Effects of Ca2+ Ions on the Localized Corrosion of Carbon Steel Influence of the Associated Anion
Abstract
1. Introduction
2. Experimental Methods
- -
- Assay 1: 2% (w/w) NaCl with 1380 ppm CaCl2 + CO2;
- -
- Assay 2: 2% (w/w) NaCl + CO2;
- -
- Assay 3: 2% (w/w) NaCl with 1360 ppm Na2CO3 + CO2;
- -
- Assay 4: 2% (w/w) NaCl with 1.250 g CaCO3 per liter of solution + CO2;
- -
- Assay 5: Solution with 1450 ppm NaCl + CO2;
- -
- Assay 6: Solution with 1380 ppm CaCl2 + CO2;
- -
- Assay 7: Solution with 1360 ppm Na2CO3 + CO2;
- -
- Assay 8: Solution with 500 ppm CaCl2 + CO2;
- -
- Assay 9: Solution with 424 ppm Na2CO3 + CO2;
- -
- Assay 10: Solution with 1.250 g CaCO3 per liter of solution + CO2.
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, S.L.; Cui, Z.D.; He, F.; Bai, Z.Q.; Zhu, S.L.; Yang, X.J. Characterization of the surface film formed from carbon dioxide corrosion on N80 steel. Mater. Lett. 2004, 58, 1076–1081. [Google Scholar] [CrossRef]
- Murata, T.; Sato, E.; Matsuhashi, R. Factors Controlling Corrosion of Steels in CO2 Saturated Environments. In Advances in CO2 Corrosion in the Oil and Gas Htdustty, Proceedings of the Corrosion 83 Symposium, Anaheim, CA, USA, 18–19 April 1983; Hausler, R.H., Ed.; National Association of Corrosion Engineers (NACE): Houston, TX, USA, 1983; Volume 1. [Google Scholar]
- De Motte, R.A.; Barker, R.; Burkle, D.; Vargas, S.M.; Neville, A. The Early Stages of FeCO3 Scale Formation Kinetics in CO2. Corrosion. Mater. Chem. Phys. 2018, 216, 102–111. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, Y.; Brown, B.; Nesic, S.; Singer, M. Investigation of Precipitation Kinetics of FeCO3 by EQCM. Corros. Sci. 2018, 141, 195–202. [Google Scholar] [CrossRef]
- Shamsa, A.; Barker, R.; Hua, Y.; Barmatov, E.; Hughes, T.L.; Neville, A. The role of Ca2+ ions on Ca/Fe carbonate products on X65 carbon steel in CO2 corrosion environments at 80 and 150 °C. Corros. Sci. 2019, 156, 58–70. [Google Scholar] [CrossRef]
- Mansoori, H.; Young, D.; Brown, B.; Singer, M. Influence of calcium and magnesium ions on CO2 corrosion of carbon steel in oil and gas production systems-a review. Nat. Gas Sci. Eng. 2018, 59, 287–296. [Google Scholar] [CrossRef]
- Jiang, X.; Zheng, Y.G.; Qu, D.R.; Ke, W. Effect of calcium ions on pitting corrosion and inhibition performance in CO2 corrosion of N80 steel. Corros. Sci. 2006, 48, 3091–3108. [Google Scholar] [CrossRef]
- Ding, C.; Gao, K.W.; Chen, C.F. Effect of Ca2+ on CO2 corrosion properties of X65 pipeline steel. Int. J. Miner. Metall. Mater. 2009, 16, 661–666. [Google Scholar]
- Tavares, L.M.; da Costa, E.M.; de Oliveira Andrade, J.J.; Hubler, R.; Huet, B. Effect of calcium carbonate on low carbon steel corrosion behavior in Effect of calcium carbonate on low carbon steel corrosion behavior in. Appl. Surf. Sci. 2015, 359, 143–152. [Google Scholar] [CrossRef]
- Navabzadeh Esmaeely, S.; Choi, Y.S.; Young, D.; Nešić, S. Effect of Calcium on the Formation and Protectiveness of Iron Carbonate Layer in CO2 Corrosion. Corrosion 2013, 69, 912–920. [Google Scholar] [CrossRef]
- Esmaeely, S.N.; Young, D.; Brown, B.; Nešić, S. Effect of Incorporation of Calcium into Iron Carbonate Protective Layers in CO2 Corrosion of Mild Steel. Corrosion 2017, 73, 238–246. [Google Scholar] [CrossRef]
- Sontvedt, T.; Eriksrud, N. Effect of Flow on CO2 Corrosion Rates in Real and Synthetic Formation Waters; National Association of Colleges and Employers (NACE): Bethlehem, PA, USA, 1987; Volume 1, pp. 20–38. [Google Scholar]
- Zhao, G.X.; Li, J.P.; Hao, S.M.; Lu, X.H.; Li, H.L. Effect of Ca2+ and Mg2+ on CO2 corrosion behavior of tube steel. J. Iron Steel Res. Int. 2005, 12, 38–42. [Google Scholar]
- Mansoori, H.; Young, D.; Brown, B.; Nesic, S.; Singer, M. Effect of CaCO3-saturated solution on CO2 corrosion of mild steel explored in a system with controlled water chemistry and well-defined mass transfer conditions. Corros. Sci. 2019, 158, 108078. [Google Scholar] [CrossRef]
- Rizzo, R.; Gupta, S.; Rogowska, M.; Ambat, R. Corrosion of carbon steel under CO2 conditions: Effect of CaCO3 precipitation on the stability of the FeCO3 protective layer. Corros. Sci. 2020, 162, 108214. [Google Scholar] [CrossRef]
- Li, J.; Sun, C.; Roostaei, M.; Mahmoudi, M.; Fattahpour, V.; Zeng, H.; Luo, J.L. Role of Ca2+ in the CO2 corrosion behavior and film characteristics of N80 steel and electroless Ni–P coating at high temperature and high pressure. Mater. Chem. Phys. 2021, 267, 124618. [Google Scholar] [CrossRef]
- Nesic, S. Effects of multiphase flow on internal CO2 corrosion of mild steel pipe lines. Energy Fuels Am. Chem. Soc. 2012, 26, 4098–4111. [Google Scholar] [CrossRef]
- Plummer, L.N.; Busenberg, E. The solubilities of calcite, aragonite an vaterite in CO2-H2O solutions between 0 and 90 °C, and an evaluation of aqueous model for the system CaCO3-CO2-H2O. Geochim. Cosmochim. 1982, 46, 1011–1040. [Google Scholar] [CrossRef]
- Stern, M.; Geary, A.L. Electrochemical Polarization I. A Theoretical Analysis of the Shape of Polarization Curves. J. Electrochem. Soc. 1957, 104, 56–63. [Google Scholar] [CrossRef]
- Sani, F.M.; Brown, B.; Belarbi, Z.; Nesic, S. An Experimental Investigation on the Effect of Salt Concentration on Uniform CO2 Corrosion; Corrosion; Paper Nº 13026; NACE International: Houston, TX, USA, 2019. [Google Scholar]
- Yanga, Y.; Browna, B.; Nešić, S.; Gennarob, M.E.; Molinas, B. Mechanical Strength and Removal of a Protective Iron Carbonate Layer Formed on Mild Steel in CO2 Corrosion; Paper No. 10383; NACE International: Houston, TX, USA, 2010. [Google Scholar]
- Di Bonaventura, M.; Brown, B.; Nešić, S.; Singer, M. Effect of Flow and Steel Microstructure on the Formation of Iron Carbonate; Paper No. 11179; NACE International: Houston, TX, USA, 2018. [Google Scholar]
- ASTMG102; Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements. ASTM International: West Conshohocken, PA, USA, 2015.
- Gomez-Villalba, L.S.; López-Arce, P.; Alvarez de Buergo, M.; Fort, R. Atomic Defects and Their Relationship to Aragonite-Calcite Transformation in Portlandite Nanocrystal Carbonation. Cryst. Growth Des. 2012, 12, 4844–4852. [Google Scholar] [CrossRef]
- Reischl, B.; Raiteri, P.; Gale, J.D.; Rohl, A.L. Atomistic Simulation of Atomic Force Microscopy Imaging of Hydration Layers on Calcite, Dolomite, and Magnesite Surfaces. J. Phys. Chem. 2019, 123, 14985–14992. [Google Scholar] [CrossRef]
- Söngen, H.; Reischl, B.; Miyata, K.; Bechstein, R.; Raiteri, P.; Rohl, A.L.; Gale, J.D.; Fukuma, T.; Kühnle, A. Resolving point defects in the hydration structure of calcite (10.4) with three-dimensional atomic force microscopy. Phys. Rev. Lett. 2018, 120, 116101. [Google Scholar] [CrossRef]
- Ross, N.L.; Reeder, R.J. High-pressure structural study of dolomite and ankerite. Am. Mineral. 1992, 77, 412–421. [Google Scholar]
- Chuliá-Jordán, R.; Santamaria-Perez, D.; Ruiz-Fuertes, J.; Otero-de-la-Roza, A.; Popescu, C. Compressibility and Phase Stability of Iron-Rich Ankerite. Minerals 2021, 11, 607. [Google Scholar] [CrossRef]
- Meyer, M. The influence of impurities on the growth rate of calcite. J. Cryst. Growth 1984, 66, 639–646. [Google Scholar] [CrossRef]
- De Leeuw, N.H. Molecular Dynamics Simulations of the Growth Inhibiting Effect of Fe2+, Mg2+, Cd2+, and Sr2+ on Calcite Crystal Growth. J. Phys. Chem. B 2002, 106, 5241–5249. [Google Scholar] [CrossRef]
- Mettler, S.; Wolthers, M.; Charlet, L.; Von Gunten, U. Sorption and catalytic oxidation of Fe(II) at the surface of calcite. Geochim. Cosmochim. Acta 2009, 73, 1826–1840. [Google Scholar] [CrossRef][Green Version]
- Gutjahr, A.; Dabringhaus, H.; Lacmann, R. Studies of the growth and dissolution kinetics of the CaCO3 polymorphs calcite and aragonite. The influence of divalent cation additives on the growth and dissolution rates. J. Cryst. Growth 1996, 158, 310–315. [Google Scholar] [CrossRef]
- Hausler, R.H. (Ed.) The mechanism of CO2 corrosion steel in hot, deep gas wells. In Advances in CO2 Corrosion in the Oil and Gas Htdustty, Proceedings of the Corrosion 83 Symposium, Anaheim, CA, USA, 18–19 April 1983; National Association of Corrosion Engineers (NACE): Houston, TX, USA, 1983; Volume 1. [Google Scholar]
- Alsalem, M.M.; Camilla, S.; Ryan, M.P.; Campbell, K.S. Understanding the Role of NaCl Concentration on the Corrosion of Carbon Steel and FeCO3 Formation in CO2 Containing Electrolytes. Ind. Eng. Chem. Res. 2021, 60, 12032–12048. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, X.; Li, W.; Li, B.; Guo, J.; Zhang, J.; Pang, Q.; Xu, Z. CO2 corrosion behavior of high-strength martensitic steel for marine riser exposed to CO2 saturated salt solution. Mater. Res. Express 2021, 8, 076517. [Google Scholar] [CrossRef]
- Jiang, X.; Nešic, S.; Huet, F. The Effect of Electrode Size on Electrochemical Noise Measurements and the Role of Chloride on Localized CO2 Corrosion of Mild Steel. In Proceedings of the CORROSION 2009, Atlanta, GA, USA, 22–26 March 2009; NACE International: Houston, TX, USA, 2009. [Google Scholar]
- Ishikawa, M.; Ichikuni, M. A Model of Crystal Defects in Calcita by Sodium and Potassium Uptake. Bull. Chem. Soc. Jpn. 1986, 59, 3809–3814. [Google Scholar] [CrossRef]
- Ishikawa, M.; Ichikuni, M. Uptake of sodium and potassium by calcite. Chem. Geol. 1984, 42, 137–146. [Google Scholar] [CrossRef]
- Duffó, G.S.; Farina, S.B. La Corrosión de Estructuras de Hormigón Armado: Principios Básicos, Monitoreo y Prevención; Editorial Académica Española: Berlín, Germany, 2016. [Google Scholar]
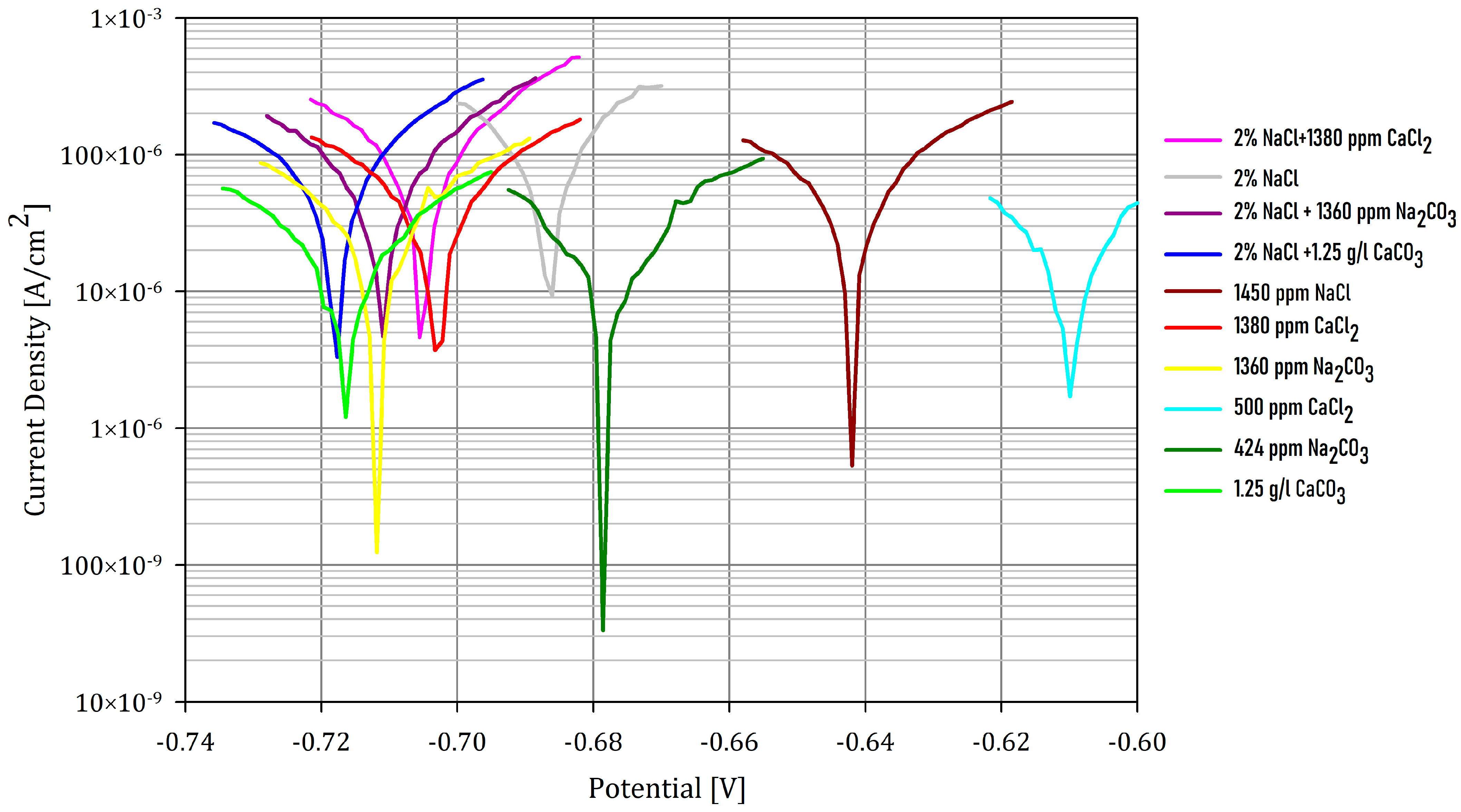
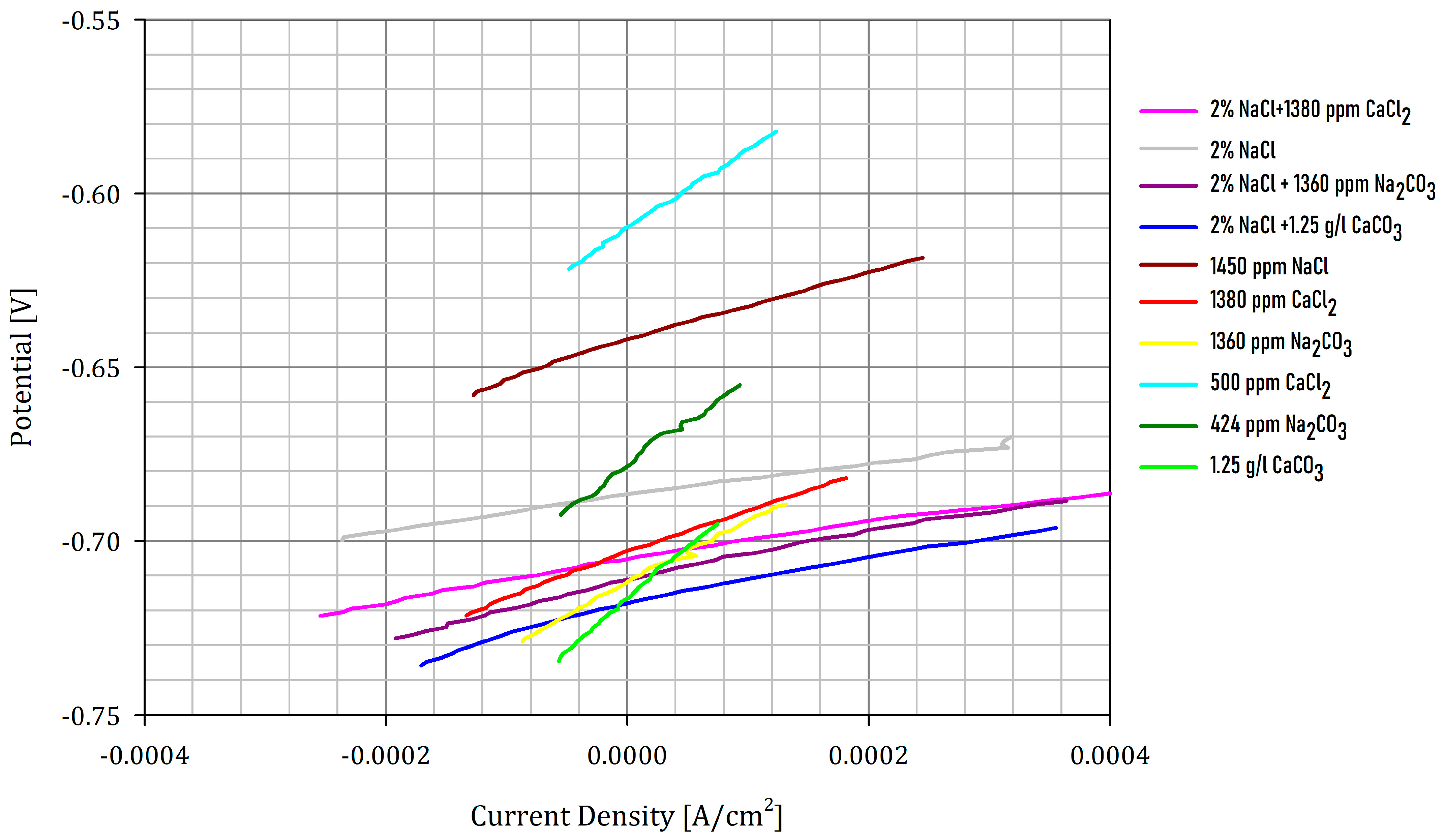
| Sample | NaCl [ppm] | CaCO3 [ppm] | CaCl2 [ppm] | Na2CO3 [ppm] | pH | Rp [Ω] | Ecorr [mV] | i [μA/cm2] | CR [mmpy] | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 20,000 | - | 1380 | - | 4.07 | 23 | −704 | 463 | 5.38 | |
| 2 | 20,000 | - | - | - | 4.38 | 25 | −686 | 431 | 5.01 | |
| 3 | 20,000 | - | - | 1360 | 6.74 | 28 | −712 | 379 | 4.41 | |
| 4 | 20,000 | 1250 | - | - | 5.71 | 31 | −719 | 349 | 4.06 | |
| 5 | 1450 | - | - | - | 4.04 | 47 | −644 | 223 | 2.59 | |
| 6 | - | - | 1380 | - | 4.03 | 53 | −703 | 201 | 2.34 | |
| 7 | - | - | - | 1360 | 6.75 | 68 | −712 | 156 | 1.81 | |
| 8 | - | - | 500 | - | 4.12 | 87 | −611 | 121 | 1.41 | |
| 9 | - | - | - | 424 | 6.34 | 98 | −678 | 101 | 1.29 | |
| 10 | - | 1250 | - | - | 5.97 | 126 | −715 | 84 | 0.97 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acosta, V.V.; Bianchi, G.L. Effects of Ca2+ Ions on the Localized Corrosion of Carbon Steel Influence of the Associated Anion. Appl. Sci. 2023, 13, 11056. https://doi.org/10.3390/app131911056
Acosta VV, Bianchi GL. Effects of Ca2+ Ions on the Localized Corrosion of Carbon Steel Influence of the Associated Anion. Applied Sciences. 2023; 13(19):11056. https://doi.org/10.3390/app131911056
Chicago/Turabian StyleAcosta, Verónica Viviana, and Gustavo Luis Bianchi. 2023. "Effects of Ca2+ Ions on the Localized Corrosion of Carbon Steel Influence of the Associated Anion" Applied Sciences 13, no. 19: 11056. https://doi.org/10.3390/app131911056
APA StyleAcosta, V. V., & Bianchi, G. L. (2023). Effects of Ca2+ Ions on the Localized Corrosion of Carbon Steel Influence of the Associated Anion. Applied Sciences, 13(19), 11056. https://doi.org/10.3390/app131911056





