Computational Study of the Physical Properties of a High Temperature Molten Salt Mixture of FLiNaK and CeF3
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Density of Molten FLiNaK
3.2. Self-Diffusion of Ions
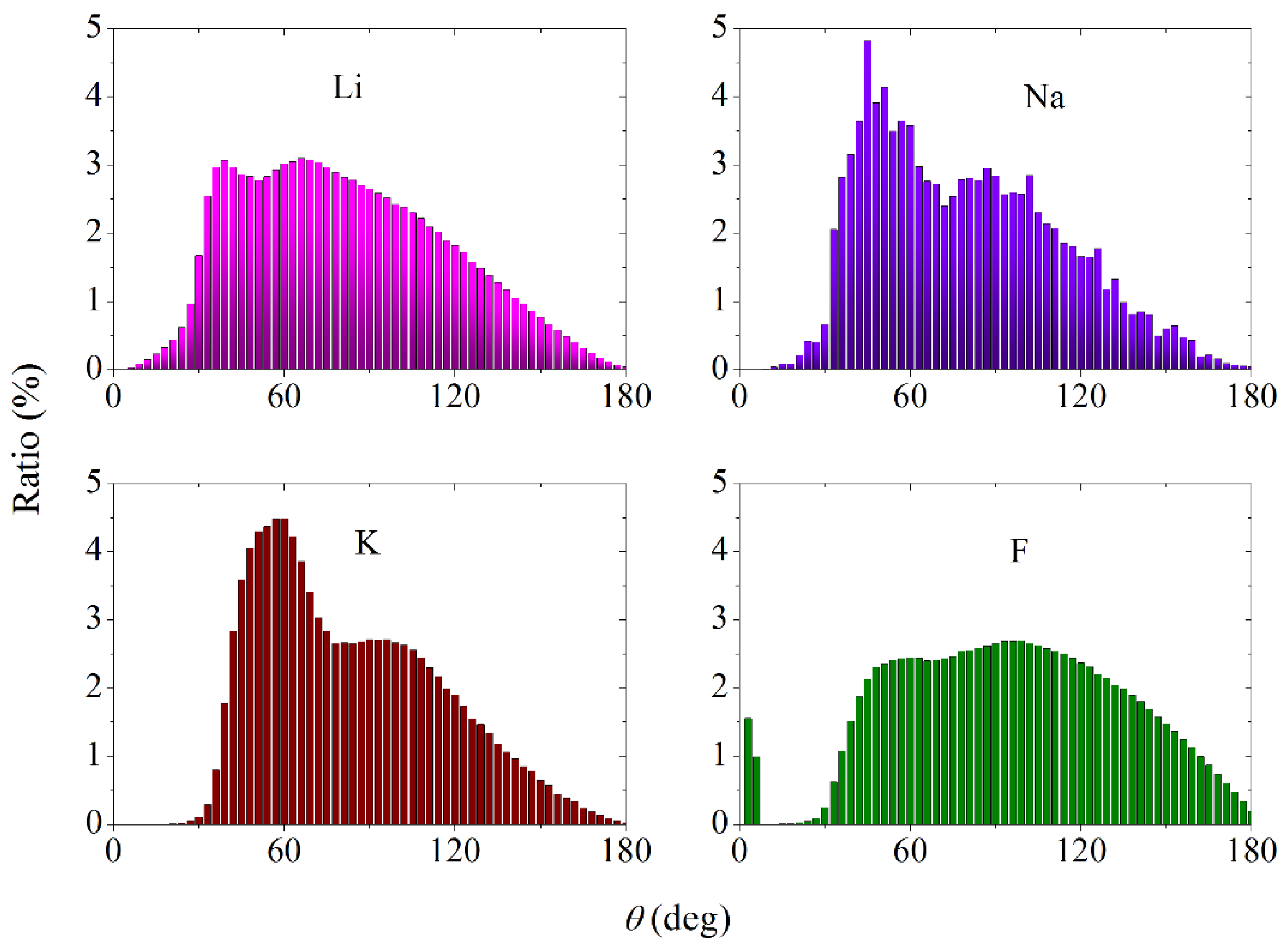
3.3. Partial Radial Distribution Functions and Coordination Numbers
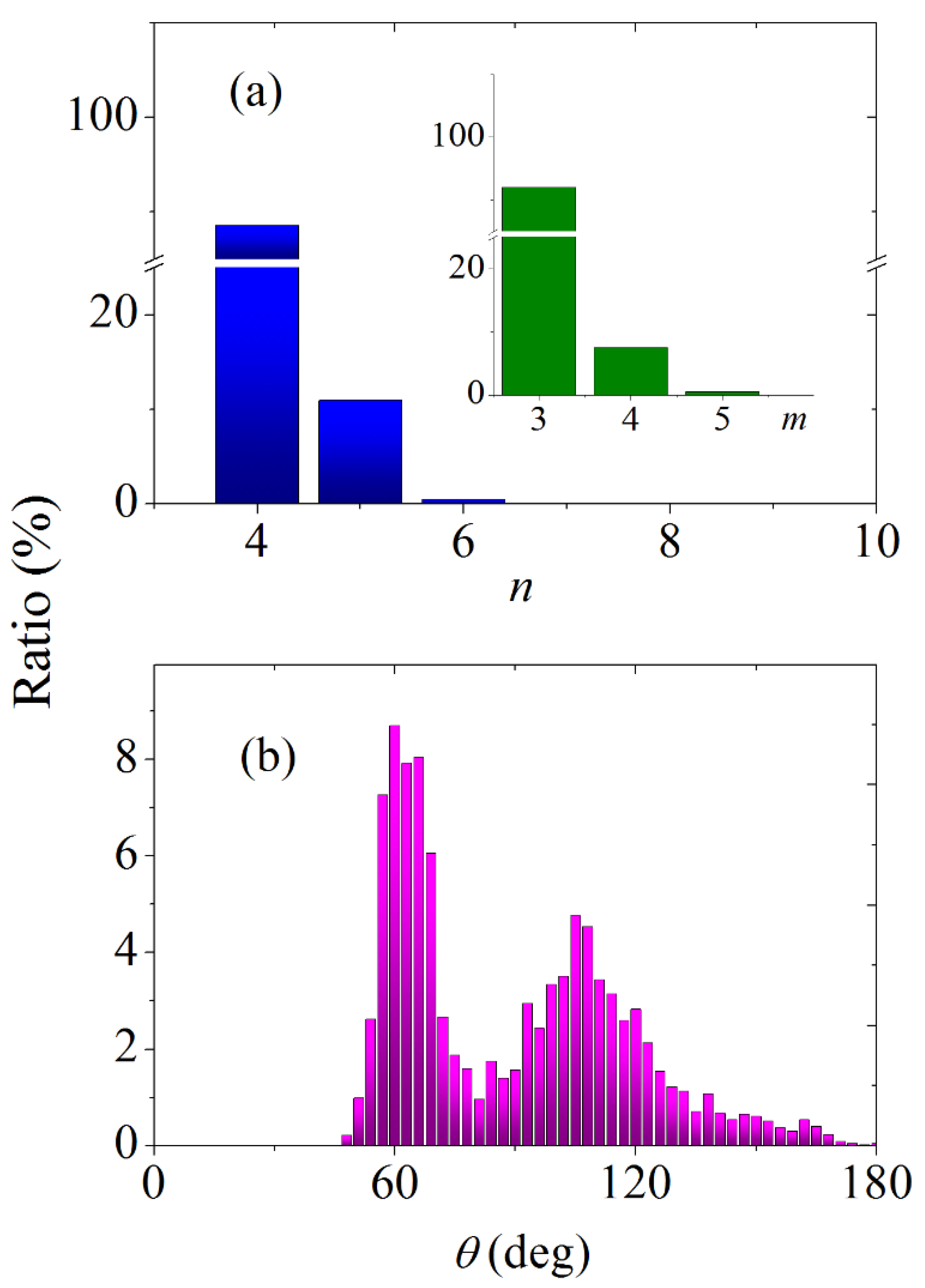
3.4. Statistical Geometry Method
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Serp, J.; Allibert, M.; Beneš, O.; Delpech, S.; Feynberg, O.; Ghetta, V.; Heuer, D.; Holcomb, D.; Ignatiev, V.; Kloosterman, J.L.; et al. The molten salt reactor (MSR) in generation IV: Overview and perspectives. Prog. Nucl. Energy 2014, 77, 308–319. [Google Scholar] [CrossRef]
- Rubiolo, P.R.; Retamales, M.T.; Ghetta, V.; Giraud, J. High temperature thermal hydraulics modeling of a molten salt: Application to a molten salt fast reactor (MSFR). ESAIM Proc. Surv. 2017, 58, 98–117. [Google Scholar] [CrossRef]
- Lizin, A.A.; Tomilin, S.V.; Gnevashov, O.E.; Gazizov, R.K.; Osipenko, A.G.; Kormilitsyn, M.V.; Baranov, A.A.; Zaharova, L.V.; Naumov, V.S.; Ponomarev, L.I. PuF3, AmF3, CeF3, and NdF3 solubility in LiF-NaF-KF melt. At. Energy 2013, 115, 11–17. [Google Scholar] [CrossRef]
- Ponomarev, L.I.; Seregin, M.B.; Mikhalichenko, A.A.; Parshin, A.P.; Zagorez, L.P. Validation of actinide fluoride simulators for studying solubility in fuel salt of molten-salt reactors. At. Energy 2012, 112, 417–422. [Google Scholar] [CrossRef]
- Winner, N.; Williams, H.; Scarlat, R.O.; Asta, M. Ab-initio simulation studies of chromium solvation in molten fluoride salts. J. Mol. Liq. 2021, 335, 116351. [Google Scholar] [CrossRef]
- Dewing, E.W. The effect of additives on activities in cryolite melts. Met. Mater. Trans. B 1989, 20, 675–677. [Google Scholar] [CrossRef]
- Sarou-Kanian, V.; Rollet, A.-L.; Salanne, M.; Simon, C.; Bessada, C.; Madden, P.A. Diffusion coefficients and local structure in basic molten fluorides: In situ NMR measurements and molecular dynamics simulations. Phys. Chem. Chem. Phys. 2009, 11, 11501–11506. [Google Scholar] [CrossRef]
- Nam, H.O.; Bengtson, A.; Vörtler, K.; Saha, S.; Sakidja, R.; Morgan, D. First-principles molecular dynamics modeling of the molten fluoride salt with Cr solute. J. Nucl. Mater. 2014, 449, 148–157. [Google Scholar] [CrossRef]
- Clark, A.D.; Lee, W.L.; Solano, A.R.; Williams, T.B.; Meyer, G.S.; Tait, G.J.; Battraw, B.C.; Nickerson, S.D. Complexation of Mo in FLiNaK Molten Salt: Insight from Ab Initio Molecular Dynamics. J. Phys. Chem. B 2020, 125, 211–218. [Google Scholar] [CrossRef]
- Maxwell, C.I. Molecular dynamics study of fission gas behaviour and solubility in molten FLiNaK salt. J. Nucl. Mater. 2022, 563, 153633. [Google Scholar] [CrossRef]
- Nosé, S. A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 1984, 81, 511–519. [Google Scholar] [CrossRef]
- Nosé, S.; Klein, M. Constant pressure molecular dynamics for molecular systems. Mol. Phys. 1983, 50, 1055–1076. [Google Scholar] [CrossRef]
- Bussi, G.; Zykova-Timan, T.; Parrinello, M. Isothermal-isobaric molecular dynamics using stochastic velocity rescaling. J. Chem. Phys. 2009, 130, 074101. [Google Scholar] [CrossRef] [PubMed]
- Delpech, S.; Merle-Lucotte, E.; Heuer, D.; Allibert, M.; Ghetta, V.; Le-Brun, C.; Doligez, X.; Picard, G. Reactor physic and reprocessing scheme for innovative molten salt reactor system. J. Fluor. Chem. 2009, 130, 11–17. [Google Scholar] [CrossRef]
- Wu, J.; Wang, J.; Ni, H.; Lu, G.; Yu, J. The influence of NaCl concentration on the (LiCl-KCl) eutectic system and temperature dependence of the ternary system. J. Mol. Liq. 2018, 253, 96–112. [Google Scholar] [CrossRef]
- Adams, D.J.; McDonald, I.R. Rigid-ion models of the interionic potential in the alkali halides. J. Phys. C Solid State Phys. 1974, 7, 2761–2775. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A modular and open-source software pro-ject for quantum simulations of materials. J. Phys. Cond. Matt. 2009, 21, 395502. [Google Scholar] [CrossRef]
- Giannozzi, P.; Andreussi, O.; Brumme, T.; Bunau, O.; Nardelli, M.B.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Cococcioni, M.; et al. Advanced capabilities for materials modelling with Quantum ESPRESSO. J. Phys. Condens. Matter 2017, 29, 465901. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Zakiryanov, D.O. Applying the Born-Mayer model to describe the physicochemical properties of FLiNaK ternary melt. Comput. Theor. Chem. 2023, 1219, 113951. [Google Scholar] [CrossRef]
- Yeh, I.-C.; Hummer, G. System-Size Dependence of Diffusion Coefficients and Viscosities from Molecular Dynamics Simulations with Periodic Boundary Conditions. J. Phys. Chem. B 2004, 108, 15873–15879. [Google Scholar] [CrossRef]
- Nijboer, B.R.A.; Ruijgrok, T.W. On the energy per particle in three- and two-dimensional Wigner lattices. J. Stat. Phys. 1988, 53, 361–382. [Google Scholar] [CrossRef]
- Karki, B.B.; Bhattari, D.; Stixrude, L. First-principles calculations of the structural dynamical, and electrical properties of liquid MgO. Phys. Rev. B 2006, 73, 174208. [Google Scholar] [CrossRef]
- Galashev, A.Y. Numerical simulation of functioning a silicene anode of a lithium-ion battery. J. Comput. Sci. 2022, 64, 101835. [Google Scholar] [CrossRef]
- Plimpton, S. Fast Parallel Algorithms for Short-Range Molecular Dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Gallagher, R.C.; Agca, C.; Russell, N.; McMurray, J.W.; Bull Ezell, N.D. Assessment of molten eutectic LiF-NaF-KF density through experimental determination and semiempirical modeling. J. Chem. Eng. Data 2022, 67, 1406–1414. [Google Scholar] [CrossRef]
- Salanne, M.; Simon, C.; Turq, P.; Madden, P.A. Heat-transport properties of molten fluorides: Determination from first-principles. J. Fluor. Chem. 2009, 130, 38–44. [Google Scholar] [CrossRef]
- Umesaki, N.; Iwamoto, N.; Tsunawaki, Y.; Ohno, H.; Furukawa, K. Self-diffusion of lithium, sodium, potassium and fluorine in a molten LiF+ NaF+ KF eutectic mixture. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1981, 77, 169–175. [Google Scholar] [CrossRef]
- Igarashi, K.; Okamoto, Y.; Mochinaga, J.; Ohno, H. X-ray diffraction study of molten eutectic LiF-NaF-KF mixture. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1988, 84, 4407–4415. [Google Scholar] [CrossRef]
- Lam, S.T.; Li, Q.-J.; Mailoa, J.; Forsberg, C.; Ballinger, R.; Li, J. The Impact of Hydrogen Valence on Its Bonding and Transport in Molten Fluoride Salts, Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. The Royal Society of Chemistry 2021. Available online: https://www.rsc.org/suppdata/d0/ta/d0ta10576g/d0ta10576g1.pdf (accessed on 8 January 2023).
- Dočkal, J.; Lísal, M.; Moučka, F. Polarizable force fields for accurate molecular simulations of aqueous solutions of electrolytes, crystalline salts, and solubility: Li+, Na+, K+, Rb+, F−, Cl−, Br−, I−. J. Mol. Liq. 2022, 362, 119659. [Google Scholar] [CrossRef]
- Philippi, F.; Welton, T. Targeted modifications in ionic liquids—From understanding to design. Phys. Chem. Chem. Phys. 2021, 23, 6993–7021. [Google Scholar] [CrossRef] [PubMed]
- Oppenheim, J.J.; Skorupskii, G.; Dincă, M. Aperiodic metal–organic frameworks. Chem. Sci. 2020, 11, 11094–11103. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.J.; Sprouster, D.; Zheng, G.; Neuefeind, J.C.; Braatz, A.D.; Mcfarlane, J.; Olds, D.; Lam, S.; Li, J.; Khaykovich, B. Complex structure of molten NaCl–CrCl3 salt: Cr–Cl octahedral network and intermediate-range order. ACS Appl. Energy Mater. 2021, 4, 3044–3056. [Google Scholar] [CrossRef]
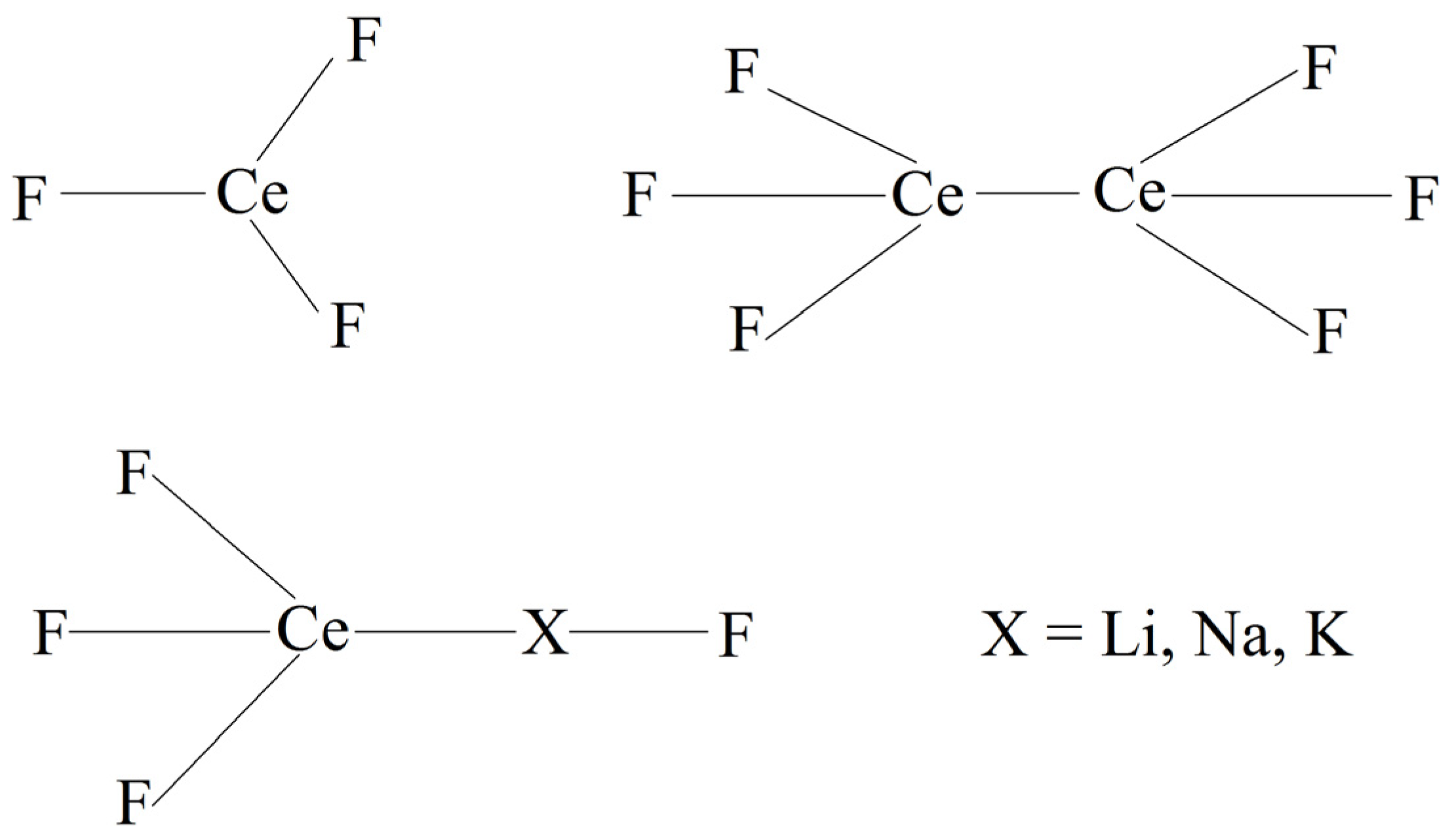

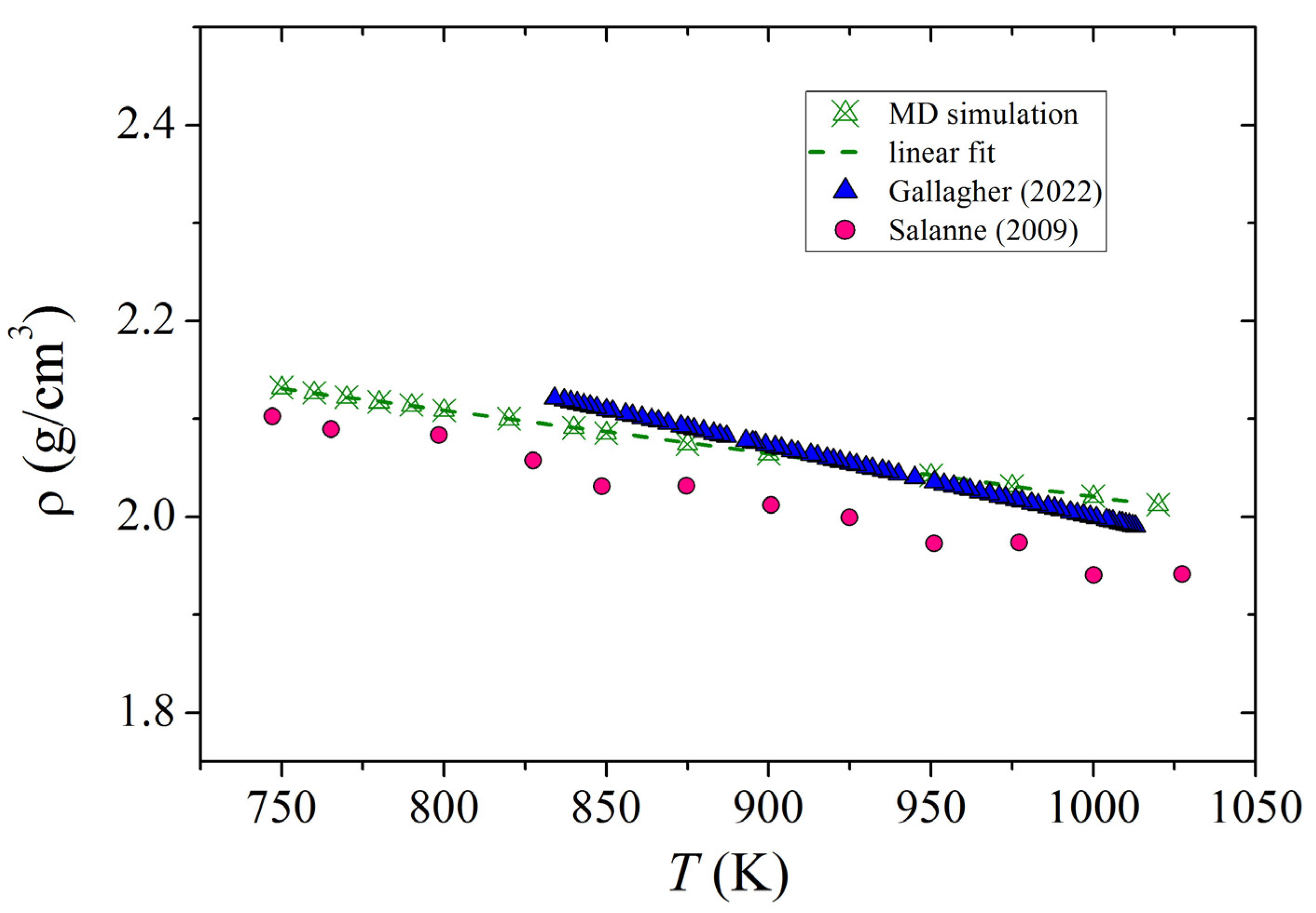
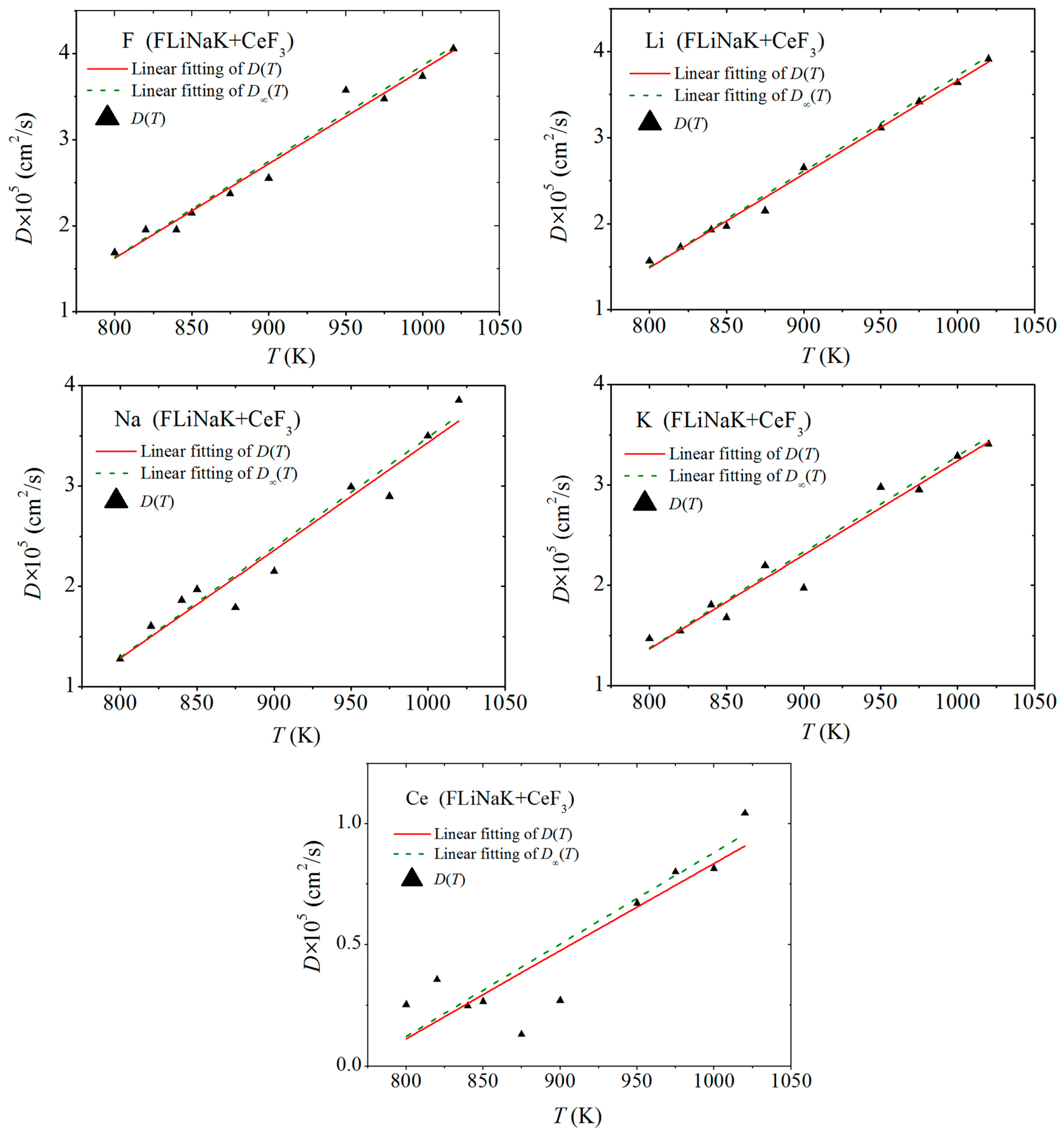

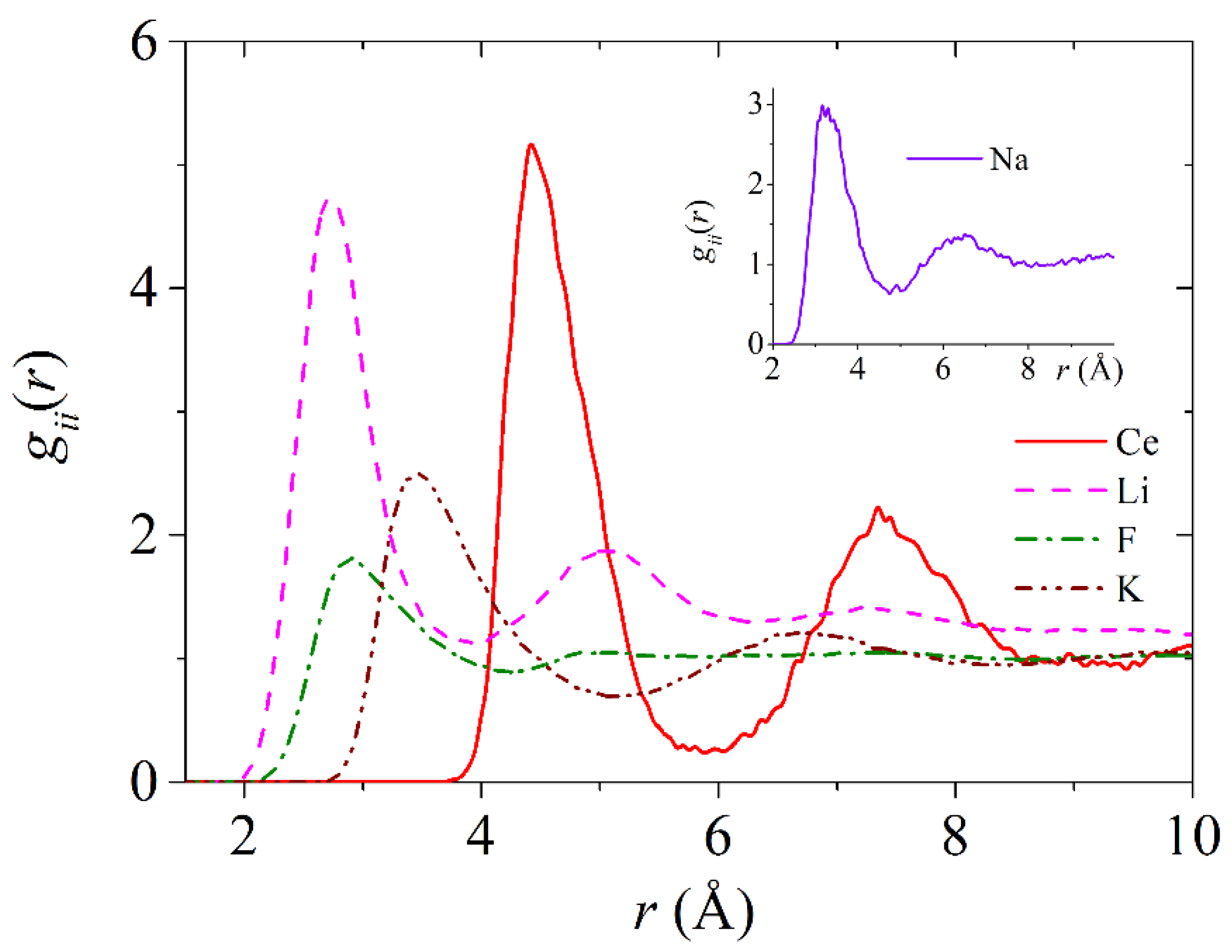

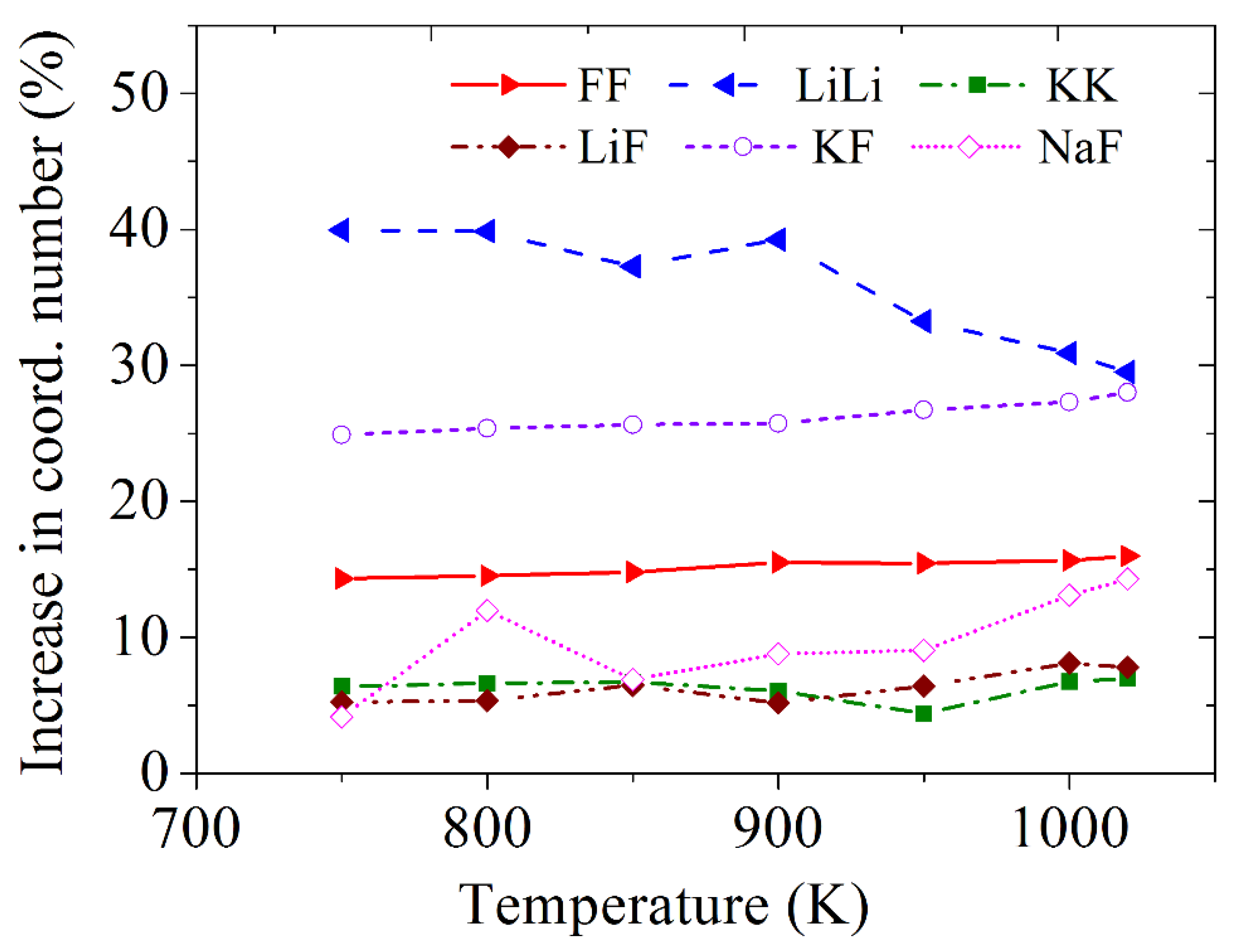
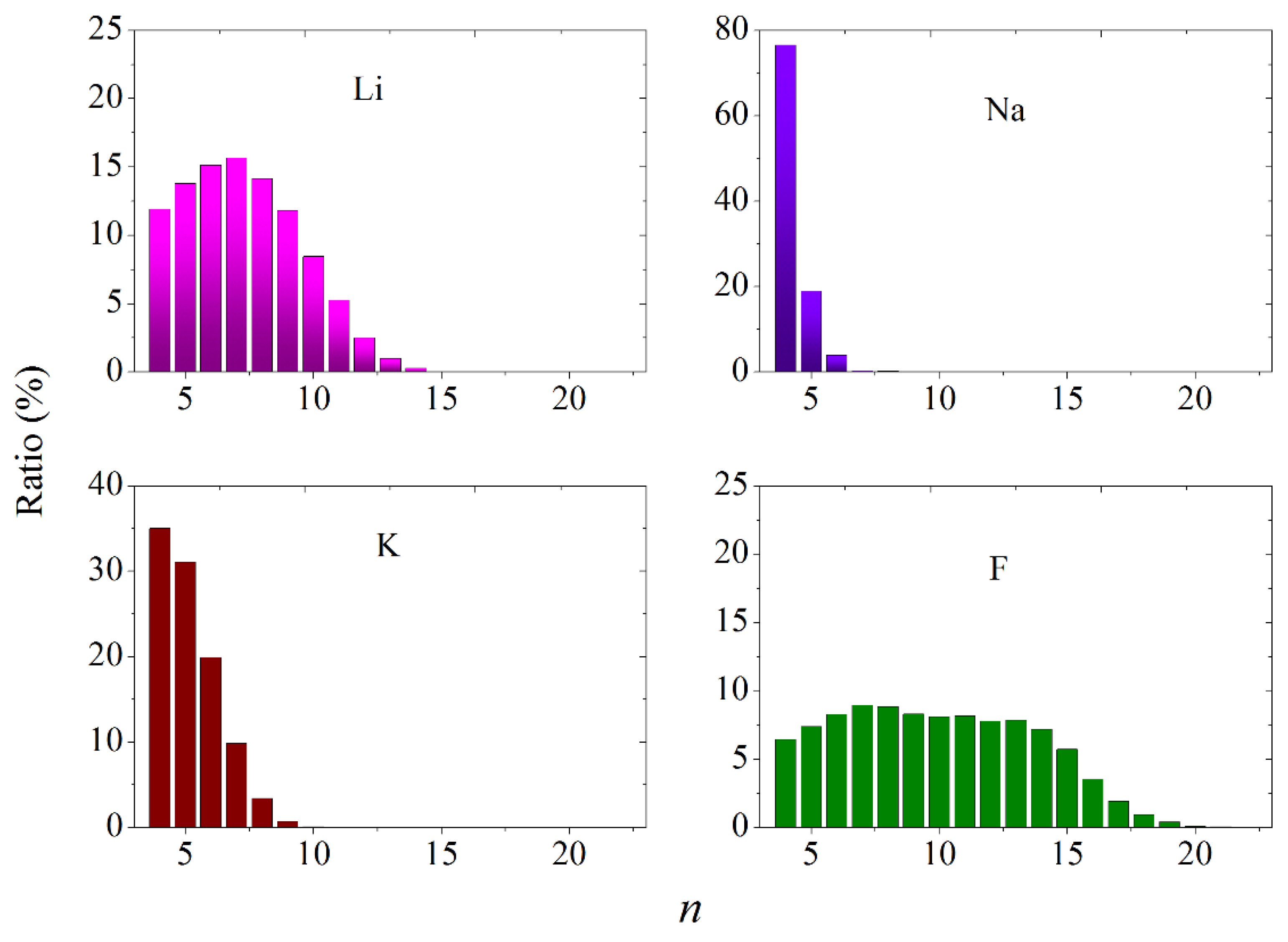
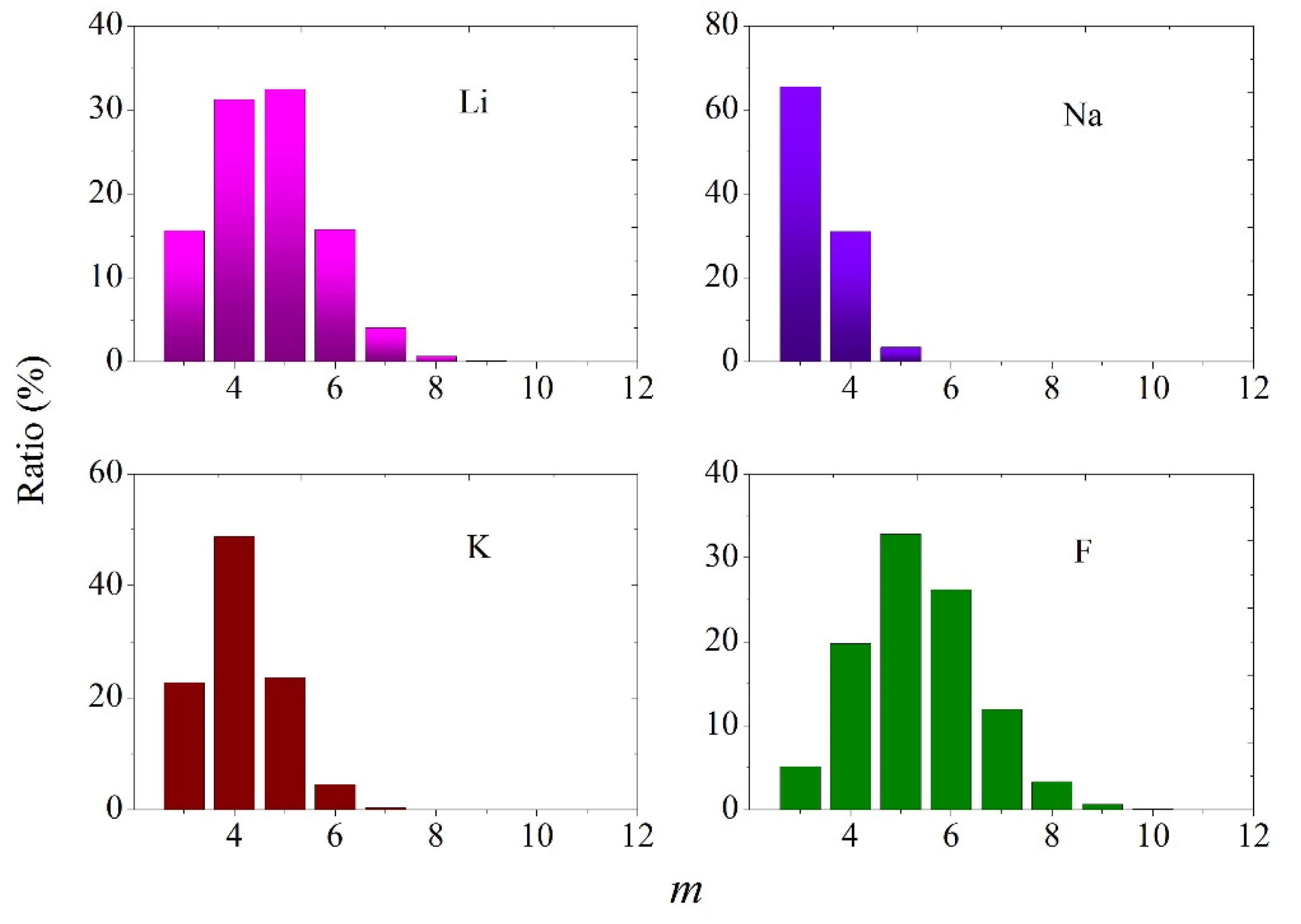
| Cerium | B, eV | ρ, Å | σ, Å |
|---|---|---|---|
| Ce–Ce | 1.357 | 0.141 | 2.02 |
| Ce–F | 1.618 | 0.3324 | 2.34 |
| Ce–Li | 0.053 | 0.137 | 1.77 |
| Ce–Na | 0.092 | 0.146 | 2.03 |
| Ce–K | 0.078 | 0.168 | 2.39 |
| T (K) | System | Li | Na | K | F | Ce |
|---|---|---|---|---|---|---|
| 800 | Pure FLiNaK | 1.82 | 1.43 | 1.45 | 1.84 | - |
| Exp. FLiNaK | 1.43 | 1.84 | 1.66 | 1.72 | - | |
| FLiNaK+Ce | 1.57 | 1.25 | 1.50 | 1.57 | 0.113 | |
| 880 | Pure FLiNaK | 2.87 | 2.47 | 2.19 | 2.92 | - |
| Exp. FLiNaK | 2.39 | 3.03 | 2.57 | 2.62 | - | |
| FLiNaK+Ce | 2.31 | 2.10 | 2.25 | 2.44 | 0.402 | |
| 950 | Pure FLiNaK | 3.80 | 3.37 | 2.84 | 3.87 | - |
| Exp. FLiNaK | (3.47) | (4.36) | (3.56) | (3.57) | - | |
| FLiNaK+Ce | 2.95 | 2.85 | 2.91 | 3.20 | 0.656 | |
| 1020 | Pure FLiNaK | 4.72 | 4.27 | 3.49 | 4.81 | - |
| Exp. FLiNaK | (4.80) | (5.48) | (4.35) | (4.34) | - | |
| FLiNaK+Ce | 3.60 | 3.60 | 3.57 | 3.97 | 0.909 |
| Parameters | Li | Na | K | F | Ce |
|---|---|---|---|---|---|
| a, 10−5 cm2/(s∙K) | 0.0092 | 0.0107 | 0.0094 | 0.0109 | 0.00362 |
| b, 10−5 cm2/s | 5.783 | 7.309 | 6.015 | 7.147 | 2.783 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galashev, A. Computational Study of the Physical Properties of a High Temperature Molten Salt Mixture of FLiNaK and CeF3. Appl. Sci. 2023, 13, 1085. https://doi.org/10.3390/app13021085
Galashev A. Computational Study of the Physical Properties of a High Temperature Molten Salt Mixture of FLiNaK and CeF3. Applied Sciences. 2023; 13(2):1085. https://doi.org/10.3390/app13021085
Chicago/Turabian StyleGalashev, Alexander. 2023. "Computational Study of the Physical Properties of a High Temperature Molten Salt Mixture of FLiNaK and CeF3" Applied Sciences 13, no. 2: 1085. https://doi.org/10.3390/app13021085
APA StyleGalashev, A. (2023). Computational Study of the Physical Properties of a High Temperature Molten Salt Mixture of FLiNaK and CeF3. Applied Sciences, 13(2), 1085. https://doi.org/10.3390/app13021085







