Abstract
The diet rich in fruits is widely recommended for health-promoting properties. Regular consumption of fruits could reduce the risk of many diseases. The fruit-based alcoholic beverages have been produced for centuries and, in many countries, are still very popular. ‘Nalewka’ is a traditional name of Polish liqueur, i.e., an alcoholic beverage made by maceration of fruits. Homemade nalewkas are often stored for a long time before consumption, which can affect the content of valuable secondary metabolites. The aim of the study was to compare the effect of long-time storage on antioxidant activity and total polyphenol, total anthocyanin, tannin as well as gallic acid content in three homemade nalewkas. Cherry, plum, and multifruit nalewka were produced in 1997 and between 2013–2019 years. The antioxidant activity was measured by the DPPH, ABTS, FRAP, and CUPRAC methods. The content of gallic acid was estimated by high-performance liquid chromatography (HPLC). The oldest nalewkas (from 1997) showed significantly lower antioxidant activity as well as a lower content of polyphenols, anthocyanins, and gallic acid compared to the later-produced nalewkas, in particular those produced in 2016–2019. In most cases, a correlation was also found between the parameters of antioxidant activity as well as the total content of polyphenols and anthocyanins. Long-term storage of nalewkas reduces the content of valuable secondary metabolites responsible for the antioxidant activity and, thus, the health properties of the beverage.
1. Introduction
A diet rich in fruits is widely recommended for its health-promoting properties. Regular consumption of fruits is correlated with the decreased risk of many civilization diseases, i.e., cardiovascular, cancer, osteoporosis, diabetes, and many others. Their valuable properties are related, among others, to their ability to deactivate reactive oxygen species (ROS) [1,2,3,4]. The uncontrolled production of ROS can lead to oxidative stress responsible for accelerated aging and the development of many diseases. ROS induces some oxidative damage to biomolecules such as lipids, nucleic acids, proteins, and carbohydrates. ROS have been implicated in more than 100 diseases, including civilization diseases, among others, heart disease, stroke, arteriosclerosis, diabetes, and cancer [5,6,7]. In order to protect the organism against such undesirable effects, it is important to provide exogenous antioxidants, obtained among others, from fruits [8,9].
Fruit-based alcoholic beverages have been produced for centuries, and they are still very popular in many countries [10,11]. Such products often containing herbs, are frequently produced at home according to well-known recipes handed down for generations. In Poland, homemade alcoholic liqueurs called ‘nalewka’ are quite popular. The first mention of this drink can be found in 18th-century literature [12]. Nalewka is a traditional Polish alcoholic beverage made by maceration (from several days to several months) of fruit, herbs, flowers, and roots, in pure ethanol or vodka, frequently with sugar addition. The method of preparation is important to obtain the proper aroma and flavor. Such beverages usually contain around 40–45% ethanol by volume and are characterized by a distinct taste, aroma, and color [13]. Traditional homemade liqueurs called nalewka do not contain artificial flavors and colorants [12]. Nalewkas based on one fruit is more popular. However, also those containing many seasonal fruits called ‘ratafia’ are willingly consumed. To obtain such beverages, popular and widely cultivated fruits, rich in valuable ingredients with antioxidant properties, are often used. During the maceration, active components of fruits are dissolved in ethanol to obtain the final product with antioxidant potential. The ethanol softens the fruits and promotes the extraction of various dyes and some other substances that give the liquor a characteristic color, flavor, and smell [14]. Many studies report the antioxidant efficacy of alcoholic beverages containing herbs and many fruits, i.e., chokeberry, black rose, blackcurrant, blackberry, raspberry, sloe, strawberry, cherry and sour cherry, elderberry, rowanberry [9], rose petals [15], walnut [16,17] or myrtle berry [18]. The antioxidant properties of such tinctures are primarily due to the beneficial chemical composition of fruits and herbs. The major bioactive compounds are especially polyphenols, responsible for their health benefits [1,2,3]. However, it should be remembered that the consumption of alcoholic beverages, especially in larger quantities, is dangerous to human health, and this unfavorable effect may outweigh any possible health-promoting properties. Therefore, it should be emphasized that nalewkas and other alcoholic beverages prepared with fruits and herbs should be used in limited amounts.
The most popular fruits used for the production of nalewkas in Poland are seasonal fruits, inter alia cherries, plums, strawberries, blackcurrants, raspberries as well as quince. They contain many valuable ingredients, among which antioxidants are particularly important.
Sour cherry (Prunus cerasus L.) is popular fruit which is widely used both fresh and processed. Cherry fruits are often used for the home or industrial production of juices, liqueurs, etc. [19]. Nowadays, around the world, there is an increasing industrial production of cherry liquors. Cherry liquor is an old alcoholic drink popular in several countries, such as Poland. In Spain it is called ‘licor de cerezas’ or ‘licor de guindas’, while in Portugal ‘grinjinha’ [14]. Cherry fruits and juice are characterized by a high content of polyphenols, anthocyanins, flavonoids, vitamins as well as beta carotene [20,21]. In traditional medicine, they have been used as prophylactic agents against Alzheimer’s disease, cardiovascular damage, and diabetes diseases [19,22,23]. The anthocyanins contained in these fruits may have anti-inflammatory effects and may provide additional supportive therapy for chronic diseases associated with oxidative stress [24].
The plum (Prunus domestica L.) is a drupe fruit that belongs to the subgenus Prunus [25]. The plum fruit contains phenolics, vitamins, minerals, and organic acids as well. The fruits of this plant are characterized primarily by a very rich phenolic composition, including such compounds as chlorogenic acid, gallic acid, dihydroxybenzoic acid, rutin, esculin, quercetin, as well as less known neruloyl-glucoside, p-coumaroyl-glucoside, vanillic acid-glucosides, 3,4-dihydroxybenzoyl-glucoside, quercetin-3-O-pentosides, quercetin-3-O-rhamnoside, quercetin-pentoside-rhamnosides [26]. In recent years, plums have been considered foods with health-promoting properties. Their antioxidant, anti-inflammatory, anti-allergic, and anti-bacterial properties, cognitive improvement, and supportive, positive effects in cardiovascular diseases have been described by many authors [25,27,28].
Strawberries (Fragaria × ananasa Duch.) are appreciated for their nutritional value, taste, and antioxidant properties, mainly due to phenolics (phenolic acid, anthocyanin, and flavonoids), vitamins (such as vitamin C) and beta-carotene content. In recent years, there has been an increasing interest in strawberry fruits due to their nutritional quality and antioxidant properties [29]. Strawberry fruits are an important source of polyphenols in the human diet and are considered a functional food [30]. Some studies reported the beneficial effects of strawberries. For example, the treatment of melanoma cells with strawberry fruit crude extracts produced a remarkable reduction in cell proliferation, paralleled with both the lowering of the intracellular levels of polyamine and the enhancement of tissue transglutaminase [31].
Blackcurrant (Ribes nigrum L.) is an important fruit-growing crop, growing on a large scale mainly in Europe and Russia. Berries are used mainly in the production of fruit juices [32]. Blackcurrant fruits are widely recognized for containing high levels of polyphenols and anthocyanins [33]. The health effects of this fruit, including antioxidant and anti-inflammatory or antibacterial activity, are well documented [33,34,35]. Fruits rich in anthocyanins also have an anti-cancer effect, which has been observed in numerous studies [36].
Raspberry fruits (Rubus idaeus L.) are a popular antioxidant, antimicrobial, and anti-inflammatory remedy used in traditional medicine. The fruits are often used to treat the common cold and fever [37]. They contain a lot of flavonoids, anthocyanins, phenolic acids, and vitamin C [38,39].
Quince (Cydonia oblonga Miller) is also a valuable fruit for the production of nalewka. Quince is a sour and hard fruit rather not suitable for consumption in a fresh state. However, it is often used to prepare jam, jelly, liqueur, or marmalade. The fruits of this plant are a source of nutrients such as carbohydrates, vitamins, and minerals, especially polyphenols, with various health-promoting properties, such as antioxidative, antibacterial, antiulcer, cardioprotective, anti-inflammatory, antidiabetic, and antiallergic [40,41].
The aim of the study was to compare the antioxidant activity and polyphenols, anthocyanin, and tannin, as well as gallic acid content in three nalewkas during, long-time storage. Three types of homemade nalewkas: cherry, plum, and multifruit nalewka called ‘ratafia’ produced in 1997 and from 2013 to 2019 were analyzed.
2. Materials and Methods
2.1. Chemicals
2,2-diphenyl-1-picrylhydrazyl (DPPH), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (trolox), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), 2,4,6-tripyridyl-s-triazine (TPTZ), cyanidin-3-glucoside, gallic acid, methanol (analytical grade) were purchased from Sigma Aldrich (Darmstadt, Germany), iron(III) chloride hexahydrate, iron(II) sulfate heptahydrate, Folin–Ciocalteu reagent were from Merck, Darmstadt (Germany), whereas 99.5% acetic acid, sodium acetate anhydrous, potassium persulfate, potassium acetate, aluminum chloride, 36% hydrochloric acid as well as ethyl alcohol (all of the analytical grade) were from Chempur, Piekary Śląskie (Poland).
2.2. Nalewkas Preparation
The fruits for the production of nalewkas are bought each year at the local market. All nalewkas were homemade and prepared according to the same recipe in all years.
To prepare plum nalewka, 1 kg of pitted plums, 250 mL of (96% v/v) ethanol, and 0.2 kg of sugar were used. For cherry nalewka, 1 kg of pitted cherries, 250 mL of (96% v/v) ethanol, and 0.2 kg of sugar were applied, whereas to prepare ‘Ratafia’ (multifruit nalewka), 0.25 kg of strawberries, 0.25 kg of redcurrants, 0.25 kg of raspberries, 0.25 kg of quince, as well as 250 mL of ethanol (96% v/v) and 0.20 kg of sugar were used.
The maceration of the nalewkas in the 5-L jars lasted 3 months. After this time, homemade nalewkas were filtered and stored in glass bottles in a cool and dark cellar.
2.3. Color Determined
The color parameters were estimated using the methodology described by Pandeya et al. [42]. The absorbance of nalewka samples was measured at 420, 520, and 620 nm. The sum of absorbances at all wavelengths was referred to as color intensities (IC′). The proportion of red, yellow, and blue (%R, %Y, %B, respectively) were determined using the following equations: %R = [(A520 × 100)/IC′]; %Y = [(A420 × 100)/IC′]; %B = [(A620 × 100)/IC′]. Each sample was prepared in triplicate, and the results were presented as arithmetical mean ± SD of three independent measurements.
2.4. Antioxidant Activity
DPPH radical scavenging activities of nalewkas were measured using a previously described technique [6,43]. Briefly, an aliquot of 150 µL of nalewka was mixed with 2850 µL of DPPH radical solution in 96% (v/v) ethanol. After 10 min of incubation in the dark at room temperature, measurement of absorbance at 517 nm against 96% (v/v) ethanol was performed using Hitachi UV-Vis Spectrophotometer U-5100 (Hitachi High-Tech Science Corporation, Tokyo, Japan). Three independent samples of each examined nalewkas were prepared. The results are presented in mg Trolox·g−1 of nalewka. Additionally, the antioxidant activity was expressed as a percentage of inhibition (%).
The potential of scavenging ABTS radical by fruit nalewkas was measured using the method previously applied by Muzykiewicz-Szymańska et al. [43,44]. Shortly, a 7 mM solution of ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)) in a 2.45 mM aqueous solution of potassium persulfate was used as a stock solution. After dissolving the components, the solution was incubated for 24 h in the dark at room temperature, then diluted with 50% (v/v) methanol to obtain a working solution of absorbance of 1.00 ± 0.02 at 734 nm. Next, 2.5 mL of working ABTS solution and 0.025 mL of a studied nalewka were introduced into the spectrophotometric cuvette. After 6 min of incubation at room temperature, absorbance was measured. Each extract was evaluated in triplicate. The results are presented in mg Trolox·g−1 of nalewka.
The ferric ion reducing potential was measured with FRAP (ferric reducing antioxidant power) method as in previous studies [44]. The working solution was prepared by mixing 1 volume of 10 mM TPTZ (dissolved in 40 mM HCl), 1 volume of 20 mM FeCl3, and 10 volumes of acetate buffer (pH 3.6). An aliquot of 2320 µL of the working solution was mixed with 80 µL of nalewka. The absorbance was measured after 15 min at 593 nm. The results are expressed as mg FeSO4·g−1 of nalewka
In order to evaluate the reducing ability of cupric ions, the CUPRAC method with volume modification was applied according to the methodology described by Roman et al. [45]. Shortly, 500 µL of 0.01 M aqueous CuCl2 solution, 500 µL of 7.5 mM neocuproine solution in 96% ethanol, 500 µL of 1 M acetate buffer (pH 7), 300 µL of distilled water and 250 µL of the extract were thoroughly mixed. After 30 min of incubation at room temperature, the absorbance was measured at 450 nm. The results are presented in mg Trolox·g−1 of nalewka.
Total polyphenol content was determined using the Folin–Ciocalteu (F-C) method [6]. Shortly, 0.15 mL of the studied sample, 0.15 mL of tenfold diluted Folin–Ciocalteu reagent, 1.35 mL of 0.01 M sodium carbonate aqueous solution, and 1.35 mL of water were added and mixed. The spectrophotometric measurement was carried out at 765 nm. The time of incubation was 15 min. The results were expressed as gallic acid equivalents (GA) in mg GA·g−1 of nalewka. Three independent measurements were made.
Total anthocyanins content (TAC) was estimated using the modified method proposed by Lee et al. [46]. The absorbance of fruit nalewka diluted in buffers at 1.0 pH (hydrochloric acid-potassium chloride) and 4.5 pH (acetic acid-sodium acetate) was measured at 510 as well as 700 nm. Results were expressed as cyjanidin-3-glucoside equivalents (mg Cy-3-Glu·mL−1 of nalewka). Three independent measurements were made.
Total tannin content (TTC) was carried out using a methodology described by Pandeya et al. [42]. Briefly, two samples of each nalewka containing 200 μL of 10-fold diluted nalewka, 300 μL of concentrated HCl, and 100 μL of distilled water were prepared. The first sample was incubated at 100 °C for 30 min, while the second sample, to which 50 μL of 96%(v/v) ethanol was added, was left at room temperature. The absorbance of samples was measured at 470, 520, and 570 nm. The differences between the absorbance at given wavelengths were presented as ΔA470, ΔA520, and ΔA570. The ΔA470 and ΔA570 were then expressed in terms of ΔA520 using the equations: ΔA520 = 1.1 × ΔA470 and ΔA520 = 1.54 × ΔA570. The TTC (mg·mL−1 of nalewka) was calculated using the following formula: TTC = 15.7 × lowest ΔA520. Each sample was prepared in triplicate, and the results were presented as the mean ± SD of three independent measurements.
2.5. Gallic Acid Determination
The concentration of gallic acid in the nalewkas was determined by high-performance liquid chromatography (HPLC) using the HPLC system from Knauer (Berlin, Germany). The tested components were separated isocratic on a 125 mm × 4 mm column filled with Hyperisil ODS, particle size 5 µm. The mobile phase consisted of 1% acetic acid, and MeOH (30:70 by vol.) was pumped at 1 mL/min. An aliquot of 20 µL of the nalewka was injected into the column. Determinations were made at a wavelength of 280 nm. Results were expressed as µg mL−1 of nalewka. All nalewkas were diluted 100-fold before injection. All the samples were analyzed three times.
All the above-mentioned parameters (p. 2.3.–2.5.) were evaluated in 2022.
2.6. Statistical Analysis
Results are presented as the mean ± standard deviation (SD). A one-way analysis of variance (ANOVA) was used. In the case of the cumulative mass, the significance of differences between individual groups was evaluated with Tukey’s test (α < 0.05). A cluster analysis was carried out to determine similarities between all years of storage of nalewkas. The Pearson test was used to demonstrate the correlation between the antioxidant activity evaluated with different methods. Statistical calculations were performed using Statistica 13 PL software (StatSoft, Kraków, Polska).
3. Results
3.1. Color Determined
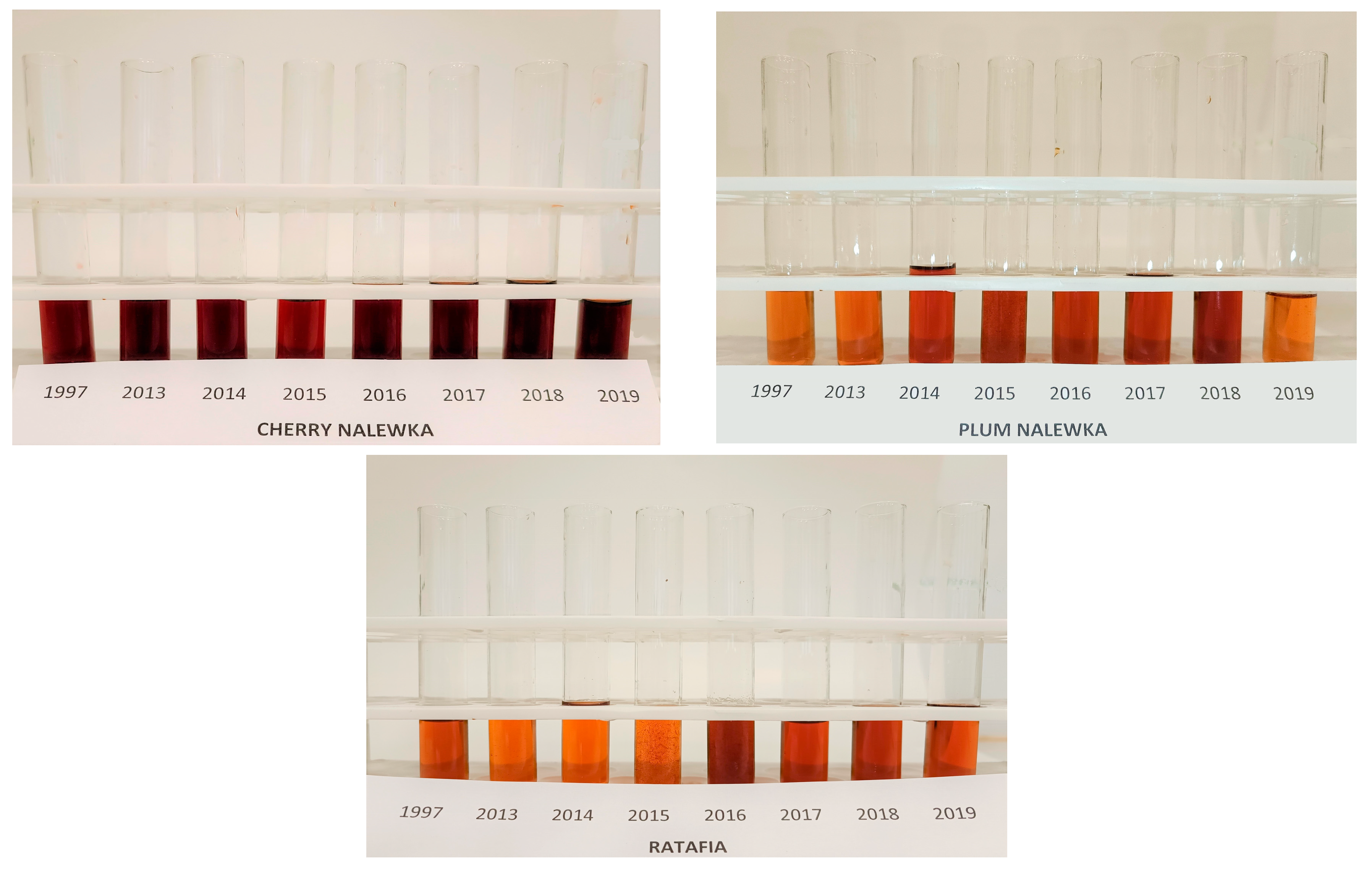
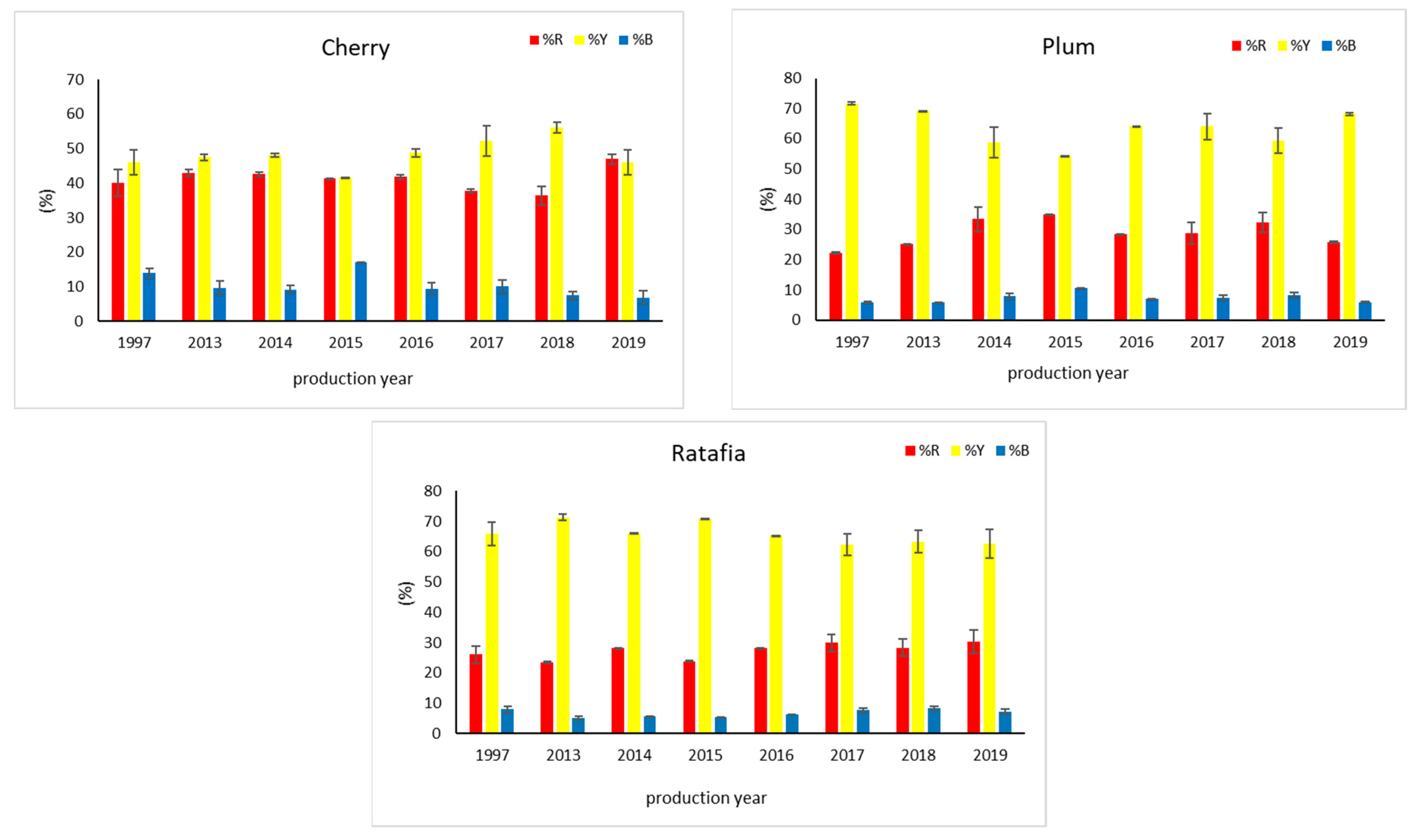
Figure 1 presents all the analyzed nalewkas obtained in different years. Figure 2 shows the individual colors’ contribution to the overall color of the analyzed nalewkas. In all the analyzed nalewkas, the largest percentage share in the primary colors was yellow, while the lowest was blue. In cherry nalewka, red color ranged, depending on the vintage, from 36.4% to 47.0%, yellow from 41.6% to 56.0%, whereas blue from 6.9% to 17.1%. In ratafia, these values were: 23.5–30.3%, 62.3–71.3%, and 5.2–8.3%, respectively, while in plum nalewka, were 22.4–35.0%, 54.3–71.7% and 5.8–10.7%, respectively (Figure 2).
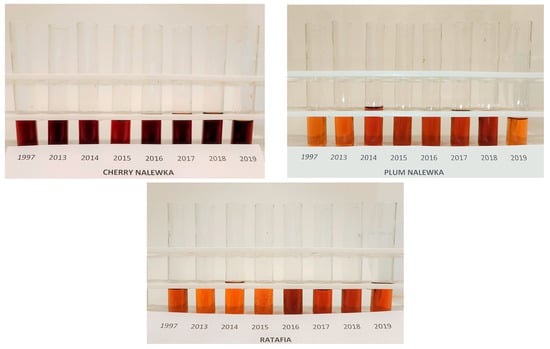
Figure 1.
The nalewkas from all years of production.
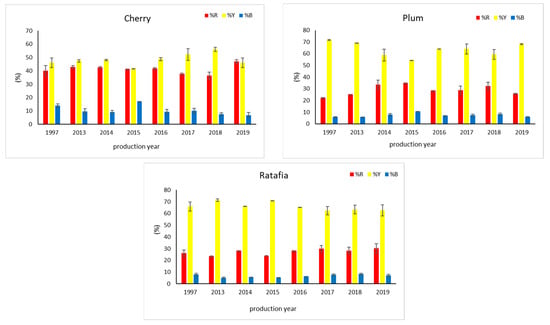
Figure 2.
The color parameters of three nalewkas produced in different years (mean ± SD, n = 3). %R—the percentage of red color, %Y—the percentage of yellow color, %B—the percentage of blue color.
3.2. Antioxidant Activity
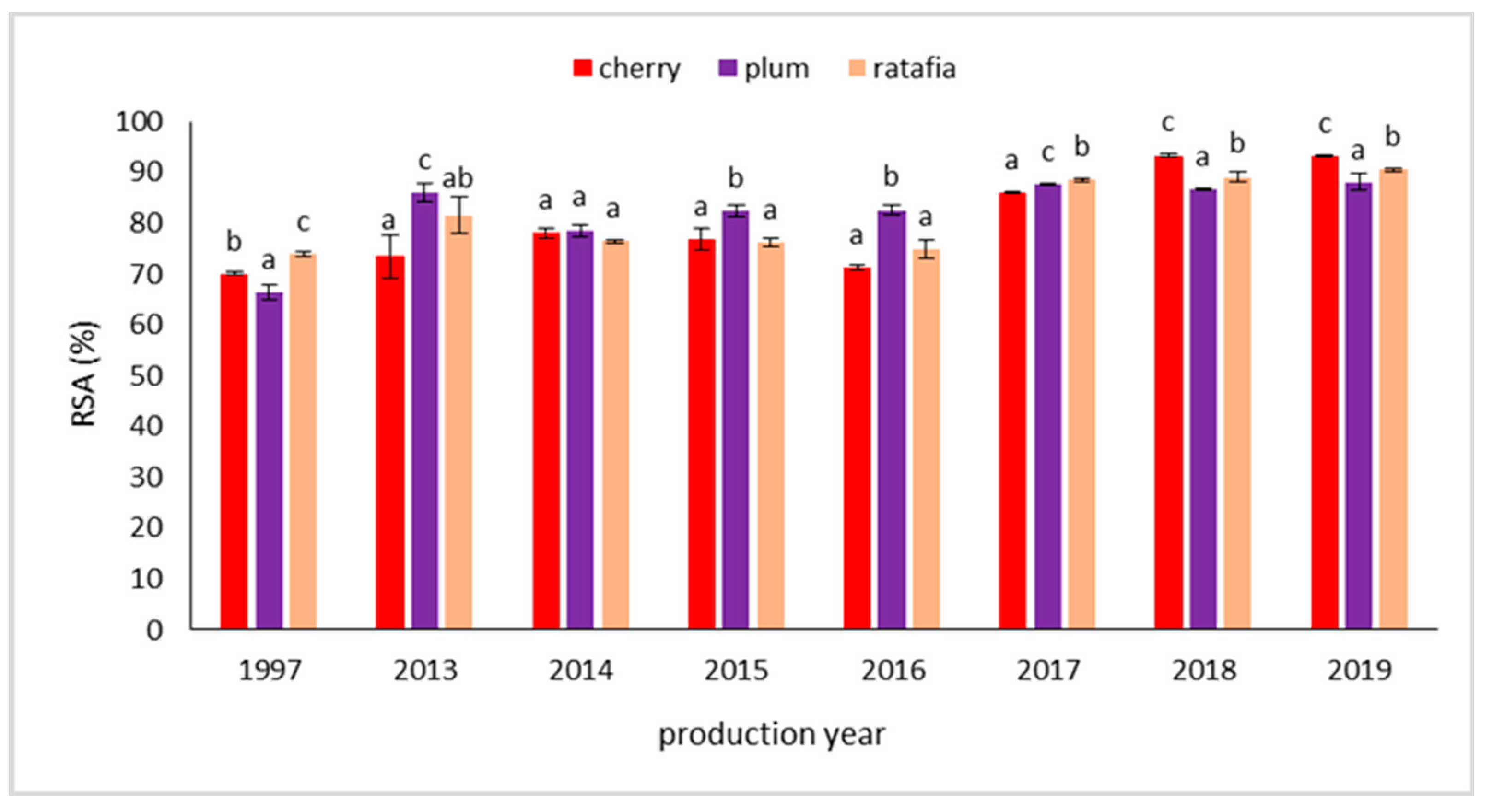
The antioxidant potential of the nalewkas measured by the DPPH, ABTS, FRAP, and CUPRAC methods are presented in Table 1, Table 2, Table 3 and Table 4. All the tested nalewkas showed antioxidant activity. However, this parameter differed depending on the type of nalewkas and production year. The antioxidant activity measured with the DPPH method ranged from 2.814 ± 0.062 mg Trolox·g−1 for plum nalewka prepared in 1997 to 4.005 ± 0.014 mg Trolox·g−1 for cherry nalewka prepared in 2018 (Table 1). Figure 3 presents a comparison of the antioxidant activity of all nalewkas in individual years, expressed as free radical scavenging activity, in %. Taking into account the individual nalewka in each year, the ability to scavenge free radicals varied depending on the fruit used. The ability to scavenge free radicals was high and ranged from 66.47 ± 1.39 for plum nalewka (the year 1997) to 93.33 ± 0.32% for cherry nalewka (the year 2018) (Figure 3). In the case ABTS technique, the antioxidant potential ranged from 2.597 ± 0.422 mg Trolox·g−1 for cherry nalewka prepared in the year 1997 to 17.249 ± 0.151 mg Trolox·g−1 for cherry nalewka prepared in the 2018 year (Table 2). It should be added that for younger nalewkas higher antioxidant activity was found for cherry and plum nalewkas produced between 2016 and 2019, whereas for ratafia only for this produced in 2019. A similar trend was also observed for the FRAP method, in which the ability to reduce ferric ions ranged from 5.870 ± 0.117 mg FeSO4·g−1 for cherry nalewka prepared in 1997 to 17.547 ± 0.069 mg FeSO4·g−1 for cherry nalewka prepared in 2018. In general, the oldest nalewka, produced in 1997, showed a significantly lower ability to reduce ferric ions compared to the whole group produced after 2013 (Table 3). Analyzing the CUPRAC method, the lowest antioxidant activity was shown by cherry nalewka produced in 1997 and the highest by ratafia produced in 2019. Antioxidant activity in these cases was 8.640 ± 0.131 and 20.732 ± 0.492 mg Trolox·g−1, respectively (Table 4).

Table 1.
The antioxidant activity of nalewkas was measured by the DPPH method (mean ± SD, n = 3). Different letters indicate significant differences between the individual years. The result was expressed in mg Trolox·g−1 of nalewka.

Table 2.
The antioxidant activity of nalewkas was measured by the ABTS method (mean ± SD, n = 3). Different letters indicate significant differences between the individual years. The result was expressed in mg Trolox·g−1 of nalewka.

Table 3.
The antioxidant activity of nalewkas was measured by the FRAP method (mean ± SD, n = 3). Different letters indicate significant differences between the individual years. The result was expressed in mg FeSO4·g−1 of nalewka.

Table 4.
The antioxidant activity of nalewkas was measured by the CUPRAC method (mean ± SD, n = 3). Different letters indicate significant differences between the individual years. The result was expressed in mg Trolox·g−1 of nalewka.
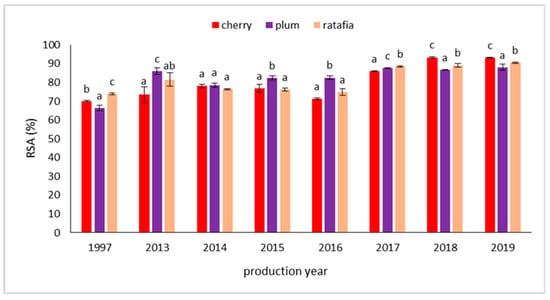
Figure 3.
Percentage of free radical scavenging by nalewkas in each year of production (mean ± SD, n = 3). The results were calculated based on the DPPH method. Different letters indicate significant differences between the nalewkas in individual years of production.
3.3. Total Polyphenol, Anthocyanin, and Tannin Content
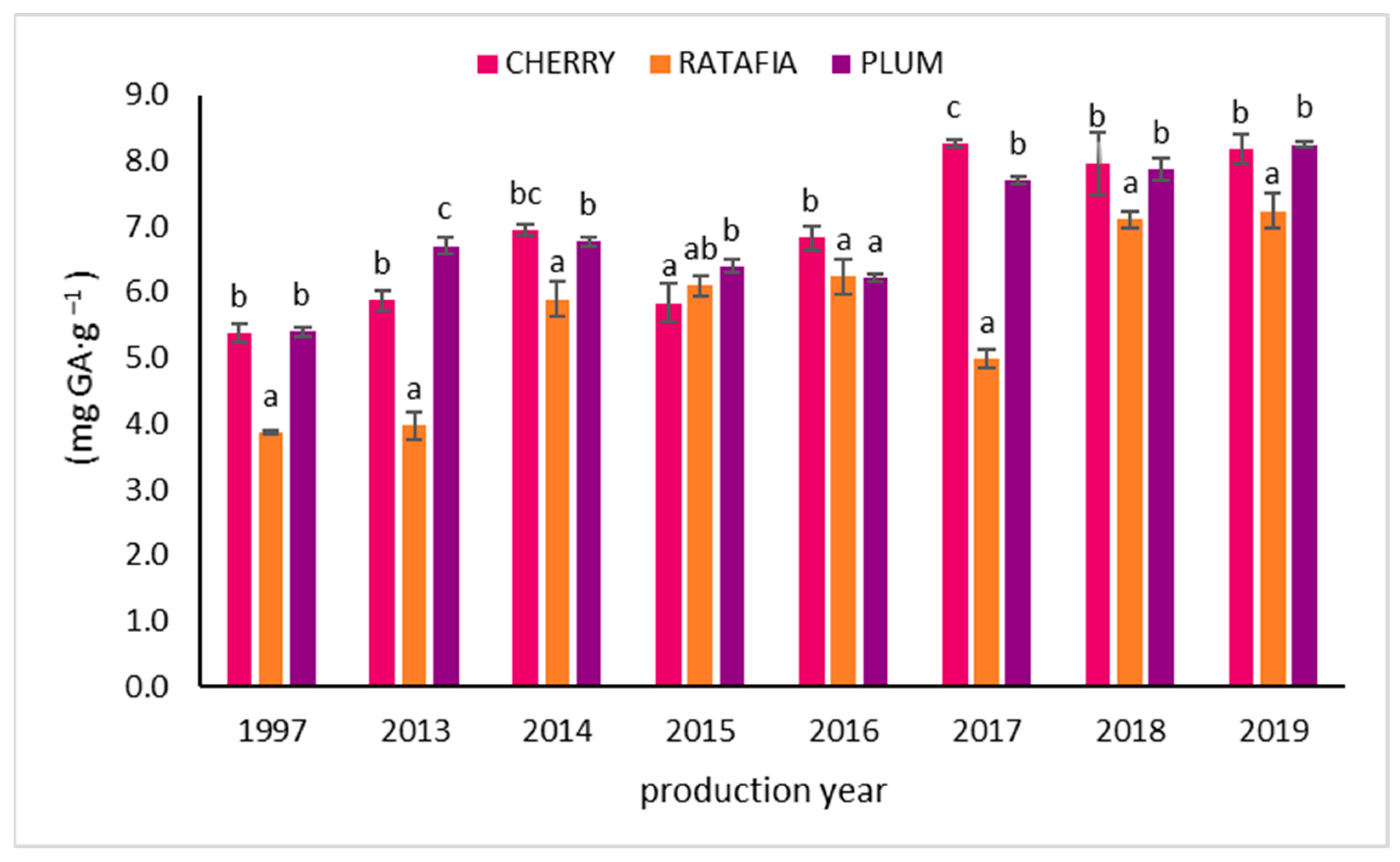
The total polyphenols content in all types of nalewkas is presented in Figure 4. This parameter ranged from 3.877 ± 0.022 mg GA·g−1 for ratafia prepared in 1997 to 8.232 ± 0.045 mg GA·g−1 for plum nalewka prepared in 2019. Similarly to the antioxidant activity measured by other methods, generally, the highest total polyphenol content was observed for the youngest nalewkas: for ratafia prepared in 2018 and 2019 and for cherry and plum nalewkas produced in 2017–2019 (Figure 4).
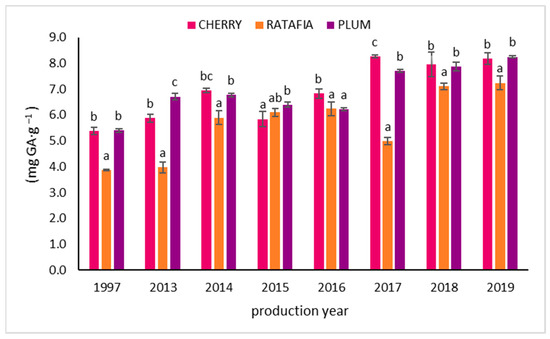
Figure 4.
The total polyphenol content of nalewkas (mean ± SD, n = 3). Different letters indicate significant differences between the nalewkas in individual years of production (α = 0.05). The result was expressed in mg GA·g−1 of nalewka; GA—gallic acid.
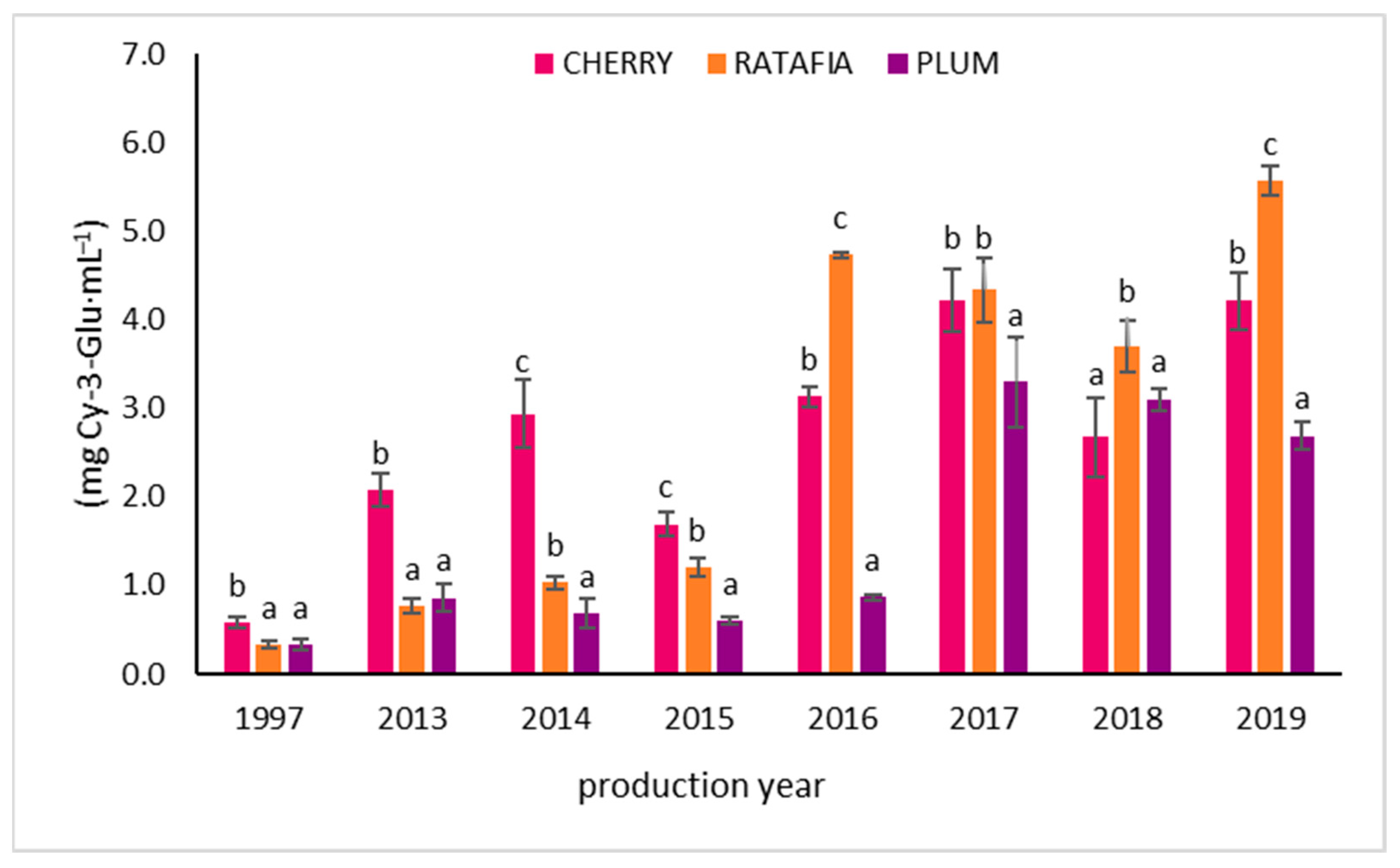
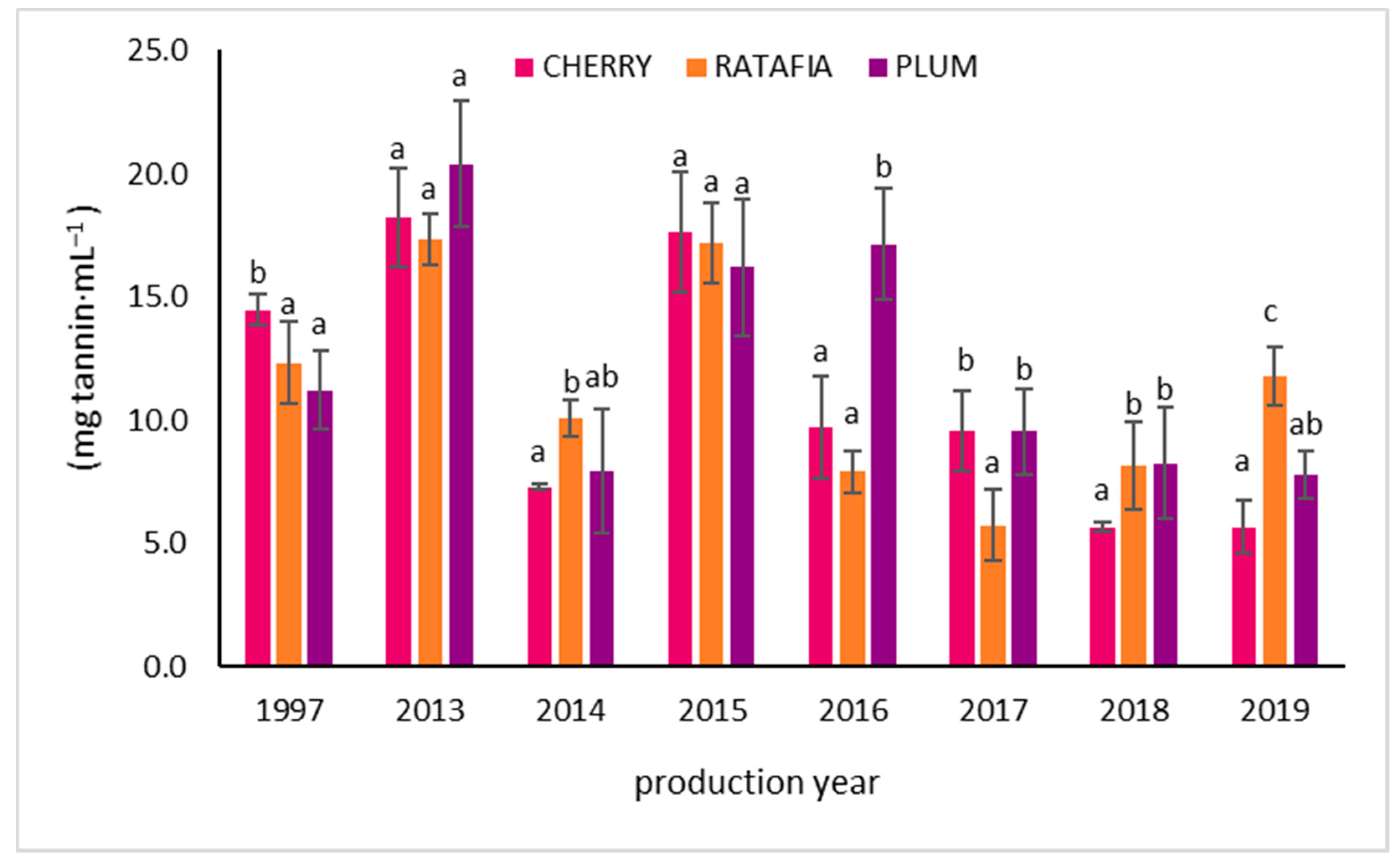
The anthocyanin content in all types of nalewkas is presented in Figure 5. This parameter ranged from 0.334 ± 0.041 mg Cy-3-Glu/1 mL for ratafia prepared in 1997 to 5.572 ± 0.170 Cy-3-Glu/1 mL for ratafia prepared in 2019. As in the case of antioxidant activity measured by other methods, generally, the highest content of anthocyanins was observed for the youngest nalewkas: for ratafia produced in 2018 and 2019 and for cherry and plum nalewka of 2017–2019 (Figure 5). In the case of ratafia, the anthocyanins content is significantly higher in the youngest nalewka produced in 2019. A different trend was observed for cherry nalewka, where the content of anthocyanins was comparable in 2013–2019. On the other hand, the ratafia produced in 2017–2019 was characterized by a higher content of these compounds compared to older samples. The tannin content is presented in Figure 6. The highest concentration of tannins was observed in nalewkas in 2013. The highest concentration of these compounds was identified in plum nalewka, while the lowest was in ratafia (20.394 ± 2.531 and 17.317 ± 1.044 mg·mL−1, respectively) (Figure 6).
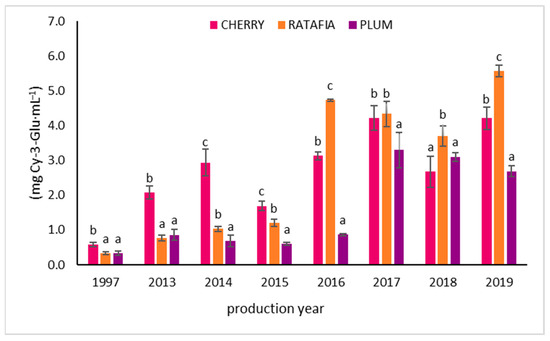
Figure 5.
The anthocyanin content of nalewkas (Mean ± SD, n = 3). Different letters indicate significant differences between the nalewkas in individual years of production (α = 0.05). The result was expressed in mg Cy-3-Glu·mL−1 of nalewka; Cy-3-Glu—cyjanidin-3-glucoside.
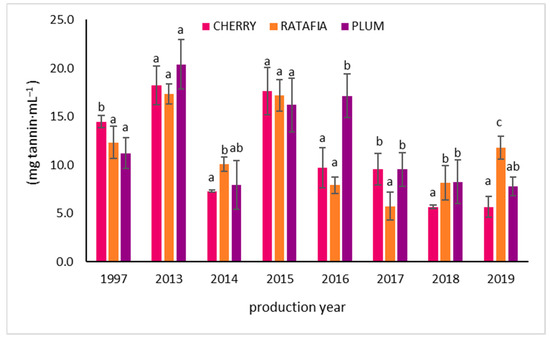
Figure 6.
The tannin content of nalewkas (mean ± SD, n = 3). Different letters indicate significant differences between the nalewkas in individual years of production (α = 0.05). The result was expressed in mg tannin·mL−1 of nalewka.
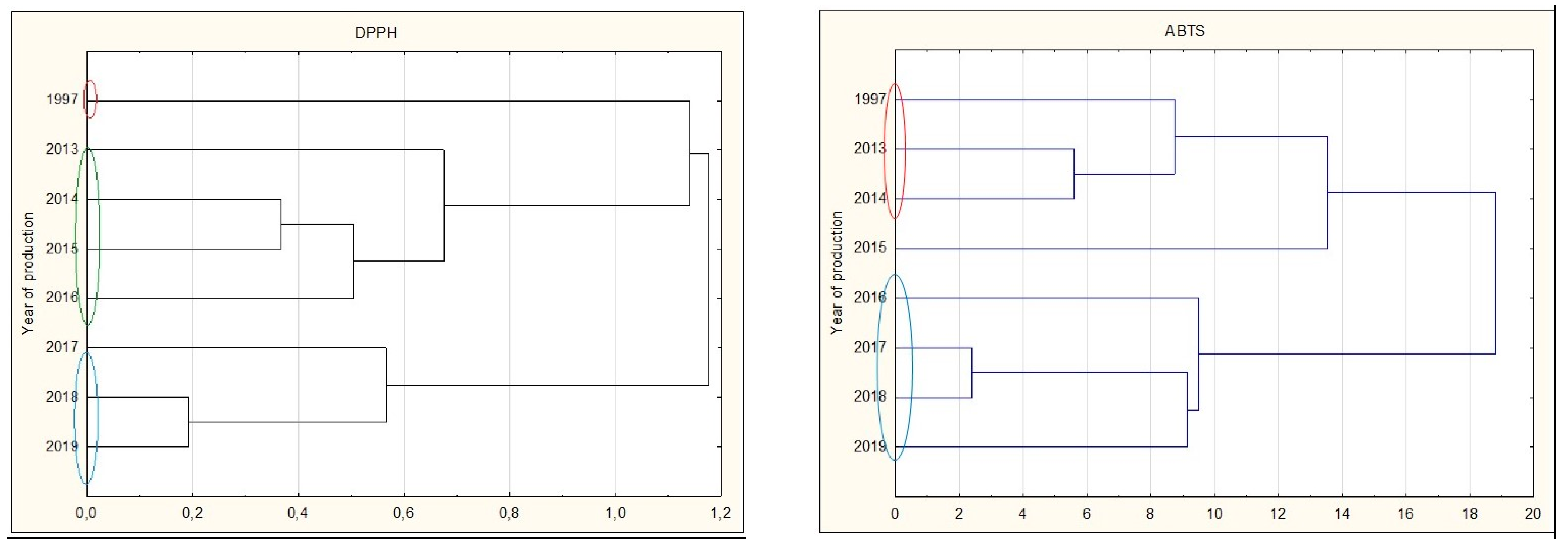
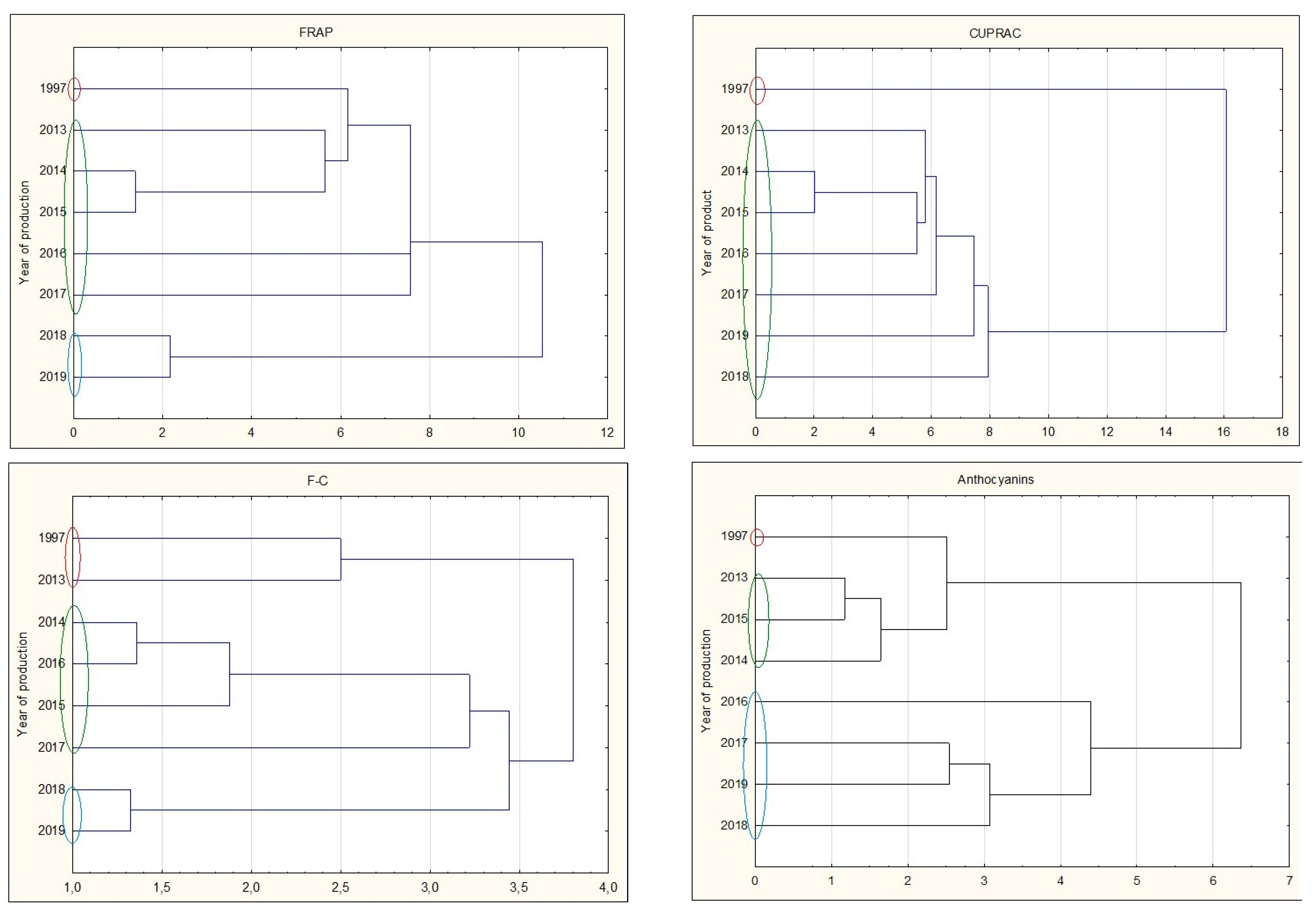
Analyzing all types of nalewkas, a significant decrease in antioxidant activity was observed during prolonged storage. The youngest nalewkas, produced in 2017–2019, have been most often characterized by significantly the highest antioxidant activity. This is also confirmed by the cluster analysis, in which individual groups with different antioxidant activities can be distinguished. The graphs show that 25-year nalewkas form a group definitely different from the other groups of younger nalewkas (Figure 7).
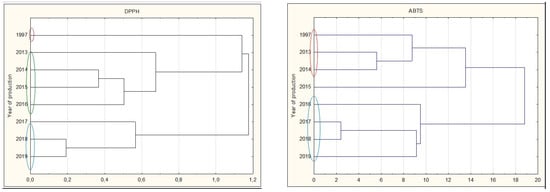
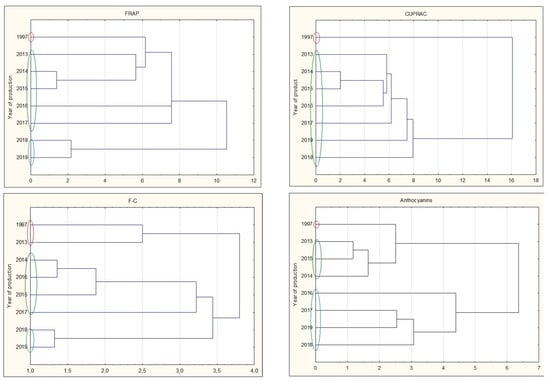
Figure 7.
Cluster analysis graph for parameters antioxidant activity, total polyphenol content, and anthocyanins for all nalewkas. Years with similar values are marked with colored circles.
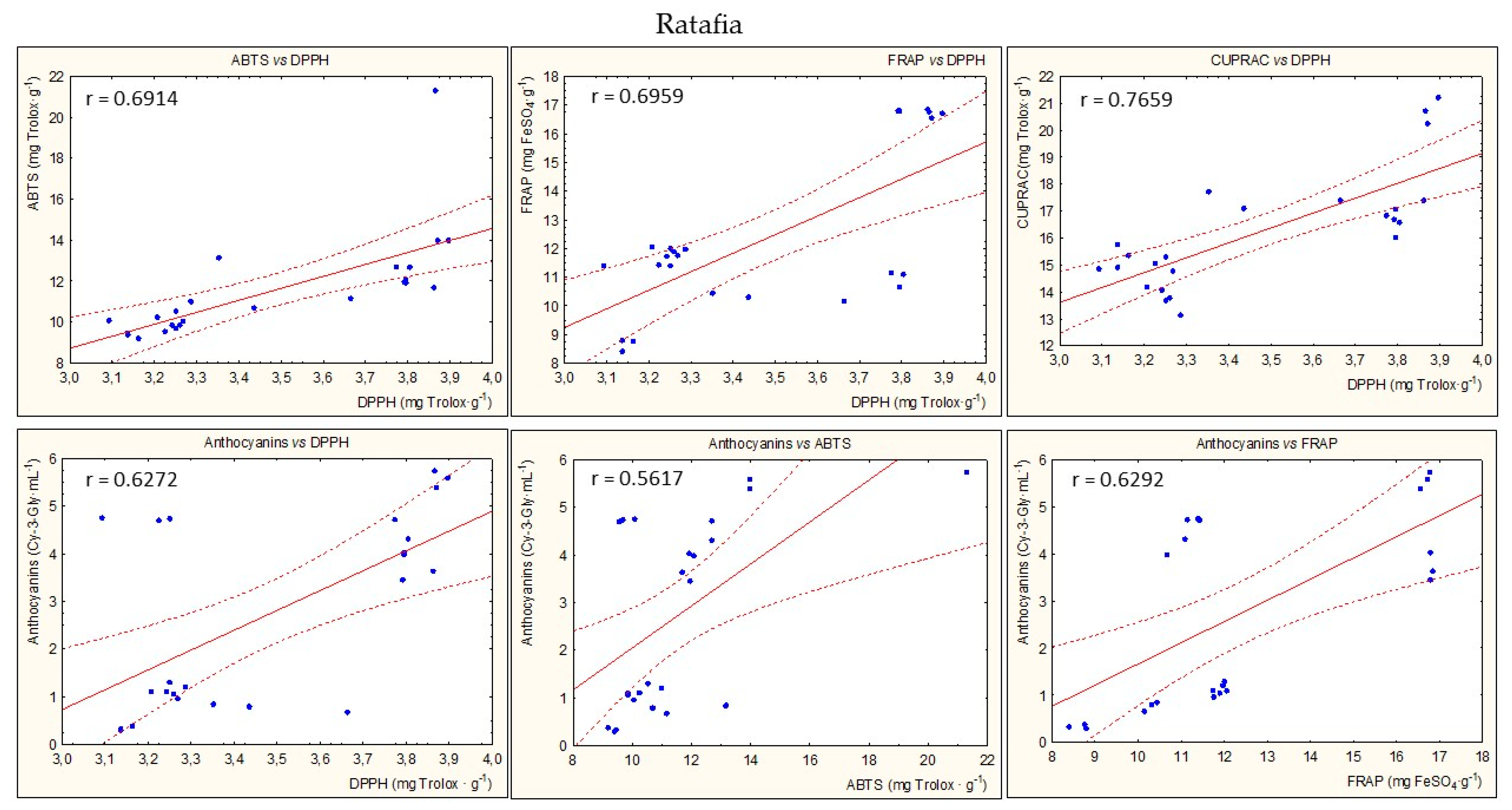
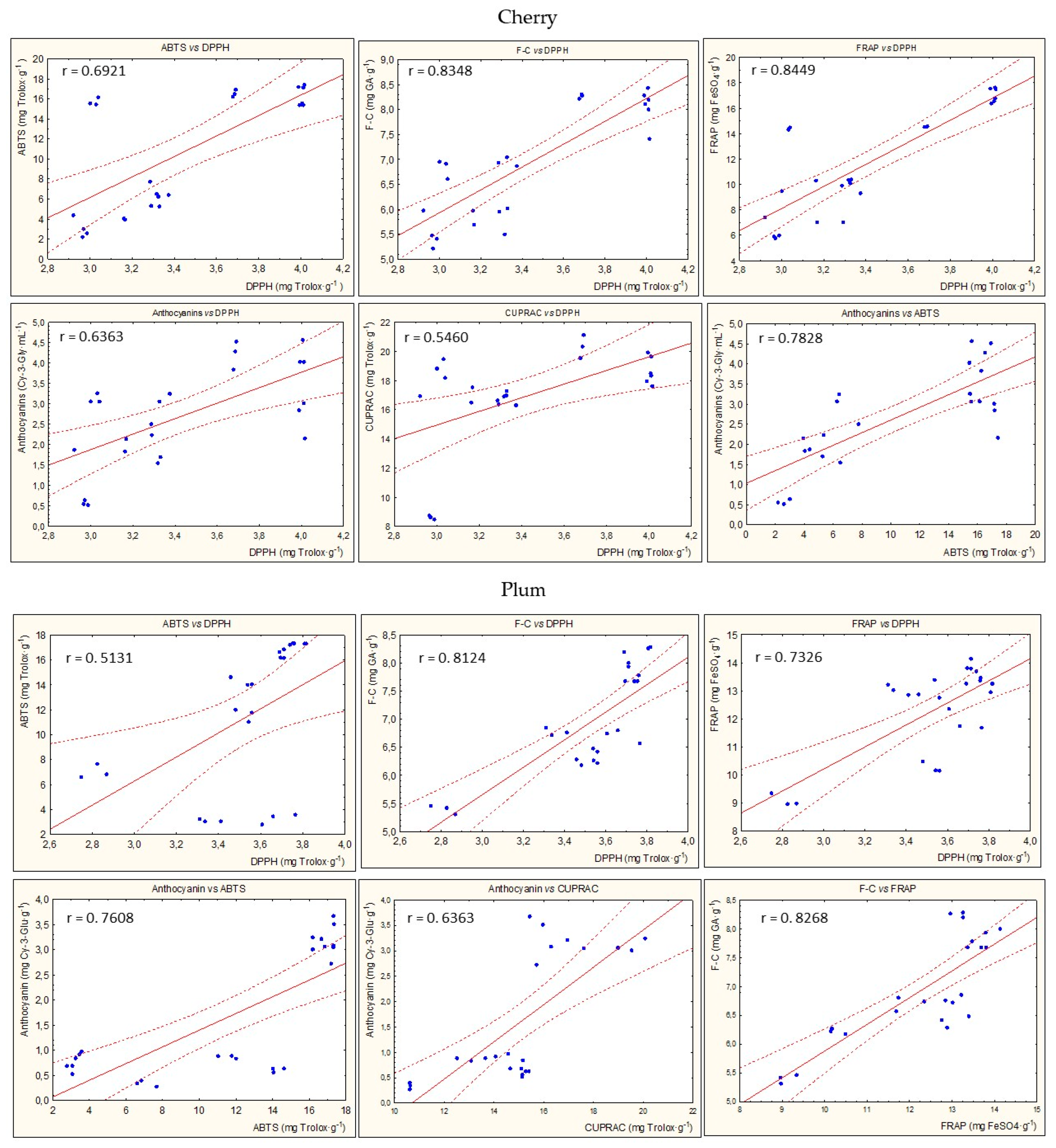
Figure 8 presents the statistically significant correlations between parameters of antioxidant potential during the entire storage period for all types of nalewkas. In most cases, a high correlation coefficient was found between the individual parameters of antioxidant activity (p < 0.05) (Figure 5).
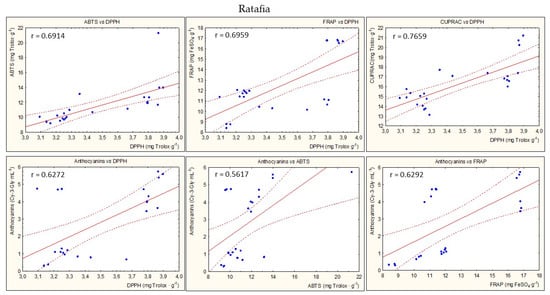
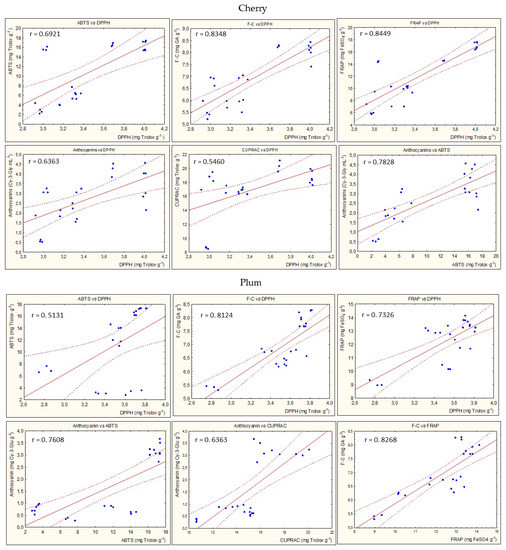
Figure 8.
Examples of the statistically significant correlations (r) between parameters of antioxidant activity for all nalewkas; p < 0.05.
3.4. Gallic Acid Content
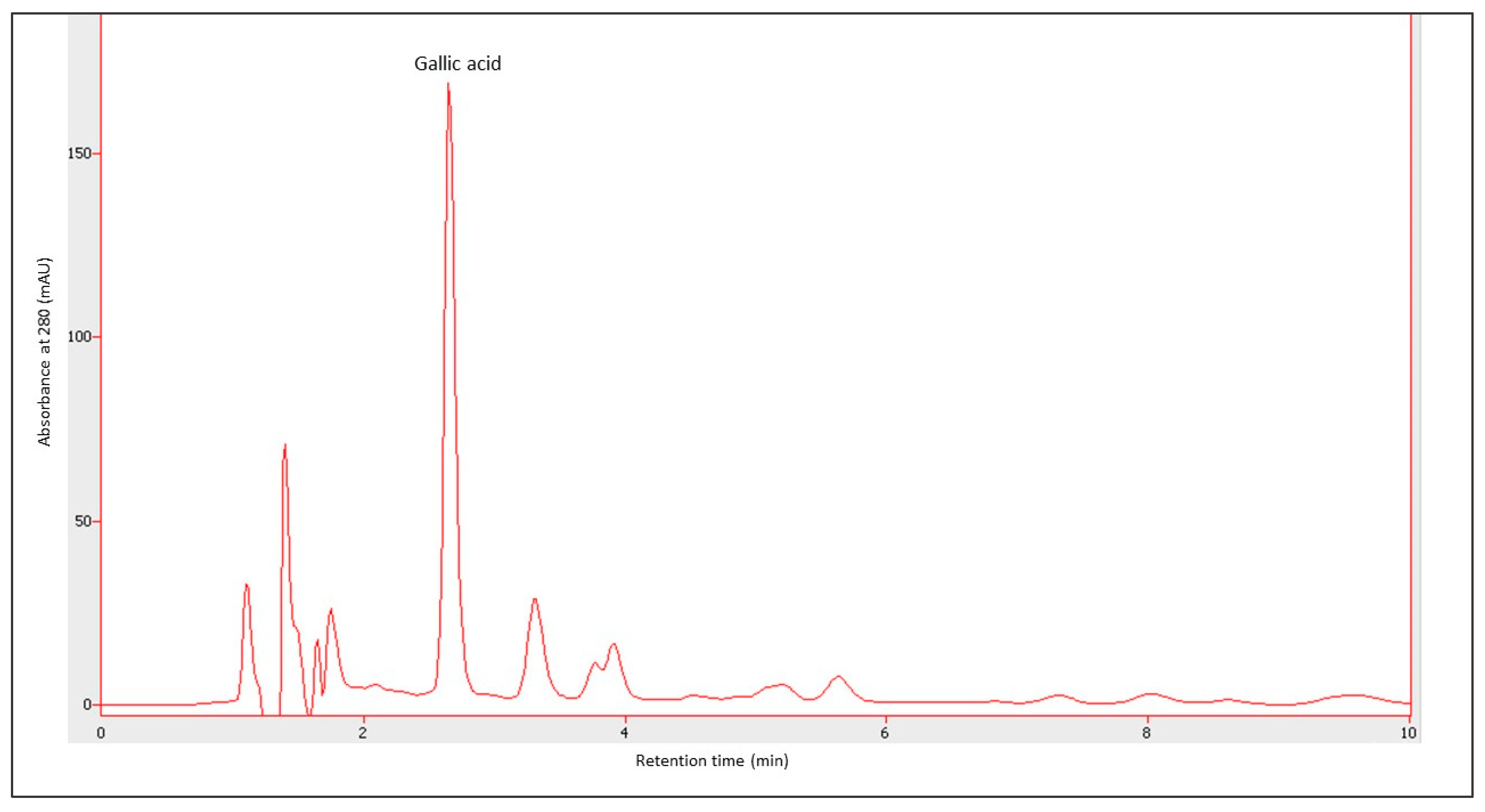
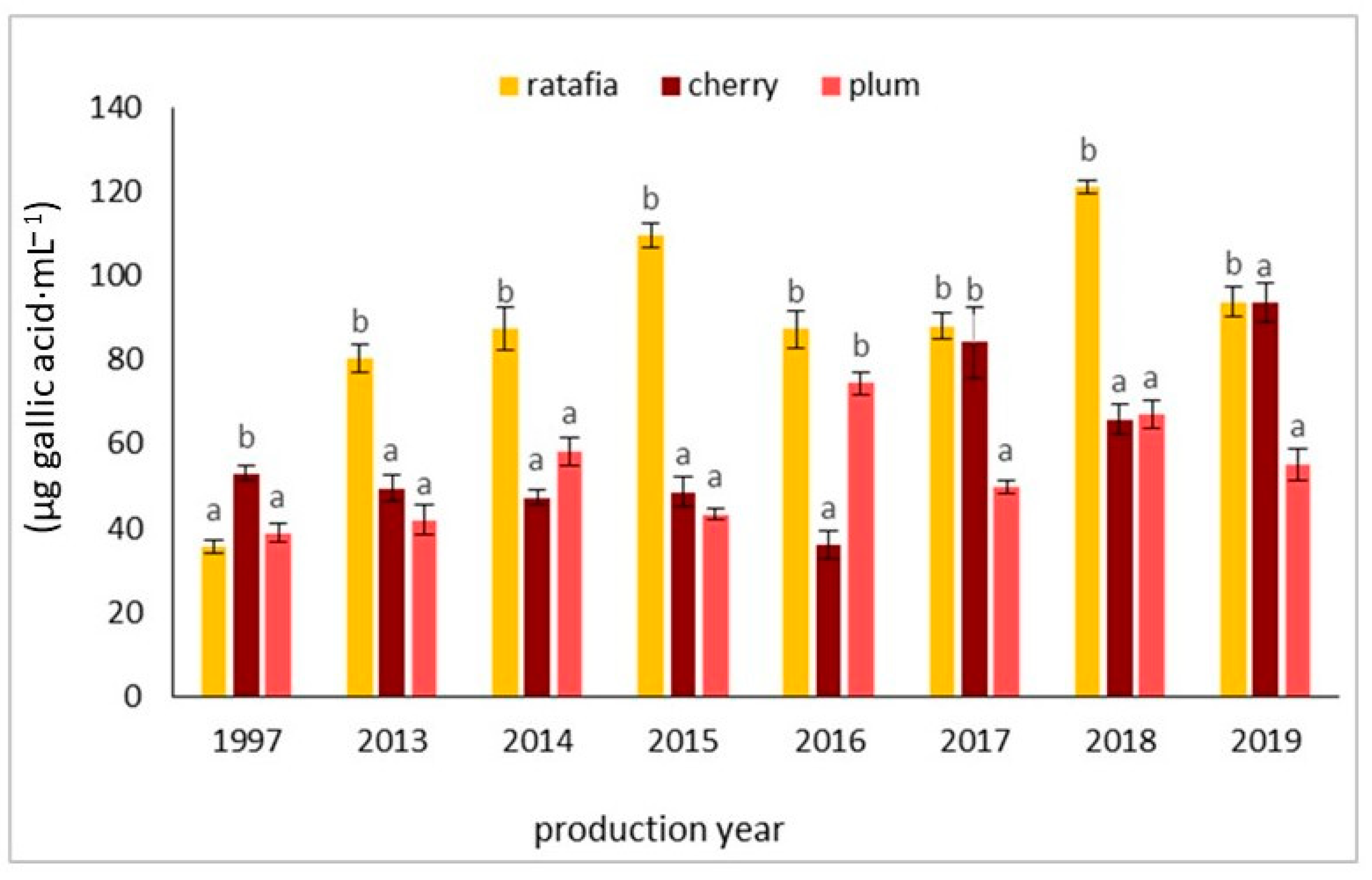
Figure 9 presents an example of an HPLC chromatogram of gallic acid, whereas Figure 10 shows the content of gallic acid determined by the HPLC method. The content of gallic acid depended on the year of production as well as on the fruit used. The concentration of this compound ranged from 35.442 ± 1.540 µg·mL−1 for the ratafia produced in 1997 to 120.985 ± 1.534 µg·mL−1 for the ratafia from 2018. Ratafia contained the highest amount of gallic acid. In most cases, a significant difference in the content of this compound was observed in comparison with other nalewkas.
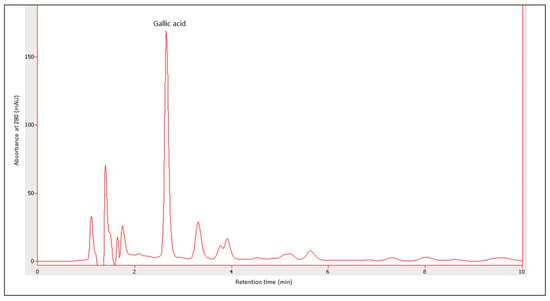
Figure 9.
An example of a chromatogram for the determination of gallic acid. A sample of plum nalewka from 2019, the sample was diluted 100 times. The retention time of gallic acid was 2.650 min.
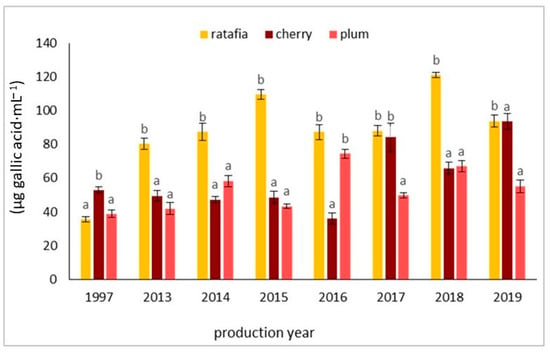
Figure 10.
The content of gallic acid in three types of nalewkas in 1997 and 2013–2019. Values are the mean of three determinations (n = 3). Different letters indicate significant differences between all types of nalewka in individual years (α = 0.05).
4. Discussion
In our study, the effect of long-term storage of homemade nalewkas containing seasonal fruits, such as cherries, plums, strawberries, currants, raspberries, and quince, on their antioxidant activity was evaluated. The antioxidant activity of the analyzed nalewkas was measured using the DPPH, ABTS, FRAP, and CUPRAC methods. The total polyphenol (TPC), anthocyanin (TAC), total tannin (TTC), and gallic acid content were also analyzed.
Nalewka is a traditional Polish alcoholic beverage made by maceration, among others, of fruit with or without the addition of sugar in pure ethanol or vodka. To obtain such beverages, popular and widely cultivated fruits rich in valuable ingredients with antioxidant properties are often used. All of our tested nalewkas showed antioxidant activity, but it depended on the year of production as well as the type of fruits used. Many authors have reported the antioxidant capacity of various natural alcoholic beverages containing, among others, fruits [9,15,47], nuts [16,17], or tea leaves [48]. The antioxidant properties of traditional Polish nalewkas were also assessed by Polak and Bartoszak. They proved the highest antioxidant activity, measured with the DPPH method for the elderberry nalewka (1045 μmol/100 mL), then for the raspberry nalewka (285 μmol/100 mL) and the cherry nalewka (133 μmol/100 mL), whereas the lowest for the cranberry nalewka (45 μmol/100 mL) [13].
Many studies report that the antioxidant activity depends on the time of storage [9,17]. This was also confirmed in our analyses, in which a significant decrease in antioxidant activity with increased storage time was observed. The youngest nalewkas, produced in 2017–2019, have been most often characterized by significantly the highest antioxidant activity. However, according to many authors, the antioxidant activity of fruit and herbal liqueurs after prolonged storage does not always decrease. Many factors can affect this parameter, such as the type of fruit or herb used, storage conditions, and the scheme of the preparation of alcoholic beverages. For example, Alamprese et al. evaluated the antioxidant activity of 17 homemade nocino liqueurs, each made in a different year (from 1977 to 2000). The authors did not show a significant difference between the antioxidant activity in the younger liqueurs compared to the older ones. DPPH radical scavenging activities of the older samples produced in 1977–1983 were similar to younger samples produced in 1996–2000, wherein the average activity expressed as Trolox equivalents for all samples was 199 μmol Trolox/100 mL [17].
Sokół-Łękowska et al. suggested that storage time can influence on activities of samples evaluated with the DPPH method and may cause either increasing or decrease of antioxidant activity. The authors compared the antioxidant capacity of liqueurs made from red fruits, such as chokeberry (Aronia melanocarpa (Michx.) Elliott), cornelian cherry (Prunus avium L.), black rose (Rosa spinosissima L.), blackcurrant (Ribes nigrum L.), blackberry (Vaccinium myrtillus L.), raspberry (Rubus idaeus L.), mahonia (Mahonia aquifolium (Pursh) Nutt.), sloe (Prunus spinose L.), strawberry (Fragaria × ananasa) and sour cherry (Cerasus vulgaris Mill.). All liqueurs were prepared using 65% (v/v) ethanol. The authors analyzed, among others, the influence of 6 months of storage at 15 °C and 30 °C on the antioxidant potential of the samples. During storage, it was observed a different decrease in antioxidant activity depending on the type of liqueurs. For example, the black rose, chokeberry, and blackcurrant liqueurs stored at a temperature of 30 °C showed a significant reduction in antioxidant activity from 20% to 50% [9]. In another study, the antioxidant capacity of cherry liqueurs analyzed by the ABTS method changed during 24 weeks of storage, probably due to sugar addition and storage temperature. The antioxidant activity before storage at 15 °C was 11.56 μmol Trolox/mL (without sugar added) and 11.77 μmol Trolox/mL (with sugar added), whereas, after storage, it decreased to 10.83 and 10.68 μmol Trolox/mL, respectively. Moreover, at 30 °C it was 12.28 μmol Trolox/mL (without sugar added) and 12.62 μmol Trolox/mL (with sugar added), while after storage—9.90 and 7.85 μmol Trolox/mL [11]. Kallithraka et al. suggest that during the storage of wine, oxidation of principal polyphenolic compounds would presumably lead to changes in the levels of antioxidants as a consequence of changes in the redox equilibrium [49].
A large group of polyphenols plays an important role in the human diet. Epidemiological studies have shown associations between the consumption of polyphenol-rich beverages and the prevention of diseases viz. cancers, diabetes, and cardiovascular [50,51]. A high polyphenols content is responsible for the strong antioxidant properties of fruit [13,50]. Polyphenols may have anti-inflammatory and/or inflammatory response stabilizing activities, which is very important in health maintenance and disease risk reduction. It turns out that diets rich in fruits are inversely associated with inflammatory stress, and the prevalence of diabetes and Alzheimer’s disease is lower with increased fruit consumption [52]. In our research, all analyzed nalewkas were characterized by a high content of total polyphenols. The total polyphenol content in our study ranged from 3.877 ± 0.022 mg GA·g−1 for ratafia prepared in 1997 to 8.232 ± 0.045 mg GA·g−1 for plum nalewka prepared in 2019. Sokół-Łękowska et al. stated that the black rose, chokeberry, sloe, and mahonia fruits liqueurs had high total polyphenol contents of 671, 329, 271, and 218 GAE in 100 mL of liqueur, respectively. On the contrary, liqueurs made from raspberries, strawberries, and cornelian cherry were characterized by significantly lower total polyphenol content: 63.4, 82.0, and 99.0 GAE in 100 mL, respectively [9]. Polak and Bartoszek analyzed various fruits nalewka containing elderberry, cranberry, rowanberry, raspberry, cherry, as well as nuts. The evaluated nalewkas were purchased in the local market or were produced at home. Authors stated that all evaluated alcoholic beverages were characterized by the content of total polyphenols—which varied from 83 mg GA/L in the case of rowanberry nalewka to 3649 mg GA/L for elderberry nalewka. Interestingly, homemade nalewka from raspberry contained over three times more total polyphenols (1811 mg GA/L) as compared with nalewka purchased locally marked (534 mg GA/L). It could be related, among others, to the different content of ethanol because nalewka produced homemade was characterized by a higher alcohol concentration of 45% (v/v), whereas commercial—25% (v/v) [13]. In our study, similarly to the antioxidant activity, the highest total polyphenol content was observed for the youngest nalewkas: 2018 and 2019 for ratafia and 2017–2019 for cherry and plum nalewkas. Regardless of the type of nalewkas, three groups can be distinguished: the group of the oldest nalewkas from 1997, the group from 2013–2017, and the last one from 2018 and 2019 characterized by the highest total polyphenol content. Many factors may contribute to the reduction in polyphenols during storage, such as storage time, temperature, and sugar content, as well as the type of fruit from which the nalewka was made [9,13]. For example, Sokół-Łękowska et al. found no significant change in the total polyphenol content of cherry liqueurs due to storage conditions. During 24 weeks of storage, the total phenolic content before storage was 1.15 mg GA/mL, whereas, after storage, it was 1.10–1.14 or 1.21–1.25 mg GA/mL in samples without and with sugar, respectively [11]. In our research, during the last 2 years of storage (2018 and 2019), no significant changes in total polyphenol were observed in all nalewkas. However, in the literature, there are not many reports on the analysis of alcoholic beverages with added fruits after 25 years of storage. Only Alapmrese et al. stated that the mean total phenol content of nocino liqueur from 1977–2000 reached 2758 mg/L and did not significantly decrease upon the liqueur aging if they were stored at 15–18 °C. The nocino liqueur is made with green, unripe walnuts and is a typical nalewka containing a very high content of tannins, which probably also affects the stability of these tinctures [17].
An important parameter in the evaluation of alcoholic fruit beverages is the color for which some secondary metabolites are responsible, among others anthocyanins. All analyzed by us nalewkas were characterized by a characteristic color. The darkest product was cherry nalewkas, which is due to the high content of anthocyanins [11]. Visually, the color of samples from different years of production was similar. Therefore, we decided to estimate the percentage share of individual colors in all nalewkas in individual years of production. The largest percentage share in the primary colors was yellow, while the lowest was blue. Pandeya et al., who studied the color parameters of Nepali wines, have made similar observations. In the wines they examined, the dominant colors were red and yellow, with a small share of blue. The authors explain this phenomenon by the fact that the proportion of red color is mainly derived from anthocyanins and decreases as anthocyanin polymerizes with tannins and other phenolic compounds, which leads to the increase in yellow color proportion. The intensification of this process was observed with the age of the wine [42]. We observed a similar tendency in our nalewkas. The nalewkas from 2019, in particular ratafia and cherry, were characterized by the highest concentration of anthocyanins and the highest share of red color, whereas the nalewkas from 1997 were characterized by the lowest concentration of anthocyanins. In the case of plum nalewka, the low concentration of anthocyanins was reflected in the color of the nalewka, in which the highest share of yellow was found. The anthocyanins, as pigments, are almost exclusively responsible for the blue, red, and purple colors in fruits [53]. Anthocyanins are natural dietary pigments that could also be involved in health effects [54]. These compounds are known to be functional compounds of a healthy diet and belong to polyphenolic organic compounds. These natural pigments are essential secondary metabolites characterized by powerful antioxidant activity [55]. In our study, the total anthocyanin content of nalewkas varied depending on the production year as well as the type of nalewka. The older nalewkas contained significantly fewer anthocyanins as compared to the younger ones. An interesting result was observed in the case of cherry nalewka, in which the content of anthocyanins remained at a similar level over the last 7 years. In the case of the other nalewkas, high content of anthocyanins was found only in the last four and three years for ratafia and plum nalewka, respectively. Sokół-Łękowska et al. in their study stated, that anthocyanins underwent a partial or completely significant degradation during 6 months of storage in various of fruit liqueurs [9]. Similarly, Kucharska et al. (2007) observed a lower content of anthocyanins in the cornelian cherry liqueur after three months of storage, wherein the color of liqueurs turned more into yellow [56]. Anthocyanins are undurable pigments, susceptible to oxidation, especially in case of ascorbic acid presence [11]. In our study, the lowest degradation of anthocyanins during storage in cherry nalewka were observed. The cherry nalewkas have a specific aroma and an intensive purplish-red color, which may correspond to its anthocyanin content during long-term storage.
In our study, we observed interesting and unusual results regarding the content of tannins. Their highest concentration was observed in liqueurs from 2013. The highest concentration of these compounds was identified in plum nalewkas, while the lowest was in ratafia (20.394 ± 2.531 mg·mL−1 and 17.317 ± 1.044 mg·mL−1, respectively). The lowest concentration of tannins was found in the youngest nalewkas. The reason for such a discrepancy was probably different climatic conditions prevailing in a given year of fruit harvest. As it is commonly known, the content of secondary metabolites and, thus, the antioxidant activity of plants is largely dependent on the weather conditions in a given year of vegetation [57].
Secondary metabolites, mainly polyphenols, are responsible for the high antioxidant activity of the fruit [58]. Many studies report a strong correlation between antioxidant activity and the content of these compounds [59], which was also confirmed in our research. Pearson’s correlation was calculated between the antioxidant activities and total polyphenol content, and anthocyanin content. In the majority of cases, a significant relationship was found between the antioxidant activity and the total content of polyphenols. Similarly, Alpresampre et al. showed a high correlation between the antioxidant activity and the content of total phenols, total tannins, and non-tannin phenolics in nocino liqueur from 1977–2000 [17].
Phenolic acids are one of the major classes of phenolic compounds, found widely in foods of plant origin. A good source of phenolic acids are fruits as well as various types of fruit-based drinks also alcoholic [11,60]. Many studies indicate that among the rich phenolic composition of fruits, the main compound with a strong antioxidant potential is gallic acid. Gallic acid is a secondary metabolite present in most plants, including fruits. This substance exhibit a range of bioactivities, including antioxidant, antimicrobial, anti-inflammatory, and anticancer [61,62,63,64,65]. It also provides efficient protection against oxidative damage caused by ROS often encountered in biological systems, including hydroxyl (HO˙), superoxide (O2˙−), and peroxyl (ROO˙) radicals as well as hydrogen peroxide (H2O2). In addition, this compound, as well as its ester derivatives (propyl gallate, octyl gallate, and lauryl gallate), are used as preservatives to prevent oxidation rancidity and spoilage [61,66]. Therefore, in our work, we decided to determine the content of gallic acid in nalewkas by the HPLC method. The content of gallic acid varied depending on the year of production as well as on the fruit used. The concentration of this compound ranged from 35.442 ± 1.540 µg·mL−1 for the ratafia produced in 1997 to 120.985 ± 1.534 µg·mL−1 for the ratafia from 2018. Generally, it was observed that gallic acid was quite stable during storage. Its content was significantly lower only in all 25-year-old nalewka, while the second group (2013–2019) most often remained at a similar level. Similarly, in the study of Sokół-Łękowska et al., the phenolic acids in sour cherry liqueurs, such as p-coumaroylquinic, neochlorogenic and chlorogenic acids after 6 months of storage, also were quite stable [11]. On the contrary, Kallithraka et al. reported a significant decrease in phenolic compounds, including gallic acid, in wines after 6 months of storage [49].
5. Conclusions
The fruit-based alcoholic beverages have been popular for a very long time. In Poland, a traditional drink of this type is nalewka, which is made by maceration of seasonal fruit in ethyl alcohol for several months. Valuable secondary metabolites contained in the fruit during such maceration get into the alcohol, which makes it a beverage with high antioxidant activity. Thus, consuming small amounts of such nalewka could have a beneficial effect on health. The antioxidants contained in the fruit are one of the most valuable exogenous compounds that eliminate the adverse effects of ROS constantly attacking the human body. In our study, we examined the antioxidant activity and the total content of polyphenols and one of the phenolic acids, i.e., gallic acid, in three types of our own fruit, nalewkas. Nalewkas stored since 1997 and produced in 2013–2019 were analyzed. The antioxidant activity was different depending on the year and the fruit used. A decrease in antioxidant activity was observed during long storage. Moreover, the long storage time of nalewkas resulted in a decrease in polyphenols content, which correlates with their antioxidant activity. The obtained results suggest that longer storage time and its conditions (e.g., temperature) result in a decrease in the antioxidant potential of nalewkas. In addition, the observed decrease in the content of anthocyanins, which could be responsible for a color change (decrease in the share of red color in favor of yellow color), also suggests an adverse effect of long-term storage on the content of biologically active compounds. Noteworthy is also the observation that tinctures from 2013 were characterized by the highest content of tannins, which probably were related to the weather conditions prevailing in this growing season.
To sum up, the antioxidant potential of nalewkas is affected by many factors, both those related to the preparation of tinctures (type of fruit, method of preparation, sugar content) as well as to storage conditions (time, temperature). Weather conditions, in particular, growing seasons, are also important, as they also affect changes in the biochemical composition of fruits. The obtained results suggest that, in general, tinctures stored for a shorter period of time (2013–2019) are richer in compounds with antioxidant activity than tinctures produced in 1997 after a 25-year storage period.
Author Contributions
Conceptualization, A.N. (Anna Nowak) and A.N. (Andrzej Nowak); methodology, A.N. (Anna Nowak), A.M.-S. and Ł.K.; software, A.N. (Anna Nowak) and A.M.-S.; formal analysis, A.N. (Anna Nowak), W.D., A.M.-S., Ł.K., J.Z.-B. and A.N. (Andrzej Nowak); writing—original draft preparation, A.N. (Anna Nowak) and A.M.-S.; writing—review and editing, A.N. (Anna Nowak), A.M.-S. and J.Z.-B.; supervision, A.K. and A.N. (Andrzej Nowak). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Swallah, M.S.; Sun, H.; Affoh, R.; Fu, H.; Yu, H. Antioxidant Potential Overviews of Secondary Metabolites (Polyphenols) in Fruits. Int. J. Food Sci. 2020, 2020, 9081686. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Xu, B.-T.; Xu, X.-R.; Qin, X.-S.; Gan, R.-Y.; Li, H.-B. Antioxidant Capacities and Total Phenolic Contents of 56 Wild Fruits from South China. Molecules 2010, 15, 8602–8617. [Google Scholar] [CrossRef]
- Fu, L.; Xu, B.-T.; Xu, X.-R.; Gan, R.-Y.; Zhang, Y.; Xia, E.-Q.; Li, H.-B. Antioxidant Capacities and Total Phenolic Contents of 62 Fruits. Food Chem. 2011, 129, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J.L.; Lloyd, B. Health Benefits of Fruits and Vegetables. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Liu, H.; Wu, X.; Weng, W.; Wang, X.; Su, J. Reactive Oxygen Species (ROS)-Responsive Biomaterials for the Treatment of Bone-Related Diseases. Front. Bioeng. Biotechnol. 2022, 9, 820468. [Google Scholar] [CrossRef]
- Nowak, A.; Klimowicz, A.; Duchnik, W.; Kucharski, Ł.; Florkowska, K.; Muzykiewicz, A.; Wira, D.; Zielonkabrzezicka, J.; Siedłowska, A.; Nadarzewska, K. Application of Green-Extraction Technique to Evaluate of Antioxidative Capacity of Wild Population of Fireweed (Epilobium angustifolium). Herba Pol. 2019, 65, 18–30. [Google Scholar] [CrossRef]
- Nowak, A.; Kojder, K.; Zielonka-Brzezicka, J.; Wróbel, J.; Bosiacki, M.; Fabiańska, M.; Wróbel, M.; Sołek-Pastuszka, J.; Klimowicz, A. The Use of Ginkgo biloba L. as a Neuroprotective Agent in the Alzheimer’s Disease. Front. Pharmacol. 2021, 12, 775034. [Google Scholar] [CrossRef]
- Jideani, A.I.O.; Silungwe, H.; Takalani, T.; Omolola, A.O.; Udeh, H.O.; Anyasi, T.A. Antioxidant-Rich Natural Fruit and Vegetable Products and Human Health. Int. J. Food Prop. 2021, 24, 41–67. [Google Scholar] [CrossRef]
- Sokół-Łętowska, A.; Kucharska, A.Z.; Wińska, K.; Szumny, A.; Nawirska-Olszańska, A.; Mizgier, P.; Wyspiańska, D. Composition and Antioxidant Activity of Red Fruit Liqueurs. Food Chem. 2014, 157, 533–539. [Google Scholar] [CrossRef]
- Egea, T.; Signorini, M.A.; Bruschi, P.; Rivera, D.; Obón, C.; Alcaraz, F.; Palazón, J.A. Spirits and Liqueurs in European Traditional Medicine: Their History and Ethnobotany in Tuscany and Bologna (Italy). J. Ethnopharm. 2015, 175, 241–255. [Google Scholar] [CrossRef]
- Sokół-Łętowska, A.; Kucharska, A.Z.; Szumny, A.; Wińska, K.; Nawirska-Olszańska, A. Phenolic Composition Stability and Antioxidant Activity of Sour Cherry Liqueurs. Molecules 2018, 23, 2156. [Google Scholar] [CrossRef] [PubMed]
- Śliwińska, M.; Wiśniewska, P.; Dymerski, T.; Wardencki, W.; Namieśnik, J. Application of Electronic Nose Based on Fast GC for Authenticity Assessment of Polish Homemade Liqueurs Called Nalewka. Food Anal. Methods 2016, 9, 2670–2681. [Google Scholar] [CrossRef]
- Polak, J.; Bartoszek, M. The Study of Antioxidant Capacity of Varieties of Nalewka, a Traditional Polish Fruit Liqueur, Using EPR, NMR and UV–Vis Spectroscopy. J. Food Comp. Anal. 2015, 40, 114–119. [Google Scholar] [CrossRef]
- Paz, I.; Pinto, G. Spectroscopic study about the kinetics of the anthocyanin pigments extraction during the maceration of cherries in liquor. Spectrosc. Lett. 2002, 35, 357–368. [Google Scholar] [CrossRef]
- Cendrowski, A.; Ścibisz, I.; Kieliszek, M.; Kolniak-Ostek, J.; Mitek, M. UPLC-PDA-Q/TOF-MS Profile of Polyphenolic Compounds of Liqueurs from Rose Petals (Rosa rugosa). Molecules 2017, 22, 1832. [Google Scholar] [CrossRef] [PubMed]
- Stampar, F.; Solar, A.; Hudina, M.; Veberic, R.; Colaric, M. Traditional Walnut Liqueur—Cocktail of Phenolics. Food Chem. 2006, 95, 627–631. [Google Scholar] [CrossRef]
- Alamprese, C. Characterization and Antioxidant Activity of Nocino Liqueur. Food Chem. 2005, 90, 495–502. [Google Scholar] [CrossRef]
- Serreli, G.; Jerković, I.; Gil, K.A.; Marijanović, Z.; Pacini, V.; Tuberoso, C.I.G. Phenolic Compounds, Volatiles and Antioxidant Capacity of White Myrtle Berry Liqueurs. Plant Foods Hum. Nutr. 2017, 72, 205–210. [Google Scholar] [CrossRef]
- Sokół-Łętowska, A.; Kucharska, A.Z.; Hodun, G.; Gołba, M. Chemical Composition of 21 Cultivars of Sour Cherry (Prunus cerasus) Fruit Cultivated in Poland. Molecules 2020, 25, 4587. [Google Scholar] [CrossRef]
- Damar, İ.; Ekşi, A. Antioxidant Capacity and Anthocyanin Profile of Sour Cherry (Prunus cerasus L.) Juice. Food Chem. 2012, 135, 2910–2914. [Google Scholar] [CrossRef]
- Serradilla, M.J.; Hernández, A.; López-Corrales, M.; Ruiz-Moyano, S.; de Guía Córdoba, M.; Martín, A. Composition of the Cherry (Prunus avium L. and Prunus cerasus L.; Rosaceae). In Nutritional Composition of Fruit Cultivars; Elsevier: Amsterdam, The Netherlands, 2016; pp. 127–147. ISBN 978-0-12-408117-8. [Google Scholar]
- Kelley, D.; Adkins, Y.; Laugero, K. A Review of the Health Benefits of Cherries. Nutrients 2018, 10, 368. [Google Scholar] [CrossRef] [PubMed]
- Cásedas, G.; Les, F.; Gómez-Serranillos, M.P.; Smith, C.; López, V. Bioactive and Functional Properties of Sour Cherry Juice (Prunus cerasus). Food Funct. 2016, 7, 4675–4682. [Google Scholar] [CrossRef] [PubMed]
- Šarić, A.; Sobočanec, S.; Balog, T.; Kušić, B.; Šverko, V.; Dragović-Uzelac, V.; Levaj, B.; Čosić, Z.; Mačak Šafranko, Ž.; Marotti, T. Improved Antioxidant and Anti-Inflammatory Potential in Mice Consuming Sour Cherry Juice (Prunus cerasus Cv. Maraska). Plant Foods Hum. Nutr. 2009, 64, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Igwe, E.O.; Charlton, K.E. A Systematic Review on the Health Effects of Plums (Prunus domestica and Prunus salicina): Health Effects of Plums (Prunus domestica and Prunus salicina). Phytother. Res. 2016, 30, 701–731. [Google Scholar] [CrossRef]
- Jaiswal, R.; Karaköse, H.; Rühmann, S.; Goldner, K.; Neumüller, M.; Treutter, D.; Kuhnert, N. Identification of Phenolic Compounds in Plum Fruits (Prunus salicina L. and Prunus domestica L.) by High-Performance Liquid Chromatography/Tandem Mass Spectrometry and Characterization of Varieties by Quantitative Phenolic Fingerprints. J. Agric. Food Chem. 2013, 61, 12020–12031. [Google Scholar] [CrossRef]
- Karasawa, K.; Miyashita, R.; Otani, H. Anti-Allergic Properties of a Fruit Extract of Prune (Prunus domestica L.) in Mite-Sensitized BALB/c Mice. Food Sci. Technol. Res. 2012, 18, 755–760. [Google Scholar] [CrossRef]
- Rop, O.; Jurikova, T.; Mlcek, J.; Kramarova, D.; Sengee, Z. Antioxidant Activity and Selected Nutritional Values of Plums (Prunus domestica L.) Typical of the White Carpathian Mountains. Sci. Hortic. 2009, 122, 545–549. [Google Scholar] [CrossRef]
- Romandini, S.; Mazzoni, L.; Giampieri, F.; Tulipani, S.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Locorotondo, N.; D’Alessandro, M.; Mezzetti, B.; Bompadre, S.; et al. Effects of an Acute Strawberry (Fragaria × ananassa) Consumption on the Plasma Antioxidant Status of Healthy Subjects. J. Berry Res. 2013, 3, 169–179. [Google Scholar] [CrossRef]
- Gasperotti, M.; Masuero, D.; Mattivi, F.; Vrhovsek, U. Overall Dietary Polyphenol Intake in a Bowl of Strawberries: The Influence of Fragaria Spp. in Nutritional Studies. J. Funct. Foods 2015, 18, 1057–1069. [Google Scholar] [CrossRef]
- Forni, C.; Braglia, R.; Mulinacci, N.; Urbani, A.; Ronci, M.; Gismondi, A.; Tabolacci, C.; Provenzano, B.; Lentini, A.; Beninati, S. Antineoplastic Activity of Strawberry (Fragaria × ananassa Duch.) Crude Extracts on B16-F10 Melanoma Cells. Mol. BioSyst. 2014, 10, 1255–1263. [Google Scholar] [CrossRef]
- Piotrowski, W.; Łabanowska, B.H.; Kozak, M. Assessment of Infestation of Selected Blackcurrant (Ribes nigrum L.) Genotypes by the Blackcurrant Leaf Midge (Dasineura tetensi Rübs.) in Poland. Insects 2021, 12, 492. [Google Scholar] [CrossRef]
- Cortez, R.E.; Gonzalez de Mejia, E. Blackcurrants (Ribes nigrum): A Review on Chemistry, Processing, and Health Benefits. J. Food Sci. 2019, 84, 2387–2401. [Google Scholar] [CrossRef] [PubMed]
- Tits, M.; Angenot, L.; Poukens, P.; Warin, R.; Dierckxsens, Y. Prodelphinidins from Ribes nigrum. Phytochemistry 1992, 31, 971–973. [Google Scholar] [CrossRef]
- Tabart, J.; Franck, T.; Kevers, C.; Pincemail, J.; Serteyn, D.; Defraigne, J.-O.; Dommes, J. Antioxidant and Anti-Inflammatory Activities of Ribes nigrum Extracts. Food Chem. 2012, 131, 1116–1122. [Google Scholar] [CrossRef]
- Bishayee, A.; Háznagy-Radnai, E.; Mbimba, T.; Sipos, P.; Morazzoni, P.; Darvesh, A.S.; Bhatia, D.; Hohmann, J. Anthocyanin-Rich Black Currant Extract Suppresses the Growth of Human Hepatocellular Carcinoma Cells. Nat. Prod. Commun. 2010, 5, 1934578X1000501. [Google Scholar] [CrossRef]
- Krauze-Baranowska, M.; Głód, D.; Kula, M.; Majdan, M.; Hałasa, R.; Matkowski, A.; Kozłowska, W.; Kawiak, A. Chemical Composition and Biological Activity of Rubus idaeus Shoots—A Traditional Herbal Remedy of Eastern Europe. BMC Complement. Altern. Med. 2014, 14, 480. [Google Scholar] [CrossRef] [PubMed]
- Bobinaitė, R.; Viškelis, P.; Venskutonis, P.R. Chemical Composition of Raspberry (Rubus spp.) Cultivars. In Nutritional Composition of Fruit Cultivars; Elsevier: Amsterdam, The Netherlands, 2016; pp. 713–731. ISBN 978-0-12-408117-8. [Google Scholar]
- Aprea, E.; Biasioli, F.; Gasperi, F. Volatile Compounds of Raspberry Fruit: From Analytical Methods to Biological Role and Sensory Impact. Molecules 2015, 20, 2445–2474. [Google Scholar] [CrossRef] [PubMed]
- Wojdyło, A.; Oszmiański, J.; Bielicki, P. Polyphenolic Composition, Antioxidant Activity, and Polyphenol Oxidase (PPO) Activity of Quince (Cydonia oblonga Miller) Varieties. J. Agric. Food Chem. 2013, 61, 2762–2772. [Google Scholar] [CrossRef] [PubMed]
- Muzykiewicz, A.; Zielonka-Brzezicka, J.; Klimowicz, A. Quince (Cydonia oblonga Mill.) as a Useful Source of Antioxidants—Antioxidant Activity Evaluation. Herba Pol. 2018, 64, 23–33. [Google Scholar] [CrossRef]
- Pandeya, A.; Rayamajhi, S.; Pokhrel, P.; Giri, B. Evaluation of Secondary Metabolites, Antioxidant Activity, and Color Parameters of Nepali Wines. Food Sci. Nutr. 2018, 6, 2252–2263. [Google Scholar] [CrossRef]
- Nowak, A.; Zagórska-Dziok, M.; Ossowicz-Rupniewska, P.; Makuch, E.; Duchnik, W.; Kucharski, Ł.; Adamiak-Giera, U.; Prowans, P.; Czapla, N.; Bargiel, P.; et al. Epilobium angustifolium L. Extracts as Valuable Ingredients in Cosmetic and Dermatological Products. Molecules 2021, 26, 3456. [Google Scholar] [CrossRef] [PubMed]
- Muzykiewicz-Szymańska, A.; Nowak, A.; Wira, D.; Klimowicz, A. The Effect of Brewing Process Parameters on Antioxidant Activity and Caffeine Content in Infusions of Roasted and Unroasted Arabica Coffee Beans Originated from Different Countries. Molecules 2021, 26, 3681. [Google Scholar] [CrossRef] [PubMed]
- Roman, B.; Muzykiewicz-Szymańska, A.; Ossowicz-Rupniewska, P.; Klimowicz, A.; Janus, E. The Application of Amino Acid Ionic Liquids as Additives in the Ultrasound-Assisted Extraction of Plant Material. RSC Adv. 2021, 11, 25983–25994. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Durst, R.W.; Wrolstad, R.E.; Eisele, T.; Giusti, M.M.; Hach, J.; Hofsommer, H.; Koswig, S.; Krueger, D.A.; Kupina, S.; et al. Determination of Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants, and Wines by the PH Differential Method: Collaborative Study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef]
- Laaksonen, O.; Kuldjärv, R.; Paalme, T.; Virkki, M.; Yang, B. Impact of Apple Cultivar, Ripening Stage, Fermentation Type and Yeast Strain on Phenolic Composition of Apple Ciders. Food Chem. 2017, 233, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Bayram, M.; Kaya, C. Effects of Different Tea Concentrations and Extraction Durations on Caffeine and Phenolics of Tea Liqueurs: Some Properties of Tea Liqueurs. Food Measur. 2018, 12, 285–291. [Google Scholar] [CrossRef]
- Kallithraka, S.; Salacha, M.I.; Tzourou, I. Changes in Phenolic Composition and Antioxidant Activity of White Wine during Bottle Storage: Accelerated Browning Test versus Bottle Storage. Food Chem. 2009, 113, 500–505. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary Intake and Bioavailability of Polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic Acids: Natural Versatile Molecules with Promising Therapeutic Applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Joseph, S.V.; Edirisinghe, I.; Burton-Freeman, B.M. Fruit Polyphenols: A Review of Anti-Inflammatory Effects in Humans. Crit. Rev. Food Sci. Nutr. 2016, 56, 419–444. [Google Scholar] [CrossRef]
- Einbond, L.S.; Reynertson, K.A.; Luo, X.-D.; Basile, M.J.; Kennelly, E.J. Anthocyanin Antioxidants from Edible Fruits. Food Chem. 2004, 84, 23–28. [Google Scholar] [CrossRef]
- Felgines, C.; Texier, O.; Garcin, P.; Besson, C.; Lamaison, J.-L.; Scalbert, A. Tissue Distribution of Anthocyanins in Rats Fed a Blackberry Anthocyanin-Enriched Diet. Mol. Nutr. Food Res. 2009, 53, 1098–1103. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Shi, Y.; Wang, R.; Su, D.; Tang, M.; Liu, Y.; Li, Z. Antioxidant Activity and Healthy Benefits of Natural Pigments in Fruits: A Review. Int. J. Mol. Sci. 2021, 22, 4945. [Google Scholar] [CrossRef]
- Kucharska, A.Z.; Sokół-Łętowska, A.; Hudko, J.; Nawirska, A. Influence of the preparation procedure on the antioxidant activity and colour of liqueurs from cornelian cherry (Cornus mas L.). Pol. J. Food Nutr. Sci. 2007, 57, 343–347. [Google Scholar]
- Haokip, S.W.; Shankar, K.; Lalrinngheta, J. Climate Change and Its Impact on Fruit Crops. J. Pharm. Phytochem. 2020, 9, 435–438. [Google Scholar]
- Afonso, S.; Oliveira, I.V.; Meyer, A.S.; Aires, A.; Saavedra, M.J.; Gonçalves, B. Phenolic Profile and Bioactive Potential of Stems and Seed Kernels of Sweet Cherry Fruit. Antioxidants 2020, 9, 1295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, Y.; Zhou, Z. Phenolic and Flavonoid Contents of Mandarin (Citrus reticulata Blanco) Fruit Tissues and Their Antioxidant Capacity as Evaluated by DPPH and ABTS Methods. J. Integr. Agric. 2018, 17, 256–263. [Google Scholar] [CrossRef]
- Mattila, P.; Hellström, J.; Törrönen, R. Phenolic Acids in Berries, Fruits, and Beverages. J. Agric. Food Chem. 2006, 54, 7193–7199. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, M.; Wu, S.; Zou, X.; Chen, X.; Ge, L.; Zhang, Q. Effects of Gallic Acid on Fermentation Parameters, Protein Fraction, and Bacterial Community of Whole Plant Soybean Silage. Front. Microbiol. 2021, 12, 662966. [Google Scholar] [CrossRef]
- Fernandes, F.H.A.; Salgado, H.R.N. Gallic Acid: Review of the Methods of Determination and Quantification. Crit. Rev. Anal. Chem. 2016, 46, 257–265. [Google Scholar] [CrossRef]
- Mei, Y.; Sun, H.; Du, G.; Wang, X.; Lyu, D. Exogenous Chlorogenic Acid Alleviates Oxidative Stress in Apple Leaves by Enhancing Antioxidant Capacity. Sci. Hortic. 2020, 274, 109676. [Google Scholar] [CrossRef]
- Sorrentino, E.; Succi, M.; Tipaldi, L.; Pannella, G.; Maiuro, L.; Sturchio, M.; Coppola, R.; Tremonte, P. Antimicrobial Activity of Gallic Acid against Food-Related Pseudomonas Strains and Its Use as Biocontrol Tool to Improve the Shelf Life of Fresh Black Truffles. Int. J. Food Microbiol. 2018, 266, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Kosuru, R.Y.; Aashique, M.; Fathima, A.; Roy, A.; Bera, S. Revealing the Dual Role of Gallic Acid in Modulating Ampicillin Sensitivity of Pseudomonas aeruginosa Biofilms. Future Microbiol. 2018, 13, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Badhani, B.; Sharma, N.; Kakkar, R. Gallic Acid: A Versatile Antioxidant with Promising Therapeutic and Industrial Applications. RSC Adv. 2015, 5, 27540–27557. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).