Real Fluid Modeling and Simulation of the Structures and Dynamics of Condensation in CO2 Flows Shocked Inside a de Laval Nozzle, Considering the Effects of Impurities
Abstract
:1. Introduction
1.1. Introduction to Carbon, Capture, Utilization and Storage (CCUS)
1.2. Introduction to Impurities in CO
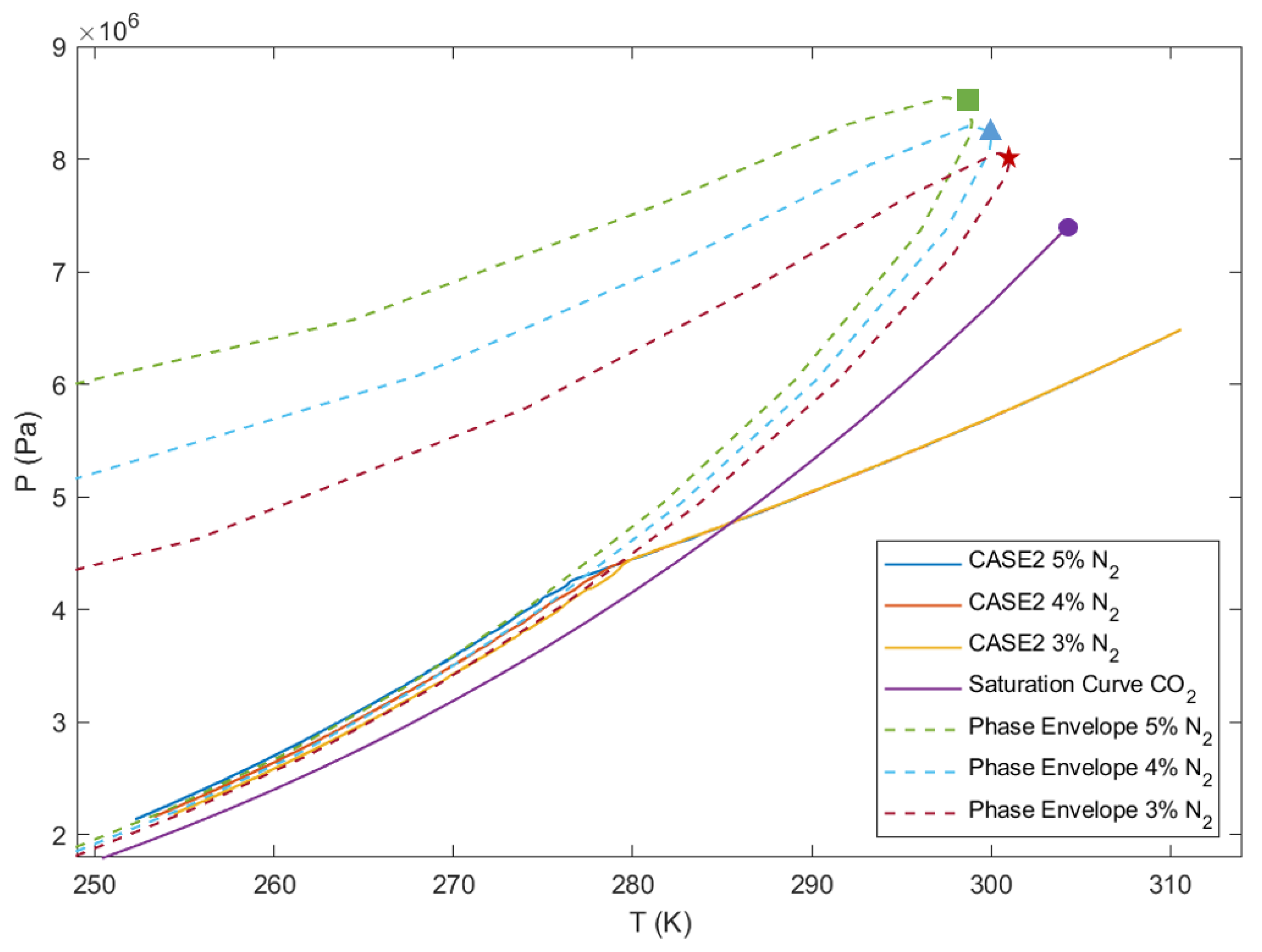
1.3. Introduction to Single- and Multi-Component PT-Diagram
1.4. Introduction to Two-Phase Flow Modeling
1.5. Introduction to the Real Fluid RFM Model
1.6. Short Overview of VLE Tabulation
1.7. Scope of the Present Study
2. Computation Model and Methodology
2.1. The Real Fluid RFM Model
2.2. Governing Equations
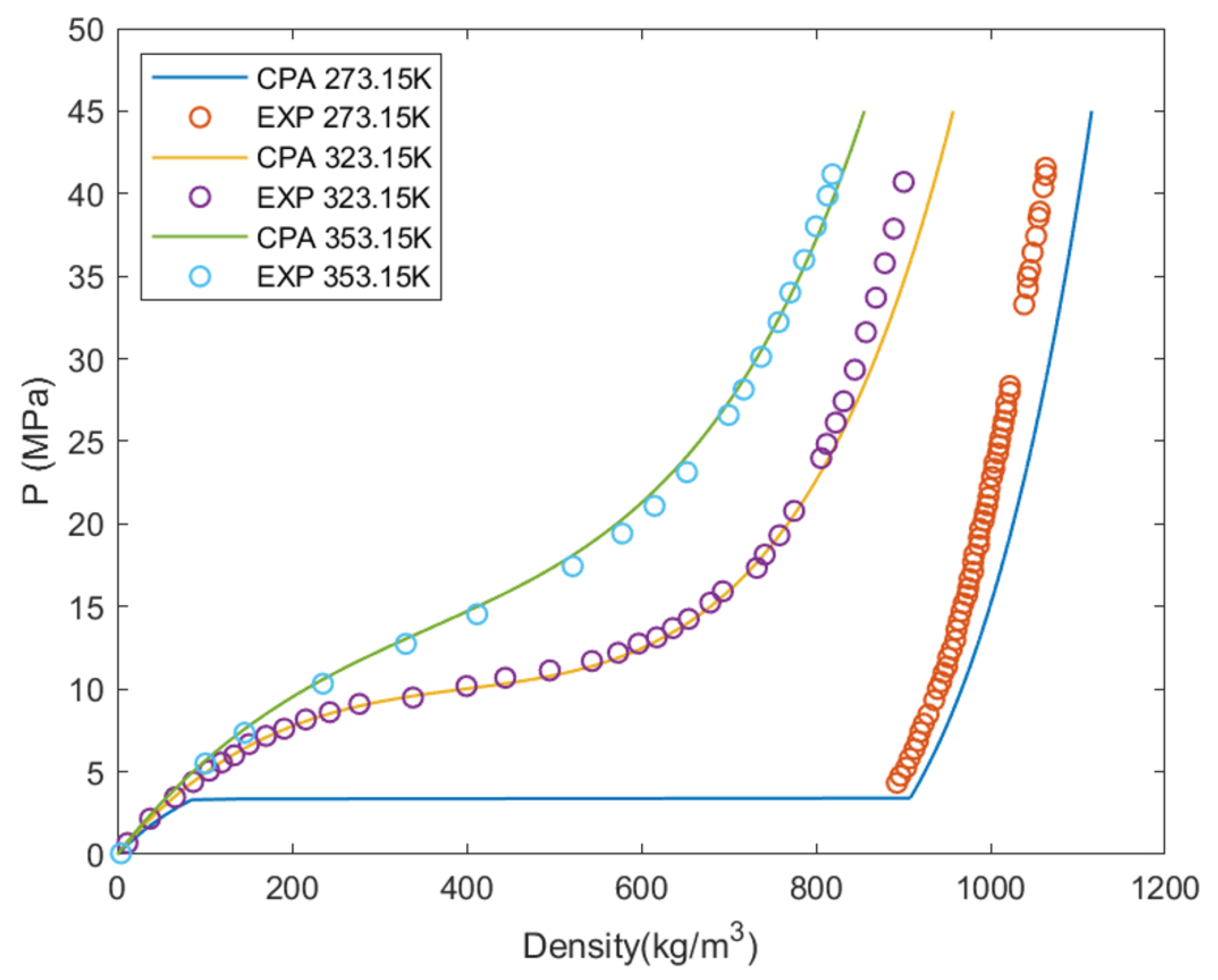
2.3. Thermodynamic Closure and Tabulation Details
2.4. Thermodynamic Validation for CO–H Configuration
3. Experimental and Computational Methods
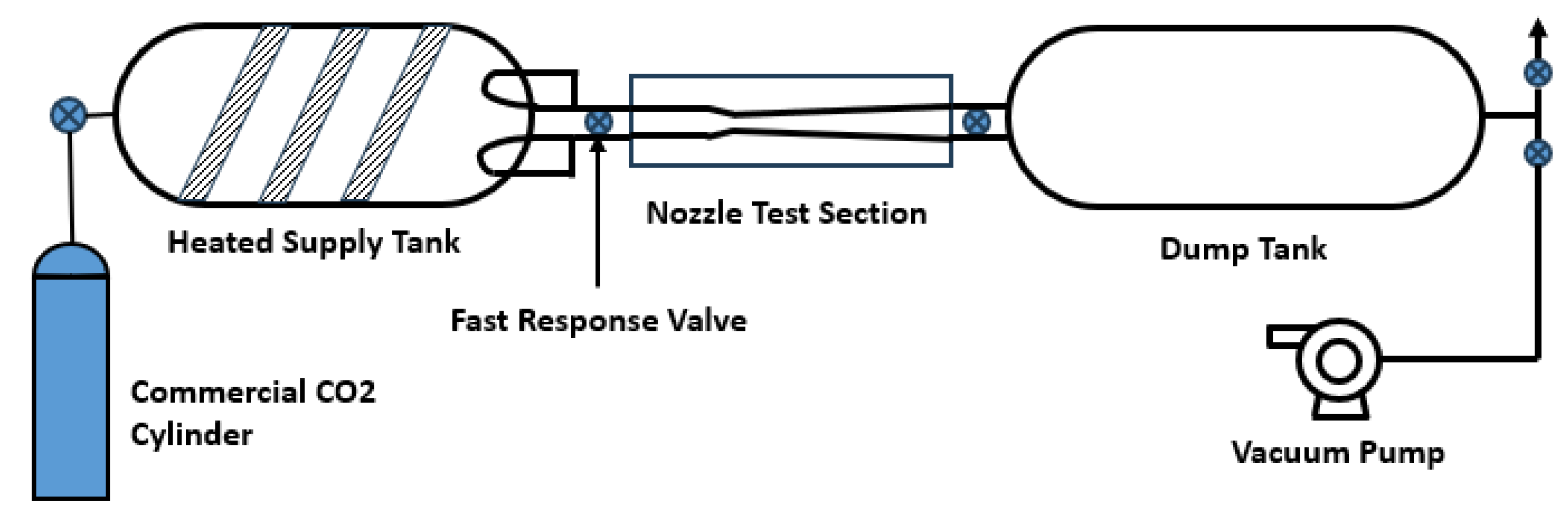
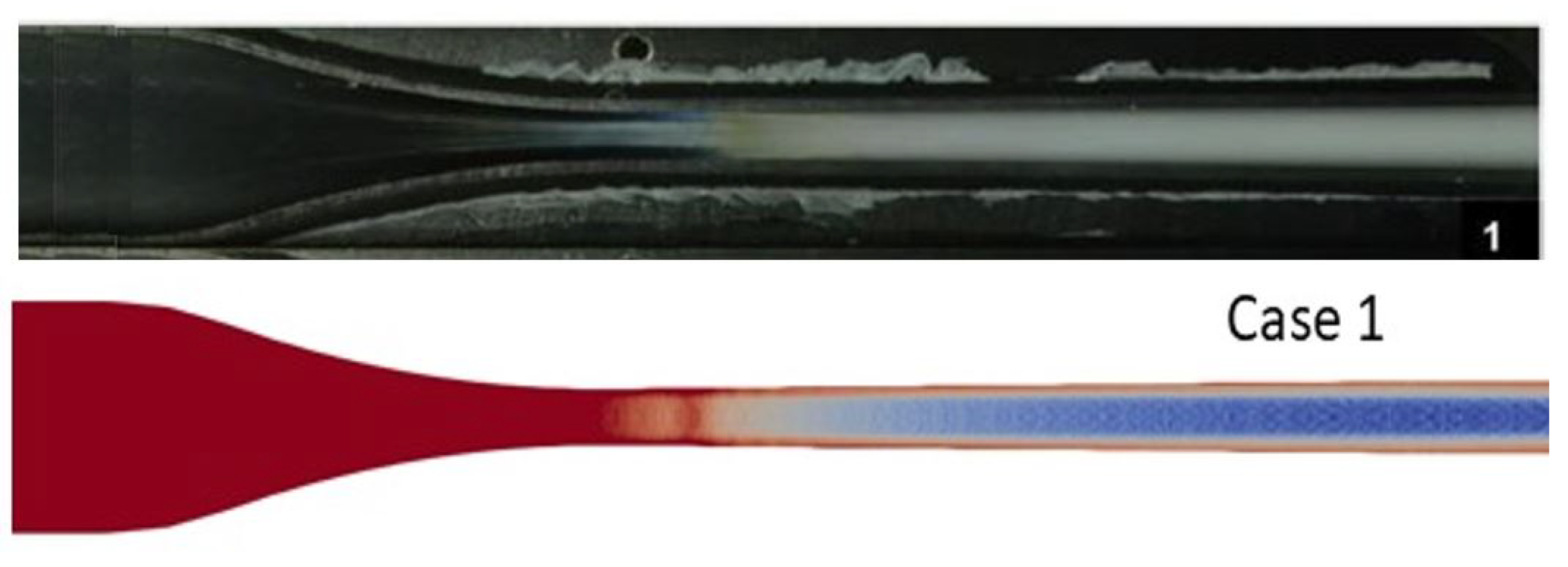
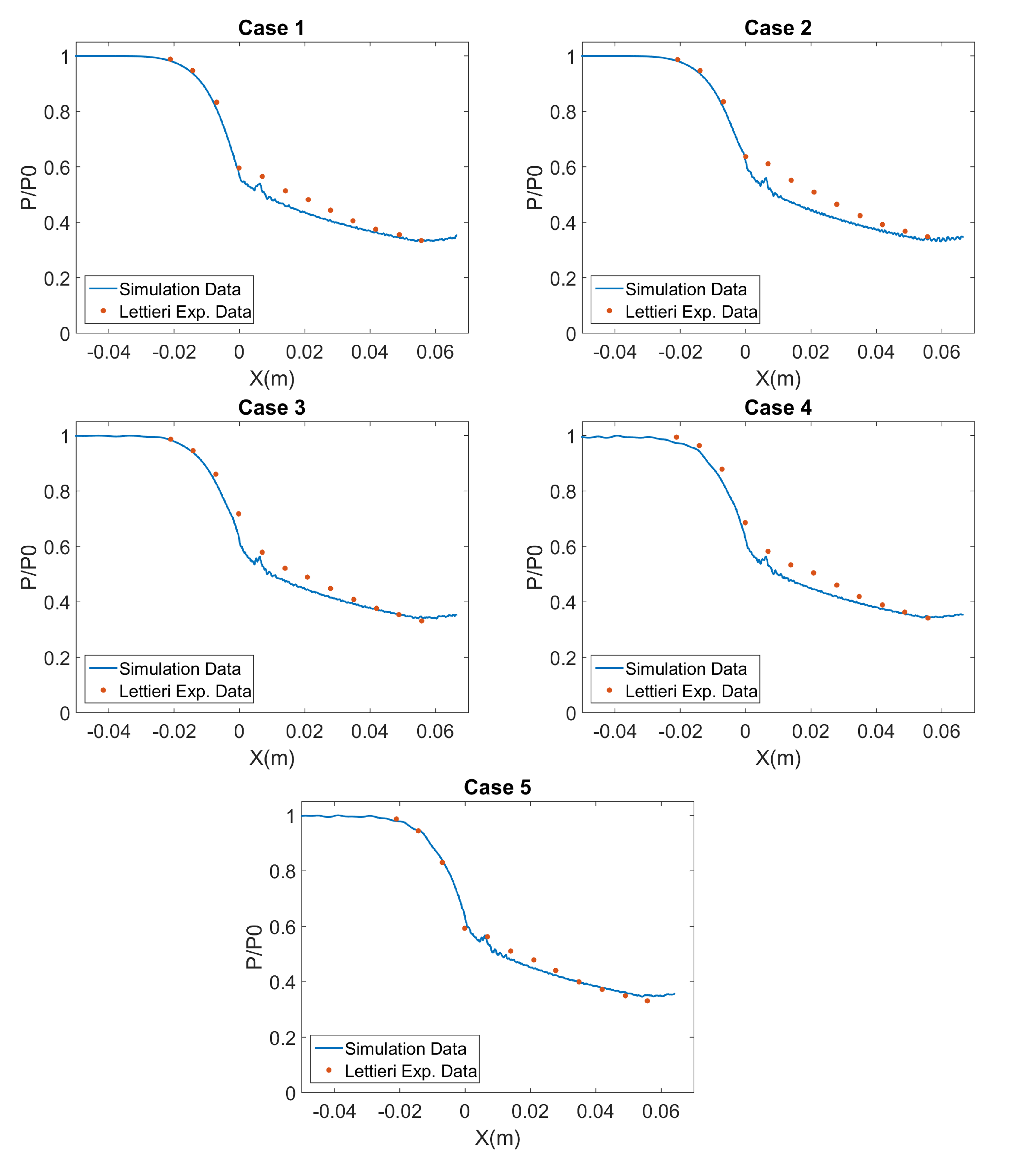
3.1. De Laval Nozzle Experiments
- The steady state pressure profiles along the nozzle;
- The shadography images of the the nozzle showing the two-phase (condensation) region.
3.2. Computational Setup
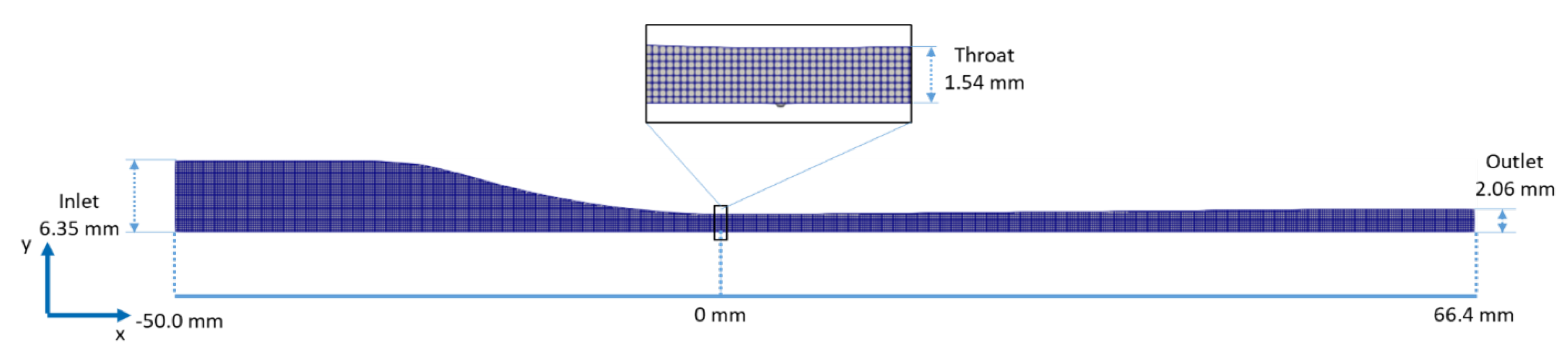
3.2.1. Computational Grid
3.2.2. Boundary Conditions
3.2.3. Initial Conditions
3.2.4. Numerical Schemes, Models and Thermodynamic Table
4. Results and Discussions
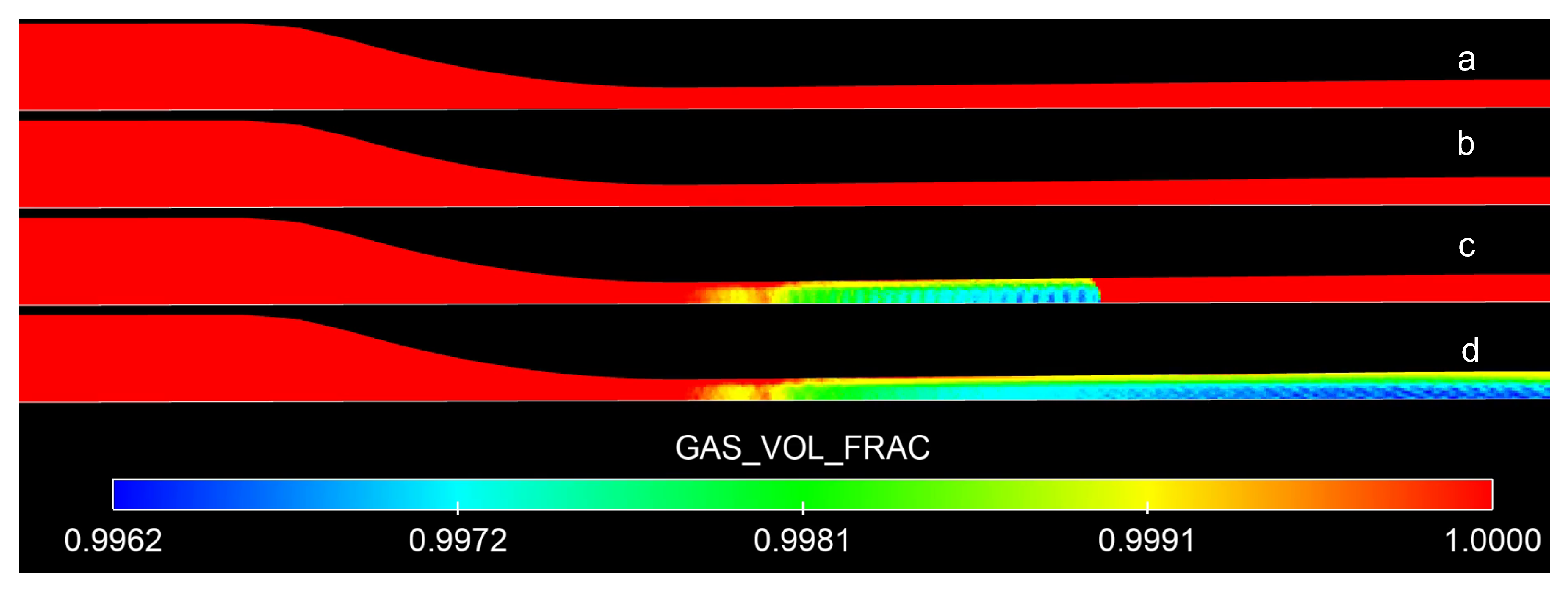
4.1. Transient Flow Characteristics
- A subsonic regime in both the converging and diverging part of the de Laval nozzle. In this first step, the flow accelerates progressively in the converging part, but it decelerates along the diverging part. The Mach number is still lower than one in the entire nozzle, and has its maximum value at the throat, as shown Figure 5b. In addition, the pressure profile is also shown in Figure 5a to progressively decrease in the converging part, but it increases along the diverging part towards (). Figure 5c and Figure 6a show that the condensation is not present in the nozzle in this subsonic regime and, consequently, the nozzle is only filled in the gas phase.
- A sonic regime where the flow has been accelerated up to a Mach number of one (see Figure 5e) happens simultaneously with the throat pressure decreasing to a critical value (approximately 3.3 MPa), as shown in Figure 5d. Figure 5f and Figure 6b show that the presence of condensation is still not observed just at the moment of meeting critical conditions.
- A supersonic/subsonic transition regime. In fact, because the pressure at the nozzle outlet ( = 1.973 MPa) is still lower than the pressure value at the throat (3.3 MPa), the flow continues to accelerate and reaches the supersonic regime in the diverging section, as shown in Figure 5h,g. In these figures, a shock wave created just after the throat, and moving towards the nozzle outlet inside the divergent part, is successfully captured when the outlet pressure is relaxed towards (). The presence of condensation is observed in Figure 6c, as well as the profiles of gas volume fraction in Figure 5i.
- A fully supersonic regime in the diverging part of the de Laval nozzle is finally obtained when the shock wave leaves the computational domain, as shown in Figure 5j,k. However, the converging part remains in the subsonic regime. The nozzle is filled with condensation from the throat to the exit downstream. Such fully supersonic acceleration of the flow through a converging–diverging nozzle is used in practical applications such as rocket engine lift-off.
4.2. CO Condensation and Shock Wave Interaction
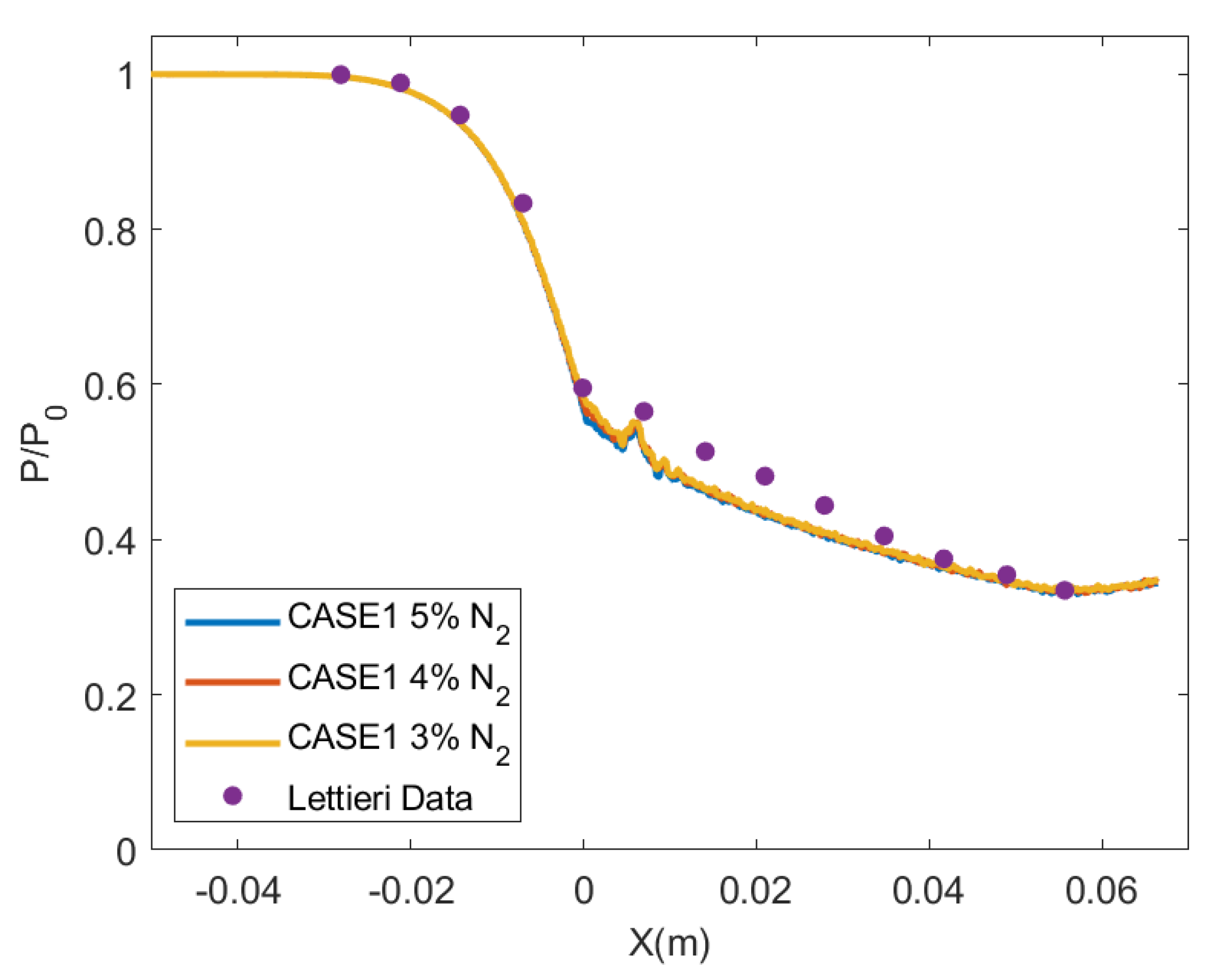
4.3. Nozzle Axial Profiles Results
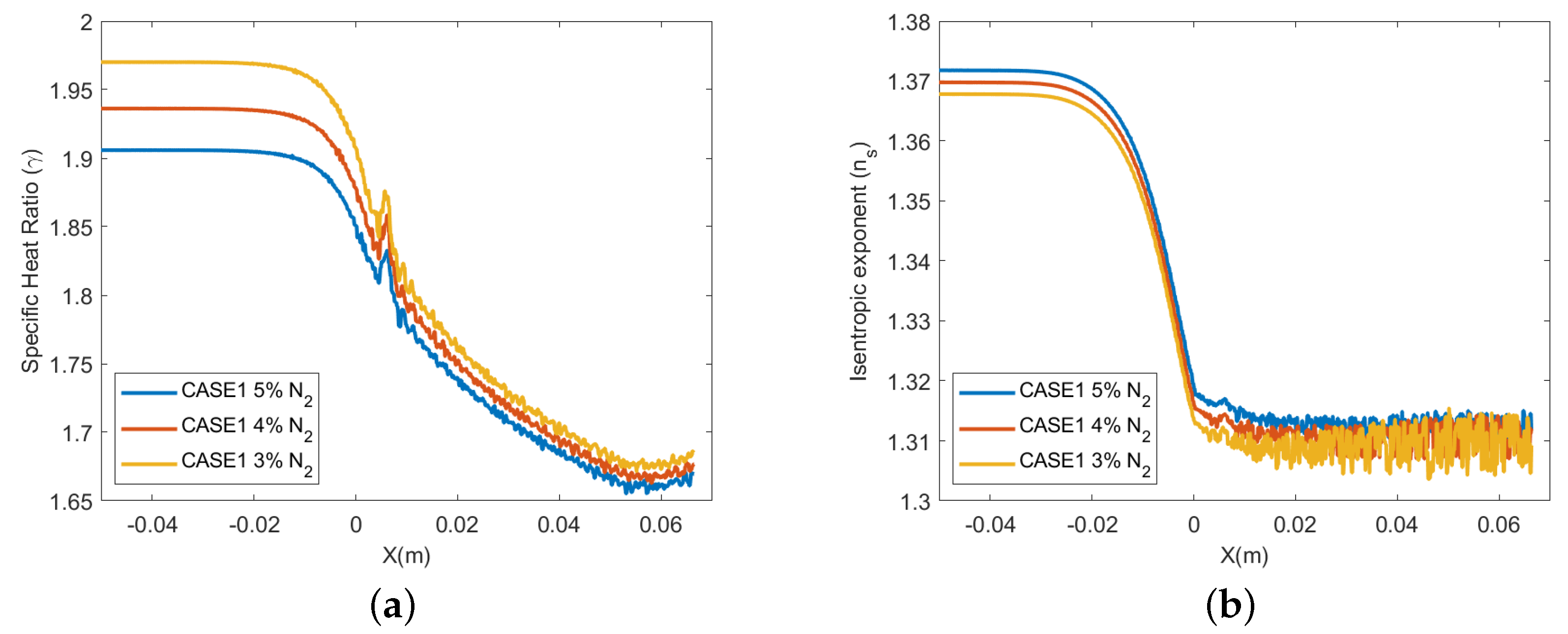
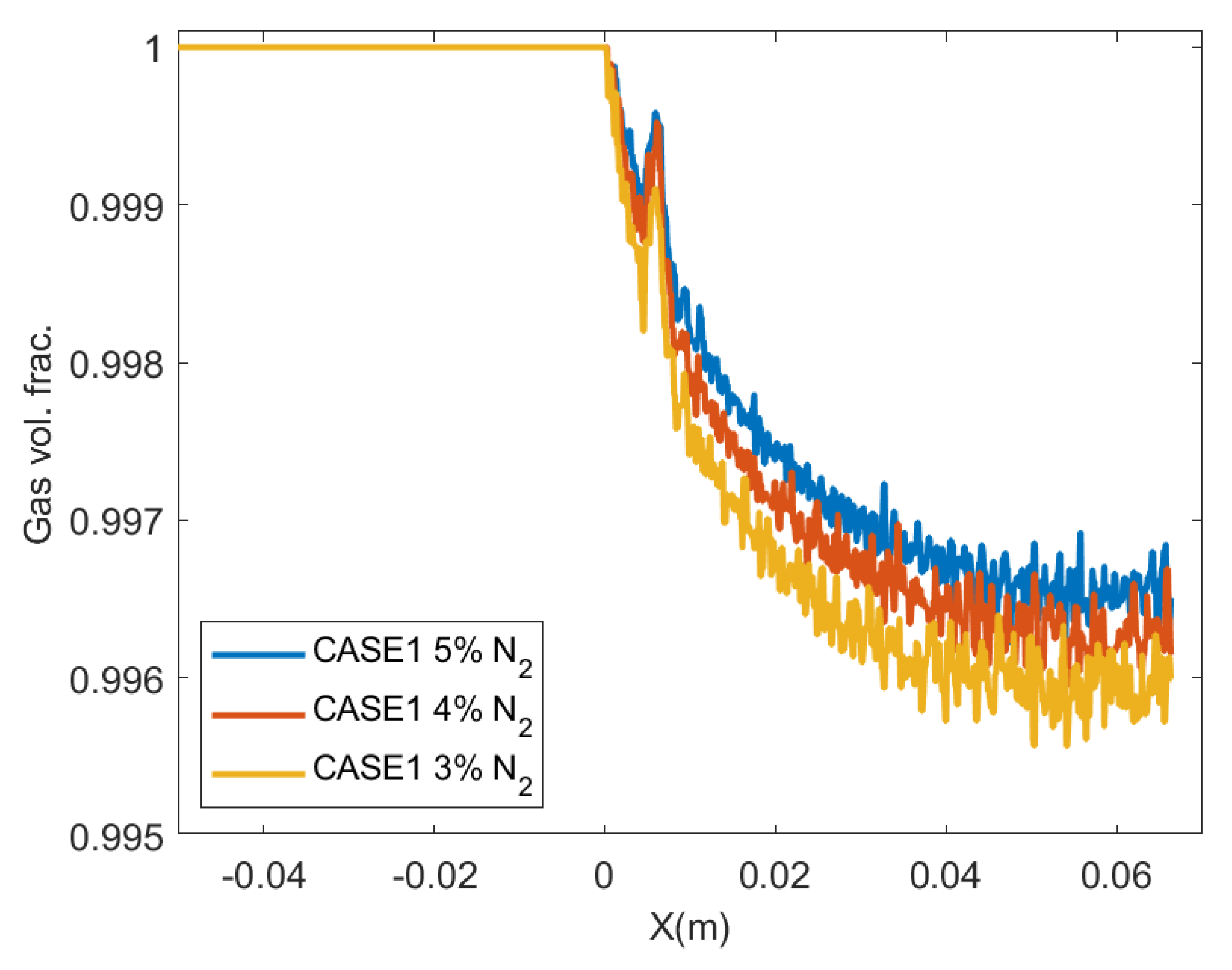
4.4. Effect of Impurities on the Amount of Condensation
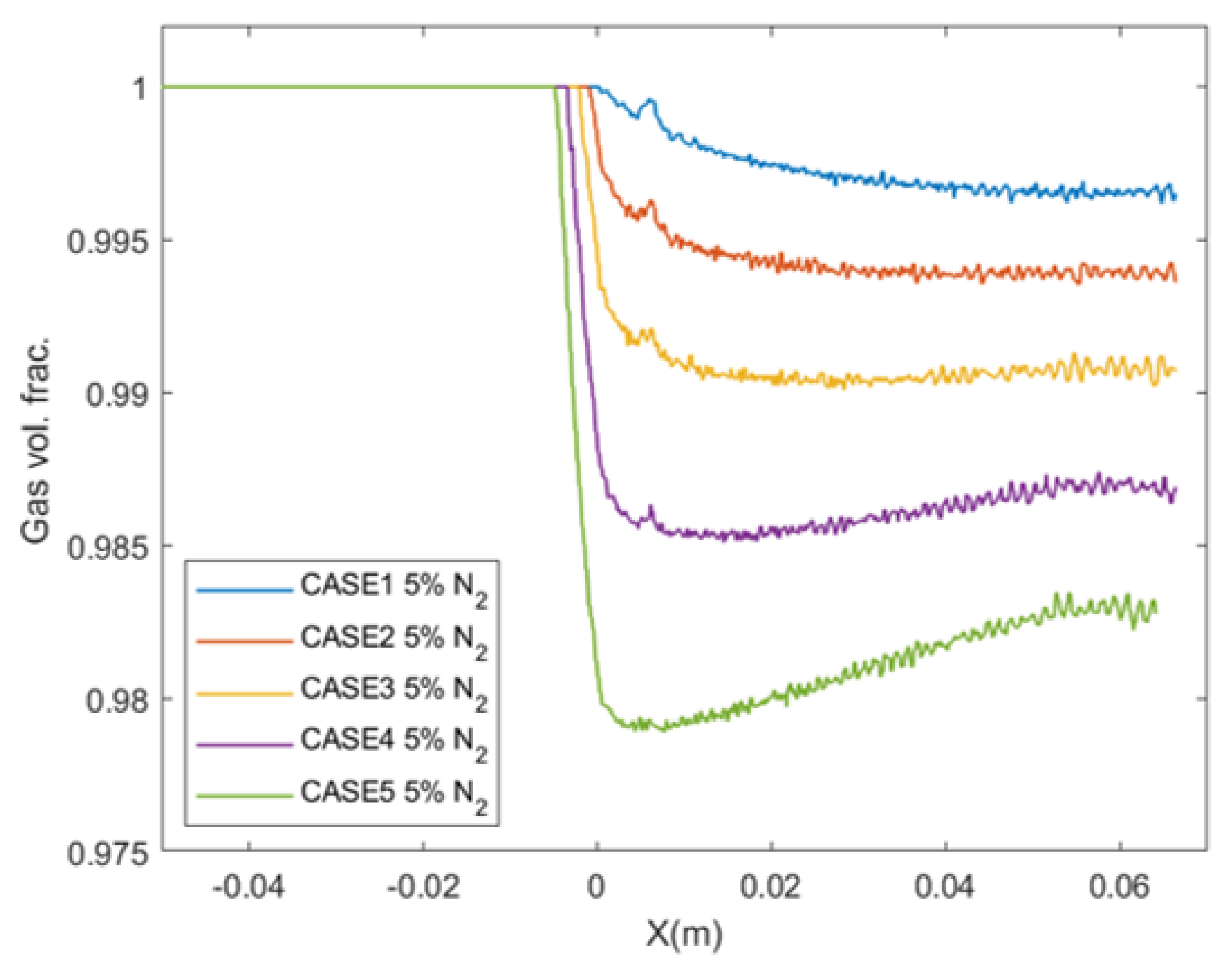
4.5. Effect of Operating Pressure on the Amount of Condensation
5. Conclusions
- The structure and dynamics of the de Laval in-nozzle flow include shock waves, condensation and the amount of condensed CO in supersonic nozzle flows.
- This amount increases as the inlet operating condition is moved close to the supercritical state.
- The effects on condensation of the inlet conditions and impurities.
- The amount of condensation increases as the amount of impurities decreases. Consequently, the present numerical study has shown that the condensation of CO observed experimentally in the de Laval nozzle can hardly be produced without adding a small amount of impurity.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Intergovernmental Panel on Climate Change (IPCC) Is the United Nations Body for Assessing the Science Related to Climate Change. Available online: https://www.ipcc.ch/ (accessed on 4 July 2023).
- Zhao, X.; Ji, G.; Liu, W.; He, X.; Anthony, E.J.; Zhao, M. Mesoporous MgO promoted with NaNO3/NaNO2 for rapid and high-capacity CO2 capture at moderate temperatures. Chem. Eng. J. 2018, 332, 216–226. [Google Scholar] [CrossRef]
- Chapoy, A.; Nazeri, M.; Kapateh, M.; Burgass, R.; Coquelet, C.; Tohidi, B. Effect of impurities on thermophysical properties and phase behaviour of a CO2-rich system in CCS. Int. J. Greenh. Gas Control. 2013, 19, 92–100. [Google Scholar] [CrossRef]
- Sterpenich, J.; Dubessy, J.; Pironon, J.; Renard, S.; Caumon, M.C.; Randi, A.; Jaubert, J.N.; Favre, E.; Roizard, D.; Parmentier, M.; et al. Role of Impurities on CO2 Injection: Experimental and Numerical Simulations of Thermodynamic Properties of Water-salt-gas Mixtures (CO2 + Co-injected Gases) Under Geological Storage Conditions. Energy Procedia 2013, 37, 3638–3645. [Google Scholar] [CrossRef]
- de Visser, E.; Hendriks, C.; Barrio, M.; Mølnvik, M.J.; de Koeijer, G.; Liljemark, S.; Le Gallo, Y. Dynamis CO2 quality recommendations. Int. J. Greenh. Gas Control. 2008, 2, 478–484. [Google Scholar] [CrossRef]
- Brown, S.; Peristeras, L.D.; Martynov, S.; Porter, R.T.J.; Mahgerefteh, H.; Nikolaidis, I.K.; Boulougouris, G.C.; Tsangaris, D.M.; Economou, I.G. Thermodynamic interpolation for the simulation of two-phase flow of non-ideal mixtures. Comput. Chem. Eng. 2016, 95, 49–57. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, Y.X. The influence of impurities on the transportation safety of an anthropogenic CO2 pipeline. Process. Saf. Environ. Prot. 2014, 92, 80–92. [Google Scholar] [CrossRef]
- Brinckman, K.W.; Hosangadi, A.; Liu, Z.; Weathers, T. Numerical Simulation of Non-Equilibrium Condensation in Supercritical CO2 Compressors. In Turbo Expo Power for Land, Sea and Air, Proceedings of the ASME Turbo Expo 2018: Turbomachinery Technical Conference and Exposition, Oslo, Norway, 11–15 June 2018; Volume 9: Oil and Gas Applications; Supercritical CO2 Power Cycles; Wind Energy; American Society of Mechanical Engineers: New York, NY, USA, 2018; p. 06172019. [Google Scholar] [CrossRef]
- Baltadjiev, N.D.; Lettieri, C.; Spakovszky, Z.S. An Investigation of Real Gas Effects in Supercritical CO2 Centrifugal Compressors. J. Turbomach. 2015, 137, 091003. [Google Scholar] [CrossRef]
- Lettieri, C.; Yang, D.; Spakovszky, Z. An Investigation of Condensation Effects in Supercritical Carbon Dioxide Compressors. J. Eng. Gas Turbines Power 2015, 137, 082602. [Google Scholar] [CrossRef]
- Lettieri, C.; Paxson, D.; Spakovszky, Z.; Bryanston-Cross, P. Characterization of Nonequilibrium Condensation of Supercritical Carbon Dioxide in a de Laval Nozzle. J. Eng. Gas Turbines Power 2018, 140, 041701. [Google Scholar] [CrossRef]
- Romei, A.; Persico, G. Computational fluid-dynamic modelling of two-phase compressible flows of carbon dioxide in supercritical conditions. Appl. Therm. Eng. 2021, 190, 116816. [Google Scholar] [CrossRef]
- Nakagawa, M.; Berana, M.S.; Kishine, A. Supersonic two-phase flow of CO2 through converging–diverging nozzles for the ejector refrigeration cycle. Int. J. Refrig. 2009, 32, 1195–1202. [Google Scholar] [CrossRef]
- Zhao, H.; Quan, S.; Dai, M.; Pomraning, E.; Senecal, P.K.; Xue, Q.; Battistoni, M.; Som, S. Validation of a Three-Dimensional Internal Nozzle Flow Model Including Automatic Mesh Generation and Cavitation Effects. J. Eng. Gas Turbines Power 2014, 136, 092603. [Google Scholar] [CrossRef]
- Banuti, D.T. Crossing the Widom-line—Supercritical pseudo-boiling. J. Supercrit. Fluids 2015, 98, 12–16. [Google Scholar] [CrossRef]
- Jafari, S.; Gaballa, H.; Habchi, C.; de Hemptinne, J.C. Towards Understanding the Structure of Subcritical and Transcritical Liquid–Gas Interfaces Using a Tabulated Real Fluid Modeling Approach. Energies 2021, 14, 5621. [Google Scholar] [CrossRef]
- Coquelet, C.; Stringari, P.; Hajiw, M.; Gonzalez, A.; Pereira, L.; Nazeri, M.; Burgass, R.; Chapoy, A. Transport of CO2: Presentation of New Thermophysical Property Measurements and Phase Diagrams. Energy Procedia 2017, 114, 6844–6859. [Google Scholar] [CrossRef]
- Hirt, C.; Nichols, B. Volume of fluid (VOF) method for the dynamics of free boundaries. J. Comput. Phys. 1981, 39, 201–225. [Google Scholar] [CrossRef]
- Osher, S.; Fedkiw, R.P. Level Set Methods: An Overview and Some Recent Results. J. Comput. Phys. 2001, 169, 463–502. [Google Scholar] [CrossRef]
- Baer, M.R.; Nunziato, J.W. A two-phase mixture theory for the deflagration-to-detonation transition (ddt) in reactive granular materials. Int. J. Multiph. Flow 1986, 12, 861–889. [Google Scholar] [CrossRef]
- Saurel, R.; Pantano, C. Diffuse-Interface Capturing Methods for Compressible Two-Phase Flows. Annu. Rev. Fluid Mech. 2018, 50, 105–130. [Google Scholar] [CrossRef]
- Cahn, J.W.; Hilliard, J.E. Free Energy of a Nonuniform System. I. Interfacial Free Energy. J. Chem. Phys. 1958, 28, 258–267. [Google Scholar] [CrossRef]
- Linstrom, P. NIST Chemistry WebBook, NIST Standard Reference Database 69; NIST: Gaithersburg, MD, USA, 2023. [Google Scholar] [CrossRef]
- Gaballa, H.; Habchi, C.; de Hemptinne, J.C. Modeling and LES of high-pressure liquid injection under evaporating and non-evaporating conditions by a real fluid model and surface density approach. Int. J. Multiph. Flow 2023, 160, 104372. [Google Scholar] [CrossRef]
- Gaballa, H.; Jafari, S.; Di-Lella, A.; Habchi, C.; de Hemptinne, J.C. A tabulated real-fluid modeling approach applied to renewable dual-fuel evaporation and mixing. In Proceedings of the International Conference on Liquid Atomization and Spray Systems (ICLASS), Edinburgh, UK, 30 August–2 September 2021; Volume 1. [Google Scholar] [CrossRef]
- Gaballa, H.; Jafari, S.; Habchi, C.; de Hemptinne, J.C. Numerical investigation of droplet evaporation in high-pressure dual-fuel conditions using a tabulated real-fluid model. Int. J. Heat Mass Transf. 2022, 189, 122671. [Google Scholar] [CrossRef]
- Kheiri, A.; Afsharpour, A. The CPA EoS application to model CO2 and H2S simultaneous solubility in ionic liquid [C2mim][PF6]. Pet. Sci. Technol. 2018, 36, 944–950. [Google Scholar] [CrossRef]
- Yang, S.; Habchi, C. Real-fluid phase transition in cavitation modeling considering dissolved non-condensable gas. Phys. Fluids 2020, 32, 032102. [Google Scholar] [CrossRef]
- Yi, P.; Yang, S.; Habchi, C.; Lugo, R. A multicomponent real-fluid fully compressible four-equation model for two-phase flow with phase change. Phys. Fluids 2019, 31, 026102. [Google Scholar] [CrossRef]
- de Lorenzo, M.; Lafon, P.; Di Matteo, M.; Pelanti, M.; Seynhaeve, J.M.; Bartosiewicz, Y. Homogeneous two-phase flow models and accurate steam-water table look-up method for fast transient simulations. Int. J. Multiph. Flow 2017, 95, 199–219. [Google Scholar] [CrossRef]
- Föll, F.; Hitz, T.; Müller, C.; Munz, C.D.; Dumbser, M. On the use of tabulated equations of state for multi-phase simulations in the homogeneous equilibrium limit. Shock Waves 2019, 29, 769–793. [Google Scholar] [CrossRef]
- Yang, S.; Yi, P.; Habchi, C. Real-fluid injection modeling and LES simulation of the ECN Spray A injector using a fully compressible two-phase flow approach. Int. J. Multiph. Flow 2020, 122, 103145. [Google Scholar] [CrossRef]
- Richards, K.J.; Senecal, P.K.; Pomraning, E. CONVERGE 3.1; Convergent Science: Madison, WI, USA, 2023. [Google Scholar]
- Peng, D.Y.; Robinson, D.B. A New Two-Constant Equation of State. Ind. Eng. Chem. Fundam. 1976, 15, 59–64. [Google Scholar] [CrossRef]
- Soave, G. Equilibrium constants from a modified Redlich-Kwong equation of state. Chem. Eng. Sci. 1972, 27, 1197–1203. [Google Scholar] [CrossRef]
- Péneloux, A.; Rauzy, E.; Fréze, R. A consistent correction for Redlich-Kwong-Soave volumes. Fluid Phase Equilibria 1982, 8, 7–23. [Google Scholar] [CrossRef]
- Redlich, O.; Kwong, J.N.S. On the thermodynamics of solutions; an equation of state; fugacities of gaseous solutions. Chem. Rev. 1949, 44, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Kontogeorgis, G.M.; Michelsen, M.L.; Folas, G.K.; Derawi, S.; von Solms, N.; Stenby, E.H. Ten Years with the CPA (Cubic-Plus-Association) Equation of State. Part 1. Pure Compounds and Self-Associating Systems. Ind. Eng. Chem. Res. 2006, 45, 4855–4868. [Google Scholar] [CrossRef]
- Oliveira, M.; Coutinho, J.; Queimada, A. Mutual solubilities of hydrocarbons and water with the CPA EoS. Fluid Phase Equilibria 2007, 258, 58–66. [Google Scholar] [CrossRef]
- Palma, A.M.; Oliveira, M.B.; Queimada, A.J.; Coutinho, J.A. Re-evaluating the CPA EoS for improving critical points and derivative properties description. Fluid Phase Equilibria 2017, 436, 85–97. [Google Scholar] [CrossRef]
- Jafari, S.; Gaballa, H.; Habchi, C.; de Hemptinne, J.C.; Mougin, P. Exploring the interaction between phase separation and turbulent fluid dynamics in multi-species supercritical jets using a tabulated real-fluid model. J. Supercrit. Fluids 2022, 184, 105557. [Google Scholar] [CrossRef]
- Menter, F.R. Two-equation eddy-viscosity turbulence models for engineering applications. AIAA J. 1994, 32, 1598–1605. [Google Scholar] [CrossRef]
- Gaballa, H.; Jafari, S.; Di-Lella, A.; Habchi, C.; de Hemptinne, J.C. A tabulated real-fluid modeling approach applied to cryogenic LN2-H2 jets evaporation and mixing at transcritical regime. In Proceedings of the International Conference on Liquid Atomization and Spray Systems (ICLASS), Edinburgh, UK, 30 August–2 September 2021; Volume 1. [Google Scholar] [CrossRef]



| Case | Inlet Total Pressure (MPa) | Inlet Total Temperature (K) | Subsonic Outlet Pressure (MPa) |
|---|---|---|---|
| 1 | 5.896 | 314.67 | 1.973 |
| 2 | 6.535 | 311.99 | 2.281 |
| 3 | 7.353 | 313.60 | 2.437 |
| 4 | 7.999 | 313.94 | 2.729 |
| 5 | 8.474 | 313.88 | 2.805 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhatia, H.; Habchi, C. Real Fluid Modeling and Simulation of the Structures and Dynamics of Condensation in CO2 Flows Shocked Inside a de Laval Nozzle, Considering the Effects of Impurities. Appl. Sci. 2023, 13, 10863. https://doi.org/10.3390/app131910863
Bhatia H, Habchi C. Real Fluid Modeling and Simulation of the Structures and Dynamics of Condensation in CO2 Flows Shocked Inside a de Laval Nozzle, Considering the Effects of Impurities. Applied Sciences. 2023; 13(19):10863. https://doi.org/10.3390/app131910863
Chicago/Turabian StyleBhatia, Harshit, and Chaouki Habchi. 2023. "Real Fluid Modeling and Simulation of the Structures and Dynamics of Condensation in CO2 Flows Shocked Inside a de Laval Nozzle, Considering the Effects of Impurities" Applied Sciences 13, no. 19: 10863. https://doi.org/10.3390/app131910863
APA StyleBhatia, H., & Habchi, C. (2023). Real Fluid Modeling and Simulation of the Structures and Dynamics of Condensation in CO2 Flows Shocked Inside a de Laval Nozzle, Considering the Effects of Impurities. Applied Sciences, 13(19), 10863. https://doi.org/10.3390/app131910863







