Abstract
This study focuses on the development and characterization of solid dispersions (SDs) of Gefitinib (GEF) to improve its aqueous solubility and therapeutic activity against lung cancer. SDs were prepared by the co-precipitation method with tocopheryl-polyethylene-glycol succinate-1000 (TPGS) (F1), sodium lauryl sulfate (SLS) (F2) and complexation of F1 with hydroxypropyl β-cyclodextrin (HP-β-CD) (F3). Optimal formulations (F1 and F3) were used against A549 cells to determine the apoptosis, expressions of p53 and caspases. F3 has shown the highest solubility (1271.21 µg/mL), followed by F1 (1003.69 µg/mL), F2 (707.81 µg/mL) and GEF pure (303.85 µg/mL) in 0.1N HCl. Dissolution at 1.2 pH significantly enhanced the release from F3 (99.19%), followed by F1 (94.76%), F2 (85.70%) and GEF pure (37.26%) during 120 min. Complexation of GEF–TPGS with HP-β-CD significantly improved drug release with high dissolution efficiency (78.57%) in 24.9 min of mean dissolution time. Differential scanning calorimetry revealed crystalline to amorphous conversion of GEF in SDs, which was confirmed by scanning electron microscopy. Fourier transform infrared and proton nuclear magnetic resonance spectral analysis revealed no interaction between GEF and excipients. The IC50 values were 2.239, 3.135 and 4.471 µM for F3, F1 and GEF pure, respectively, against A549 cells. Increased expressions of p53 (5.9-, 4.6- and 3.04-fold), caspase-3 (5.38-, 3.78- and 3.01-fold) and caspase-9 (5.35-, 3.76- and 2.47-fold) in the case of F3, F1 and GEF pure, respectively, as compared to the untreated A549 cells indicated improved apoptotic potential of the SDs. TPGS SDs and their complexation with HP-β-CD improved the solubility, dissolution and efficacy of GEF against A549 cells. So, they can be a suitable alternative to the conventional GEF formulations against non-small-cell lung cancers.
1. Introduction
Gefitinib (GEF; MW: 446.9 g/mole), a water-insoluble anilinoquinazoline derivative, is the epidermal growth factor receptor (EGFR) inhibitor and belongs to the family of tyrosine kinase inhibitor. It promotes cell cycle arrest and apoptosis, leading to inhibition of angiogenesis and cell invasion. The angiogenic-pathway is an important target to inhibit tumor growth [1]. It mediates the anti-angiogenesis effect by reducing the expression of pro-angiogenic genes, cell-proliferation and vascular density [2]. It is used to treat non-small-cell lung cancer (NSCLC), especially in patients who already have received chemotherapy [1,3,4,5]. It has poor oral bioavailability (not more than 60%) because of its biopharmaceutical and physicochemical properties [6]. It exhibits poor aqueous solubility (approximately 0.3 mg/mL in ethanol and 0.5 mg/mL in 1:1 v/v solution of DMSO and PBS (pH 7.2) [7]. After a single oral dose of 50 mg GEF, only 12.1% of the drug was present in the blood after 240 h post-dose [8]. The absorption of GEF through the oral route is limited due to its low dissolution rate (only around 41.13% from pure-GEF) [8,9,10]; hence, it shows high variability in blood concentration and causes low bioavailability [11]. In addition to the low oral bioavailability, only 40–45% of the plasma concentration was found to act on the target tumors [12].
Therefore, it was mandatory to develop a formulation with better solubility, dissolution and absorption to enhance the oral bioavailability of GEF [5,13]. The present study focuses on the formulation of GEF with different excipients for evaluation of its impact on their solubility and dissolution rate. TPGS and SLS were used as surfactants to prepare the solid dispersions of GEF. The optimal composition was further encaged into HP β-CD to form ternary SDs.
TPGS and SLS are non-ionic and anionic surfactants, respectively, which were found to have potential to increase the solubility and dissolution rate of various poorly soluble drugs. These surfactants have shown somewhat mechanistic simulation towards the in vivo solubilization and absorption of some drugs [14,15,16]. TPGS is generally regarded as a safe listed excipient that can be taken orally long-term even at high doses [16]. Additionally, vitamin-E-TPGS 1000 (where 1000 denotes the molecular weight of the polyethylene glycol chain) is an FDA-approved drug-solubilizer for oral, topical, nasal and vaginal delivery systems [16,17]. Cyclodextrin, especially HP β-CD, has been reported to be safe and biocompatible [14,18]. It is considered as a good solubilizer and bio-enhancer that forms an inclusion complex to improve the solubility and bioavailability of poorly soluble drugs [18]. It has served as an outstanding complexing agent to enhance the solubility, stability and bioavailability of GEF [5,10].
In this study, TPGS and SLS surfactant-based binary solid dispersions (SDs) of GEF were prepared. The addition of HP β-CD occurred for the optimal binary SDs to increase the aqueous solubility of GEF. The physicochemical interaction between GEF and the excipients was studied by FTIR and NMR spectroscopy. The morphological changes in the SDs were assessed by SEM, while the flow property was checked by Carr’s index and Hausner ratio determination. The DSC was performed to determine the crystalline to amorphous conversion of GEF. The saturation solubility of the SDs was checked at different pH conditions, and dissolution tests were completed in 0.1N HCl (pH 1.2). Finally, the selected binary SDs and ternary SDs were evaluated for cell viability study using the lung cancer cells (A549) to evaluate apoptotic activity comparing GEF pure and untreated cells. The levels of p53 and caspases were also determined to substantiate their apoptotic properties.
2. Experimental
2.1. Materials
The Gefitinib (≥98% purity by HPLC), D-α-Tocopherol polyethylene glycol 1000 succinate (TPGS), sodium lauryl sulfate (SLS), (2-Hydroxypropyl)-β-cyclodextrin (HP β-CD) and the HPLC-grade solvents were purchased from Sigma Aldrich (St. Louis, MO, USA). All other chemicals and solvents of analytical grade were used as received. The human p53, human caspase-3 and human caspase-9 ELISA Kits were purchased from Abcam (Boston, MA, USA).
2.2. Methods
2.2.1. Quantitative Analysis of GEF
The GEF was quantified by high-performance liquid chromatography (HPLC) with UV detection at 254 nm [5,19]. The Waters® HPLC system (Milford, MA, USA) fitted with binary pump, automated sampling system and UV detector was used. The system was monitored by Breeze 2 Software (Build No. 2154, Copyright 2008 Waters® Corporation (Milford, MA, USA). A reverse phase C18 column (250 mm × 4.6 mm, 5 µm) SunFire by Waters® at ambient temperature was used for elution of GEF. The mobile phase consisting of acetonitrile and ammonium acetate (1%, w/v) at 60:40 (v/v) was isocratically pumped at 1 mL/min of flow rate. The injection volume was 30 μL with a total run time of 10 min. The GEF (10 mg) was dissolved in 100 mL of acetonitrile to obtain a stock solution of 100 µg/mL concentration. The calibration standards of GEF were prepared by serial dilution of stock solution with the mobile mixture to obtain a concertation range of 5–50 μg/mL.
2.2.2. Preparation of Solid Dispersions of GEF
The solid dispersions (SDs) of the GEF with SLS and TPGS were prepared by the co-precipitation method [20,21,22]. The detailed compositions of the SDs were mentioned in Table 1. Briefly, the optimal amount of GEF was dissolved in 40 mL mixture of acetone and methanol (at 24/16, v/v). The surfactants (SLS and TPGS) with and without HP β-CD were dissolved in 40 mL of purified water separately. The surfactant with HP β-CD solution was added slowly into the GEF organic solution at magnetic stirring (700 rpm). The stirring was continued for 24 h at ambient temperature for complete evaporation of the organic solvents. The resulting suspensions were iced up at −80 °C and freeze-dried by “FreeZone-4.5 Freeze Dry System (Labconco Corporation, Kansas City, MO, USA)” at −50 °C for 48 h at 0.02 mbar vacuum to get the SDs [23,24]. After freeze-drying, the relative yield (%) of SDs was calculated using total initial weights of all and the final weight of SDs. The dried samples were then stored at 2–8 °C for other experiments.

Table 1.
Amounts of GEF, surfactant and complexing agent to formulate the GEF SDs.
2.2.3. Saturation Solubility
The saturation solubility of GEF pure and GEF SDs was checked in the phosphate buffer saline (pH 5.6 and pH 7.2) and 0.1 N hydrochloric acid (HCl) of pH 1.2. An excess amount of GEF and its SDs was tried to dissolve in 10 mL of each of the above solvents in small beakers (set of three for each product) [25]. The mixtures placed for 72 h in an orbital shaking water bath at 100 oscillations/minute maintained at 37 ± 0.5 °C. The mixes were kept at room temperature for a further 24 h to reach equilibrium and facilitate the settling of the undissolved drug. The supernatant was obtained, filtered using 0.45 µm pore size Millipore filtration unit and then diluted as necessary to analyze the GEF by HPLC-UV as mentioned above.
2.2.4. In Vitro Dissolution Study
The dissolution tests of GEF pure, F1, F2 and F3 were performed in 0.1 N HCl (pH 1.2) as dissolution media. The freeze-dried samples, equivalent to 20 mg of GEF, were filled in hard gelatin capsules and put in the dissolution apparatus containing 900 mL of dissolution medium stirred at 100 rpm and maintained at 37 ± 0.5 °C [26]. Aliquots of (2 mL each) were collected at predetermined time intervals (at 0, 5, 10, 15, 30, 45, 60, 90 and 120 min) and replaced with an equal volume of the fresh medium (pre-warmed at 37 ± 0.5 °C) to retain the sink good condition. The withdrawn samples were centrifuged (6000 rpm for 5 min), diluted appropriately (if needed) and GEF concentration was determined by HPLC-UV [5,19].
2.2.5. Characterization of GEF SDs
Based on the saturation solubility and dissolution test performance out of three SDs, only F1 and F3 were chosen for further characterization. The lyophilized products and the individual excipients were analyzed by differential scanning calorimetry (DCS), nuclear magnetic resonance (NMR), scanning electron microscopy (SEM) and Fourier transform infrared spectroscopy (FTIR). Moreover, the samples were subjected to micromeritics to evaluate their fundamental and derived properties.
Differential Scanning Calorimetry (DSC)
Differential scanning calorimetry (DSC) was conducted for the physical state analysis of the products by “DSC-8000 (Perkin Elmer Instruments, Shelton, CT, USA)”. The analysis was conducted under an inert N2 purging (20 mL/min) at the heating rate of 10 °C/min in the range of 30–300 °C temperature. “The DSC instrument was calibrated with 100% pure Indium (Melting point 156.6 °C and 6.80 cal/g heat of fusion)”. Around 5 mg of each sample to be tested were crimped in the standard aluminum pans and lid for the analysis, whereas a similar empty pan was put in the reference cell. The thermograms of GEF, excipients, lyophilized solid dispersions and the inclusion complex were recorded and analyzed through the PYRIS V-11 software attached to the instrument.
Fourier Transform Infrared (FTIR) Spectroscopy
The FTIR spectra of GEF-pure, individual excipients, freeze-dried SDs and the inclusion complexes were recorded by using a Perkin Elmer (FTIR). Each material was taken and kept in the sample holder to analyze the spectra across 4000 to 400 cm−1 wavenumber by 3 scans and 2 cm−1 resolutions. The IR spectra of pure sample were compared with the IR spectra of prepared formulations to evaluate the changes and shifting in the wavenumbers of the respective functional groups.
Nuclear Magnetic Resonance (NMR)
To assess the structural changes in the GEF after formulation into SDs, the proton NMR (1H NMR) was used. The study was carried out for GEF pure, F1, physical mixture of GEF, TPGS and HP β–CD (PM) and F3 to compare the NMR spectra of each. The samples were assessed by 1H NMR (functioning at 700 MHz frequency) (Bruker NMR; Switzerland) spectroscopy. Tetramethylsilane (TMS) was used as versatile internal standard, while deuterated dimethyl sulfoxide (DMSO) served as the solvent for this study.
Morphology by Scanning Electron Microscopy (SEM)
The morphology of GEF pure, PM, F1 and F3 was assessed through SEM. The SEM analysis “(Zeiss EVO LS10, Cambridge, UK)” was completed using the gold-sputter technique. Herein, the gold coating was completed on the dried samples using the “Q-150R Sputter Unit” of “Quorum Technologies Ltd. (East Sussex, UK)”. The coating was conducted for 1 min at 20 mA current in an argon atmosphere. Finally, the imaging was completed at working distance of 15 mm, accelerating voltage of 10–20 kV and the magnification was 500–2000 times.
Micromeritics and Flow Property
The GEF physical mixture and the prepared SDs were evaluated for different parameters of micromeritics, such as Carr’s index, Hausner ratio and angle of repose [27]. These variables describe the flow behavior of dried granular materials and powdered samples. For the samples having ideal flow properties, the value must be less than 15% for Carr’s index and <1.25 for Hausner ratio. These parameters can be calculated through the tapped and bulk densities of the sample. The angle of repose was determined by the funnel method. The samples were passed from funnel to a flat surface to form a cone, and then the height (h) and radius (r) of the cone were measured. The sample shows the value as excellent flow (˂30°), good flow (˂31–35°), fair flow (˂36–40°) and poor flow (˂41–55°) [28]. The above parameters were calculated by the equations (Equations (1)–(3)) as follows:
2.3. In Vitro Cytotoxicity
Based on the above characteristics, especially the saturation solubility and dissolution tests, the GEF–TPGS SDs (F1) were found superior to GEF–SLS SDs (F2). Therefore, only F1 combination was chosen for further complexation with HP β–CD as F3. Hence, the in vitro cytotoxicity studies were carried out for F1 and F3 as compared to GEF pure on A549 cell lines.
2.3.1. Maintenance and Culture of A549 Cells
The non-small-cell lung cancer (NSCLC) cell lines i.e., A549 cells, were procured from the “American Type Culture Collection (ATCC, Manassas, VA, USA)”. The cells were seeded and grown in Dulbecco’s modified Eagle’s medium (DMEM, UFC Biotech, Riyadh, Saudi Arabia) at cell density of 5 × 104/mL in 50 cm2 tissue culture flasks. The cells were incubated at 37 °C for 24 h in CO2 incubator, which was humidified with 5% CO2. Further, 10% fetal bovine serum (FBS) by Alpha Chemika, Mumbai, India, and a 1% solution of Penicillin and Streptomycin (each at 100 IU/mL) by ThermoFischer Scientific, Waltham, MA, USA, were added to the medium as supplements. The medium was also supplemented with Amphotericin-B (Gibco®, New York, NY, USA) at 250 ng/mL and L-glutamine at 1% (BioWest, Riverside, MO, USA). The 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTT) was procured from Sigma Aldrich (St. Louis, MO, USA).
2.3.2. Preparation of Aqueous Solutions of GEF Pure, F1 and F3
The varying concentrations (0.195, 0.39, 0.78, 1.56, 3.125, 6.25, 12.5, 25, 50 and 100 µM) of GEF pure, F1 and F3 were prepared. The concentrations of GEF from 0.195 to 100 µM were used as per the reported studies [29,30]. Accurately weighed amount of GEF pure (4.469 mg) and equivalent amounts of SDs were dissolved in 100 mL of DMEM using DMSO (200 µL) as co-solvent to obtain 100 µM stock solutions and kept at −20 °C for further use. The concentration of DMSO was 0.2% v/v in the stock solution, which was further reduced in the different dilutions as the stocks were diluted by fresh DMEM prior to each experiment.
2.3.3. MTT Assay on A549 Cells and Morphological Changes
This assay is a colorimetric technique to determine the viable cell counts, which is based on the reduction of MTT by the cells to produce a soluble product (formazan, soluble in the DMEM and detected through UV spectrophotometry). Through predominantly mitochondria-mediated apoptosis, this assay approximates the viability of the cells. Here, it was applied to determine the anticancer potential of F1 and F3 in comparison to GEF pure. Around 150 μL cultured A549 cells (≈3 × 103 cells/well density) from the exponential growth phase was seeded in 96-well flat-bottomed plates (Corning Inc., New York, NY, USA) and left untouched for 24 h. Thereafter, the cells in the wells were washed two times with phosphate-buffered saline (PBS) and replaced with DMEM medium (supplemented by 10% FBS) with varying concentrations of GEF pure, F1 and F3. The control wells where only DMEM medium (with drug) was added were maintained to check the survival and viable cells in the culture medium.
The treated and untreated (control) cells were then incubated at 37 °C in CO2 incubator for 72 h. After incubation, 10 µL of MTT (5 mg/mL) was added into each well and incubated further at 37 °C for 4 h. The viable cells reduced the MTT (yellow color) to formazan crystals (dark color). The medium was pipetted out carefully from each well to avoid disruption of the monolayer and discarded. Then, DMSO (150 µL) was added into the wells and the plate was allowed to be shaken slowly at ambient temperature for 10 min to dissolve the formazan crystals [31]. The optical density (OD) of the appeared dark color was measured using a Synergy HT spectrophotometric microplate reader (BioTek Instruments, Winooski, VT, USA) at 570 nm [30,31,32,33]. Each concentration was poured in six wells and the experiment was performed in triplicate. The proliferation or relative cell viability (%) was articulated as percentage of growth in comparison to the control (untreated cells) by Equation (4):
The data were plotted as percentage cell viability versus log concentration of GEF, normalized relative to the viable control cells (assuming as 100%). The IC50 values of each GEF product were determined against the A549 cells. With the aid of GraphPad Prism-5 (GraphPad Software, San Diego, CA, USA), the data were represented graphically. The curves were fitted by non-linear regression model symbolized as “Y” as expressed in Equation (5) with the sigmoidal dose responses.
The phase contrast inverted microscope (Olympus CLX 41) was used to observe the morphological changes in the treated A549 cells. The A549 cells (≈3 × 103 cells/well density) per well were seeded in 24-well plates with DMEM and incubated for 24 h at 37 °C with 5% CO2 supply. Thereafter, cells were treated with the GEF formulations (considering 3.135 µM GEF concentration for each). All the treated and untreated cells were incubated for 72 h at above-mentioned conditions. After 72 h of incubation, microscopic observation was done and the photographs were captured to see any apoptotic morphological changes [31,32].
2.3.4. p53 and Caspases Activity
The p53 and caspases activity was determined by Enzyme Linked immunosorbent Assay (ELISA) kits by following the reported studies and protocol of the manufacturer [34,35]. The A549 cells at 5 × 103 cells/well density was seeded in 96-flat-bottomed-well plates. The GEF pure, F1 and F3 aqueous solutions were used to treat the cells in the wells. The GEF concentration for these experiments was kept 3.125 µM, which was near the IC50 value of pure drug (3.986 µM) in the present investigation. The treated A549 cells were kept at ambient temperature to equilibrate. The untreated cells were also kept at ambient temperature and those served as control. Following that, 100 µL of each antibody was added to the corresponding wells that were already containing of the culture medium (100 µL in each). The plates had been covered and incubated at room temperature for 2–3 min while being stirred at 500 rpm. The optical density (OD) was measured at 405 nm by a microplate reader (Synergy HT, BioTek Instruments, Winooski, VT, USA) 1 h after incubation.
2.4. Statistical Data Analysis
Unless otherwise stated, the mean of three readings along with the standard deviation (mean ± SD) were used to represent the data. The graphs were produced using “GraphPad Prism: V5 (GraphPad Software, Inc., San Diego, CA, USA)”. Following the completion of the statistical analysis, the findings were compared using Student’s t-test and “p < 0.05” was taken into consideration as statistical significance.
3. Results and Discussion
3.1. HPLC Analysis of GEF
The adopted HPLC method [19] was found to provide linear calibration curves at these concentrations with a correlation coefficient (R2) of 0.9997 and regressed equation of “y = 101,575x − 80,100”. The retention time for GEF was 6.545 min. The sensitivity in terms of limit of detection (LOD) and limit of quantification (LOQ) of this method was reported as 1.3 and 3.9 µg/mL, respectively. The accuracy values in terms of recovery (%) of quality control samples were 98.5–101.2% with 1.09–1.56% of RSD (%), while the RSD (%) values of peak area were 0.23–1.22% and 0.12–1.56% for intra-day and inter-day precision, respectively. As the RSD (%) values of peak areas were less than 2%, the method was considered as accurate and reproducible [19]. These results established a linearity between the peak area and GEF concentrations. The adopted method was successful for the quantification of GEF in the present study.
3.2. Formulation Development
The compositions of the SDs with GEF and other excipients in detail were summarized in Table 1. The co-precipitation method was successful to prepare the SDs of GEF using TPGS and SLS as a surfactant and HP β-CD as a complexing agent. The formulations were developed with varying concentrations of the surfactants and HP β-CD. The concentration of GEF was kept constant. For each group, we have developed three formulations. Based on the value of percentage yield (%) and cumulative drug release (%), the optimal one from each group (such as F1, F2 and F3) was selected for further investigation. The formulation development for optimization purposes has been supplied as Supplementary Material (Table S1 and Figure S1). For the optimal formulations, the relative yield (%) of GEF SDs was less (61.75 ± 4.86%) in the case of F2 (SLS as surfactant) as compared to F1 (86.54 ± 5.52%), where TPGS was used as a surfactant in the present work. Therefore, the complexation of GEF SDs with HP β-CD was completed only for the F1 composition (where TPGS was used as a surfactant). The yield (%) of F3 (GEF–TPGS with HP β-CD SDs) was found to be 82.55 ± 3.95%. The yield of F3 was slightly less as compared to F1, which was expected due to the increase in total mass of this formulation.
The percentage yield values indicated that the type of surfactant has an important role in the formation of SDs of lipophilic molecules, which greatly depends on the inherent property of the surfactants and the nature of the interaction between SDs and surfactants [36]. The higher yield of GEF SDs with TPGS in the present investigation might be attributed to the non-ionic nature of TPGS with the hydrophilic–lipophilic balance (HLB) value of 13.2 and low critical micelle concentration (CMC, 0.02%, w/w) [37]. The amphiphilic nature of the hydrophilic polar head and lipophilic alkyl end of TPGS makes it an ideal biomaterial to develop different drug delivery systems, including the SDs, which would be able to improve the solubility, drug release pattern, absorption and bioavailability of the drug [38].
In contrast, the SLS is an anionic surfactant with high HLB (≈40.0) and CMC (0.24%, w/w) [36,39], resulting in lower yield of GEF SDs. The electrostatic interaction due to the pKa values (pKa1 5.4 and pKa2 7.2) of dibasic molecule (GEF) and ionization of SLS might be responsible for this phenomenon. Moreover, the hydrophobic interactions (i.e., log p value 4.85, polar surface area, conformational flexibility and HLB, etc.) might be the reasons. Therefore, the surfactants (TPGS and SLS) used as stabilizers to prepare SDs of GEF in the present investigation critically affected the physicochemical properties and stability of GEF, which was also reported previously [40]. Further studies to elucidate the exact mechanism for the higher yield of GEF SDs using TPGS as a stabilizer are presently underway.
3.3. Saturation Solubility
The saturation solubility was demonstrated for GEF pure, F1 and F3 in different solvents having pH values simulating gastric fluid (pH 1.2), the slightly acidic microenvironment of cancers (pH 5.6) and small intestine fluid (pH 7.2). The pH of the microenvironment of cancers or malignant tumors is slightly acidic and varies between 5.6 and 6.8. The reason for this acidic microenvironment is attributed to glycolysis, inadequate blood flow and hypoxia in the tumor cells [41]. Table 2 represents the saturation solubility (in linear and logarithmic) of GEF (μg/mL) at different pH values. The results were suggesting the pH dependent solubility of GEF and GEF SDs. It was found that GEF was comparatively very much soluble at 1.2 pH (gastric environment) compared to pH 5.6 (tumor microenvironment) and pH 7.2 (intestinal environment). The relatively low saturation solubility at neutral pH is attributed to the aqueous-insoluble or poor aqueous soluble property of GEF, which was due to the fact that GEF is a dibasic molecule with two pKa values (5.4 and 7.2). The enhanced saturation solubility of GEF SDs at acidic pH is suggesting, such that the SDs of GEF prepared with TPGS (F1) will have better absorption of the drug through GIT (especially in the stomach part) as compared to the SDs of GEF prepared with SLS (F2). Among the three optimal SDs, the F3 (the complexation of F1 with HP β-CD) has shown the highest solubility (F3 > F1 > F2 > GEF pure) at 1.2 pH. Moreover, use of TPGS as a stabilizer would improve the oral bioavailability of GEF from SDs to overcome the multidrug resistance as TPGS is a potent P-glycoprotein (P-gp) inhibitor [38]. Thus, we can postulate that the complexation of GEF SDs prepared with TPGS will provide the highest absorption of GEF in the GIT, which in turn would improve the overall bioavailability of the drug.

Table 2.
Solubility of GEF SDs and GEF pure in different solvents. The values represent the mean and standard deviation of three measurements.
3.4. Dissolution Test
Figure 1 shows the dissolution profiles of GEF from GEF pure and solid dispersions of GEF (F1, F2 and F3) at pH 1.2 (0.1 N HCl). The 0.1 N HCl solution was used as release medium because it simulates the gastric environmental pH conditions. GEF pure was used as a reference product in this study. The low pH release medium was used for dissolution tests because, being a weak base with two pKa values (pKa1 5.4 and pKa2 7.2), GEF ionizes completely with high aqueous solubility [25].
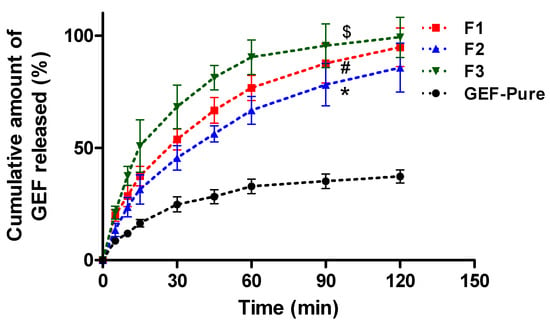
Figure 1.
Dissolution test and release profile of GEF from GEF pure and GEF SDs in 0.1 N HCl as release medium (pH 1.2). The data were provided as the mean and standard deviation (mean ± SD) of three measurements, where “*” p < 0.005, F3 against F1, F2 and GEF pure; “#” p < 0.005, F1 against F2 and GEF pure; “$” p < 0.005, F2 against GEF pure.
The GEF pure depicted poor release due to its poor aqueous solubility. The GEF pure showed only 37.26 ± 2.89% of cumulative drug release till 2 h. The study suggested a quick release of GEF from the GEF SDs prepared with TPGS and SLS. The maximum GEF release was achieved within 60 min from the SDs. The presence of surfactants (TPGS in F1) and SLS (in F2) was able to reduce the interfacial tension between the drug and release medium, leading to enhanced GEF solubility. Among TPGS and SLS, the SDs prepared with TPGS (F1) showed faster release than the SDs prepared with SLS (F2). Within 5 min, a significant difference in the GEF release was observed between F1 and F2. Around 19.92 ± 1.01% drug was released from F1 (GEF–TPGS SDs), whereas only 13.38 ± 1.16% drug release was found from F2 (GEF–SLS SDs) within 5 min. As the time increases, a gradual increase in GEF release was achieved from both the SDs (F1 and F2). The F1 showed a maximum release of GEF (94.76 ± 3.45%), while F2 released around 85.71 ± 4.38% at 120 min.
The dissolution of GEF–TPGS SDs (F1) was further controlled by the addition of HP β-CD as a carrier. For this, the F1 was further incorporated into the HP β-CD as F3 to modify the GEF release pattern. After incorporation of HP β-CD, the release pattern of GEF was significantly improved. The formation of the inclusion complex further enhanced the release behavior of GEF. Around 90.32 ± 3.09% and 99.18 ± 3.61% of the drug was released from F3 within 60 min and 120 min, respectively. Overall, the dissolution profiles showed that TPGS has a higher dissolution rate in comparison to the intrinsic dissolution of GEF in 0.1N HCl as compared to the SLS as the GEF–SLS SDs (F2) had the lowest effect on the dissolution of GEF. This might be due to the degradation of SLS in the strong acidic environment of 0.1N HCl. The utilization of TPGS would further facilitate the intestinal permeability of GEF as TPGS also serves as a penetration enhancer in association with its p-glycoproteins’ efflux inhibition activity. Thus, improved aqueous solubility and intestinal penetration would lead to enhanced oral bioavailability of the poorly soluble anticancer agent [42].
The in vitro release data were further evaluated by computing the dissolution efficiency (%DE) and mean dissolution time (MDT). The %DE (Figure 2a) and MDT values (Figure 2b) for the developed GEF carriers were significantly improved as compared to the suspension of GEF pure. It was evident that all the examined developed drug delivery systems in the present investigation exhibited faster dissolution of GEF than the GEF pure.
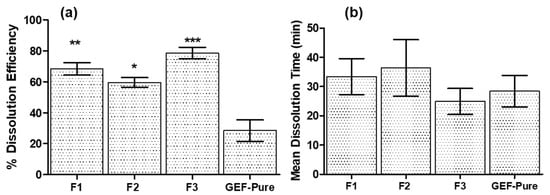
Figure 2.
(a) Dissolution efficiencies (DE%) with 95% confidence intervals of different GEF SDs, where “***” p < 0.005 represents the significant improvement in DE% of F3 against all other GEF products; “**” p < 0.005 represents the significant improvement in DE% of F1 against F2 and GEF pure and “*” p < 0.005 represents the significant improvement in DE% of F2 against GEF pure only. (b) Mean dissolution time (MDT) with 95% confidence intervals for different GEF SDs.
The DE% and MDT of GEF pure were only around 28.46 ± 2.78% and 28.40 ± 2.15 min, respectively. In the case of the F1 (GEF–TPGS) and F2 (GEF–SLS), better dissolution properties were observed as compared to the GEF pure. The DE and MDT of GEF–TPGS (F1) were 68.32 ± 1.64% and 33.42 ± 2.47 min, respectively, while those of (GEF–SLS) F2 were 59.64 ± 1.25% and 36.37 ± 3.88 min. Thus, the dissolution parameters for F1 (GEF–TPGS) were superior to F2 (GEF–SLS) because of better release profile in the case of F1, which was attributed to the good stabilizing property of TPGS. In the case of GEF–TPGS with HP β-CD (F3), both the dissolution parameters were significantly improved. It represented around 78.57 ± 1.53% of DE and 24.90 ± 1.79 min of MDT. The improvement in the dissolution parameters for F3 might be associated with the high solubility and complexation of GEF with TPGS and HP β-CD, respectively. Thus, the use of a ternary system in the present study confirms the synergistic effect on the solubility of GEF when used in combination with HP β-CD [22,43].
3.5. FTIR Spectroscopy
The FTIR spectroscopy of individual ingredients (GEF pure, TPGS, SLS and HP β-CD) of GEF SDs (F1, F2 and GEF–TPGS SDs complexed with HP β-CD (F3)) was investigated for the shifting in the wavenumber (if any) of functional groups, as represented in Figure 3. The GEF belongs to the class of quinazolines, a tyrosine kinase inhibitor of the epidermal growth factor receptor (EGFR). The secondary (2°) amine of the drug depicted sharp stretching vibration at 3391.34 cm−1. The aromatic C-H and C=N stretching vibration of the quinazoline ring exhibited frequencies at 2952.92 cm−1 and 2815.33 cm−1, respectively. The C–N stretching vibration of the morpholine ring was exhibited at 1390 cm−1. The halogen functional groups illustrate their stretching vibrational peaks at 1498.95 cm−1 for fluoro and 842.97 cm−1 for chloro groups. The C–O–C stretching vibration of the pure drug was observed at 1119.25 cm−1. The displayed frequency of the carrier TPGS was observed at 2878.13 cm−1, which beheld the stretching vibration for the ester group. The other functional groups of the carrier exhibited stretching vibrations at 1345.75 and 1100.69 cm−1 for C=C and C–O–C moieties. The HP β-CD displayed its stretching vibration at 3287.60 cm−1 and 1018.62 cm−1 for cyclic hydroxyl and C–O–C moieties, which was also noted during the FTIR spectroscopy of HP β-CD at of 4000 and 450 cm−1 scanning range [44].
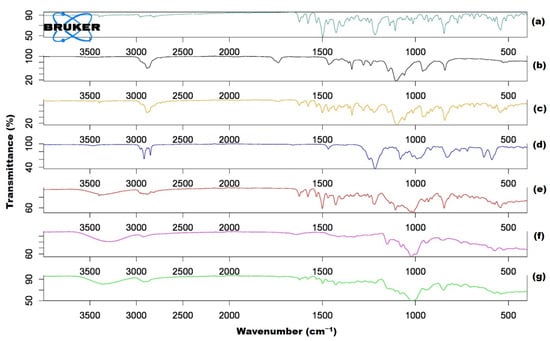
Figure 3.
The FTIR spectra of individual ingredients and solid dispersions. GEF pure (a); TPGS alone (b); F1 (c); SLS alone (d); F2 (e); HP β-CD alone (f) and F3 (g).
The binary complex (GEF with TPGS) attributed their stretching vibration to the slight variation in the peak at 3393.71 cm−1 for 2°-amine of the GEF-pure. This finding exhibited that there was no potential intermolecular interaction between GEF and TPGS in its binary complex, which was also noted in the previous studies such as the complexation of desloratadine with HP β-CD [45], quercetin with methyl-β-CD [46] and fluconazole with β-CD [47]. Halogen peaks were also observed in the binary complex with a drastic change in their stretching vibration peaks at 1462.14 cm−1 for the fluoro and 838.57 cm−1 for chloro group in comparison to the peaks of the GEF pure. The stretching vibration of the C–N moiety of the morpholine ring also exhibited drastic changes in their peaks at 1344.09 cm−1 when correlated with the pure drug. The binary complex also exhibited carrier stretching vibration peaks with a slight change at 2877.68 cm−1 for the ester moiety. It is worth mentioning that no peaks were observed for the C=N stretching vibration of the quinazoline ring, which may be assumed to be merged with the peaks of the carrier’s ester moiety. The IR spectral peak analysis confirmed the formation of a binary complex in the form of GEF SDs, which was also reported during the development of SDs of GEF with polyethylene glycol-4000, GEF with Kollidon® and polyethylene glycol-4000 as well as GEF with Soluplus® and polyethylene glycol-4000 [26]. The pattern of IR spectra for the GEF–TPGS HP β-CD inclusion complex (F3) confirmed the encaging of GEF–TPGS SDs inside the bucket-like cavity of HP β-CD, as substantiated by the host–guest complexation of desloratadine with HP β-CD [48].
The IR spectroscopy of GEF, TPGS and HP β-CD physical mixture exhibited stretching vibration peaks at 3391.26 cm−1 for 2° amine with an insignificant change as compared with GEF pure. The C=N stretching vibration peak of the quinazoline ring shifted slightly at 2819.86 cm−1. The halogen groups also exhibited variation in their stretching vibrations at 1463.22 cm−1 for fluoro and 843.47 cm−1 for chloro moieties. The C-O-C exhibited merged stretching vibrations at 1100.15 cm−1 for GEF pure and for TPGS. The HP β-CD depicted prominent C-O-C stretching vibrations at 1020.02 cm−1. The other peaks of TPGS were also observed with slight changes in their frequency at 2881.4 cm−1 for the ester and 1343.93 cm−1 for C=C aromatic moieties.
For the complexation of GEF-TPGS with HP β-CD (F3), the stretching vibrations were observed mostly for GEF pure with slightly changed frequencies. The stretching vibrations for 2° amine were shifted at 3364.57 cm−1 and for C-H at 2920.19 cm−1 as compared to GEF pure. The halogens exhibited shifting in their frequencies at 1498.80 cm−1 (for fluoro) and 849.93 cm−1 (for chloro) as compared to GEF pure. C-N stretching of the morpholine ring for F3 was found at 1387.84 cm−1. The C-O-C peaks exhibited the peak values for drugs and carriers at 1017.07 cm−1, endorsing the development of the inclusion complex as a ternary formulation. The pattern of IR spectra for the GEF–TPGS HP β-CD inclusion complex (F3) confirmed the encaging of GEF–TPGS SDs inside the bucket-like cavity of HP β-CD, as substantiated by the host–guest complexation of desloratadine with HP β-CD [48].
3.6. DSC Analysis
The overlay DSC thermograms of GEF pure, SLS alone, TPGS alone, HP β-CD alone, GEF–TPGS SDs (F1), GEF–SLS SDs (F2) and GEF–TPGS HP β-CD inclusion complex (F3) were represented in Figure 4. The DSC thermogram of GEF pure exhibited a sharp endotherm at 195.82 °C, which corresponds to the melting endotherm of GEF. The thermograms of used excipients such as SLS (104.15 °C), TPGS (36.24 °C) and HP β-CD (133.10 °C) have shown their characteristic melting peaks. The GEF–SLS has shown a small peak of endotherm at 193.01 °C very near to the melting point of GEF pure (195.82°), which was attributed to the less crystalline state of GEF in the GEF–SLS SDs form. In the case of GEF–TPGS SDs (35.23 °C), no melting peak was observed for GEF at around 195.82 °C. This suggested the complete solubilization of GEF in TPGS aqueous solution. In the case of the GEF–TPGS HP β-CD inclusion complex, the sharp crystalline peak of GEF was almost diminished and a very small intense peak at 193.87 °C was found, while a less intense broad pelting peak at 120.11 °C was found, which was due to the presence of HP β-CD.
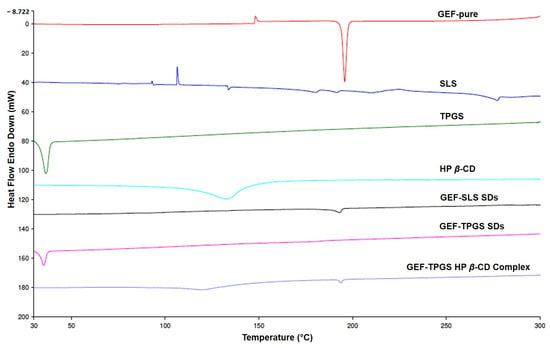
Figure 4.
The overlay DSC thermograms of GEF pure, SLS alone, TPGS alone, HP β–CD alone, GEF–TPGS SDs (F1), GEF–SLS SDs (F2) and F1 complexed with HP β–CD (F3).
The reduction in the peak intensity of GEF in the case of GEF–SLS SDs might be associated with the incomplete solubilization of GEF, which can be further substantiated by the lower yield (%) of GEF SDs when SLS was the stabilizer [26]. In contrast, when the GEF SDs were prepared with TPGS as a stabilizer, the thermogram did not show an endothermic peak of GEF at around the melting endotherm of GEF, which was attributed to the good molecular dispersion of GEF in the GEF–TPGS SDs. The broad peak of endotherm for HP β-CD near to 133 °C might be due to its thermal degradation, which was also reported during the development of the HP-β-CD inclusion complex of lipophilic drugs, such as desloratadine [45] and posaconazole [49]. Overall, the reduction in and absence of endothermic peak intensity of GEF in the SDs and HP-β-CD-complex manifested the increased solubilization of the drug due to its amorphous conversion from crystalline state. These findings suggested the feasible manifestation of an inclusion complex between the GEF–TPGS SDs and HP β-CD. Moreover, the diminished sharp melting endotherm of GEF in inclusion complex indicated the amorphous conversion of GEF (in GEF–TPGS SDs) during its complexation with HP β-CD and confirms the formation of the complex, which could be substantiated by previous reports [45,50].
3.7. Proton NMR
The chemical structure of a molecule is very helpful to interpret its proton NMR, so the structure of GEF was represented in Figure 5. The proton NMR of GEF pure (Figure 6a), GEF–TPGS SDs, i.e., F1 (Figure 6b), the physical mixture (PM) of GEF, TPGS and HP β-CD (Figure 6c) and the GEF–TPGS SDs complexed with HP β-CD i.e., F3 (Figure 6d) were evaluated to investigate the formation of the complexes. The GEF belongs to the class of quinazolines. The proton NMR values for the GEF pure exhibited at δ scale a chemical shift of 9.57 ppm, which corresponds to the deshielded proton of aromatic hydrogens. It also exhibited multiple peaks at δ 7.22 ppm to 8.51 ppm for the quinazoline ring. The peaks for halogens viz., fluoro- and chloro- were also observed at δ 4.20 and 3.59 ppm. Singlet methoxy peaks were observed at δ 3.95 ppm. The morpholine peaks were observed in the range of δ 2.4 ppm to 2.51 ppm. The singlet amine peak was observed at δ 2.10 ppm and the aliphatic methyl peak was observed at δ 2.01 ppm. Now, switching to our binary complex or SDs of GEF with TPGS (F1), the spectra exhibited the peaks of GEF-pure without any significant changes in the peaks, along with the peaks of TPGS at δ 3.52 ppm and 3.34 ppm, which exhibited methylene peaks.
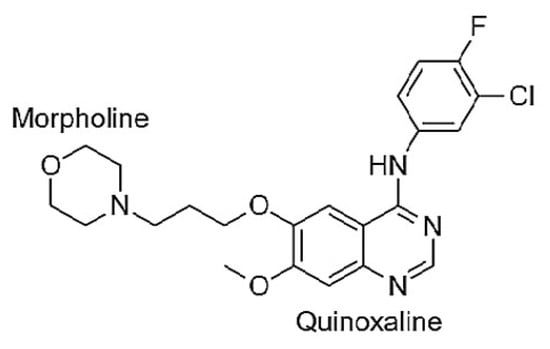
Figure 5.
Molecular structure of GEF [N-(3-Chloro-4-fluorophenyl)-7-methoxy-6-(3-morpholinopropoxy) quinazolin-4-amine].
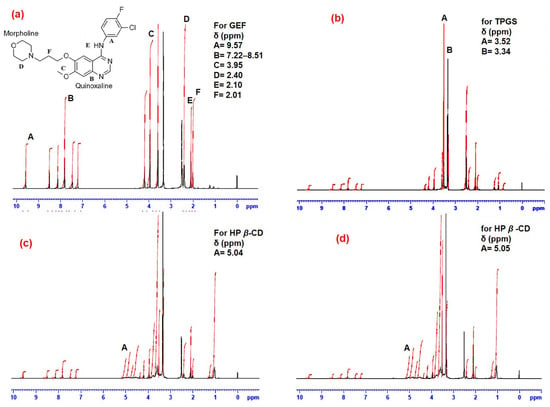
Figure 6.
The proton NMR of GEF pure (a); the binary complex or GEF–TPGS SDs as F1 (b); physical mixture (PM) of GEF, TPGS and HP β-CD (c) and the complex of GEF–TPGS SDs with HP β-CD as F3 (d).
Additionally, the proton NMR confirms the formation of a ternary complex of the physical mixture (GEF–TPGS complexed with HP β-CD (F3)) by having the peaks of GEF-pure, TPGS, with the slightly altered methylene peak at δ 3.52 ppm and 3.35 ppm. The HP β-CD peaks were observed at δ 5.04 ppm, which represents the peaks of position 1 of the glucose moiety. Finally, the formulation (F3) exhibited similar peaks for the pure drug but a slight change in the HP β-CD peaks at δ 5.05 ppm. In the formulation, other peaks were also observed in the range of δ 4.36 ppm to 4.85 ppm and 1.03 ppm to 1.25 ppm, which confirms the formation of the ternary complex. In reference to the above spectral values, it was concluded that the formation of GEF–TPGS with HP β-CD (F3) as a ternary complex occurred successfully.
3.8. Morphological Characterization by SEM
The morphological examination by scanning electron microscopy (SEM) has shown the shape of GEF-pure was altered after its conversion into solid dispersions (Figure 7). The SEM imaging were done for GEF–pure, GEF–TPGS SDs (F1), the physical mixture of GEF, TPGS and HP β–CD and GEF–TPGS complexed with HP β–CD (F3). The SEM images of pure drug appeared as thin and elongated crystal aggregates as shown in Figure 7a, while GEF–TPGS SDs (F1) only exhibited one component (Figure 7b) [51]. The SEM image of pure HP β-CD (Figure 7c) appeared as almost hollow and spherical in shape with numerous visible cavities of different sizes on the surface. A similar structural morphology of HP β-CD was also reported previously [52]. The physical mixture and the inclusion complex had a typical shape with a fluffy structure, which was morphologically different from the pure HP β-CD.

Figure 7.
SEM images of pure GEF pure (a); GEF–TPGS SDs (b); HP β-CD pure (c); physical mixture of GEF–TPGS and HP β-CD (d) as well as the inclusion complex of GEF–TPGS SDs and HP β-CD as F3 (e).
There was an obvious difference between the morphology of GEF–TPGS SDs from the GEF pure. The distinguished amorphous structure of smaller dimensions as compared to the GEF-pure has replaced the shapes and patterns of crystalline GEF. The addition of HP β–CD to GEF–TPGS SDs (i.e., F3) showed that further morphological and crystalline changes offer compelling proof that most of the drug molecules were converted to amorphous form (Figure 7d). The surfaces of GEF–TPGS SDs complexed with HP β–CD (F3) were irregular, spongy and composed of homogeneously distributed amorphous aggregates (Figure 7e). It is beneficial and interesting that the amorphous substances dissolve rapidly as compared to its crystalline counterpart because of its increased internal energy and molecular mobility [53]. The findings of the SEM imaging support the results of dissolution study. The solid dispersion prepared by solvent evaporation followed by freeze-drying of the products illustrated the highest solubility and dissolution due to the more amorphous nature of the drug.
3.9. Flow Properties
To examine the flow characteristics of the powder sample, the samples were assessed for Carr’s index, Hausner ratio and angle of repose. The prepared GEF–TPGS SDs complexed with HP β–CD (F3) had superior flow characteristics compared to F1 and F2. The values of Carr’s index and Hausner ratio for the GEF–TPGS SDs complexed with HP β–CD (F3) were found to be 9.7 and 1.1, respectively, whereas, GEF–TPGS SDs (F1) and GEF–SLS SDs (F2) showed values of 15.2 and 14.8 for Carr’s index and 1.31 and 1.37 for Hausner ratio, respectively. The GEF–TPGS SDs complexed with HP β-CD had a significantly lower value than the other two samples, which meant that they had better flow properties. The values were discovered to be within the norm [54]. The angle of repose value was found to be 22.1° for the ternary inclusion complex (F3), whereas GEF–TPGS (F1) and GEF–SLS (F2) depicted 27.2° and 29.1°, respectively. The values of micromeritics parameters of F3 were within the standard limit, thus depicting excellent flow properties.
3.10. MTT Assay and Morphological Changes
The percentages of cells that survive in relation to various concentrations of GEF formulations were represented in Figure 8. The sigmoidal curve for the MTT assay shows the IC50 values of GEF-containing formulations and the percentage viability (%) of treated A549 cells. Both the formulations (F1 and F3) illustrated remarkable reductions in the cell viability as indicated by the IC50 values (2.239, 3.135 and 4.471 µM for F3, F1 and GEF pure). The MTT assay has shown that the GEF pure, F1 and F3 had significantly reduced the population of A549 cells in dose-dependent way as represented by the sigmoid curve (Figure 8). However, no significant differences in the cell viability were noted between the pure-GEF and F1, while the F3-treated A549 cells showed significant (p < 0.05) cell deaths. The changes in the treated A549 cells were more prominent when treated with F3, which was due to the complexation of GEF–TPGS SDs with HP β-CD (F3). The caging of the GEF SDs into HP β-CD further enhanced the phase solubility of GEF, which in turn has shown improved cytotoxic potential regarding GEF [55].
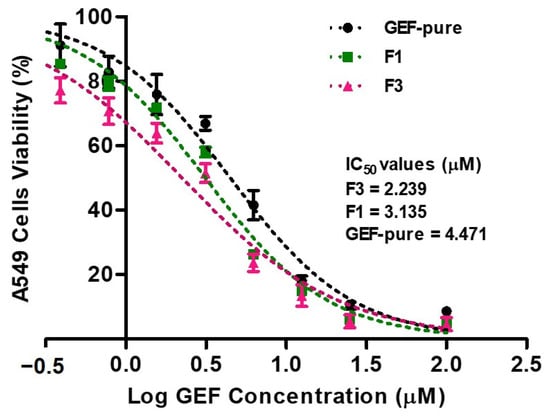
Figure 8.
The MTT assay showed that GEF pure and GEF SDs (F1 and F3) significantly decreased the living population of A549 cells in dose dependent mode by assuming 100% of the cells were viable in the untreated (control) group. However, no significant differences in viability were noted between the pure-GEF and F1, while F3-treated A549 cells showed significant (p < 0.05) cell deaths. The coefficient of correlation (R2) values for GEF pure, F1 and F3 were 0.9823, 0.9825 and 0.9692, respectively. The data were reported as the mean and standard deviation of three measurements (mean ± SD, n = 3).
The encaging of the GEF into HP β-CD with TPGS further enhanced the solubility of the drug, which in turn has shown improved cytotoxic potential of GEF [55]. In addition to the MTT assay, the morphological changes in the cells (observed under microscopy) also support the cytotoxic potential of the developed formulations against the treated cells. The microscopic observations (Figure 9) endorsed the cytotoxic properties of GEF and F1 and F3. The morphological changes in the A549 cells were found after their treatment with GEF formulations. The IC50 value of 3.135 µM of GEF for A549 cells was chosen as the treatment dose for this experiment. The altered morphological topographies, such as blebbing of the membrane (blister-like), the shrinkage of the cells, cellular granularity, collapsing or condensation of nucleus and cellular volume, disintegration and break-up as well as morphological changes in cell surfaces, were considered as the standard apoptotic indicators [31,32,56].
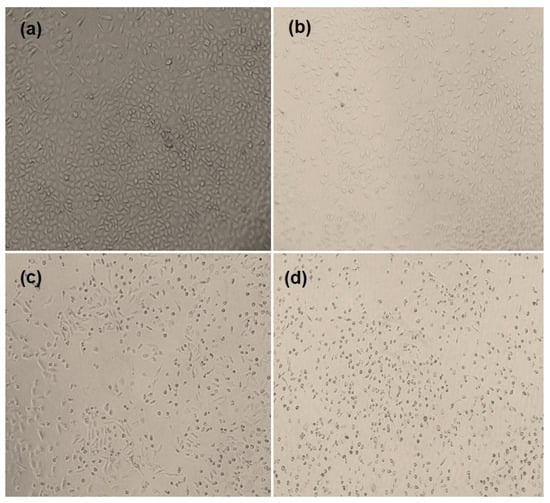
Figure 9.
The phase contrast images of morphological changes in A549 cells observed under phase contrast microscope after exposing them to different GEF formulations. Control or untreated cells (a); GEF-pure-treated cells (b); F1-treated cells (c) and F3-treated cells (d).
The apoptosis and appearance of dead cell debris indicated a marked cytotoxic potential of GEF on A549 cells, where the treated cells have shown maximum morphological changes, such as blister-like blebbing of the membrane, shrinkage of the cells and condensation of the nucleus with F3 relative to F1 as compared to GEF pure at a particular drug concentration (F3 > F1 > GEF pure). The obvious changes in the morphology of the A549 cells treated with F3 followed by F1 might be associated with high solubility of the lipophilic drug (GEF) in the used media due to conversion of pure GEF to solid dispersions. The distinct morphological changes in the cells treated by F3 followed by F1 were due to the high phase solubility of GEF in solid dispersion states, which might increase the accumulation of GEF into the nucleus of the treated cells.
3.11. p53 and Caspases Activity
The process of apoptosis is primarily accomplished by the activation of caspases (caspase-3 and caspase-9) and the expression of p53 protein, which specifically take down a number of intracellular components into pieces without harming or inflaming the neighboring normal cells [35,57]. The p53 is a well-known tumor suppressor protein that demonstrates a crucial role in cell cycle regulation and DNA repair. When DNA damage occurs, p53 is activated and induces either cell cycle arrest or apoptosis depending on the severity of the damage. Caspase-3 and caspase-9 are also the key players in the apoptotic pathway, which is initiated by various signals, including oxidative stress, DNA damage or cytokine withdrawal. Once activated, these caspases cleave specific cellular components and cause the typical morphological changes in the apoptotic cells. Thus, aiming the p53 protein and caspases (caspase-3 and caspase-9) was explored as a potential therapeutic approach for the treatment of cancers.
In the current study, GEF pure, F1 and F3 have shown significantly (p < 0.05) higher levels of p53 expression than the untreated cells (control), as represented in Figure 10a. This demonstrated that F3 > F1 > GEF pure effectively induced apoptosis in A549 cells, as evidenced by the fact that p53 expression was 5.9-, 4.6- and 3.04-fold higher in the treated groups with F3, F1 and GEF pure, respectively, than that of control group. The results of the current investigation were consistent with the earlier study, which demonstrated that p53 increased GEF-mediated growth inhibition and apoptosis in NSCLC [35,57].
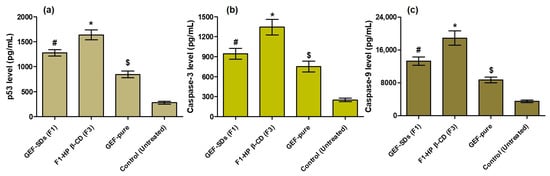
Figure 10.
Effects of F3, F1 and GEF pure on the overexpression of p53 level (a); caspase-3 level (b) and caspase-9 level (c) levels in A549 cells. The concentration of GEF for all these experiments was kept at 3.125 µM. The data were the mean and standard deviations of three measurements (mean ± SD, n = 3). The * p < 0.05 indicates the significantly improved activities of F3 on A549 cells as compared to F1, GEF pure and untreated cells (control). The # p < 0.05 signifies the enhanced activities of F1 on A549 cells as compared to GEF pure and untreated cells (control). The $ p < 0.05 suggests the increased activities of GEF pure on A549 cells as compared to untreated cells (control).
Additionally, F3-, F1- and GEF-pure-treated A549 cells had significantly higher expressions of caspase-3 (Figure 10b) and caspase-9 (Figure 10c) than the untreated cells (control), which serves as another piece of evidence of the powerful anti-apoptotic effects of GEF formulations. This was also observed in macrophages during GEF-induced apoptosis, which was mediated by caspase-3 [58]. The caspase-3 expression increased around 5.38-, 3.78- and 3.01-fold in comparison to untreated (control) cells, whereas the caspase-9 expression increased approximately 5.35-, 3.76- and 2.47-fold in F3-, F1- and GEF-pure-treated cells. These results can be supported by a prior demonstration in which si-MIR31HG lncRNAs were transfected into PC9-R cells, leading to noticeably higher levels of caspase-3 and caspase-9 [59].
The higher level of caspase-3, caspase-9 and p53 expressions in the GEF SDs-treated cells compared to the GEF-pure-treated cells is due to the high aqueous solubility of SDs, which could result in increased drug concentration surrounding the treated cells. The higher drug concentration in F3 might enhance the anticancer activity against the NSCLC (A549) because of reduced p-glycoprotein (Pgp) efflux activity of TPGS [60]. This hypothesis can be supported by a previous report where HP-sulfobutyl β-CD improved the edaravone oral bioavailability by modifying the drug-efflux pump of enterocytes, where the Pgp-efflux activity of HP-sulfobutyl β-CD was realized through meddling with the lipid bundle and depletion of cholesterol of the enterocytes membrane [61], while the enhanced anticancer activity of F1 was attributed to the increased endocytosis of the highly soluble GEF SDs by A549 cells [62]. Moreover, the presence of HP β-CD in the case of F3 exerted a synergistic effect to GEF as HP β-CD itself has promising anticancer effects [63]. The HP β-CD acts by reducing the intracellular cholesterol, which leads to cell cycle arrest at the G2/M phase and apoptosis, where the intracellular DNA damage remains hard to fix [63,64]. In conclusion, GEF SDs and their HP β-CD inclusion complex have improved efficacy, which may be the reason why they were successfully apoptotic to the A549 cells.
4. Conclusions
In the present research, the solid dispersions (SDs) of GEF were prepared with SLS and TPGS by co-precipitation and solvent evaporation, followed by a freeze-drying technique. The SDs developed using TPGS (as a surfactant) and complexed with HP β-CD (F3) have shown remarkable improvement in the saturation solubility and dissolution efficiency of GEF than that of F1 (GEF–TPGS SDs), F2 (GEF–SLS SDs) and GEF pure. There were no GEF surfactants or carrier interaction in the prepared SDs, as confirmed by the FTIR and NMR spectral analysis. The results of micromeritics depicted ideal flow properties for the developed formulations (F3 > F1 > F2). The results of DSC and SEM studies demonstrated that GEF was successfully converted into amorphous state, which could improve the performance of the drugs in the developed formulations. The MTT assay against A549 cells illustrated remarkable reduction in the cell viability in a dose-dependent manner, as indicated by the IC50 values (2.239, 3.135 and 4.471 µM for F3, F1 and GEF pure). The apoptosis was further supported by determining the p53, caspase-3 and caspase activities. The expressions of p53 were approximately 5.9-, 4.6- and 3.04-fold higher; for caspase-3, they were 5.38-, 3.78- and 3.01-fold higher and, for caspase-9, they were 5.35-, 3.76- and 2.47-fold higher in the case of F3-, F1- and GEF-pure-treated cells, respectively, as compared to untreated A549 cells. Conclusively, the developed TPGS SDs and their complexation with HP β-CD have improved physical characteristics, solubility, dissolution efficiency with short mean dissolution time and efficacy with successful apoptotic activity against A549 cells. The developed formulations can be a suitable alternative to the conventional formulations of GEF in treating non-small-cell lung cancers. Further, determining the in vivo efficacy of the developed SDs is warranted to check their actual pharmacokinetics and pharmacodynamics in animal models.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app131910859/s1. Figure S1. Dissolution test and release profile of GEF from different GEF-SDs in 0.1 N HCl as release medium (pH 1.2). The data were the mean and standard deviation (Mean ± SD) of three measurements. Where (a) representing the drug release profile from SDs prepared with TPGS (Group-F1), (b) the drug release profile from SDs prepared with SLS (Group-2F) and (c) the drug release profile from GEF-SD complexed with HP β-CD (Group-F3). Table S1. The formulation of GEF-SDs with varying amounts of surfactants for optimization purpose.
Author Contributions
Conceptualization, M.A.K. and A.K.A.; Methodology, M.A.K., A.A.A., S.S.A., M.S.K. and A.K.A.; Software, S.S.A.; Validation, A.A.A., S.S.A., M.S.K. and A.K.A.; Formal analysis, M.A.K., A.A.A., S.S.A. and A.K.A.; Investigation, A.A.A., S.S.A., M.S.K. and A.K.A.; Resources, A.K.A. and M.A.; Data curation, M.A.K. and M.S.K.; Writing—original draft, A.A.A. and M.S.K.; Writing—review & editing, M.A.K. and M.A.; Supervision, M.A.K. and M.A.; Project administration, M.A.K. and M.A.; Funding acquisition, M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Researchers Supporting Project number (RSPD2023R726), King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not required.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors extend their appreciation to the Researchers Supporting Project number (RSPD2023R726), King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gontijo, S.M.; Guimarães, P.P.; Viana, C.T.; Denadai, Â.M.; Gomes, A.D.; Campos, P.P.; Andrade, S.P.; Sinisterra, R.D.; Cortés, M.E. Erlotinib/hydroxypropyl-β-cyclodextrin inclusion complex: Characterization and in vitro and in vivo evaluation. J. Incl. Phenom. Macrocycl. Chem. 2015, 83, 267–279. [Google Scholar] [CrossRef]
- Mahller, Y.Y.; Vaikunth, S.S.; Currier, M.A.; Miller, S.J.; Ripberger, M.C.; Hsu, Y.-H.; Mehrian-Shai, R.; Collins, M.H.; Crombleholme, T.M.; Ratner, N.; et al. Oncolytic HSV and erlotinib inhibit tumor growth and angiogenesis in a novel malignant peripheral nerve sheath tumor xenograft model. Mol. Ther. 2007, 15, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Chellappan, D.K.; Tambuwala, M.M.; Bakshi, H.A.; Dua, K.; Dureja, H. Central composite designed formulation, characterization and in vitro cytotoxic effect of erlotinib loaded chitosan nanoparticulate system. Int. J. Biol. Macromol. 2019, 141, 596–610. [Google Scholar] [CrossRef]
- Reddy, B.P.; Reddy, K.R.; Reddy, R.R.; Reddy, D.M.; Rao, T.S. Hydrated Form of Erlotinib Free Base and a Process for Preparation of Erlotnb Hydrochloride Polymorph Forma Substantially Free of Polymorph Formb. U.S. Patent No. 8,669,265, 11 March 2014. [Google Scholar]
- Phillip Lee, Y.H.; Sathigari, S.; Jean Lin, Y.J.; Ravis, W.R.; Chadha, G.; Parsons, D.L.; Rangari, V.K.; Wright, N.; Babu, R.J. Gefitinib-cyclodextrin inclusion complexes: Physico-chemical characterization and dissolution studies. Drug Dev. Ind. Pharm. 2009, 35, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.J.; Kim, Y.; Jeon, J.Y.; Park, S.J.; Kwak, Y.G.; Kim, M.G. Pharmacokinetic properties and bioequivalence of gefitinib 250 mg in healthy Korean male subjects. Transl. Clin. Pharmacol. 2021, 29, 171–179. [Google Scholar] [CrossRef]
- Ciardiello, F.; Caputo, R.; Bianco, R.; Damiano, V.; Pomatico, G.; De Placido, S.; Bianco, A.R.; Tortora, G. Antitumor effect and potentiation of cytotoxic drugs activity in human cancer cells by ZD-1839 (Iressa), an epidermal growth factor receptor-selective tyrosine kinase inhibitor. Clin. Cancer Res. 2000, 6, 2053–2063. [Google Scholar]
- McKillop, D.; Hutchison, M.; Partridge, E.; Bushby, N.; Cooper, C.; Clarkson-Jones, J.; Herron, W.; Swaisland, H. Metabolic disposition of gefitinib, an epidermal growth factor receptor tyrosine kinase inhibitor, in rat, dog and man. Xenobiotica 2004, 34, 917–934. [Google Scholar] [CrossRef]
- Fan, L.; Hu, L.; Yang, B.; Fang, X.; Gao, Z.; Li, W.; Sun, Y.; Shen, Y.; Wu, X.; Shu, Y.; et al. Erlotinib promotes endoplasmic reticulum stress-mediated injury in the intestinal epithelium. Toxicol. Appl. Pharmacol. 2014, 278, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Gidwani, B.; Vyas, A.; Kaur, C.D. Investigation of inclusion behaviour of gefitinib with epichlorohydrin-β-cyclodextrin polymer: Preparation of binary complex, stoichiometric determination and characterization. J. Pharm. Biomed. Anal. 2018, 160, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.; Pyo, Y.C.; Kim, D.H.; Lee, S.E.; Kim, J.K.; Park, J.S. Overview of the Manufacturing Methods of Solid Dispersion Technology for Improving the Solubility of Poorly Water-Soluble Drugs and Application to Anticancer Drugs. Pharmaceutics 2019, 11, 132. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.M.; Grandis, J.R. Pharmacokinetic and pharmacodynamic properties of EGFR inhibitors under clinical investigation. Cancer Treat. Rev. 2004, 30, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Devasari, N.; Dora, C.P.; Singh, C.; Paidi, S.R.; Kumar, V.; Sobhia, M.E.; Suresh, S. Inclusion complex of erlotinib with sulfobutyl ether-beta-cyclodextrin: Preparation, characterization, in silico, in vitro and in vivo evaluation. Carbohydr. Polym. 2015, 134, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Altamimi, M.A.; Elzayat, E.M.; Alhowyan, A.A.; Alshehri, S.; Shakeel, F. Effect of β-cyclodextrin and different surfactants on solubility, stability, and permeability of hydrochlorothiazide. J. Mol. Liq. 2018, 250, 323–328. [Google Scholar] [CrossRef]
- Crison, J.R.; Weiner, N.D.; Amidon, G.L. Dissolution media for in vitro testing of water-insoluble drugs: Effect of surfactant purity and electrolyte on in vitro dissolution of carbamazepine in aqueous solutions of sodium lauryl sulfate. J. Pharm. Sci. 1997, 86, 384–388. [Google Scholar] [CrossRef]
- Yan, A.; Von Dem Bussche, A.; Kane, A.B.; Hurt, R.H. Tocopheryl Polyethylene Glycol Succinate as a Safe, Antioxidant Surfactant for Processing Carbon Nanotubes and Fullerenes. Carbon. N. Y. 2007, 45, 2463–2470. [Google Scholar] [CrossRef] [PubMed]
- Constantinides, P.P.; Han, J.; Davis, S.S. Advances in the use of tocols as drug delivery vehicles. Pharm. Res. 2006, 23, 243–255. [Google Scholar] [CrossRef]
- Loftsson, T.; Jarho, P.; Masson, M.; Järvinen, T. Cyclodextrins in drug delivery. Expert Opin. Drug Deliv. 2005, 2, 335–351. [Google Scholar] [CrossRef]
- Kumar, P.; Mangla, B.; Beg, S.; Afzal, O.; Altamimi, A.S.A.; Almalki, W.H.; Ullah, S.N.M.N.; Aggarwal, G. Optimization and validation of stability indicating RP-HPLC method for the quantification of gefitinib in bulk drug and nanoformulations: An application towards in vitro and ex vivo performance evaluation. Arab. J. Chem. 2022, 15, 104333. [Google Scholar] [CrossRef]
- Douroumis, D.; Bouropoulos, N.; Fahr, A. Physicochemical characterization of solid dispersions of three antiepileptic drugs prepared by solvent evaporation method. J. Pharm. Pharmacol. 2007, 59, 645–653. [Google Scholar] [CrossRef]
- Fitriani, L.; Haqi, A.; Zaini, E. Preparation and characterization of solid dispersion freeze-dried efavirenz-polyvinylpyrrolidone K-30. J. Adv. Pharm. Technol. Res. 2016, 7, 105–109. [Google Scholar] [CrossRef]
- Cheirsilp, B.; Rakmai, J. Inclusion complex formation of cyclodextrin with its guest and their applications. Biol. Eng. Med. 2016, 2, 1–6. [Google Scholar] [CrossRef]
- Suarez, D.F.; Consuegra, J.; Trajano, V.C.; Gontijo, S.M.; Guimaraes, P.P.; Cortes, M.E.; Denadai, A.L.; Sinisterra, R.D. Structural and thermodynamic characterization of doxycycline/beta-cyclodextrin supramolecular complex and its bacterial membrane interactions. Colloids Surf. B Biointerfaces 2014, 118, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Kalam, M.A.; Alshamsan, A. Poly (d, l-lactide-co-glycolide) nanoparticles for sustained release of tacrolimus in rabbit eyes. Biomed. Pharmacother. 2017, 94, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, W.W.; Fletcher, J.; Khoder, M.; Alany, R.G. Solid Dispersions of Gefitinib Prepared by Spray Drying with Improved Mucoadhesive and Drug Dissolution Properties. AAPS PharmSciTech 2022, 23, 48. [Google Scholar] [CrossRef]
- Alshehri, S.; Alanazi, A.; Elzayat, E.M.; Altamimi, M.A.; Imam, S.S.; Hussain, A.; Alqahtani, F.; Shakeel, F. Formulation, In Vitro and In Vivo Evaluation of Gefitinib Solid Dispersions Prepared Using Different Techniques. Processes 2021, 9, 1210. [Google Scholar] [CrossRef]
- Shah, R.B.; Tawakkul, M.A.; Khan, M.A. Comparative evaluation of flow for pharmaceutical powders and granules. AAPS PharmSciTech 2008, 9, 250–258. [Google Scholar] [CrossRef]
- Clayton, J. Chapter 17—An Introduction to Powder Characterization. In Handbook of Pharmaceutical Wet Granulation; Narang, A.S., Badawy, S.I.F., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 569–613. [Google Scholar]
- Engelman, J.A.; Jänne, P.A.; Mermel, C.; Pearlberg, J.; Mukohara, T.; Fleet, C.; Cichowski, K.; Johnson, B.E.; Cantley, L.C. ErbB-3 mediates phosphoinositide 3-kinase activity in gefitinib-sensitive non-small cell lung cancer cell lines. Proc. Natl. Acad. Sci. USA 2005, 102, 3788–3793. [Google Scholar] [CrossRef]
- Mukohara, T.; Engelman, J.A.; Hanna, N.H.; Yeap, B.Y.; Kobayashi, S.; Lindeman, N.; Halmos, B.; Pearlberg, J.; Tsuchihashi, Z.; Cantley, L.C.; et al. Differential Effects of Gefitinib and Cetuximab on Non–small-cell Lung Cancers Bearing Epidermal Growth Factor Receptor Mutations. JNCI J. Natl. Cancer Inst. 2005, 97, 1185–1194. [Google Scholar] [CrossRef] [PubMed]
- Alshetaili, A.S. Gefitinib loaded PLGA and chitosan coated PLGA nanoparticles with magnified cytotoxicity against A549 lung cancer cell lines. Saudi J. Biol. Sci. 2021, 28, 5065–5073. [Google Scholar] [CrossRef]
- Alshememry, A.; Kalam, M.A.; Almoghrabi, A.; Alzahrani, A.; Shahid, M.; Khan, A.A.; Haque, A.; Ali, R.; Alkholief, M.; Binkhathlan, Z. Chitosan-coated poly (lactic-co-glycolide) nanoparticles for dual delivery of doxorubicin and naringin against MCF-7 cells. J. Drug Deliv. Sci. Technol. 2022, 68, 103036. [Google Scholar] [CrossRef]
- Zhao, Z.Q.; Yu, Z.Y.; Li, J.; Ouyang, X.N. Gefitinib induces lung cancer cell autophagy and apoptosis via blockade of the PI3K/AKT/mTOR pathway. Oncol. Lett. 2016, 12, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Alali, A.S.; Kalam, M.A.; Ahmed, M.M.; Aboudzadeh, M.A.; Alhudaithi, S.S.; Anwer, M.K.; Fatima, F.; Iqbal, M. Nanocrystallization Improves the Solubilization and Cytotoxic Effect of a Poly (ADP-Ribose)-Polymerase-I Inhibitor. Polymers 2022, 14, 4827. [Google Scholar] [CrossRef] [PubMed]
- Rho, J.K.; Choi, Y.J.; Ryoo, B.-Y.; Na, I.I.; Yang, S.H.; Kim, C.H.; Lee, J.C. p53 enhances Gefitinib-induced growth inhibition and apoptosis by regulation of Fas in non–small cell lung cancer. Cancer Res. 2007, 67, 1163–1169. [Google Scholar] [CrossRef]
- Yen, C.-W.; Kuhn, R.; Hu, C.; Zhang, W.; Chiang, P.-C.; Chen, J.Z.; Hau, J.; Estevez, A.; Nagapudi, K.; Leung, D.H. Impact of surfactant selection and incorporation on in situ nanoparticle formation from amorphous solid dispersions. Int. J. Pharm. 2021, 607, 120980. [Google Scholar] [CrossRef] [PubMed]
- Ke, W.-T.; Lin, S.-Y.; Ho, H.-O.; Sheu, M.-T. Physical characterizations of microemulsion systems using tocopheryl polyethylene glycol 1000 succinate (TPGS) as a surfactant for the oral delivery of protein drugs. J. Control. Release 2005, 102, 489–507. [Google Scholar] [CrossRef]
- Zhang, Z.; Tan, S.; Feng, S.S. Vitamin E TPGS as a molecular biomaterial for drug delivery. Biomaterials 2012, 33, 4889–4906. [Google Scholar] [CrossRef]
- Sulek, M.W.; Wasilewski, T.; Kurzydłowski, K.J. The effect of concentration on lubricating properties of aqueous solutions of sodium lauryl sulfate and ethoxylated sodium lauryl sulfate. Tribol. Lett. 2010, 40, 337–345. [Google Scholar] [CrossRef]
- Sherif, A.Y.; Harisa, G.I.; Alanazi, F.K.; Nasr, F.A.; Alqahtani, A.S. PEGylated SLN as a promising approach for lymphatic delivery of gefitinib to lung cancer. Int. J. Nanomed. 2022, 17, 3287–3311. [Google Scholar] [CrossRef]
- Lin, B.; Chen, H.; Liang, D.; Lin, W.; Qi, X.; Liu, H.; Deng, X. Acidic pH and High-H(2)O(2) Dual Tumor Microenvironment-Responsive Nanocatalytic Graphene Oxide for Cancer Selective Therapy and Recognition. ACS Appl. Mater. Interfaces 2019, 11, 11157–11166. [Google Scholar] [CrossRef]
- Godugu, C.; Doddapaneni, R.; Patel, A.R.; Singh, R.; Mercer, R.; Singh, M. Novel Gefitinib Formulation with Improved Oral Bioavailability in Treatment of A431 Skin Carcinoma. Pharm. Res. 2016, 33, 137–154. [Google Scholar] [CrossRef]
- Mennini, N.; Maestrelli, F.; Cirri, M.; Mura, P. Analysis of physicochemical properties of ternary systems of oxaprozin with randomly methylated-ss-cyclodextrin and l-arginine aimed to improve the drug solubility. J. Pharm. Biomed. Anal. 2016, 129, 350–358. [Google Scholar] [CrossRef]
- Parmar, K.R.; Patel, K.A.; Shah, S.R.; Sheth, N.R. Inclusion complexes of lamotrigine and hydroxy propyl β-cyclodextrin: Solid state characterization and dissolution studies. J. Incl. Phenom. Macrocycl. Chem. 2009, 65, 263–268. [Google Scholar] [CrossRef]
- Yurtdas Kirimlioglu, G. Host-guest inclusion complex of desloratadine with 2-(hydroxy) propyl-beta-cyclodextrin (HP-beta-CD): Preparation, binding behaviors and dissolution properties. J. Res. Pharm. 2020, 24, 693–707. [Google Scholar]
- Güleç, K.; Demirel, M. Characterization and antioxidant activity of quercetin/methyl-β-cyclodextrin complexes. Curr. Drug Deliv. 2016, 13, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Yurtdaş, G.; Demirel, M.; Genç, L. Inclusion complexes of fluconazole with β-cyclodextrin: Physicochemical characterization and in vitro evaluation of its formulation. J. Incl. Phenom. Macrocycl. Chem. 2011, 70, 429–435. [Google Scholar] [CrossRef]
- Demirel, M.; Yurtdaş, G.; Genç, L. Inclusion complexes of ketoconazole with beta-cyclodextrin: Physicochemical characterization and in vitro dissolution behaviour of its vaginal suppositories. J. Incl. Phenom. Macrocycl. Chem. 2011, 70, 437–445. [Google Scholar] [CrossRef]
- Tang, P.; Ma, X.; Wu, D.; Li, S.; Xu, K.; Tang, B.; Li, H. Posaconazole/hydroxypropyl-β-cyclodextrin host–guest system: Improving dissolution while maintaining antifungal activity. Carbohydr. Polym. 2016, 142, 16–23. [Google Scholar] [CrossRef]
- Ol’khovich, M.V.; Sharapova, A.V.; Perlovich, G.L.; Skachilova, S.Y.; Zheltukhin, N.K. Inclusion complex of antiasthmatic compound with 2-hydroxypropyl-β-cyclodextrin: Preparation and physicochemical properties. J. Mol. Liq. 2017, 237, 185–192. [Google Scholar] [CrossRef]
- Maragos, S.; Archontaki, H.; Macheras, P.; Valsami, G. Effect of cyclodextrin complexation on the aqueous solubility and solubility/dose ratio of praziquantel. AAPS PharmSciTech 2009, 10, 1444–1451. [Google Scholar] [CrossRef]
- Michalska, P.; Wojnicz, A.; Ruiz-Nuño, A.; Abril, S.; Buendia, I.; León, R. Inclusion complex of ITH12674 with 2-hydroxypropyl-β-cyclodextrin: Preparation, physical characterization and pharmacological effect. Carbohydr. Polym. 2017, 157, 94–104. [Google Scholar] [CrossRef]
- Bera, H.; Chekuri, S.; Sarkar, S.; Kumar, S.; Muvva, N.B.; Mothe, S.; Nadimpalli, J. Novel pimozide-β-cyclodextrin-polyvinylpyrrolidone inclusion complexes for Tourette syndrome treatment. J. Mol. Liq. 2016, 215, 135–143. [Google Scholar] [CrossRef]
- Lebrun, P.; Krier, F.; Mantanus, J.; Grohganz, H.; Yang, M.; Rozet, E.; Boulanger, B.; Evrard, B.; Rantanen, J.; Hubert, P. Design space approach in the optimization of the spray-drying process. Eur. J. Pharm. Biopharm. 2012, 80, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, J.; Hu, H.; Xu, S.; Xu, L.; Chen, E. Gefitinib encapsulation based on nano-liposomes for enhancing the curative effect of lung cancer. Cell Cycle 2020, 19, 3581–3594. [Google Scholar] [CrossRef] [PubMed]
- Alanazi, A.M.; Khan, A.A.; Rehman, M.T.; Jabeen, M.; Algrain, N.; Baig, M.H. Biophysical interactions, docking studies and cytotoxic potential of a novel propofol-linolenate: A multi-technique approach. J. Biomol. Struct. Dyn. 2020, 38, 2389–2401. [Google Scholar] [CrossRef]
- Jung, S.; Kim, D.H.; Choi, Y.J.; Kim, S.Y.; Park, H.; Lee, H.; Choi, C.-M.; Sung, Y.H.; Lee, J.C.; Rho, J.K. Contribution of p53 in sensitivity to EGFR tyrosine kinase inhibitors in non-small cell lung cancer. Sci. Rep. 2021, 11, 19667. [Google Scholar] [CrossRef]
- Noguchi, T.; Sekiguchi, Y.; Kudoh, Y.; Naganuma, R.; Kagi, T.; Nishidate, A.; Maeda, K.; Ishii, C.; Toyama, T.; Hirata, Y.; et al. Gefitinib initiates sterile inflammation by promoting IL-1beta and HMGB1 release via two distinct mechanisms. Cell Death Dis. 2021, 12, 49. [Google Scholar] [CrossRef]
- Wang, B.; Jiang, H.; Wang, L.; Chen, X.; Wu, K.; Zhang, S.; Ma, S.; Xia, B. Increased MIR31HG lncRNA expression increases gefitinib resistance in non-small cell lung cancer cell lines through the EGFR/PI3K/AKT signaling pathway. Oncol. Lett. 2017, 13, 3494–3500. [Google Scholar] [CrossRef]
- Arima, H.; Yunomae, K.; Hirayama, F.; Uekama, K. Contribution of P-glycoprotein to the enhancing effects of dimethyl-beta-cyclodextrin on oral bioavailability of tacrolimus. J. Pharmacol. Exp. Ther. 2001, 297, 547–555. [Google Scholar] [PubMed]
- Rong, W.T.; Lu, Y.P.; Tao, Q.; Guo, M.; Lu, Y.; Ren, Y.; Yu, S.Q. Hydroxypropyl-sulfobutyl-beta-cyclodextrin improves the oral bioavailability of edaravone by modulating drug efflux pump of enterocytes. J. Pharm. Sci. 2014, 103, 730–742. [Google Scholar] [CrossRef]
- Stearns, R.C.; Paulauskis, J.D.; Godleski, J.J. Endocytosis of ultrafine particles by A549 cells. Am. J. Respir. Cell Mol. Biol. 2001, 24, 108–115. [Google Scholar] [CrossRef]
- Yokoo, M.; Kubota, Y.; Motoyama, K.; Higashi, T.; Taniyoshi, M.; Tokumaru, H.; Nishiyama, R.; Tabe, Y.; Mochinaga, S.; Sato, A.; et al. 2-Hydroxypropyl-beta-Cyclodextrin Acts as a Novel Anticancer Agent. PLoS ONE 2015, 10, e0141946. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Zhang, X.; Yu, P.; Chen, X.; Lu, P.; Li, M.; Liu, X.; Li, Z.; Wei, F.; Wang, K.; et al. Cinobufagin Induces Cell Cycle Arrest at the G2/M Phase and Promotes Apoptosis in Malignant Melanoma Cells. Front. Oncol. 2019, 9, 853. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).