Antioxidant Properties of Selenium Nanoparticles Synthesized Using Tea and Herb Water Extracts
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Tea, Herbal Samples, and Supplement
2.3. Chromatographic Analysis of Polyphenols in the Studied Infusions
2.4. The Synthesis and Characterization of Obtained SeNPs
2.5. Antioxidant Activity Measurements
2.6. Statistical Analysis
3. Results and Discussion
3.1. HPLC Analysis ad UV–Vis Spectroscopy of Used Plant Extracts
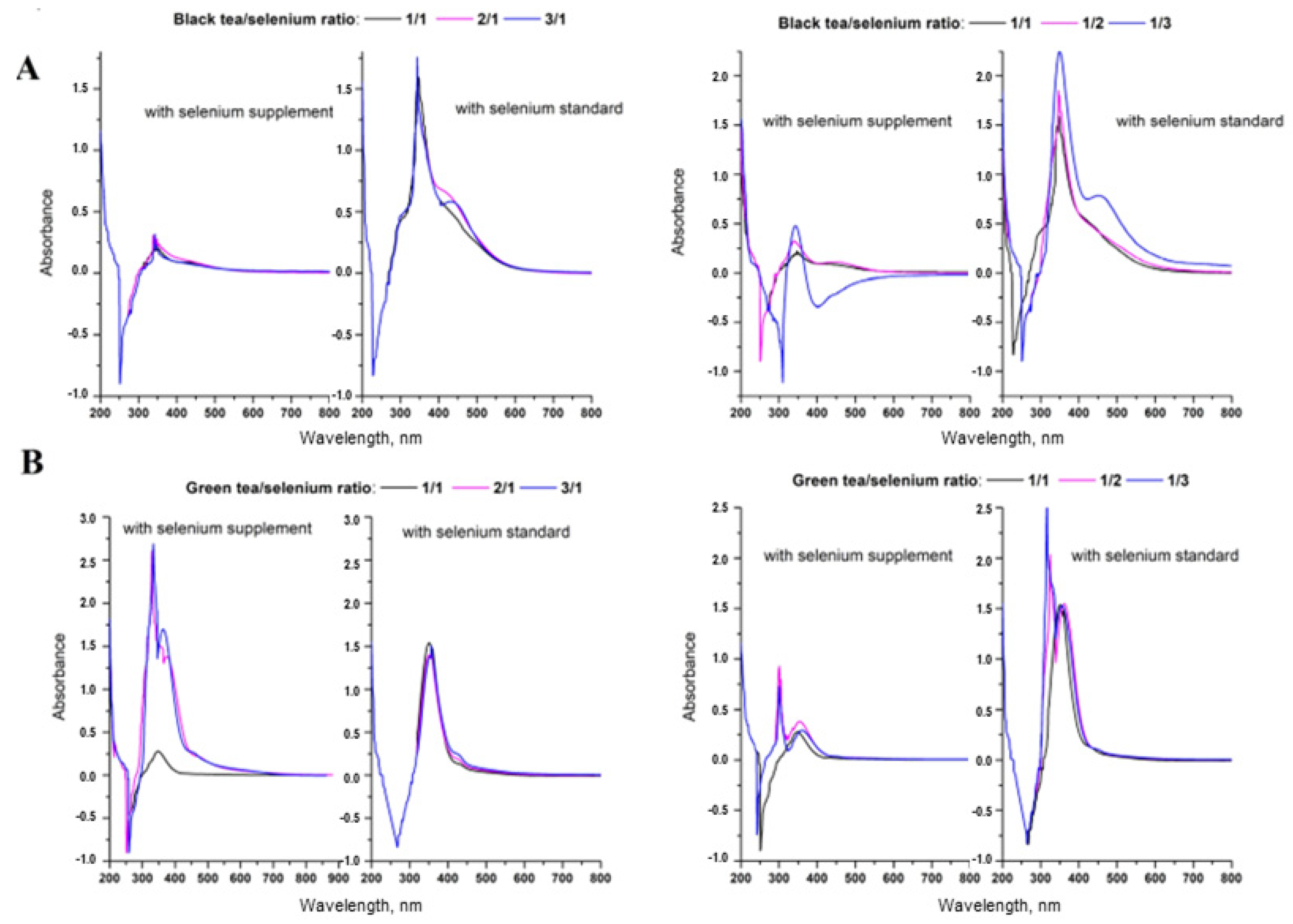
3.2. Synthesis and Characterization of SeNPs
3.3. Antioxidant Activity of Obtained SeNPs
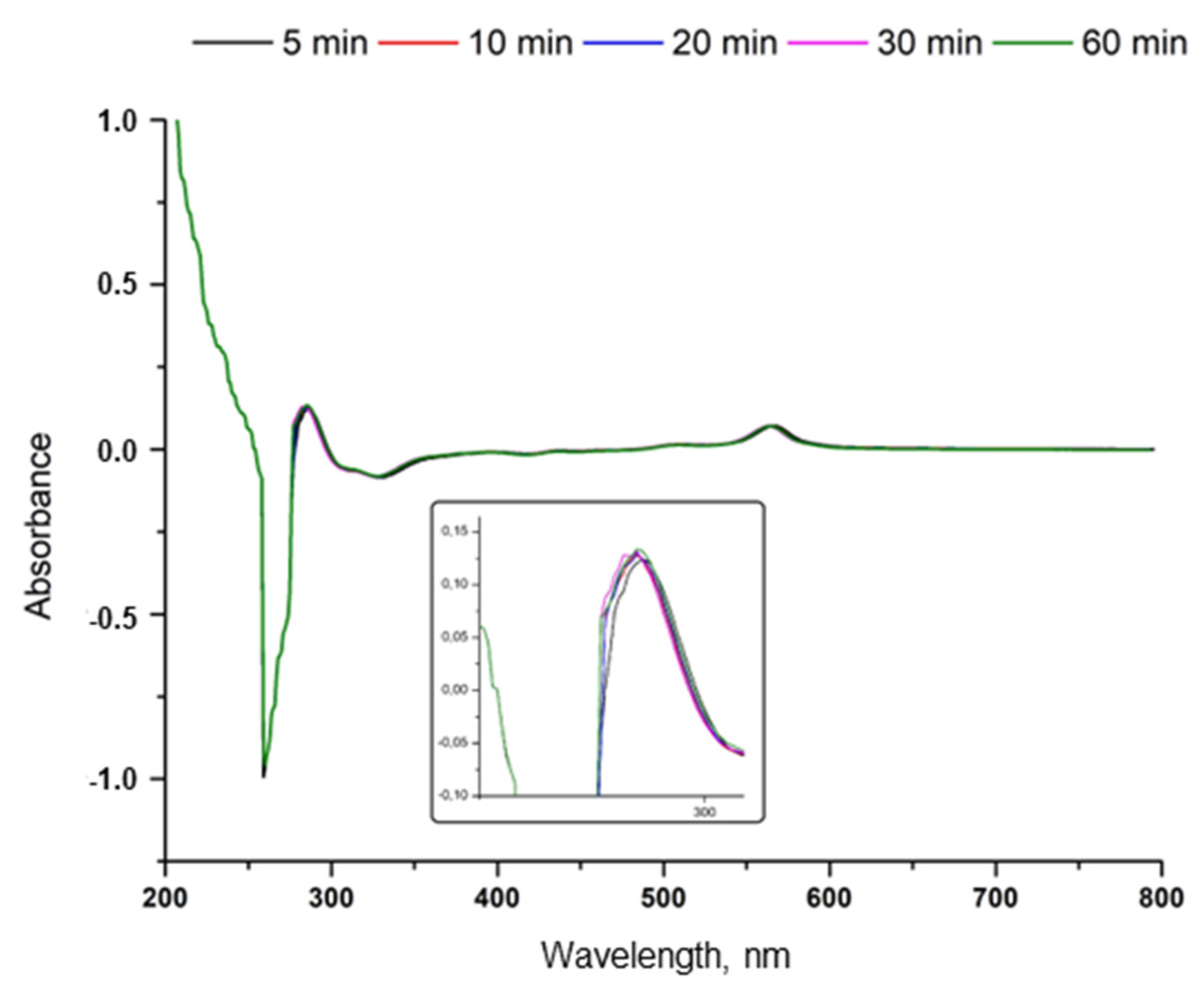
3.4. Synthesis of SeNPs in a Cup of Tea
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kieliszek, M. Selenium–fascinating microelement, properties and sources in food. Molecules 2019, 24, 1298. [Google Scholar] [CrossRef]
- Fairweather-Tait, S.J.; Bao, Y.; Broadley, M.R.; Collings, R.; Ford, D.; Hesketh, J.E.; Hurst, R. Selenium in human health and disease. Antioxid. Redox Signal. 2018, 14, 1337–1383. [Google Scholar] [CrossRef]
- Handy, D.E.; Loscalzo, J. The role of glutathione peroxidase-1 in health and disease. Free Radic. Biol. Med. 2022, 188, 146–161. [Google Scholar] [CrossRef]
- Razaghi, A.; Poorebrahim, M.; Sarhan, D.; Björnstedt, M. Selenium stimulates the antitumour immunity: Insights to future research, Eur. J. Cancer 2021, 155, 256–267. [Google Scholar] [CrossRef]
- Wan, J.; Zhang, M.; Adihikari, B. Advances in selenium-enriched foods: From the farm to the fork. Trends Food Sci. Technol. 2018, 76, 1–5. [Google Scholar] [CrossRef]
- Adadi, P.; Barakova, N.V.; Muravyov, K.Y.; Krivoshapkina, E.F. Designing selenium functional foods and beverages: A review. Food Res. Int. 2019, 120, 708–725. [Google Scholar] [CrossRef]
- Zakeri, N.; Kelishadi, M.R.; Asbaghi, O.; Naeini, F.; Afsharfar, M.; Mirzadeh, E.; Naserizadeh, S.K. Selenium supplementation and oxidative stress: A review. Pharm. Nutr. 2021, 17, 100263. [Google Scholar] [CrossRef]
- Hadrup, N.; Ravn-Haren, G. Absorption, distribution, metabolism and excretion (ADME) of oral selenium from organic and inorganic sources: A review. J. Trace Elem. Med. Biol. 2021, 67, 126801. [Google Scholar] [CrossRef]
- Nayak, V.; Singh, K.R.B.; Singh, A.K.; Singh, R.P. Potentialities of selenium nanoparticles in biomedical science. New J. Chem. 2021, 45, 2849–2878. [Google Scholar] [CrossRef]
- Kumar, P.; Mahajan, P.; Kaur, R.; Gautam, S. Nanotechnology and its challenge in the food sector: A review. Mater. Today Chem. 2020, 17, 100332. [Google Scholar] [CrossRef]
- Kondaparthi, P.; Flora, S.J.S.; Naqvi, S. Selenium nanoparticles: An insight on its pro-oxidant and antioxidant properties. Front. Nanosci. Nanotech. 2019, 6, 1–5. [Google Scholar] [CrossRef]
- Menon, S.; Shrudhi Devi, K.S.; Agarval, H.; Shanmugam, V.K. Efficacy of biogenic selenium nanoparticles from an extract of ginger towards evaluation on anti-microbial and anti-oxidant activities. Colloids Interface Sci. Commun. 2019, 29, 1–8. [Google Scholar] [CrossRef]
- Zhao, G.; Wu, X.; Chen, P.; Zhang, L.; Yang, C.S.; Zhan, J. Selenium nanoparticles are more efficient than sodium selenite in producing reactive oxygen species and hyper-accumulation of selenium nanoparticles in cancer cells generates potent therapeutic effects. Free Radic. Biol. Med. 2018, 126, 55–66. [Google Scholar] [CrossRef]
- Kuršvietiene, L.; Mongirdienė, A.; Bernatonienė, J.; Šulinskienė, J.; Štanevičienė, I. Selenium anticancer properties and impast on cellular redox status. Antioxidants 2020, 9, 80. [Google Scholar] [CrossRef]
- Gharbav, M.; Johari, B.; Mousazadeh, N.; Rahimi, B.; Leilan, M.P.; Eslami, S.S.; Sharafi, A. Hybrid of niosomes and bio-synthesized selenium nanoparticles as a novel approach in drug delivery for cancer treatment. Mol. Biol. Rep. 2020, 47, 6517–6529. [Google Scholar] [CrossRef]
- Bisht, N.; Phalswal, P.; Khanna, P.K. Selenium nanoparticles: A review on synthesis and biomedical applications. Mater. Adv. 2022, 3, 1415–1431. [Google Scholar] [CrossRef]
- Korde, P.; Ghotekar, S.; Pagar, T.; Pansambal, S.; Oza, R.; Mane, D. Plant extract assisted eco-benevolent synthesis of selenium nanoparticles-a review on plant parts involved, characterization and their recent applications. J. Chem. Rev. 2020, 2, 157–168. [Google Scholar]
- Pyrzynska, K.; Sentkowska, A. Biosynthesis of selenium nanoparticles using plant extracts. J. Nanostruct. Chem. 2022, 12, 467–480. [Google Scholar] [CrossRef]
- Marslin, G.; Siram, K.; Maqbool, Q.; Selvakesavan, R.K.; Kruszka, D.; Kachlicki, P.; Franklin, G. Secondary metabolites in the green synthesis of metallic nanoparticles. Materials 2018, 11, 940. [Google Scholar] [CrossRef]
- Asimuddin, M.; Shaik, M.R.; Adil, S.F.; Siddigui, M.R.H.; Alwarthan, A.; Jamil, K.; Khan, M. Azadirachta indica based biosynthesis of silver nanoparticles and evaluation of their antibacterial and cytotoxic effects. J. King Saud. Univ. Sci. 2020, 32, 648–656. [Google Scholar] [CrossRef]
- Ali, S.G.; Ansari, M.A.; Jamal, Q.M.S.; Almatroudi, A.; Alzohairy, M.A.; Alomary, M.N.; Rehman, S.; Mahadevamurthy, M.; Jalal, M.; Khan, H.M.; et al. Butea monosperma seed extract mediated biosynthesis of ZnO NPs and their antibacterial, antibiofilm and anti-quorum sensing potentialities. Arab. J. Chem. 2021, 14, 103044. [Google Scholar] [CrossRef]
- Saif, S.; Tahir, A.; Asim, T.; Chen, Y.; Khan, M.; Adil, S.F. Green synthesis of ZnO hierarchical microstructures by Cordia myxa and their antibacterial activity. Saudi J. Biol. Sci. 2019, 26, 1364–1371. [Google Scholar] [CrossRef]
- Khan, M.; Albalawi, G.H.; Shaik, M.R.; Khan, M.; Adil, S.F.; Kuniyil, M.; Alkhathalan, H.Z.; Al-Warthan, A.; Siddiqui, M.R.H. Miswak mediated green synthesized palladium nanoparticles as effective catalysis for Suzuki coupling reactions in aqueous media. J. Saudi Chem. Soc. 2017, 21, 450–457. [Google Scholar] [CrossRef]
- Bai, K.; Hong, B.; He, J.; Hong, Z.; Tan, R. Preparation and antioxidant properties of selenium nanoparticles-loaded chitosan microspheres. Int. J. Nanomed. 2017, 12, 4527–4539. [Google Scholar] [CrossRef] [PubMed]
- Sentkowska, A.; Biesaga, M.; Pyrzynska, K. Effects of operation parameters on HILIC separation of flavonoids on zwitterionic column. Talanta 2013, 115, 284–290. [Google Scholar] [CrossRef]
- Smirnoff, N.; Cumbes, Q.J. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry 1987, 28, 1057–1060. [Google Scholar] [CrossRef]
- Peterson, J.; Dwyer, J.; Bhagwat, S.; Haytowitz, D.; Holden, J.; Eldridge, A.L.; Beecher, G.; Aladesanmi, J. Major flavonoids in dry tea. J. Food Compos. Anal. 2005, 18, 487–501. [Google Scholar] [CrossRef]
- Sing, A.K.; Seth, A.P.; Husain, M.M.; Madhavan, S.; Mukhtar, H.; Maheshwari, R.K. Green tea constituent epigallocatechin-3-gallate inhibits angiogenic differentiation of human endothelial cells. Arch. Biochem. Biophys. 2002, 401, 29–37. [Google Scholar] [CrossRef]
- Li, S.; Lo, C.Y.; Pan, M.H.; Lai, C.S.; Ho, C.T. Black tea: Chemical analysis and stability. Food Funct. 2013, 4, 10–18. [Google Scholar] [CrossRef]
- Sentkowska, A.; Biesaga, M.; Pyrzynska, K. Effects of brewing process on phenolic compounds and antioxidant activity of herbs. Food Sci. Biotechnol. 2016, 25, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.; John, J.A.; Shahidi, F. Polyphenol composition and antioxidant potential of mint leaves. Food Prod. Process. Nutr. 2019, 1, 1. [Google Scholar] [CrossRef]
- Pękal, A.; Dróżdż, P.; Biesaga, M.; Pyrzynska, K. Evaluation of the antioxidant properties of fruit and flavoured black teas. Eur. J. Nutr. 2011, 50, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Masłowski, M.; Aleksieiev, A.; Miedzianowska, J.; Strzelec, K. Potential application of peppermint (Mentha piperita L.), german chamomile (Matricaria chamomilla L.) and yarrow (Achillea millefolium L.) as active fillers in natural rubber biocomposites. Int. J. Mol. Sci. 2021, 22, 7530. [Google Scholar] [CrossRef]
- Beigi, M.; Torki-Harchegani, M.; Pirbalouti, A.G. Quantity and chemical composition of essential oil of peppermint (Mentha piperita L.) leaves under different drying methods. Int. J. Food Prop. 2018, 21, 267–276. [Google Scholar] [CrossRef]
- Kazemi, M. Chemical Composition and Antimicrobial Activity of Essential Oil of Matricaria recutita. Int. J. Food Prop. 2014, 18, 1784–1792. [Google Scholar] [CrossRef]
- Wyrostek, J.; Kowalski, R.; Pankiewicz, U.; Solarska, E. Estimation of the Content of Selected Active Substances in Primary and Secondary Herbal Brews by UV-VIS and GC-MS Spectroscopic Analyses. J. Anal. Methods Chem. 2020, 2020, 8891855. [Google Scholar] [CrossRef] [PubMed]
- Giovannetti, R.; Alibabaei, L.; Zannotti, M.; Ferraro, S.; Petetta, L. HPLC-DAD-ESI/MS Identification of Light Harvesting and Light Screening Pigments in the Lake Sediments at Edmonson Point. Sci. World J. 2013, 2013, 741906. [Google Scholar] [CrossRef] [PubMed]
- Atobe, S.; Saga, K.; Maeyama, H.; Fujiwara, K.; Okada, S.; Imou, K. Culture of the green microalga Botryococcus braunii Showa with LED irradiation eliminating violet light enhances hydrocarbon production and recovery. Biosci. Biotechnol. Biochem. 2014, 78, 1765–1771. [Google Scholar] [CrossRef]
- Fernandez, P.L.; Martin, M.J.; Gonzalez, A.G.; Pablos, F. HPLC determination of catechins and caffeine in tea. Differentiation of green, black and instant teas. Analyst 2000, 125, 421–425. [Google Scholar] [CrossRef]
- Roberts, E.A.H.; Smith, R.F. Spectrophotometric measurements of theaflavins and thearubigins in black tea liquors in assessments of quality in teas. Analyst 1961, 20, 141–151. [Google Scholar] [CrossRef]
- Palacios-Morillo, A.; Alcazar, A.; de Pablos, F.; Jurando, J.M. Differentiation of tea varieties using UV-Vis spectra and pattern recognition techniques. Spectrochim. Acta Part A Mol. Biomol. Spec. 2013, 103, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Sentkowska, A.; Pyrzyńska, K. Does the type matter? Verification of different tea types’ potential in the synthesis of SeNPs. Antioxidants 2022, 11, 2489. [Google Scholar] [CrossRef]
- Lin, Z.H.; Chris-Wang, C.R. Evidence on the size-dependent absorption spectral evolution of selenium nanoparticles. Mater. Chem. Phys. 2005, 92, 591–594. [Google Scholar] [CrossRef]
- Sentkowska, A.; Pyrzynska, K. The influence of synthesis conditions on the antioxidant activity of selenium nanoparticles. Molecules 2022, 27, 2486. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Dutta, T.; Kim, K.H.; Rawat, M.; Samddar, P.; Kumar, P. Green synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 84. [Google Scholar] [CrossRef]
- Vyas, J.; Rana, S. Antioxidants activity and green synthesis of selenium nanoparticles using Allium sativum extract. Int. J. Phytomed. 2017, 9, 634–641. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Qiu, W.Y.; Sun, L.; Ding, Z.C.; Ya, J.K. Preparation, characterization, and antioxidant capacities of selenium nanoparticles stabilized using polysaccharide-protein complexes from Corbicula fluminea. Food Biosen. 2018, 26, 177–184. [Google Scholar] [CrossRef]
- Bardaweel, S.K.; Gul, M.; Alzweiri, M.; Ishaqat, A.; ALSalamat, H.A.; Bashatwah, R.M. Reactive Oxygen Species: The dual role in physiological and pathological conditions of the human body. Eurasian J. Med. 2018, 50, 93–201. [Google Scholar] [CrossRef]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS sources in physiological and pathological conditions. Oxid. Med. Cell. Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef]
- Sandhir, R.; Yadav, A.; Sunkaria, A.; Singhal, N. Nano-antioxidants: An emerging strategy for intervention against neurodegenerative conditions. Neurochem. Int. 2015, 89, 26315960. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed]
- Castro-Alves, V.C.; Cordenunsi, B.R. Total soluble phenolic compounds quantification is not as simple as it seems. Food Anal. Meth. 2015, 8, 873–884. [Google Scholar] [CrossRef]
- Makarov, V.V.; Love, A.J.; Sinitsyna, O.V.; Makarova, S.S.; Yaminsky, I.V.; Taliansky, M.E.; Kalinina, N.O. “Green” nanotechnologies: Synthesis of metal nanoparticles using plants. Acta Nature 2014, 6, 35–44. [Google Scholar] [CrossRef]
- He, X.; Li, J.; Zhao, W.; Liu, R.; Zhang, L.; Kong, X. Chemical fingerprint analysis for quality control and identification of Ziyang green tea by HPLC. Food Chem. 2015, 171, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Sentkowska, A. Content of selenoaminoacids and catechins in Chinese green teas. Eur. Res. Technol. 2021, 247, 613–622. [Google Scholar] [CrossRef]

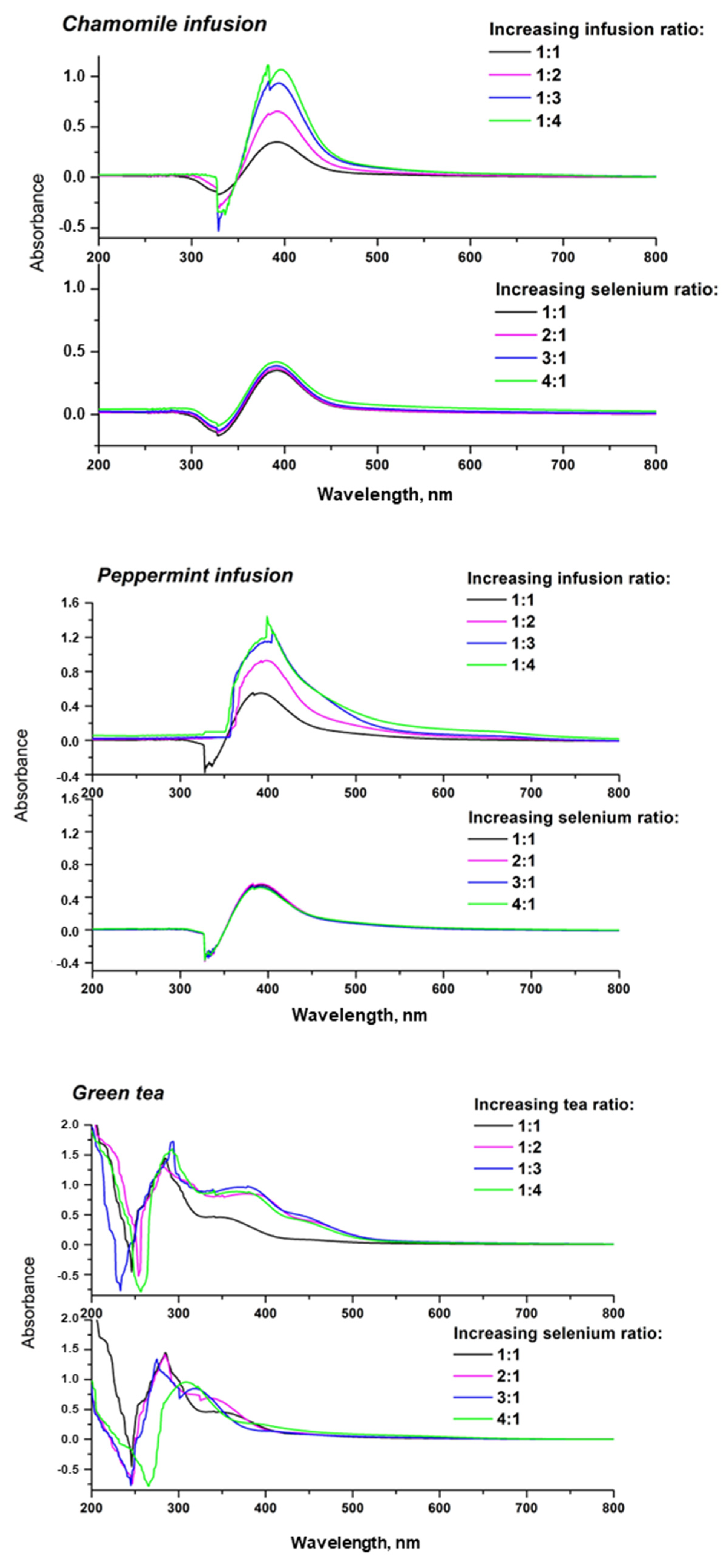
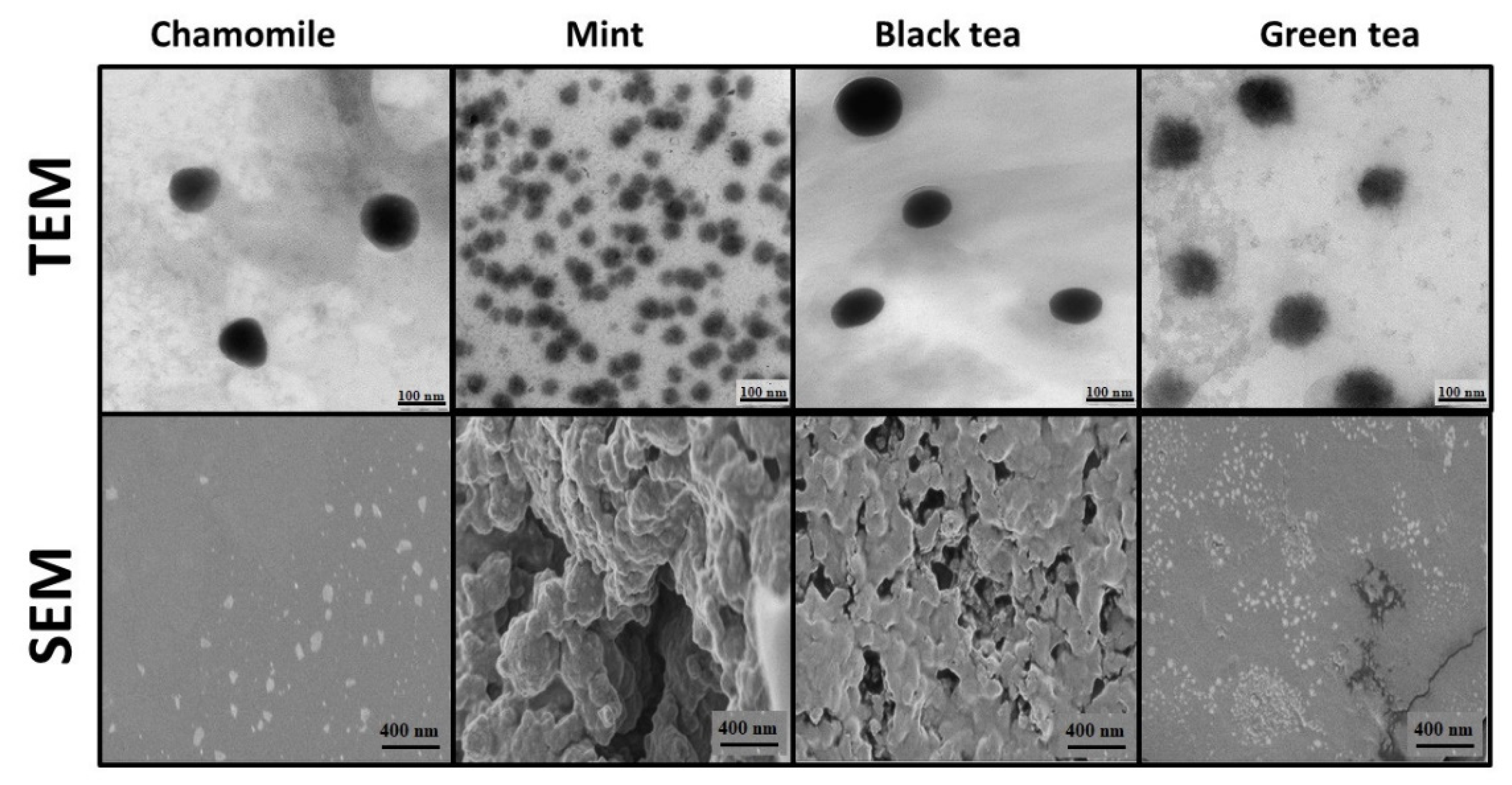
| Compound | Herbal Extracts | Camelia sinesis Extracts | ||
|---|---|---|---|---|
| Chamomile | Mint | Black Tea | Green Tea | |
| Polyphenolic acids | ||||
| Gallic | 0.150 ± 0.006 a | 0.080 ± 0.003 b | 4.76 ± 0.183 c | 2.45 ± 0.110 d |
| Caffeic | 0.101 ± 0.005 a | 0.265 ± 0.013 b | <LOD | <LOD |
| pHBA | 12.1 ± 0.461 a | 23.1 ± 0.875 b | 68.9 ± 2.20 c | 38.5 ± 1.05 d |
| Chlorogenic | 0.963 ± 0.040 a | 0.201 ± 0.01 b | 1.25 ± 0.020 c | 2.23 ± 0.100 d |
| p-coumaric | 1.52 ± 0.070 a | 1.39 ± 0.070 b | 9.26 ± 0.400 c | 2.61 ± 0.105 d |
| Protocatechuic | 0.401 ± 0.015 a | 1.06 ± 0.05 b | 0.608 ± 0.030 c | 0.365 ± 0.018 d |
| Flavonoids | ||||
| Rutin | 1.12 ± 0.010 a | <LOD | 18.7 ± 0.870 b | 35.7 ± 1.12 c |
| Catechin | <LOD | <LOD | 1.30 ± 0.060 a | 39.3 ± 1.33 b |
| Epicatechin | <LOD | <LOD | 0.330 ± 0.016 a | 1.64 ± 0.080 b |
| EGCG | 3.91 ± 0.121 a | 1.69 ± 0.084 b | 23.7 ± 1.15 c | 90.1 ± 2.31 d |
| Luteolin | 0.442 ± 0.020 a | 0.138 ± 0.007 b | <LOD | <LOD |
| Apigenin | 1.24 ± 0.060 a | <LOD | <LOD | <LOD |
| Quercitrin | <LOD | <LOD | <LOD | 0.100 ± 0.004 a |
| Ascorbic acid | 25.6 ± 1.20 a | 17.2 ± 0.75 b | 58.3 ± 2.60 c | 31.4 ± 1.32 d |
| Tea Infusions | ||
|---|---|---|
| Selenium/Infusion Ratio | Black Tea | Green Tea |
| 1:1 | 0.317 | 0.253 |
| 1:2 | 0.943 | 0.920 |
| 1:3 | 0.644 | 0.909 |
| 1:4 | 0.260 | 0.911 |
| 2:1 | 0.105 | 0.129 |
| 3:1 | 0.541 | 0.815 |
| 4:1 | 0.881 | 0.818 |
| Herbal infusions | ||
| Selenium/infusion ratio | Chamomile | Mint |
| 1:1 | 0.481 | 0.944 |
| 1:2 | 0.890 | 0.130 |
| 1:3 | 0.751 | 0.146 |
| 1:4 | 0.829 | 0.154 |
| 2:1 | 0.109 | 0.082 |
| 3:1 | 0.322 | 0.109 |
| 4:1 | 0.920 | 0.115 |
| FC Method [mg GA/L] | OH· Assay [%] | |
|---|---|---|
| Black tea extract | 597.1 ± 7.23 a | 65.6 ± 2.71 a |
| SeNPs synthesized with black tea extract | ||
| Selenium/infusion ratio | ||
| 1:1 | 69.41 ± 2.751 b | 97.3 ± 3.21 b |
| 1:2 | 163.9 ± 6.703 c | 95.0 ± 1.40 c |
| 1:3 | 216.0 ± 8.840 d | 91.7 ± 3.57 d |
| 1:4 | 278.3 ± 9.231 e | 85.9 ± 4.04 e |
| 2:1 | 82.61 ± 3.633 f | 94.2 ± 2.84 c |
| 3:1 | 63.21 ± 3.070 g | 85.0 ± 2.89 e |
| 4:1 | 57.74 ± 2.520 h | 77.0 ± 3.17 f |
| Green tea extract | 679.0 ± 8.75 a | 70.8 ± 2.00 a |
| SeNPs synthesized with green tea extract | ||
| Selenium/infusion ratio | ||
| 1:1 | 68.11 ± 2.50 b | 99.8 ± 3.40 b |
| 1:2 | 163.1 ± 6.41 c | 96.8 ± 4.08 c |
| 1:3 | 234.8 ± 9.31 d | 78.3 ± 2.80 d |
| 1:4 | 346.6 ± 11.0 e | 100 ± 2.75 b |
| 2:1 | 66.41 ± 2.11 b | 97.0 ± 3.21 c |
| 3:1 | 61.3 ± 3.03 e | 84.6 ± 3.70 e |
| 4:1 | 62.93 ± 2.50 e | 45.6 ± 1.99 f |
| Chamomile extract | 89.94 ± 4.21 a | 33.9 ± 1.36 a |
| SeNPs synthesized with chamomile extract | ||
| Selenium/infusion ratio | ||
| 1:1 | 14.77 ± 0.63 b | 65.7 ± 2.43 b |
| 1:2 | 25.57 ± 1.07 c | 80.6 ± 3.37 c |
| 1:3 | 33.09 ± 0.75 d | 92.5 ± 3.18 d |
| 1:4 | 37.94 ± 1.63 e | 99.1 ± 4.01 e |
| 2:1 | 20.31 ± 0.95 f | 71.9 ± 2.71 f |
| 3:1 | 12.83 ± 0.533 g | 92.8 ± 2.57 d |
| 4:1 | 15.57 ± 0.75 b | 97.9 ± 3.87 g |
| Mint extract | 354.7 ± 9.85 a | 53.87 ± 2.04 a |
| SeNPs synthesized with mint extract | ||
| Selenium/infusion ratio | ||
| 1:1 | 86.11 ± 3.21 b | 94.4 ± 2.33 b |
| 1:2 | 71.44 ± 1.63 c | 91.1 ± 3.61 c |
| 1:3 | 110.7 ± 4.50 d | 79.6 ± 1.95 d |
| 1:4 | 134.7 ± 5.07 e | 99.9 ± 3.70 e |
| 2:1 | 35.94 ± 1.21 f | 86.6 ± 3.11 f |
| 3:1 | 31.34 ± 0.95 g | 94.3 ± 1.75 b |
| 4:1 | 34.69 ± 1.11 f | 99.9 ± 2.20 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sentkowska, A.; Pyrzyńska, K. Antioxidant Properties of Selenium Nanoparticles Synthesized Using Tea and Herb Water Extracts. Appl. Sci. 2023, 13, 1071. https://doi.org/10.3390/app13021071
Sentkowska A, Pyrzyńska K. Antioxidant Properties of Selenium Nanoparticles Synthesized Using Tea and Herb Water Extracts. Applied Sciences. 2023; 13(2):1071. https://doi.org/10.3390/app13021071
Chicago/Turabian StyleSentkowska, Aleksandra, and Krystyna Pyrzyńska. 2023. "Antioxidant Properties of Selenium Nanoparticles Synthesized Using Tea and Herb Water Extracts" Applied Sciences 13, no. 2: 1071. https://doi.org/10.3390/app13021071
APA StyleSentkowska, A., & Pyrzyńska, K. (2023). Antioxidant Properties of Selenium Nanoparticles Synthesized Using Tea and Herb Water Extracts. Applied Sciences, 13(2), 1071. https://doi.org/10.3390/app13021071







