An Observational Study on Changes in the Oral and Gut Microbiota through Professional Mechanical Tooth Cleaning, including Tooth-Brushing Instructions in Patients with Multi-Bracket Appliances
Abstract
1. Introduction
2. Materials and Methods
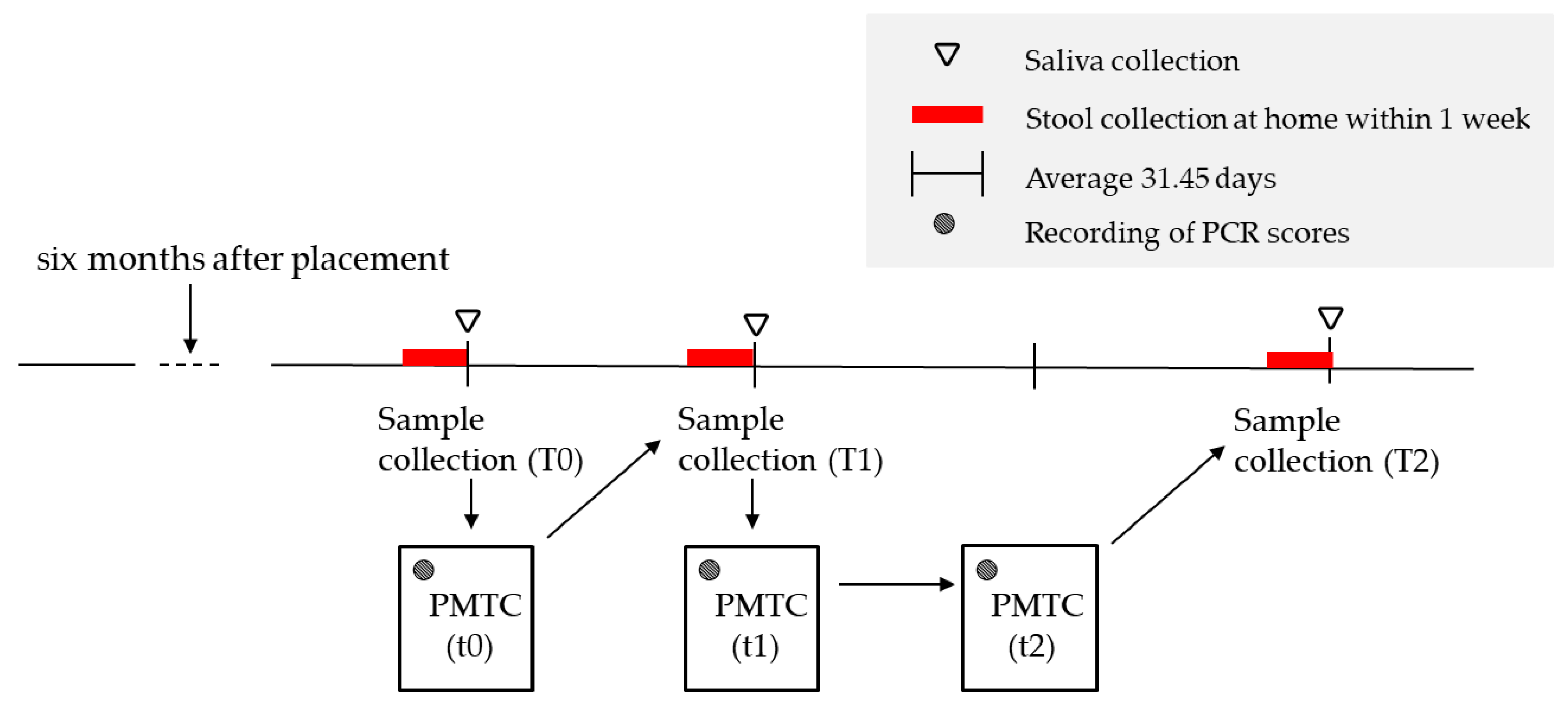
2.1. Study Design and Settings
2.1.1. Ethical Statement
2.1.2. Participants
2.1.3. Sample Collection and Storage
2.1.4. PMTC
2.1.5. PMTC Flow
- Stain teeth with wires in place to visualize any plaque or debris (2 TONE; Kulzer Denta Ltd., Hanau, Germany).
- Rinse their mouth with water three times.
- Measure O’Leary Plaque Control Record (PCR) [24] using the 6-point method.
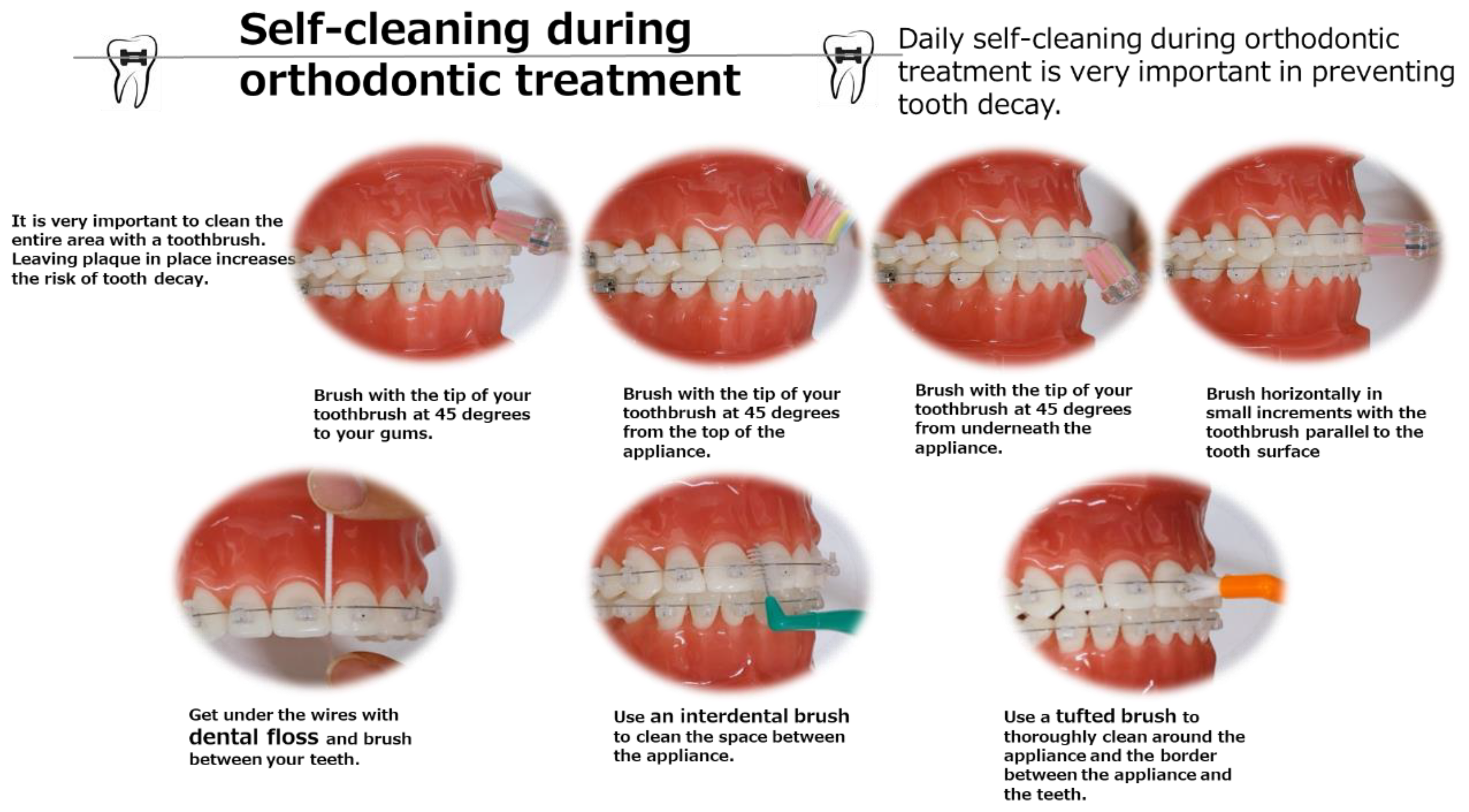
- Perform oral cleaning instructions (TBI) using jaw models (TOMY INTERNATIONAL Inc., Tokyo, Japan), toothbrushes, tufted brushes, and interdental brushes.
- Remove upper and lower wires.
- Attach a scaler brush (Mini Taper; Ci Medical Co., Ltd., Ishikawa, Japan) to the tip of the air scaler (GC Co., Ltd., Tokyo, Japan) to clean periodontal pockets.
- Remove tartar using an ultrasonic scaler (Solfy; Morita Co., Ltd., Tokyo, Japan).
- Attach dental prophy brush (Ci Polishing Brush Flat Soft; Ci Medical Co., Ltd., Ishikawa, Japan) to the contra handpiece (GC Co., Ltd., Tokyo, Japan) and use dental paste (NEW PROCLEAR MEDIUM FINE; Ci Medical Co., Ltd., Ishikawa, Japan) to remove plaque and stain from the tooth surfaces.
- Clean interdental spaces with floss.
- Patients were provided instructions to follow the oral hygiene procedure at home.
2.2. Microbial DNA Extraction
2.3. Microbial Community Analysis
2.4. Biodiversity and Statistical Analysis
2.5. Statistical Analysis
3. Results
3.1. Oral Microbiota
3.1.1. Bacterial Composition Ratio
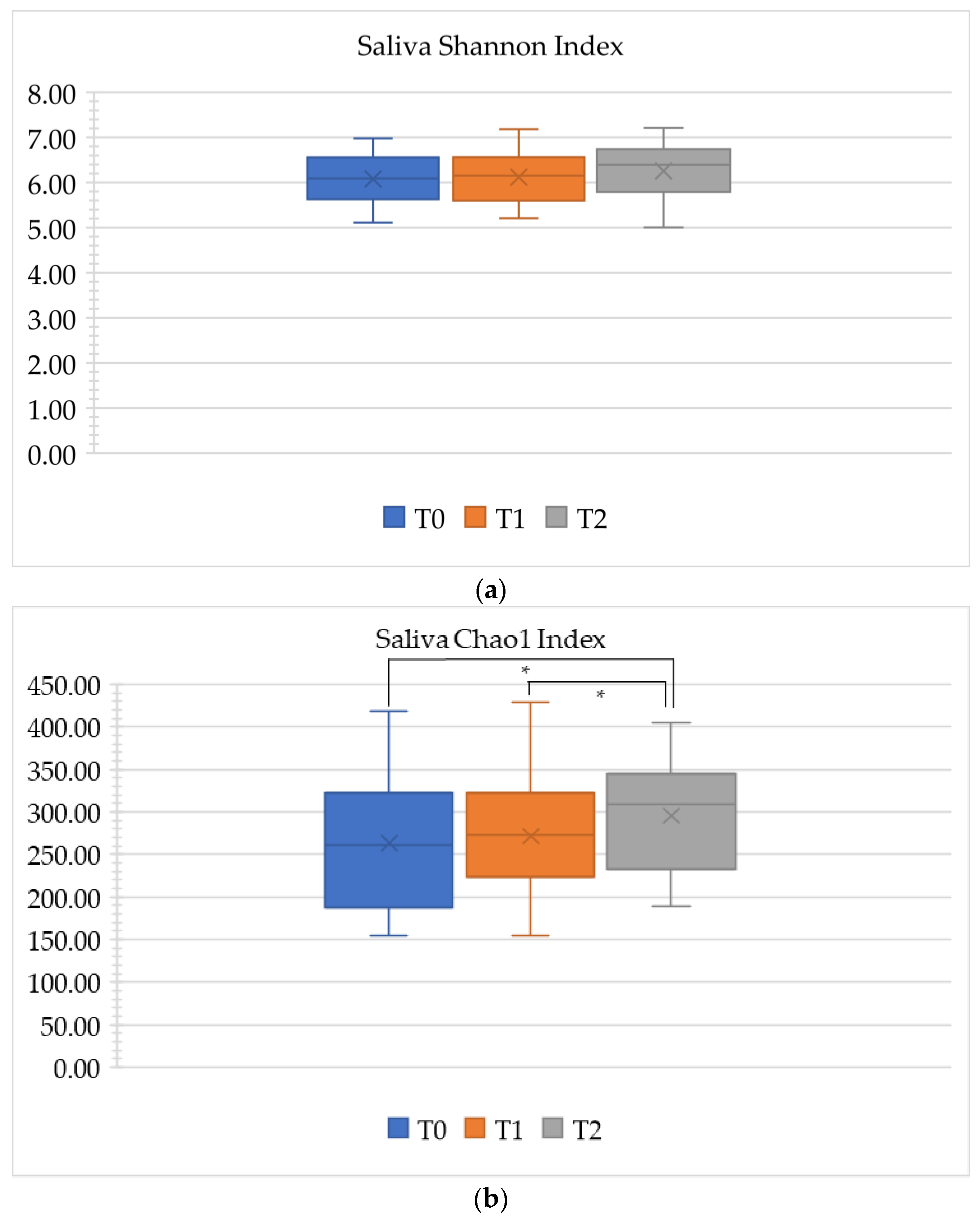
3.1.2. Alpha Diversity
3.2. Gut Microbiota
3.2.1. Comparison of Bacterial Composition
3.2.2. Duration and Gut Microbiota
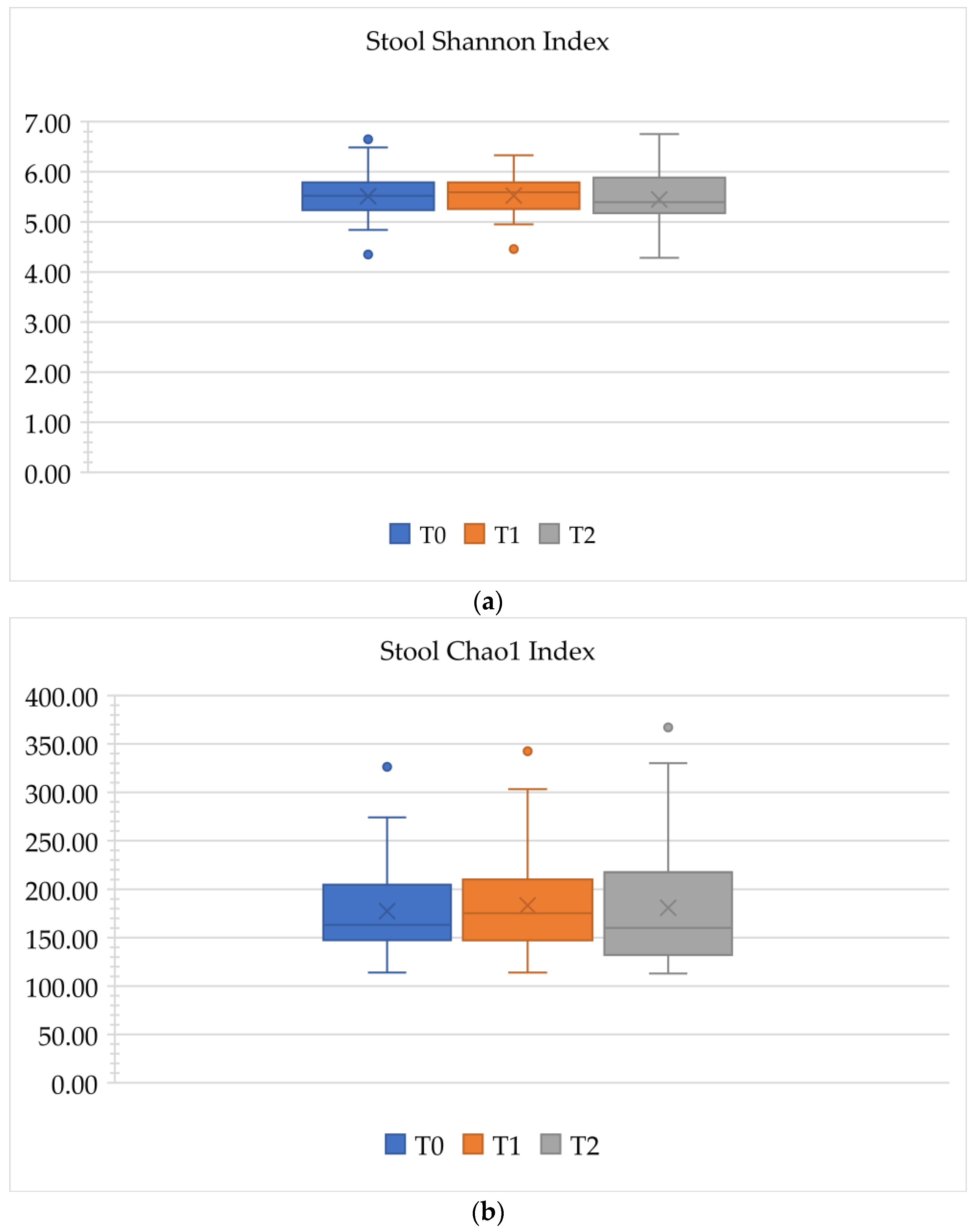
3.2.3. Alpha Diversity
3.3. PCR Score
4. Discussion
4.1. Summary
4.2. Multi-Bracket-PMTC-Microbiota
4.3. Gut Microbiota
4.3.1. Treatment Duration and Gut Microbiota
4.3.2. Oral and Gut Microbiota
4.4. Conditions for Participant Selection
4.4.1. Age
4.4.2. Number of Teeth
4.4.3. Race
4.4.4. Skeletal Pattern, Vertical Jaw Relationship, and Occlusal Relationship
4.5. Mouth Breathing
4.6. Method
4.7. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PMTC | professional mechanical tooth cleaning |
| TBI | including tooth-brushing instructions |
| S. mutans | Streptococcus mutans |
| SD | standard deviation |
| PCR | plaque control record |
| OHI | oral health education and oral hygiene instruction |
| BMI | body mass index |
References
- Lucchese, A.; Bondemark, L.; Marcolina, M.; Manuelli, M. Changes in oral microbiota due to orthodontic appliances: A systematic review. J. Oral. Microbiol. 2018, 10, 1476645. [Google Scholar] [CrossRef] [PubMed]
- Ristic, M.; Svabic, M.V.; Sasic, M.; Zelic, O. Effects of fixed orthodontic appliances on subgingival microflora. Int. J. Dent. Hyg. 2008, 6, 129–136. [Google Scholar] [CrossRef]
- Thornberg, M.J.; Riolo, C.S.; Bayirli, B.; Riolo, M.L.; Van Tubergen, E.A.; Kulbersh, R. Periodontal pathogen levels in adolescents before, during, and after fixed orthodontic appliance therapy. Am. J. Orthod. Dentofac. Orthop. 2009, 135, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Julien, K.C.; Buschang, P.H.; Campbell, P.M. Prevalence of white spot lesion formation during orthodontic treatment. Angle Orthod. 2013, 83, 641–647. [Google Scholar] [CrossRef]
- Heymann, G.C.; Grauer, D. A contemporary review of white spot lesions in orthodontics. J. Esthet. Restor. Dent. 2013, 25, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, E.; Geraci, J.; Sachse, S.; Rödel, J.; Pfister, W.; Löffler, B.; Wagner, Y.; Eigenthaler, M.; Wolf, M. Qualitative and quantitative changes in the oral bacterial flora occur shortly after implementation of fixed orthodontic appliances. Am. J. Orthod. Dentofac. Orthop. 2019, 156, 735–744. [Google Scholar] [CrossRef]
- Mummolo, S.; Nota, A.; Albani, F.; Marchetti, E.; Gatto, R.; Marzo, G.; Quinzi, V.; Tecco, S. Salivary levels of Streptococcus mutans and Lactobacilli and other salivary indices in patients wearing clear aligners versus fixed orthodontic appliances: An observational study. PLoS ONE 2020, 15, e0228798. [Google Scholar] [CrossRef]
- Kado, I.; Hisatsune, J.; Tsuruda, K.; Tanimoto, K.; Sugai, M. The impact of fixed orthodontic appliances on oral microbiome dynamics in Japanese patients. Sci. Rep. 2020, 10, 21989. [Google Scholar] [CrossRef]
- Pan, S.; Liu, Y.; Zhang, L.; Li, S.; Zhang, Y.; Liu, J.; Wang, C.; Xiao, S. Profiling of subgingival plaque biofilm microbiota in adolescents after completion of orthodontic therapy. PLoS ONE 2017, 12, e0171550. [Google Scholar] [CrossRef]
- Uchino, Y.; Goto, Y.; Konishi, Y.; Tanabe, K.; Toda, H.; Wada, M.; Kita, Y.; Beppu, M.; Mori, S.; Hijioka, H.; et al. Colorectal Cancer patients have four specific bacterial species in oral and gut microbiota in common—A metagenomic comparison with healthy subjects. Cancers 2021, 13, 3332. [Google Scholar] [CrossRef]
- Chen, B.-Y.; Lin, W.-Z.; Li, Y.-L.; Bi, C.; Du, L.-J.; Liu, Y.; Zhou, L.-J.; Liu, T.; Xu, S.; Shi, C.-J.; et al. Roles of oral microbiota and oral-gut microbial transmission in hypertension. J. Adv. Res. 2023, 43, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Olsen, I.; Yamazaki, K. Can oral bacteria affect the microbiome of the gut? J. Oral. Microbiol. 2019, 11, 1586422. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Chavakis, T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat. Rev. Immunol. 2021, 21, 426–440. [Google Scholar] [CrossRef]
- de Oliveira, C.; Watt, R.; Hamer, M. Toothbrushing, inflammation, and risk of cardiovascular disease: Results from Scottish Health Survey. BMJ 2010, 340, c2451. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ma, J.-B.; Wang, B.; Zhang, X.; Yin, Y.-L.; Bai, H. Alterations of the oral microbiome in patients treated with the Invisalign system or with fixed appliances. Am. J. Orthod. Dentofac. Orthop. 2019, 156, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Liu, M.; Chen, W.; Zhang, H.; Bai, Y.; Ren, W. Salivary microbial changes during the first 6 months of orthodontic treatment. PeerJ 2020, 8, e10446. [Google Scholar] [CrossRef] [PubMed]
- Boyd, R.L. Longitudinal evaluation of a system for self-monitoring plaque control effectiveness in orthodontic patients. J. Clin. Periodontol. 1983, 10, 380–388. [Google Scholar] [CrossRef]
- Awartani, F.; Atassi, F. Oral hygiene status among orthodontic patients. J. Contemp. Dent. Pract. 2010, 11, 25–32. [Google Scholar] [CrossRef]
- Zhao, R.; Huang, R.; Long, H.; Li, Y.; Gao, M.; Lai, W. The dynamics of the oral microbiome and oral health among patients receiving clear aligner orthodontic treatment. Oral. Dis. 2020, 26, 473–483. [Google Scholar] [CrossRef]
- Jing, D.; Hao, J.; Shen, Y.; Tang, G.; Lei, L.; Zhao, Z. Effect of fixed orthodontic treatment on oral microbiota and salivary proteins. Exp. Ther. Med. 2019, 17, 4237–4243. [Google Scholar] [CrossRef]
- Zivkovic-Sandic, M.; Popovic, B.; Carkic, J.; Nikolic, N.; Glisic, B. Changes in subgingival microflora after placement and removal of fixed orthodontic appliances. Srp. Arh. Za Celok. Lek. 2014, 142, 301–305. [Google Scholar] [CrossRef]
- Takayasu, L.; Suda, W.; Takanashi, K.; Iioka, E.; Kurokawa, R.; Shindo, C.; Hattori, Y.; Yamashita, N.; Nishijima, S.; Oshima, K.; et al. Circadian oscillations of microbial and functional composition in the human salivary microbiome. DNA Res. 2017, 24, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Hosomichi, K.; Kim, Y.-I.; Hikita, Y.; Tajima, A.; Yamaguchi, T. Comprehensive Genetic Exploration of Fused Teeth by Whole Exome Sequencing. Appl. Sci. 2022, 12, 11899. [Google Scholar] [CrossRef]
- O’Leary, T.J.; Drake, R.B.; Naylor, J.E. The plaque control record. J. Periodontol. 1972, 43, 38. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Nambu, T.; Tanimoto, H.; Iwata, N.; Yoshikawa, K.; Okinaga, T.; Yamamoto, K. Interdental Plaque Microbial Community Changes under In Vitro Violet LED Irradiation. Antibiotics 2021, 10, 1348. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; Mcmurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Chen, T.; Yu, W.-H.; Izard, J.; Baranova, O.V.; Lakshmanan, A.; Dewhirst, F.E. The Human Oral Microbiome Database: A web accessible resource for investigating oral microbe taxonomic and genomic information. Database 2010, 6, baq013. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef]
- Yang, F.; Zeng, X.; Ning, K.; Liu, K.-L.; Lo, C.-C.; Wang, W.; Chen, J.; Wang, D.; Huang, R.; Chang, X.; et al. Saliva microbiomes distinguish caries-active from healthy human populations. ISME J. 2011, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ku, C.; Xu, J.; Saxena, D.; Caufield, P. Survey of oral microbial diversity using PCR-based denaturing gradient gel electrophoresis. J. Dent. Res. 2005, 84, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Zaghrisson, B.U.; Zachrisson, S. Caries incidence and oral hygiene during orthodontic treatment. Scand. J. Dent. Res. 1971, 79, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Jongsma, M.A.; Pelser, F.D.H.; van der Mei, H.C.; Atema-Smit, J.; van de Belt-Gritter, B.; Busscher, H.J.; Ren, Y. Biofilm formation on stainless steel and gold wires for bonded retainers in vitro and in vivo and their susceptibility to oral antimicrobials. Clin. Oral. Investig. 2013, 17, 1209–1218. [Google Scholar] [CrossRef][Green Version]
- Hagg, U.; Kaveewatcharanont, P.; Samaranayake, Y.H.; Samaranayake, L.P. The effect of fixed orthodontic appliances on the oral carriage of Candida species and Enterobacteriaceae. Eur. J. Orthod. 2004, 26, 623–629. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Chapple, I.L.C.; Jepsen, S.; Sanz, M. Primary and secondary prevention of periodontal and peri-implant diseases: Introduction to, and objectives of the 11th European Workshop on Periodontology consensus conference. J. Clin. Periodontol. 2015, 42 (Suppl. 16), S1–S4. [Google Scholar] [CrossRef]
- Dimenäs, S.L.; Jönsson, B.; Andersson, J.S.; Lundgren, J.; Petzold, M.; Abrahamsson, I.; Abrahamsson, K.H. A person-centred, theory-based, behavioural intervention programme for improved oral hygiene in adolescents: A randomized clinical field study. J. Clin. Periodontol. 2022, 49, 378–387. [Google Scholar] [CrossRef]
- Smiech-Slomkowska, G.; Jablonska-Zrobek, J. The effect of oral health education on dental plaque development and the level of caries-related Streptococcus mutans and Lactobacillus spp. Eur. J. Orthod. 2007, 29, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Shimpo, Y.; Nomura, Y.; Sekiya, T.; Arai, C.; Okada, A.; Sogabe, K.; Hanada, N.; Tomonari, H. Effects of the dental caries preventive procedure on the white spot lesions during orthodontic treatment—An open label randomized controlled trial. J. Clin. Med. 2022, 11, 854. [Google Scholar] [CrossRef] [PubMed]
- Soldo, M.; Matijević, J.; Ivanišević, A.M.; Čuković-Bagić, I.; Marks, L.; Borić, D.N.; Krmek, S.J. Impact of oral hygiene instructions on plaque index in adolescents. Cent. Eur. J. Public. Health 2020, 28, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Crielaard, W.; Zaura, E.; Schuller, A.A.; Huse, S.M.; Montijn, R.C.; Keijser, B.J. Exploring the oral microbiota of children at various developmental stages of their dentition in the relation to their oral health. BMC Med. Genom. 2011, 4, 22. [Google Scholar] [CrossRef]
- Duran-Pinedo, A.E.; Chen, T.; Teles, R.; Starr, J.R.; Wang, X.; Krishnan, K.; Frias-Lopez, J. Community-wide transcriptome of the oral microbiome in subjects with and without periodontitis. ISME J. 2014, 8, 1659–1672. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the healthy gut microbiota composition? a changing ecosystem across age, environment, diet, and diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Larsen, N.; Vogensen, F.K.; Van Den Berg, F.W.J.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sørensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef] [PubMed]
- Koliada, A.; Syzenko, G.; Moseiko, V.; Budovska, L.; Puchkov, K.; Perederiy, V.; Gavalko, Y.; Dorofeyev, A.; Romanenko, M.; Tkach, S.; et al. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 2017, 17, 120. [Google Scholar] [CrossRef] [PubMed]
- Waters, J.L.; Ley, R.E. The human gut bacteria Christensenellaceae are widespread, heritable, and associated with health. BMC Biol. 2019, 17, 83. [Google Scholar] [CrossRef]
- Gnanasambandam, V.; Gnaneswar, S.M. Effects of orthodontic treatment on body mass index, food habits and self-esteem of patients: A prospective single-arm cohort study. J. Taibah Univ. Med. Sci. 2022, 17, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Yaoita, F.; Watanabe, K.; Kimura, I.; Miyazawa, M.; Tsuchiya, S.; Kanzaki, M.; Tsuchiya, M.; Tan-No, K. Impact of habitual chewing on gut motility via microbiota transition. Sci. Rep. 2022, 12, 13819. [Google Scholar] [CrossRef] [PubMed]
- Nakano, K.; Nomura, R.; Matsumoto, M.; Ooshima, T. Roles of oral bacteria in cardiovascular diseases—From molecular mechanisms to clinical cases: Cell-surface structures of novel serotype k Streptococcus mutans strains and their correlation to virulence. J. Pharmacol. Sci. 2010, 113, 120–125. [Google Scholar] [CrossRef]
- Arimatsu, K.; Yamada, H.; Miyazawa, H.; Minagawa, T.; Nakajima, M.; Ryder, M.I.; Gotoh, K.; Motooka, D.; Nakamura, S.; Iida, T.; et al. Oral pathobiont induces systemic inflammation and metabolic changes associated with alteration of gut microbiota. Sci. Rep. 2014, 4, 4828. [Google Scholar] [CrossRef]
- Nakajima, M.; Arimatsu, K.; Kato, T.; Matsuda, Y.; Minagawa, T.; Takahashi, N.; Ohno, H.; Yamazaki, K. Oral administration of P. gingivalis induces dysbiosis of gut microbiota and impaired barrier function leading to dissemination of enterobacteria to the liver. PLoS ONE 2015, 10, e0134234. [Google Scholar] [CrossRef]
- Ohkusa, T.; Yoshida, T.; Sato, N.; Watanabe, S.; Tajiri, H.; Okayasu, I. Commensal bacteria can enter colonic epithelial cells and induce proinflammatory cytokine secretion: A possible pathogenic mechanism of ulcerative colitis. J. Med. Microbiol. 2009, 58, 535–545. [Google Scholar] [CrossRef]
- Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Cueva, C.; Silva, M.; Pinillos, I.; Bartolomé, B.; Moreno-Arribas, M.V. Interplay between dietary polyphenols and oral and gut microbiota in the development of colorectal cancer. Nutrients 2020, 12, 625. [Google Scholar] [CrossRef]
- Kovatcheva-Datchary, P.; Nilsson, A.; Akrami, R.; Lee, Y.S.; De Vadder, F.; Arora, T.; Hallen, A.; Martens, E.; Björck, I.; Bäckhed, F. Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of Prevotella. Cell Metab. 2015, 22, 971–982. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.-T.; Huang, S.-L.; Yang, H.-W.; Kao, C.-C.; Wei, C.-C.; Huang, Y.-F. Oral microbiota in xerostomia patients—A preliminary study. J. Dent. Sci. 2022, 17, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Jiang, S.; Koh, D.; Hsu, C.-Y.S. Salivary biomarkers for dental caries. Periodontol. 2000 2016, 70, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; He, J.; Xue, J.; Wang, Y.; Li, K.; Zhang, K.; Guo, Q.; Liu, X.; Zhou, Y.; Cheng, L.; et al. Oral cavity contains distinct niches with dynamic microbial communities. Environ. Microbiol. 2015, 17, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Vale, G.C.; Mayer, M.P.A. Effect of probiotic Lactobacillus rhamnosus by-products on gingival epithelial cells challenged with Porphyromonas gingivalis. Arch. Oral. Biol. 2021, 128, 105174. [Google Scholar] [CrossRef] [PubMed]
- Seminario-Amez, M.; Lopez-Lopez, J.; Estrugo-Devesa, A.; Ayuso-Montero, R.; Jane-Salas, E. Probiotics and oral health: A systematic review. Med. Oral. Patol. Oral. Cir. Bucal. 2017, 22, e282–e288. [Google Scholar] [CrossRef]
- Butera, A.; Pascadopoli, M.; Pellegrini, M.; Gallo, S.; Zampetti, P.; Cuggia, G.; Scribante, A. Domiciliary Use of Chlorhexidine vs. Postbiotic Gels in Patients with Peri-Implant Mucositis: A Split-Mouth Randomized Clinical Trial. Appl. Sci. 2022, 12, 2800. [Google Scholar] [CrossRef]
- Seidel, C.L.; Gerlach, R.G.; Weider, M.; Wölfel, T.; Schwarz, V.; Ströbel, A.; Schmetzer, H.; Bogdan, C.; Gölz, L. Influence of probiotics on the periodontium, the oral microbiota and the immune response during orthodontic treatment in adolescent and adult patients (ProMB Trial): Study protocol for a prospective, double-blind, controlled, randomized clinical trial. BMC Oral. Health 2022, 22, 148. [Google Scholar] [CrossRef] [PubMed]
| Clinical Characteristics | Data |
|---|---|
| Age (year), mean ± SD | 25.7 ± 4.3 |
| Sex, n (%) | |
| Male Female | 1 (4.2) 23 (95.8) |
| Treatment duration (year), mean ± SD | 11.2 ± 5.0 |
| T0 | T1 | T2 | T0–T1–T2 ANOVA FDR p-Value | |
|---|---|---|---|---|
| (a) Phylum level | ||||
| Firmicutes | 29.86% | 29.93% | 29.01% | 0.70 |
| Bacteroidetes | 27.49% | 27.34% | 27.04% | 0.95 |
| Fusobacteria | 11.85% | 11.63% | 12.64% | 0.70 |
| Proteobacteria | 19.64% | 19.39% | 19.65% | 0.99 |
| Saccharibacteria (TM7) | 3.21% | 3.38% | 3.21% | 0.93 |
| Actinobacteria | 7.01% | 7.10% | 7.18% | 0.98 |
| (b) Genus level | ||||
| Streptococcus | 13.51% | 12.98% | 13.18% | 0.83 |
| Prevotella | 15.91% | 16.00% | 15.51% | 0.91 |
| Fusobacterium | 6.83% | 6.85% | 6.58% | 0.89 |
| Leptotrichia | 5.01% | 4.78% | 6.05% | 0.70 |
| Haemophilus | 5.12% | 5.16% | 5.28% | 0.96 |
| Veillonella | 5.54% | 6.12% | 5.49% | 0.70 |
| Saccharibacteria (TM7)[G-1] | 2.08% | 2.40% | 2.18% | 0.78 |
| Alloprevotella | 5.07% | 4.90% | 4.42% | 0.74 |
| Neisseria | 8.06% | 8.28% | 7.22% | 0.70 |
| Porphyromonas | 3.82% | 3.95% | 4.26% | 0.89 |
| Rothia | 4.15% | 4.11% | 3.88% | 0.91 |
| Gemella | 1.95% | 1.59% | 1.65% | 0.70 |
| Granulicatella | 1.40% | 1.26% | 1.14% | 0.70 |
| Serratia | 3.33% | 3.17% | 1.64% | 0.76 |
| Campylobacter | 1.57% | 1.68% | 2.02% | 0.70 |
| Oribacterium | 1.33% | 1.65% | 1.18% | 0.70 |
| Capnocytophaga | 2.02% | 1.73% | 1.82% | 0.85 |
| T0 | T1 | T2 | T0–T1–T2 ANOVA FDR p-Value | |
|---|---|---|---|---|
| (a) Phylum level | ||||
| Firmicutes | 48.58% | 48.97% | 50.80% | 0.59 |
| Bacteroidota | 39.01% | 36.91% | 37.75% | 0.61 |
| Actinobacteriota | 6.36% | 7.62% | 5.99% | 0.59 |
| Proteobacteria | 3.10% | 2.62% | 2.55% | 0.59 |
| (b) Genus level | ||||
| Blautia | 7.41% | 7.11% | 8.73% | 0.59 |
| Bacteroides | 30.99% | 29.42% | 29.91% | 0.60 |
| Faecalibacterium | 8.68% | 7.25% | 9.38% | 0.59 |
| Bifidobacterium | 5.54% | 6.68% | 5.02% | 0.59 |
| Parabacteroides | 3.18% | 3.19% | 3.36% | 1.00 |
| Streptococcus | 2.06% | 2.40% | 2.16% | 0.80 |
| Akkermansia | 1.42% | 2.73% | 1.03% | 0.59 |
| Alistipes | 1.74% | 1.96% | 1.70% | 0.72 |
| Phylum | Class | Order | Family | Genus | Comparison Period | Changes in the Short-Treatment Duration Group | Changes in the Long-Treatment Duration Group | p-Value |
|---|---|---|---|---|---|---|---|---|
| Firmicutes | Clostridia | Christensenellales | T1–T2 | 0.08% | −0.34% | 0.01 * | ||
| Christensenellaceae | T1–T2 | 0.08% | −0.34% | 0.01 * | ||||
| Christensenellaceae_ R-7_group | T1–T2 | 0.09% | −0.34% | 0.01 * | ||||
| Bacteroidota | Bacteroidia | Bacteroidales | Prevotellaceae | T0–T1 | 0.00% | −1.47% | 0.01 * | |
| T1–T2 | −0.01% | 1.21% | 0.01 * |
| Mean ± SD | Friedman Test | Wilcoxson Test | |||||
|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T0–T1–T2 | T0–T1 | T1–T2 | T0–T2 | |
| PCR Score | 58.6 ± 16.7 | 38.0 ± 14.9 | 29.5 ± 16.9 | 1.88 × 10−8 * | 2.84 × 10−5 * | 5.83 × 10−3 * | 1.19 × 10−6 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okuwaki, S.; Hosomichi, K.; Tajima, A.; Yamaguchi, T. An Observational Study on Changes in the Oral and Gut Microbiota through Professional Mechanical Tooth Cleaning, including Tooth-Brushing Instructions in Patients with Multi-Bracket Appliances. Appl. Sci. 2023, 13, 10843. https://doi.org/10.3390/app131910843
Okuwaki S, Hosomichi K, Tajima A, Yamaguchi T. An Observational Study on Changes in the Oral and Gut Microbiota through Professional Mechanical Tooth Cleaning, including Tooth-Brushing Instructions in Patients with Multi-Bracket Appliances. Applied Sciences. 2023; 13(19):10843. https://doi.org/10.3390/app131910843
Chicago/Turabian StyleOkuwaki, Satoko, Kazuyoshi Hosomichi, Atsushi Tajima, and Tetsutaro Yamaguchi. 2023. "An Observational Study on Changes in the Oral and Gut Microbiota through Professional Mechanical Tooth Cleaning, including Tooth-Brushing Instructions in Patients with Multi-Bracket Appliances" Applied Sciences 13, no. 19: 10843. https://doi.org/10.3390/app131910843
APA StyleOkuwaki, S., Hosomichi, K., Tajima, A., & Yamaguchi, T. (2023). An Observational Study on Changes in the Oral and Gut Microbiota through Professional Mechanical Tooth Cleaning, including Tooth-Brushing Instructions in Patients with Multi-Bracket Appliances. Applied Sciences, 13(19), 10843. https://doi.org/10.3390/app131910843







