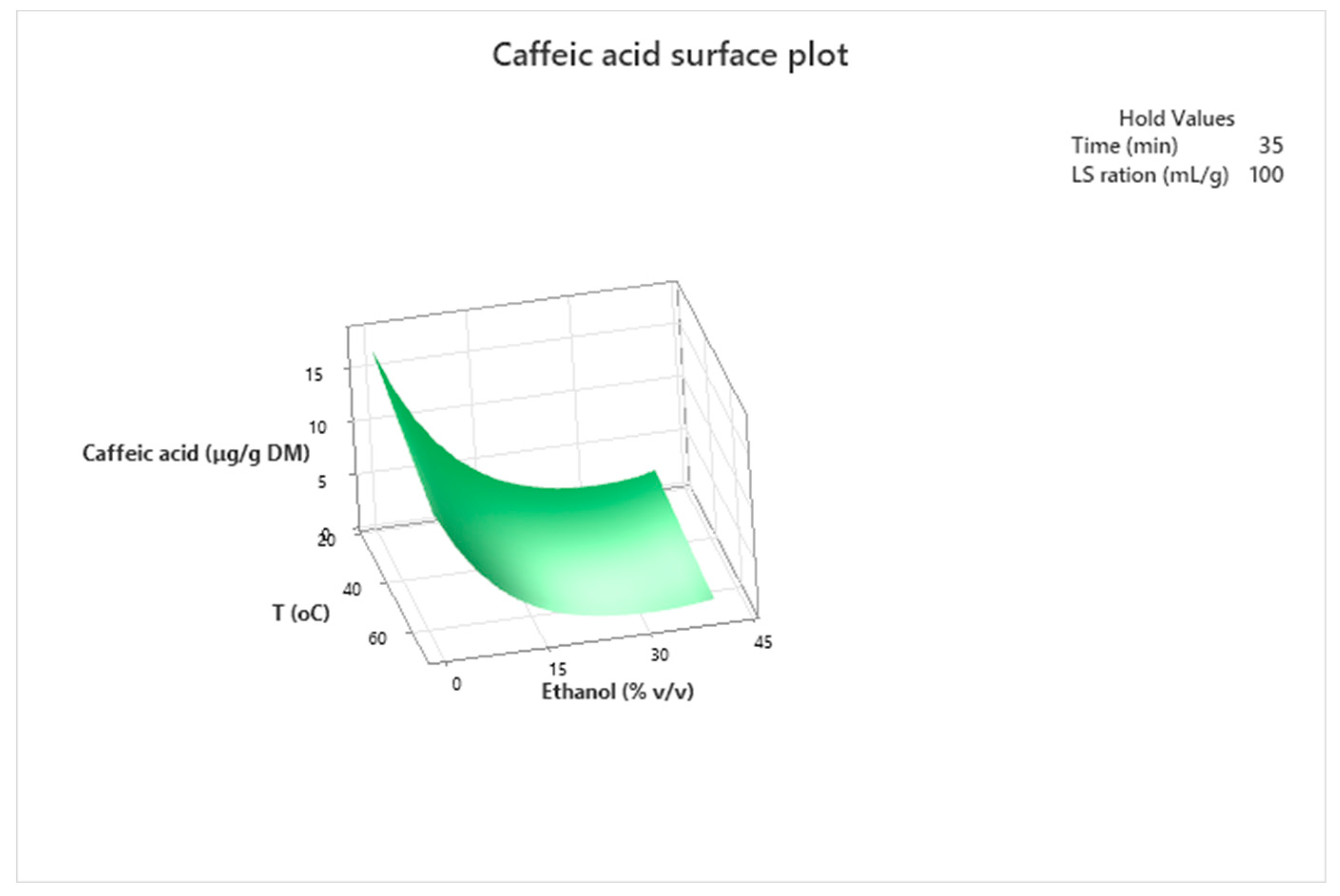
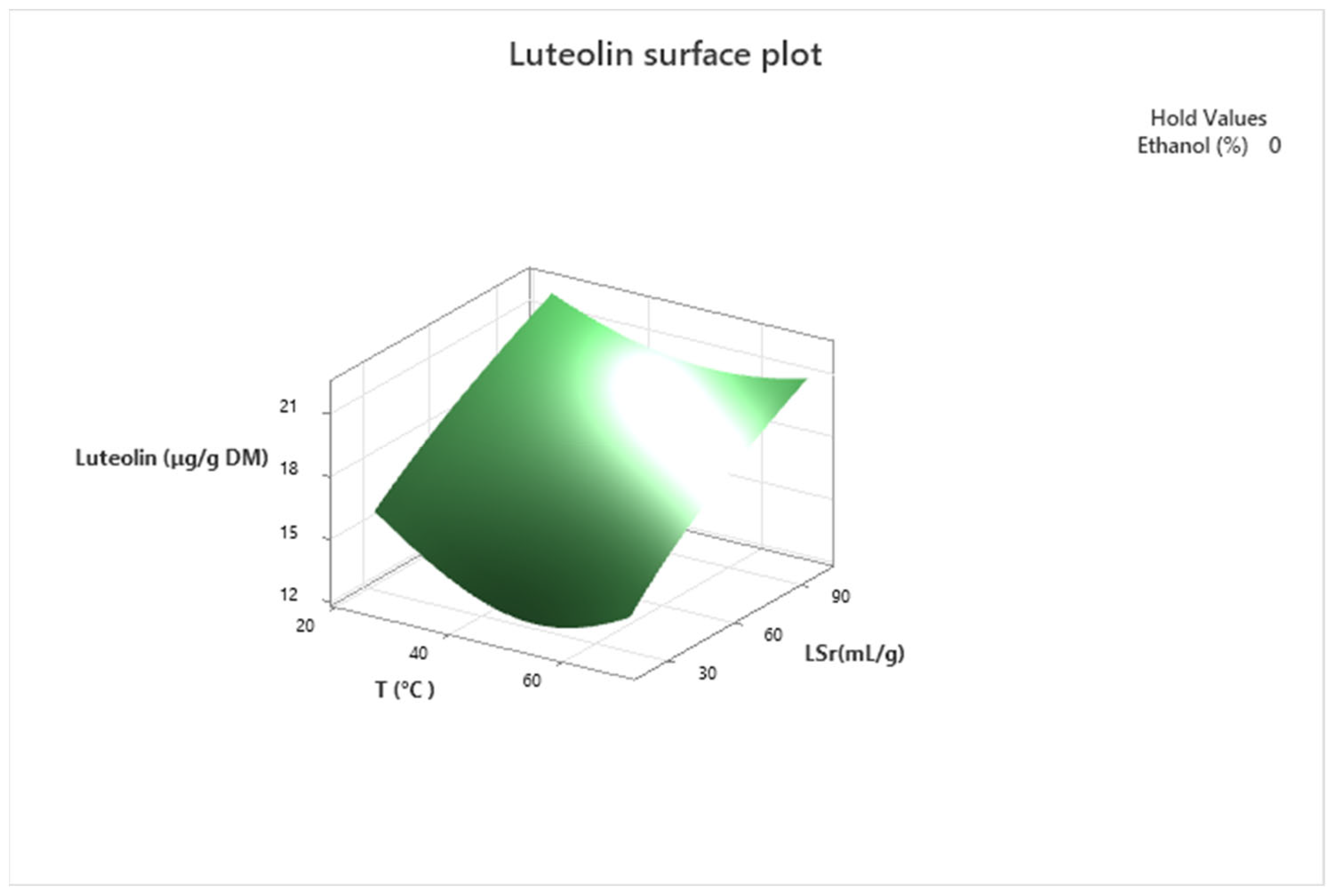
3.3. Optimization of the Extraction Conditions
3.4. Verification of the Models
3.5. Antioxidan Activity Evaluation and Total Phenolic Determination
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- López, M.G.; Sánchez-Mendoza, I.R.; Choa-Alejo, N. Comparative Study of Volatile Components and Fatty Acids of Plants and in Vitro Cultures of Parsley (Petroselinum crispum (Mill) Nym Ex Hill). J. Agric. Food Chem. 1999, 47, 3292–3296. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, V.S.; Chávez, D.W.H.; Paiva, P.R.F.; Gamallo, O.D.; Castro, R.N.; Sawaya, A.C.H.F.; Sampaio, G.R.; Torres, E.A.F.D.S.; Saldanha, T. Parsley (Petroselinum crispum Mill.): A Source of Bioactive Compounds as a Domestic Strategy to Minimize Cholesterol Oxidation during the Thermal Preparation of Omelets. Food Res. Int. 2022, 156, 111199. [Google Scholar] [CrossRef] [PubMed]
- Dziki, D.; Hassoon, W.H.; Biernacka, B.; Gawlik-Dziki, U. Dried and Powdered Leaves of Parsley as a Functional Additive to Wheat Bread. Appl. Sci. 2022, 12, 7930. [Google Scholar] [CrossRef]
- Mouhoubi, K.; Boulekbache-Makhlouf, L.; Madani, K.; Palatzidi, A.; Perez-Jimenez, J.; Mateos-Aparicio, I.; Garcia-Alonso, A. Phenolic Compounds and Antioxidant Activity Are Differentially Affected by Drying Processes in Celery, Coriander and Parsley Leaves. Int. J. Food Sci. Technol. 2022, 57, 3467–3476. [Google Scholar] [CrossRef]
- Şener, G.; Karakadıoglu, G.; Ozbeyli, D.; Ede, S.; Yanardag, R.; Sacan, O.; Aykac, A. Petroselinum crispum Extract Ameliorates Scopolamine-Induced Cognitive Dysfunction: Role on Apoptosis, Inflammation and Oxidative Stress. Food Sci. Hum. Wellness 2022, 11, 1290–1298. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Fernandes, Â.; Finimundy, T.C.; Polyzos, N.; Pinela, J.; Ivanov, M.; Soković, M.; Ferreira, I.C.F.R.; Barros, L. The Bioactivities and Chemical Profile of Turnip-Rooted Parsley Germplasm. Horticulturae 2022, 8, 639. [Google Scholar] [CrossRef]
- Arsenov, D.; Župunski, M.; Pajević, S.; Nemeš, I.; Simin, N.; Alnuqaydan, A.M.; Watson, M.; Aloliqi, A.A.; Mimica-Dukić, N. Roots of Apium Graveolens and Petroselinum crispum—Insight into Phenolic Status against Toxicity Level of Trace Elements. Plants 2021, 10, 1785. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Ghasemzadeh, N. Flavonoids and Phenolic Acids: Role and Biochemical Activity in Plants and Human. J. Med. Plant Res. 2011, 5, 6697–6703. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Parama, D.; Daimari, E.; Girisa, S.; Banik, K.; Harsha, C.; Dutta, U.; Kunnumakkara, A.B. Rationalizing the Therapeutic Potential of Apigenin against Cancer. Life Sci. 2021, 267, 118814. [Google Scholar] [CrossRef]
- Głowacki, R.; Furmaniak, P.; Kubalczyk, P.; Borowczyk, K. Determination of Total Apigenin in Herbs by Micellar Electrokinetic Chromatography with UV Detection. J. Anal. Methods Chem. 2016, 2016, 3827832. [Google Scholar] [CrossRef]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef] [PubMed]
- Cizmarova, B.; Hubkova, B.; Bolerazska, B.; Marekova, M.; Birkova, A. Caffeic Acid: A Brief Overview of Its Presence, Metabolism, and Bioactivity. Bioact. Compd. Health Dis. 2020, 3, 74–81. [Google Scholar] [CrossRef]
- Lin, Y.; Shi, R.; Wang, X.; Shen, H.-M. Luteolin, a Flavonoid with Potential for Cancer Prevention and Therapy. Curr. Cancer Drug Targets 2008, 8, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Shang, P.; Li, D. Luteolin: A Flavonoid That Has Multiple Cardio-Protective Effects and Its Molecular Mechanisms. Front. Pharmacol. 2017, 8, 692. [Google Scholar] [CrossRef]
- Gadi, D.; Bnouham, M.; Aziz, M.; Ziyyat, A.; Legssyer, A.; Legrand, C.; Lafeve, F.F.; Mekhfi, H. Parsley Extract Inhibits in Vitro and Ex Vivo Platelet Aggregation and Prolongs Bleeding Time in Rats. J. Ethnopharmacol. 2009, 125, 170–174. [Google Scholar] [CrossRef]
- Adsersen, A.; Gauguin, B.; Gudiksen, L.; Jäger, A.K. Screening of Plants Used in Danish Folk Medicine to Treat Memory Dysfunction for Acetylcholinesterase Inhibitory Activity. J. Ethnopharmacol. 2006, 104, 418–422. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Zhang, H.; Dzah, C.S.; Zandile, M.; Duan, Y.; Ma, H.; Luo, X. Advances in Ultrasound Assisted Extraction of Bioactive Compounds from Cash Crops–A Review. Ultrason. Sonchem. 2018, 48, 538–549. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of Phenolic Compounds: A Review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef]
- ICH Topic Q 2 (R1) Validation of Analytical Prcedures: Text and Methodology Step 5 Note for Guidance on Validation of Analytical Prcedures: Text and Methodology (CPMP/ICH/381/95) Approval by CPMP November 1994 Date for Coming into Operation. 1995. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q-2-r1-validation-analytical-prcedures-text-methodology-step-5_en.pdf (accessed on 4 December 2022).
- Miller, N.J.; Rice-Evans, C.; Davies, M.J.; Gopinathan, V.; Milner, A. A Novel Method for Measuring Antioxidant Capacity and Its Application to Monitoring the Antioxidant Status in Premature Neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of ‘Antioxidant Power’: The FRAP Assay. Anal. Bichem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Özyürek, M.; Güçlü, K.; Apak, R. The Main and Modified CUPRAC Methods of Antioxidant Measurement. TrAC-Trends Anal. Chem. 2011, 30, 652–664. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Cicalteu Reagent; Academic Press: Cambridge, MA, USA, 1999; Volume 299. [Google Scholar]
- Vo, T.P.; Nguyen, L.N.H.; Le, N.P.T.; Mai, T.P.; Nguyen, D.Q. Optimization of the Ultrasonic-Assisted Extraction Prcess to Obtain Total Phenolic and Flavonoid Compounds from Watermelon (Citrullus lanatus) Rind. Curr. Res. Food Sci. 2022, 5, 2013–2021. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.V.; Sengar, A.S.; Sunil, C.K.; Rawson, A. Ultrasonication-A Green Technology Extraction Technique for Spices: A Review. Trends Food Sci. Technol. 2021, 116, 975–991. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound Assisted Extraction (UAE) of Bioactive Compounds from Fruit and Vegetable Processing by-Products: A Review. Ultrason. Sonchem. 2021, 70, 105325. [Google Scholar] [CrossRef] [PubMed]
- Muñiz-Márquez, D.B.; Martínez-Ávila, G.C.; Wong-Paz, J.E.; Belmares-Cerda, R.; Rodríguez-Herrera, R.; Aguilar, C.N. Ultrasound-Assisted Extraction of Phenolic Compounds from Laurus nobilis L. and Their Antioxidant Activity. Ultrason. Sonchem. 2013, 20, 1149–1154. [Google Scholar] [CrossRef]
- Mahindrakar, K.V.; Rathod, V.K. Ultrasonic Assisted Aqueous Extraction of Catechin and Gallic Acid from Syzygium Cumini Seed Kernel and Evaluation of Total Phenolic, Flavonoid Contents and Antioxidant Activity. Chem. Eng. Process.-Process Intensif. 2020, 149, 107841. [Google Scholar] [CrossRef]
- Zhang, L.-L.; Xu, M.; Wang, Y.-M.; Wu, D.-M.; Chen, J.-H. Optimizing Ultrasonic Ellagic Acid Extraction Conditions from Infructescence of Platycarya Strobilacea Using Response Surface Methodology. Molecules 2010, 15, 7923–7932. [Google Scholar] [CrossRef]
- Silva, E.M.; Rogez, H.; Larondelle, Y. Optimization of Extraction of Phenolics from Inga Edulis Leaves Using Response Surface Methodology. Sep. Purif. Technol. 2007, 55, 381–387. [Google Scholar] [CrossRef]
- Zhong, K.; Wang, Q. Optimization of Ultrasonic Extraction of Polysaccharides from Dried Longan Pulp Using Response Surface Methodology. Carbohydr. Polym. 2010, 80, 19–25. [Google Scholar] [CrossRef]
- Hugo, P.-C.; Gil-Chávez, J.; Sotelo-Mundo, R.R.; Namiesnik, J.; Gorinstein, S.; González-Aguilar, G.A. Antioxidant Interactions between Major Phenolic Compounds Found in ‘Ataulfo’ Mango Pulp: Chlorogenic, Gallic, Protcatechuic and Vanillic Acids. Molecules 2012, 17, 12657–12664. [Google Scholar] [CrossRef]
- Kim, D.-O.; Lee, K.W.; Lee, H.J.; Lee, C.Y. Vitamin C Equivalent Antioxidant Capacity (VCEAC) of Phenolic Phytchemicals. J. Agric. Food Chem. 2002, 50, 3713–3717. [Google Scholar] [CrossRef] [PubMed]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant Activity of Dietary Polyphenols as Determined by a Modified Ferric Reducing/Antioxidant Power Assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef] [PubMed]
- Vasco, C.; Ruales, J.; Kamal-Eldin, A. Total Phenolic Compounds and Antioxidant Capacities of Major Fruits from Ecuador. Food Chem. 2008, 111, 816–823. [Google Scholar] [CrossRef]
- Liberal, Â.; Fernandes, Â.; Polyzos, N.; Petropoulos, S.A.; Dias, M.I.; Pinela, J.; Petrović, J.; Soković, M.; Ferreira, I.C.F.R.; Barros, L. Bioactive Properties and Phenolic Compound Profiles of Turnip-Rooted, Plain-Leafed and Curly-Leafed Parsley Cultivars. Molecules 2020, 25, 5606. [Google Scholar] [CrossRef]
- Tang, E.L.-H.; Rajarajeswaran, J.; Fung, S.; Kanthimathi, M.S. Petroselinum crispum Has Antioxidant Properties, Protects against DNA Damage and Inhibits Proliferation and Migration of Cancer Cells. J. Sci. Food Agric. 2015, 95, 2763–2771. [Google Scholar] [CrossRef]
- Shi, J.; Nawaz, H.; Pohorly, J.; Mittal, G.; Kakuda, Y.; Jiang, Y. Extraction of Polyphenolics from Plant Material for Functional Foods-Engineering and Technology. Food Rev. Int. 2005, 21, 139–166. [Google Scholar] [CrossRef]
- Ali, F.; Rahul; Naz, F.; Jyoti, S.; Siddique, Y.H. Health Functionality of Apigenin: A Review. Int. J. Food Prop. 2017, 20, 1197–1238. [Google Scholar] [CrossRef]
- Taheri, Y.; Sharifi-Rad, J.; Antika, G.; Yilmaz, Y.B.; Tumer, T.B.; Abuhamdah, S.; Chandra, S.; Saklani, S.; Kiliç, C.S.; Sestito, S.; et al. Paving Luteolin Therapeutic Potentialities and Agro-Food-Pharma Applications: Emphasis on in Vivo Pharmacological Effects and Bioavailability Traits. Oxid. Med. Cell. Longev. 2021, 2021, 1987588. [Google Scholar] [CrossRef] [PubMed]
- Vinceković, M.; Viskić, M.; Jurić, S.; Giacometti, J.; Bursać Kovačević, D.; Putnik, P.; Donsì, F.; Barba, F.J.; Režek Jambrak, A. Innovative Technologies for Encapsulation of Mediterranean Plants Extracts. Trends Food Sci. Technol. 2017, 69, 1–12. [Google Scholar] [CrossRef]


| Factor Levels and Range | ||||
|---|---|---|---|---|
| Factors | Codes | −1 | 0 | 1 |
| Temperature (°C) | X1 | 25 | 47.5 | 70 |
| Time (min) | X2 | 10 | 25 | 40 |
| 1 LS ratio (mL/g) | X3 | 20 | 60 | 100 |
| Ethanol (%, v/v) | X4 | 0 | 20 | 40 |
| Run | Independent Factors | Luteolin (μg/g) 2 | Caffeic Acid (μg/g) | Apigenin/(μg/g) | |||
|---|---|---|---|---|---|---|---|
| X1 Temperature (°C) | X2 Time (min) | X3 Ethanol (% v/v) | X4 1 LS Ratio mL/g Parsley | ||||
| 1 | 47.5 (0) | 10 (−1) | 20 (0) | 20 | 12.2 | 2.2 | 7.1 |
| 2 | 25 (−1) | 40 (+1) | 20 (0) | 60 | 18.3 | 0.46 | 9.3 |
| 3 | 47.5 (0) | 25 (0) | 20 (0) | 60 | 8.8 | 0.92 | 5.5 |
| 4 | 47.5 (0) | 10 (−1) | 20 (0) | 20 | 13.2 | 0.95 | 7.3 |
| 5 | 47.5 (0) | 40 (+1) | 0 (−1) | 60 | 19.5 | 14.8 | 9.6 |
| 6 | 25 (−1) | 25 (0) | 20 (0) | 100 | 21.2 | 3.4 | 10.8 |
| 7 | 47.5 (0) | 10 (−1) | 20 (0) | 100 | 15.6 | 2.2 | 9.8 |
| 8 | 47.5 (0) | 40 (+1) | 20 (0) | 100 | 21.7 | 1.9 | 10.1 |
| 9 | 70 (+1) | 25 (0) | 20 (0) | 20 | 19.9 | 8.4 | 10.1 |
| 10 | 47.5 (0) | 40 (+1) | 20 (0) | 100 | 22.4 | 3.5 | 9.9 |
| 11 | 47.5 (0) | 25 (0) | 20 (0) | 60 | 8.0 | 1.1 | 5.2 |
| 12 | 70 (+1) | 10 (−1) | 20 (0) | 60 | 21.3 | 3.8 | 9.8 |
| 13 | 25 (−1) | 10 (−1) | 20 (0) | 60 | 16.9 | 0.8 | 9.0 |
| 14 | 47.5 (0) | 25 (0) | 0 (−1) | 20 | 14.0 | 10.5 | 4.5 |
| 15 | 47.5 (0) | 10 (−1) | 40 (+1) | 60 | 19.8 | 2.3 | 10.1 |
| 16 | 70 (+1) | 25 (0) | 20 (0) | 20 | 19.3 | 7.9 | 10.2 |
| 17 | 70 (+1) | 25 (0) | 20 (0) | 100 | 24.8 | 1.6 | 12.0 |
| 18 | 47.5 (0) | 40 (+1) | 0 (−1) | 60 | 18.0 | 14.3 | 12.4 |
| 19 | 70 (+1) | 25 (0) | 40 (+1) | 60 | 21.1 | 1.7 | 10.5 |
| 20 | 47.5 (0) | 25 (0) | 20 (0) | 60 | 7.8 | 1.0 | 5.1 |
| 21 | 47.5 (0) | 25 (0) | 20 (0) | 60 | 8.0 | 1.1 | 5.1 |
| 22 | 25 (−1) | 10 (−1) | 20 (0) | 60 | 17.0 | 1.0 | 9.6 |
| 23 | 47.5 (0) | 40 (+1) | 20 (0) | 20 | 12.2 | 1.0 | 7.5 |
| 24 | 47.5 (0) | 10 (−1) | 20 (0) | 20 | 14.4 | 1.1 | 7.3 |
| 25 | 47.5 (0) | 25 (0) | 0 (−1) | 100 | 21.0 | 15.7 | 17.1 |
| 26 | 47.5 (0) | 25 (0) | 40 (+1) | 20 | 14.2 | 1.7 | 7.8 |
| 27 | 70 (+1) | 25 (0) | 20 (0) | 20 | 20.1 | 7.3 | 10.2 |
| 28 | 47.5 (0) | 10 (−1) | 0 (−1) | 60 | 19.3 | 10.9 | 8.9 |
| 29 | 47.5 (0) | 25 (0) | 40 (+1) | 100 | 22.4 | 2.4 | 13.4 |
| 30 | 25 (−1) | 25 (0) | 40 (+1) | 60 | 19.5 | 1.4 | 9.6 |
| 31 | 47.5 (0) | 25 (0) | 20 (0) | 60 | 18.6 | 1.2 | 7.6 |
| 32 | 70 (+1) | 25 (0) | 0 (−1) | 60 | 11.5 | 11.3 | 4.7 |
| 33 | 47.5 (0) | 25 (0) | 40 (+1) | 20 | 15.1 | 1.7 | 8.0 |
| 34 | 70 (+1) | 25 (0) | 20 (0) | 100 | 24.2 | 1.3 | 11.6 |
| 35 | 47.5 (0) | 25 (0) | 0 (−1) | 100 | 22.5 | 16.1 | 18.6 |
| 36 | 70 (+1) | 25 (0) | 20 (0) | 100 | 24.6 | 1.9 | 12.1 |
| 37 | 47.5 (0) | 10 (−1) | 0 (−1) | 60 | 19.3 | 10.6 | 11.2 |
| 38 | 25 (−1) | 40 (+1) | 20 (0) | 60 | 18.1 | 0.44 | 9.7 |
| 39 | 25 (−1) | 25 (0) | 20 (0) | 20 | 14.8 | 0.78 | 7.8 |
| 40 | 25 (−1) | 25 (0) | 20 (0) | 20 | 14.2 | 0.90 | 8.1 |
| 41 | 47.5 (0) | 25 (0) | 0 (−1) | 20 | 14.4 | 10.6 | 5.8 |
| 42 | 47.5 (0) | 25 (0) | 40 (+1) | 100 | 22.5 | 2.5 | 13.2 |
| 43 | 47.5 (0) | 40 (+1) | 20 (0) | 20 | 15.3 | 3.4 | 7.3 |
| 44 | 25 (−1) | 25 (0) | 20 (0) | 100 | 21.5 | 3.1 | 11.0 |
| 45 | 70 (+1) | 25 (0) | 0 (−1) | 60 | 11.4 | 14.1 | 4.1 |
| 46 | 47.5 (0) | 25 (0) | 20 (0) | 60 | 23.3 | 3.3 | 11.2 |
| 47 | 70 (+1) | 40 (+1) | 20 (0) | 60 | 22.5 | 2.4 | 10.5 |
| 48 | 70 (+1) | 25 (0) | 40 (+1) | 60 | 21.6 | 1.4 | 10.6 |
| 49 | 25 (−1) | 25 (0) | 40 (+1) | 60 | 19.5 | 1.5 | 9.5 |
| 50 | 47.5 (0) | 10 (−1) | 40 (+1) | 60 | 19.9 | 2.4 | 9.9 |
| 51 | 70 (+1) | 40 (+1) | 20 (0) | 60 | 20.6 | 1.7 | 10.1 |
| 52 | 25 (−1) | 10 (−1) | 20 (0) | 60 | 17.4 | 0.5 | 9.4 |
| 53 | 47.5 (0) | 10 (−1) | 20 (0) | 100 | 15.3 | 2.1 | 10.0 |
| 54 | 25 (−1) | 25 (0) | 0 (−1) | 60 | 19.6 | 9.2 | 18.6 |
| 55 | 25 (−1) | 40 (+1) | 20 (0) | 60 | 17.8 | 0.42 | 9.9 |
| 56 | 47.5 (0) | 25 (0) | 0 (−1) | 100 | 23.4 | 15.4 | 19.3 |
| 57 | 70 (+1) | 10 (−1) | 20 (0) | 60 | 21.1 | 2.5 | 10.0 |
| 58 | 47.5 (0) | 25 (0) | 0 (−1) | 20 | 14.7 | 10.0 | 5.9 |
| 59 | 47.5 (0) | 40 (+1) | 20 (0) | 20 | 16.3 | 1.4 | 8.5 |
| 60 | 47.5 (0) | 10 (−1) | 40 (+1) | 60 | 19.4 | 2.2 | 10.1 |
| 61 | 70 (+1) | 10 (−1) | 20 (0) | 60 | 21.0 | 2.4 | 10.0 |
| 62 | 70 (+1) | 25 (0) | 0 (−1) | 60 | 11.4 | 13.6 | 4.3 |
| 63 | 47.5 (0) | 25 (0) | 20 (0) | 60 | 20.4 | 3.3 | 10.3 |
| 64 | 47.5 (0) | 10 (−1) | 20 (0) | 100 | 15.3 | 2.1 | 10.0 |
| 65 | 25 (−1) | 25 (0) | 0 (−1) | 60 | 18.9 | 10.9 | 21.0 |
| 66 | 47.5 (0) | 40 (+1) | 40 (+1) | 60 | 19.3 | 2.0 | 9.7 |
| 67 | 47.5 (0) | 40 (+1) | 40 (+1) | 60 | 19.0 | 1.7 | 9.9 |
| 68 | 47.5 (0) | 25 (0) | 20 (0) | 60 | 19.8 | 1.2 | 8.3 |
| 69 | 70 (+1) | 25 (0) | 40 (+1) | 60 | 21.0 | 1.4 | 9.1 |
| 70 | 47.5 (0) | 40 (+1) | 20 (0) | 100 | 19.9 | 2.5 | 9.6 |
| 71 | 25 (−1) | 25 (0) | 20 (0) | 20 | 15.3 | 1.2 | 7.5 |
| 72 | 47.5 (0) | 25 (0) | 20 (0) | 60 | 17.6 | 2.6 | 8.5 |
| 73 | 70 (+1) | 40 (+1) | 20 (0) | 60 | 21.3 | 2.4 | 10.3 |
| 74 | 25 (−1) | 25 (0) | 0 (−1) | 60 | 19.1 | 10.3 | 20.1 |
| 75 | 25 (−1) | 25 (0) | 40 (+1) | 60 | 18.9 | 1.1 | 9.7 |
| 76 | 47.5 (0) | 40 (+1) | 0 (−1) | 60 | 20.6 | 16.0 | 13.1 |
| 77 | 47.5 (0) | 40 (+1) | 40 (+1) | 60 | 19.0 | 1.9 | 9.7 |
| 78 | 47.5 (0) | 25 (0) | 40 (+1) | 100 | 22.6 | 2.3 | 13.5 |
| 79 | 25 (−1) | 25 (0) | 20 (0) | 100 | 20.5 | 2.4 | 11.2 |
| 80 | 47.5 (0) | 10 (−1) | 0 (−1) | 60 | 19.2 | 11.2 | 11.8 |
| 81 | 47.5 (0) | 25 (0) | 40 (+1) | 20 | 14.9 | 1.2 | 7.3 |
| 1 Apigenin | 1 Caffeic Acid | 1 Luteolin | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Source | 2 DF | F-Value | p-Value | DF | F-Value | p-Value | DF | F-Value | p-Value |
| Model | 7 | 14.56 | 0.000 | 8 | 105.45 | 0.000 | 5 | 15.77 | 0.000 |
| Linear | 3 | 15.40 | 0.000 | 4 | 132.21 | 0.000 | 3 | 21.54 | 0.000 |
| T (°C) (X1) | 1 | 4.95 | 0.029 | 1 | 37.98 | 0.000 | 1 | 8.54 | 0.005 |
| Time (min) (X2) | - | - | - | 1 | 0.16 | 0.688 | - | - | - |
| LS ratio (mL/g) (X3) | 1 | 40.36 | 0.000 | 1 | 2.13 | 0.148 | 1 | 53.01 | 0.000 |
| Ethanol (%) (X4) | 1 | 0.90 | 0.345 | 1 | 488.56 | 0.000 | 1 | 3.06 | 0.084 |
| Square | 2 | 6.01 | 0.004 | 2 | 130.70 | 0.000 | 1 | 6.82 | 0.011 |
| T (°C)*T (°C) (X12) | 1 | 4.41 | 0.039 | - | - | - | 1 | 6.82 | 0.011 |
| LSr (mL/g)*LSr (mL/g) (X32) | - | - | - | 1 | 12.29 | 0.001 | - | - | - |
| Ethanol (%)*Ethanol (%) (X42) | 1 | 9.76 | 0.003 | 1 | 261.31 | 0.000 | - | - | - |
| 2-Way Interaction | 2 | 21.83 | 0.000 | 2 | 26.70 | 0.000 | 1 | 7.44 | 0.008 |
| T (°C)*Ethanol (%) (X1X4) | 1 | 36.56 | 0.000 | - | - | - | 1 | 7.44 | 0.008 |
| LSr (mL/g)*Ethanol (%) (X3X4) | 1 | 7.11 | 0.009 | - | - | - | - | - | - |
| T (°C)*LSr (mL/g) (X1X3) | - | - | - | 1 | 49.01 | 0.000 | - | - | - |
| Time (min)*Ethanol (%) (X2X4) | - | - | - | 1 | 4.39 | 0.040 | - | - | - |
| Error | 73 | 72 | 75 | ||||||
| Lack-of-Fit | 67 | 1.03 | 0.550 | 66 | 0.74 | 0.756 | 69 | 0.45 | 0.947 |
| Pure Error | 6 | 6 | 6 | ||||||
| Total | 80 | 80 | 80 | ||||||
| Phenolic | 1 Predictive Equations | |
|---|---|---|
| Apigenin | (2) | Y λa = 3.21 − 0.04X1 + 0.01X3 − 0.04X4 + 0.0002 X12 + 0.0004X42 + 0.0008 X1X4 − 0.0002 X3X4 |
| Caffeic acid | (3) | Yλca = 1.02 + 0.04 X1 + 0.01X2 + 0.01X3 − 0.11X4 + 0.0001 X32 + 0.0021 X42 − 0.0005 X1X3 − 0.0005 X2X4 |
| Luteolin | (4) | (Y(λl−1))/(λl × g^(λl−1)) = 9.80 − 0.34 X1 + 0.08 X3 − 0.19 X4 + 0.0032 X12 + 0.0048 X2X4 |
| 1 Independent Factors | Predicted Values | Experimental Values | Desirability |
|---|---|---|---|
| Luteolin (μg/gDM) | 24.54 a | 23.92 ± 1.86 a | 0.98223 |
| Caffeic acid (μg/gDM) | 18.48 a | 19.10 ± 0.75 a | 1.0000 |
| Apigenin (μg/gDM) | 23.53 a | 25.29 ± 1.82 a | 1.0000 |
| 1 Parameters | Apigenin Optimized Extract | Caffeic Acid Optimized Extract | Luteolin Optimized Extract |
|---|---|---|---|
| DPPH (nmol TE) | 638.5 ± 21.3 a | 510.5 ± 19.4 b | 638.3 ± 24.4 a |
| ABTS (nmol TE) | 687.7 ± 20.3 a | 550.4 ± 18.1 b | 687.8 ± 22.3 a |
| FRAP (nmol TE) | 114.4 ± 3.1 a | 115.4 ± 3.2 a | 118.8 ± 2.9 a |
| CUPRAC (nmol TE) | 13.2 ± 0.6 a | 13.3 ± 0.4 a | 14.5 ± 0.8 a |
| TPC (mg GAE) | 39.4 ± 0.8 a | 38.8 ± 0.5 a | 39.9 ± 0.7 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michalaki, A.; Karantonis, H.C.; Kritikou, A.S.; Thomaidis, N.S.; Dasenaki, M.E. Ultrasound-Assisted Extraction of Specific Phenolic Compounds from Petroselinum crispum Leaves Using Response Surface Methodology and HPLC-PDA and Q-TOF-MS/MS Identification. Appl. Sci. 2023, 13, 798. https://doi.org/10.3390/app13020798
Michalaki A, Karantonis HC, Kritikou AS, Thomaidis NS, Dasenaki ME. Ultrasound-Assisted Extraction of Specific Phenolic Compounds from Petroselinum crispum Leaves Using Response Surface Methodology and HPLC-PDA and Q-TOF-MS/MS Identification. Applied Sciences. 2023; 13(2):798. https://doi.org/10.3390/app13020798
Chicago/Turabian StyleMichalaki, Afroditi, Haralabos C. Karantonis, Anastasia S. Kritikou, Nikolaos S. Thomaidis, and Marilena E. Dasenaki. 2023. "Ultrasound-Assisted Extraction of Specific Phenolic Compounds from Petroselinum crispum Leaves Using Response Surface Methodology and HPLC-PDA and Q-TOF-MS/MS Identification" Applied Sciences 13, no. 2: 798. https://doi.org/10.3390/app13020798
APA StyleMichalaki, A., Karantonis, H. C., Kritikou, A. S., Thomaidis, N. S., & Dasenaki, M. E. (2023). Ultrasound-Assisted Extraction of Specific Phenolic Compounds from Petroselinum crispum Leaves Using Response Surface Methodology and HPLC-PDA and Q-TOF-MS/MS Identification. Applied Sciences, 13(2), 798. https://doi.org/10.3390/app13020798







