Phytogenic Synthesis and Characterization of Silver Metallic/Bimetallic Nanoparticles Using Beta vulgaris L. Extract and Assessments of Their Potential Biological Activities
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Beta vulgaris Extract
2.2. Biosynthesis of Silver Metallic/Bimetallic Nanoparticles
2.3. Instruments and Chemical Features of NPs
2.4. Phytochemical Analysis
2.5. Biological Assessment
2.5.1. Antioxidant Activity
2.5.2. Antibacterial Activity
2.5.3. Cytotoxic Activity
Tumor and Normal Cell Lines
MTT Assay
3. Results and Discussion
3.1. Identification of Metallic/Bimetallic Nanoparticles
3.1.1. UV-Visible Spectra
3.1.2. FTIR Spectral Analyses
3.1.3. TEM Investigation
3.1.4. Zeta Potential Analyses
3.2. Phytochemical Analysis
3.3. Biological Assessments
3.3.1. Antioxidant Activity
3.3.2. Antibacterial Activity
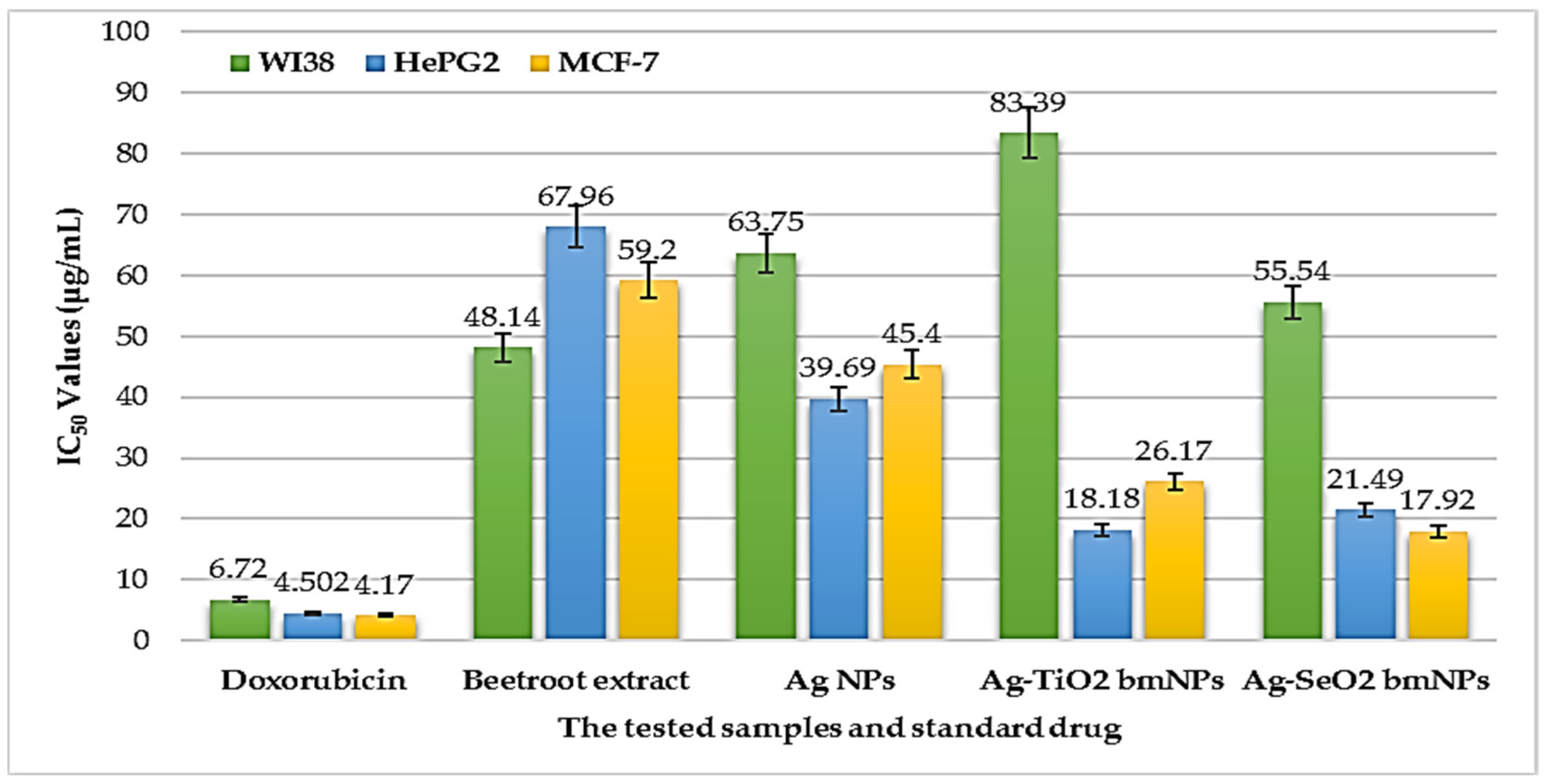
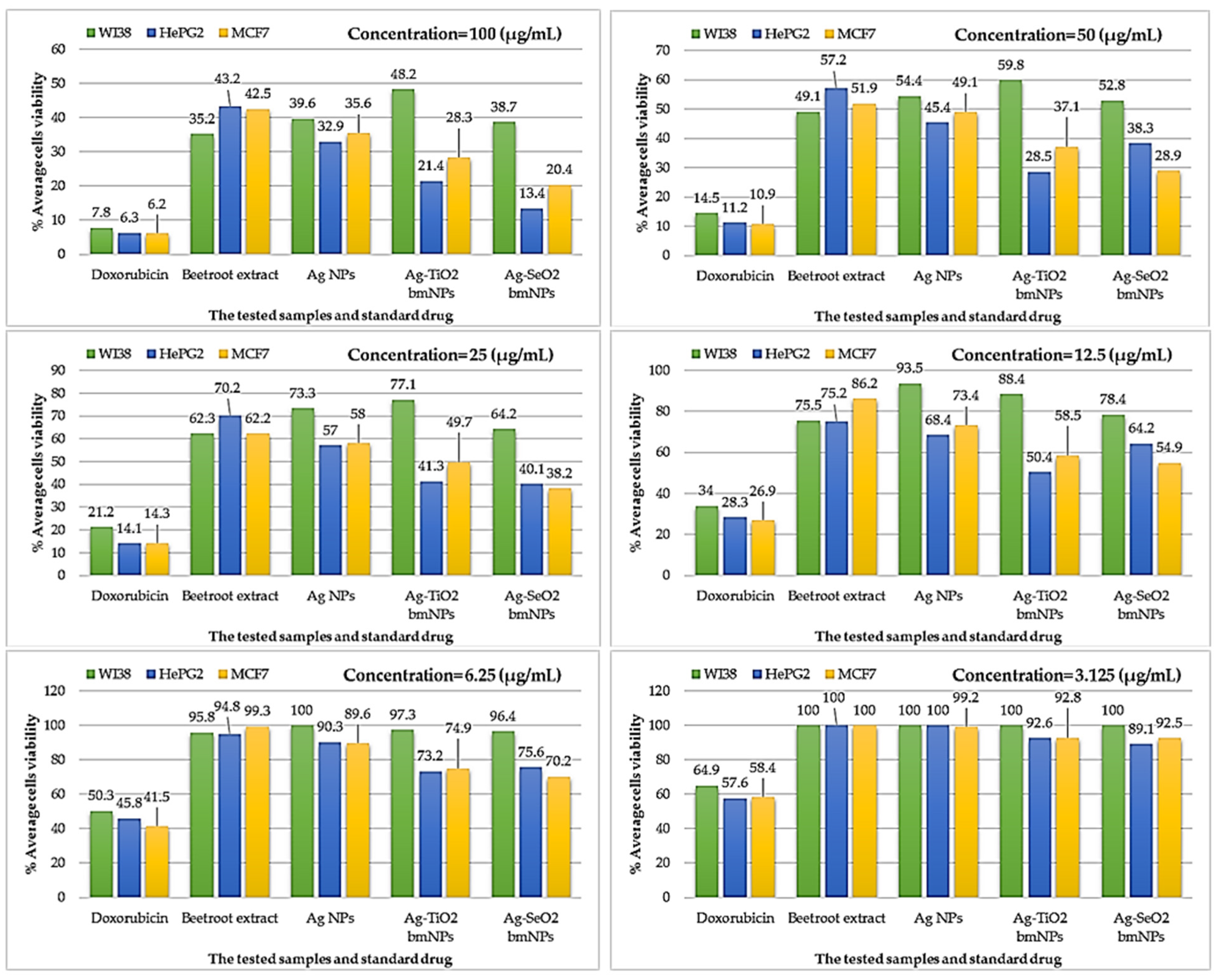
3.3.3. Cytotoxic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, V.K.; Sayes, C.M.; Guo, B.; Pillai, S.; Parsons, J.G.; Wang, C.; Yan, B.; Ma, X. Interactions between silver nanoparticles and other metal nanoparticles under environmentally relevant conditions: A review. Sci. Total Environ. 2019, 653, 1042–1051. [Google Scholar] [CrossRef]
- Bäuerlein, P.S.; Emke, E.; Tromp, P.; Hofman, J.A.; Carboni, A.; Schooneman, F.; de Voogt, P.; van Wezel, A.P. Is there evidence for man-made nanoparticles in the Dutch environment? Sci. Total Environ. 2017, 576, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Ahn, E.C.; Wong, H.-S.P.; Pop, E. Carbon nanomaterials for non-volatile memories. Nat. Rev. Mater. 2018, 3, 18009. [Google Scholar] [CrossRef]
- Yu, X.; Cheng, H.; Zhang, M.; Zhao, Y.; Qu, L.; Shi, G. Graphene-based smart materials. Nat. Rev. Mater. 2017, 2, 17046. [Google Scholar] [CrossRef]
- Tong, T.; Wilke, C.M.; Wu, J.; Binh, C.T.T.; Kelly, J.J.; Gaillard, J.-F.o.; Gray, K.A. Combined toxicity of nano-ZnO and nano-TiO2: From single-to multinanomaterial systems. Environ. Sci. Technol. 2015, 49, 8113–8123. [Google Scholar] [CrossRef]
- Wimmer, A.; Kalinnik, A.; Schuster, M. New insights into the formation of silver-based nanoparticles under natural and semi-natural conditions. Water Res. 2018, 141, 227–234. [Google Scholar] [CrossRef]
- Manfra, L.; Rotini, A.; Bergami, E.; Grassi, G.; Faleri, C.; Corsi, I. Comparative ecotoxicity of polystyrene nanoparticles in natural seawater and reconstituted seawater using the rotifer Brachionus plicatilis. Ecotoxicol. Environ. Saf. 2017, 145, 557–563. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, B.; Hower, J.; Schindler, M.; Winkler, C.; Brandt, J.; Di Giulio, R.; Ge, J.; Liu, M.; Fu, Y. Discovery and ramifications of incidental Magnéli phase generation and release from industrial coal-burning. Nat. Commun. 2017, 8, 194. [Google Scholar] [CrossRef]
- Goodwin Jr, D.G.; Adeleye, A.S.; Sung, L.; Ho, K.T.; Burgess, R.M.; Petersen, E.J. Detection and quantification of graphene-family nanomaterials in the environment. Environ. Sci. Technol. 2018, 52, 4491–4513. [Google Scholar] [CrossRef]
- Schindler, M.; Hochella, M.F. Sequestration of Pb–Zn–Sb-and As-bearing incidental nanoparticles by mineral surface coatings and mineralized organic matter in soils. Environ. Sci. Process. Impacts 2017, 19, 1016–1027. [Google Scholar] [CrossRef]
- Hochella, M.F.; Spencer, M.G.; Jones, K.L. Nanotechnology: Nature’s gift or scientists’ brainchild? Environ. Sci. Nano 2015, 2, 114–119. [Google Scholar] [CrossRef]
- Wilke, C.M.; Wunderlich, B.; Gaillard, J.-F.; Gray, K.A. Synergistic bacterial stress results from exposure to nano-Ag and nano-TiO2 mixtures under light in environmental media. Environ. Sci. Technol. 2018, 52, 3185–3194. [Google Scholar] [CrossRef]
- Kesharwani, P.; Gorain, B.; Low, S.Y.; Tan, S.A.; Ling, E.C.S.; Lim, Y.K.; Chin, C.M.; Lee, P.Y.; Lee, C.M.; Ooi, C.H. Nanotechnology based approaches for anti-diabetic drugs delivery. Diabetes Res. Clin. Pract. 2018, 136, 52–77. [Google Scholar] [CrossRef]
- Saravanan, M.; Barik, S.K.; MubarakAli, D.; Prakash, P.; Pugazhendhi, A. Synthesis of silver nanoparticles from Bacillus brevis (NCIM 2533) and their antibacterial activity against pathogenic bacteria. Microb. Pathog. 2018, 116, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Oves, M.; Aslam, M.; Rauf, M.A.; Qayyum, S.; Qari, H.A.; Khan, M.S.; Alam, M.Z.; Tabrez, S.; Pugazhendhi, A.; Ismail, I.M. Antimicrobial and anticancer activities of silver nanoparticles synthesized from the root hair extract of Phoenix dactylifera. Mater. Sci. Eng. C 2018, 89, 429–443. [Google Scholar] [CrossRef] [PubMed]
- Samuel, M.S.; Jose, S.; Selvarajan, E.; Mathimani, T.; Pugazhendhi, A. Biosynthesized silver nanoparticles using Bacillus amyloliquefaciens; Application for cytotoxicity effect on A549 cell line and photocatalytic degradation of p-nitrophenol. J. Photochem. Photobiol. B Biol. 2020, 202, 111642. [Google Scholar] [CrossRef]
- Mehdizadeh, S.; Ghasemi, N.; Ramezani, M. The synthesis of silver nanoparticles using Beetroot extract and its antibacterial and catalytic activity. Eurasian Chem. Commun. 2019, 1, 545–558. [Google Scholar]
- Tajik, S.; Beitollahi, H.; Dourandish, Z.; Mohammadzadeh Jahani, P.; Sheikhshoaie, I.; Askari, M.B.; Salarizadeh, P.; Nejad, F.G.; Kim, D.; Kim, S.Y. Applications of non-precious transition metal oxide nanoparticles in electrochemistry. Electroanalysis 2022, 34, 1065–1091. [Google Scholar] [CrossRef]
- Babu, M.G.; Gunasekaran, P. Production and structural characterization of crystalline silver nanoparticles from Bacillus cereus isolate. Colloids Surf. B Biointerfaces 2009, 74, 191–195. [Google Scholar] [CrossRef]
- Powar, N.; Patel, V.; Pagare, P.; Pandav, R. Cu nanoparticle: Synthesis, characterization and application. Chem. Methodol 2019, 3, 457–480. [Google Scholar]
- Song, J.Y.; Kim, B.S. Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess Biosyst. Eng. 2009, 32, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Gardea-Torresdey, J.L.; Gomez, E.; Peralta-Videa, J.R.; Parsons, J.G.; Troiani, H.; Jose-Yacaman, M. Alfalfa sprouts: A natural source for the synthesis of silver nanoparticles. Langmuir 2003, 19, 1357–1361. [Google Scholar] [CrossRef]
- Ahmad, N.; Sharma, S. Green synthesis of silver nanoparticles using extracts of Ananas comosus. Green Sustain. Chem. 2012, 2, 141–147. [Google Scholar] [CrossRef]
- Ajitha, B.; Reddy, Y.A.K.; Jeon, H.-J.; Ahn, C.W. Synthesis of silver nanoparticles in an eco-friendly way using Phyllanthus amarus leaf extract: Antimicrobial and catalytic activity. Adv. Powder Technol. 2018, 29, 86–93. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Chung, I.-M.; Samynathan, R.; Chandar, S.H.; Venkidasamy, B.; Sarkar, T.; Rebezov, M.; Gorelik, O.; Shariati, M.A.; Simal-Gandara, J. A comprehensive review of beetroot (Beta vulgaris L.) bioactive components in the food and pharmaceutical industries. Crit. Rev. Food Sci. Nutr. 2022, 1–33. [Google Scholar] [CrossRef]
- Hadipour, E.; Taleghani, A.; Tayarani-Najaran, N.; Tayarani-Najaran, Z. Biological effects of red beetroot and betalains: A review. Phytother. Res. 2020, 34, 1847–1867. [Google Scholar] [CrossRef]
- Singh, C.; Mehata, A.K.; Priya, V.; Malik, A.K.; Setia, A.; Suseela, M.N.L.; Vikas; Gokul, P.; Samridhi; Singh, S.K. Bimetallic Au–Ag nanoparticles: Advanced nanotechnology for tackling antimicrobial resistance. Molecules 2022, 27, 7059. [Google Scholar] [CrossRef]
- Elemike, E.E.; Onwudiwe, D.C.; Nundkumar, N.; Singh, M.; Iyekowa, O. Green synthesis of Ag, Au and Ag-Au bimetallic nanoparticles using Stigmaphyllon ovatum leaf extract and their in vitro anticancer potential. Mater. Lett. 2019, 243, 148–152. [Google Scholar] [CrossRef]
- Saeed, A.; Akhtar, M.; Zulfiqar, S.; Hanif, F.; Alsafari, I.A.; Agboola, P.O.; Haider, S.; Warsi, M.F.; Shakir, I. Thiamine-functionalized silver–copper bimetallic nanoparticles-based electrochemical sensor for sensitive detection of anti-inflammatory drug 4-aminoantipyrine. Chem. Pap. 2022, 76, 2721–2731. [Google Scholar] [CrossRef]
- Imraish, A.; Abu Thiab, T.; Al-Awaida, W.; Al-Ameer, H.J.; Bustanji, Y.; Hammad, H.; Alsharif, M.; Al-Hunaiti, A. In vitro anti-inflammatory and antioxidant activities of ZnFe2O4 and CrFe2O4 nanoparticles synthesized using Boswellia carteri resin. J. Food Biochem. 2021, 45, e13730. [Google Scholar] [CrossRef]
- Hewitt, R.E.; Chappell, H.F.; Powell, J.J. Small and dangerous? Potential toxicity mechanisms of common exposure particles and nanoparticles. Curr. Opin. Toxicol. 2020, 19, 93–98. [Google Scholar] [CrossRef]
- Angelé-Martínez, C.; Nguyen, K.V.T.; Ameer, F.S.; Anker, J.N.; Brumaghim, J.L. Reactive oxygen species generation by copper (II) oxide nanoparticles determined by DNA damage assays and EPR spectroscopy. Nanotoxicology 2017, 11, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Khalil, A.T.; Ali, M.; Iqbal, J.; Ali, W.; Alarifi, S.; Shinwari, Z.K. Green-synthesized silver nanoparticles induced apoptotic cell death in MCF-7 breast cancer cells by generating reactive oxygen species and activating caspase 3 and 9 enzyme activities. Oxidative Med. Cell. Longev. 2020, 2020, 1215395. [Google Scholar] [CrossRef] [PubMed]
- Abdelhafez, O.H.; Ali, T.F.S.; Fahim, J.R.; Desoukey, S.Y.; Ahmed, S.; Behery, F.A.; Kamel, M.S.; Gulder, T.A.; Abdelmohsen, U.R. Anti-inflammatory potential of green synthesized silver nanoparticles of the soft coral Nephthea sp. supported by metabolomics analysis and docking studies. Int. J. Nanomed. 2020, 15, 5345–5360. [Google Scholar] [CrossRef]
- Helmy, E.T.; Ayyad, M.A.; Ali, M.A.; Mohamedbakr, H.; Pan, J.H. Biochemical, Histological Changes, Protein Electrophoretic Pattern, and Field Application of CuPb–Ferrite/TiO2 Nanocomposites for Controlling Terrestrial Gastropod Eobania vermiculata (Müller). J. Agric. Food Chem. 2023, 71, 6626–6634. [Google Scholar] [CrossRef] [PubMed]
- Kamli, M.R.; Srivastava, V.; Hajrah, N.H.; Sabir, J.S.; Ali, A.; Malik, M.A.; Ahmad, A. Phytogenic fabrication of Ag–Fe Bimetallic nanoparticles for cell cycle arrest and apoptosis signaling pathways in Candida auris by generating oxidative stress. Antioxidants 2021, 10, 182. [Google Scholar] [CrossRef]
- Soni, M.; Mehta, P.; Soni, A.; Goswami, G.K. Green nanoparticles: Synthesis and applications. IOSR J. Biotechnol. Biochem. 2018, 4, 78–83. [Google Scholar]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The Folin–Ciocalteu assay revisited: Improvement of its specificity for total phenolic content determination. Anal. Methods 2013, 5, 5990–5999. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. Research on antioxidant activity of flavonoids from natural materials. Food Chem 1999, 64, e9. [Google Scholar]
- Aberoumand, A. Nutritional evaluation of edible Portulaca oleracia as plant food. Food Anal. Methods 2009, 2, 204–207. [Google Scholar] [CrossRef]
- Kitts, D.D.; Wijewickreme, A.N.; Hu, C. Antioxidant properties of a North American ginseng extract. Mol. Cell. Biochem. 2000, 203, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Parejo, I.; Codina, C.; Petrakis, C.; Kefalas, P. Evaluation of scavenging activity assessed by Co (II)/EDTA-induced luminol chemiluminescence and DPPH·(2,2-diphenyl-1-picrylhydrazyl) free radical assay. J. Pharmacol. Toxicol. Methods 2000, 44, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Belyagoubi-Benhammou, N.; Belyagoubi, L.; Gismondi, A.; Di Marco, G.; Canini, A.; Atik Bekkara, F. GC/MS analysis, and antioxidant and antimicrobial activities of alkaloids extracted by polar and apolar solvents from the stems of Anabasis articulata. Med. Chem. Res. 2019, 28, 754–767. [Google Scholar] [CrossRef]
- Zerrad, A.; Anissi, J.; Ghanam, J.; Sendide, K.; El Hassouni, M. Antioxidant and antimicrobial activities of melanin produced by a Pseudomonas balearica strain. J. Biotechnol. Lett. 2014, 5, 87–94. [Google Scholar]
- Denizot, F.; Lang, R. Rapid colorimetric assay for cell growth and survival: Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Crane, R.A.; Scott, T.B. Nanoscale zero-valent iron: Future prospects for an emerging water treatment technology. J. Hazard. Mater. 2012, 211, 112–125. [Google Scholar] [CrossRef]
- Argentiere, S.; Cella, C.; Cesaria, M.; Milani, P.; Lenardi, C. Silver nanoparticles in complex biological media: Assessment of colloidal stability and protein corona formation. J. Nanopart. Res. 2016, 18, 253. [Google Scholar] [CrossRef]
- Lankoff, A.; Sandberg, W.J.; Wegierek-Ciuk, A.; Lisowska, H.; Refsnes, M.; Sartowska, B.; Schwarze, P.E.; Meczynska-Wielgosz, S.; Wojewodzka, M.; Kruszewski, M. The effect of agglomeration state of silver and titanium dioxide nanoparticles on cellular response of HepG2, A549 and THP-1 cells. Toxicol. Lett. 2012, 208, 197–213. [Google Scholar] [CrossRef]
- Bae, E.; Lee, B.-C.; Kim, Y.; Choi, K.; Yi, J. Effect of agglomeration of silver nanoparticle on nanotoxicity depression. Korean J. Chem. Eng. 2013, 30, 364–368. [Google Scholar] [CrossRef]
- Hebbalalu, D.; Lalley, J.; Nadagouda, M.N.; Varma, R.S. Greener techniques for the synthesis of silver nanoparticles using plant extracts, enzymes, bacteria, biodegradable polymers, and microwaves. ACS Sustain. Chem. Eng. 2013, 1, 703–712. [Google Scholar] [CrossRef]
- Hendry, G.A.F.; Houghton, J. Natural Food Colorants; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1996; pp. 40–79. [Google Scholar]
- Bokern, M.; Heuer, S.; Wray, V.; Witte, L.; Macek, T.; Vanek, T.; Strack, D. Ferulic acid conjugates and betacyanins from cell cultures of Beta vulgaris. Phytochemistry 1991, 30, 3261–3265. [Google Scholar] [CrossRef]
- Jackman, R.; Smith, J. Anthocyanins and betalains. In Natural Food Colorants; Springer: Berlin/Heidelberg, Germany, 1996; pp. 244–309. [Google Scholar]
- Kujala, T.S.; Loponen, J.M.; Klika, K.D.; Pihlaja, K. Phenolics and betacyanins in red beetroot (Beta v ulgaris) root: Distribution and effect of cold storage on the content of total phenolics and three individual compounds. J. Agric. Food Chem. 2000, 48, 5338–5342. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Al-Asfar, A.; Zaheer, Z.; Aazam, E.S. Eco-friendly green synthesis of Ag@ Fe bimetallic nanoparticles: Antioxidant, antimicrobial and photocatalytic degradation of bromothymol blue. J. Photochem. Photobiol. B Biol. 2018, 185, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Ghosh, R.; Mandal, P. Biogenic synthesis of silver nanoparticles using S1 genotype of Morus alba leaf extract: Characterization, antimicrobial and antioxidant potential assessment. SN Appl. Sci. 2019, 1, 498. [Google Scholar] [CrossRef]
- de Castro, D.T.; do Nascimento, C.; Alves, O.L.; de Souza Santos, E.; Agnelli, J.A.M.; Dos Reis, A.C. Analysis of the oral microbiome on the surface of modified dental polymers. Arch. Oral Biol. 2018, 93, 107–114. [Google Scholar] [CrossRef]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The antibacterial mechanism of silver nanoparticles and its application in dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef]
- Staats, K.; Pilz, M.; Sun, J.; Boiadjieva-Scherzer, T.; Kronberger, H.; Tobudic, S.; Windhager, R.; Holinka, J. Antimicrobial potential and osteoblastic cell growth on electrochemically modified titanium surfaces with nanotubes and selenium or silver incorporation. Sci. Rep. 2022, 12, 8298. [Google Scholar] [CrossRef]
- Filipović, N.; Ušjak, D.; Milenković, M.T.; Zheng, K.; Liverani, L.; Boccaccini, A.R.; Stevanović, M.M. Comparative study of the antimicrobial activity of selenium nanoparticles with different surface chemistry and structure. Front. Bioeng. Biotechnol. 2021, 8, 624621. [Google Scholar] [CrossRef]
- Boey, A.; Ho, H.K. All roads lead to the liver: Metal nanoparticles and their implications for liver health. Small 2020, 16, 2000153. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, T.R.; van den Hengel, S.K.; Raza, B.G.; Rutjes, S.A.; de Roda Husman, A.M.; Peijnenburg, W.J.; Roesink, H.E.D.; de Vos, W.M. Surface chemistry-dependent antiviral activity of silver nanoparticles. Nanotechnology 2021, 32, 365101. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Yadav, K.; Jagadevan, S. A comprehensive review on green synthesis of nature-inspired metal nanoparticles: Mechanism, application and toxicity. J. Clean. Prod. 2020, 272, 122880. [Google Scholar] [CrossRef]
- Feng, X.; Chen, A.; Zhang, Y.; Wang, J.; Shao, L.; Wei, L. Central nervous system toxicity of metallic nanoparticles. Int. J. Nanomed. 2015, 10, 4321–4340. [Google Scholar]
- Stankic, S.; Suman, S.; Haque, F.; Vidic, J. Pure and multi metal oxide nanoparticles: Synthesis, antibacterial and cytotoxic properties. J. Nanobiotechnol. 2016, 14, 73. [Google Scholar] [CrossRef] [PubMed]
- Selim, Y.; Azb, M.; Ragab, I.; Abd El-Azim, M. Green synthesis of zinc oxide nanoparticles using aqueous extract of Deverra tortuosa and their cytotoxic activities. Sci. Rep. 2020, 10, 3445. [Google Scholar] [CrossRef]
- Akter, M.; Sikder, M.T.; Rahman, M.M.; Ullah, A.A.; Hossain, K.F.B.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A systematic review on silver nanoparticles-induced cytotoxicity: Physicochemical properties and perspectives. J. Adv. Res. 2018, 9, 1–16. [Google Scholar] [CrossRef]
- Lewinski, N.; Colvin, V.; Drezek, R. Cytotoxicity of nanoparticles. Small 2008, 4, 26–49. [Google Scholar] [CrossRef]
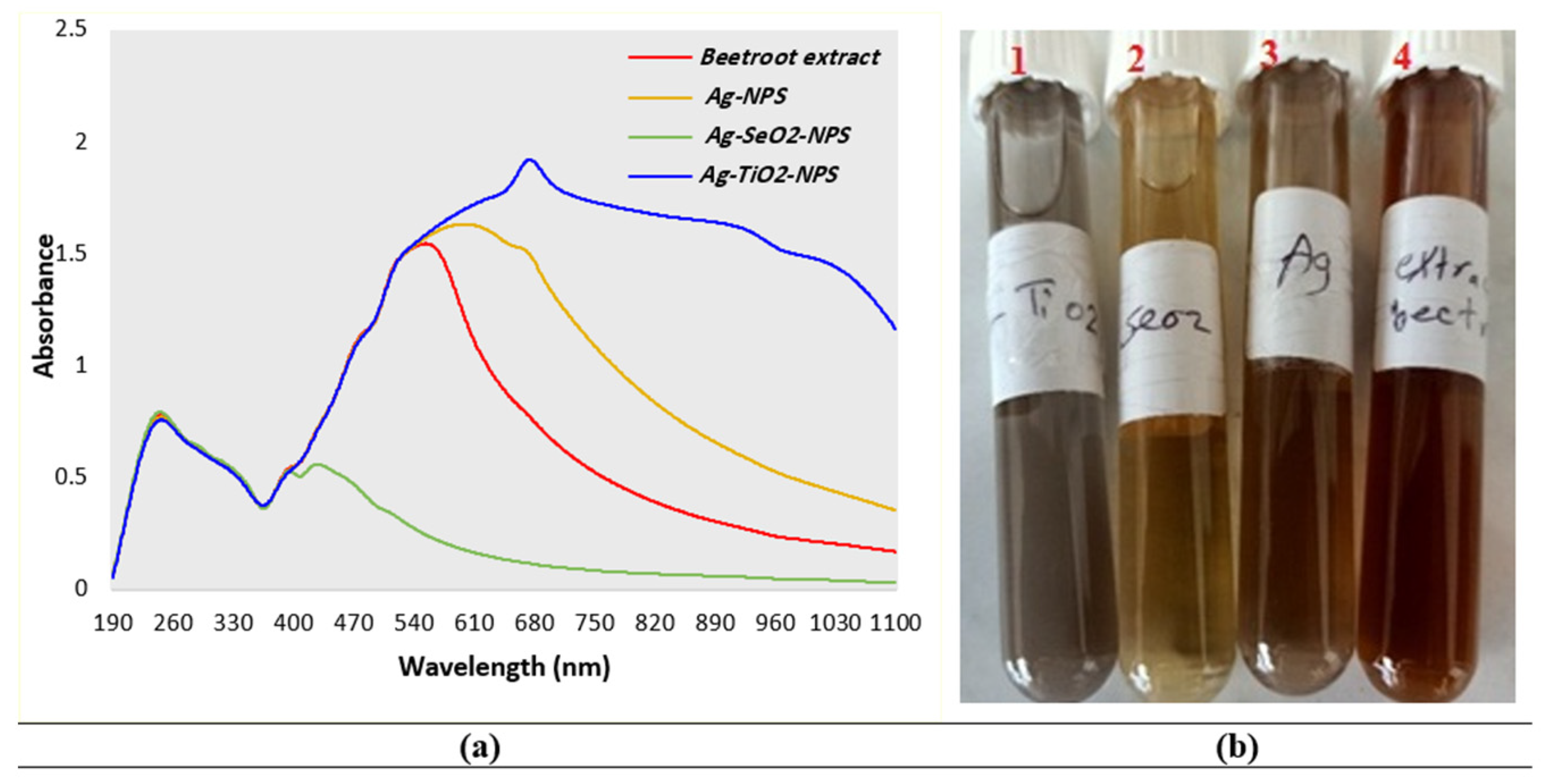
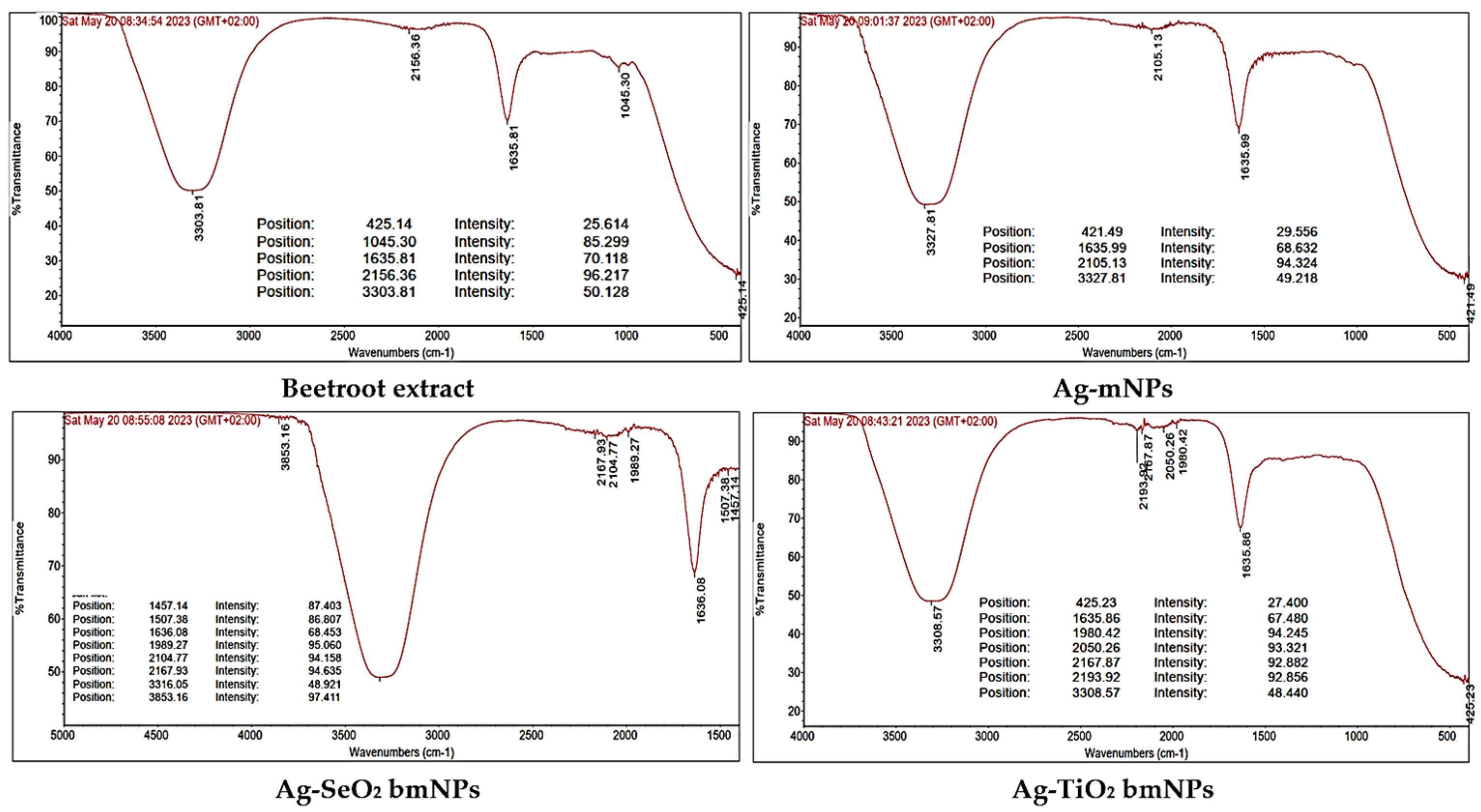
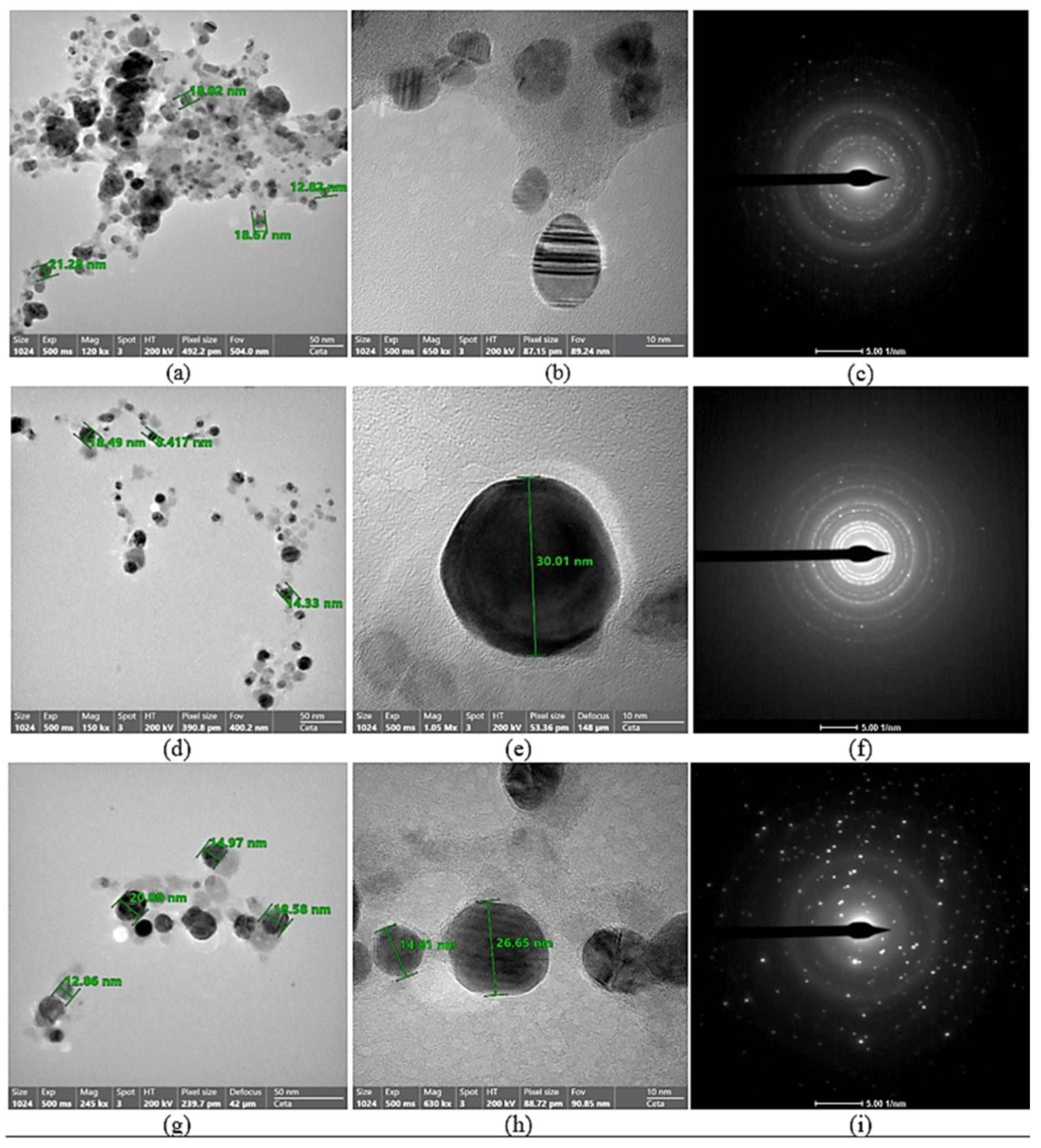


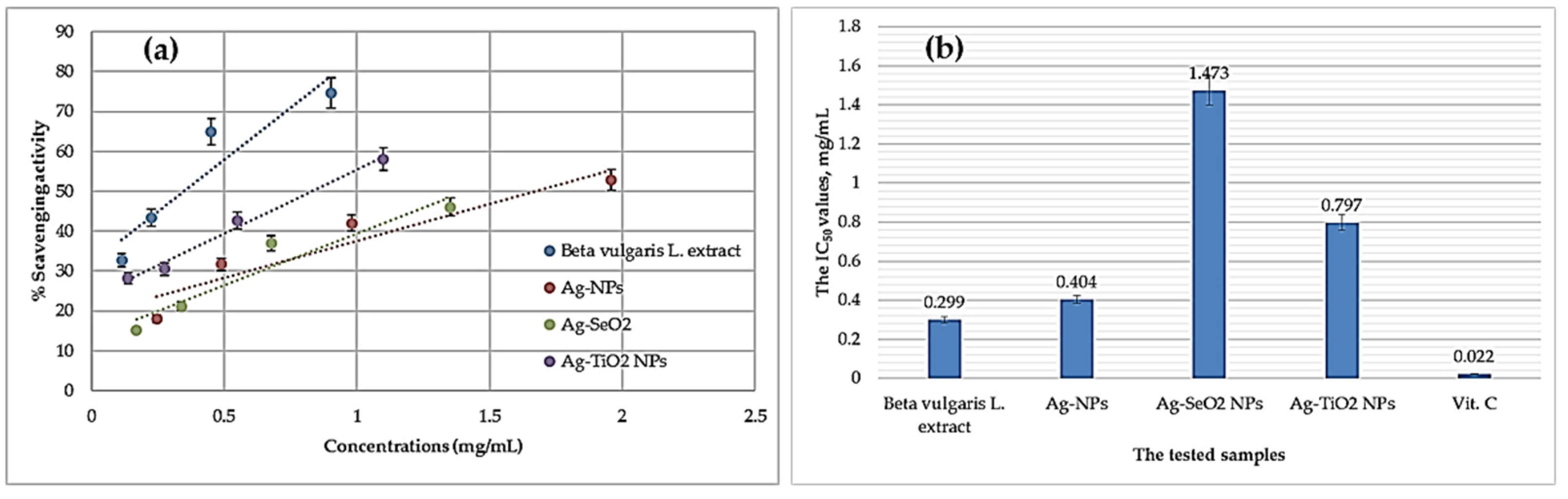

| Material | Total Antioxidant Capacity (mg AAE/100 gm DS) (a) |
|---|---|
| Plant extract | 2388.524 ± 7.226 |
| Ag-mNPs | 1326.292 ± 8.835 |
| Ag-SeO2 bmNPs | 497.994 ± 2.364 |
| Ag-TiO2 bmNPs | 1142.639 ± 5.901 |
| Bacterium | Nanoparticle | Hallow Zone Diameter (mm) |
|---|---|---|
| S. aureus | Ag-NPs | 16.7 ± 1.1 |
| Ag-SeO2-NPs | 37.7 ± 2.1 | |
| Ag-TiO2-NPs | 21.3 ± 0.6 | |
| E. faecalis | Ag-NPs | 14.0 ± 1.3 |
| Ag-SeO2-NPs | 34.7 ± 1.6 | |
| Ag-TiO2-NPs | 20.0 ± 2.6 | |
| E. coli | Ag-NPs | 16.7 ± 0.8 |
| Ag-SeO2-NPs | 11.7 ± 0.6 | |
| Ag-TiO2-NPs | 11.0 ± 1.0 | |
| S. enterica | Ag-NPs | 19.7 ± 2.1 |
| Ag-SeO2-NPs | 32.7 ± 1.2 | |
| Ag-TiO2-NPs | 25.0 ± 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elattar, K.M.; Ghoniem, A.A.; Al-Otibi, F.O.; El-Hersh, M.S.; Helmy, Y.A.; Saber, W.I.A. Phytogenic Synthesis and Characterization of Silver Metallic/Bimetallic Nanoparticles Using Beta vulgaris L. Extract and Assessments of Their Potential Biological Activities. Appl. Sci. 2023, 13, 10110. https://doi.org/10.3390/app131810110
Elattar KM, Ghoniem AA, Al-Otibi FO, El-Hersh MS, Helmy YA, Saber WIA. Phytogenic Synthesis and Characterization of Silver Metallic/Bimetallic Nanoparticles Using Beta vulgaris L. Extract and Assessments of Their Potential Biological Activities. Applied Sciences. 2023; 13(18):10110. https://doi.org/10.3390/app131810110
Chicago/Turabian StyleElattar, Khaled M., Abeer A. Ghoniem, Fatimah O. Al-Otibi, Mohammed S. El-Hersh, Yosra A. Helmy, and WesamEldin I. A. Saber. 2023. "Phytogenic Synthesis and Characterization of Silver Metallic/Bimetallic Nanoparticles Using Beta vulgaris L. Extract and Assessments of Their Potential Biological Activities" Applied Sciences 13, no. 18: 10110. https://doi.org/10.3390/app131810110
APA StyleElattar, K. M., Ghoniem, A. A., Al-Otibi, F. O., El-Hersh, M. S., Helmy, Y. A., & Saber, W. I. A. (2023). Phytogenic Synthesis and Characterization of Silver Metallic/Bimetallic Nanoparticles Using Beta vulgaris L. Extract and Assessments of Their Potential Biological Activities. Applied Sciences, 13(18), 10110. https://doi.org/10.3390/app131810110







