Abstract
Endometriosis is a chronic condition characterized by the presence of abnormal endometrial tissue outside the uterus. These misplaced cells are responsible for inflammation, symptoms, scar tissue and adhesions. Endometriosis manifests mainly in three patterns: superficial peritoneal lesions (SUP), ovarian endometriomas (OMA) and deep infiltrating endometriosis (DIE). It also exhibits atypical and extremely rare localization. The updated 2022 guidelines of the ESHRE recommend using both ultrasound and magnetic resonance imaging (MRI) as first-line diagnostic tests. Currently, MRI provides a more complete view of the pelvis anatomy. The aim of our review is to provide radiologists with a “map” that can help them in reporting pelvic MRI scans in patients with endometriosis. We will illustrate the usual and unusual localizations of endometriosis (categorized into compartments) using post-operative imaging, and we will focus on the role of MRI, the main sequences and the use of contrast agents.
1. Introduction
Endometriosis is defined as a benign chronic inflammation caused by the abnormal presence of endometrial glands and stroma outside the uterus. The ectopic endometrial cells on the surface of other organs, such as the ovaries (endometrioma), uterus (adenomyosis), tube, intestine, vagina, and bladder, undergo the same modifications of the physiological uterine endometrium during the menstrual cycle; this can generate an inflammatory state with symptoms and the formation of scar tissue and adhesions that, if neglected, can also cause infertility [1].
Several theories exist concerning the origins of endometriosis, with the most widely accepted one suggesting that endometrial cells shed during menstruation make their way through the fallopian tubes into the abdominal cavity. This leads to their implantation on the peritoneum and the surface of the pelvic organs [2]. However, this hypothesis does not rule out alternative explanations, including a potential genetic factor, the spread of endometrial cells through the bloodstream and lymphatic system, or the metaplastic theory. Specifically, the latter theory proposes that both endometrial and peritoneal cells originate from a shared embryological precursor [3]. Therefore, it suggests that peritoneal cells have the potential to undergo a transformation into endometrial cells for reasons that are currently unknown [4]. The peak incidence occurs between the ages of 25 and 35, but the disease can also occur in lower age groups. Diagnosis of the condition is not always immediate and may often involve a prolonged diagnostic journey [5].
Endometriosis can be asymptomatic. When symptomatic, it usually manifests with pelvic pain, especially in the peri-menstrual phase, painful menstruation (dysmenorrhea), pain during sexual intercourse (dyspareunia), urinary urgency, hematuria or pain during defecation, sometimes together with the presence of blood in the faeces [1].
The updated 2022 guidelines of the European Society for Human Reproduction and Embryology (ESHRE) recommend using both ultrasound and magnetic resonance imaging (MRI) as first-line diagnostic testing. It is important to be aware, however, that negative imaging does not exclude the disease. If strong clinical suspicion persists, patients are directed to a laparoscopic examination with diagnostic and therapeutic purposes [6].
Several studies in the literature have confirmed the accuracy of MRI in the diagnosis of deep infiltrating endometriosis (DIE) and in the evaluation of its extension.
A prospective study by Bazot et al. showed a sensitivity and specificity of the technique of 90.30% and 91%, respectively [7].
In a further study, performed at the University of Udine, 44 women with clinical or ultrasound suspicion of pelvic endometriosis underwent MRI and then an operational laparoscopy. The results showed a 95% match between the proposed extension of the disease in MRI and the confirmed staging at laparoscopy; this shows that MRI plays a fundamental role, as a non-invasive diagnostic examination, in the assessment of the extent of the disease in patients who are candidates for surgery [8].
The modern therapeutic management of endometriosis involves a medical and/or surgical approach, although in recent years, new treatment and management proposals have been considered. For instance, interventional radiology has played a role in the management of selected cases of endometriosis predominantly of the abdominal wall with ablative procedures. Cryoablation in these cases offers advantages over surgery, especially with regard to the reduction in intra and post-procedural pain [9,10].
The aim of this review is to summarize the typical and atypical localizations of the disease using post-operative imaging to focus on the role of MRI as the technical protocol, and to provide a conclusion projected towards new discoveries and new applications in the field of diagnosis and treatment of endometriosis.
2. Indications for MRI
Nowadays, in the literature there is no consensus on the indications of MRI for patients dealing with pelvic disorders in premenopausal age. MRI is often required in cases of dysmenorrhea, dyspareunia, difficulty conceiving, evaluation of chronic pelvic pain, or unrecognized adnexal masses [11]. However, the most relevant indication for MRI in this age range remains the evaluation and staging of deep pelvic endometriosis¸ defined as at least one structure involved, such as the uterosacral ligaments, vagina, rectosigmoid colon, rectovaginal septum or bladder, for a distance of 5 mm or more.
In the literature, the value of MRI as a first-line clinical technique, compared to transvaginal ultrasound, is not confirmed [12]; in fact, several studies underline that the first approach method in the evaluation of suspected endometriosis should remain trans-vaginal ultrasound, especially for ovarian and bladder endometriosis and for rectosigmoid endometriotic lesions [13].
MRI is able to recognize past hemorrhagic content in endometrioma, and owing to its large field of view (FOV), spatial resolution and contrast imaging, can identify multiple dislocated endometrial implants [14]. It is, therefore, often requested as a second-line technique in the case of still symptomatic patients without any ultrasound findings [15], for patients undergoing surgery for pre-operative staging and mapping of the implants, or in case of difficult diagnosis [16]. Only a few recent studies suggest the use of MRI as a triage test in the diagnosis of rectosigmoid colon endometriosis and as a first-line examination in women with a high suspicion of intestinal endometriosis [17].
2.1. Procedure and Patient Preparation
There is no consensus regarding patient preparation [11]: fasting is the most suggested for evaluation of DIE imaging for at least three to six hours before the exam. It is also recommended to refrain from voiding for 1 h before the MRI, so as to reach a correct angle of the uterus and displace the small bowel [14], and to improve the detection of small implants in the anterior compartment. An extremely filled bladder could in fact cause spastic movements of the detrusor muscle with consequent artifacts [8].
Bowel preparation before the examination is not routinely suggested. When required, it consists of two doses of an oral laxative the day before imaging, or an enema; the patient should also follow a low-residue dietary regimen on the day before and on the day of the MRI [18].
The supine position is recommended, but for those who suffer from claustrophobia, the prone position is accepted [19].
Just before the MRI, it is recommended to perform an injection of 10 mg or 20 mg of butylescopolamine, an anti-peristaltic agent, to reduce bowel peristalsis. Intravenous administration is preferred to intra-muscular [20].
Vaginal opacification is considered an option in the valuation of deep pelvic endometriosis. Some studies describe a better visualization of small implants in the middle compartment, while others do not find any advantage. Usually, when performed, 20 mL of sonographic gel is infused into the vagina to distend the fornix [12]. Rectal opacification is suggested as an option: some studies consider this practice as useless and uncomfortable for the patient, causing movement artifacts and an increase in bowel peristalsis, which may cause blurring of the images. Moreover, the non-dilated colon above may become spastic. In addition, bowel wall retracting, a valuable sign of the presence of an endometriotic lesion, is likely to disappear when the rectum is distended [20]. Regarding timing, some authors underline the need to perform the exam between the 8th and 12th day of the menstrual cycle so as to obtain a spontaneous T1-weighted imaging (T1WI) signal intensity of blood in the days before the exam [21]. Others argue that it is best to perform the exam in the post-ovulatory phase to aid interpretation, while others suggest avoiding the menstrual phase due to the thickness of the junctional zone leading to inappropriate diagnosis [22].
2.2. Technical Requirements and Protocols of Acquisition
A 1.5 Tesla (T) or more MRI system is strongly recommended. Only a few centers have published some interesting analyses using a 3.0 T, but there is still a lack of information on the comparison of the images obtained by the two systems and, therefore, there is no recommendation for using a specific device or another [23].
Pelvic phased array coils give a higher signal-to-noise ratio (SNR) than a body coil; some centers use endocavitary coil in addition to the pelvic array, although this is not convenient in terms of costs and acceptability [24].
The MRI protocol consists of a T2-weighted imaging (T2WI) sequence without fat suppression [7], which is considered the best for detecting pelvic endometriosis implants. At least two orthogonal planes must be performed (sagittal and axial). An axial T2WI acquisition from the renal hila to the pubic bone, allowing the visualization of kidneys and the right iliac fossa, should be recommended. In addition, a thin oblique section 2D T2WI sequence may be used to detect uterosacral and parametrial implants [25].
Three-dimensional T2WI sequences are reported as a potential option in some studies. In fact, some authors describe a new technique called “CUBE”, a coronal single-slab 3D fast spin echo T2WI MRI able to reduce time acquisition with superior image quality and better 3D reconstruction (multi-planar reconstruction). These improvements are similar to 2D fast spin echo T2WI MRI in the evaluation of DIE locations [24].
T1WI MRI sequences with or without fat suppression are recommended for the study of adnexal endometriosis and for active foci of DIE [26,27].
There is no recommendation regarding diffusion-weighted imaging (DWI) sequences, but some authors suggest that it can be performed to differentiate endometriomas from hemorrhagic cysts, with lower apparent diffusion coefficient (ADC) values in the endometriomas [23].
There is no consensus on the use of intravenous contrast agent in the evaluation of DIE, but it is recommended as an “option” in the evaluation of adnexal endometriosis; it can be useful for recognizing abdominal wall endometriosis, or in the differentiation of an endometrioma from a luteal ovarian cyst or tubo-ovarian abscess due to an intense wall enhancement [28,29].
Some authors show that it can be useful to recognize mural nodules, which are probably malignant in ovarian lesions [30]. Finally, in patients with the simultaneous presence of endometriosis and pelvic inflammatory disease, a strong wall enhancement within an adnexal mass is useful to distinguish the two pathologies.
Recently, few studies reported the utility of using T2-star-weighted imaging (T2*WI) to better characterize endometrioma: this sequence is highly sensitive for hemosiderin as it can detect the hemosiderin deposits in endometrioma caused by ectopic endometrium’s cyclic hemorrhage, appearing hypointense. This hypointensity can be classified into four types of patterns: eggshell, nodular, multinodular, and planar all along the cyst walls.
Consequently, hypointensity in T2*WI can be useful in distinguishing endometrioma from mucinous cystadenomas [31].
With regard to artificial intelligence (AI), several studies have recently demonstrated the role of AI in increasing diagnostic and research efficacy and outcome prediction in endometriosis; a recent literature review analyzed the most recent published studies on endometriosis and AI, identifying six major categories to build diagnostic and predictive AI models (biomarkers, such as plasma biomarkers of menstrual cycle, miRNA, components of endometrial fluid, clinical symptoms, genetic variables and metabolite spectra, and MRI), showing a good diagnostic and predictive capacity in assessing endometriosis [32].
Another recent review compared two algorithms of AI medical image segmentation and the nursing care given to patients in pre- and post-surgical assistance. The results show how the new algorithm proposed is more specific and more sensitive to helping radiologists detect ovarian endometriosis by speeding up the process of diagnosis, nursing assistance and recovery of the patients, and also reducing the incidence of post-operative complications [33].
3. Localization
Endometriosis manifests mainly in three patterns: superficial peritoneal lesions (SUP), ovarian endometriomas (OMA) and deep infiltrating endometriosis (DIE). In the case of DIE, there are implants, usually multiple, that infiltrate the tissues more than 5 mm from the surface of the peritoneum or in the case of organs, such as the bladder or intestine, reaching the muscularis propria. Generally, the symptoms are more severe [34].
It is very common that an extended condition of deep endometriosis is associated with ovarian endometrioma, an adenomyosis condition, and the infiltration of endometrial cells into the myometrium (the smooth muscle forming the wall of the uterus) [35].
There are also atypical and extremely rare localizations of endometriosis (lungs, pleura, liver, post-operative scars, inguinal region, or even the brain), which require a careful evaluation [36].
Below we will illustrate the main sites of endometriosis implantation in order to provide the radiologist with a “map” that can help in correct MRI reporting (Table 1).

Table 1.
The table schematically represents the main localizations of DIE, MRI sign and their appearance in each of the sequences [7,8,14,16].
3.1. Superficial Peritoneal Lesions (SUP)
Superficial endometriosis is the most common form of endometriosis and is present in at least 8 out of 10 women diagnosed with endometriosis.
The lesions are located superficially on the peritoneum and across pelvic organs like ovaries and uterine ligaments.
Compared to deep endometriosis, women have less marked symptoms and medical therapy may be sufficient to control symptoms and ensure a good quality of life [37].
Flat lesions are not easy to detect with MRIs, except if they present hemorrhagic components detected as hyperintense on fat-suppressed T1WI or T1WI. When even an MRI is not adequately sensitive to screen superficial endometriosis, diagnostic laparoscopy followed by histological confirmation remains the gold standard [38].
3.2. Deep Infiltrating Endometriosis (DIE)
DIE occurs if the endometrial tissue has penetrated at least 5 mm beyond the surface of the peritoneum. It is the most aggressive form of endometriosis, often associated with a symptomatology that may not benefit from medical therapy alone. DIE is often associated with conditions of infertility. The role of the radiologist and the MRI, in this case, is to provide indications to the surgeon on the location of the lesions and their extension in order to correctly plan surgery.
Recently, the ENDOVALIRM group has examined the literature and has established an MRI lexicon consensus in the management of DIE [39].
According to them, the pelvis should be divided into the following nine compartments, with each one containing specific structures:
- -
- Right and left anterolateral compartments: distal round ligament;
- -
- Anterocentral: proximal round ligament and bladder;
- -
- Right and left mediolateral: parametrium, ureter, uterine artery and pelvic wall (external iliac and/or obturator vessels);
- -
- Mediocentral: torus, proximal uterosacral ligaments, posterior vaginal fornix, rectovaginal septum, anterior mesorectum and external adenomyosis;
- -
- Right and left posterolateral: distal uterosacral ligaments, sacro-rectal-genital septum and pelvic wall (sacral roots, sciatic nerve, internal iliac vessels);
- -
- Posterocentral: rectum and rectosigmoid junction.
They also assigned a tenth compartment to extrapelvic lesions (sigmoid colon, cecum, ileum, appendix, extrapelvic ureters, abdominal wall and inguinal regions).
In our review, we focus on the main structures involved in DIE, specifically on related symptomatology and MRI appearance.
3.2.1. Bladder and Urinary Tract
Urinary tract endometriosis affects up to 15% of women with DIE and the bladder is the most frequently involved, especially the bladder dome, followed by the vescico-uterine pouch and the bladder base [14,39,40].
Apart from pain, women with endometriosis of the urinary tract may experience the following symptoms: dysuria, urgency, frequency and, in some cases, hematuria [41].
Transvaginal ultrasound and MRI are the methods of choice for a correct diagnosis and evaluation of disease extension; in particular, the MRI plays a predominant role in preoperative planning [40].
The most common sign of MRI is a diffused or localized bladder wall thickening involving the muscularis layer; more rarely, lesions infiltrate the mucosa and protrude into the lumen [41].
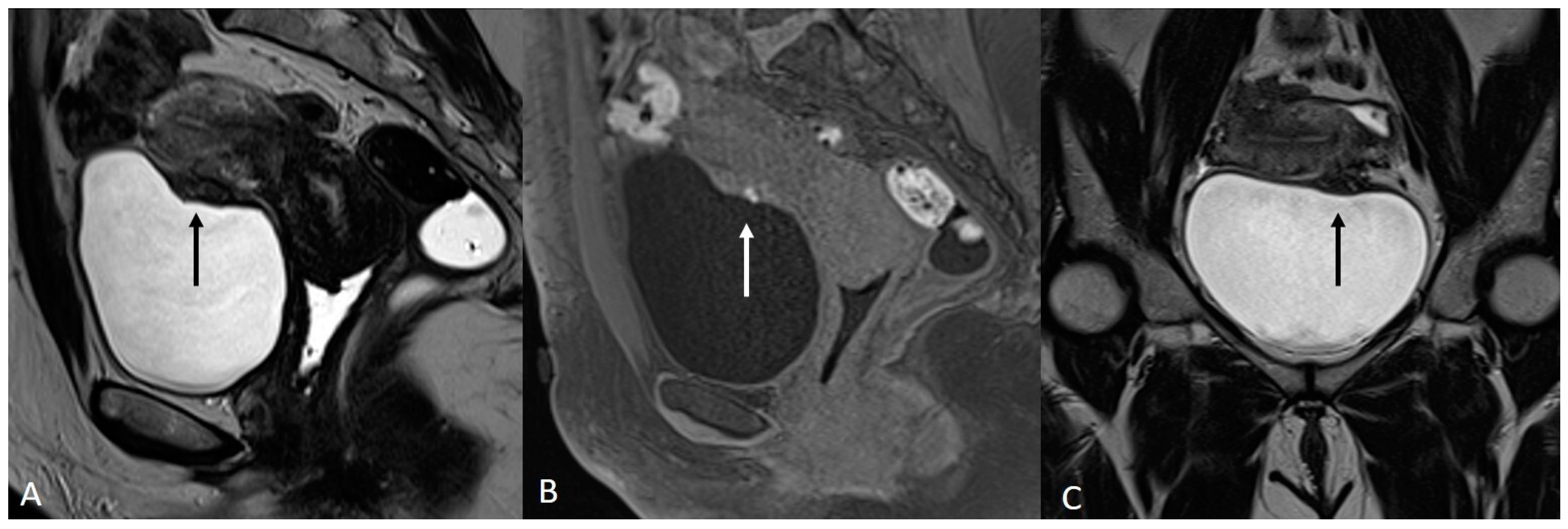
In order to perform a correct evaluation, the bladder must be at a degree of repletion suitable for the correct study of its walls and its lumen: those nodules or masses are responsible for the loss of the normal hypointense signal of the muscularis layer on T2WI. Hemorrhagic content may also be seen as hyperintense foci on T1WI, and it is also possible to observe hyperintense cystic areas on T2WI [7,14] (Figure 1).
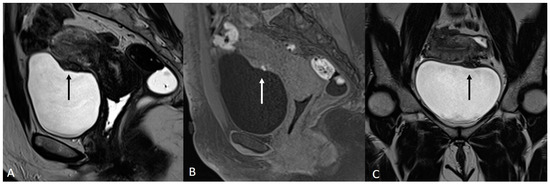
Figure 1.
Localization of endometriosis in the vesico-uterine pouch in a 42-year-old female. (A) Sagittal T2WI (black arrow); (B) Sagittal fat-suppressed T1WI (white arrow); (C) Coronal T2WI (black arrow).
In the case of a mural mass protruding into the lumen, mucosal or submucosal edema is well depicted on T2WI [40].
From the perspective of a surgical approach, it is necessary to assess the distance between the lesion and the ureteral meatus. In fact, the involvement of the ureters requires a more aggressive approach, which in severe and extensive cases leads to ureteral resection–reimplantation or even nephrectomy. Ureters may appear dilated caused by either extrinsic compression or intrinsic infiltration [42].
3.2.2. Round Ligaments
The round ligaments are rope-like bands of connective tissue that hold the uterus in place. There is one on either side, and their proximal attachment is the antero-lateral uterine serosa, which then terminates in the mons pubis through the inguinal canal. In normal conditions, they present a low intensity signal on T2WI [39].
Endometriosis may cause thickening of round ligaments. On an MRI, the lesions demonstrate low signal intensity associated with small cystic areas on T2WI and high-signal-intensity spots of hemorrhagic components on T1WI.
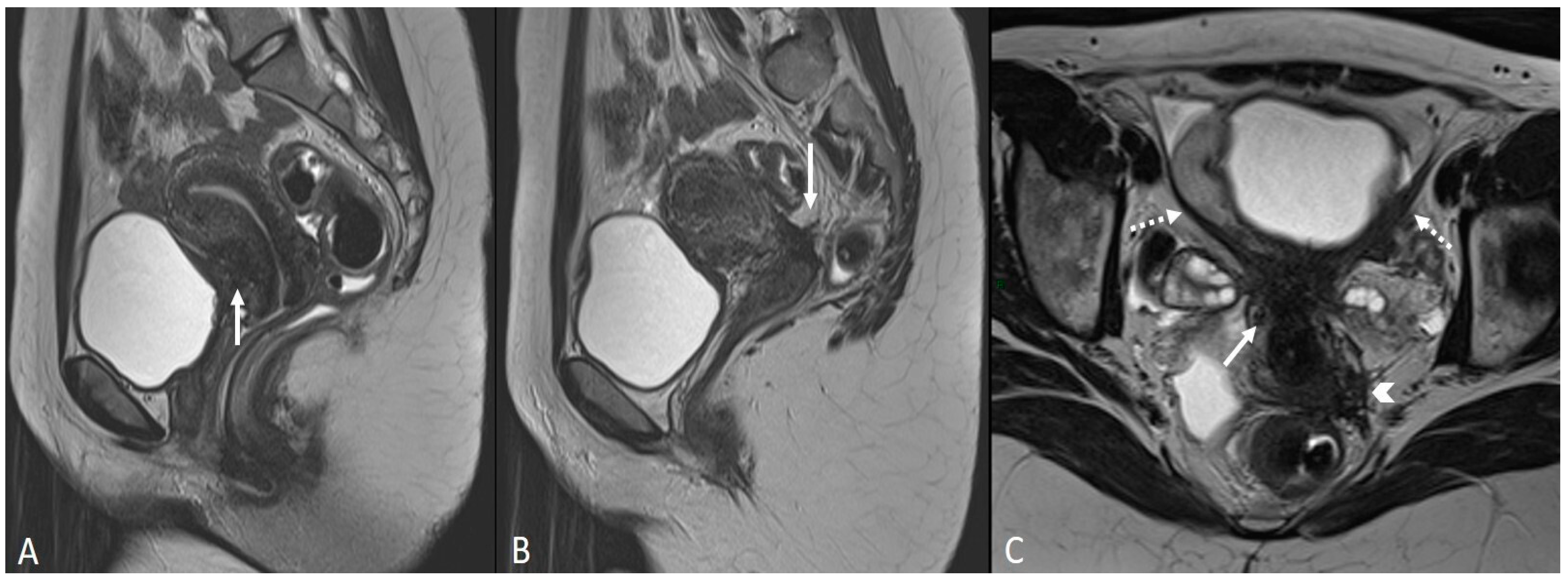
In other cases, the involvement of the round ligament can be appreciated as irregular contours with a nodular aspect [43] (Figure 2).
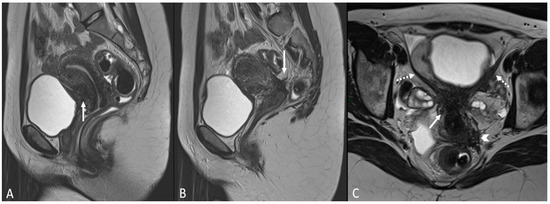
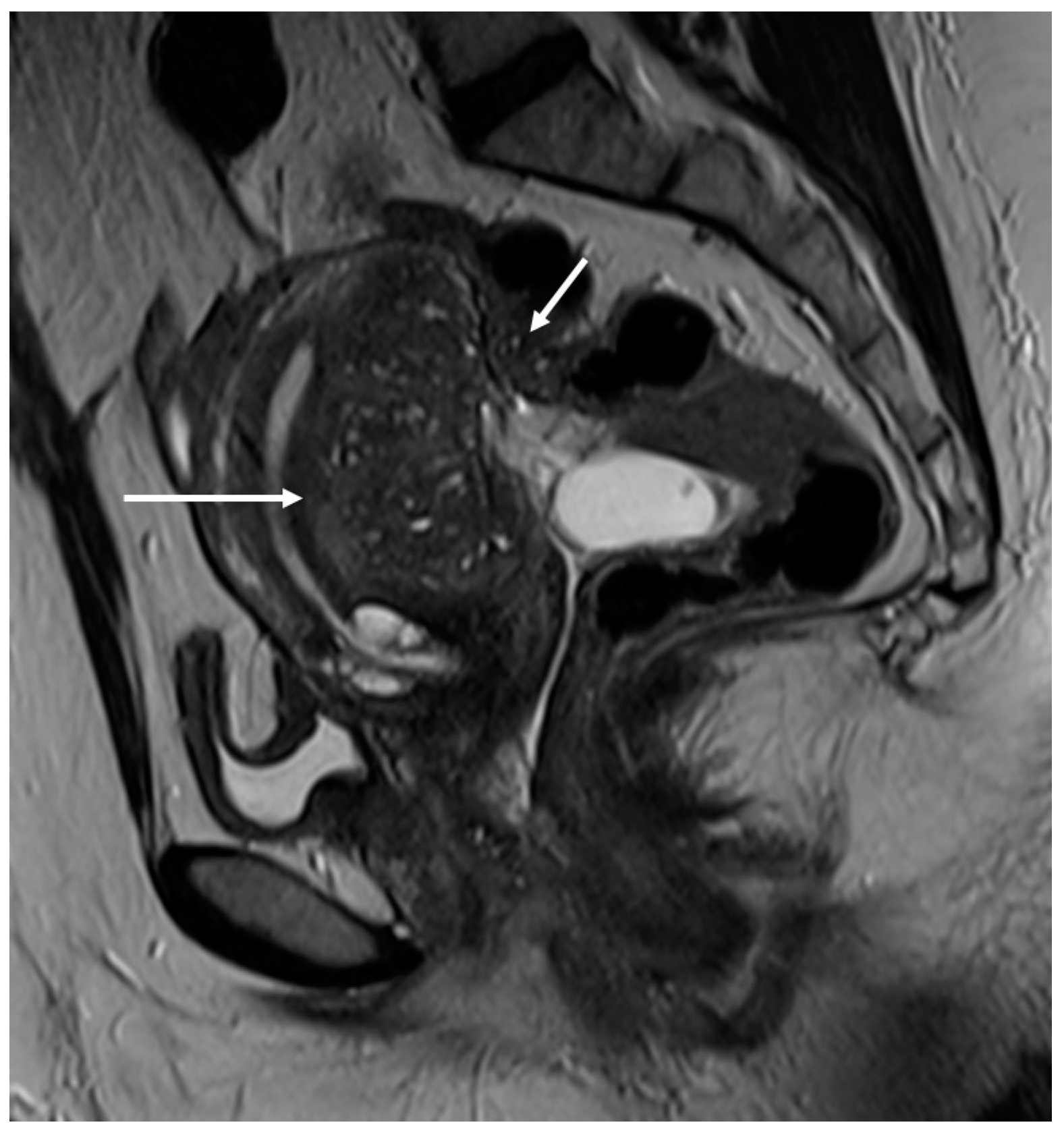
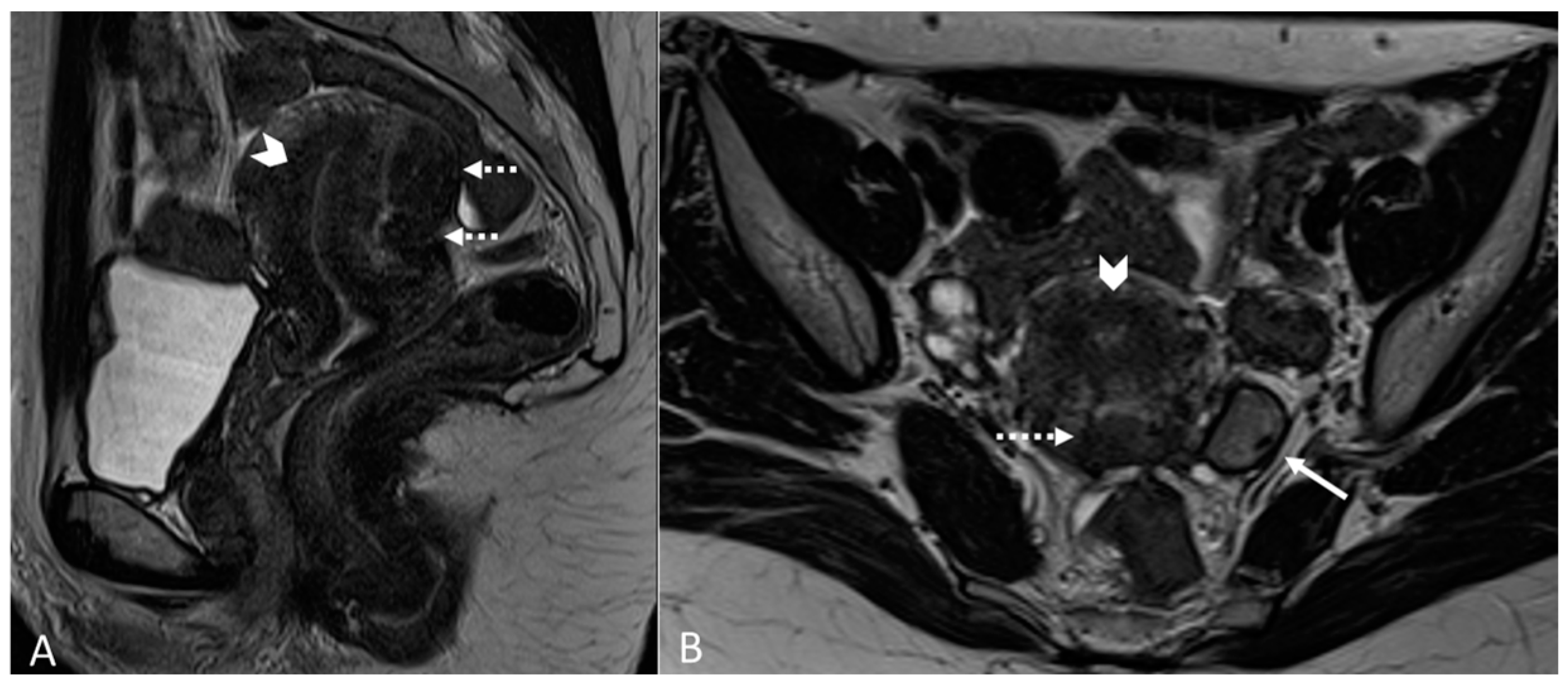
Figure 2.
Deep infiltrating endometriosis of the anterior and posterior compartments in 33-year-old female with dysmenorrhea, chronic pelvic pain, dyspareunia and dyschezia. (A) Sagittal T2WI. Evidence of external anterior adenomyosis and plaque of the vesico-uterine pouch (white arrow); (B) Sagittal T2WI. Retrocervical localization of DIE (white arrow); (C) Axial T2WI. Left uterosacral ligament (USL) localization (white arrowhead), plaque of vesico-uterine pouch (white arrow) with involvement of the round ligaments, particularly on the left (white dotted arrows), and adhesions with the ovaries (retraction of the broad ligaments).
The surgical approach can vary according to the proximal or distal involvement [39].
3.2.3. Torus Uterinus and Sacrouterine Ligaments
The uterosacral ligaments, also known as rectouterine ligaments, extend from the uterus to the sacrum; specifically, they are attached to the cervix creating the torus uterinus and posteriorly to the presacral tissues [43]. The ligaments consist of smooth muscle, connective and adipose tissue, and they are a common site of endometriosis associated with dyspareunia.
In the literature, there is still a high variability regarding the diagnostic presentation of disease in the uterosacral ligaments, which is likely to lead to an over-diagnosis at that level.
Bazot et al. and the European Society of Urogenital Radiology (ESUR) consider the irregular margins, thickening and asymmetry as parameters of diagnosis, while Kinkel et al. referred to a specific thickness (>9 mm) as a criterion for endometriotic involvement [11].
Rousset et al. and the ENDOVALIRM group, in order to avoid false positive findings, prefer to use the following morphologic abnormalities in at least two planes as criteria of disease: asymmetry between each other, irregular or nodular margin, a spiculated or retracted aspect, and/or thickness of more than 5 mm. In the case of hemorrhagic spots, a diagnosis is made without any other alteration needed [39].
On T2WI, a hypo/isointense signal compared to myometrium may indicate the presence of endometriosis. Hemorrhagic foci corresponding to a high signal on fat-suppressed T1WI and/or T1WI and cystic alterations may also be seen as hyperintense cavities on T2WI [14].
Involvement of the proximal portion of ligaments (distance less than 2 cm from the cervix) may, in some cases, be associated with thickening at the level of the torus uterinus; in such cases, it is essential to report any mass or thickening in the upper portion of the posterior cervix, as this could cause the surgeon to proceed with a laparoscopic trans-vaginal approach [7] (Figure 2, Figure 3 and Figure 4).
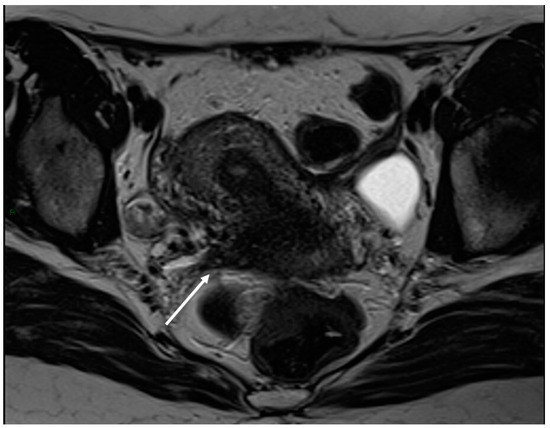
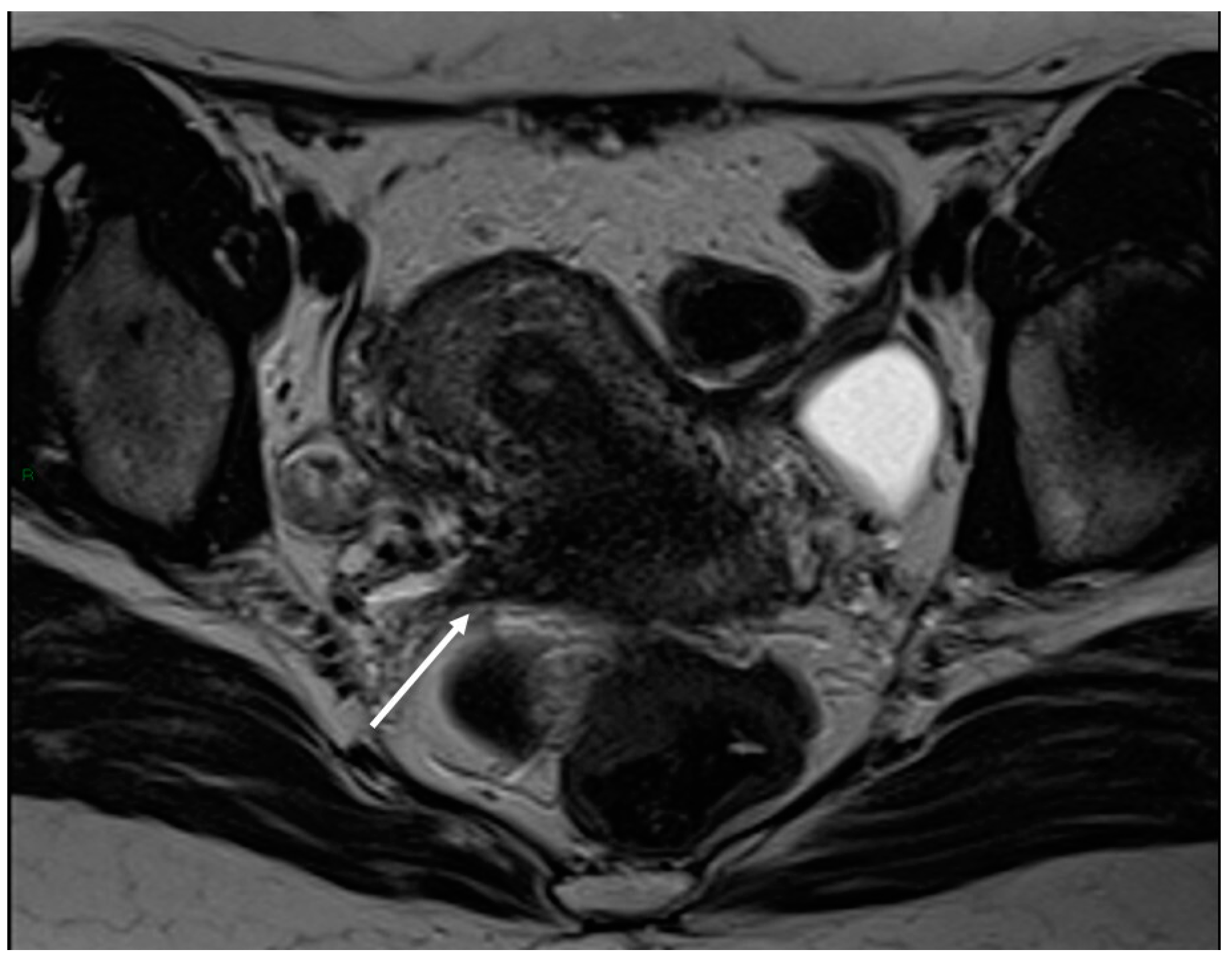
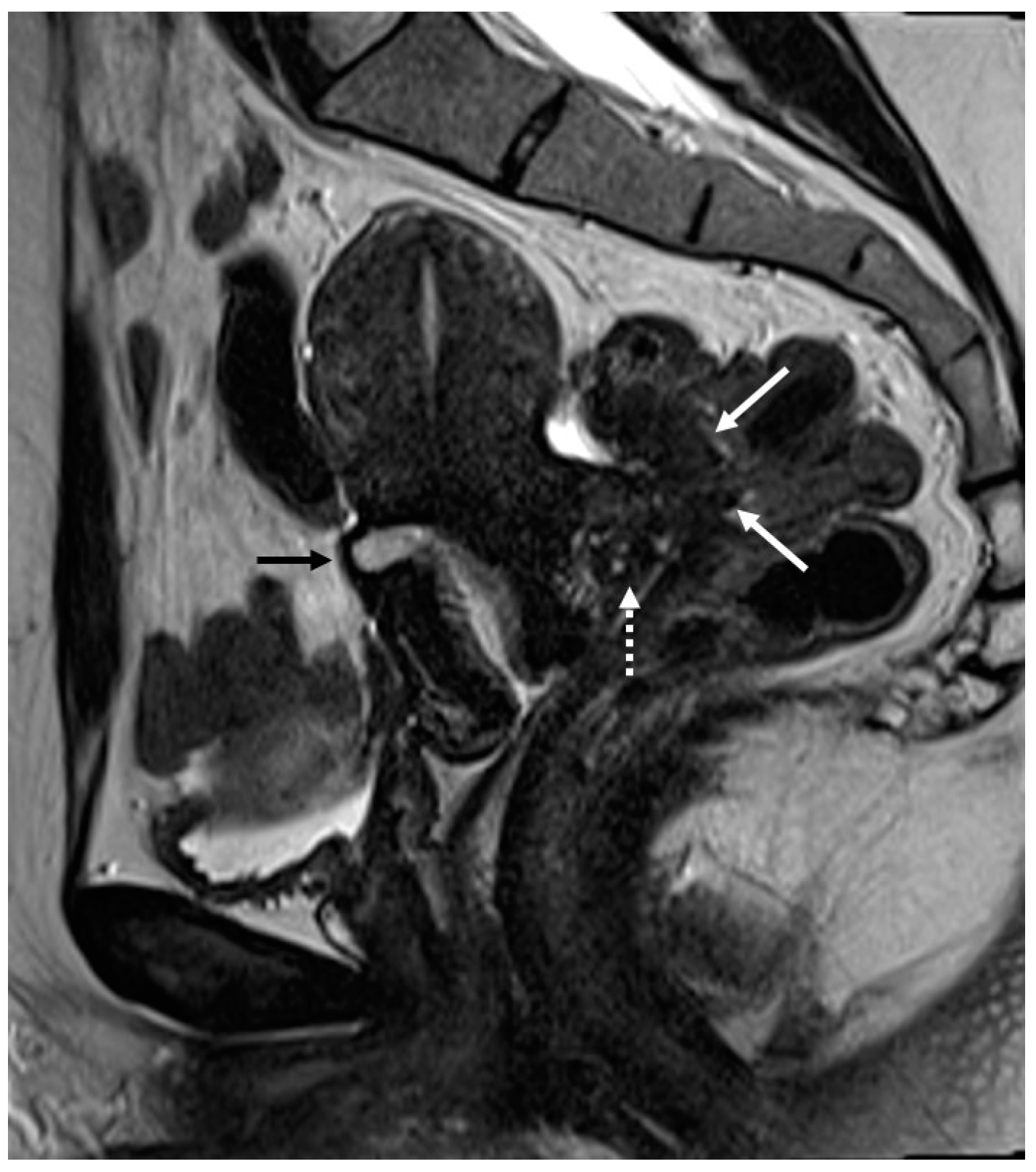
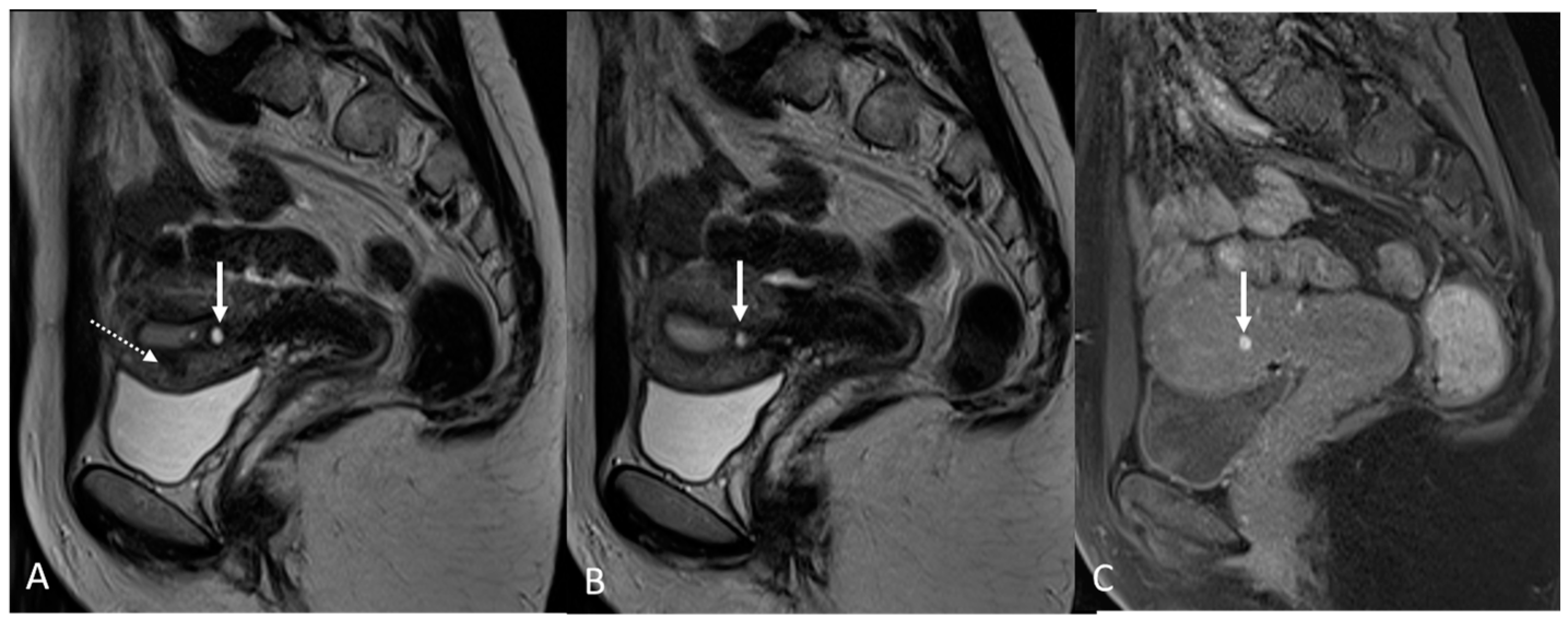
Figure 3.
Right uterosacral ligament localization in a 43-year-old female. The right uterosacral ligament appears thickened at uterine insertion (white arrow).
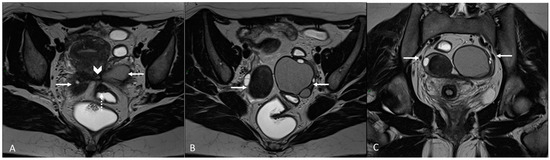
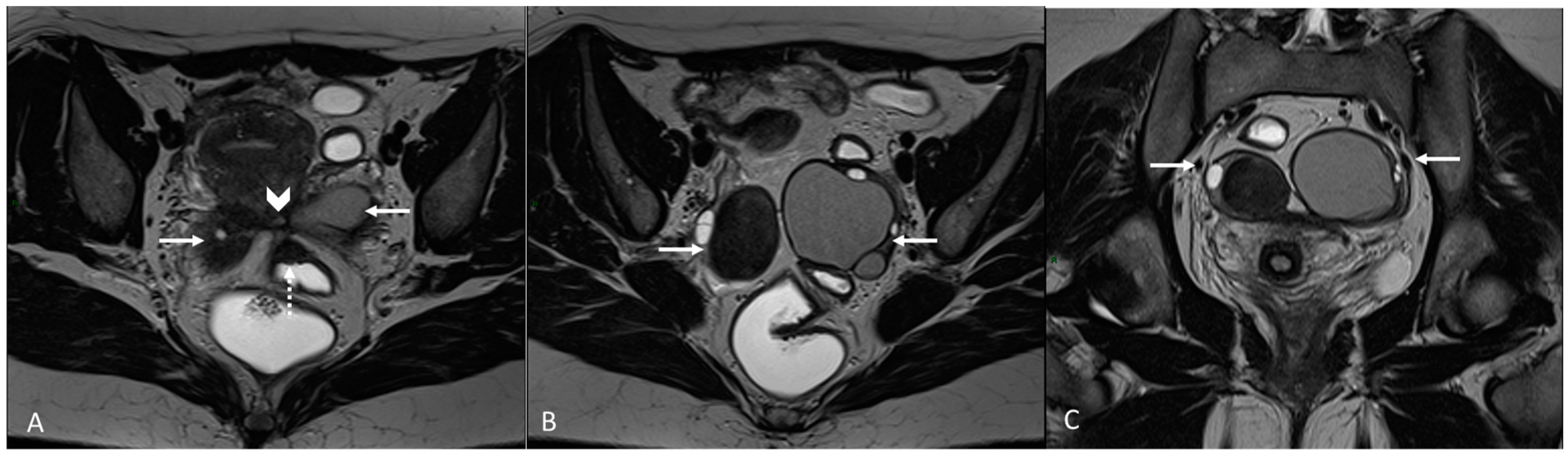
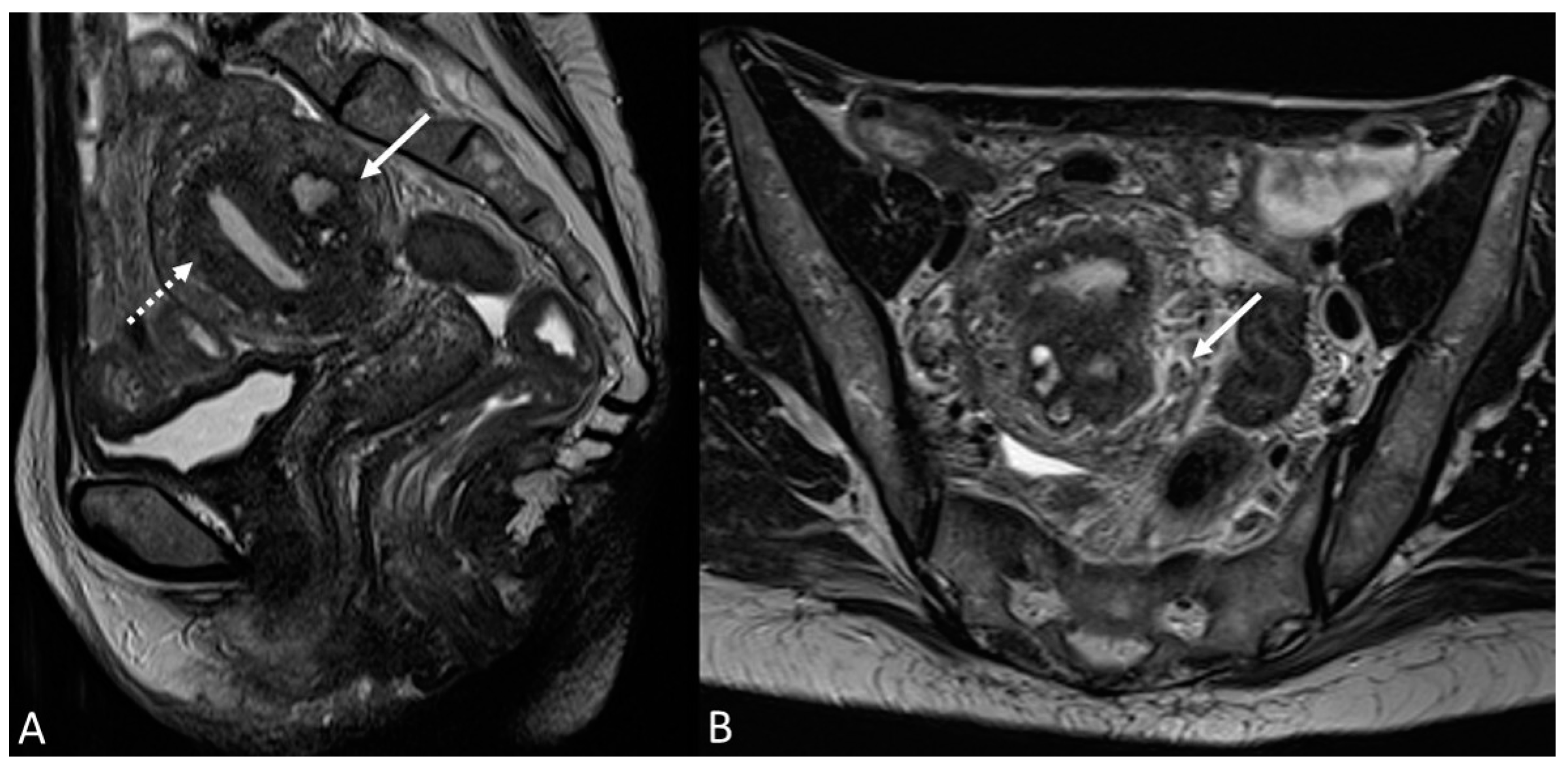
Figure 4.
Retrocervical endometriosis plaque, intestinal localization, kissing ovaries with bilateral ovarian endometriomas. (A,B) Axial T2WI; (C) Coronal T2WI. Hypointense retrocervical plaque (white arrowhead in (A)) localized in the uterine torus with involvement of the utero-sacral ligaments. Adhesions involve both ovaries with bilateral endometriomas (white arrows), which are prolapsed in a retro-uterine position and adhered to the utero-sacral ligaments and the recto-sigmoid junction. There is also an eccentric anterior parietal thickening in the recto-sigmoid junction suggestive of intestinal endometriosis lesion (white dotted arrow in (A)).
In patients with retroflexion of the uterus, evaluation of the cervical uterosacral ligament insertion may be particularly difficult at MRI.
3.2.4. Rectovaginal Septum
Endometriosis of the rectovaginal septum is often associated with the involvement of uterosacral ligaments, torus uterinus, posterior vaginal fornix and rectum [44].
A gynecological examination with vaginal exploration is sometimes the first step in recognizing the presence of palpable or visible plaques at this level [45].
With an MRI, it is possible to detect nodules or masses located between the vagina and the rectum, below the peritoneal reflection, with hypointense signal on T2WI, and with cystic components [7].
It is also important for detecting the involvement of the posterior fornix of the vagina, to allow for optimal surgical planning. Nodule or thickening of the posterior vaginal wall are the main signs of endometriosis localization detected with MRI [39].
In more extensive disease, it is possible to observe uterine retractile retroflexion and adhesions, which may result in frozen pelvis: a distortion of pelvic anatomy in which organs are attached together and cannot move freely or be separated during surgery.
3.2.5. Rectosigmoid Colon
The presence of endometriosis nodules in the context of the rectal wall generally causes pain (dyspareunia and chronic pain localized posteriorly), dyskinesia and proctorragia, the intensity of which depends on the degree of infiltration. Sometimes, rectal endometriosis is associated with an extensive involvement of the recto-vaginal septum with fibrosis that fills the Douglas pouch [41].
In more than half the cases, rectal lesions are associated with a second intestinal localization.
The rectum and rectum–sigmoid junction are the most involved sites due to their intraperitoneal position and proximity to the uterus and ovaries [35]. Other intestinal localizations will be described in the following paragraph on atypical localizations.
On an MRI, lesions appear as irregular thickness or nodules within the intestinal serosa or muscularis propria, hypointense at T2WI. They rarely affect the submucosa or the mucosa, and if infiltrated, we can observe edema and swelling in the area [7] (Figure 5 and Figure 6).
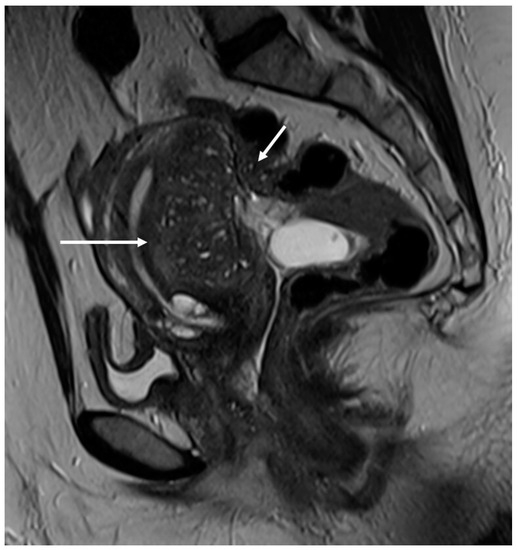
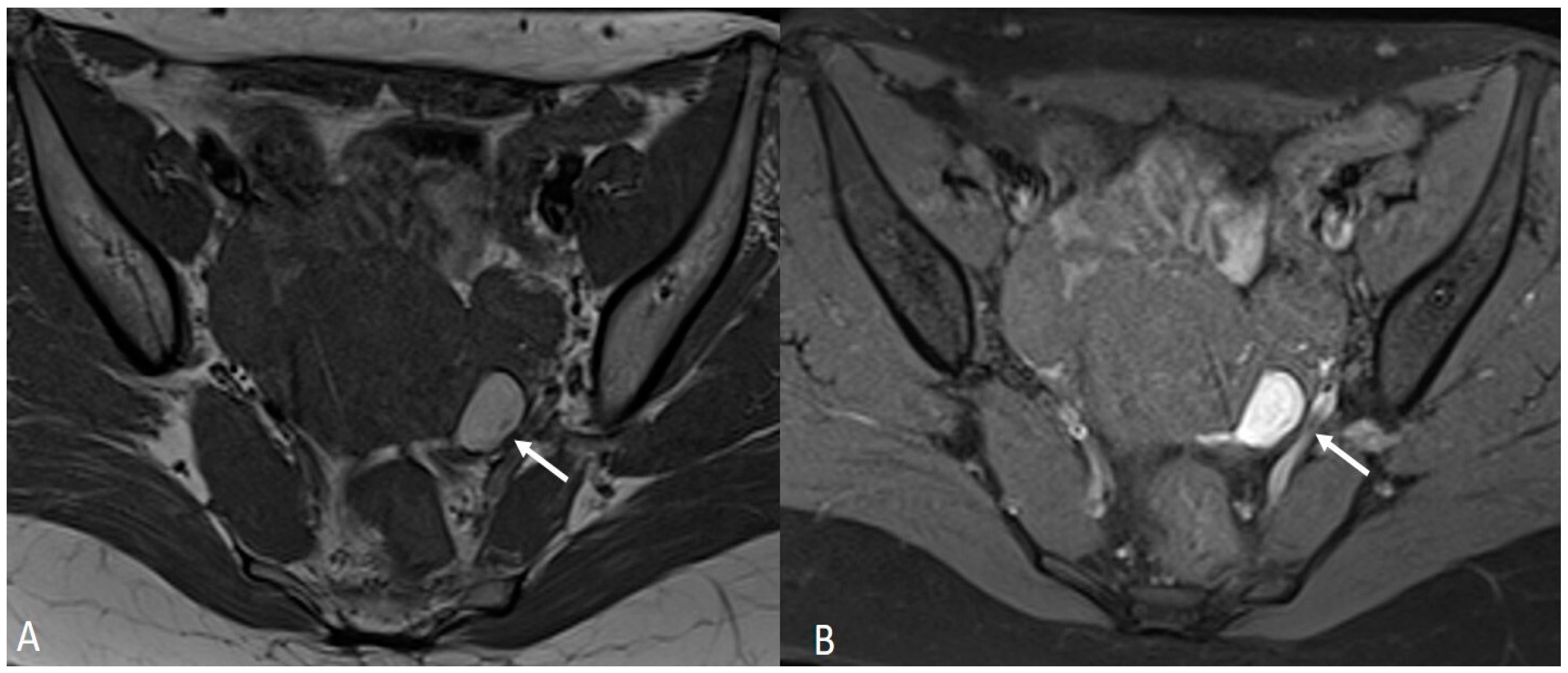
Figure 5.
External adenomyosis and intestinal endometriosis localization in 49-year-old female with story of multiple laparoscopy surgery. Sagittal T2WI shows a hypointense ill-defined subserosal mass in the posterior myometrium with hyperintense foci (long white arrow), suggestive for external adenomyosis, that cause retracting phenomena towards the anterior wall of the rectum. Additionally, there is deep endometriosis of the posterior compartment with hypointense nodule involving the serous and muscular layers of the anterior wall of the rectum (short white arrow).
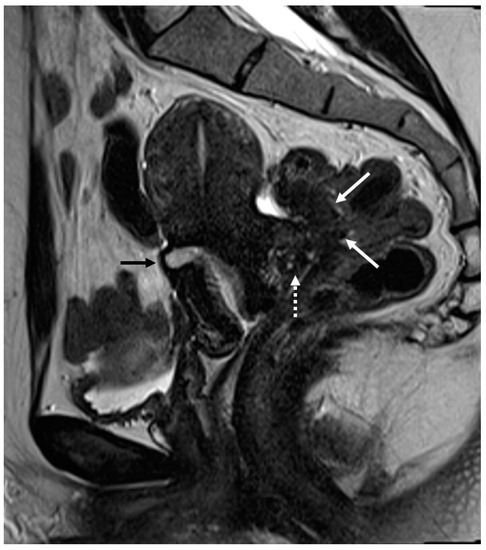
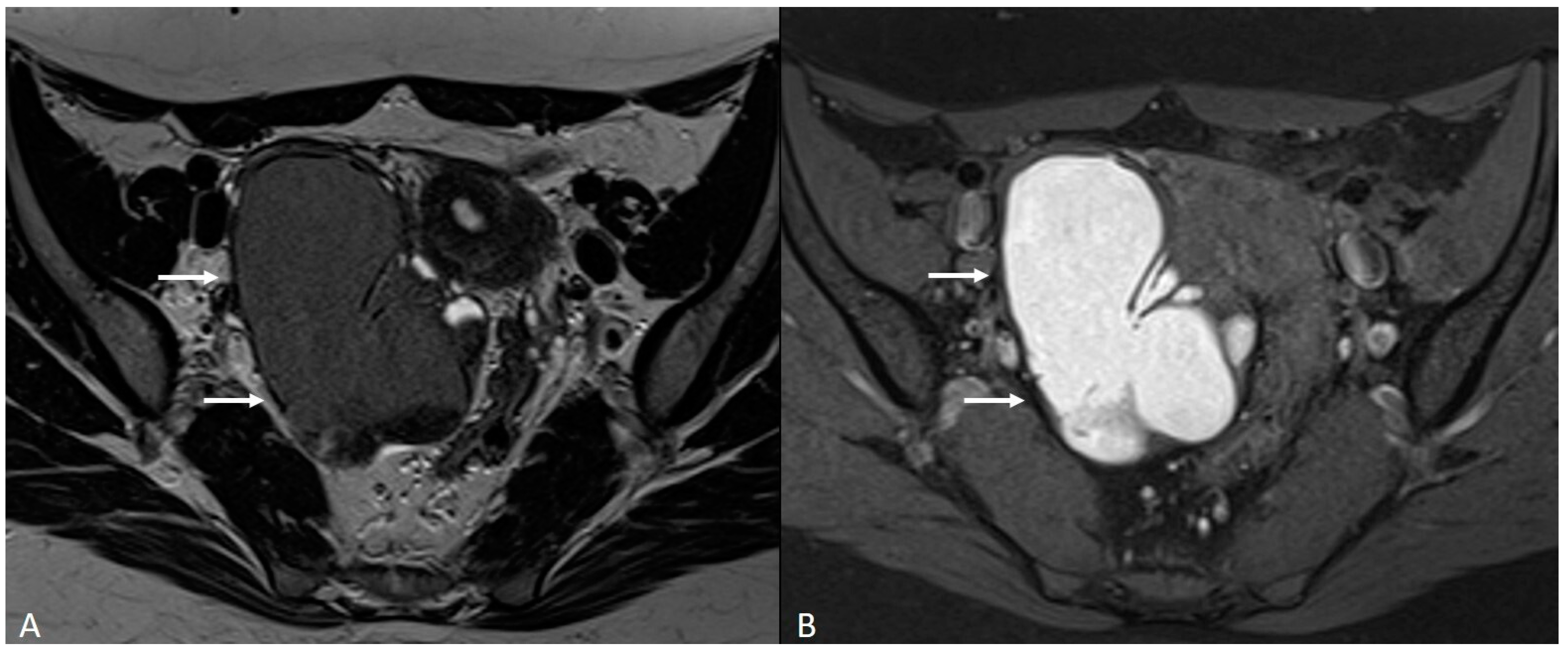
Figure 6.
Retrocervical localization, intestinal mushroom cap sign and isthmocele in a 40-years old female. Retrocervical endometriosis localization (white dotted arrow), intestinal “mushroom cap sign” (white arrows) and isthmocele (black arrow).
What is also important for the radiologist to report, besides the depth and length of the plaques, is the distance between the lesion and the anal margin, as this can affect the type of surgery to be conducted [46]. In severe cases, the MRI shows a circular involvement resulting in a clinical condition of occlusive or sub-occlusive stenosis [47].
4. Ovarian Endometrioma
The endometrioma, also known as “chocolate cysts”, develops when superficial endometriotic lesions invade the surface of the ovaries. As a result, the blood produced at each menstrual cycle fails to flow properly and accumulates in the context of the ovary, forming a cyst that can grow over time reaching up to 10–20 cm in exceptional cases.
They are often responsible for adhesions and, therefore, dislocation and fixity of the ovaries. The typical symptoms of an endometriosis cyst are pain and dysmenorrhea. In some cases, the presence of cysts interferes with proper ovulation, leading to reduced chances of conception and/or an increased risk of ectopic pregnancy [48,49].
Rupture of an endometrioma is considered a medical emergency with severe pain, dark blood loss from the vagina, fever, nausea, and vomiting.
Treatment can be pharmacological or surgical. Typically, small cysts require a medical approach, while large cysts require surgery [50].
On an MRI, endometriomas appear as cystic lesions with different signal characteristics according to the age of the blood and the type of hemoglobin present.
Generally, they appear hyperintense on fat-suppressed T1WI and T1WI (Figure 7 and Figure 8). The hyperintensity on fat-suppressed T1WI helps differentiate endometriomas from dermoid cyst and teratoma, which usually contain fat [51]. On T2WI, a variable signal can be obtained: a hypointense signal can affect variable portions of the cyst, sometimes also presenting a stratification, until a complete loss of the signal. This is called the shading sign and is correlated to the different state of hemoglobin degradation [52] (Figure 8). The T2 dark spot sign refers to hypointense spots in the wall of the cyst due to the presence of macrophages (Figure 9).
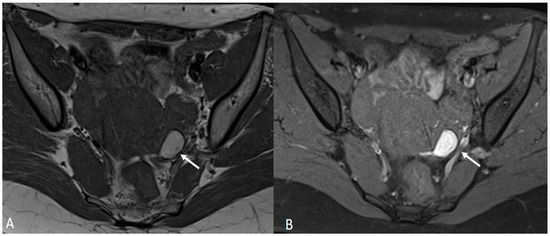
Figure 7.
Ovarian endometrioma in a 37-year-old female. (A) Axial T1WI; (B) Axial fat-suppressed T1WI. Typical aspect of an ovarian endometrioma (white arrows).
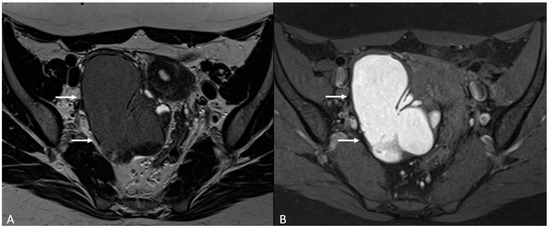
Figure 8.
Right tubo-ovarian endometriosis in a 25-year-old female patient with reported localized abdominal pain in the right iliac fossa, which increases intensity during the menstrual cycle. (A) Axial T2WI; (B) Axial fat-suppressed T1WI. Enlarged right adnexal cyst with incomplete septa denoting dilated tube (white arrows). The cystic content shows low signal intensity on T2WI and high signal intensity on fat-suppressed T1WI, consistent with hemorrhagic fluid.
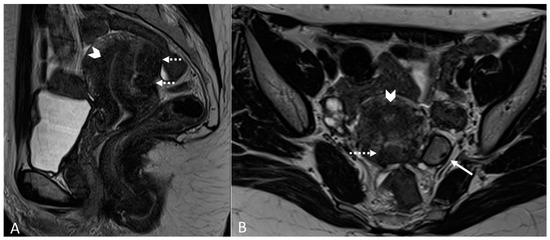
Figure 9.
Diffuse internal and external adenomyosis in a 37-year-old female. (A) Sagittal T2WI; (B) Axial T2WI. Diffuse internal adenomyosis as demonstrated by diffuse hypertrophy of the JZ (white arrowhead in (A,B)), subserosal hypointense ill-defined mass in the posterior myometrium consistent with external adenomyosis (white dotted arrows in (A,B)), with associated deep endometriosis of posterior compartment. Left endometrioma with T2 dark spot sign (white arrow in (B)).
Contrast agents may exclude the presence of enhancing nodules that can be indicative of malignant transformation. MRI is also a valuable aid in achieving a proper differential diagnosis between endometrioma and other ovarian cystic lesions, such as mature cystic teratoma, hemorrhagic cyst, cystadenoma, cystadenofibroma and malignant cystic neoplasm. We can distinguish the main ovarian cystic lesions based on the following magnetic resonance characteristics [53].
Mature cystic teratoma is macroscopically characterized by a multicystic mass containing hair, teeth, and/or skin. Occasionally, a solid component known as Rokitansky’s nodule can be observed within it [54,55]. The abundant presence of adipose tissue allows for a differential diagnosis from endometriomas, as a signal reduction will be observed in sequences with adipose signal suppression [56].
Hemorrhagic ovarian cysts (HOCs) develop when bleeding occurs within a follicle or follicular cyst. On an MRI, hemorrhagic cysts exhibit high signal intensity on pre-contrast fat-suppressed T1WI and low signal intensity on T2WI. They do not show enhancement after gadolinium administration [54]. In some cases, a correct differential diagnosis can be aided by considering the patient’s clinical presentation, which may involve acute abdominal symptoms, contrasting with the chronic and cyclic pain associated with endometriomas [57].
Cystadenoma and cystadenofibroma are benign ovarian tumors that can exhibit either serous or mucinous characteristics. They may manifest as cystic formations containing fluid or hematic contents, accompanied by the presence of septations and/or enhancement of the cyst wall following contrast administration [56,58].
Malignant cystic neoplasm typically demonstrates signal intensity distinct from fat and blood, and they may be accompanied by features such as lymphadenopathy, abdominal effusion, and peritoneal implants. The clinical presentation and laboratory tests can also resemble those of a malignant condition [57,59].
5. Adenomyosis
Adenomyosis is a disease characterized by the presence of endometrial tissue (glands and stroma) in the context of the myometrium. It is much more common in pluripares and in women undergoing surgery of the uterus [60]. Generally, women complain of extremely severe pain during menstruation and suffer from abdominal cramps, abundant and irregular menstruation, fertility problems and adverse pregnancy outcomes [61,62].
Over the years, authors have proposed different classifications that could collate the different facets of adenomyosis. Among the most recent and complete classifications is the one proposed by Kobaiashi et al., which specifically selected five parameters to describe the disease: (1) the affected area (internal adenomyosis or external adenomyosis); (2) the pattern (diffuse or focal); (3) the size; (4) the location; and (5) the concomitance of other localization of endometriosis or other gynecological pathologies [63].
In order to discuss internal endometriosis, it is essential to introduce the concept of the junctional zone. The junctional zone represents the intermediate layer of the myometrium, which typically exhibits a low signal intensity on T2WI sequences, and appears as a continuous structure under normal physiological conditions [64,65]. The thickness of the junctional zone can vary throughout a woman’s life based on factors such as age and hormonal therapies. Additionally, it undergoes slight changes on a monthly basis in accordance with the different phases of the menstrual cycle [66,67].
The agreement among the authors is that a thickening of the junction zone (>12 mm) serves as an indirect indication of internal adenomyosis. Nevertheless, according to the recent literature, this particular sign cannot be considered conclusive evidence of the disease on its own. It is, therefore, advised to assess two additional parameters for a comprehensive evaluation [68]:
- -
- The parameter known as “ratiomax” corresponds to the ratio between JZmax and the total thickness of the myometrium, with a value exceeding 40% typically considered an acceptable diagnostic parameter [68];
- -
- The parameter called “JZ differential” (JZdiff) measures the maximum and minimum thickness difference between the anterior and posterior uterine walls, where a value greater than 5 mm is indicative [68].
The aforementioned criteria serve as indirect indicators that can aid radiologists in reporting cases of internal adenomyosis (Figure 9). On the other hand, direct criteria include the observation of cystic areas (hyperintense on T2WI) and/or hemorrhagic foci (hyperintense on fat-suppressed T1WI and T1WI) within the inner layer of the myometrium [67,69] (Figure 10).
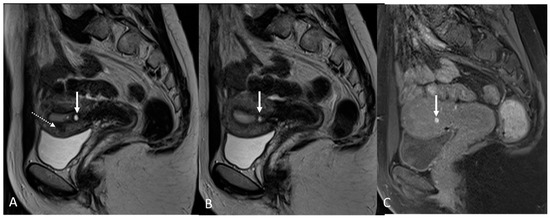
Figure 10.
Focal internal adenomyosis and focal uterine contraction in 36-year-old female with dysmenorrhea, chronic pelvic pain, dyspareunia and dyschezia. (A) Sagittal T2WI; (B) Sagittal T2WI; (C) Sagittal fat-suppressed T1WI. Isolated subendometrial cyst with hemorrhagic content without JZ hypertrophy (white arrow), consistent with focal internal adenomyosis, and a transient uterine contraction in the anterior myometrium (white dotted arrow in (A)).
The ENDOVALIRM group considers external adenomyosis as part of mediocentral localization of DIE, both in anterior and posterior subtypes. The external adenomyosis corresponds to an extrinsic involvement of the outer myometrial layers. It is more common that external adenomyosis involves the posterior compartment in association with a severe involvement of the rectum–vaginal space [39].
On T2WI, an irregular thickening of the subserosal myometrium is observed, sometimes in association with hyperintense spots that correspond to cystic components. Another fundamental criterion is the presence of blood components, which can be evaluated in the sequences weighed on T1 [69,70] (Figure 5 and Figure 9).
However, there are instances where adenomyosis can be localized, known as adenomyoma. It is crucial to accurately differentiate adenomyosis from uterine fibroids, as fibroids often cause more noticeable distortion of the uterine contour and exhibit greater differentiation from the surrounding myometrium. This distinction may also be aided by the presence of a pseudocapsule in fibroids [71].
It is also important for differential diagnosis, even with transient uterine contractions. During uterine contractions, a temporary localized area of decreased signal intensity can occur along with thickening of the junctional zone. This natural process may mimic the appearance of focal or diffuse adenomyosis. If there is uncertainty regarding the nature of a bulge or a region of low intensity in the myometrium, it becomes the responsibility of the radiologist to repeat the imaging after an appropriate interval, typically within 20 to 45 min [72,73] (Figure 10).
On T2WI, adenomyoma presents as an ill-defined hypointense mass within the endometrial wall generally without involving the junctional zone. It may present a heterogeneous aspect due to the high-intensity cystic components (on T2WI) or hemorrhagic cavities > 5 mm (hyperintense on T1WI) [71] (Figure 11).
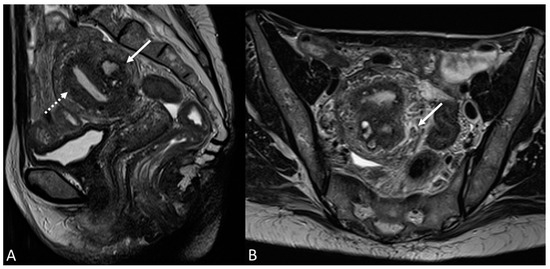
Figure 11.
Cystic adenomyoma and diffuse adenomyosis in a young female. (A) Sagittal T2WI; (B) Axial T2WI. Cystic adenomyoma of the posterior uterine wall (white arrow) and diffuse internal adenomyosis (white dotted arrow in (A)).
Bazot et al. proposed a classification based on the three main subtypes of adenomyoma: (1) intramural adenomyoma; (2) submucosal adenomyoma; and (3) subserous adenomyoma [74].
Intramural adenomyoma can manifest in two variants: the solid variant and the cystic variant. The cystic variant is characterized by a higher presence of fluid and, in some instances, even hemorrhagic components. It is less common compared to the solid variant and tends to occur more frequently in women over 30 years of age [75].
The submucosal adenomyoma appears instead as an ill-defined mass that can protrude into the uterine cavity, resembling a polypoid mass. The subserous adenomyoma, on the other hand, is characterized by a poorly defined mass with small cystic elements located in the subserosal region [67,76].
6. Atypical Site
Extrapelvic endometriosis is extremely rare and is generally associated with extensive pelvic involvement. The diagnosis is often delayed over the years because the symptoms are non-specific and, in any case, the definitive confirmation is often only obtained with post-operative histological analysis [77]. The catamenial nature of the symptoms, a cyclical pain, worsening during the menstrual cycle may suggest diagnosis, especially in young women when other more common differential diagnoses were excluded [78].
6.1. Soft Tissues Localization
Abdominal endometriosis is often associated with a cutaneous scar from a gynecologic procedure, notably from a cesarean section. Lesions are generally located in subcutaneous soft tissues or inside the muscle layer [79] (Figure 12).
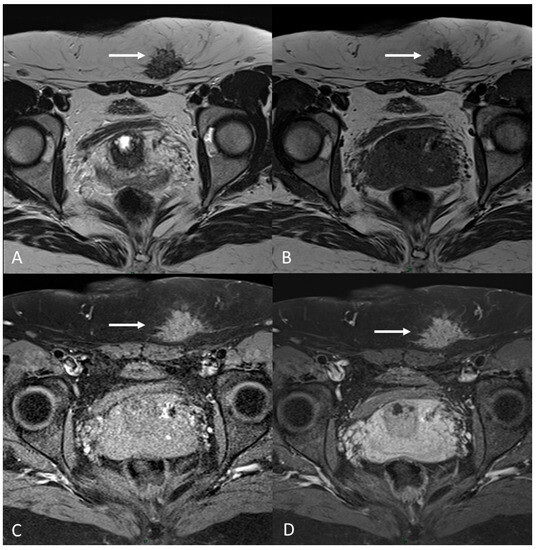
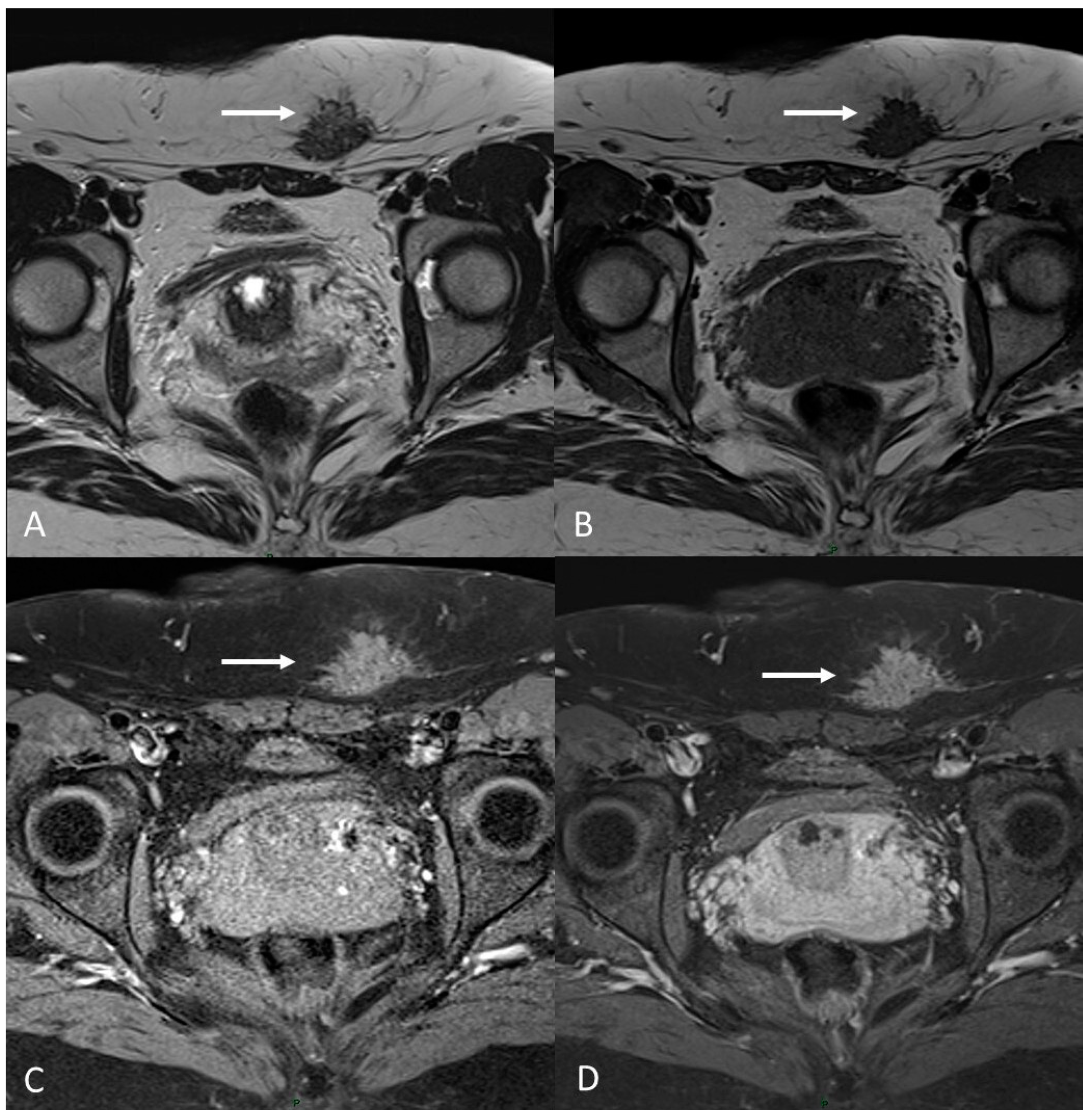
Figure 12.
C-section scar endometriosis in a 38-year-old female with subcutaneous nodule causing increasing pain during menstrual cycle. (A,B) Axial T2WI, T1WI. Nodular hypointense lesion with spiculated margins in subcutaneous soft tissues of abdominal wall; (C) Axial fat-suppressed T1WI. The lesion shows small hyperintense foci; (D) Axial T1WI post-contrast medium. The lesion shows homogenous contrast enhancement (white arrows).
The patient often realizes the presence of a mass or an abnormal thickening at the site of the scar. Ultrasound is generally the first level diagnostic test, useful to exclude much more common alterations such as lipomas or hernias, followed by a magnetic resonance exam [80].
MRI increases the specificity for diagnosis while detecting hemorrhagic spots within the fibrotic tissue.
The other atypical site of cutaneous endometriosis is the umbilicus, followed by the inguinal region [81].
In the literature, there are also much rarer cases of disease localization within the context of striated muscles. For example, Chiaramonte et al. described the case of a 38-year-old woman with endometriosis of the external oblique muscle, complicated by the onset of deep venous thrombosis of the iliac vein [82].
The risk of such localizations, especially if asymptomatic or associated with nonspecific symptoms, is the delay in diagnosis and consequently in disease management, with the risk of developing complications [83].
6.2. Gastrointestinal Localization
The bowel is the most common extragenital site in about a third of women with DIE. The associated symptoms can be multiple and nonspecific, such as constipation, diarrhea, melena, hematochezia, tenesmus, abdominal discomfort or meteorism. Excluding the rectum and the rectosigmoid junction, the most affected sites are the appendix, cecum and distal ileum. The enteric nodules generally leave the mucosa intact [84].
6.3. Thoracic Endometriosis
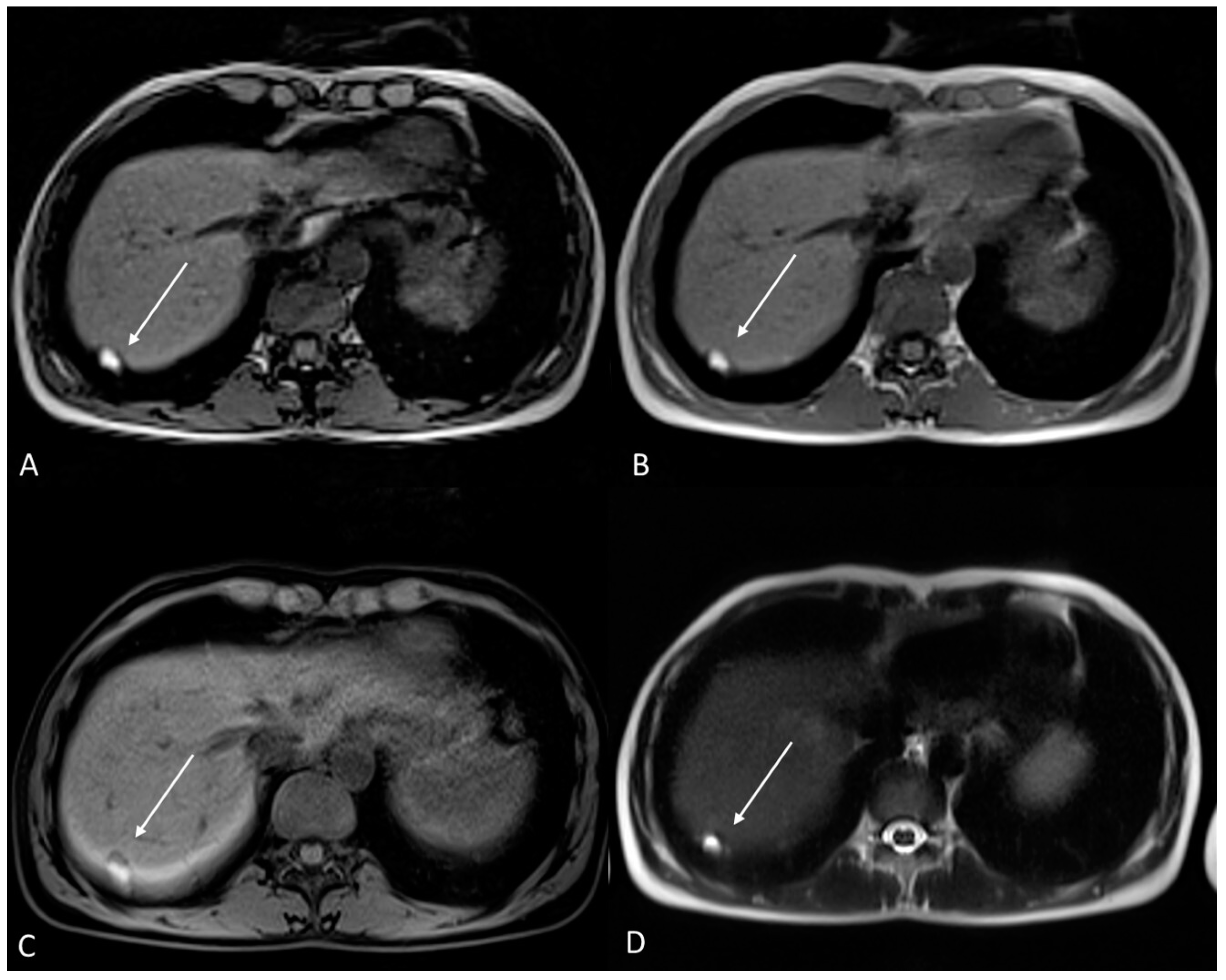
Thoracic and pleuric endometriosis are the most frequent extrapelvic manifestations, which are called thoracic endometriosis syndrome (TES). The pleuric disease could manifest as pneumo/hemo-thorax or pneumomediastinum associated with chest pain, dyspnea and sometimes hemoptysis. The pulmonary form of the disease presents as catamenial hemoptysis, pulmonary nodules or diaphragm foci [85] (Figure 13).
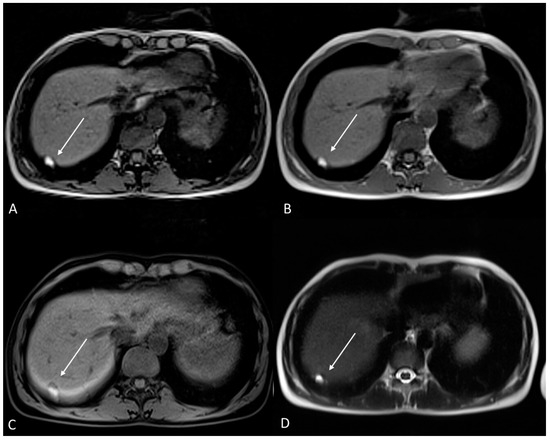
Figure 13.
Diaphragmatic endometriosis in a 33-year-old female with right shoulder pain during menstrual cycle. (A) Axial T1WI out of phase; (B) Axial T1WI in phase; (C) Axial fat-suppressed T1WI; (D) Axial T2WI. Atypical localization of endometriosis at the level of diaphragm on the right. The lesion has high signal intensity on T1WI and on T2WI due to hemorrhagic content (white arrows).
Pericardial endometriosis is reported only in a few cases in the literature.
6.4. Neural Involvement
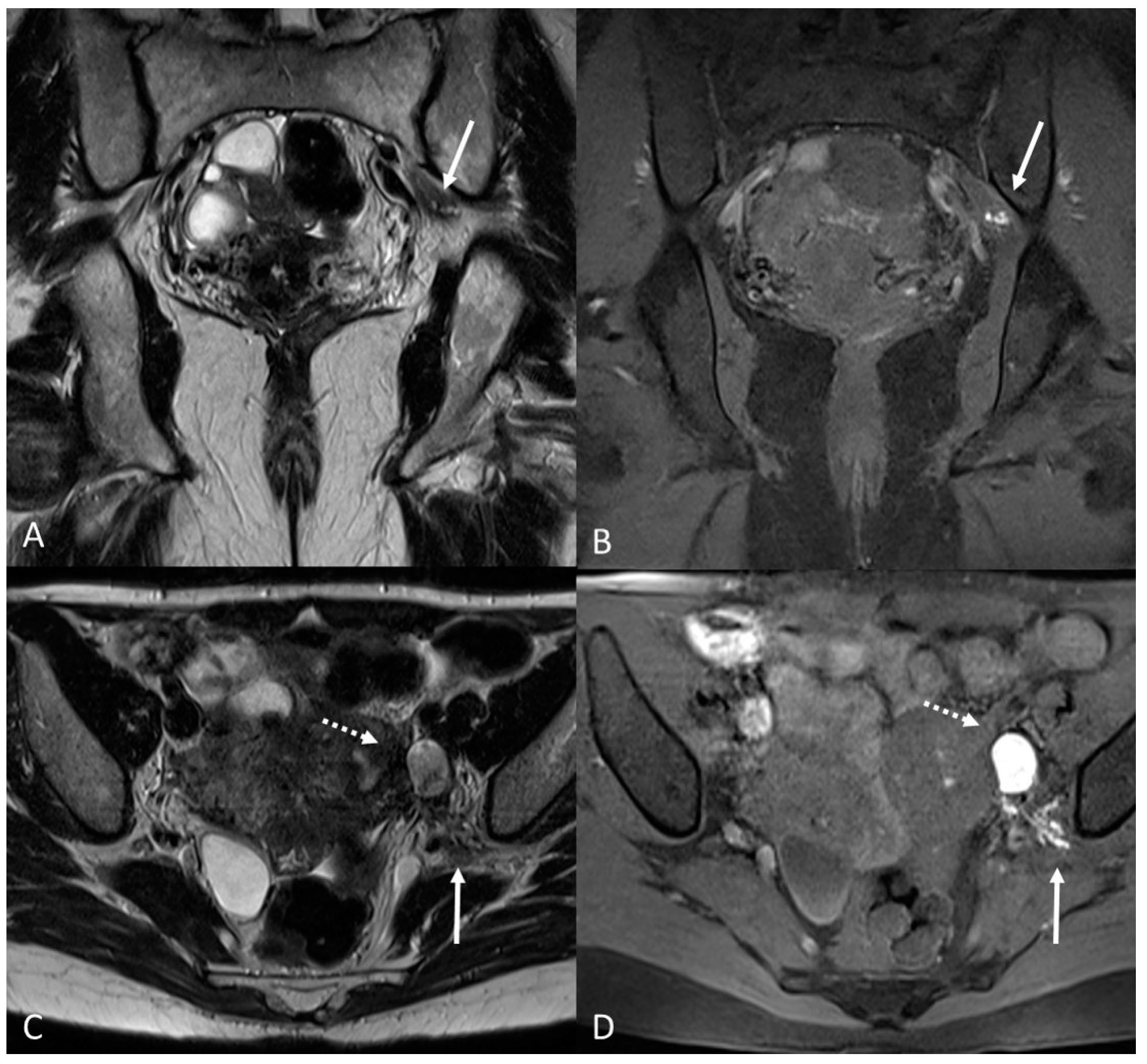
There are also cases of involvement of peripheral nerve plexuses of the pelvic region, such as sciatic, obturator, femoral, and pudendal nerves and their branches (Figure 14).
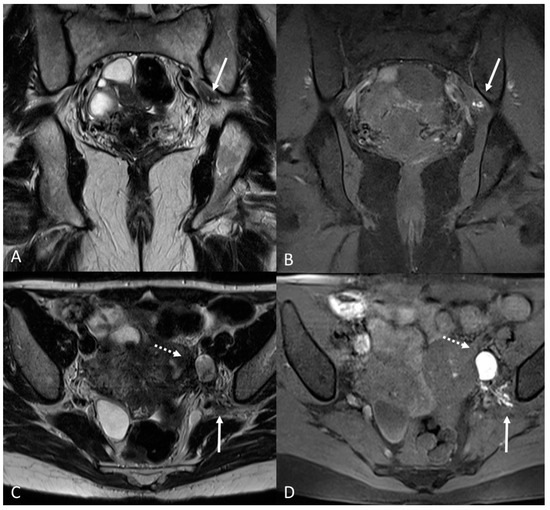
Figure 14.
Localization of the left sciatic nerve and para-uterine endometriotic nodule in a 43-year-olf female with a previously diagnosed endometriosis and surgery approach, accepted at the emergency department for reported left lumbar pain, which radiates to the left side and is associated with episodes of vomiting. (A) Coronal T2WI. Involvement of peripheral nerve plexuses of the pelvic region (sciatic nerve) appearing as a hypointense plaque (white arrow); (B) Coronal fat-suppressed T1WI. The lesion shows small hematic foci (white arrow); (C) Axial T2WI. Para-uterine endometriotic nodule (white dotted arrow) shows variable intensity ranging from low intensity (referred to as shading) to intermediate or high intensity; (D) Axial T1WI. Para-uterine endometriotic nodule with hyperintense signal (white dotted arrow).
MRI is the best imaging modality to investigate neural involvement [78].
Endometriosis lesions in the central nervous system are even rarer [86].
7. Post-Operative Imaging
Treatment approaches have to be assessed based on multiple factors.
Medical therapy is definitely the first-line therapeutic option for patients with manageable pelvic pain and no desire for immediate pregnancy. It consists of combined oral contraceptives, progesterone or estrogen. In the second line, analogs of GnRH can be used. Medical therapy also includes specific pain medications and can often be a combination of drugs [87].
On the other hand, surgery is necessary in case of symptoms not controlled by medical therapy, massive or growing endometriomas, infertility or important involvement of the abdominal organs with high risk for systemic complications. In women with infertility, the opportunity for assisted reproduction technologies before performing surgery should be considered [88].
The approach is generally laparoscopical with minimally invasive techniques, and it consists of the identification and removal of endometriotic foci and the restoration of the correct pelvic anatomy [35].
The laparotomy approach is reserved for rare cases where lesions are much more extensive.
Post-operative MRI can be useful both to document the success of the surgery with normal post-operative findings and to highlight some paraphysiological conditions such as the presence of fibrosis, adhesions or even alterations of the pelvic anatomy that can contribute to symptoms or disorders. It is also possible that a CT scan or MRI may be necessary to assess post-operative complications or the presence of recurring or residual disease [89].
In this paragraph, we will explore the main signs in post-operative imaging in order to provide the radiologist with an indication to evaluate MRIs of women with extensive and complex endometriosis undergoing surgery (Figure 15 and Figure 16).
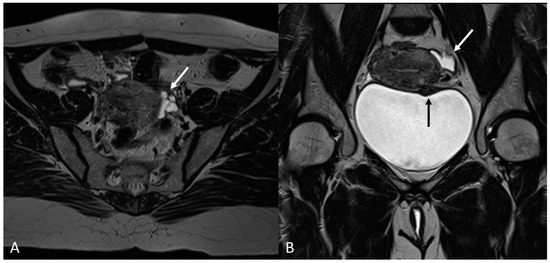
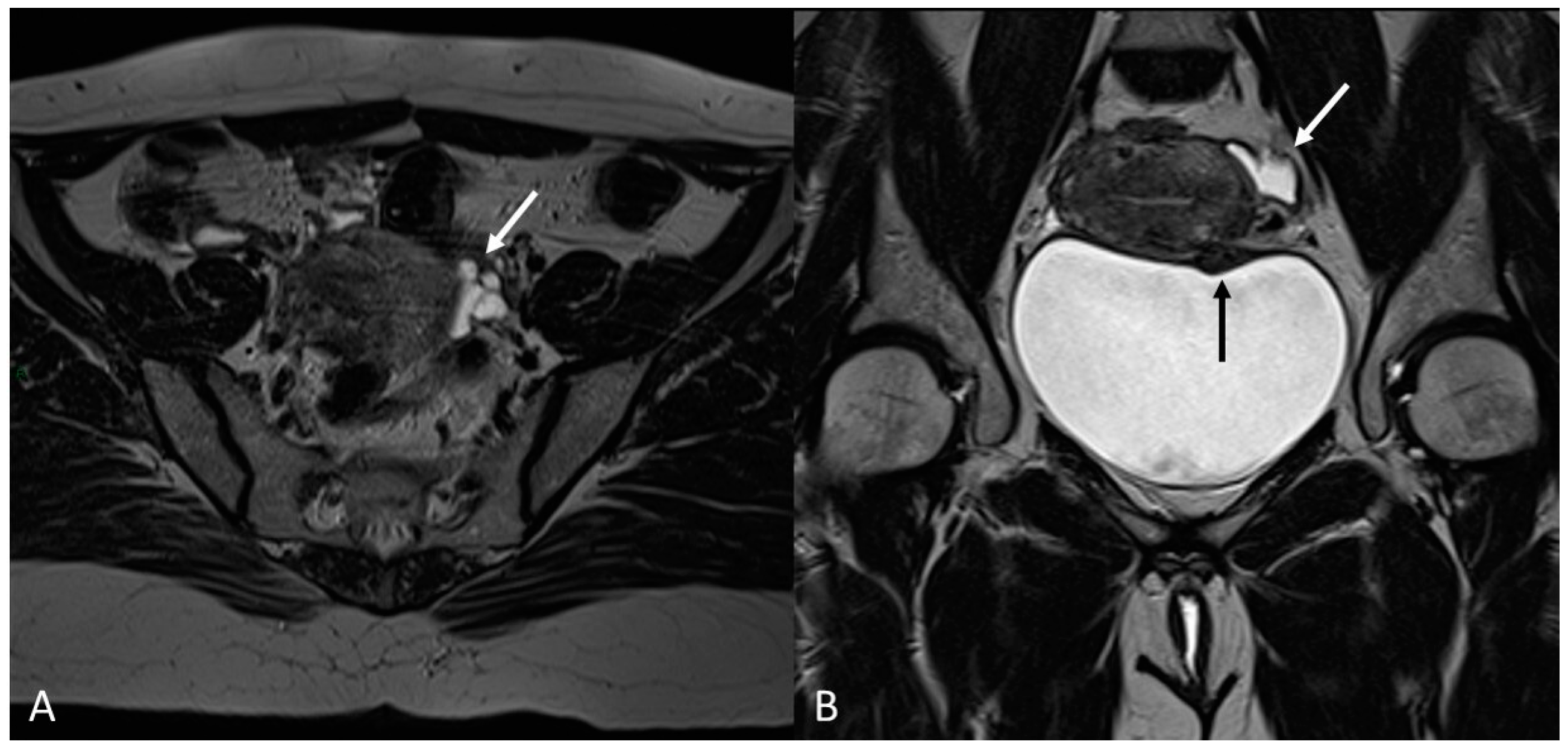
Figure 15.
Para-uterine fluid collection in a 43-year-old female who underwent several gynecological surgeries for deep infiltrative endometriosis. (A) Axial T2WI. Compartmentalized hyperintense collection in the left para-uterine area after pelvic surgery (white arrow). (B) Coronal T2WI. Endometriotic localization of the vescico-uterine pouch (black arrow).
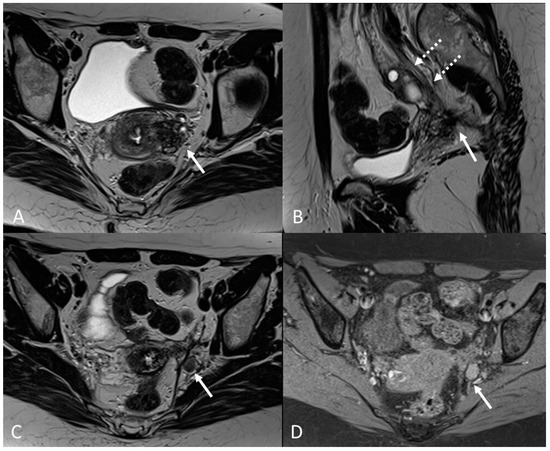
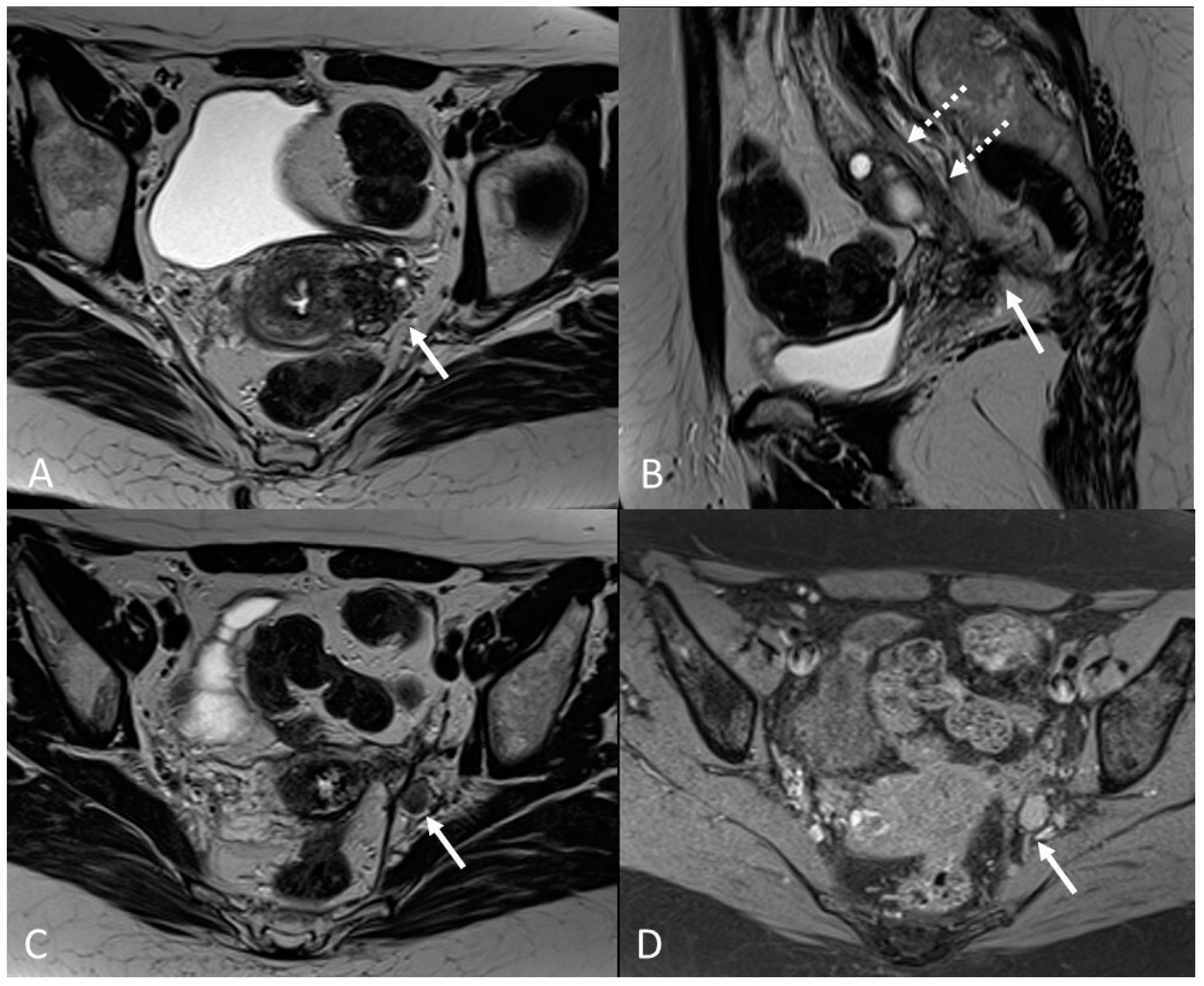
Figure 16.
Localization of endometriosis in the left parametrium and ureteral stenosis in 49-year-old female who underwent supracervical hysterectomy, bilateral salpingectomy and mild vaginal bleeding and abdominal pain. (A) Axial T2WI; (B) Sagittal T2WI. Endometriotic localization of the left parametrium (white arrow), with irregular margins and heterogeneous signal intensity. Tractions towards the sigmoid colon, left pararectal fascia, anterior pelvic peritoneal reflection and adnexa. Ureteral stenosis caused by the parametrial plaque (dotted arrows in (B)). (C) Axial T2WI; (D) Axial fat-suppressed T1WI. Endometriotic nodule of the left pelvic wall (white arrow).
According to Chamié et al., the first pelvic post-operative MRI should be performed after 6 months. It could be anticipated in selected cases of women who are undergoing medically assisted procreation for infertility [89,90].
One of the main challenges for the radiologist is to make a correct differential diagnosis between post-operative fibrosis and recurring/residual disease.
MRI can highlight the difference between these two conditions: fibrotic scars or plaques appear as bands of hypointensity on T2WI, without areas of cystic degeneration, blood foci (hyperintense in T1WI) or even nodular thickenings that are otherwise typical signs of endometriotic lesions [89].
The posterior compartment is the most involved in cases of DIE and therefore often a location of surgical resections.
MRI after surgery may show an alteration of the physiological morphology of the pelvis due to the presence of adhesions and sometimes because of the obliterations of anatomical spaces and pouch without a fat tissue interface between the bladder, uterus, vagina and rectum [91].
Thinning of the uterine or vaginal wall may also be observed; this alteration can lead to an increased risk of perforation or rupture of the aforementioned organs.
The surgical management of intestinal endometriosis is a prerogative of both gynecology and general surgery. A careful pre-operative evaluation is essential to plan the best possible surgery, in order to be as radical as possible without compromising the quality of life of patients [92].
As described above, endometrial lesions tend to be located primarily in the rectal or sigmoid tract. Resections can be segmental, discoid, or more extensive.
The presence of free intraperitoneal fluid or gas associated with severe symptomatology may indicate post-operative complications such as perforation or fistula.
Surgery for the treatment of endometrioma should also be mentioned.
Some studies have also documented the possible reduction in the reserve of oocytes; therefore, the patient must be informed, and appropriate assessments must be carried out in pre-operative planning [48].
On an MRI, the ovary treated may appear smaller than the contralateral one. Hypointense alterations on T1WI and T2WI may indicate fibrotic sequelae, which can lead to adhesions and dislocation of ovaries [93].
The residues of the disease lead to the regrowth of the endometriotic cyst in the same place where it was removed.
In the case of recurrence, endometrioma may occur in the same ovary or in the contralateral one [94].
To conclude, for the urgent management of early post-operative complications, contrast CT scans provide the radiologist with an initial assessment. The most worrying conditions include hemorrhage, infection, and visceral perforation, as well as abscesses, signs of tubo-ovarian inflammation, and the existence of abdominal free gas [95].
Cystography CT or a CT urography protocols are also suitable for assessing complications of the anterior compartment, such as a fistula between the bladder and vagina or laceration of the bladder or ureters.
8. Conclusions
The aim of our study is to report the importance of MRI for the diagnoses of endometriosis, both for its usual locations and for the unusual ones, such as thoracic and pleuric endometriosis or neural involvement.
For the diagnostic process, it is fundamental to have an adequate protocol in order to detect the lesions, including a T2-weighted sequence in at least two orthogonal planes (sagittal and axial), a T1-weighted sequence with or without fat suppression to detect adnexal endometriosis and active foci of deep infiltrating endometriosis, and a T2-star-weighted imaging sequence for the detection of hemosiderin.
Furthermore, the evolving role of AI in the diagnosis of endometriosis is a promising avenue. In fact, it could be a valuable tool to assist radiologists in identifying and characterizing lesions, showcasing the substantial impact on clinical practice in terms of accuracy and efficiency.
Author Contributions
Conceptualization, C.L.P.; methodology, C.L.P. and M.S.; software, C.L.P., L.C. and M.S.; validation, C.L.P., M.S. and C.D.C.N.; formal analysis, C.L.P. and M.S.; investigation, C.L.P., L.C., M.S. and A.M.D.N.; resources, L.C. and A.M.D.N.; data curation, L.C. and A.M.D.N.; writing—original draft preparation, C.L.P., L.C., M.S. and A.M.D.N.; writing—review and editing, C.L.P., L.C., M.S. and A.M.D.N.; visualization, C.L.P., M.S. and C.D.C.N.; supervision, C.L.P. and M.S.; project administration, C.L.P., M.S., E.F., R.F.G. and B.B.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Horne, A.W.; Missmer, S.A. Pathophysiology, Diagnosis, and Management of Endometriosis. BMJ 2022, 379, e070750. [Google Scholar] [CrossRef] [PubMed]
- Koninckx, P.R.; Ussia, A.; Adamyan, L.; Wattiez, A.; Gomel, V.; Martin, D.C. Pathogenesis of Endometriosis: The Genetic/Epigenetic Theory. Fertil. Steril. 2019, 111, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Amro, B.; Ramirez Aristondo, M.E.; Alsuwaidi, S.; Almaamari, B.; Hakim, Z.; Tahlak, M.; Wattiez, A.; Koninckx, P.R. New Understanding of Diagnosis, Treatment and Prevention of Endometriosis. Int. J. Environ. Res. Public Health 2022, 19, 6725. [Google Scholar] [CrossRef] [PubMed]
- Koninckx, P.R.; Fernandes, R.; Ussia, A.; Schindler, L.; Wattiez, A.; Al-Suwaidi, S.; Amro, B.; Al-Maamari, B.; Hakim, Z.; Tahlak, M. Pathogenesis Based Diagnosis and Treatment of Endometriosis. Front. Endocrinol. 2021, 12, 745548. [Google Scholar] [CrossRef]
- Taylor, H.S.; Kotlyar, A.M.; Flores, V.A. Endometriosis Is a Chronic Systemic Disease: Clinical Challenges and Novel Innovations. Lancet 2021, 397, 839–852. [Google Scholar] [CrossRef]
- Yu, E.H.; Joo, J.K. Commentary on the New 2022 European Society of Human Reproduction and Embryology (ESHRE) Endometriosis Guidelines. Clin. Exp. Reprod. Med. 2022, 49, 219–224. [Google Scholar] [CrossRef]
- Bazot, M.; Darai, E.; Hourani, R.; Thomassin, I.; Cortez, A.; Uzan, S.; Buy, J.-N. Deep Pelvic Endometriosis: MR Imaging for Diagnosis and Prediction of Extension of Disease. Radiology 2004, 232, 379–389. [Google Scholar] [CrossRef]
- Zanardi, R.; Del Frate, C.; Zuiani, C.; Bazzocchi, M. Staging of Pelvic Endometriosis Based on MRI Findings versus Laparoscopic Classification according to the American Fertility Society. Abdom. Imaging 2003, 28, 733–742. [Google Scholar] [CrossRef]
- Kim, C.H.; Lee, D.Y.; Moon, D.H.; Lee, S. Percutaneous Cryoablation of Multiple Pulmonary Endometriosis. J. Chest Surg. 2021, 54, 75–78. [Google Scholar] [CrossRef]
- Welch, B.T.; Ehman, E.C.; VanBuren, W.M.; Cope, A.G.; Welch, T.L.; Woodrum, D.A.; Kurup, A.N.; Burnett, T.L. Percutaneous Cryoablation of Abdominal Wall Endometriosis: The Mayo Clinic Approach. Abdom. Radiol. 2020, 45, 1813–1817. [Google Scholar] [CrossRef]
- Bazot, M.; Bharwani, N.; Huchon, C.; Kinkel, K.; Cunha, T.M.; Guerra, A.; Manganaro, L.; Buñesch, L.; Kido, A.; Togashi, K.; et al. European Society of Urogenital Radiology (ESUR) Guidelines: MR Imaging of Pelvic Endometriosis. Eur. Radiol. 2017, 27, 2765–2775. [Google Scholar] [CrossRef] [PubMed]
- Bazot, M.; Thomassin, I.; Hourani, R.; Cortez, A.; Darai, E. Diagnostic Accuracy of Transvaginal Sonography for Deep Pelvic Endometriosis. Ultrasound Obstet. Gynecol. Off. 2004, 24, 180–185. [Google Scholar] [CrossRef]
- Mais, V.; Guerriero, S.; Ajossa, S.; Angiolucci, M.; Paoletti, A.M.; Melis, G.B. The Efficiency of Transvaginal Ultrasonography in the Diagnosis of Endometrioma. Fertil. Steril. 1993, 60, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Chamié, L.P.; Blasbalg, R.; Pereira, R.M.A.; Warmbrand, G.; Serafini, P.C. Findings of Pelvic Endometriosis at Transvaginal US, MR Imaging, and Laparoscopy. Radiographics 2011, 31, E77–E100. [Google Scholar] [CrossRef]
- Guerriero, S.; Ajossa, S.; Orozco, R.; Perniciano, M.; Jurado, M.; Melis, G.B.; Alcazar, J.L. Accuracy of Transvaginal Ultrasound for Diagnosis of Deep Endometriosis in the Rectosigmoid: Systematic Review and Meta-Analysis. Ultrasound Obstet. Gynecol. 2016, 47, 281–289. [Google Scholar] [CrossRef]
- Kinkel, K.; Chapron, C.; Balleyguier, C.; Fritel, X.; Dubuisson, J.B.; Moreau, J.F. Magnetic Resonance Imaging Characteristics of Deep Endometriosis. Hum. Reprod. 1999, 14, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Nisenblat, V.; Bossuyt, P.M.M.; Farquhar, C.; Johnson, N.; Hull, M.L. Imaging Modalities for the Non-Invasive Diagnosis of Endometriosis. Cochrane Database Syst. Rev. 2016, 2, CD009591. [Google Scholar] [CrossRef] [PubMed]
- Ciggaar, I.A.; Henneman, O.D.F.; Oei, S.A.; Vanhooymissen, I.J.; Blikkendaal, M.D.; Bipat, S. Bowel Preparation in MRI for Detection of Endometriosis: Comparison of the Effect of an Enema, no Additional Medication and Intravenous Butylscopolamine on Image Quality. Eur. J. Radiol. 2022, 149, 110222. [Google Scholar] [CrossRef]
- Yang, R.K.; Roth, C.G.; Ward, R.J.; de Jesus, J.O.; Mitchell, D.G. Optimizing Abdominal MR Imaging: Approaches to Common Problems. Radiographics 2010, 30, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Gutzeit, A.; Binkert, C.A.; Koh, D.-M.; Hergan, K.; von Weymarn, C.; Graf, N.; Patak, M.A.; Roos, J.E.; Horstmann, M.; Kos, S.; et al. Evaluation of the Anti-Peristaltic Effect of Glucagon and Hyoscine on the Small Bowel: Comparison of Intravenous and Intramuscular Drug Administration. Eur. Radiol. 2012, 22, 1186–1194. [Google Scholar] [CrossRef]
- Fiaschetti, V.; Crusco, S.; Meschini, A.; Cama, V.; Di Vito, L.; Marziali, M.; Piccione, E.; Calabria, F.; Simonetti, G. Deeply Infiltrating Endometriosis: Evaluation of Retro-Cervical Space on MRI after Vaginal Opacification. Eur. J. Radiol. 2012, 81, 3638–3645. [Google Scholar] [CrossRef] [PubMed]
- Tamai, K.; Togashi, K.; Ito, T.; Morisawa, N.; Fujiwara, T.; Koyama, T. MR Imaging Findings of Adenomyosis: Correlation with Histopathologic Features and Diagnostic Pitfalls. Radiographics 2005, 25, 21–40. [Google Scholar] [CrossRef] [PubMed]
- Hottat, N.; Larrousse, C.; Anaf, V.; Noël, J.-C.; Matos, C.; Absil, J.; Metens, T. Endometriosis: Contribution of 3.0-T Pelvic MR Imaging in Preoperative Assessment–Initial Results. Radiology 2009, 253, 126–134. [Google Scholar] [CrossRef] [PubMed]
- McCauley, T.R.; McCarthy, S.; Lange, R. Pelvic Phased Array Coil: Image Quality Assessment for Spin-Echo MR Imaging. Magn. Reson. Imaging 1992, 10, 513–522. [Google Scholar] [CrossRef]
- Bazot, M.; Gasner, A.; Ballester, M.; Daraï, E. Value of Thin-Section Oblique Axial T2-Weighted Magnetic Resonance Images to Assess Uterosacral Ligament Endometriosis. Hum. Reprod. 2011, 26, 346–353. [Google Scholar] [CrossRef]
- Cornfeld, D.M.; Israel, G.; McCarthy, S.M.; Weinreb, J.C. Pelvic Imaging Using a T1W Fat-Suppressed Three-Dimensional Dual Echo Dixon Technique at 3T. J. Magn. Reson. Imaging 2008, 28, 121–127. [Google Scholar] [CrossRef]
- Togashi, K.; Nishimura, K.; Kimura, I.; Tsuda, Y.; Yamashita, K.; Shibata, T.; Nakano, Y.; Konishi, J.; Konishi, I.; Mori, T. Endometrial Cysts: Diagnosis with MR Imaging. Radiology 1991, 180, 73–78. [Google Scholar] [CrossRef]
- Suzuki, S.; Yasumoto, M.; Matsumoto, R.; Andoh, A. MR Findings of Ruptured Endometrial Cyst: Comparison with Tubo-Ovarian Abscess. Eur. J. Radiol. 2012, 81, 3631–3637. [Google Scholar] [CrossRef]
- Onbas, O.; Kantarci, M.; Alper, F.; Kumtepe, Y.; Durur, I.; Ingec, M.; Gursan, N.; Okur, A. Nodular Endometriosis: Dynamic MR Imaging. Abdom. Imaging 2007, 32, 451–456. [Google Scholar] [CrossRef]
- Tanaka, Y.O.; Yoshizako, T.; Nishida, M.; Yamaguchi, M.; Sugimura, K.; Itai, Y. Ovarian Carcinoma in Patients with Endometriosis: MR Imaging Findings. AJR Am. J. Roentgenol. 2000, 175, 1423–1430. [Google Scholar] [CrossRef]
- Takahashi, N.; Yoshino, O.; Maeda, E.; Naganawa, S.; Harada, M.; Koga, K.; Hiraike, O.; Nakamura, M.; Tabuchi, T.; Hori, M.; et al. Usefulness of T2 Star-Weighted Imaging in Ovarian Cysts and Tumors. J. Obstet. Gynaecol. Res. 2016, 42, 1336–1342. [Google Scholar] [CrossRef] [PubMed]
- Sivajohan, B.; Elgendi, M.; Menon, C.; Allaire, C.; Yong, P.; Bedaiwy, M.A. Clinical Use of Artificial Intelligence in Endometriosis: A Scoping Review. NPJ Digit. Med. 2022, 5, 109. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Xie, H.; Lin, J.; Wang, Y.; Yin, Y. Diagnosis and Nursing Intervention of Gynecological Ovarian Endometriosis with Magnetic Resonance Imaging under Artificial Intelligence Algorithm. Comput. Intell. Neurosci. 2022, 2022, 3123310. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Nicholes, K.; Shih, I.-M. The Origin and Pathogenesis of Endometriosis. Annu. Rev. Pathol. 2020, 15, 71–95. [Google Scholar] [CrossRef] [PubMed]
- Quesada, J.; Härmä, K.; Reid, S.; Rao, T.; Lo, G.; Yang, N.; Karia, S.; Lee, E.; Borok, N. Endometriosis: A Multimodal Imaging Review. Eur. J. Radiol. 2023, 158, 110610. [Google Scholar] [CrossRef]
- Bulun, S.E.; Yilmaz, B.D.; Sison, C.; Miyazaki, K.; Bernardi, L.; Liu, S.; Kohlmeier, A.; Yin, P.; Milad, M.; Wei, J. Endometriosis. Endocr. Rev. 2019, 40, 1048–1079. [Google Scholar] [CrossRef]
- Reis, F.M.; Santulli, P.; Marcellin, L.; Borghese, B.; Lafay-Pillet, M.-C.; Chapron, C. Superficial Peritoneal Endometriosis: Clinical Characteristics of 203 Confirmed Cases and 1292 Endometriosis-Free Controls. Reprod. Sci. 2020, 27, 309–315. [Google Scholar] [CrossRef]
- Rolla, E. Endometriosis: Advances and Controversies in Classification, Pathogenesis, Diagnosis, and Treatment. F1000Research 2019, 8, F1000 Faculty Rev-529. [Google Scholar] [CrossRef]
- Rousset, P.; Florin, M.; Bharwani, N.; Touboul, C.; Monroc, M.; Golfier, F.; Nougaret, S.; Thomassin-Naggara, I.; ENDOVALIRM Group. Deep Pelvic Infiltrating Endometriosis: MRI Consensus Lexicon and Compartment-Based Approach from the ENDOVALIRM Group. Diagn. Interv. Imaging 2023, 104, 95–112. [Google Scholar] [CrossRef]
- Rousset, P.; Bischoff, E.; Charlot, M.; Grangeon, F.; Dubernard, G.; Paparel, P.; Lega, J.-C.; Golfier, F. Bladder Endometriosis: Preoperative MRI Analysis with Assessment of Extension to Ureteral Orifices. Diagn. Interv. Imaging 2021, 102, 255–263. [Google Scholar] [CrossRef]
- Falcone, T.; Flyckt, R. Clinical Management of Endometriosis. Obstet. Gynecol. 2018, 131, 557–571. [Google Scholar] [CrossRef]
- Sherman, A.K.; MacLachlan, L.S. A Review of Urinary Tract Endometriosis. Curr. Urol. Rep. 2022, 23, 219–223. [Google Scholar] [CrossRef]
- Kaniewska, M.; Gołofit, P.; Heubner, M.; Maake, C.; Kubik-Huch, R.A. Suspensory Ligaments of the Female Genital Organs: MRI Evaluation with Intraoperative Correlation. Radiographics 2018, 38, 2195–2211. [Google Scholar] [CrossRef]
- Del Frate, C.; Girometti, R.; Pittino, M.; Del Frate, G.; Bazzocchi, M.; Zuiani, C. Deep Retroperitoneal Pelvic Endometriosis: MR Imaging Appearance with Laparoscopic Correlation. Radiographics 2006, 26, 1705–1718. [Google Scholar] [CrossRef] [PubMed]
- Gerges, B.; Li, W.; Leonardi, M.; Mol, B.W.; Condous, G. Meta-Analysis and Systematic Review to Determine the Optimal Imaging Modality for the Detection of Uterosacral Ligaments/Torus Uterinus, Rectovaginal Septum and Vaginal Deep Endometriosis. Hum. Reprod. Open 2021, 2021, hoab041. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P.; Sergenti, G.; Buggio, L.; Frattaruolo, M.P.; Dridi, D.; Berlanda, N. Advances in the Medical Management of Bowel Endometriosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2021, 71, 78–99. [Google Scholar] [CrossRef] [PubMed]
- Bazot, M.; Kermarrec, E.; Bendifallah, S.; Daraï, E. MRI of Intestinal Endometriosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2021, 71, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Cranney, R.; Condous, G.; Reid, S. An Update on the Diagnosis, Surgical Management, and Fertility Outcomes for Women with Endometrioma. Acta Obstet. Gynecol. Scand. 2017, 96, 633–643. [Google Scholar] [CrossRef]
- Cecchino, G.N.; García-Velasco, J.A. Endometrioma, Fertility, and Assisted Reproductive Treatments: Connecting the Dots. Curr. Opin. Obstet. Gynecol. 2018, 30, 223–228. [Google Scholar] [CrossRef]
- Kaponis, A.; Taniguchi, F.; Azuma, Y.; Deura, I.; Vitsas, C.; Decavalas, G.O.; Harada, T. Current Treatment of Endometrioma. Obstet. Gynecol. Surv. 2015, 70, 183–195. [Google Scholar] [CrossRef]
- Kido, A.; Himoto, Y.; Moribata, Y.; Kurata, Y.; Nakamoto, Y. MRI in the Diagnosis of Endometriosis and Related Diseases. Korean J. Radiol. 2022, 23, 426–445. [Google Scholar] [CrossRef]
- Dias, J.L.; Veloso Gomes, F.; Lucas, R.; Cunha, T.M. The Shading Sign: Is It Exclusive of Endometriomas? Abdom. Imaging 2015, 40, 2566–2572. [Google Scholar] [CrossRef]
- Wolfman, W.; Thurston, J.; Yeung, G.; Glanc, P. Guideline No. 404: Initial Investigation and Management of Benign Ovarian Masses. J. Obstet. Gynaecol. Can. 2020, 42, 1040–1050.e1. [Google Scholar] [CrossRef]
- Singla, V.; Dawadi, K.; Singh, T.; Prabhakar, N.; Srinivasan, R.; Suri, V.; Khandelwal, N. Multiparametric MRI Evaluation of Complex Ovarian Masses. Curr. Probl. Diagn. Radiol. 2021, 50, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Forstner, R.; Thomassin-Naggara, I.; Cunha, T.M.; Kinkel, K.; Masselli, G.; Kubik-Huch, R.; Spencer, J.A.; Rockall, A. ESUR Recommendations for MR Imaging of the Sonographically Indeterminate Adnexal Mass: An Update. Eur. Radiol. 2017, 27, 2248–2257. [Google Scholar] [CrossRef] [PubMed]
- Sadowski, E.A.; Maturen, K.E.; Rockall, A.; Reinhold, C.; Addley, H.; Jha, P.; Bharwani, N.; Thomassin-Naggara, I. Ovary: MRI Characterisation and O-RADS MRI. Br. J. Radiol. 2021, 94, 20210157. [Google Scholar] [CrossRef]
- Biggs, W.S.; Marks, S.T. Diagnosis and Management of Adnexal Masses. Am. Fam. Physician 2016, 93, 676–681. [Google Scholar] [PubMed]
- Modesitt, S.C.; Pavlik, E.J.; Ueland, F.R.; DePriest, P.D.; Kryscio, R.J.; van Nagell, J.R. Risk of Malignancy in Unilocular Ovarian Cystic Tumors Less than 10 Centimeters in Diameter. Obstet. Gynecol. 2003, 102, 594–599. [Google Scholar] [CrossRef]
- Gershenson, D.M. Management of Borderline Ovarian Tumours. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 41, 49–59. [Google Scholar] [CrossRef]
- Dason, E.S.; Maxim, M.; Sanders, A.; Papillon-Smith, J.; Ng, D.; Chan, C.; Sobel, M. Guideline No. 437: Diagnosis and Management of Adenomyosis. J. Obstet. Gynaecol. Can. 2023, 45, 417–429.e1. [Google Scholar] [CrossRef]
- Vannuccini, S.; Petraglia, F. Recent Advances in Understanding and Managing Adenomyosis. F1000Research 2019, 8, F1000 Faculty Rev-283. [Google Scholar] [CrossRef] [PubMed]
- Schrager, S.; Yogendran, L.; Marquez, C.M.; Sadowski, E.A. Adenomyosis: Diagnosis and Management. Am. Fam. Physician 2022, 105, 33–38. [Google Scholar] [PubMed]
- Kobayashi, H.; Matsubara, S. A Classification Proposal for Adenomyosis Based on Magnetic Resonance Imaging. Gynecol. Obstet. Investig. 2020, 85, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Lacheta, J. Uterine Adenomyosis: Pathogenesis, Diagnostics, Symptomatology and Treatment. Ceska Gynekol. 2019, 84, 240–246. [Google Scholar] [PubMed]
- Bourdon, M.; Santulli, P.; Marcellin, L.; Maignien, C.; Maitrot-Mantelet, L.; Bordonne, C.; Plu Bureau, G.; Chapron, C. Adenomyosis: An Update Regarding Its Diagnosis and Clinical Features. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 102228. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Bazot, M.; Tsatoumas, M.; Munro, M.G.; Reinhold, C. MRI of Adenomyosis: Where Are We Today? Can. Assoc. Radiol. J. 2023, 74, 58–68. [Google Scholar] [CrossRef]
- Celli, V.; Dolciami, M.; Ninkova, R.; Ercolani, G.; Rizzo, S.; Porpora, M.G.; Catalano, C.; Manganaro, L. MRI and Adenomyosis: What Can Radiologists Evaluate? Int. J. Environ. Res. Public Health 2022, 19, 5840. [Google Scholar] [CrossRef]
- Nougaret, S.; Sbarra, M.; Robbins, J. Imaging Spectrum of Benign Uterine Disease and Treatment Options. Radiol. Clin. N. Am. 2020, 58, 239–256. [Google Scholar] [CrossRef]
- Chapron, C.; Vannuccini, S.; Santulli, P.; Abrão, M.S.; Carmona, F.; Fraser, I.S.; Gordts, S.; Guo, S.-W.; Just, P.-A.; Noël, J.-C.; et al. Diagnosing Adenomyosis: An Integrated Clinical and Imaging Approach. Hum. Reprod. Update 2020, 26, 392–411. [Google Scholar] [CrossRef]
- Bordonné, C.; Puntonet, J.; Maitrot-Mantelet, L.; Bourdon, M.; Marcellin, L.; Dion, E.; Plu-Bureau, G.; Santulli, P.; Chapron, C. Imaging for Evaluation of Endometriosis and Adenomyosis. Minerva Obstet. Gynecol. 2021, 73, 290–303. [Google Scholar] [CrossRef]
- Song, S.E.; Sung, D.J.; Park, B.J.; Kim, M.J.; Cho, S.B.; Kim, K.A. MR Imaging Features of Uterine Adenomyomas. Abdom. Imaging 2011, 36, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Ozsarlak, O.; Schepens, E.; de Schepper, A.M.; Deckers, F.; Parizel, P.M.; Campo, R. Transient Uterine Contraction Mimicking Adenomyosis on MRI. Eur. Radiol. 1998, 8, 54–56. [Google Scholar] [CrossRef]
- Zhai, J.; Vannuccini, S.; Petraglia, F.; Giudice, L.C. Adenomyosis: Mechanisms and Pathogenesis. Semin. Reprod. Med. 2020, 38, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Bazot, M.; Daraï, E. Role of Transvaginal Sonography and Magnetic Resonance Imaging in the Diagnosis of Uterine Adenomyosis. Fertil. Steril. 2018, 109, 389–397. [Google Scholar] [CrossRef]
- Manta, L.; Suciu, N.; Constantin, A.; Toader, O.; Popa, F. Focal Adenomyosis (Intramural Endometriotic Cyst) in a very Young Patient—Differential Diagnosis with Uterine Fibromatosis. J. Med. Life 2016, 9, 180–182. [Google Scholar] [PubMed]
- Takeuchi, M.; Matsuzaki, K.; Harada, M. MR Manifestations of Uterine Polypoid Adenomyoma. Abdom. Imaging 2015, 40, 480–487. [Google Scholar] [CrossRef]
- Andres, M.P.; Arcoverde, F.V.L.; Souza, C.C.C.; Fernandes, L.F.C.; Abrão, M.S.; Kho, R.M. Extrapelvic Endometriosis: A Systematic Review. J. Minim. Invasive Gynecol. 2020, 27, 373–389. [Google Scholar] [CrossRef]
- Chamié, L.P.; Ribeiro, D.M.F.R.; Tiferes, D.A.; de Macedo Neto, A.C.; Serafini, P.C. Atypical Sites of Deeply Infiltrative Endometriosis: Clinical Characteristics and Imaging Findings. Radiographics 2018, 38, 309–328. [Google Scholar] [CrossRef]
- Raffi, L.; Suresh, R.; McCalmont, T.H.; Twigg, A.R. Cutaneous Endometriosis. Int. J. Women Dermatol. 2019, 5, 384–386. [Google Scholar] [CrossRef]
- Youssef, A.T. The Ultrasound of Subcutaneous Extrapelvic Endometriosis. J. Ultrason. 2020, 20, e176–e180. [Google Scholar] [CrossRef]
- Dalkalitsis, A.; Salta, S.; Tsakiridis, I.; Dagklis, T.; Kalogiannidis, I.; Mamopoulos, A.; Daniilidis, A.; Athanasiadis, A.; Navrozoglou, I.; Paschopoulos, M.; et al. Inguinal Endometriosis: A Systematic Review. Taiwan. J. Obstet. Gynecol. 2022, 61, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Chiaramonte, R.; Castorina, S.; Castorina, E.G.; Panarello, A.; Antoci, S.A.M. Thrombosis of Iliac Vessels, a Rare Complication of Endometriosis: Case Report and Review of Literature. J. Adv. Res. 2017, 8, 1–5. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, M.; Chen, K.; Fong, Y.F. A Rare Case of Endometriosis Invading External Iliac Vein Causing Deep Vein Thrombosis. Am. J. Obstet. Gynecol. 2019, 220, 113–114. [Google Scholar] [CrossRef]
- Ferrero, S.; Stabilini, C.; Barra, F.; Clarizia, R.; Roviglione, G.; Ceccaroni, M. Bowel Resection for Intestinal Endometriosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2021, 71, 114–128. [Google Scholar] [CrossRef] [PubMed]
- Nezhat, C.; Lindheim, S.R.; Backhus, L.; Vu, M.; Vang, N.; Nezhat, A.; Nezhat, C. Thoracic Endometriosis Syndrome: A Review of Diagnosis and Management. JSLS 2019, 23, e2019.00029. [Google Scholar] [CrossRef]
- Elefante, C.; Brancati, G.E.; Oragvelidze, E.; Lattanzi, L.; Maremmani, I.; Perugi, G. Psychiatric Symptoms in Patients with Cerebral Endometriosis: A Case Report and Literature Review. J. Clin. Med. 2022, 11, 7212. [Google Scholar] [CrossRef] [PubMed]
- Kalaitzopoulos, D.R.; Samartzis, N.; Kolovos, G.N.; Mareti, E.; Samartzis, E.P.; Eberhard, M.; Dinas, K.; Daniilidis, A. Treatment of Endometriosis: A Review with Comparison of 8 Guidelines. BMC Women Health 2021, 21, 397. [Google Scholar] [CrossRef]
- Bonavina, G.; Taylor, H.S. Endometriosis-Associated Infertility: From Pathophysiology to Tailored Treatment. Front. Endocrinol. 2022, 13, 1020827. [Google Scholar] [CrossRef]
- Guerra, A.; Daraï, E.; Osório, F.; Setúbal, A.; Bendifallah, S.; Loureiro, A.; Thomassin-Naggara, I. Imaging of Postoperative Endometriosis. Diagn. Interv. Imaging 2019, 100, 607–618. [Google Scholar] [CrossRef]
- Chamié, L.P.; Ribeiro, D.M.F.R.; Ribeiro, G.M.P.A.R.; Serafini, P.C. Postoperative Imaging Findings after Laparoscopic Surgery for Deeply Infiltrating Endometriosis. Abdom. Radiol. 2020, 45, 1847–1865. [Google Scholar] [CrossRef]
- Tang, X.; Ling, R.; Gong, J.; Mei, D.; Luo, Y.; Li, M.; Xu, J.; Ma, L. Deep Infiltrating Endometriosis MR Imaging with Surgical Correlation. Quant. Imaging Med. Surg. 2018, 8, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Darici, E.; Salama, M.; Bokor, A.; Oral, E.; Dauser, B.; Hudelist, G. Different Segmental Resection Techniques and Postoperative Complications in Patients with Colorectal Endometriosis: A Systematic Review. ACTA Obstet. Gynecol. Scand. 2022, 101, 705–718. [Google Scholar] [CrossRef] [PubMed]
- Burnett, T.L.; Feldman, M.K.; Huang, J.Q. The Role of Imaging as a Guide to the Surgical Treatment of Endometriosis. Abdom. Radiol. 2020, 45, 1840–1846. [Google Scholar] [CrossRef]
- Surgical Therapy of Ovarian Endometrioma: Recurrence and Pregnancy Rates—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/25392619/ (accessed on 19 July 2023).
- Singh, S.S.; Gude, K.; Perdeaux, E.; Gattrell, W.T.; Becker, C.M. Surgical Outcomes in Patients with Endometriosis: A Systematic Review. J. Obstet. Gynaecol. Can. 2020, 42, 881–888.e11. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).