Cytotoxic Evaluation of Effective Ecoproduce (EEP) as a Potential Root Canal Irrigant: A Preliminary In Vitro Study
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Preparation of Test Materials and Control Solutions
2.3. Cell Culture
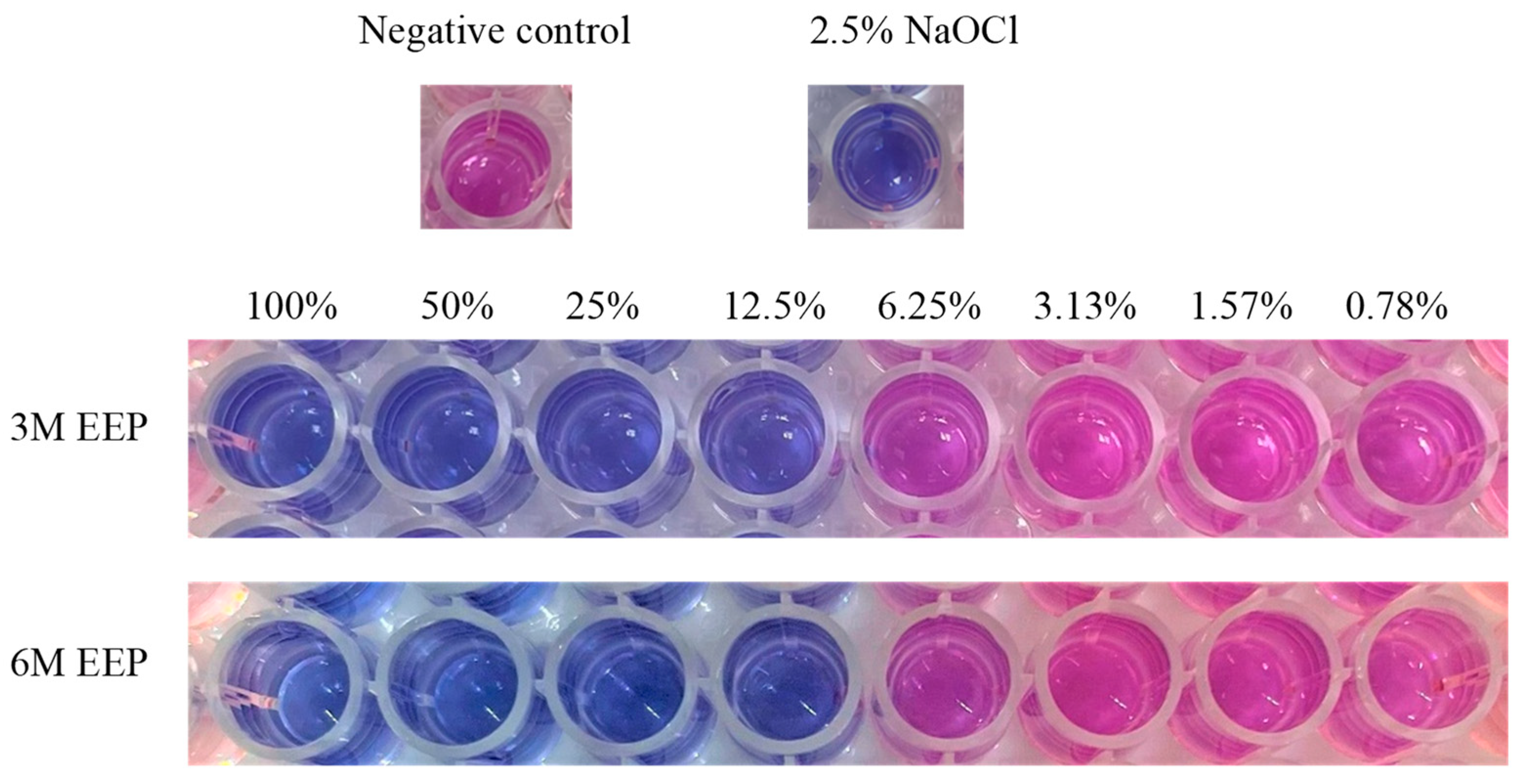
2.4. Alamar Blue Cytotoxic Assay
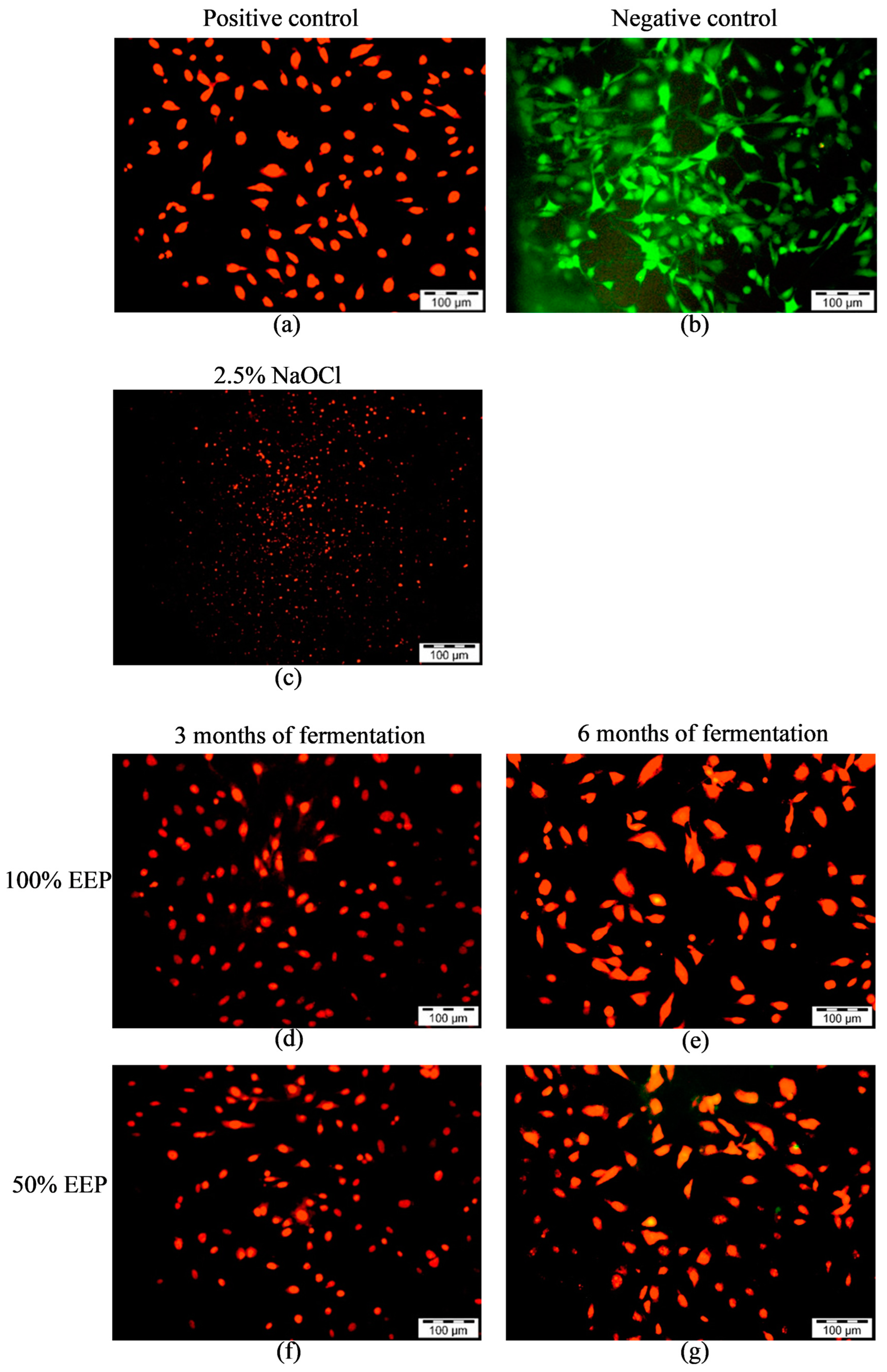
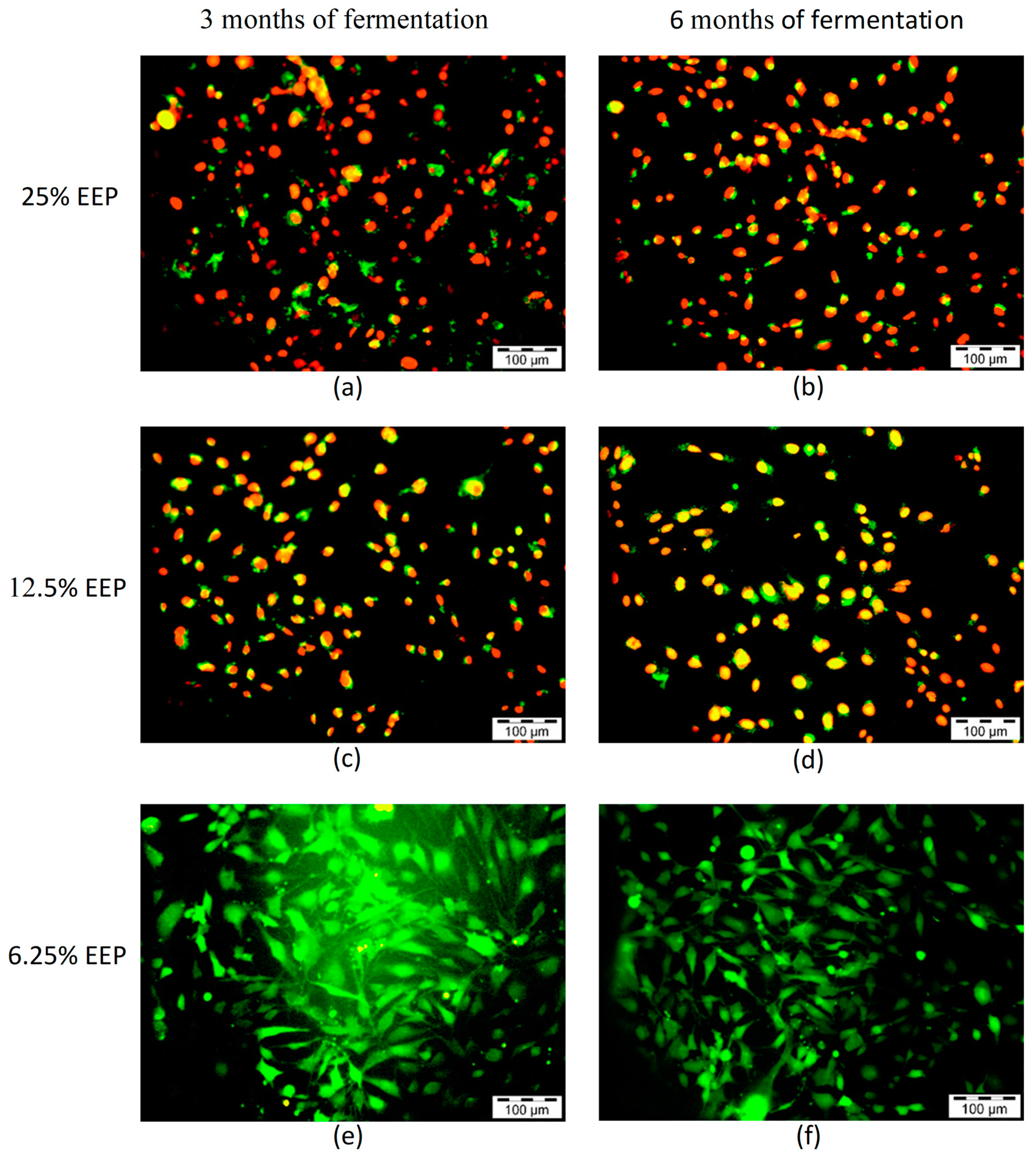
2.5. Live and Dead Cell Assay
2.6. TEM Analysis
- i.
- Cells exposed to culture medium only (untreated negative control);
- ii.
- Cells exposed to 3 M 12.5% EEP (lowest concentration that was cytotoxic);
- iii.
- Cells exposed to 3 M 6.25% EEP (highest concentration that was non-cytotoxic);
- iv.
- Cells exposed to 6 M 12.5% EEP (lowest concentration that was cytotoxic);
- v.
- Cells exposed to 6 M 6.25% EEP (highest concentration that was non-cytotoxic).
2.7. Data Analysis
3. Results
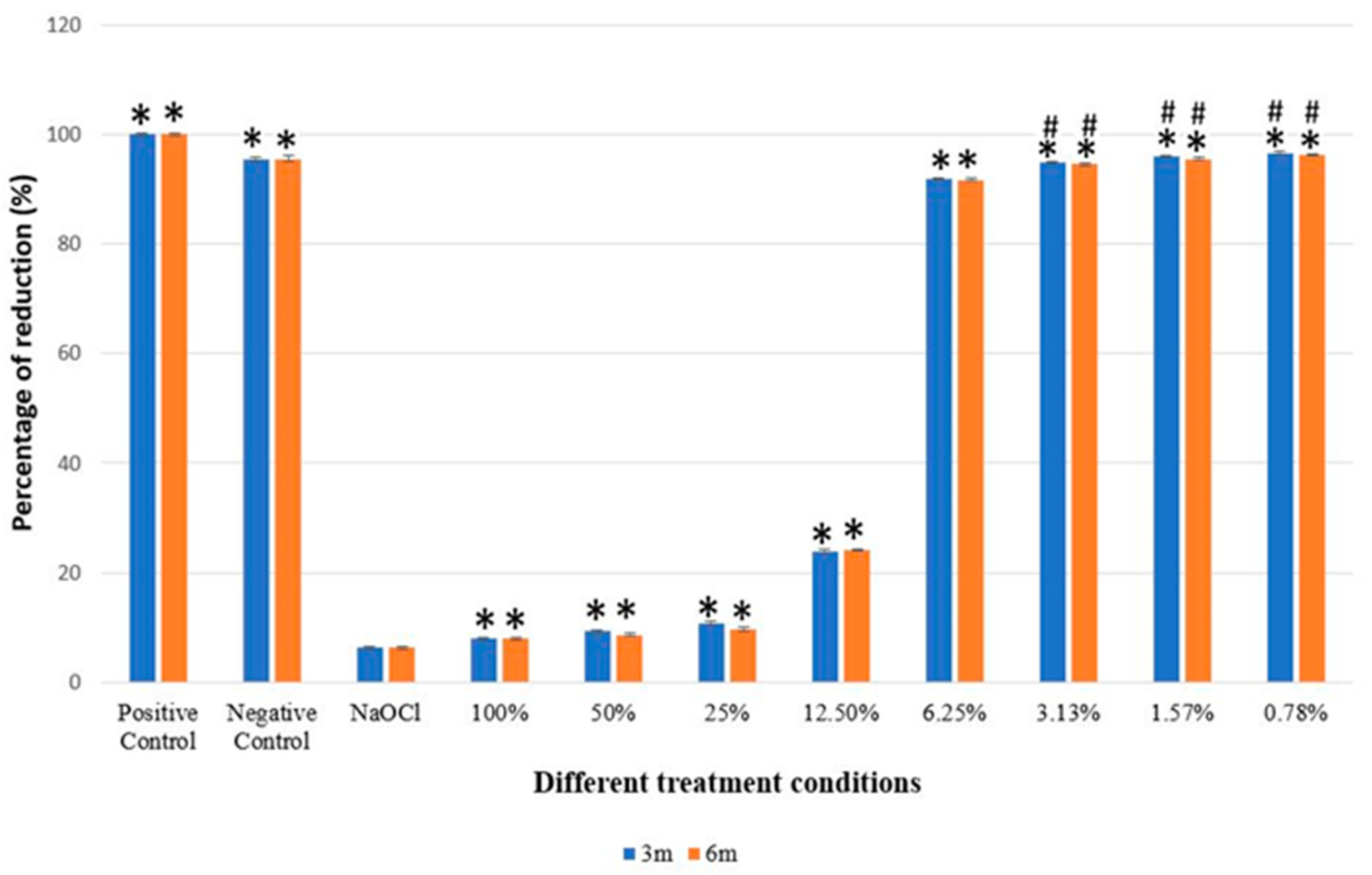
3.1. Alamar Blue Assay
3.2. The Effects of Different Concentrations and Fermentation Periods on the Cytotoxicity of EEP Extracts
3.3. Live and Dead Cell Assay
3.4. TEM Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wong, J.; Manoil, D.; Näsman, P.; Belibasakis, G.N.; Neelakantan, P. Microbiological Aspects of Root Canal Infections and Disinfection Strategies: An Update Review on the Current Knowledge and Challenges. Front. Oral Health 2021, 2, 672887. [Google Scholar] [CrossRef] [PubMed]
- Galler, K.M.; Weber, M.; Korkmaz, Y.; Widbiller, M.; Feuerer, M. Inflammatory Response Mechanisms of the Dentine–Pulp Complex and the Periapical Tissues. Int. J. Mol. Sci. 2021, 22, 1480. [Google Scholar] [CrossRef] [PubMed]
- Cimilli, H.; Karacayli, U.; Şişman, N.; Kartal, N.; Mumcu, G. Comparison of the oral health-related quality of life and dental pain in symptomatic irreversible pulpitis and pericoronitis. J. Dent. Sci. 2012, 7, 250–260. [Google Scholar] [CrossRef]
- Pesaressi, E.; Villena, R.S.; Frencken, J.E. Dental caries and oral health-related quality of life of 3-year-olds living in Lima, Peru. Int. J. Paed. Dent. 2020, 30, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Sjamsudin, E.; Manurung, B.; Arumsari, A.; Maulina, T. The management of septic shock and Ludwig’s angina: A case report of a life-threatening condition. SAGE Open Med. Case Rep. 2020, 8, 2050313X20930909. [Google Scholar] [CrossRef]
- Ruksakiet, K.; Hanák, L.; Farkas, N.; Hegyi, P.; Sadaeng, W.; Czumbel, L.M.; Sang-Ngoen, T.; Garami, A.; Mikó, A.; Varga, G.; et al. Antimicrobial Efficacy of Chlorhexidine and Sodium Hypochlorite in Root Canal Disinfection: A Systematic Review and Meta-analysis of Randomized Controlled Trials. J. Endod. 2020, 46, 1032–1041.e7. [Google Scholar] [CrossRef]
- Zehnder, M. Root canal irrigants. J. Endod. 2006, 32, 389–398. [Google Scholar] [CrossRef]
- Neelakantan, P.; Romero, M.; Vera, J.; Daood, U.; Khan, A.U.; Yan, A.; Cheung, G.S.P. Biofilms in Endodontics—Current Status and Future Directions. Int. J. Mol. Sci. 2017, 18, 1748. [Google Scholar] [CrossRef]
- Haapasalo, M.; Shen, Y.; Wang, Z.; Gao, Y. Irrigation in endodontics. Br. Dent. J. 2014, 216, 299–303. [Google Scholar] [CrossRef]
- Lopes, R.M.V.; Marins, F.C.; Belladonna, F.G.; Souza, E.M.; De-Deus, G.; Lopes, R.T.; Silva, E.J.N.L. Untouched Canal Areas and Debris Accumulation after Root Canal Preparation With Rotary and Adaptive Systems. Aust. Endod. J. 2018, 44, 260–266. [Google Scholar] [CrossRef]
- Boutsioukis, C.; Arias-Moliz, M.T. Present status and future directions—Irrigants and irrigation methods. Int. Endod. J. 2022, 55, 588–612. [Google Scholar] [CrossRef]
- Walker, A. A definite and dependable therapy for pulpless teeth. J. Am. Dent. Assoc. 1936, 23, 1418–1425. [Google Scholar] [CrossRef]
- Gonçalves, L.S.; Rodrigues, R.C.V.; Junior, C.V.A.; Soares, R.G.; Vettore, M.V. The Effect of Sodium Hypochlorite and Chlorhexidine as Irrigant Solutions for Root Canal Disinfection: A Systematic Review of Clinical Trials. J. Endod. 2016, 42, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Neelakantan, P.; Herrera, D.R.; Pecorari, V.G.A.; Gomes, B.P.F.A. Endotoxin Levels after Chemomechanical Preparation of Root Canals with Sodium Hypochlorite or Chlorhexidine: A Systematic Review of Clinical Trials and Meta-Analysis. Int. Endod. J. 2019, 52, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Heling, I.; Rotstein, I.; Dinur, T.; Szweclevine, Y.; Steinberg, D. Bactericidal and cytotoxic effects of sodium hypochlorite and sodium dichloroisocyanurate solutions in vitro. J. Endod. 2001, 27, 278–280. [Google Scholar] [CrossRef] [PubMed]
- Guivarc’H, M.; Ordioni, U.; Ahmed, H.M.A.; Cohen, S.; Catherine, J.-H.; Bukiet, F. Sodium Hypochlorite Accident: A Systematic Review. J. Endod. 2017, 43, 16–24. [Google Scholar] [CrossRef]
- Chung, I.; Ryu, H.; Yoon, S.-Y.; Ha, J.C. Health Effects of Sodium Hypochlorite: Review of Published Case Reports. Environ. Anal. Health Toxicol. 2022, 37, e2022006. [Google Scholar] [CrossRef]
- Park, J.S.; Min, J.H.; Kim, H.; Lee, S.W. Esophageal perforation and mediastinitis after suicidal ingestion of 4.5% sodium hydrochlorite bleach. Clin. Toxicol. 2011, 49, 765–766. [Google Scholar] [CrossRef]
- Drews, D.-J.; Nguyen, A.D.; Diederich, A.; Gernhardt, C.R. The Interaction of Two Widely Used Endodontic Irrigants, Chlorhexidine and Sodium Hypochlorite, and Its Impact on the Disinfection Protocol during Root Canal Treatment. Antibiotics 2023, 12, 589. [Google Scholar] [CrossRef]
- Almadi, E.M.; Almohaimede, A.A.; Ou, J.; Chen, H.; Li, L.; Zhao, L.; Nie, N. Natural Products in Endodontics. Saudi Med. J. 2018, 39, 124–130. [Google Scholar] [CrossRef]
- Vitali, F.C.; Andrada, A.C.; Cardoso, H.C.d.L.; Xavier-Junior, G.F.; Teixeira, C.d.S.; Salles, L.P.; Lia, E.N.; Massignan, C. Does the use of natural products for endodontic therapy in primary teeth have sufficient evidence for clinical practice? A scoping review. Clin. Oral Investig. 2022, 26, 6043–6060. [Google Scholar] [CrossRef] [PubMed]
- Susila, A.V.; Sai, S.; Sharma, N.; Balasubramaniam, A.; Veronica, A.K.; Nivedhitha, S. Can Natural Irrigants Replace Sodium Hypochlorite? A Systematic Review. Clin. Oral Investig. 2023, 27, 1831–1849. [Google Scholar] [CrossRef] [PubMed]
- Arun, C.; Sivashanmugam, P. Investigation of Biocatalytic Potential of Garbage Enzyme and Its Influence On Stabilization of Industrial Waste Activated Sludge. Process Saf. Environ. Prot. 2015, 94, 471–478. [Google Scholar] [CrossRef]
- Rahman, S.; Haque, I.; Goswami, R.C.D.; Barooah, P.; Sood, K.; Choudhury, B. Characterization and FPLC Analysis of Garbage Enzyme: Biocatalytic and Antimicrobial Activity. Waste Biomass-Valorization 2021, 12, 293–302. [Google Scholar] [CrossRef]
- Ng, M.Y.; Shafiei, Z.; Rahman, A.; Sockalingam, S.N.M.; Zakaria, A.S.I.; Mahyuddin, A. Antibacterial effects of effective ecoproduce on Enterococcus faecalis: An in vitro Study. J. Int. Dent. Med. Res. 2020, 13, 861–867. [Google Scholar]
- Mavani, H.A.K.; Tew, I.M.; Wong, L.; Yew, H.Z.; Mahyuddin, A.; Ghazali, R.A.; Pow, E.H.N. Antimicrobial Efficacy of Fruit Peels Eco-Enzyme against Enterococcus faecalis: An In Vitro Study. Int. J. Environ. Res. Public Health 2020, 17, 5107. [Google Scholar] [CrossRef]
- Sai, S.; Abisha, V.M.J.; Mahalakshmi, K.; Veronica, A.K.; Susila, A.V. Treasure from Trash—Is Ecoenzyme the New Panacea in Conservative Dentistry and Endodontics? J. Conserv. Dent. 2023, 26, 176–181. [Google Scholar]
- Arun, C.; Sivashanmugam, P. Identification and optimization of parameters for the semi-continuous production of garbage enzyme from pre-consumer organic waste by green RP-HPLC method. Waste Manag. 2015, 44, 28–33. [Google Scholar] [CrossRef]
- Tallei, T.E.; Fatimawali; Niode, N.J.; Alsaihati, W.M.; Salaki, C.L.; Alissa, M.; Kamagi, M.; Rabaan, A.A. Antibacterial and Antioxidant Activity of Ecoenzyme Solution Prepared from Papaya, Pineapple, and Kasturi Orange Fruits: Experimental and Molecular Docking Studies. J. Food Process Preserv. 2023, 2023, 5826420. [Google Scholar] [CrossRef]
- Istanti, A.; Utami, S.W. Utilization of household waste into eco-enzyme in gitik village, rogojampi district, banyuwangi. War. Pengabdi. 2022, 16, 30–43. [Google Scholar] [CrossRef]
- Ng, M.Y. Antibacterial and Anti-Biofilm Effects of Effective Eco-Produce on Enterococcus Faecalis. Master’s Thesis, The National University of Malaysia, Kuala Lumpur, Malaysia, 28 August 2019. [Google Scholar]
- Arun, C.; Sivashanmugam, P. Study on Optimization of Process Parameters for Enhancing the Multi-Hydrolytic Enzyme Activity in Garbage Enzyme Produced From Preconsumer Organic Waste. Bioresour. Technol. 2017, 226, 200–210. [Google Scholar] [CrossRef] [PubMed]
- American Type Culture Collection. 2023. ATCC MC3T3-E1 Subclone 14. Available online: https://www.atcc.org/products/crl-25 (accessed on 13 January 2023).
- American Type Culture Collection. 2022. ATCC Animal Cell Culture Guide. Available online: https://www.atcc.org/-/media/resources/culture-guides/animal-cell-cultureguide.pdf (accessed on 13 May 2022).
- Freshney, R.I. Culture of Animal Cells: A Manual of Basic Technique and Specialized Applications, 7th ed.; Wiley-Blackwell: New York, NY, USA, 2015. [Google Scholar]
- Valanezhad, A.; Odatsu, T.; Abe, S.; Watanabe, I. Bone formation ability and cell viability enhancement of MC3T3-E1 cells by Ferrostatin-1 a ferroptosis inhibitor of cancer cells. Int. J. Mol. Sci. 2021, 22, 12259. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Meng, L.; Wang, F.; Hu, X.; Yu, Y. Sodium fluoride induces apoptosis and autophagy via the endoplasmic reticulum stress pathway in MC3T3-E1 osteoblastic cells. Mol. Cell. Biochem. 2019, 454, 77–85. [Google Scholar] [CrossRef]
- ISO/EN10993-5; Biological Evaluation of Medical Devices—Part 5: Tests for Cytotoxicity: In Vitro Methods. International Organization for Standardization: Geneva, Switzerland, 2009.
- Thermo Scientific. 2012. Instructions for AlamarBlueTM Cell Viability Assay Reagent. Available online: https://assets.fishersci.com/TFSAssets/LSG/manuals/MAN0011850_alamarBlue_Cell_Viability_Asy_Reag_UG.pdf?_ga=2.154220465.825288967.1689556985-1078133582.1689556985 (accessed on 8 July 2023).
- Zachari, M.A.; Chondrou, P.S.; Pouliliou, S.E.; Mitrakas, A.G.; Abatzoglou, I.; Zois, C.E.; Koukourakis, M.I. Evaluation of The Alamarblue Assay for Adherent Cell Irradiation Experiments. Dose-Response 2013, 12, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Al-Nasiry, S.; Geusens, N.; Hanssens, M.; Luyten, C.; Pijnenborg, R. The use of Alamar Blue assay for quantitative analysis of viability, migration and invasion of choriocarcinoma cells. Hum. Reprod. 2007, 22, 1304–1309. [Google Scholar] [CrossRef]
- Cintra, L.T.A.; Benetti, F.; Queiroz, O.d.A.; Lopes, J.M.d.A.; de Oliveira, S.H.P.; Araújo, G.S.; Gomes-Filho, J.E. Cytotoxicity, biocompatibility, and biomineralization of the new high-plasticity MTA material. J. Endod. 2017, 43, 774–778. [Google Scholar] [CrossRef]
- Tinari, A.; Giammarioli, A.M.; Manganelli, V.; Ciarlo, L.; Malorni, W. Chapter One Analyzing Morphological and Ultrastructural Features in Cell Death. Methods Enzym. 2008, 442, 1–26. [Google Scholar] [CrossRef]
- Longhin, E.M.; El Yamani, N.; Rundén-Pran, E.; Dusinska, M. The alamar blue assay in the context of safety testing of nanomaterials. Front. Toxicol. 2022, 4, 981701. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Sari, G.; Ozdal, T.; Capanoglu, E. Guidelines for Cell Viability Assays. Food Front. 2020, 1, 332–349. [Google Scholar] [CrossRef]
- Rampersad, S.N. Multiple applications of alamar blue as an indicator of metabolic function and cellular health in cell viability bioassays. Sensors 2012, 12, 12347–12360. [Google Scholar] [CrossRef]
- Najdawi, Z.; Abu-Asab, M. An Ultrastructural Perspective on Cell Death. Jordan Med. J. 2022, 56. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Faculty Opinions recommendation of Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, P.A.; Coelho, M.S.; Kato, A.S.; Fontana, C.E.; Bueno, C.E.; Pedro-Rocha, D.G. Cytotoxicity assessment of 1% peracetic acid, 2.5% sodium hypochlorite and 17% EDTA on FG11 and FG15 human fibroblasts. Acta Odontol. Latinoam. AOL 2018, 31, 11–15. [Google Scholar] [PubMed]
- Ahmad, Z.H.; Alkahtany, S.M.; Anil, S. An in vitro Evaluation of the Cytotoxicity of Varying Concentrations of Sodium Hypochlorite on Human Mesenchymal Stem Cells. J. Contemp. Dent. Pract. 2014, 15, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Böhle, S.; Röhner, E.; Zippelius, T.; Jacob, B.; Matziolis, G.; Rohe, S. Cytotoxic effect of sodium hypochlorite (Lavanox 0.08%) and chlorhexidine gluconate (Irrisept 0.05%) on human osteoblasts. Eur. J. Orthop. Surg. Traumatol. 2022, 32, 81–89. [Google Scholar] [CrossRef]
- Galluzzi, L.; Maiuri, M.C.; Vitale, I.; Zischka, H.; Castedo, M.; Zitvogel, L.; Kroemer, G. Cell death modalities: Classification and pathophysiological implications. Cell Death Differ. 2007, 14, 1237–1243. [Google Scholar] [CrossRef]
- Aydin, Z.U.; Akpinar, K.E.; Hepokur, C.; Erdönmez, D. Assessment of toxicity and oxidative DNA damage of sodium hypochlorite, chitosan and propolis on fibroblast cells. Braz. Oral Res. 2018, 32, e119. [Google Scholar] [CrossRef]
- Vikas, O.; Mridul, U. Bioconversion of Papaya Peel Waste in to Vinegar Using Acetobacter Aceti. Int. J. Sci. Res. 2014, 3, 409–411. [Google Scholar]
- Vama, L.; Cherekar, M.N.J. Production, Extraction and Uses of Eco-Enzyme Using Citrus Fruit Waste: Wealth from Waste. Asian J. Microbiol. Biotech. Environ. Sci. 2020, 22, 346–351. [Google Scholar]
- Matsuo, M.; Sasaki, N.; Saga, K.; Kaneko, T. Cytotoxicity of flavonoids toward cultured normal human cells. Biol. Pharm. Bull. 2005, 28, 253–259. [Google Scholar] [CrossRef]
- Perveen, S.; Al-Taweel, A.M. Phenolic Compounds from the Natural Sources and Their Cytotoxicity. In Phenolic Compounds—Natural Sources, Importance and Applications, 1st ed.; Soto-Hernández, M., Palma-Tenango, M., Garcia-Mateos, M.d.R., Eds.; InTech Open: London, UK, 2017. [Google Scholar]
- Chaves, S.R.; Rego, A.; Martins, V.M.; Santos-Pereira, C.; Sousa, M.J.; Côrte-Real, M. Regulation of Cell Death Induced by Acetic Acid in Yeasts. Front. Cell Dev. Biol. 2021, 9, 642375. [Google Scholar] [CrossRef] [PubMed]
- Kocyigit, A.; Koyuncu, I.; Dikilitas, M.; Bahadori, F.; Turkkan, B. Cytotoxic, genotoxic and apoptotic effects of naringenin-oxime relative to naringenin on normal and cancer cell lines. Asian Pac. J. Trop. Biomed. 2016, 6, 872–880. [Google Scholar] [CrossRef]
- Stompor, M.; Uram, Ł.; Podgórski, R. In Vitro Effect of 8-Prenylnaringenin and Naringenin on Fibroblasts and Glioblastoma Cells-Cellular Accumulation and Cytotoxicity. Molecules 2017, 22, 1092. [Google Scholar] [CrossRef]
- Rego, A.; Mendes, F.; Costa, V.; Chaves, S.R.; Côrte-Real, M. Pkh1p-Ypk1p and Pkh1p-Sch9p Pathways Are Activated by Acetic Acid to Induce a Mitochondrial-Dependent Regulated Cell Death. Oxidative Med. Cell Longev. 2020, 2020, 7095078. [Google Scholar] [CrossRef] [PubMed]
- Snigirevskaya, E.S.; Komissarchik, Y.Y. Ultrastructural traits of apoptosis. Cell Biol. Int. 2019, 43, 728–738. [Google Scholar] [CrossRef]
- Lu, W.; Yu, C.R.; Lien, H.; Sheu, G.; Cherng, S. Cytotoxicity of naringenin induces Bax-mediated mitochondrial apoptosis in human lung adenocarcinoma A549 cells. Environ. Toxicol. 2020, 35, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- Sena, N.T.; Gomes, B.P.F.A.; Vianna, M.E.; Berber, V.B.; Zaia, A.A.; Ferraz, C.C.R.; Souza-Filho, F.J. In vitro antimicrobial activity of sodium hypochlorite and chlorhexidine against selected single-species biofilms. Int. Endod. J. 2006, 39, 878–885. [Google Scholar] [CrossRef]
- Giardino, L.; Ambu, E.; Savoldi, E.; Rimondini, R.; Cassanelli, C.; Debbia, E.A. Comparative evaluation of antimicrobial efficacy of sodium hypochlorite, MTAD, and tetraclean against enterococcus faecalis biofilm. J. Endod. 2007, 33, 852–855. [Google Scholar] [CrossRef]
- Chai, W.L.; Hamimah, H.; Abdullha, M. Evaluation of antibacterial efficacy of antibiotics and calcium hydroxide against Enterococcus faecalis biofilm in dentin. Sains Malays. 2013, 42, 73–80. [Google Scholar]
- Tirali, R.E.; Bodur, H.; Sipahi, B.; Sungurtekin, E. Evaluation of the antimicrobial activities of chlorhexidine gluconate, sodium hypochlorite and octenidine hydrochloride in vitro. Aust. Endod. J. 2013, 39, 15–18. [Google Scholar] [CrossRef]
- Ghivari, S.B.; Bhattacharya, H.; Bhat, K.G.; Pujar, M. Antimicrobial activity of root canal irrigants against biofilm forming pathogens- An in vitro study. J. Conserv. Dent. 2017, 20, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Czekanska, E.M.; Stoddart, M.J.; Ralphs, J.R.; Richards, R.G.; Hayes, J.S. A Phenotypic Comparison of Osteoblast Cell Lines Versus Human Primary Osteoblasts for Biomaterials Testing. J. Biomed. Mater. Res. Part A 2014, 102, 2636–2643. [Google Scholar] [CrossRef] [PubMed]
- Nagendrababu, V.; Kishen, A.; Murray, P.E.; Nekoofar, M.H.; Figueiredo, J.A.P.; Priya, E.; Jayaraman, J.; Pulikkotil, S.J.; Camilleri, J.; Silva, R.M.; et al. PRIASE 2021 guidelines for reporting animal studies in Endodontology: A consensus-based development. Int. Endod. J. 2021, 54, 848–857. [Google Scholar] [CrossRef]
- Iorio, R.A.; Del Duca, S.; Calamelli, E.; Pula, C.; Lodolini, M.; Scamardella, F.; Pession, A.; Ricci, G. Citrus allergy from pollen to clinical symptoms. PLoS ONE 2013, 8, e53680. [Google Scholar] [CrossRef] [PubMed][Green Version]

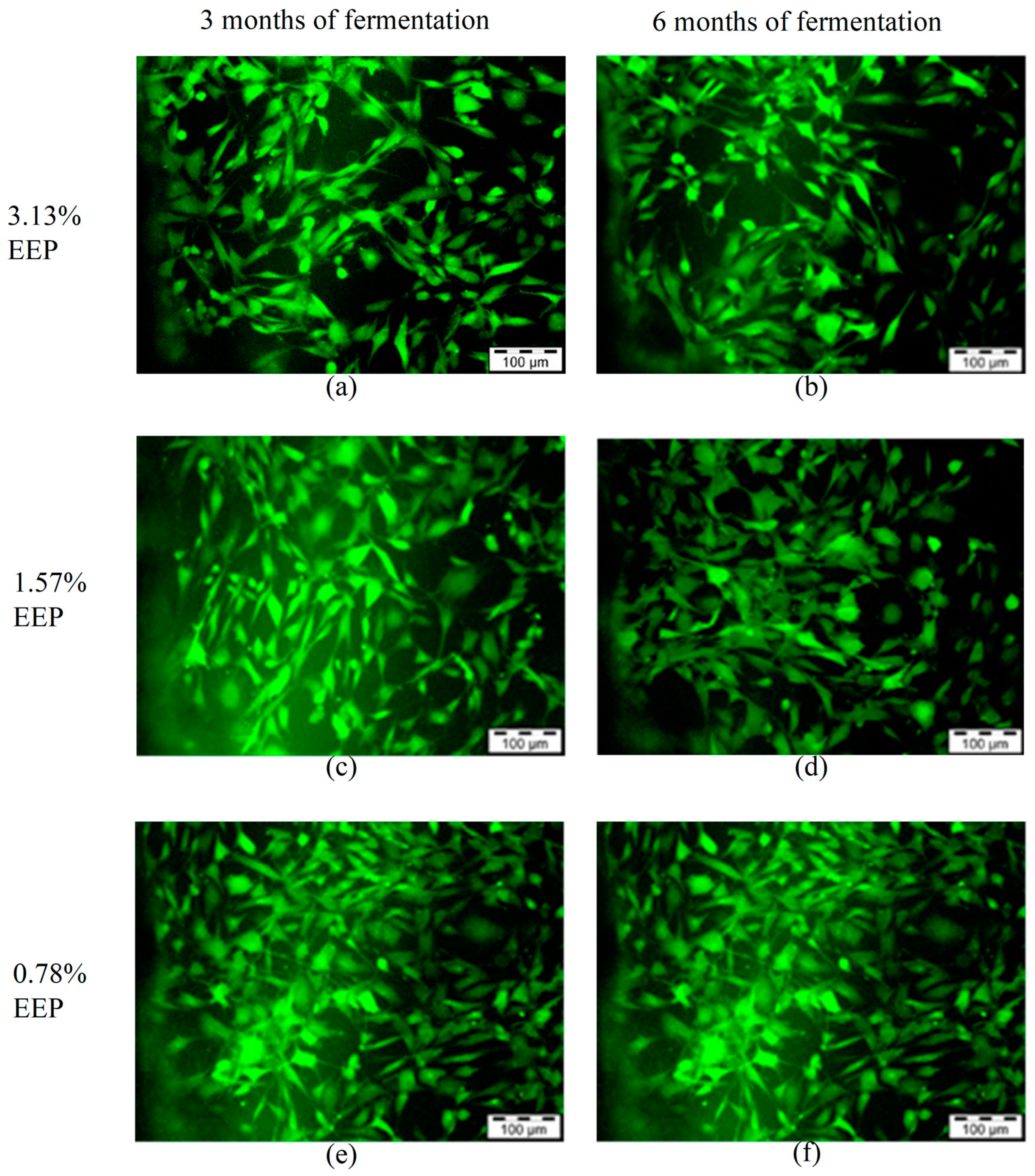
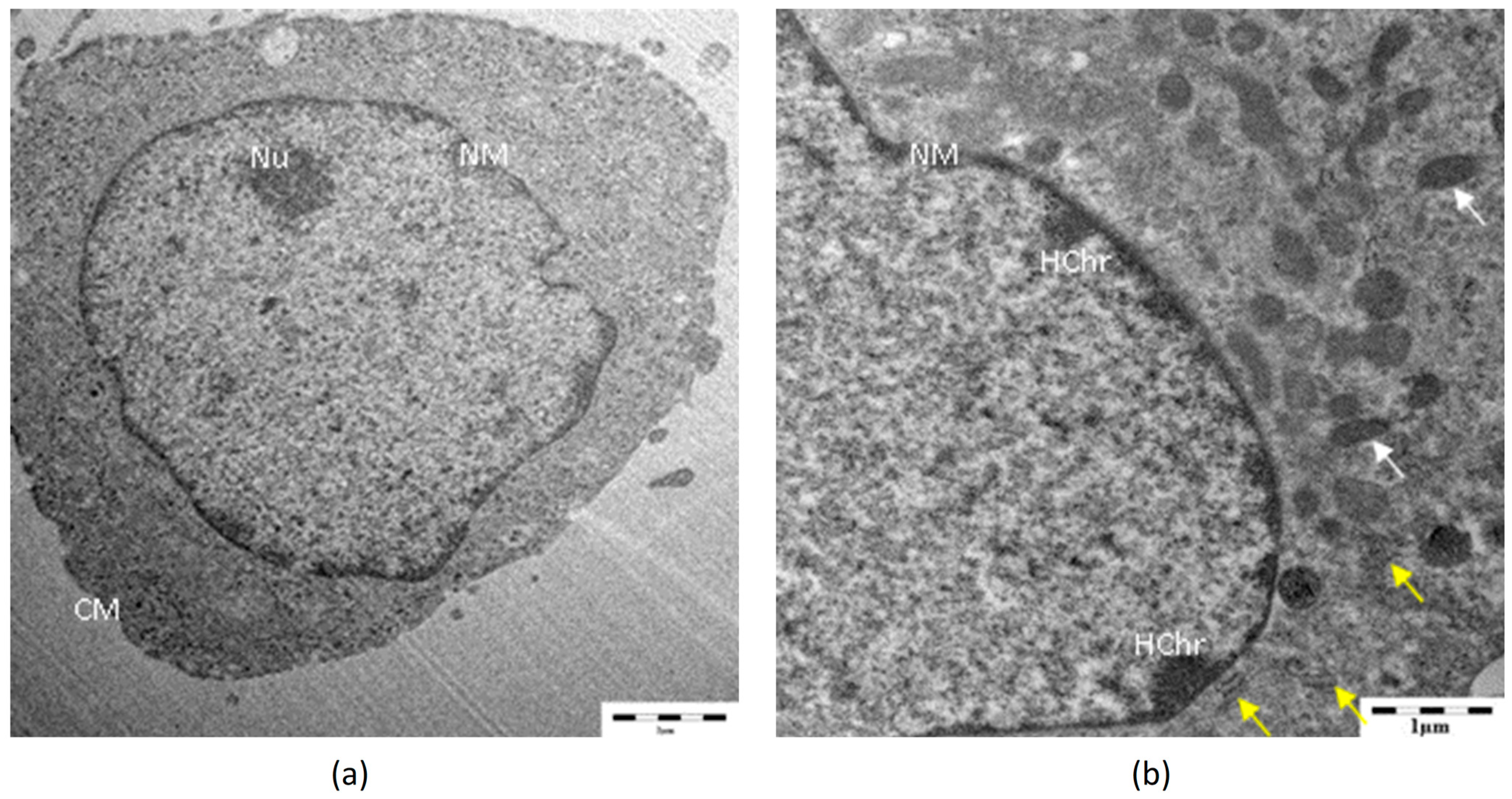
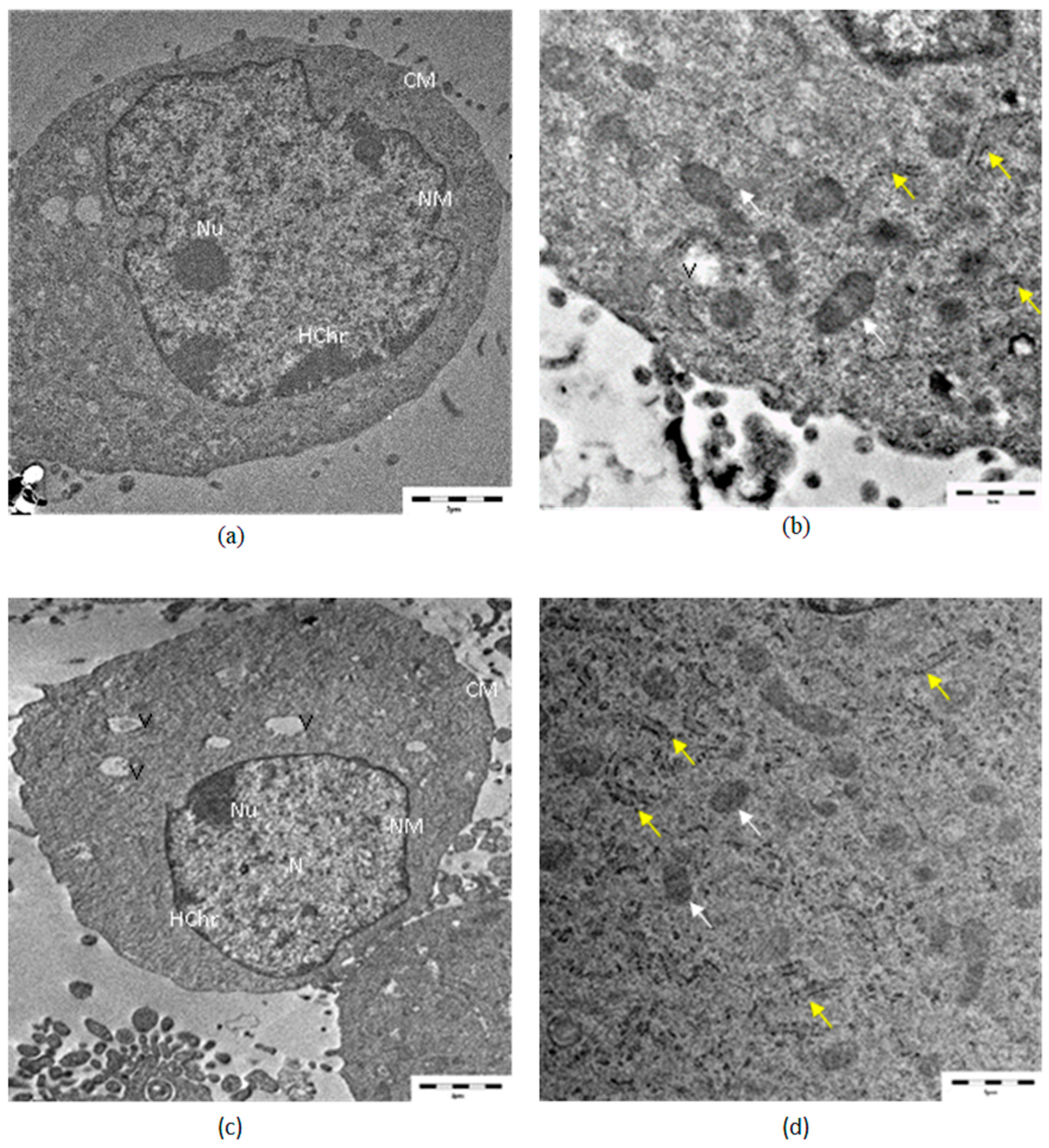
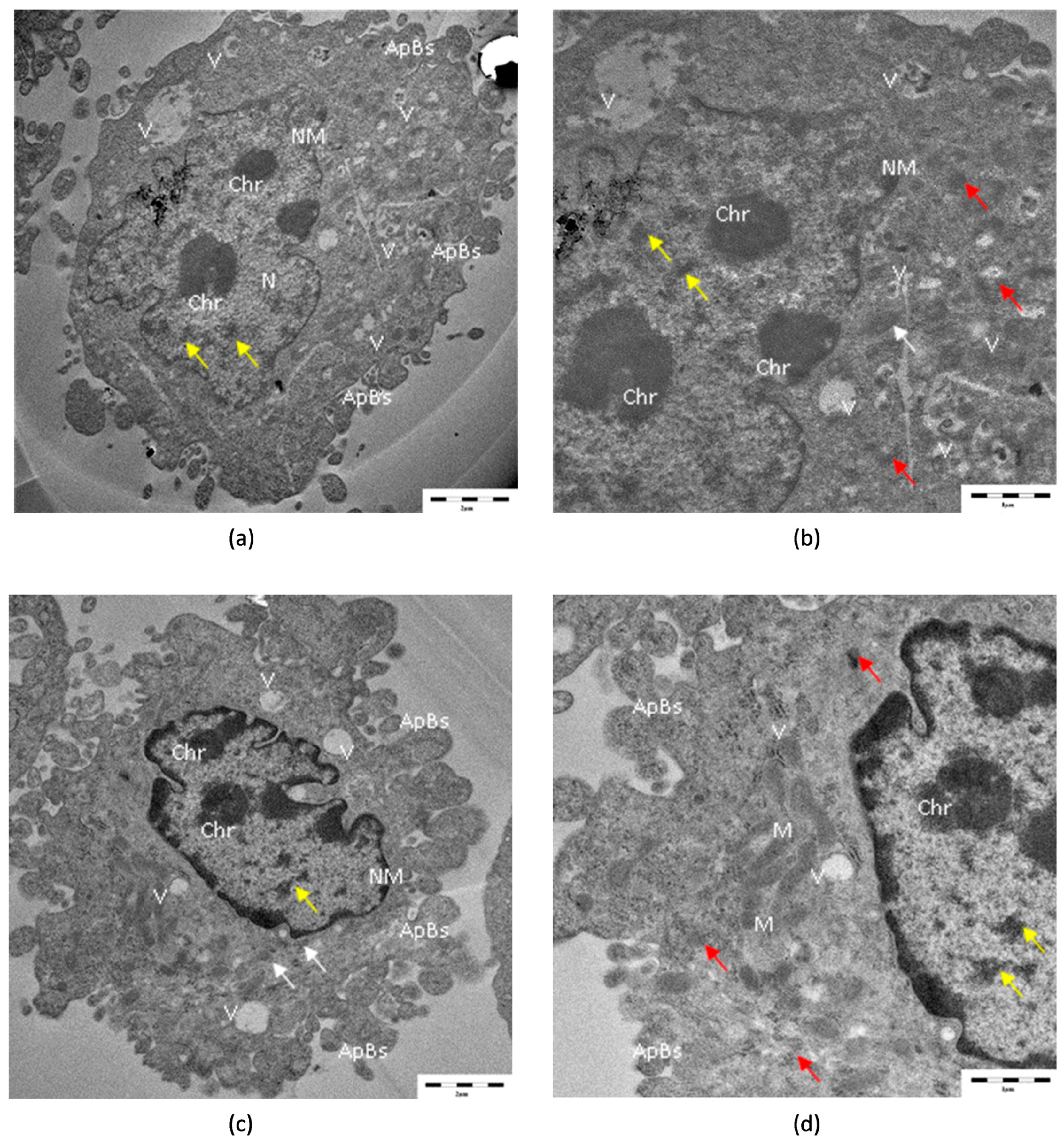
| Concentrations | Relative Cell Viability (% of Control) | |
|---|---|---|
| 3 Months of Fermentation (Mean ± SE) | 6 Months of Fermentation (Mean ± SE) | |
| 100% EEP | 8.25 ±0.25 | 8.28 ± 0.28 |
| 50% EEP | 9.65 ± 0.39 | 9.03 ± 0.27 |
| 25% EEP | 11.28 ± 0.39 | 10.14 ± 0.36 |
| 12.5% EEP | 24.97 ± 0.33 | 25.24 ± 0.26 |
| 6.25% EEP | 96.20 ± 0.21 * | 96.06 ± 0.30 * |
| 3.13% EEP | 99.35 ± 0.23 * | 99.02 ± 0.32 * |
| 1.57% EEP | 100.49 ± 0.24 * | 100.03 ± 0.31 * |
| 0.78% EEP | 101.13 ± 0.23 * | 100.82 ± 0.24 * |
| Interaction between Different Treatment Groups and Fermentation Periods | ||
|---|---|---|
| Two-Way Mixed ANOVA | F = 0.724, p = 0.713 | |
| Main Effect | Different Treatment Groups F = 38,354.39, p < 0.001 * | Fermentation Periods F = 3.408, p = 0.068 |
| 3 Months of Fermentation | 6 Months of Fermentation | |||||
|---|---|---|---|---|---|---|
| Source | Percentage of Reduction (Mean ± SE) | Pairwise Comparison with Negative Control | Percentage of Reduction (Mean ± SE) | Pairwise Comparison with Negative Control | ||
| Mean Difference (Mean ± SE) | p-Value | Mean Difference (Mean ± SE) | p-Value | |||
| 100% EEP | 7.89 ± 0.35 | 87.68 ± 0.45 | 0.001 * | 7.91 ± 0.27 | 87.62 ± 0.44 | <0.001 * |
| 50% EEP | 9.22 ± 0.38 | 86.34 ± 0.45 | <0.001 * | 8.62 ± 0.26 | 86.91 ± 0.44 | <0.001 * |
| 25% EEP | 10.78 ± 0.37 | 84.78 ± 0.45 | <0.001 * | 9.69 ± 0.35 | 85.84 ± 0.44 | <0.001 * |
| 12.5% EEP | 23.86 ± 0.31 | 71.70 ± 0.45 | <0.001 * | 24.11 ± 0.25 | 71.42 ± 0.44 | <0.001 * |
| 6.25% EEP | 91.93 ± 0.20 | 3.64 ± 0.45 | <0.001 * | 91.73 ± 0.29 | 3.76 ± 0.44 | <0.001 * |
| 3.13% EEP | 94.94 ± 0.22 | 0.62 ± 0.45 | 0.993 | 94.60 ± 0.31 | 0.94 ± 0.44 | 0.597 |
| 1.57% EEP | 96.03 ± 0.25 | −0.47 ± 0.45 | 0.996 | 95.56 ± 0.30 | −0.03 ± 0.44 | 1.00 |
| 0.78% EEP | 96.65 ± 0.22 | −1.08 ± 0.45 | 0.400 | 96.31 ± 0.23 | −0.78 ± 0.44 | 0.82 |
| NaOCl | 95.56 ± 0.38 | 89.31 ± 0.45 | <0.001 * | 95.53 ± 0.50 | 89.28 ± 0.44 | <0.001 * |
| Positive control | 100.00 ± 0.34 | −4.44 ± 0.45 | <0.001 * | 100.00 ± 0.27 | −4.47 ± 0.44 | <0.001 * |
| 3 Months of Fermentation | 6 Months of Fermentation | |||||
|---|---|---|---|---|---|---|
| Source | Percentage of Reduction (Mean ± SE) | Pairwise Comparison with NaOCl | Percentage of Reduction (Mean ± SE) | Pairwise Comparison with NaOCl | ||
| Mean Difference (Mean ± SE) | p-Value | Mean Difference (Mean ± SE) | p-Value | |||
| 100% EEP | 7.89 ± 0.35 | −1.63 ± 0.45 | <0.021 * | 7.91 ± 0.27 | −1.67 ± 0.44 | 0.013 * |
| 50% EEP | 9.22 ± 0.38 | −2.97 ± 0.45 | <0.001 * | 8.62 ± 0.26 | −2.37 ± 0.44 | <0.001 * |
| 25% EEP | 10.78 ± 0.37 | −4.52 ± 0.45 | <0.001 * | 9.69 ± 0.35 | −3.43 ± 0.44 | <0.001 * |
| 12.5% EEP | 23.86 ± 0.31 | −17.61 ± 0.45 | <0.001 * | 24.11 ± 0.25 | −17.86 ± 0.44 | <0.001 * |
| 6.25% EEP | 91.93 ± 0.20 | −85.67 ± 0.45 | <0.001 * | 91.73 ± 0.29 | −85.52 ± 0.44 | <0.001 * |
| 3.13% EEP | 94.94 ± 0.22 | −88.68 ± 0.45 | <0.001 * | 94.60 ± 0.31 | −88.34 ± 0.44 | <0.001 * |
| 1.57% EEP | 96.03 ± 0.25 | −89.78 ± 0.45 | <0.001 * | 95.56 ± 0.30 | −89.31 ± 0.44 | <0.001 * |
| 0.78% EEP | 96.65± 0.22 | −90.39 ± 0.45 | <0.001 * | 96.31 ± 0.23 | −90.06 ± 0.44 | <0.001 * |
| Negative control | 95.56 ± 0.38 | −89.31 ± 0.45 | <0.001 * | 95.53 ± 0.50 | −89.28 ± 0.44 | <0.001 * |
| Positive control | 100.00 ± 0.34 | −93.74 ± 0.45 | <0.001 * | 100.00 ± 0.27 | −93.75 ± 0.44 | <0.001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hung, W.K.; Mahyuddin, A.; Sockalingam, S.N.M.P.; Shafiei, Z.; Abdul Rahman, M.; Mahamad Apandi, N.I.; Abdul Ghani, Z.D.F.; Zakaria, A.S.I. Cytotoxic Evaluation of Effective Ecoproduce (EEP) as a Potential Root Canal Irrigant: A Preliminary In Vitro Study. Appl. Sci. 2023, 13, 10125. https://doi.org/10.3390/app131810125
Hung WK, Mahyuddin A, Sockalingam SNMP, Shafiei Z, Abdul Rahman M, Mahamad Apandi NI, Abdul Ghani ZDF, Zakaria ASI. Cytotoxic Evaluation of Effective Ecoproduce (EEP) as a Potential Root Canal Irrigant: A Preliminary In Vitro Study. Applied Sciences. 2023; 13(18):10125. https://doi.org/10.3390/app131810125
Chicago/Turabian StyleHung, Wong Kiong, Alida Mahyuddin, S. Nagarajan M. P. Sockalingam, Zaleha Shafiei, Mariati Abdul Rahman, Nurul Inaas Mahamad Apandi, Zuleen Delina Fasya Abdul Ghani, and Ahmad Shuhud Irfani Zakaria. 2023. "Cytotoxic Evaluation of Effective Ecoproduce (EEP) as a Potential Root Canal Irrigant: A Preliminary In Vitro Study" Applied Sciences 13, no. 18: 10125. https://doi.org/10.3390/app131810125
APA StyleHung, W. K., Mahyuddin, A., Sockalingam, S. N. M. P., Shafiei, Z., Abdul Rahman, M., Mahamad Apandi, N. I., Abdul Ghani, Z. D. F., & Zakaria, A. S. I. (2023). Cytotoxic Evaluation of Effective Ecoproduce (EEP) as a Potential Root Canal Irrigant: A Preliminary In Vitro Study. Applied Sciences, 13(18), 10125. https://doi.org/10.3390/app131810125







