Physiological Effects of Hydrolyzed Skim Milk and Probiotics on Osteoporosis Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animal Study
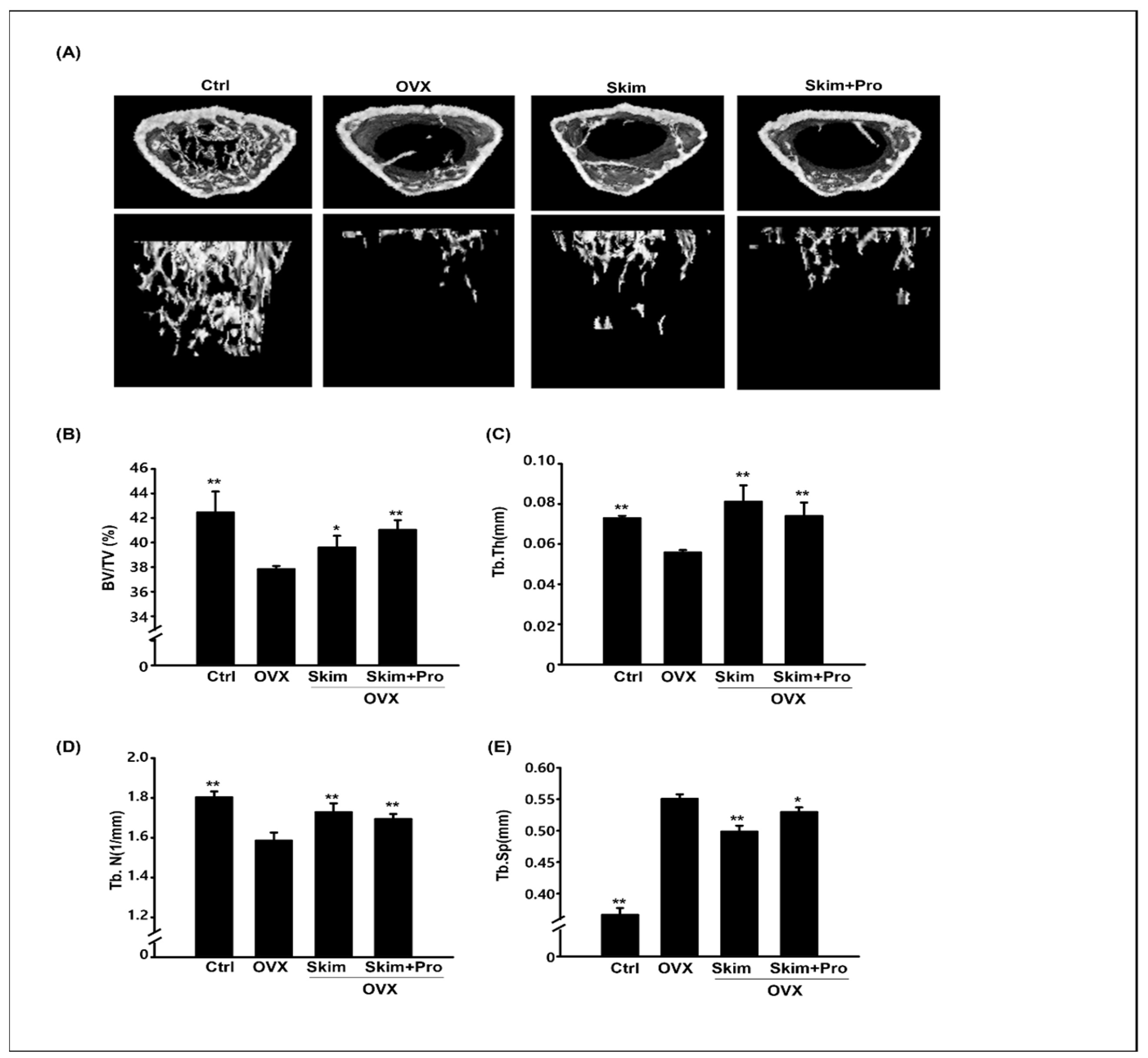
2.3. Micro-CT Analysis
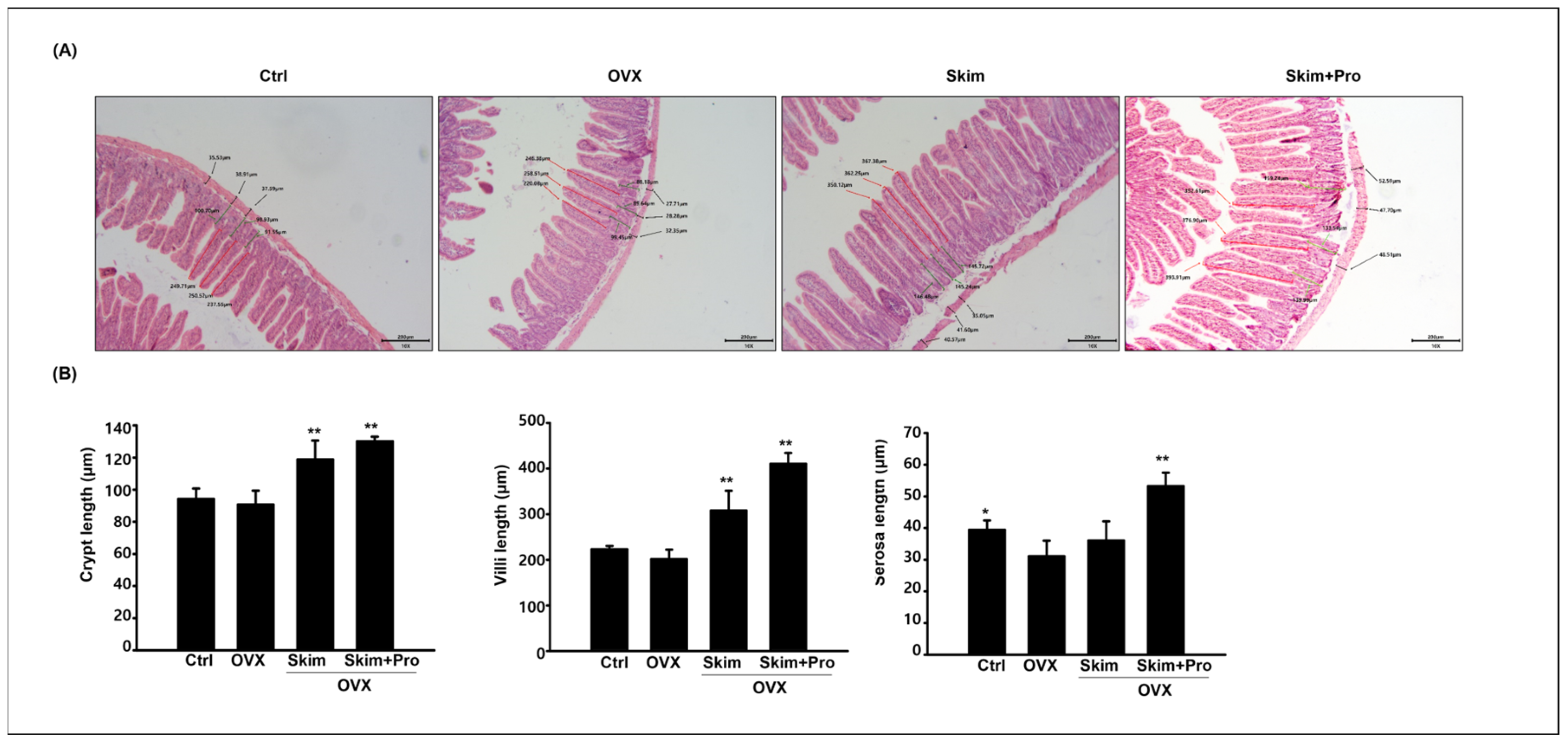
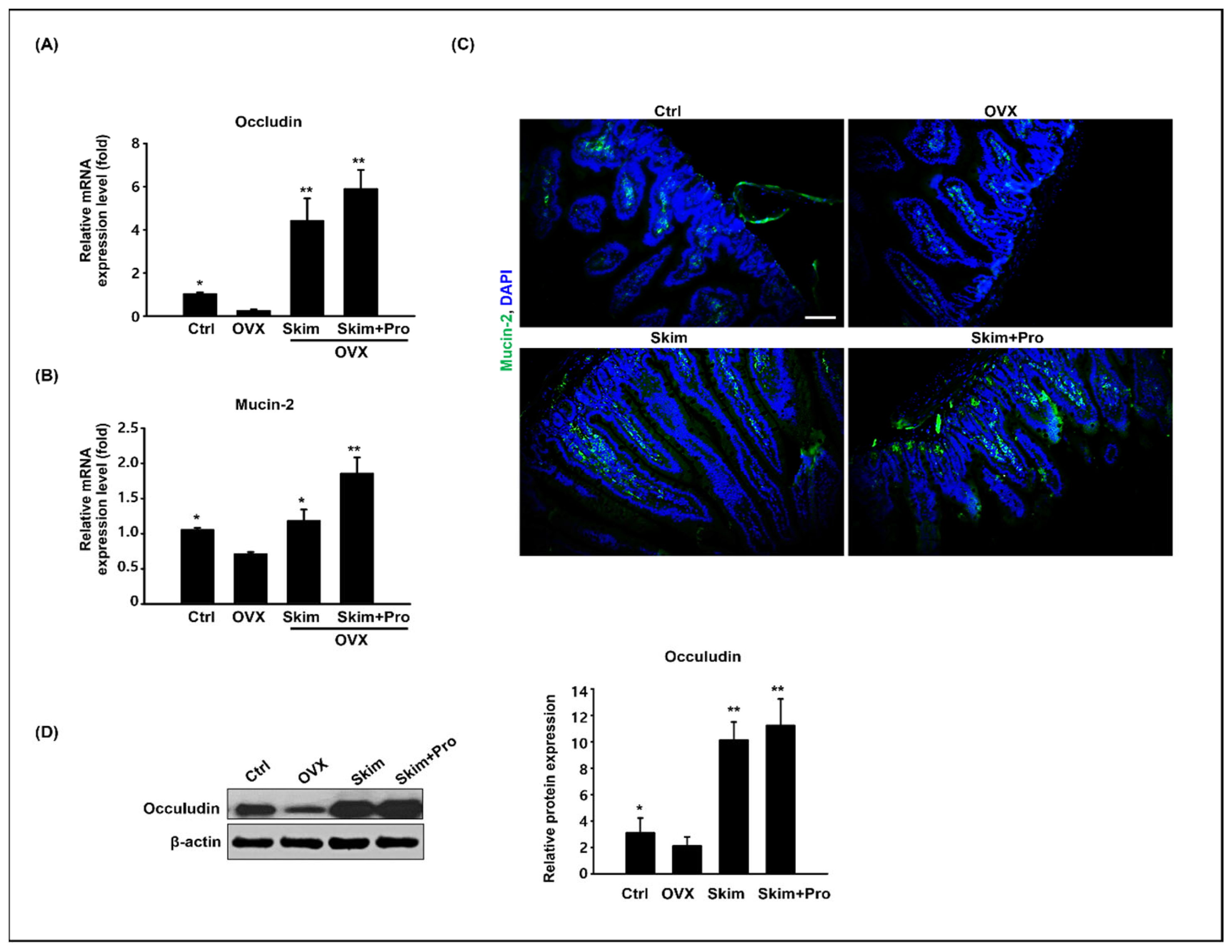
2.4. Histological Preparation and Immunostaining
2.5. Quantitative Real-Time PCR
2.6. Western Blotting
2.7. Statistical Analysis
3. Results and Discussion
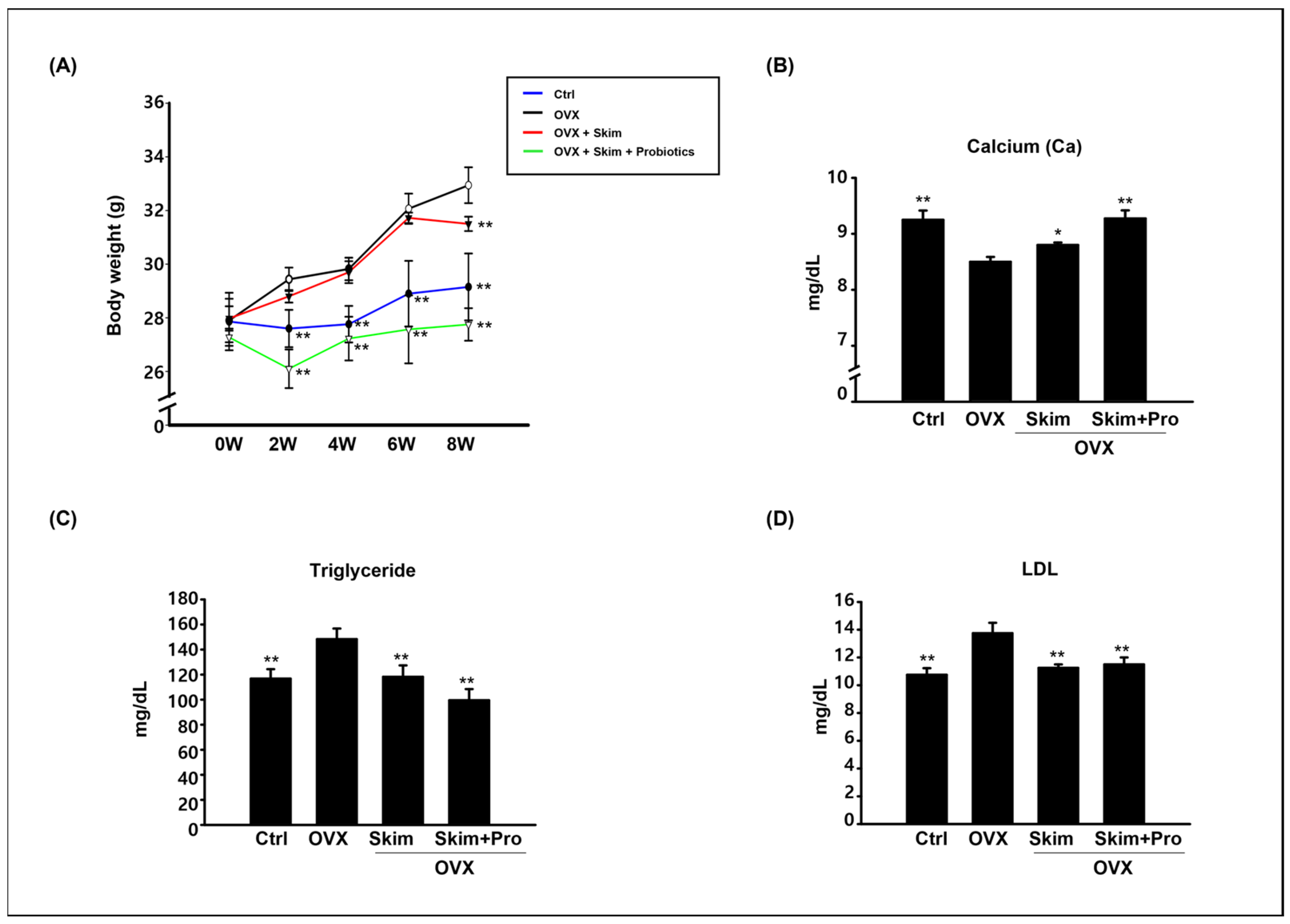
3.1. Physiological Effect of Skim Milk and Probiotics on Osteoporosis Models
3.2. Attenuation of the OVX-Driven Effects of Skim Milk and Probiotics
3.3. Effect of Skim Milk and Probiotics on the Small Intestine
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Eastell, R.; O’Neill, T.W.; Hofbauer, L.C.; Langdahl, B.; Reid, I.R.; Gold, D.T.; Cummings, S.R. Postmenopausal osteoporosis. Nat. Rev. Dis. Primers 2016, 2, 16069. [Google Scholar] [CrossRef] [PubMed]
- Rosen, C.J. The Epidemiology and Pathogenesis of Osteoporosis; MDText.com, Inc.: South Dartmouth, MA, USA, 2020. [Google Scholar]
- Armas, L.A.; Recker, R.R. Pathophysiology of osteoporosis: New mechanistic insights. Endocrinol. Metab. Clin. N. Am. 2012, 41, 475–486. [Google Scholar] [CrossRef]
- Janiszewska, M.; Kulik, T.; Dziedzic, M.; Żołnierczuk-Kieliszek, D.; Barańska, A. Osteoporosis as a social problem- pathogenesis, symptoms and risk factors of postmenopausal osteoporosis. Probl. Hig. Epidemiol. 2015, 96, 106–114. [Google Scholar]
- Cymet, T.C.; Wood, B.; Orbach, N. Osteoporosis. J. Am. Osteopath. Assoc. 2000, 100, S9–S15. [Google Scholar]
- Fiona, E.A.M.; Liam, M.; Alison, G.; George, D.; Neville, C.E.; Rob, V.H.; Boreham, C.; Ralston, S.H. Genetic and environmental determinants of peak bone mass in young men and women. J. Bone Miner. Res. 2002, 17, 1273–1279. [Google Scholar]
- Ankit, D.; Sameer, A.; Prasoon, K.; Vishal, K.; Deba, P.D.; Sanjay, K.B. High prevalence of vitamin D deficiency and osteoporosis in patients with fragility fractures of hip: A pilot study. J. Clin. Orthop. Trauma. 2019, 10, 1097–1100. [Google Scholar]
- Verduci, E.; D’Elios, S.; Cerrato, L.; Comberiati, P.; Calvani, M.; Palazzo, S.; Martelli, A.; Landi, M.; Trikamjee, T.; Peroni, D.G. Cow’s Milk Substitutes for Children: Nutritional Aspects of Milk from Different Mammalian Species, Special Formula and Plnat-Based Beverages. Nutrients 2019, 11, 1739. [Google Scholar] [CrossRef]
- Fardellone, P. The effect of milk consumption on bone and fracture incidence, an update. Aging Clin. Exp. Res. 2019, 31, 759–764. [Google Scholar] [CrossRef]
- Heidi, J.K.; Jane, C.K.; Bruce, P.L. Milk intake during childhood and adolescence, adult bone density, and osteoporotic fractures in US women. Am. J. Clin. Nutr. 2003, 77, 257–265. [Google Scholar]
- Aviv, F.; Sarah, L.M.; Lindsay, K.E.; Caeley, L.; Raylene, A.R.; Ronald, F.Z. Skim milk powder enhances trabecular bone architecture compared with casein or whey in diet-induced obese rats. Nutrition 2012, 28, 331–335. [Google Scholar]
- Ratajczak, A.E.; Zawada, A.; Rychter, A.M.; Dobrowolska, A.; Krela-Kaźmierczak, I. Milk and dairy products: Good or bad for human bone? Practical dietary recommendations for the prevention and management of osteoporosis. Nutrients 2021, 13, 1329. [Google Scholar] [CrossRef] [PubMed]
- Jing, Z.; Katherine, J.M.; Regina, I.; Ormond, A.M.; Robert, A.B.; Laura, R.M. Loss of bone and Wnt10b expression in male type 1 diabetic mice is blocked by the probiotic Lactobacillus reuteri. Endocrinology 2015, 153, 3169–3182. [Google Scholar]
- Xu, B.; Ling, S.; Xu, X.; Liu, X.; Wang, A.; Zhou, Y.; Luo, Y.; Li, W.; Yao, X. A New Formulation of Probiotics Attenuates Calcipotriol-Induced Dermatitis by Inducing Regulatory Dendritic Cells. Front. Immunol. 2021, 12, 775018. [Google Scholar] [CrossRef] [PubMed]
- Butera, A.; Pascadopoli, M.; Pellegrini, M.; Gallo, S.; Zampetti, P.; Guggia, G.; Scribante, A. Domiciliary Use of Chlorhexidine vs. Postbiotic Gels in Patients with Peri-Implant Mucositis: A Split-Mouth Randomized Clinical Trial. Appl. Sci. 2022, 12, 2800. [Google Scholar] [CrossRef]
- Zhou, B.; Jin, G.; Pang, X.; Mo, Q.; Bao, J.; Liu, T.; Wu, J.; Xie, R.; Liu, X.; Liu, J.; et al. Lactobacillus rhamnosus GG colonization in early life regulates gut-brain axis and relieves anxiety-like behavior in adulthood. Pharmacol. Res. 2022, 177, 106090. [Google Scholar] [CrossRef] [PubMed]
- Inada, M.; Matsumoto, C.; Miyaura, C. Animal models for bone and joint disease. Ovariectomized and orchidectomized animals. Clin. Calcium 2011, 21, 164–170. [Google Scholar] [PubMed]
- Chiang, S.S.; Pan, T.M. Antiosteoporotic effects of Lactobacillus-fermented soy skim milk on bone mineral density and the microstructure of femoral bone in ovariectomized mice. J. Agric. Food Chem. 2011, 59, 7734–7742. [Google Scholar] [CrossRef]
- Yang, L.C.; Lin, S.W.; Li, I.C.; Chen, Y.P.; Tzu, S.Y.; Chou, W.; Chen, C.C.; Lin, W.C.; Chen, Y.L.; Lin, W.H. Lactobacillus plantarum GKM3 and Lactobacillus paracasei GKS6 supplementation ameliorates bone loss in ovariectomized mice by promoting osteoblast differentiation and inhibiting osteoclast formation. Nutrients 2020, 12, 1914. [Google Scholar] [CrossRef]
- Park, H.J.; Lee, W.; Zhang, M.; Hong, K.; Park, C.; Kim, J.; Song, H. Evaluation of resmethrin toxicity to neonatal testes in organ culture. Toxicol. Sci. 2020, 173, 53–64. [Google Scholar] [CrossRef]
- Lee, W.Y.; Park, H.J. Toxicology of cerium oxide nanoparticles on neonatal testicular development in mouse organ culture. Reprod. Toxicol. 2022, 111, 120–128. [Google Scholar] [CrossRef]
- Cao, J.J.; Gregoire, B.R. A high-fat diet increases body weight and circulating estradiol concentration but does not improve bone structural properties in ovariectomized mice. Nutr. Res. 2016, 36, 320–327. [Google Scholar] [CrossRef]
- Li, J.Y.; Chassaing, B.; Tyagi, A.M.; Vaccaro, C.; Luo, T.; Adams, J.; Darby, T.M.; Weitzmann, M.N.; Mulle, J.G.; Gewirtz, A.T.; et al. Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J. Clin. Investig. 2016, 126, 2049–2063. [Google Scholar] [CrossRef]
- Park, I.S.; Lee, H.W.; Ryuk, J.A.; Ko, B.S. Effects of an aqueous extract of dangguijagyagsan on serum lipid levels and blood flow improvement in ovariectomized rats. Evid. Based Complement. Alternat Med. 2014, 2014, 497836. [Google Scholar] [CrossRef]
- Tunick, M.H.; Van Hekken, D.L. Dairy products and health: Recent insights. J. Agric. Food Chem. 2015, 63, 9381–9388. [Google Scholar] [CrossRef]
- Amdekar, S.; Kumar, A.; Sharma, P.; Singh, R.; Singh, V. Lactobacillus protected bone damage and maintained the antioxidant status of liver and kidney homogenates in female Wistar rats. Mol. Cell Biochem. 2012, 368, 155–165. [Google Scholar] [CrossRef]
- Britton, R.A.; Irwin, R.; Quach, D.; Schaefer, L.; Zhang, J.; Lee, T.; Parameswaran, N.; McCabe, L.R. Probiotic L. reuteri treatment prevents bone loss in a menopausal ovariectomized mouse model. J. Cell Physiol. 2014, 229, 1822–1830. [Google Scholar] [CrossRef] [PubMed]
- Kok, C.R.; Hutkins, R. Yogurt and other fermented foods as sources of health-promoting bacteria. Rev. Nutr. Rev. 2018, 76, 1–15. [Google Scholar] [CrossRef]
- Butel, M. Probiotics, gut microbiota and health. Med. Mal. Infect. 2013, 44, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Tu, Y.; Jia, X.; Du, Q.; Zheng, X.; Yuan, Q.; Zheng, L.; Zhou, X.; Xu, X. Probiotics ameliorate alveolar bone loss by regulating gut microbiota. Cell Prolif. 2021, 54, e13075. [Google Scholar] [CrossRef] [PubMed]
- Izadi, H.; Arshami, J.; Golian, A.; Raji, M.R. Effects of chicory root powder on growth performance and histomorphometry of jejunum in broiler chicks. Vet. Res. Forum 2013, 4, 169–174. [Google Scholar]
- Pupa, P.; Apiwatsiri, P.; Sirichokchatchawan, W.; Pirarat, N.; Maison, T.; Koontanatechanon, A.; Prapasarakul, N. Use of Latobacillus plantarum (strains 22F and 25F) and Pediococcus acidilactici (strain 72N) as replacements for antibiotic-growth promotants in pigs. Sci. Rep. 2021, 11, 12028. [Google Scholar] [CrossRef]
- Awad, W.A.; Ghareeb, K.; Abdel-Raheem, S.; Böhm, J. Effects of dietary inclusion of probiotic and synbiotic on growth performance, organ weights, and intestinal histomorphology of broiler chickens. Poult. Sci. 2009, 88, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Kaminsky, L.W.; Al-Sadi, R.; Ma, T.Y. IL-1β and the intestinal epithelial tight junction barrier. Front. Immunol. 2021, 12, 767456. [Google Scholar] [CrossRef]
- Madsen, K.; Cornish, A.; Soper, P.; McKaigney, C.; Jijon, H.; Yachimec, C.; Doyle, J.; Jewell, L.; De Simone, C. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology 2001, 121, 580–591. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.E.V.; Thomsson, K.A.; Hansson, G.C. Proteomic analysis of the two mucus layers of the colon barrier reveal that their main component, the Muc2 mucin, is strongly bound to the Fcgbp protein. J. Proteome Res. 2009, 8, 3549–3557. [Google Scholar] [CrossRef] [PubMed]
- Brett, F.; Kris, C.; Bruce, A.V. Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS Pathog. 2010, 6, e1000902. [Google Scholar]
- Maria, V.S.; Barbara, A.; Adrianus, C.; Anna, V.; Jules, P.P.M.; Johannes, B.V.G.; Hans, A.B.; Jan, D.; Isabelle, V.S.; Ingrid, B.R.; et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 2006, 131, 117–129. [Google Scholar]
- Mack, D.R.; Ahrne, S.; Hyde, K.; Wei, S.; Hollingsworth, M.A. Extracellular MUC3 mucin secretion follows adherence of Lactobacillus strains to intestinal epithelial cells in vitro. Gut 2003, 52, 827–833. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, S.; Whang, K.; Kim, Y.; Oh, S. Inhibition of Escherichia coli O157:H7 attachment by interactions between lactic acid bacteria and intestinal epithelial cells. J. Microbiol. Biotechnol. 2008, 18, 1278–1285. [Google Scholar]
- Saitou, M.; Furuse, M.; Sasaki, H.; Schulzke, J.D.; Fromm, M.; Takano, H.; Noda, T.; Tsukita, S. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol. Biol. Cell 2000, 11, 4131–4142. [Google Scholar] [CrossRef]
- AI-Sadi, R.; Khatib, K.; Guo, S.; Ye, D.; Youssef, M.; Ma, T. Occludin regulates macromolecule flux across the intestinal epithelial tight junction barrier. Am. J. Physiol.-Gastrointest. Liver Physiol. 2011, 300, G1054–G1064. [Google Scholar] [CrossRef] [PubMed]
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Occludin | 5′-ATGTCCGGCCGATGCTCTC-3′ | 5′-TTTGGCTGCTCTTGGGTCTGTAT-3′ |
| Mucin 2 | 5′-GCCTGTTTG ATAGCTGCTATGTGCC-3 | 5′-GTTCCGCCAGTCAATGCAGACAC-3 |
| GAPDH | 5′-GTCGGTGTGAACGGATTTG-3 | 5′-CTTGCCGTGGGTAGAGTCAT-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shim, H.-W.; Lee, W.-Y.; Kim, H.W.; Park, J.-k.; Cho, K.; Yeo, J.M.; Park, H.-J. Physiological Effects of Hydrolyzed Skim Milk and Probiotics on Osteoporosis Models. Appl. Sci. 2023, 13, 10424. https://doi.org/10.3390/app131810424
Shim H-W, Lee W-Y, Kim HW, Park J-k, Cho K, Yeo JM, Park H-J. Physiological Effects of Hydrolyzed Skim Milk and Probiotics on Osteoporosis Models. Applied Sciences. 2023; 13(18):10424. https://doi.org/10.3390/app131810424
Chicago/Turabian StyleShim, Heyon-Woo, Won-Yong Lee, Hyoun Wook Kim, Jin-ki Park, Kwanghyun Cho, Joon Mo Yeo, and Hyun-Jung Park. 2023. "Physiological Effects of Hydrolyzed Skim Milk and Probiotics on Osteoporosis Models" Applied Sciences 13, no. 18: 10424. https://doi.org/10.3390/app131810424
APA StyleShim, H.-W., Lee, W.-Y., Kim, H. W., Park, J.-k., Cho, K., Yeo, J. M., & Park, H.-J. (2023). Physiological Effects of Hydrolyzed Skim Milk and Probiotics on Osteoporosis Models. Applied Sciences, 13(18), 10424. https://doi.org/10.3390/app131810424







