Novel Fused Core Chromophore Incorporating Spirofluorene and Anthracene Groups for Sky-Blue Emission and Solution-Processed White Devices
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Information
2.2. Synthesis
2.2.1. 5-Bromoanthraquinone (1)
2.2.2. 1-Bromoanthracene (2)
2.2.3. 1-Anthracene Boronic Acid (3)
2.2.4. 1-(2-Bromo-phenyl)-anthracene (4)
2.2.5. 2,7-Dibromo-spiro-fluorene[3,4]naphthalene (5)
2.2.6. Spiro-fluorene[3,4]naphthalene (6)
2.2.7. Spiro-fluorene[3,4]-5-bromonapthalene (7)
2.2.8. 2,7-Bis-[1,1′;3′,1″]terphenyl-spiro-fluorene[3,4]naphthalene (TP-AFF-TP)
2.2.9. Spiro-fluorene[3,4]-5-terphenylnaphthalene (TP-ASF)
3. Results and Discussion
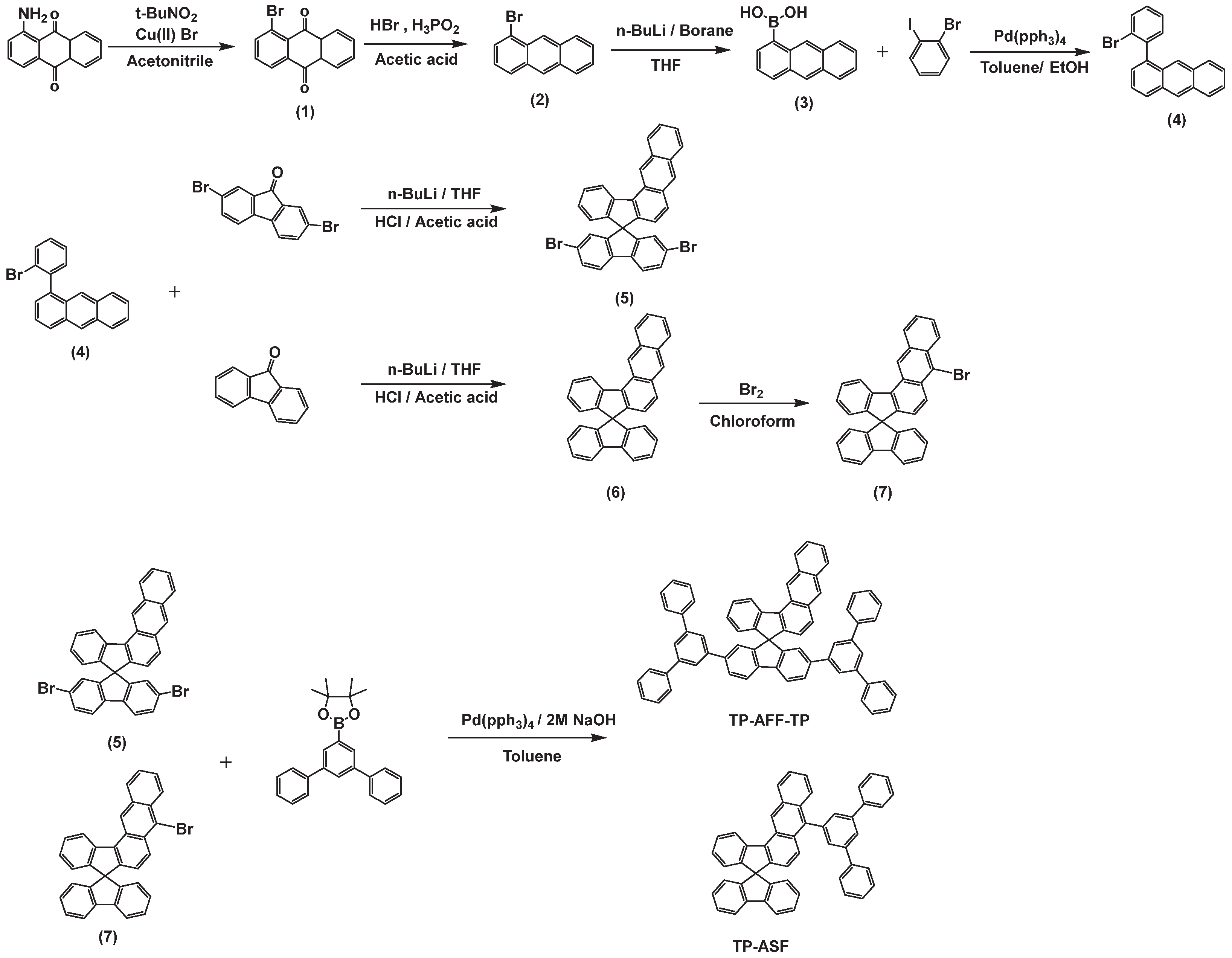
3.1. Molecular Design, Synthesis, and Characterization
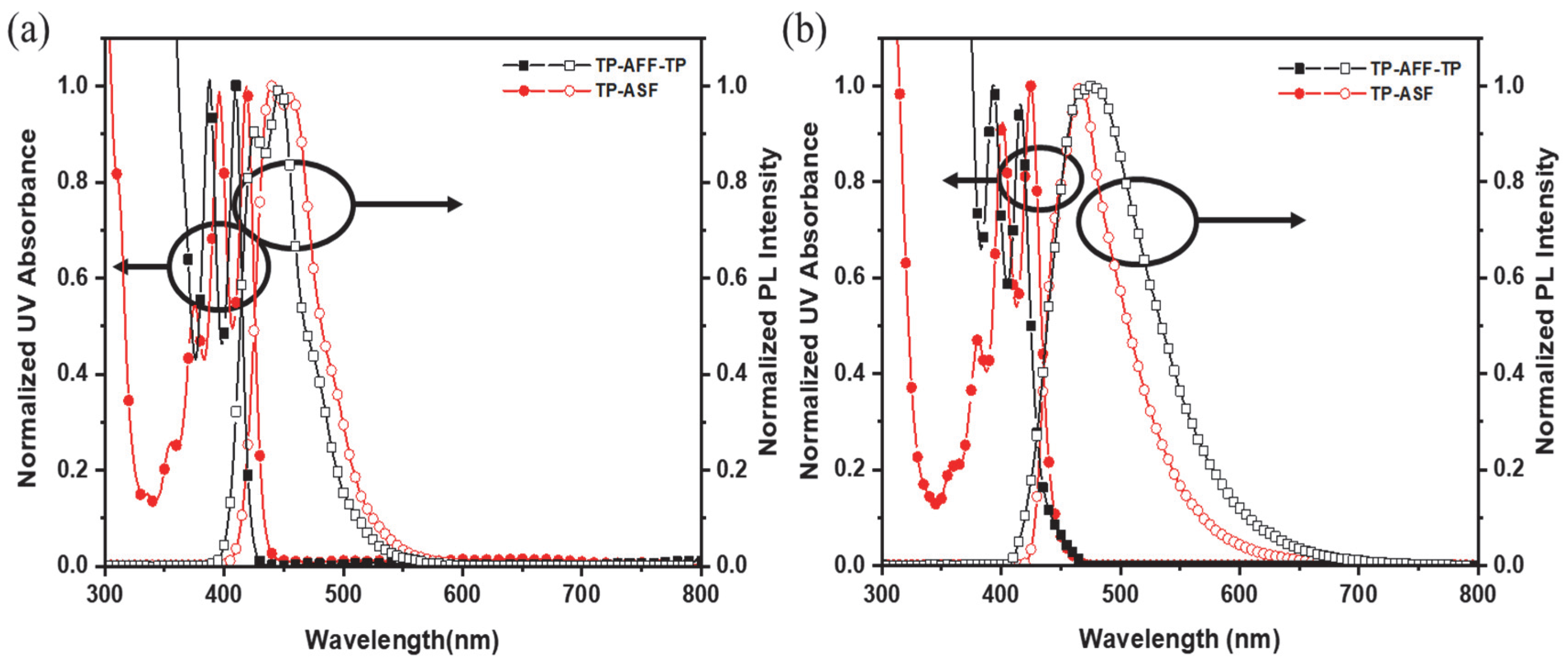
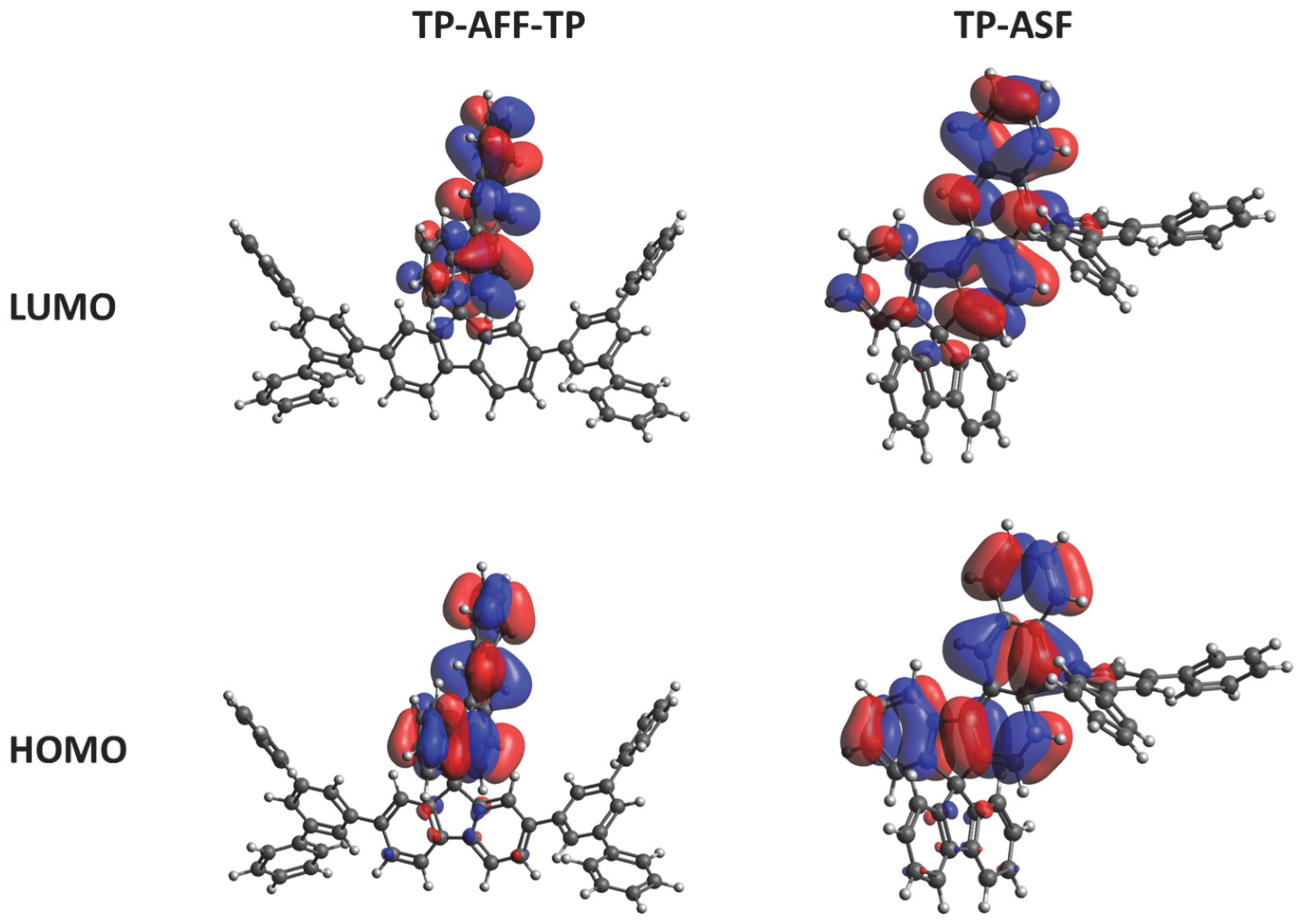
3.2. Photophysical Properties
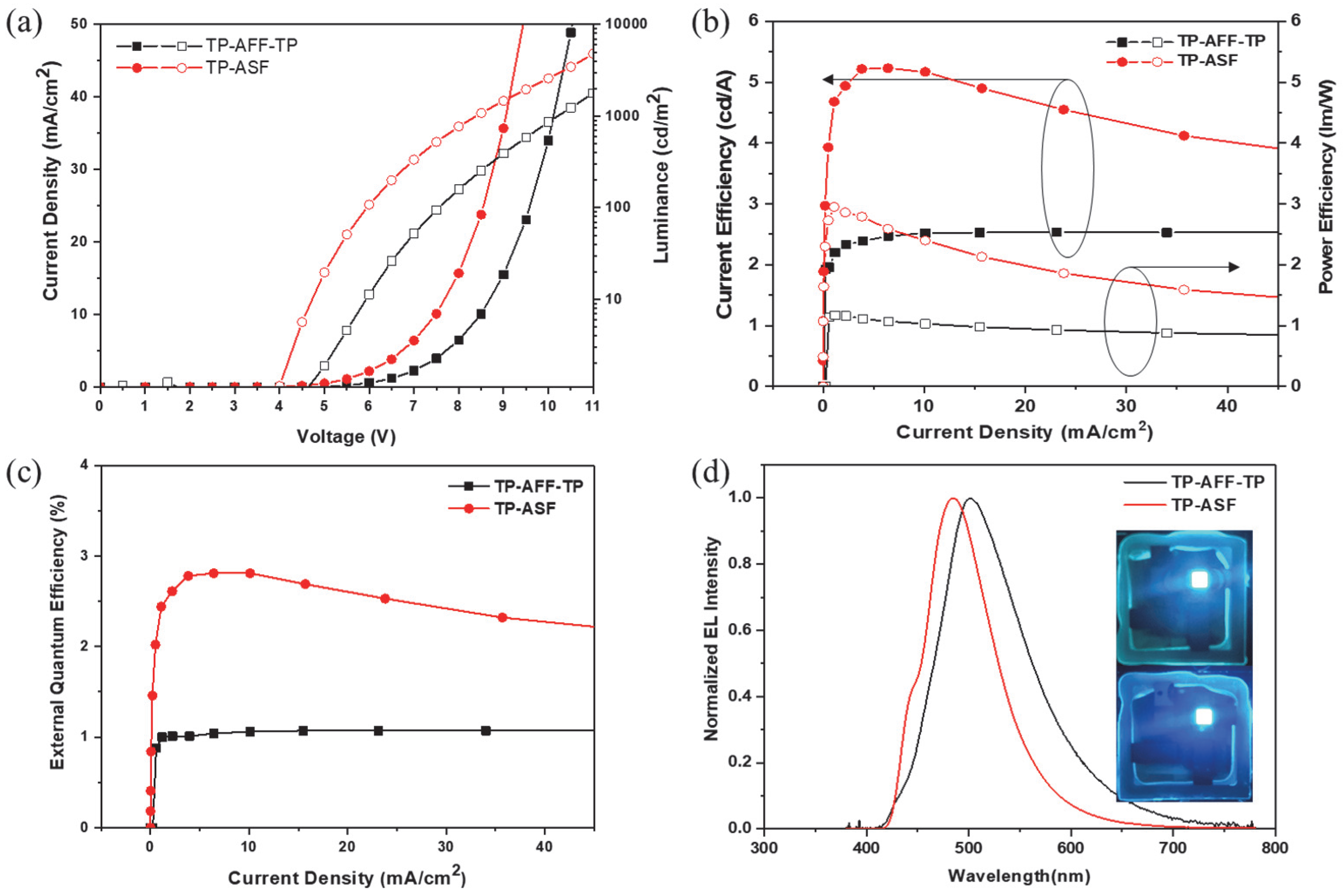
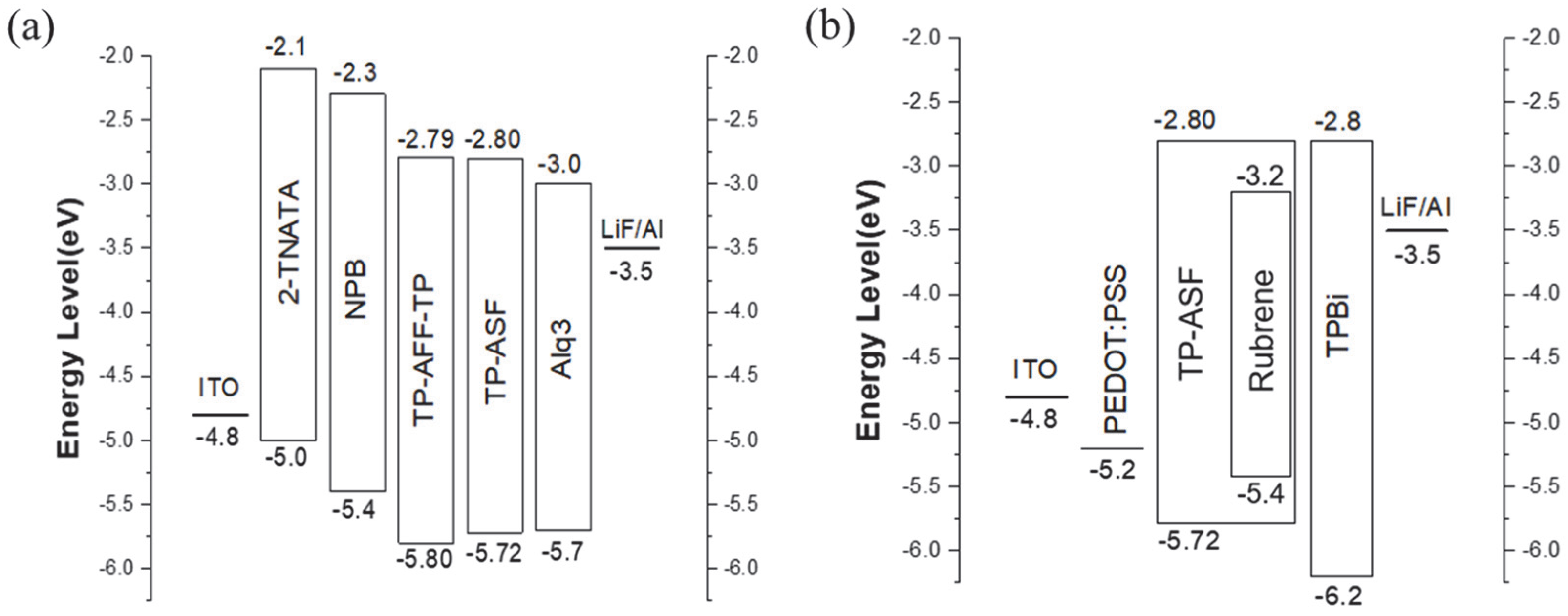
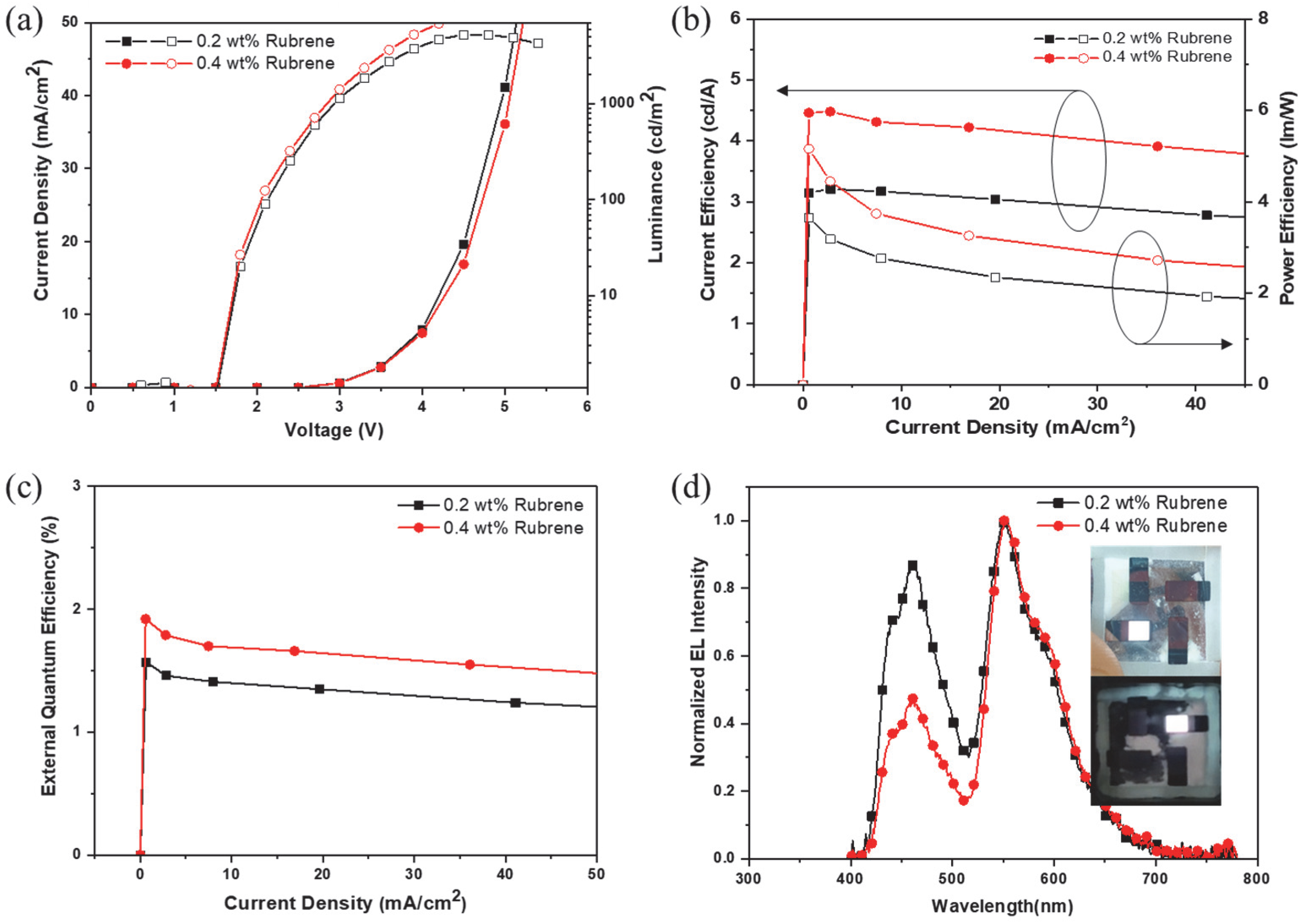
3.3. Electroluminescence Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pickup, D.F.; Yi, H.; Kun, H.; Iraqi, A.; Stevenson, M.; Lidzey, D.G. Alternating 2, 7-and 3, 6-linked carbazole copolymers as wide band gap energy transfer donors. Thin Solid Films. 2009, 517, 2840–2844. [Google Scholar] [CrossRef]
- Liu, J.; Guo, X.; Bu, L.; Xie, Z.; Cheng, Y.; Geng, Y.; Wang, L.; Jing, X.; Wang, F. White Electroluminescence from a Single-Polymer System with Simultaneous Two-Color Emission: Polyfluorene Blue Host and Side-Chain-Located Orange Dopant. Adv. Funct. Mater. 2007, 17, 1917–1925. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kwon, Y.J.; Woo, J.W.; Moon, D.K. Synthesis and characterization of fluorine–thiophene-based π-conjugated polymers using coupling reaction. J. Ind. Eng. Chem. 2008, 14, 810–817. [Google Scholar] [CrossRef]
- Kang, I.; Hong, J.A.; Kim, R.; Jang, J.W.; Hwang, J.; Kim, J.H.; Kwon, S.K.; Kim, Y.H. Synthesis and characterization of a new π-conjugated polymer with cyanoacrylate side chain for organic thin film transistors. Macromolecular. Res. 2013, 21, 450–455. [Google Scholar] [CrossRef]
- Lee, J.Y.; Choi, M.H.; Moon, D.K.; Haw, J.R. Synthesis of fluorene-and anthracene-based π-conjugated polymers and dependence of emission range and luminous efficiency on molecular weight. J. Ind. Eng. Chem. 2010, 16, 395–400. [Google Scholar] [CrossRef]
- Zhu, X.; Li, Y.; Wu, Z.; Lin, C.; Ma, D.; Zhao, Z.; Tang, B.Z. Anthracene-based bipolar deep-blue emitters for efficient white OLEDs with ultra-high stabilities of emission color and efficiency. J. Mater. Chem. 2021, 9, 5198–5205. [Google Scholar] [CrossRef]
- Noda, T.; Sasabe, H.; Owada, T.; Sugiyama, R.; Arai, A.; Kumada, K.; Tsuneyama, H.; Saito, Y.; Kido, J. Constructing Soluble Anthracene-Based Blue Emitters Free of Electrically Inert Alkyl Chains for Efficient Evaporation-and Solution-Based OLEDs. ChemPlusChem 2022, 87, e202100517. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Lee, J.; Kang, I.; Chu, H.Y.; Lee, J.I.; Kwon, S.K.; Kim, Y.H. Highly rigid and twisted anthracene derivatives: A strategy for deep blue OLED materials with theoretical limit efficiency. J. Mater. Chem. 2012, 22, 2695–2700. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Xu, C.; Wang, W.Z.; Duan, L.M.; Li, Z.; Zhao, B.T.; Ji, B.M. High-color-purity and high-efficiency non-doped deep-blue electroluminescent devices based on novel anthracene derivatives. New J. Chem. 2012, 36, 662–667. [Google Scholar] [CrossRef]
- Lim, H.; Woo, S.J.; Ha, Y.H.; Kim, Y.H.; Kim, J.J. Breaking the Efficiency Limit of Deep-Blue Fluorescent OLEDs Based on Anthracene Derivatives. Adv. Mater. 2022, 34, 2100161. [Google Scholar] [CrossRef]
- Wu, C.H.; Chien, C.H.; Hsu, F.M.; Shih, P.I.; Shu, C.F. Efficient non-doped blue-light-emitting diodes incorporating an anthracene derivative end-capped with fluorene groups. J. Mater. Chem. 2009, 19, 1464–1470. [Google Scholar] [CrossRef]
- Lai, X.S.L.; Tong, Q.X.; Chan, M.Y.; Ng, T.W.; Lo, M.F.; Lee, S.T.; Lee, C.S. Distinct electroluminescent properties of triphenylamine derivatives in blue organic light-emitting devices. J. Mater. Chem. 2011, 21, 1206–1211. [Google Scholar] [CrossRef]
- Hosokawa, C.; Higashi, H.; Nakamura, H.; Kusumoto, T. Highly efficient blue electroluminescence from a distyrylarylene emitting layer with a new dopant. Appl. Phys. Lett. 1995, 67, 3853. [Google Scholar] [CrossRef]
- Kim, B.J.; Park, Y.I.; Lee, J.H.; Yokoyama, D.; Lee, J.H.; Kido, J.; Park, J.W. Synthesis and electroluminescence properties of highly efficient blue fluorescence emitters using dual core chromophores. J. Mater. Chem. C 2013, 1, 432. [Google Scholar] [CrossRef]
- Lee, J.H.; Jung, H.C.; Shin, H.G.; Kim, J.H.; Yokoyama, D.; Nishimura, H.; Wakamiya, A.; Park, J.W. Excimer emission based on the control of molecular structure and intermolecular interactions. J. Mater. Chem. C 2016, 4, 2784–2792. [Google Scholar] [CrossRef]
- Chan, C.Y.; Tanaka, M.; Lee, Y.T.; Wong, Y.W.; Nakanotani, H.; Hatakeyama, T.; Adachi, C. Stable pure-blue hyperfluorescence organic light-emitting diodes with high-efficiency and narrow emission. Nat. Photonics 2021, 15, 203–207. [Google Scholar] [CrossRef]
- Naveen, K.R.; Lee, H.N.; Braveenth, R.; Karthik, D.; Yang, K.J.; Hwang, S.J.; Kwon, J.H. Achieving high efficiency and pure blue color in hyperfluorescence organic light emitting diodes using organo-boron based emitters. Adv. Funct. Mater. 2022, 32, 2110356. [Google Scholar] [CrossRef]
- Woo, J.Y.; Park, M.H.; Jeong, S.H.; Kim, Y.H.; Kim, B.J.; Lee, T.W.; Han, T.H. Advances in Solution-Processed OLEDs and their Prospects for Use in Displays. Adv. Mater. 2022, 2207454. [Google Scholar] [CrossRef]
- Aizawa, N.; Pu, Y.J.; Watanabe, M.; Chiba, T.; Ideta, K.; Toyota, N.; Igarashi, M.; Suzuri, Y.; Sasabe, H.; Kido, J. Solution-processed multilayer small-molecule light-emitting devices with high-efficiency white-light emission. Nat. Commun. 2014, 5, 5756. [Google Scholar] [CrossRef]
- Duan, L.; Hou, L.; Lee, T.W.; Qiao, J.; Zhang, D.; Dong, G.; Wang, L.; Qiu, Y. Solution processable small molecules for organic light-emitting diodes. J. Mater. Chem. 2010, 20, 6392–6407. [Google Scholar] [CrossRef]
- Xie, G.; Li, X.; Chen, D.; Wang, Z.; Cai, X.; Chen, D.; Li, Y.; Liu, K.; Cao, Y.; Su, S.J. Evaporation- and Solution-Process-Feasible Highly Efficient Thianthrene-9,9′,10,10′-Tetraoxide-Based Thermally Activated Delayed Fluorescence Emitters with Reduced Efficiency Roll-Off. Adv. Mater. 2016, 28, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Kido, J.; Kimura, M.; Nagai, K. Multilayer White Light-Emitting Organic Electroluminescent Device. Science 1995, 267, 1333. [Google Scholar] [CrossRef] [PubMed]
- Craft, A.; Grimsdale, A.C.; Holmes, A.B. Electroluminescent conjugated polymers—Seeing polymers in a new light. Angew. Chem. Int. Ed. 1998, 37, 402–428. [Google Scholar] [CrossRef]
- Anthony, J.E. The larger acenes: Versatile organic semiconductors. Angew. Chem. Int. Ed. 2008, 47, 452–483. [Google Scholar] [CrossRef]
- Brunner, K.; Dijken, A.V.; Börner, H.; Bastiaansen, J.J.A.M.; Kiggen, N.M.M.; Langeveld, B.M.W. Carbazole Compounds as Host Materials for Triplet Emitters in Organic Light-Emitting Diodes: Tuning the HOMO Level without Influencing the Triplet Energy in Small Molecules. J. Am. Chem. Soc. 2004, 126, 6035–6042. [Google Scholar] [CrossRef]
- Zheng, L.; Urian, R.C.; Liu, U.; Jen, A.K.Y.; Pu, L. A binaphthyl-based conjugated polymer for light-emitting diodes. Chem. Mater. 2000, 12, 13–15. [Google Scholar] [CrossRef]

| Solution a | Film b | HOMO d (eV) | LUMO (eV) | Band Gap (eV) | Td (°C) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| λAbs (nm) | λPL (nm) | FWHM c (nm) | λAbs (nm) | λPL (nm) | FWHM c (nm) | |||||
| TP-AFF-TP | 388,410 | 426, 447 | 55 | 393,416 | 477 | 96 | −5.80 | −2.79 | 3.01 | 481 |
| TP-ASF | 375, 396,419 | 439, 456 | 57 | 381, 401,425 | 467 | 68 | −5.72 | −2.80 | 2.92 | 407 |
| Materials | Von (V) | LE (cd/A) | PE (lm/W) | CIE (x, y) | ELmax (nm) | FWHM (nm) |
|---|---|---|---|---|---|---|
| TP-AFF-TP | 8.41 | 2.55 | 1.03 | (0.25, 0.45) | 504 | 96 |
| TP-ASF | 7.50 | 5.17 | 2.39 | (0.17, 0.31) | 484 | 80 |
| Materials | Von (V) | LE (cd/A) | PE (lm/W) | CIE (x, y) | ELmax (nm) |
|---|---|---|---|---|---|
| TP-ASF: 0.2 wt% Rubrene | 4.08 | 3.13 | 2.69 | (0.30, 0.34) | 463, 549 |
| TP-ASF: 0.4 wt% Rubrene | 4.12 | 4.24 | 3.60 | (0.36,0.40) | 463, 549 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Kang, S.; Park, S.; Kwon, H.; Lee, H.; Lee, K.; Park, J. Novel Fused Core Chromophore Incorporating Spirofluorene and Anthracene Groups for Sky-Blue Emission and Solution-Processed White Devices. Appl. Sci. 2023, 13, 10154. https://doi.org/10.3390/app131810154
Park S, Kang S, Park S, Kwon H, Lee H, Lee K, Park J. Novel Fused Core Chromophore Incorporating Spirofluorene and Anthracene Groups for Sky-Blue Emission and Solution-Processed White Devices. Applied Sciences. 2023; 13(18):10154. https://doi.org/10.3390/app131810154
Chicago/Turabian StylePark, Sangwook, Seokwoo Kang, Sunwoo Park, Hyukmin Kwon, Hayoon Lee, Kiho Lee, and Jongwook Park. 2023. "Novel Fused Core Chromophore Incorporating Spirofluorene and Anthracene Groups for Sky-Blue Emission and Solution-Processed White Devices" Applied Sciences 13, no. 18: 10154. https://doi.org/10.3390/app131810154
APA StylePark, S., Kang, S., Park, S., Kwon, H., Lee, H., Lee, K., & Park, J. (2023). Novel Fused Core Chromophore Incorporating Spirofluorene and Anthracene Groups for Sky-Blue Emission and Solution-Processed White Devices. Applied Sciences, 13(18), 10154. https://doi.org/10.3390/app131810154






