Automatic Needle Route Proposal in Preoperative Neck CT for Injection Laryngoplasty
Abstract
1. Introduction
- We propose the first automatic method to compute the optimal needle route in neck CT for TIL based on critical structure segmentation and minimal cost route search.
- We perform a comprehensive evaluation on neck CT scans of 136 patients, which is carried out by an expert laryngologist.
2. Methodology
2.1. Vocal Fold Localization
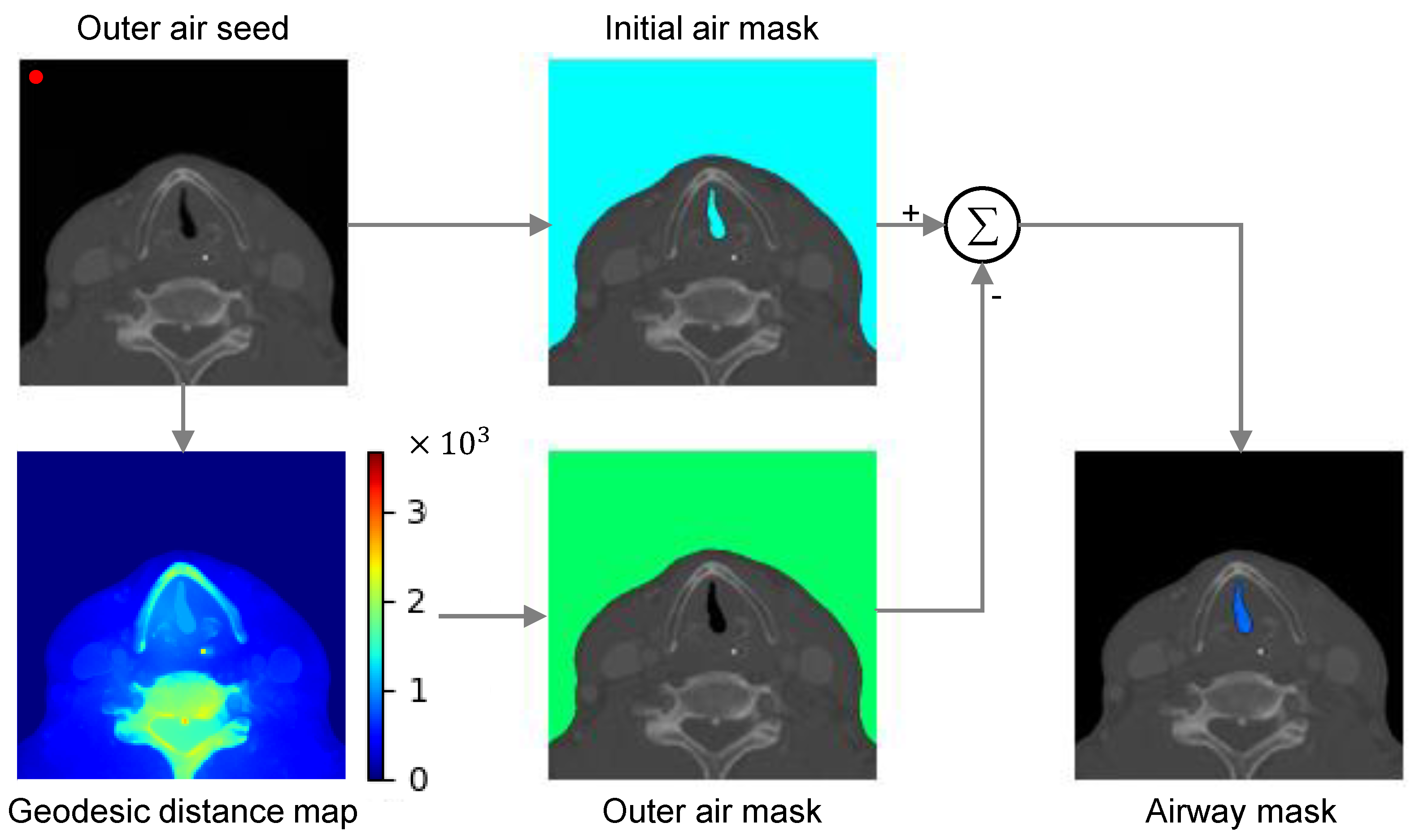
2.2. Critical Region Segmentation
- i
- Automatic localization of the foreground seed is difficult in our task because the airway location is inconsistent across patients due to anatomic and scan environment variation.
- ii
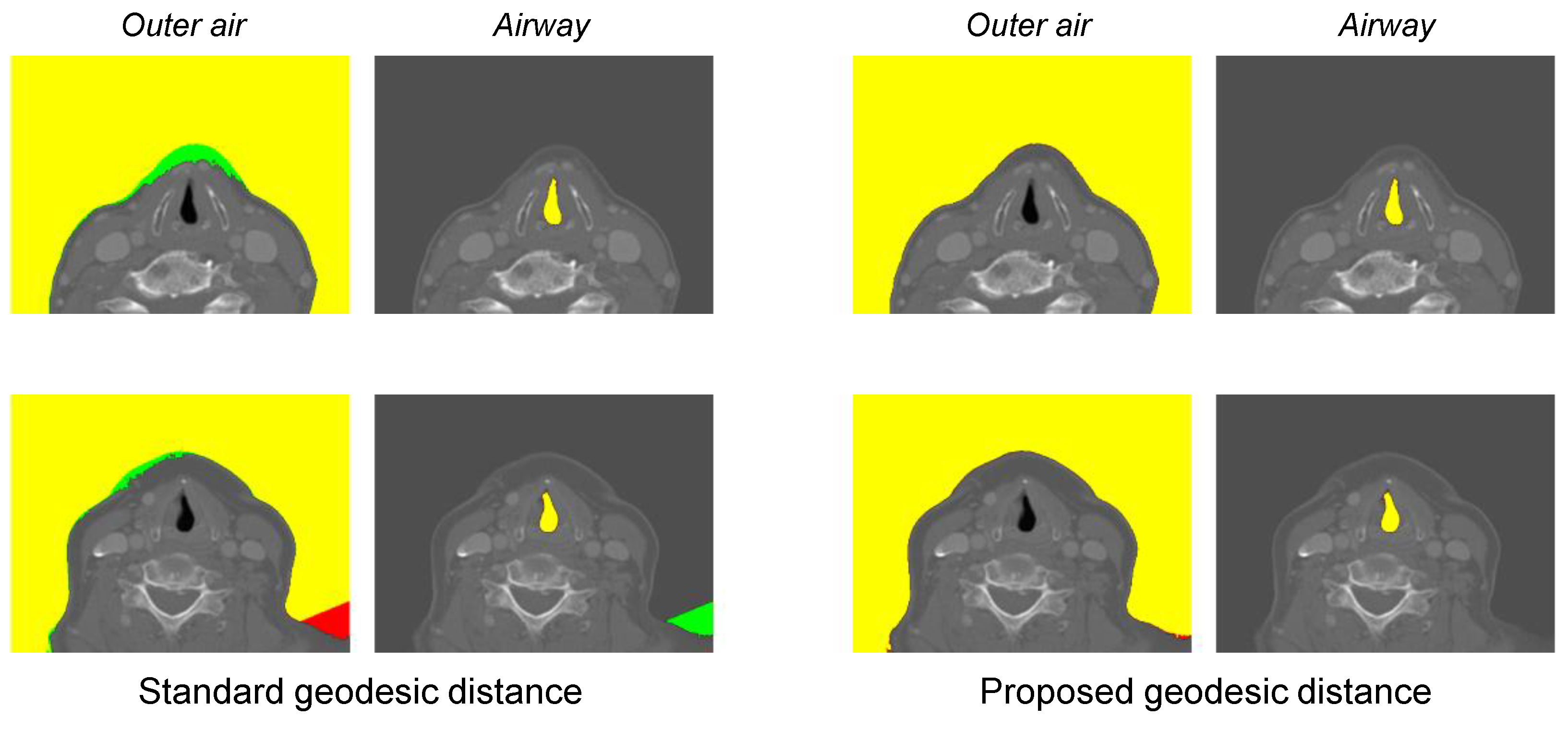
- Optimal geodesic distance threshold for airway segmentation can vary because of the patient-specific anatomic variation.
2.3. Search Space
2.4. Optimal Needle Route Search
3. Experimental Results
3.1. Data and Evaluation Method
3.2. Vocal Fold Localization Performance
3.3. Critical Region Segmentation Performance
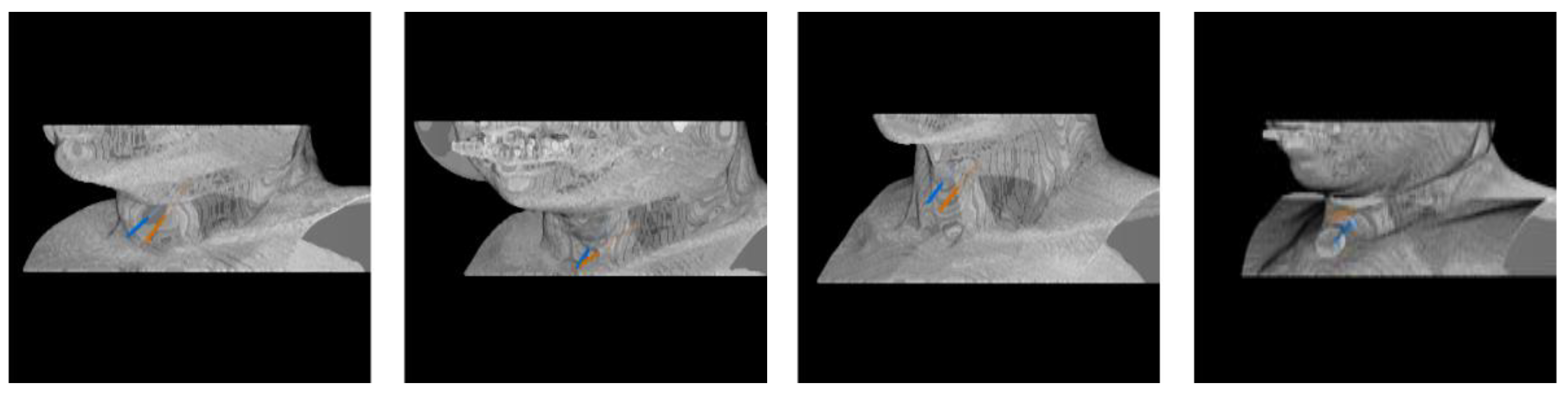
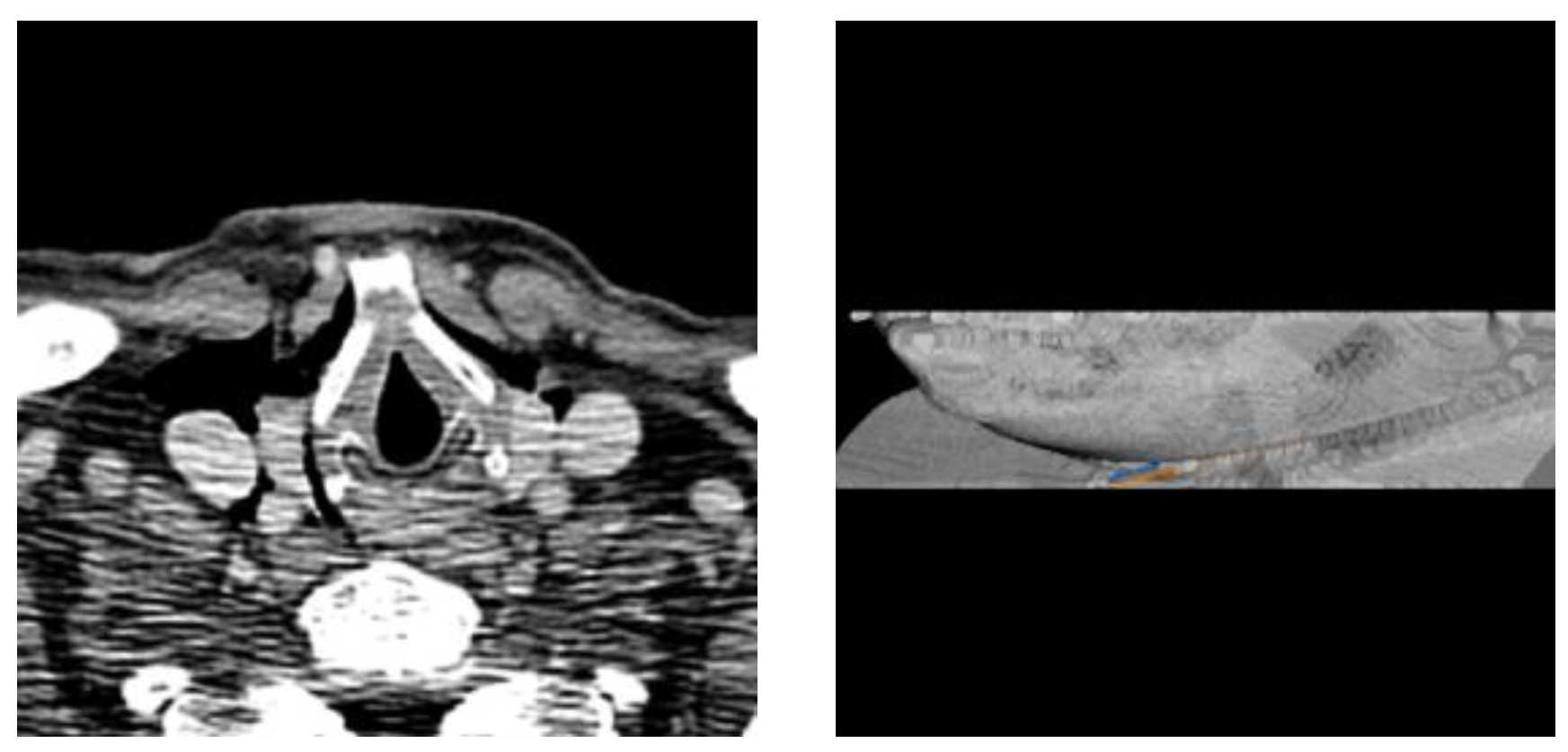
3.4. Needle Route Accuracy
3.5. Limitations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LVF | Left vocal fold |
| RVF | Right vocal fold |
| RL | Reinforcement learning |
References
- Tsai, M.S.; Yang, Y.H.; Liu, C.Y.; Lin, M.H.; Chang, G.H.; Tsai, Y.T.; Li, H.Y.; Tsai, Y.H.; Hsu, C.M. Unilateral vocal fold paralysis and risk of pneumonia: A nationwide population-based cohort study. Otolaryngol. Head Neck Surg. 2018, 158, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Chhetri, D.K.; Jamal, N. Percutaneous injection laryngoplasty. Laryngoscope 2014, 124, 742. [Google Scholar] [CrossRef] [PubMed]
- Nasir, Z.M.; Azman, M.; Baki, M.M.; Mohamed, A.S.; Kew, T.Y.; Zaki, F.M. A proposal for needle projections in transcutaneous injection laryngoplasty using three-dimensionally reconstructed CT scans. Surg. Radiol. Anat. 2021, 43, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, A.L.; Haddad, G.; Haydar, A.; Hamade, R. The 3D printing of the paralyzed vocal fold: Added value in injection laryngoplasty. J. Voice 2018, 32, 499–501. [Google Scholar] [CrossRef] [PubMed]
- Nerurkar, N.K.; Marwah, R.; Mahajan, S. Role of CT Scan in Decision Making Prior to Approximation Laryngoplasty. Indian J. Otolaryngol. Head Neck Surg. 2012, 64, 201–204. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, M.; Ang, C.; Andreadis, K.; Shin, J.; Rameau, A. An Open-Source Three-Dimensionally Printed Laryngeal Model for Injection Laryngoplasty Training. Laryngoscope 2021, 131, E890–E895. [Google Scholar] [CrossRef] [PubMed]
- Abdullah Al, W.; Cha, W.; Yun, I.D. Reinforcement Learning Based Vocal Fold Localization in Preoperative Neck CT for Injection Laryngoplasty. Appl. Sci. 2022, 13, 262. [Google Scholar] [CrossRef]
- Criminisi, A.; Sharp, T.; Blake, A. Geos: Geodesic image segmentation. In Proceedings of the Computer Vision—ECCV 2008: 10th European Conference on Computer Vision, Marseille, France, 12–18 October 2008; Proceedings, Part I 10. Springer: Berlin/Heidelberg, Germany, 2008; pp. 99–112. [Google Scholar]
- Kwak, C.; Jang, J.; Yoon, H. Facial Landmark Localization Robust on the Eyes with Position Regression Network. In Proceedings of the 2020 17th International Conference on Ubiquitous Robots (UR), Kyoto, Japan, 22–26 June 2020; pp. 130–133. [Google Scholar]
- Payer, C.; Štern, D.; Bischof, H.; Urschler, M. Integrating spatial configuration into heatmap regression based CNNs for landmark localization. Med. Image Anal. 2019, 54, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Abdullah Al, W.; Yun, I.D. Partial Policy-Based Reinforcement Learning for Anatomical Landmark Localization in 3D Medical Images. IEEE Trans. Med. Imaging 2020, 39, 1245–1255. [Google Scholar] [CrossRef] [PubMed]
- Ghesu, F.C.; Georgescu, B.; Zheng, Y.; Grbic, S.; Maier, A.; Hornegger, J.; Comaniciu, D. Multi-scale deep reinforcement learning for real-time 3D-landmark detection in CT scans. IEEE Trans. Pattern Anal. Mach. Intell. 2019, 41, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Alansary, A.; Le Folgoc, L.; Vaillant, G.; Oktay, O.; Li, Y.; Bai, W.; Passerat-Palmbach, J.; Guerrero, R.; Kamnitsas, K.; Hou, B.; et al. Automatic View Planning with Multi-scale Deep Reinforcement Learning Agents. In Proceedings of the Medical Image Computing and Computer Assisted Intervention—MICCAI 2018: 21st International Conference, Granada, Spain, 16–20 September 2018; Proceedings, Part I. Springer International Publishing: Cham, Switzerland, 2018; pp. 277–285. [Google Scholar]
- DenOtter, T.D.; Schubert, J. Hounsfield Unit; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Yushkevich, P.A.; Piven, J.; Cody Hazlett, H.; Gimpel Smith, R.; Ho, S.; Gee, J.C.; Gerig, G. User-Guided 3D Active Contour Segmentation of Anatomical Structures: Significantly Improved Efficiency and Reliability. Neuroimage 2006, 31, 1116–1128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wu, S.; Wu, W.; Gao, H.; Zhou, Z. Computer-assisted needle trajectory planning and mathematical modeling for liver tumor thermal ablation: A review. Math. Biosci. Eng. 2019, 16, 4846–4872. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, Y.; Shen, N.; Feng, L.; Ran, Z.; Deng, Z. A practical pretreatment planning method of multiple puncturing for thermal ablation surgery. Biocybern. Biomed. Eng. 2020, 40, 1469–1485. [Google Scholar] [CrossRef]
- Schumann, C.; Bieberstein, J.; Trumm, C.; Schmidt, D.; Bruners, P.; Niethammer, M.; Hoffmann, R.T.; Mahnken, A.H.; Pereira, P.L.; Peitgen, H.O. Fast automatic path proposal computation for hepatic needle placement. In Proceedings of the Medical Imaging 2010: Visualization, Image-Guided Procedures, and Modeling, San Diego, CA, USA, 14–16 February 2010; Volume 7625, pp. 478–487. [Google Scholar]
- Seitel, A.; Engel, M.; Sommer, C.M.; Radeleff, B.A.; Essert-Villard, C.; Baegert, C.; Fangerau, M.; Fritzsche, K.H.; Yung, K.; Meinzer, H.P.; et al. Computer-assisted trajectory planning for percutaneous needle insertions. Med. Phys. 2011, 38, 3246–3259. [Google Scholar] [CrossRef] [PubMed]


| Attribute | Description |
|---|---|
| Total cases | 136 |
| Age | years |
| Sex | Male: 69, female: 67 |
| Paresis cases | Unilateral: 23, bilateral: 6 |
| Avg. volume dimension | |
| Avg. voxel dimension |
| Vocal Fold | Observer 1 | Oberver 2 |
|---|---|---|
| Right | ||
| Left | ||
| Right (paresis) | ||
| Left (paresis) |
| Localization Accuracy | ||
|---|---|---|
| Method | Right Fold | Left Fold |
| RL (proposed) | ||
| Deep Coordinate Regression [9] | ||
| Deep Heatmap Regression [10] | ||
| Standard GD * | Proposed GD * | |
|---|---|---|
| Outer air | ||
| Airway |
| Observer 1 | Oberver 2 | |||
|---|---|---|---|---|
| Needle Route | Intensity Sum Cost | Max Intensity Cost | Intensity Sum Cost | Max Intensity Cost |
| Right | ||||
| Left | ||||
| Right * | ||||
| Left * | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdullah Al, W.; Cha, W.; Yun, I.D. Automatic Needle Route Proposal in Preoperative Neck CT for Injection Laryngoplasty. Appl. Sci. 2023, 13, 10554. https://doi.org/10.3390/app131810554
Abdullah Al W, Cha W, Yun ID. Automatic Needle Route Proposal in Preoperative Neck CT for Injection Laryngoplasty. Applied Sciences. 2023; 13(18):10554. https://doi.org/10.3390/app131810554
Chicago/Turabian StyleAbdullah Al, Walid, Wonjae Cha, and Il Dong Yun. 2023. "Automatic Needle Route Proposal in Preoperative Neck CT for Injection Laryngoplasty" Applied Sciences 13, no. 18: 10554. https://doi.org/10.3390/app131810554
APA StyleAbdullah Al, W., Cha, W., & Yun, I. D. (2023). Automatic Needle Route Proposal in Preoperative Neck CT for Injection Laryngoplasty. Applied Sciences, 13(18), 10554. https://doi.org/10.3390/app131810554






