Abstract
As one of the important traditional medicinal plants listed in the Chinese pharmacopoeia, Vernonia anthelmintica (L.) Willd has been shown to possess various biological activities. In this study, we characterized culturable endophytic bacteria associated with the medicinal plant V. anthelmintica collected from Hotan within the Xinjiang autonomous region of China. Bacterial endophytes were identified via 16S rRNA gene sequence analysis and compared to similar sequences from the GenBank. Isolated strains exhibited 99.08–100% similarity to Bacillus haynesii XJB-5, Bacillus proteolyticus XJB-16, Bacillus halotolerans XJB-35, Bacillus safensis XJB-71, Pseudomonas punonensis XJB-7, Lysinibacillus fusiformis XJB-17, Streptococcus lutetiensis XJB-66, Leclercia adecarboxylata XJB-12, Paenibacillus alvei XJB-14, and Pantoea agglomerans XJB-62. The ethyl acetate extracts of the bacterial endophytes demonstrated various pharmacological properties, such as antimicrobial, cytotoxic, antidiabetic, and antioxidant activity, according to a melanin content assay and have shown tyrosinase activity in murine B16 cells. A crude extract of B. halotolerans XJB-35 displayed more powerful biological activities than other bacterial endophytes; therefore, this strain was studied further in order to select the optimized parameters for enhancing the synthesis of bioactive compounds. The optimal culture medium was found to be nutrient broth (NB) medium, using peptone as its carbon source and yeast extract as its nitrogen source. A 24 h incubation time produced the optimal conditions for the maximum growth of B. halotolerans XJB-35 and the production of bioactive compounds. Moreover, we investigated the volatile chemical component of the dichloromethane fraction using GC-MS analysis. Our findings provide valuable information regarding the synthesize of bioactive natural products by B. halotolerans XJB-35 for use by the medicinal and pharmaceutical industries.
1. Introduction
Bacterial endophytes can be found in all medicinal plant species investigated so far as well as in the intracellular compartment of the host [1,2]. Endophytic microorganisms live in relationships with their host plants to enhance plant growth and improve plant nutrition gain [3,4]. Moreover, they can confer abiotic and biotic stress tolerance and protect against attack from pathogens [5,6]. The production of a large amount of unique and structurally diverse pharmacologically active natural products by endophytes is the cause and triggers such action [7,8,9]. Bioactive natural products from plants, microorganisms and animals are important because they are used in medicine as vital therapeutic drugs. In the last decade, a large number of studies have included medicines and pharmaceutical agents because of the development, spread, and threat of various diseases affecting human health—for example, diabetes mellitus, cancer, neurodegenerative diseases, and various infectious diseases [10,11]. New structurally complex and diverse secondary metabolites with high anticancer, antimicrobial, antioxidant, and anti-inflammatory properties have been isolated from endophytic microorganisms, and they are significant tools for the discovery of novel drug leads in medicinal chemistry [12,13,14,15]. Endophytic microorganisms associated with medicinal plants may produce similar secondary metabolites and possess similar pharmacological activities [16,17]. The bioactive natural products synthesized by medicinal plants might effectively affect their association with endophytic microorganisms [18]. In our previous study [19,20], we investigated the biodiversity of endophytic bacteria and fungi associated with V. anthelmintica roots and flowers. However, we investigated the pharmacological properties of crude ethyl acetate extracts as well as individual secondary metabolites [19]. The indole derivatives, diketopiperazines, dihydrocinnamic, isocoumarins, lactone derivatives, and phenolic compounds have been obtained from the most active endophytic bacteria, Pantoea ananatis, and fungus, Aspergillus terreus XJA8 [21]. Their antimicrobial activity has been evaluated against S. aureus, E. coli, and C. albicans, and the effects of melanin synthesis and tyrosinase activity on B16 cells have also been studied [21]. In the last few years, endophytic bacteria have become a natural source with high potential for the synthesis of bioactive natural products for pharmaceutical and medicinal chemistry. Therefore, this study focused on (a) isolating and molecularly identifying bioactive endophytic bacteria associated with the V. anthelmintica stem; (b) determining the antimicrobial, cytotoxic, antidiabetic, antioxidant, and melanin synthesis and tyrosinase activity of the crude extracts; (c) optimizing the culture conditions for the most active bacterial endophyte, B. halotolerans XJB-35; and (d) identifying the volatile chemical component of compounds produced by B. halotolerans XJB-35 that display high pharmacological activities. To the best of our knowledge, this is the first study of the synergetic properties of the chemical composition of endophytic bacteria associated with the medicinal plant V. anthelmintica to evaluate their potential as pharmacological agents. Our findings can be interpreted as a natural tool for discovering new drugs for vitiligo, human pathogens, and cancer cures in pharmacology and medicinal chemistry.
2. Materials and Methods
2.1. Isolation and Purification V. anthelmintica Endophytes
Generally, the isolation of endophytes can be using the surface sterilization method previously described [21]. The stem sections of the healthy plant (10 g fresh weight) were separated from the plant body and washed in sterile water. They were then washed a second time in sterile water and surface-sterilized by placing the stem tissue in 90% ethanol for 2 min and 10% sodium hypochlorite for 2 min. After that, they were washed in sterile double-distilled water and dried for 5 min on a sterile paper towel. After sterilization, approximately 5–10 g of fresh stems was cut off into small parts and properly crushed in a sterile mortar and pestle [22]. The crushed tissue (1 g) was transferred into plastic tubes with 9 mL sterile phosphate buffered saline (PBS) and shaken for 1 min using a Vortex Biosan B-1. The plant tissue, diluted to 101–105, was spread on nutrient agar (NA) (BD, Difco Laboratories, Detroit, MI, USA) media and incubated for four days at 28 °C. After incubation, morphologically distinct colonies were selected and carefully held onto nutrient agar plates via streaking and then incubated for another four days before checking the purity of the isolates. After purification, pure isolates were transferred to NA media and used for further studies.
2.2. Molecular Identification of the Endophytic Isolates
2.2.1. DNA Isolation
Molecular identification of isolated endophytic bacteria was performed after DNA extraction using methods described in a previous study with some modifications [23]. The DNA was extracted from pure isolated bacterial strains, displaced into Eppendorf tubes (2 mL), and mixed with a Biosan B-1 Vortex and 1.5 mL of sterile MQ-water for 10 s. The tubes with bacterial suspension were incubated at 90 °C for 20 min in a dry block heater (IKA Works, Inc., Wilmington, NC, USA) and centrifuged at 8050× g force for 5 min. Isolated DNA was stored at –20 °C use for PCR in the future. The existence of DNA was investigated and calculated via horizontal gel electrophoresis (0.8% agarose) with a NanoDrop™ One spectrophotometer (Thermo Fisher Scientific Inc., Waltham, MA, USA).
2.2.2. 16S rRNA Gene Amplification
The genomic DNA was extracted using phenol, and the extraction was performed according to the previously used method [24]. PCR amplification of the 16S rRNA gene was sequenced with the following primers: 27F (5′-GAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-GAAAGGAGGTGATCCAGCC-3′) (Sigma-Aldrich, St. Louis, MO, USA) [25]. Each PCR reaction (final volume 25 μL) mix consisted of 1 μL (15–40 ng) DNA, 5 μL 5×OneTaq standard reaction buffer (BioLabs, New England), 1 μL 0.1% bovine serum albumin (TaKaRa Bio Inc., USA), 0.5 μL 10 mM dNTP mix (Thermo Scientific, Louis, MO, USA), 0.5 μL 10 mM primer 16SF (Merck, Louis, MO, USA), 0.5 μL 10 mM primer 16SR (Merck, Louis, MO, USA) (25 μmol/mL), 0.125 μL One Taq polymerase (BioLabs, New England), and 16.375 μL Milli-Q water. The PCRs were subjected to the following: a primary warming step for 30 s at 94 °C; 30 cycles of denaturation for 15 s at 94 °C, annealing for 30 s at 55 °C, and enlargement for 1.5 min at 68 °C; and a final step of 20 min at 68 °C. The PCR was analyzed using a PTC-200 thermocycler (Bio-Rad Laboratories, Louis, MO, USA).
2.2.3. Restriction Fragment Length Polymorphism (RFLP) Analysis
To ascertainment the difference between the isolated bacterial strains that were similar in their size, color, and shape, we conducted RFLP analysis of PCR amplicons of the 16S rRNA gene as described by Jinneman et al. (1996) [26]. The fragments of digested PCR-amplified products were investigated via agarose gel electrophoresis on a 0.8% containing GelRed. After the electrophoresis, the gel was visualized using a digital gel imaging system (Gel-Doc XR TM+, Bio-Rad Laboratories, USA) to identify identical isolates and reduce the number of strains to be sequenced. All 16S rDNA sequences of endophytic bacteria were compared to known species from GenBank databases by using BLASTN.
2.2.4. Sequencing and Phylogenetic Analysis
The PCR products were purified by using the USB® ExoSAP-IT® PCR Product clean-up Kit (Affymetrix, USB® Products, USA), and sequencing was screened using an ABI PRISM BigDye 3.1 Terminator Cycle Sequencing Ready Reaction Kit (Applied Biosystems, USA). All results were analyzed and edited using Chromas software v2.6.5. Corrected sequences were manually merged using EMBOSS Explorer (http://emboss.bioinformatics.nl/, accessed on 17 July 2023). Then, the sequences obtained in the current study were compared with GenBank database at the NCBI (http://www.ncbi.nlm.nih.gov/, accessed on 17 July 2023) using the BLAST progrem. The original sequences were edited aligned using Clustal Omega (https://www.ebi.ac.uk/Tools/msa/clustalo/, accessed on 17 July 2023), and a FASTA-format file was used to construct the phylogenetic tree. The phylogenetic creation was inferred using neighbor joining [27]. The neighbor-joining analyses were conducted in the MEGA 6 [28] software with bootstrap values determined from 500 replicate runs [29]. The evolutionary distances were measured using the maximum composite likelihood method [30] and were in the units of the number of base change-over per site. This analysis involved 40 nucleotide sequences. There were a total of 1604 positions in the final dataset.
2.3. Incubation and Extraction of Bacterial Culture
All ten selected bacterial endophytes were incubated in Erlenmeyer flasks (1 L) containing 500 mL of nutrient broth (NB) fermentative media (5 g/L peptone; 3 g/L yeast extract; 5 g/L NaCl; and distilled water, pH 7.4) for three days at 28 °C on a shaker at 160 rpm. During the incubation process, in the fermentation medium, bacterial endophytes synthesize biologically active compounds. After incubation, the bacterial culture was centrifuged at 4293× g force at 4 °C for 15 min. Thereafter, the cell-free supernatant was extracted with the organic solvent ethyl acetate using a separatory funnel and dried in a rotavator until a dry crude extract was obtained. The crude ethyl acetate extract was stored at 40 °C for further investigation of chemical composition and biological activity.
2.4. Biological Activities of Endophytic Bacteria
The crude ethyl acetate of endophytic bacteria was dissolved in DMSO, and pharmacological properties such as antimicrobial, cytotoxic, antidiabetic, antioxidant, melanin synthesis, and tyrosinase activity were measured.
2.4.1. Antimicrobial Activity
An in vitro antimicrobial assay of the crude extracts of endophytic bacteria was screened against three pathogenic microorganisms. The gram-positive bacteria Staphylococcus aureus (ATCC6538), Gram-negative bacteria Escherichia coli (ATCC11229) and fungi Candida albicans (ATCC10231) were used in the screening test of antimicrobial activity using the paper disk method, as described in previous studies [21,24]. The inhibition zone was assumed by determining the diameter of the bacterial growth inhibition zone. Ampicillin and amphotericin B (Gibco-BRL, Grand Island, NY, USA) were used as positive controls.
2.4.2. Melanin Content Assay and Tyrosinase Activity
Cell culture: The B16 murine melanoma cell lines (B16F10) were collected from the Chinese Academy of Sciences, China (CAS). The cells were incubated in Dulbecco’s modified Eagle’s medium (DMEM, Gibco Life Technologies, Waltham, MA, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS). Penicillin G (100 U/mL) and streptomycin (100 mg/mL) (Gibco-BRL, Grand Island, NY, USA) were added at 37 °C in a humidified atmosphere of 5% CO2.
Melanin measurement: The effects of melanin synthesis of secondary metabolites from the total extracts of bacterial endophytes isolated from the V. anthelmintica stem were screened in accordance with the procedure described by Rustamova et al. [19,21]. B16 cells were seeded at a density of 2 × 105 cells per well in a 6-well plate and incubated for 24 h. Then, test samples were added to the individual wells. The cells were incubated for 48 h, then washed twice with ice-cold PBS. Consequently, they were lysed at low temperature, and harvested cells were centrifuged at 8050× g force for 20 min and were simultaneously investigated using proteinaceous supernatant; after that, the pellet was dissolved by adding 1 N sodium hydroxide and incubated at 80 °C for 1 h. Into a 96-well microplate was in placed 150 mL of each lysate and evaluated spectrophotometrically at 405 nm using a multi-plate reader. The protein content of each sample was calculated using a BCA Protein Assay Kit (Biomed, Beijing, China). The melanin amount was normalized to the cellular protein concentration. Melanin content was determined according to the following formula:
where S is the absorbance of the cells treated by the samples and B is the absorbance of the wells containing untreated cells. Each measurement was carried out in triplicate.
Tyrosinase activity assay: Analysis of the effects of bioactive natural products from the ethyl acetate extracts of endophytic bacteria associated with the V. anthelmintica stem on tyrosinase activity was performed according to the previously described method [19,21] with some modification. Firstly, the B16 murine melanoma cells were seeded in a 6-well plate at a concentration of 2 × 105 cells per well and inoculated for 24 h. Then, 50 µg/mL of each bacterial extract was added to individual wells and incubated overnight. After incubation, cells were washed with ice-cold PBS twice and lysed with 1% Triton X-100 solution containing a 1% sodium deoxycholate for 30 min at −80 °C, and each lysate was centrifuged at 12,000× g for 15 min. After determining and regulating the protein concentration of the supernatants, 90 µL of the supernatant and 10 µL of a freshly prepared substrate solution (10 µM 8-MOP) were transferred in a 96-well plate and incubated at 37 °C for 60 min. The optical densities of samples were measured at 490 nm, and 8-methoxypsoralen (MOP) was used as a positive control.
2.4.3. Cytotoxic Activity
To screen the anticancer activity of the secondary metabolites of crude ethyl acetate extracts of endophytic bacterial isolates, the MTT (3-(4,5-dimethylthiazolyl)-diphenyl tetrazolium bromide) assay method was performed as described by Rustamova et al. [19]. Firstly, we prepared the samples using 50 mg ethyl acetate extracts of bacteria dissolved in 1 mL of DMSO. In vitro anticancer screening used MCF-7 (breast cancer), HeLa (cervical cancer), and HT-29 (colon cancer) cells. MCF-7 and HeLa cells were purchased from the Shanghai Cell Bank (Shanghai, China), and HT-29 cells were obtained from Procell Life Science and Technology Co., Ltd. (Wuhan, China). The significant anticancer drug doxorubicin (DOX) was used as a positive control and purchased from BBI Inc. (Shanghai, China). Three human cancer MCF-7, HeLa, and HT-29 cell lines were particularly seeded in aliquots of 200 µL in 96-well plates at a density of 3–103 cells per well. After incubation at 37 °C, 95% moisture, and 5% CO2 for 24 h, cells were treated with 30 µM of the crude extracts of endophytes and kept for 48 h. After that, into each well was placed 20 µL of 5 mg/mL MTT and incubated at 37 °C for 4 h. Then, the supernatant was removed, and 200 µL of DMSO was added to each well. Finally, the multiwall plates were shaken for 10 min to completely dissolve the test samples. Absorbance was read at 540 nm using an enzyme-linked immunosorbent assay reader (SpectraMax M5, Molecular Devices, USA). The IC50 values were measured using the inhibition rate.
Here, C is the OD of the control group, S is the OD of the experimental group, and B is the OD of the blank.
2.4.4. Anti-Diabetic Activity (PTP-1B Inhibition Assay)
The evaluation of tyrosine phosphatase 1B (PTP1B) inhibition activity was measured using a previously described method with a slight modification [20].
2.4.5. Antioxidant Activity (DPPH Radical-Scavenging Activity)
The 2,2′-diphenyl-1-picrylhydrazyl (DPPH) radical-scavenging activity of the natural products of crude ethyl acetate extracts of endophytic bacteria was evaluated according to our previous study [20]. In each well of a 96-well microplate, a 100 μL sample (50 mg/mL concentration) and 100 μL of a freshly prepared 0.2 mM DPPH solution were added. After that, the microplate was left in the dark for 30 min at room temperature. The absorbance was recorded at 517 nm, and the IC50 values were determined. Ascorbic acid was used as a positive control. The inhibition rate (IR) of the positive control and samples was calculated using the following formula:
where A0 is the absorbance of the blank and A1 is the absorbance of the test sample.
2.5. Optimization of Culture Condition
We investigated the effect of different culture media, such as carbon and nitrogen sources, and incubation times on the growth and synthesis of bioactive natural products produced by the most active endophytic bacteria B. halotolerans XJB-35. In particular, of the ten selected bacterial endophytes, strain B. halotolerans XJB-35 highlighted the powerful biological activities. Therefore, that strain was grown in various media namely, nutrient broth (NB); N-free broth; De Man, Rogosa, and Sharpe broth (MRS); LB broth; and tryptic soy dextrose (TSD) broth (Table 1). The medium in which the bacterial strain B. halotolerans XJB-35 showed maximum growth, and the production of bioactive products was selected as the optimal medium for further use. The total extract of the endophytic bacteria that grew in different media was analyzed in terms of their chemical composition using GC-MS.

Table 1.
Chemical composition of culture medium.
2.6. The Effect of Incubation Time on the Synthese Natural Product by Most Active Endophytic Bacteria
The incubation time plays a significant role in endophytic micro-organisms’ growth and in secondary metabolites’ production [31,32]. In order to study the effect of different incubation times on the production of natural products by the selected bacterial strain, cultivation was performed for 24 h to 240 h. The extracts were obtained using ethyl acetate, and the yield of the natural product synthesized was determined.
2.7. Scanning Electron Microscopy (SEM) Analysis of Endophytic Bacteria
The scanning electron microscopy (SEM) of the most-active isolated bacterium B. halotolerans XJB-35 was treated according to the method described by Nongkhlaw et al. [33]. After incubation, the surface of the bacteria was directly coated with gold using an iron sputter coater (Hitachi E-1045, Japan), and the appearance of the surface of the bacteria including their shape and color the diameter of the colony, and the colony reversal was observed under a scanning electron microscope (Zeiss Supra 55 VP, Germany).
2.8. High-Performace Liquid Chromatography (HPLC) Analysis
Extracts of bacterial isolates were analyzed with analytical HPLC using a DIONEX UltiMate 3000 instrument (Thermo, Waltham, MA, USA) combined with a Sunfire C18 column (4.6 × 250 mm, 5 µm) (Waters, USA) and a Sunfire C18 guard column (4.6 × 20 mm, 5 µm) (Waters, Waltham, MA, USA). Isgradient solvent systems: A was 0.2% (v/v) formic acid (HCOOH) in water; mobile phase B was acetonitrile (ACN). The elution profile was: 0–10 min 100% B (isocratic), 1–86 min 10–100% B (acetonitrile). Analytical-grade solvents (Baishi Chemical Co., Ltd., Tianjin, China) were used for column chromatography and HPLC-grade solvents (Merck, Germany) were used for HPLC analysis. The flow rate was 1 mL/min, and the injection volume was 10 μL. UV chromatograms were recorded at 210, 254, and 330 nm.
2.9. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
B. halotolerans XJB-35, the most bioactive endophytic bacteria, was incubated in several liquid media, such as NB, N-free broth, MRS, LB, and TSD, with various contents. After incubation, the natural products synthesized by the endophytic bacteria in media of different compositions were extracted using the polar organic solvent ethyl acetate. Then, 10 mg of the total extracts of each sample were dissolved in dichloromethane, and the volatile chemical composition of B. halotolerans XJB-35 was subjected to gas chromatography–mass spectrometry (GC-MS) analysis, as previously reported [34]. The components identification of peaks was performed using on the basis of their retention time (RT) and by comparing the spectra to a stored MS library (W8N05ST and NIST08).
2.10. Statistical Analysis
All data analysis was performed with GraphPad Prism using three replicate values in one-way subcolumn analysis of variance (ANOVA) and using Tukey’s test to determine statistical significance. A p-value of <0.05 was considered to be statistically significant. The correlation index was calculated using the Pearson coefficient (ρ).
3. Results and Discussion
3.1. Isolation and Identification of Endophytic Bacteria
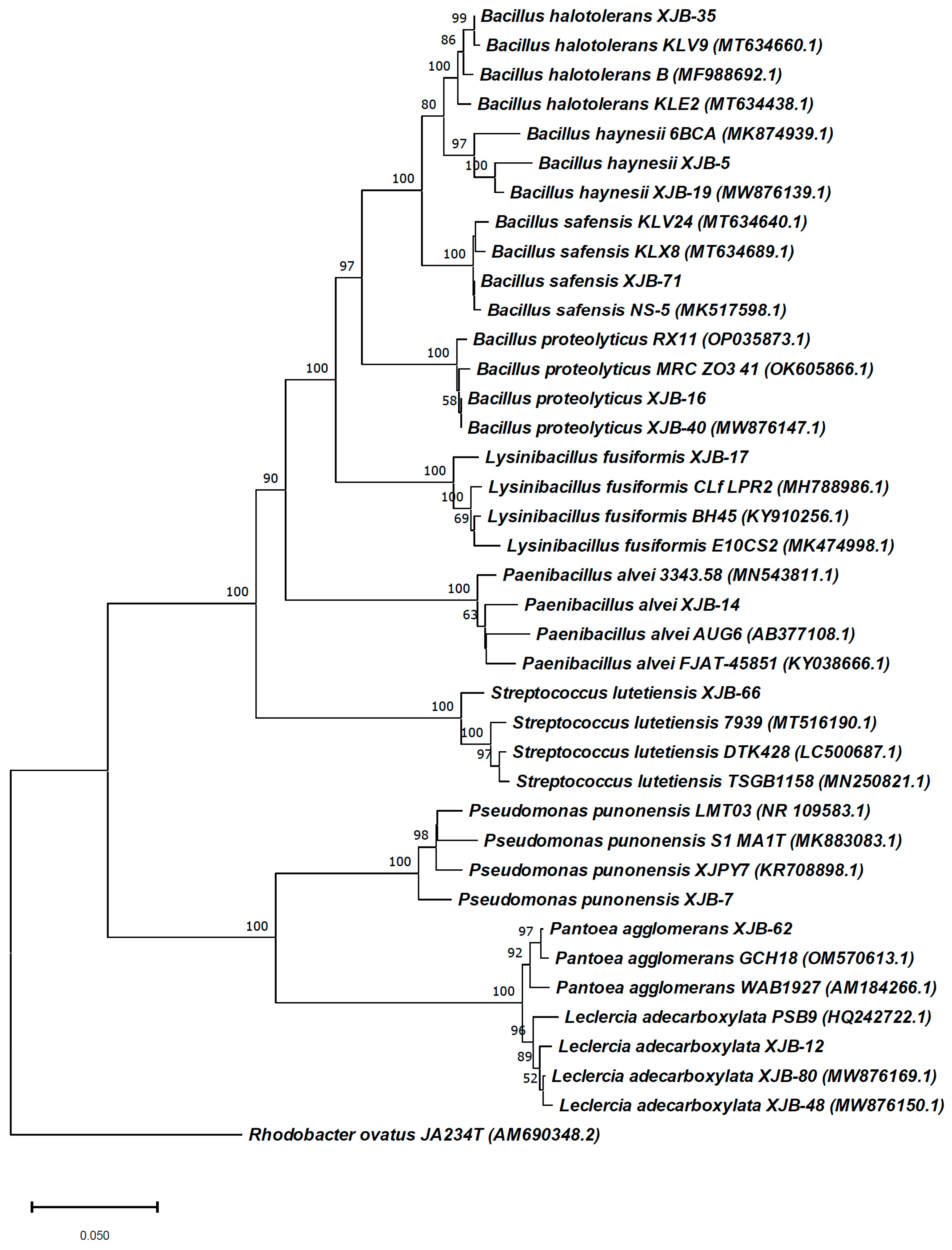
A total of 30 endophytic bacteria were isolated from the healthy plant tissues of V. anthelmintica. Among them, 10 isolates were identified and selected for future investigation (Figure 1). The primary screening of these crude extracts showed satisfactory antimicrobial activity. All selected bacterial strains were identified using the basic local alignment search tool (BLAST) and compared to similar strains from GenBank in NCBI, registered under accession numbers MW820297, MW876136, MW876143, MW876161, MW876130, MW876137, MW876158, MW876133, MW876135, and MW876157 (www.ncbi.nlm.nih.gov) (Figure 2 and Table 2). The most numerous were species of the order Bacillus genera (B. haynesii XJB-5, B. proteolyticus XJB-16, B. halotolerans XJB-35, B. safensis XJB-71,). The remaining strains belonged to the Pseudomonas (P. punonensis XJB-7), Lysinibacillus (L. fusiformis XJB-17), Streptococcus (S. lutetiensis XJB-66), Leclercia (L. adecarboxylata XJB-12), Paenibacillus (P. alvei XJB-14) and Pantoea (P. agglomerans XJB-62) genera. In our previous work [24], we isolated other species belonging to the Bacillus genera namely B. megaterium VERA2, B. endophyticus VERA6, and B. atrophaeus VERA9 from the root of V. anthelmintica. Moreover, others of strains P. chlororaphis VERA3, P. kilonensis VERA 4, and P. ananatis VERA8 were isolated and identified, and they belong to the Pseudomonas and Pantoea genera associated with the V. anthelmintica root.

Figure 1.
Isolated bacterial endophytes of V. anthelmintica stem.
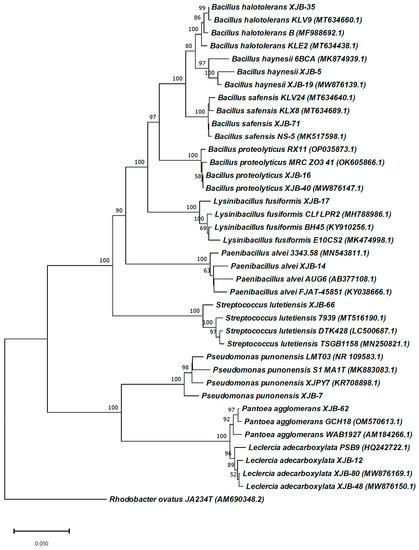
Figure 2.
The phylogenetic tree of bacterial endophytes isolated from V. anthelmintica and their closest relatives from the GenBank of NCBI.

Table 2.
Sequence similarities of bacterial endophytes isolated from the stem of V. anthelmintica with sequences registered in GenBank.
3.2. Antimicrobial Activity
Plant endophytic bacteria are excellent candidates for antibiotic agents against human pathogenic microorganisms. Previous reports have demonstrated that Bacillus has displayed antimicrobial activity against several pathogenic microorganisms [24]. For example, B. subtilis YRL02, B. licheniformis YRL03, and B. subtilis YRL07 inhibited moderate antifungal activity against the plant pathogens Phytophyhora capsii, Fusarium oxysporum, Rhizoctonia solani, and Phythium ultimun [35]. Christina et al. [36] reported that several endophytic bacteria associated with plants have shown significant antimicrobial activity. Other researchers found that terpenes compounds synthesized by endophytic microorganisms displayed powerful antimicrobial activity [37,38]. For example, novel sesquiterpene derivatives obtained from the endophytic fungus Bipolaris eleusines showed potent antibacterial activities against Alternaria solani, with MIC values of 8 and 16 g/mL, respectively [39]. Others diterpenoids were isolated from the fungal strain Drechmeria sp. and exhibited significant inhibition of C. albicans, with an MIC value of 12.5 µg/mL [40]. In our previous studies, we have reported the antibacterial and antifungal activity of total ethyl acetate extracts as well as pure secondary metabolites of the endophytic bacteria and fungus associated with V. anthelmintica roots [19,21]. In the present study, 10 bacterial strains were screened for in vitro antimicrobial activity against three human pathogenic bacteria (E. coli and S. aureus) and one fungus (C. albicans). The results showed that the majority of tested strains exhibited activity against all pathogens. The crude extract of B. halotolerans XJB-35 was assayed and demonstrated strong to moderate antibacterial and antifungal activity against E. coli, S. aureus, and C. albicans with an inhibition zone of 17.5, 18, and 10.5 mm, respectively. Three species of Bacillus, B. haynesii XJB-5, B. proteolyticus XJB-16, and B. safensis XJB-71 also demonstrated activity against all pathogens. S. lutetiensis XJB-66 showed satisfactory antifungal and higher antibacterial activity against C. albicans, E. coli, and S. aureus with inhibition zones of 9.5, 15 and 17 mm, respectively (Table 3). The crude extract of L. adecarboxylata XJB-12 significantly inhibited S. aureus, with an inhibition zone of 18 mm. Our investigation suggests that antibiotics synthesized by bacterial endophytes, particularly Bacillus strains, are active against the screened pathogens. Bacillus are a potential source due to their structurally diverse range of bioactive products, including novel antibiotics. The advantage of using bacterial endophytes as antimicrobial treatments is that they can often be cultured and produced after isolation from their host plant. Moreover, endophytic bacteria are rich natural sources of antimicrobial active natural compounds.

Table 3.
In vitro antimicrobial activity (zone of inhibition) of crude extracts of endophytic bacteria from the medicinal plant V. anthelmintica.
3.3. Melanin Content Assay and Tyrosinase Activity
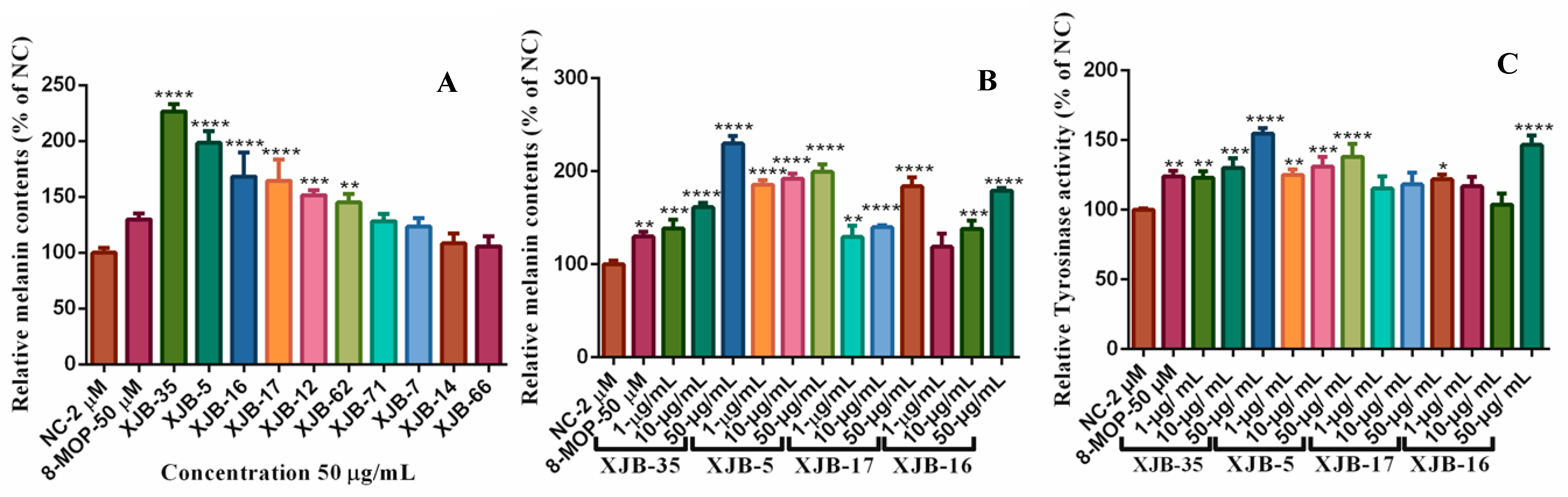
The biosynthesis of melanin plays an important role in the pigmentation of the skin [41]. Vitiligo is a disease caused by depigmentation of cells in the epidermis and decreased melanin biosynthesis in skin epidermal cells, a complex process regulated by tyrosinase enzymes [42]. Many natural products isolated from the medicinal plant V. anthelmintica have been used for the treatment of pigmentation disorders [43,44]. Previous studies have reported that crude extracts and pure natural compounds obtained from endophytic microorganisms of V. anthelmintica are involved in the regulation of melanin synthesis and tyrosinase activity [19,45]. In the present study, we investigated the influence of secondary metabolites produced by endophytic bacteria in the stem of V. anthelmintica. Our results showed that crude extracts of only six endophytic bacteria, B. halotolerans XJB-35, B. haynesii XJB-5, B. proteolyticus XJB-16, L. fusiformis XJB-17, L. adecarboxylata XJB-12, and P. agglomerans XJB-62, demonstrated increased melanin synthesis (226.1 ± 16.57%, 197.5 ± 18.65%, 168.3 ± 17.5%, 164.5 ± 15.68%, 152.7 ± 7.989%, and 145.6 ± 7.373%, respectively) in murine B16 cell, at a concentration of 50 µM, compared with the positive control 8-MOP (129.9 ± 4.179%). According to the testing data (Figure 3A and Table 4), only four bacterial endophytes exhibited stronger increased melanin synthesis and tyrosinase activity at different concentrations compared to the positive control (8-MOP 50 µM, 129.9 ± 4.179%). Therefore, we treated them again with various concentrations. The crude extract of B. halotolerans XJB-35 exhibited powerful melanin synthesis inhibition with 1µg/mL, 138.6 ± 7.638%; 10 µg/mL, 161.5 ± 3.751%; and 50 µg/mL, 229.9 ± 6.737% concentrations, respectively. The endophytic bacteria B. haynesii XJB-5 (1 µg/mL, 185.6 ± 12.51%; 10 µg/mL, 191.5 ± 76.61%; and 50 µg/mL, 198.2 ± 18.65%) (Figure 4), L. fusiformis XJB-17 (1 µg/mL, 129.3 ± 10.02%; 10 µg/mL, 139.8 ± 1.822%; 50 µg/mL, 183.8 ± 7.762%), and B. proteolyticus XJB-16 (1 µg/mL, 118.8 ± 11.50%; 10 µg/mL, 138.1 ± 7.450%; 50 µg/mL, 179.0 ± 2.288%) increased melanin synthesis by up to 1.5 fold compared with 8-MOP (50 µM, 132.0 ± 2.818%) (Figure 3B and Table 5) depending on the concentration. Four selected bacterial strains also increased tyrosinase activity as well as melanin synthesis in a dose-dependent manner (Figure 3C). All results were compared with the positive control 8-MOP (50 µM, 124.1 ± 3.172%).
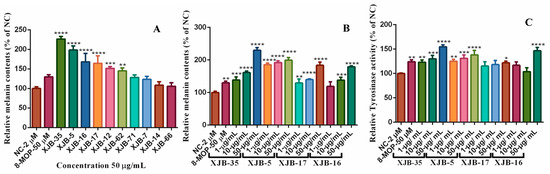
Figure 3.
(A) Effect of the crude ethyl acetate extracts of endophytic bacteria on melanin content and tyrosinase activity in B16 melanoma cells. (B,C) melanin synthesis and tyrosinase activity of cells treated with different concentrations of crude extracts. Note: * Compared to the blank control group (NC), p < 0.05; ** compared to the blank control group (NC), p < 0.01; *** compared to the blank control group (NC), p < 0.001; **** compared to the blank control group (NC), p < 0.0001.

Table 4.
Effects of melanin synthesis of secondary metabolites of endophytic bacteria in B16 cells (n = 3).
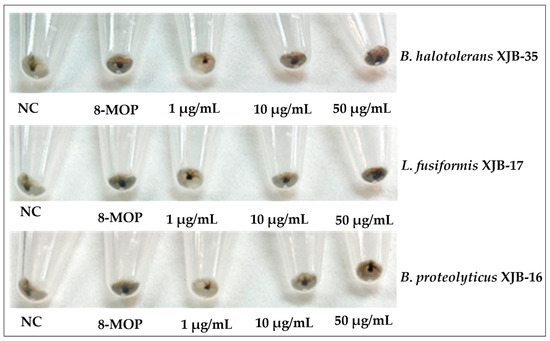
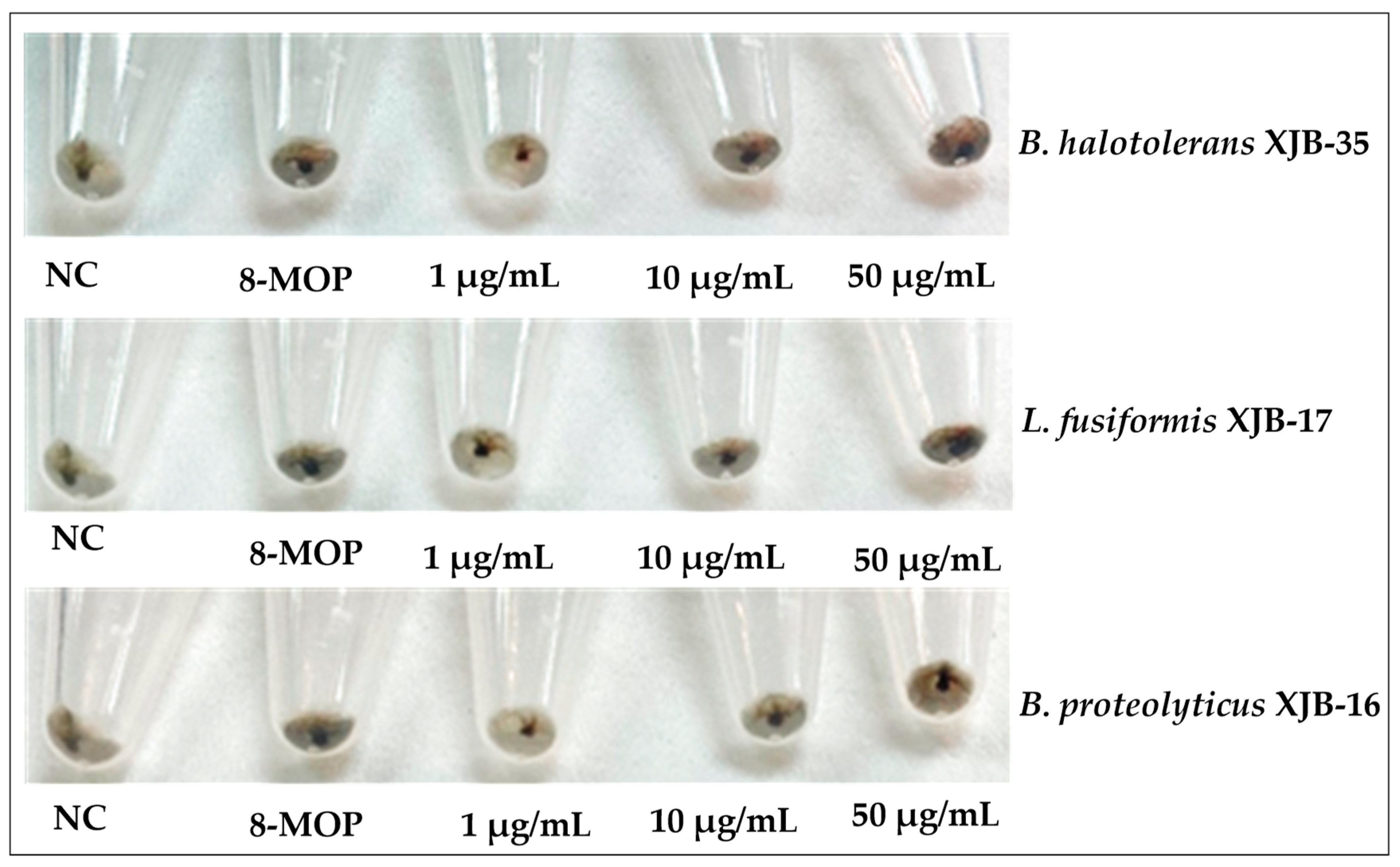
Figure 4.
Melanin content of B16 cells treated with different concentrations (1, 10, and 50 µM) of secondary metabolites for 48 h. DMSO (50 µM) was used as the vehicle control and 8-MOP (50 µM) as the positive control.

Table 5.
Effects of melanin synthesis and tyrosinase activity of secondary metabolites in B16 cells treated with various concentrations (n = 3).
3.4. Cytotoxic Activity
Over the last decade, natural compounds with anticancer properties obtained from endophytic bacteria and fungi have been widely used in pharmacology and medicinal chemistry [10,12]. The cytotoxic natural products obtained from the endophytes of Aspergillus versicolor demonstrated significant cytotoxic activity against the mouse lymphoma L5178Y cell line [46]. Jian Xiao found that new anticancer metabolites from Botryosphaeria dothidea KJ-1 exhibited powerful cytotoxicity activity against HCT 116 cancer cells [47]. Other authors have reported the anticancer activity of four novel natural compounds produced by Aspergillus terreus [48]. In the present study, we focused on the anticancer activity of secondary metabolites produced by bacterial endophytes associated with V. anthelmintica. The results revealed that the crude extract of B. halotolerans XJB-35 demonstrated in vitro cytotoxic activity against the human cancer cell lines HT-29, MCF-7 and HeLa, exhibiting IC50 values of 15.07 μg/mL ± 0.34, 19.05 μg/mL ± 0.90, and 11.39 μg/mL ± 0.23, respectively. However, B. safensis XJB-71 failed to demonstrate cytotoxic activity against the human HT-29 and MCF-7 cancer cell lines; IC50 values were 23.45 μg/mL ± 0.15 and 28.72 μg/mL ± 0.34, respectively. Crude extracts of S. lutetiensis XJB-66 and P. alvei XJB-14 also displayed satisfactory activity against HT-29, MCF-7, and HeLa with IC50 values of 36.5 μg/mL ± 1.10, 33.9 μg/mL ± 0.78, 22.09 μg/mL ± 0.00, 38.6 μg/mL ± 1.054, 29.99 μg/mL ± 0.89, and 25.29 μg/mL ± 0.15, respectively (Table 6).

Table 6.
Cytotoxic activity of secondary metabolites of crude extracts of bacterial endophytes associated with V. anthelmintica (n = 3).
3.5. Antidiabetic Activity of the Natural Products Synthesis by Endophytic Bacteria
Diabetes is a serious metabolic disorder that occurs when the pancreas does not produce insulin, resulting in hyperglycemia. It has become a global problem for human health in recent years. Many natural drugs obtained from medicinal plants and endophytes have been used for the treatment of this disease [49,50]. The alkaloid compounds were obtained from the mangrove fungal endophyte Aspergillus sp., which exhibited significant antidiabetic activity against the PTP1B enzyme [49]. The fungal strain Penicillium canescens produced bioactive secondary metabolites that showed inhibitory activity against a-glucosidase [51]. We have also measured the inhibitory effects of the crude extract of endophytic bacteria against PTP1B activity in vitro. The crude extracts of B. halotolerans XJB-35, P. punonensis XJB-7, L. adecarboxylata XJB-12, and P. alvei XJB-14 demonstrated a powerful inhibitory effect on PTP1B, with an IC50 of 4.93 ± 0.29, 6.18 ± 1.45, 8.08 ± 0.81, and 7.62 ± 0.46 µg/mL, respectively (Table 7). P. agglomerans XJB-62 did not show any inhibitory activity against PTP-1B. All results were compared with the positive control PTP1B inhibitor (1.46 ± 0.40 µg/mL).

Table 7.
PTP1B enzyme inhibition activity of the selected endophytic bacteria crude extracts (n = 3).
3.6. Antioxidant Activity of the Natural Products’ Synthesis by Endophytic Bacteria
The endophytes are pharmacologically rich sources for the production of natural products with biological activity, such as antioxidant activity [52]. For example, Silva et al. reported secondary metabolites isolated from the endophytic fungus Botryosphaeria fabicerciana with antioxidant activity [53]. The extracts of the endophytic bacteria Bacillus siamensis HMB1 and Bacillus aryabhattai HMD4 have demonstrated antioxidant activity [54]. Sharma et al. studied novel natural compounds isolated from the endophytic fungus Diaporthe sp. [55] that displayed significant DPPH scavenging activity. Sun et al. [56] investigated potential antioxidant activity of natural products synthesized by several species of Bacillus. According to our previous study, the culturable endophytic bacteria associated with the V. anthelmintica root showed antioxidant activity. In the current study, we investigated the DPPH radical scavenging activity of extracts of endophytic bacteria. Our results showed that B. halotolerans XJB-35, B. safensis XJB-71 and L. adecarboxylata XJB-12 demonstrated moderate antioxidant activity with an IC50 value of 35.453 ± 3.25, 56.41 ± 0.7 and 36.21 ± 0.3 μg/mL, respectively (Table 8). Ascorbic acid (vitamin C) was used as the positive control.

Table 8.
Antioxidant activity of ethyl acetate crude extract of bacterial endophytes (n = 3).
3.7. The Effect of Cultivation Time and Medium Content on the Natural Products Synthesis of Endophytic Bacteria
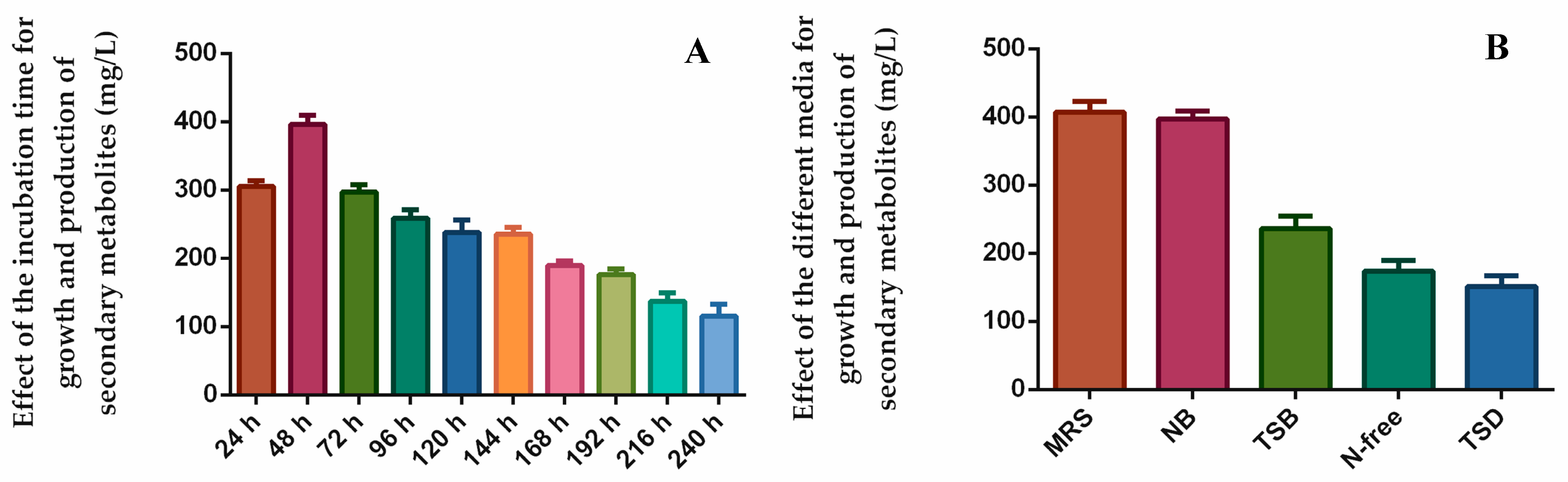
The effect of different physico-chemical parameters, including pH, temperature, incubation time, and different carbon and nitrogen sources, on the bioactive secondary metabolite production by endophytes has been reported by other researchers [57,58]. The following can be shown as an example of these: Yi et al. [32] found the optimal culture conditions for the growth and antifungal activity of the endophytic bacterium strain BT4. According to this study, bacteria reached their maximum growth and produced antifungal secondary metabolites at 48 h and pH 7.5, and the optimal carbon and nitrogen sources were starch and yeast extracts. The effect of optimal culture conditions on the synthesis of bioactive natural compounds by the endophyte strain Geosmithia pallida was also investigated by Deka et al. [59]. In the present study, we optimized the culture conditions of the most active endophytic bacterium, B. halotolerans XJB-35, for maximum growth and produced bioactive secondary metabolites. The effect of incubation time on growth and the yield of natural products is shown in Figure 5A. B. halotolerans XJB-35 reached maximum growth at 48 h and produced metabolites at a concentration of 395 mg/L. B. halotolerans XJB-35 reached its highest growth and maximum production of bioactive natural products in MRS and NB media at 400 and 396 mg/L, respectively (Figure 5B).
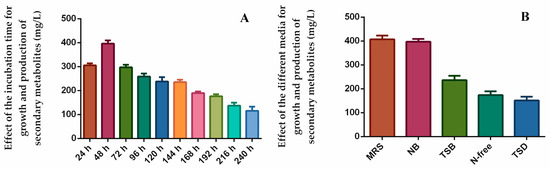
Figure 5.
Effect of incubation time (A) and different media (B) on growth and produced secondary metabolites by the most active endophytic bacteria, B. halotolerans XJB-35.
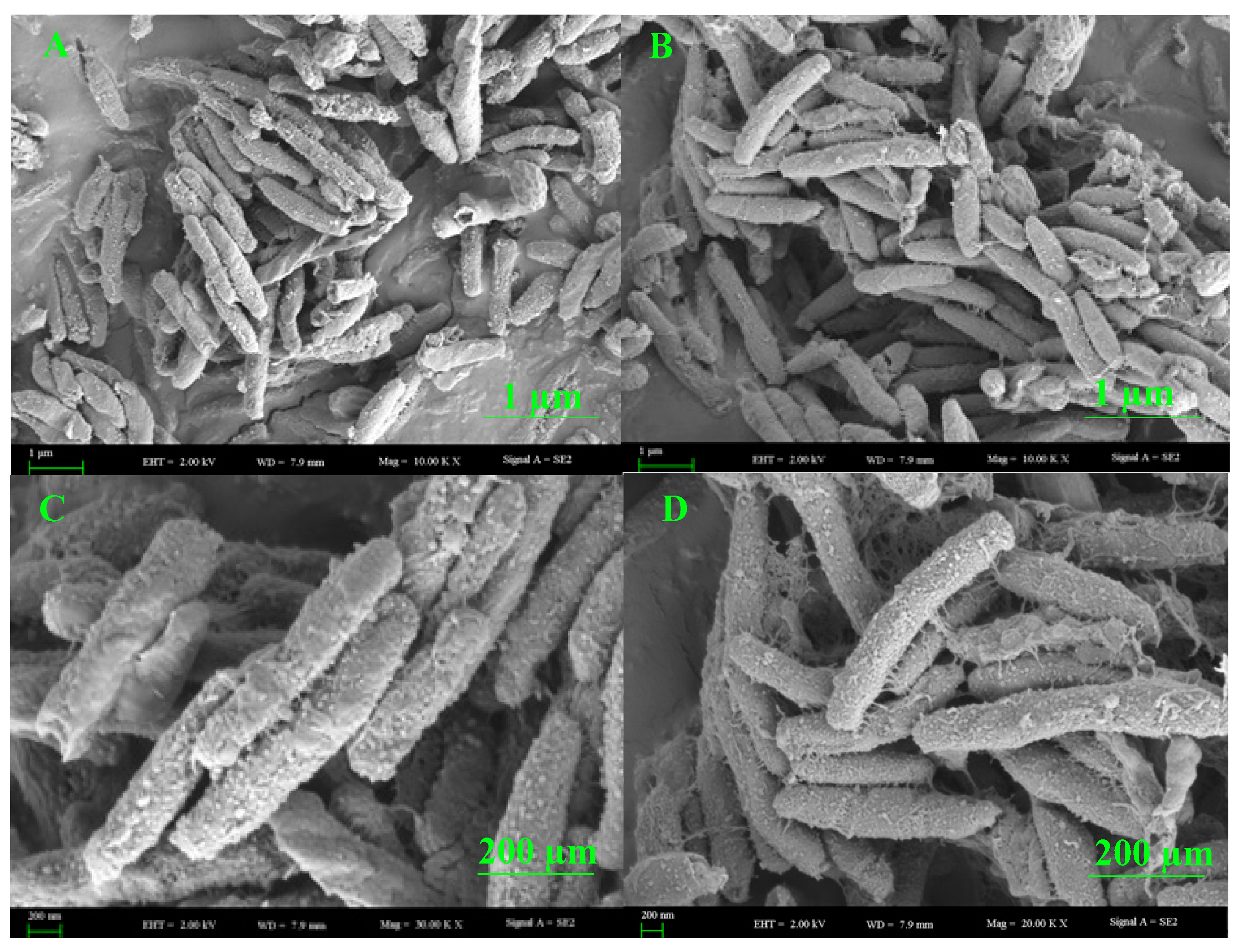
3.8. SEM Analysis of the Endophytic Bacteria B. halotolerans XJB-35
The morphological structure of the colonizing bacterial strain was visualized by SEM. The structure of the surface of the most active bacterial endophyte, the B. halotolerans XJB-35 colony, could be seen in different sizes via scanning electron microscopy (Figure 6).
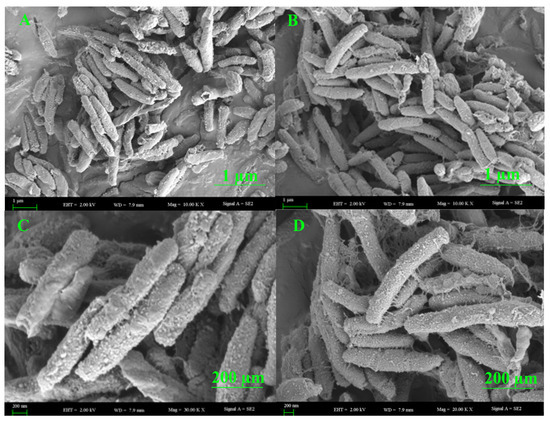
Figure 6.
Lower to higher magnification SEM images of colonies surfaces of the most active endophytic bacteria, B. halotolerans XJB-35, from a plate cultured with agar medium. Scale bars = 1 (A,B) and 200 (C,D) µm.
3.9. Secondary Metabolites Produced by the Most Active Endophytic Bacteria B. halotolerans XJB-35 on Different Culture Media HPLC Analysis
Endophytes are an unconventional source of bioactive natural products. Endophytes are now well-known to biosynthesize novel bioactive natural compounds with significant biological activities, and there have been reports of a large number of potential natural products derived from endophytes [60]. The chemical properties of secondary metabolites synthesized by endophytes directly depend on the composition of the media in which they grow. They may synthesize dissimilar compounds in culture media with different contents. This has been proven in our research study. We analyzed secondary metabolites using HPLC, which produced the most bioactive bacterium, B. halotolerans XJB-35, in different culture media, such as NB, N-free, TSD, LB, and MRS (Figures S20–S24). Accordingly, peaks with retention times at 16.5, 20, and 30 min were synthesized in all the culture media (Figure S31). In both LB and TSD media, the peak absorption at 42.5 and 65 min was not observed in the other media. It is also worth noting that the peak at 32.5 min in MRS was not found in the other cultural media (Figure S20). Also worthy of special attention is that, unlike fungus, the incubation time did not affect the qualitative quality of the compounds synthesized (Figure S18). It is possible that the yield of products decreased once the incubation time exceeded 48 h. The HPLC analysis is an initial screening tool for the identification of the various components of crude extracts. The use of HPLC as the sole tool of identifying the bioactive metabolites in the crude extracts is limited. The determined spectra of compounds show that endophytes produce various metabolites in different culture media.
3.10. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
The volatile chemical composition of the most active bacterial endophyte, B. halotolerans XJB-35, was analyzed using GC-MS when it was grown on different culture media. A total of 39 non-polar compounds were identified by retention time and comparing the spectra to a stored MS library (W8N05ST and NIST08). Six major compounds were identified from the extract with the NB medium (Figure S26), namely p-xylene, orcinol, 1,3-benzenediol, 4,5-dimethyl-, 2,2′-isopropylidenebis(5 methylfuran), 9-octadecenoic acid (Z)-, methyl ester, pyrrolo[1,2-a]pyrazine-1,4dione, and hexahydro-3-(phenylmethyl)-. Six major features from the N-free medium (Figure S27) were obtained at RT 3.741, 17.223, 17.512, 19.517, 19.848, and 20.111, suggesting the presence of p-xylene, hexadecanoic acid, methyl ester, pyrrolo[1,2-a]pyrazine-1,4dione, hexahydro-3-(2-methylpropyl)-, 9-octadecenoic acid (Z)-, methyl ester, heptadecanoic acid, 16-methyl-, methyl ester, and methyl 10-trans,12-cis octadecadienoate (Table 9). Many types of non-polar compounds were produced in the MRS medium (Figure S30). Approximately 26 compounds were identified; among them, 12 peaks represented major compounds, including butanoic acid, 3-methyl-, butanoic acid, 2-methyl-, benzeneacetic acid, methyl ester, benzeneacetic acid, diethyltrisulphide, pyrrolo[1,2-a]pyrazine-1,4dione, hexahydro-3-(2-methylpropyl)-, dibutyl phthalate, 9-octadecenoic acid (Z)-, methyl ester, 1,4,7,10,13,16-hexaoxacyclooctadecane, pyrrolo[1,2-a]pyrazine-1,4dione, hexahydro-3-(phenylmethyl)-, 9-octadecenamide, (Z)-, and eicosane. It is worth noting that compounds 9-octadecenoic acid (Z)-, methyl ester, pyrrolo[1,2-a]pyrazine-1,4dione, hexahydro-3-(2-methylpropyl)-, pyrrolo[1,2-a]pyrazine-1,4dione, and hexahydro-3-(phenylmethyl)- were major components in all of the media.

Table 9.
The volatile chemical composition of the most active endophytic bacteria, B. halotolerans XJB-35.
The GC-MS analysis is an important technique for the investigation of the chemical composition of the secondary metabolites produced by various endophytic microorganisms. Some volatiles compounds produced by various fungi and bacteria have been previously identified. For instance, dibutyl phthalate, p-xylene, and phenylethyl alcohol were produced by the most bioactive endophytic bacteria, B. subtilis EGY16 [61]. Eicosane and benzene derivatives were produced by Aspergillus clavatonanicus and Phytophthora palmivora, respectively, as antimicrobial compounds [62,63]. Butanoic acid, 2-methyl- identified from the dicloromethene fraction of endophytic bacteria Colletotrichum falcatum and fungus Trichoderma sp., has been reported for antiproliferative and antimicrobial activity [64,65]. Similarly, other such compounds, i.e., pyrrolo[1,2-a]pyrazine-1,4 dione, hexahydro-, pyrrolo[1,2-a]pyrazine-1,4dione, and hexahydro-3-(2-methylpropyl)-, have been previously reported by other researchers [66]. In our previous study, the major compounds butanoic acid, 3-methyl-, butanoic acid, 2-methyl-, p-xylene, pyrrolo[1,2-a]pyrazine-1,4dione, hexahydro-3-(2 methylpropyl)-, and 9-octadecenoic acid (Z)- methyl ester have already been reported for their pharmacological potential [19].
Our results showed that endophytic bacteria can produce different secondary metabolites in different media. Our research is continuous, and in the future, we will isolate individual compounds from the ethyl acetate fraction and will evaluate their biological activity. This will make it possible to understand exactly which compounds displayed pharmacological properties in the total extract.
4. Conclusions
In conclusion, the observed results contribute to our knowledge on diversity, antimicrobial, cytotoxic, antidiabetic, antioxidant, melanin content assay, and tyrosinase activity in murine B16 cells of crude extracts of endophytic bacteria isolated from the stem of V. anthelmintica. Overall, our results highlighted the powerful biological activities of the endophytic bacterial strain B. halotolerans XJB-35. Moreover, the present study determined the optimal culture conditions for the growth and production of bioactive natural products of the most active bacterium, B. halotolerans XJB-35. The optimal culture conditions were an MRS medium and incubation time of 48 h to increase the secondary metabolite yield. B. halotolerans XJB-35 has the potential to be an ideal bioactive agent in the biosynthesis of pharmacologically active natural compounds. This bio-based tool indicated that the secondary metabolites of endophytic bacteria could be a novel bacterial resource for new drug discovery for the treatment of various diseases. This study investigated the synergetic properties of the crude extract compositions, evaluated on their melanin synthesis, antimicrobial, antioxidant, antidiabetic, and cytotoxic activity. These results suggest that the pharmacological role of the V. anthelmintica derived bacterial endophytes, in particular individual secondary metabolites of most active endophytic bacteria remains to be investigated in future studies. Our laboratory is continuing to pursue this research.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app13179797/s1, Figure S1: Main approach of this work; Figure S2: HPLC Analysis of natural products synthesized by endophytic bacteria B. haynesii XJB-5; Figure S3: HPLC Analysis of natural products synthesized by endophytic bacteria P. punonensis XJB-7; Figure S4: HPLC Analysis of natural products synthesized by endophytic bacteria L. fusiformis XJB-17; Figure S5: HPLC Analysis of natural products synthesized by endophytic bacteria B. halotolerans XJB-35; Figure S6: HPLC Analysis of natural products synthesized by endophytic bacteria B. safensis XJB-71; Figure S7: HPLC Analysis of natural products synthesized by endophytic bacteria S. lutetiensis XJB-66; Figure S8: HPLC Analysis of natural products synthesized by endophytic bacteria L. adecarboxylata XJB-12; Figure S9: HPLC Analysis of natural products synthesized by most active endophytic bacteria B. halotolerans XJB-35 on 24 h; Figure S10: HPLC Analysis of natural products synthesized by most active endophytic bacteria B. halotolerans XJB-35 on 48 h; Figure S11. HPLC Analysis of natural products synthesized by most active endophytic bacteria B. halotolerans XJB-35 on 72 h; Figure S12. HPLC Analysis of natural products synthesized by most active endophytic bacteria B. halotolerans XJB-35 on 96 h; Figure S13. HPLC Analysis of natural products synthesized by most active endophytic bacteria B. halotolerans XJB-35 on 120 h; Figure S14. HPLC Analysis of natural products synthesized by most active endophytic bacteria B. halotolerans XJB-35 on 144 h; Figure S15. HPLC Analysis of natural products synthesized by most active endophytic bacteria B. halotolerans XJB-35 on 168 h; Figure S16. HPLC Analysis of natural products synthesized by most active endophytic bacteria B. halotolerans XJB-35 on 192 h; Figure S17. HPLC Analysis of natural products synthesized by most active endophytic bacteria B. halotolerans XJB-35 on 216 h; Figure S18. HPLC Analysis of natural products synthesized by most active endophytic bacteria B. halotolerans XJB-35 on different incubation times; Figure S19. HPLC Analysis of natural products synthesized by most active endophytic bacteria B. halotolerans XJB-35 on different incubation times; Figure S20. HPLC Analysis of natural products synthesized by most active endophytic bacteria B. halotolerans XJB-35 on MRS media; Figure S21. HPLC Analysis of natural products synthesized by most active endophytic bacteria B. halotolerans XJB-35 on TSD media; Figure S22. HPLC Analysis of natural products synthesized by most active endophytic bacteria B. halotolerans XJB-35 on N-Free media; Figure S23. HPLC Analysis of natural products synthesized by most active endophytic bacteria B. halotolerans XJB-35 on TSB media; Figure S24. HPLC Analysis of natural products synthesized by most active endophytic bacteria B. halotolerans XJB-35 on different media; Figure S25. HPLC Analysis of natural products synthesized by most active endophytic bacteria B. halotolerans XJB-35 on different media; Figure S26. Volatile chemical component of most active endophytic bacteria B. halotolerans XJB-35 on different NB media; Figure S27. Volatile chemical component of most active endophytic bacteria B. halotolerans XJB-35 on different N-free media; Figure S28. Volatile chemical component of most active endophytic bacteria B. halotolerans XJB-35 on different TSD media; Figure S29. Volatile chemical component of most active endophytic bacteria B. halotolerans XJB-35 on different TSB media; Figure S30. Volatile chemical component of most active endophytic bacteria B. halotolerans XJB-35 on different MRS media; Figure S31. HPLC analysis of secondary metabolites produced by the most-active endophytic bacteria B. halotolerans XJB-35 on different culture media.
Author Contributions
N.L. conducted all experiments, lab work; N.R. conducted all experiments, lab work, prepared the manuscript, and edited; H.-X.N. analyzed data; P.P. collected plant samples; A.Y. considered the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the West Light Foundation of the Chinese Academy of Sciences grant (2019-FPGGRC-004), the Foreign Young Talents Program, grant (QN2022045004L), and the Central Asian Drug Discovery and Development center of the Chinese Academy of Science (CAM202101).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article and supplementary material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Verma, A.; Gupta, P.; Rai, N.; Tiwari, R.K.; Kumar, A.; Salvi, P.; Kamble, S.C.; Singh, S.K.; Gautam, V. Assessment of Biological Activities of Fungal Endophytes Derived Bioactive Compounds Isolated from Amoora rohituka. J. Fungi 2022, 8, 285. [Google Scholar] [CrossRef]
- Rustamova, N.; Litao, N.; Bozorov, K.; Sayyed, R.; Aisa, H.A.; Yili, A. Plant-associated endophytic fungi: A source of structurally diverse and bioactive natural products. Plant Cell Biotechnol. Mol. Biol. 2022, 23, 1–19. [Google Scholar] [CrossRef]
- Kushwaha, P.; Kashyap, P.L.; Srivastava, A.K.; Tiwari, R.K. Plant growth promoting and antifungal activity in endophytic Bacillus strains from pearl millet (Pennisetum glaucum). Braz. J. Microbiol. 2020, 51, 229–241. [Google Scholar] [CrossRef]
- Rustamova, N.; Bozorov, K.; Efferth, T.; Yili, A. Novel secondary metabolites from endophytic fungi: Synthesis and biological properties. Phytochem. Rev. 2020, 19, 425–448. [Google Scholar] [CrossRef]
- Tyagi, J.; Chaudhary, P.; Mishra, A.; Khatwani, M.; Dey, S.; Varma, A. Role of Endophytes in Abiotic Stress Tolerance: With Special Emphasis on Serendipita indica. Int. J. Environ. Res. 2022, 16, 62. [Google Scholar] [CrossRef]
- Narayanan, Z.; Glick, B.R. Secondary Metabolites Produced by Plant Growth-Promoting Bacterial Endophytes. Microorganisms 2022, 10, 2008. [Google Scholar] [CrossRef]
- Liu, S.-S.; Huang, R.; Zhang, S.-P.; Xu, T.-C.; Hu, K.; Wu, S.-H. Antimicrobial secondary metabolites from an endophytic fungus Aspergillus polyporicola. Fitoterapia 2022, 162, 105297. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Segaran, G.; Sathiavelu, M. Chapter 4—Antimicrobial metabolites from endophytic microorganisms and its mode of action. In Biocontrol Mechanisms of Endophytic Microorganisms; Radhakrishnan, E.K., Kumar, A., Aswani, R., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 75–88. [Google Scholar]
- Siebatcheu, E.C.; Wetadieu, D.; Youassi Youassi, O.; Bedine Boat, M.A.; Bedane, K.G.; Tchameni, N.S.; Sameza, M.L. Secondary metabolites from an endophytic fungus Trichoderma erinaceum with antimicrobial activity towards Pythium ultimum. Nat. Prod. Res. 2022, 37, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Vasundhara, M.; Reddy, M.S.; Kumar, A. Chapter 18—Secondary Metabolites from Endophytic Fungi and Their Biological Activities. In New and Future Developments in Microbial Biotechnology and Bioengineering; Gupta, V.K., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 237–258. [Google Scholar]
- Gupta, S.; Choudhary, M.; Singh, B.; Singh, R.; Dhar, M.K.; Kaul, S. Diversity and biological activity of fungal endophytes of Zingiber officinale Rosc. with emphasis on Aspergillus terreus as a biocontrol agent of its leaf spot. Biocatal. Agric. Biotechnol. 2022, 39, 102234. [Google Scholar] [CrossRef]
- Debbab, A.; Aly, A.H.; Proksch, P. Bioactive secondary metabolites from endophytes and associated marine derived fungi. Fungal Divers. 2011, 49, 1–12. [Google Scholar] [CrossRef]
- Tang, Z.; Qin, Y.; Chen, W.; Zhao, Z.; Lin, W.; Xiao, Y.; Chen, H.; Liu, Y.; Chen, H.; Bu, T.; et al. Diversity, Chemical Constituents, and Biological Activities of Endophytic Fungi Isolated from Ligusticum chuanxiong Hort. Front. Microbiol. 2021, 12, 771000. [Google Scholar] [CrossRef]
- Gao, H.; Li, G.; Lou, H.-X. Structural Diversity and Biological Activities of Novel Secondary Metabolites from Endophytes. Molecules 2018, 23, 646. [Google Scholar] [CrossRef]
- Xian, P.-J.; Liu, S.-Z.; Wang, W.-J.; Yang, S.-X.; Feng, Z.; Yang, X.-L. Undescribed specialised metabolites from the endophytic fungus Emericella sp. XL029 and their antimicrobial activities. Phytochemistry 2022, 202, 113303. [Google Scholar] [CrossRef]
- Hagag, A.; Abdelwahab, M.F.; Abd El-kader, A.M.; Fouad, M.A. The endophytic Aspergillus strains: A bountiful source of natural products. J. Appl. Microbiol. 2022, 132, 4150–4169. [Google Scholar] [CrossRef]
- Wen, J.; Okyere, S.K.; Wang, S.; Wang, J.; Xie, L.; Ran, Y.; Hu, Y. Endophytic Fungi: An Effective Alternative Source of Plant-Derived Bioactive Compounds for Pharmacological Studies. J. Fungi 2022, 8, 205. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Wani, A.K.; Dhanjal, D.S.; Mukherjee, S. Insights into the beneficial roles of dark septate endophytes in plants under challenging environment: Resilience to biotic and abiotic stresses. World J. Microbiol. Biotechnol. 2022, 38, 79. [Google Scholar] [CrossRef]
- Niu, L.; Rustamova, N.; Ning, H.; Paerhati, P.; Lu, C.; Yili, A. Diversity and Biological Activities of Endophytic Fungi from the Flowers of the Medicinal Plant Vernonia anthelmintica. Int. J. Mol. Sci. 2022, 23, 11935. [Google Scholar] [CrossRef]
- Rustamova, N.; Gao, Y.; Zhang, Y.; Yili, A. Biological Activity of Endophytic Fungi from the Roots of the Medicinal Plant Vernonia anthelmintica. Microorganisms 2020, 8, 586. [Google Scholar] [CrossRef] [PubMed]
- Rustamova, N.; Bobakulov, K.; Litao, N.; Nuerxiati, R.; Wali, A.; Setzer, W.N.; Yili, A. Secondary Metabolites and their Biological Activities from Endophytic Fungal Strain Aspergillus terreus XJA8 Associated with Vernonia anthelmintica. J. Biol. Act. Prod. Nat. 2022, 12, 421–435. [Google Scholar] [CrossRef]
- Mora-Ruiz, M.D.R.; Font-Verdera, F.; Díaz-Gil, C.; Urdiain, M.; Rodríguez-Valdecantos, G.; González, B.; Orfila, A.; Rosselló-Móra, R. Moderate halophilic bacteria colonizing the phylloplane of halophytes of the subfamily Salicornioideae (Amaranthaceae). Syst. Appl. Microbiol. 2015, 38, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Dashti, A.A.; Jadaon, M.M.; Abdulsamad, A.M.; Dashti, H.M. Heat treatment of bacteria: A simple method of DNA extraction for molecular techniques. Kuwait Med. J. 2009, 41, 117–122. [Google Scholar]
- Rustamova, N.; Wubulikasimu, A.; Mukhamedov, N.; Gao, Y.; Egamberdieva, D.; Yili, A. Endophytic Bacteria Associated with Medicinal Plant Vernonia anthelmintica: Diversity and Characterization. Curr. Microbiol. 2020, 77, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. Res. 1991, 173, 697–703. [Google Scholar] [CrossRef]
- Jinneman, K.C.; Wetherington, J.H.; Adams, A.M.; Johnson, J.M.; Tenge, B.J.; Dang, N.-L.; Hill, W.E. Differentiation of Cyclospora sp. and Eimeria spp. by Using the Polymerase Chain Reaction Amplification Products and Restriction Fragment Length Polymorphisms. Food and Drug Administration Laboratory Information Bulletin LIB No. 4044. 1996. Available online: http://vm.cfsan.fda.gov/%E2%88%BCmow/kjcs19c.html (accessed on 17 July 2023).
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Tamura, K.; Nei, M.; Kumar, S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc. Natl. Acad. Sci. USA 2004, 101, 11030–11035. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Wang, S.; Li, Y.; Zheng, M.; Xi, X.; Cao, H.; Cui, X.; Guo, H.; Han, C. Yield enhancement strategies of rare pharmaceutical metabolites from endophytes. Biotechnol. Lett. 2018, 40, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.-J.; Li, Y.-S.; Xia, B.; Li, W.-P.; Pang, L.; Tong, Z.-D. Optimization of medium composition and culture conditions for antifungal activity of a tomato endophytic bacterium. Biol. Control. 2015, 82, 69–75. [Google Scholar] [CrossRef]
- Nongkhlaw, F.M.W.; Joshi, S.R. Microscopic study on colonization and antimicrobial property of endophytic bacteria associated with ethnomedicinal plants of Meghalaya. J. Microsc. Ultrastruct. 2017, 5, 132–139. [Google Scholar]
- Pan, Y.; Jin, H.; Yang, S.; Liu, H. Changes of volatile organic compounds and bioactivity of Alternaria brassicae GL07 in different ages. J. Basic Microbiol. 2019, 59, 713–722. [Google Scholar] [CrossRef]
- Seo, W.T.; Lim, W.J.; Kim, E.J.; Yun, H.D.; Lee, Y.H.; Cho, K.M. Endophytic Bacterial Diversity in the Young Radish and Their Antimicrobial Activity against Pathogens. J. Korean Soc. Appl. Biol. Chem. 2010, 53, 493–503. [Google Scholar] [CrossRef]
- Christina, A.; Christapher, V.; Bhore, S.J. Endophytic bacteria as a source of novel antibiotics: An overview. Pharmacogn. Rev. 2013, 7, 11–16. [Google Scholar]
- Wang, P.; Yu, J.-H.; Zhu, K.; Wang, Y.; Cheng, Z.-Q.; Jiang, C.-S.; Dai, J.-G.; Wu, J.; Zhang, H. Phenolic bisabolane sesquiterpenoids from a Thai mangrove endophytic fungus, Aspergillus sp. xy02. Fitoterapia 2018, 127, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Li, G.; Sun, M.; Yang, X.; Xu, J. A new antimicrobial sesquiterpene isolated from endophytic fungus Cytospora sp. from the Chinese mangrove plant Ceriops tagal. Nat. Prod. Res. 2018, 34, 1404–1408. [Google Scholar] [CrossRef]
- He, J.; Yang, M.-S.; Wang, W.-X.; Li, Z.-H.; Elkhateeb, W.A.M.; Wen, T.-C.; Ai, H.-L.; Feng, T. Anti-phytopathogenic sesquiterpenoid-xanthone adducts from potato endophytic fungus Bipolaris eleusines. RSC Adv. 2019, 9, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.-C.; Wang, Y.-L.; Zhang, T.-Y.; Chen, Z.-J.; Yang, T.-M.; Wu, Y.-Y.; Sun, C.-P.; Ma, X.-C.; Zhang, Y.-X. Indole diterpenoids from the endophytic fungus Drechmeria sp. as natural antimicrobial agents. Phytochemistry 2018, 148, 21–28. [Google Scholar] [CrossRef]
- Liu-Smith, F.; Meyskens, F.L. Molecular mechanisms of flavonoids in melanin synthesis and the potential for the prevention and treatment of melanoma. Mol. Nutr. Food Res. 2016, 60, 1264–1274. [Google Scholar] [CrossRef]
- Li, H.-R.; Habasi, M.; Xie, L.-Z.; Aisa, H.A. Effect of Chlorogenic Acid on Melanogenesis of B16 Melanoma Cells. Molecules 2014, 19, 12940–12948. [Google Scholar] [CrossRef]
- Turak, A.; Maimaiti, Z.; Ma, H.; Aisa, H.A. Pseudo-disesquiterpenoids from seeds of Vernonia anthelmintica and their biological activities. Phytochem. Lett. 2017, 21, 163–168. [Google Scholar] [CrossRef]
- Maimaiti, Z.; Turak, A.; Aisa, H.A. Two new compounds from the seeds of Vernonia anthelmintica. J. Asian Nat. Prod. Res. 2017, 19, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Rustamova, N.; Bobakulov, K.; Begmatov, N.; Turak, A.; Yili, A.; Aisa, H.A. Secondary metabolites produced by endophytic Pantoea ananatis derived from roots of Baccharoides anthelmintica and their effect on melanin synthesis in murine B16 cells. Nat. Prod. Res. 2021, 35, 796–801. [Google Scholar] [CrossRef]
- Ebada, S.S.; El-Neketi, M.; Ebrahim, W.; Mándi, A.; Kurtán, T.; Kalscheuer, R.; Müller, W.E.G.; Proksch, P. Cytotoxic secondary metabolites from the endophytic fungus Aspergillus versicolor KU258497. Phytochem. Lett. 2018, 24, 88–93. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, Q.; Gao, Y.-Q.; Tang, J.-J.; Zhang, A.-L.; Gao, J.-M. Secondary Metabolites from the Endophytic Botryosphaeria dothidea of Melia azedarach and Their Antifungal, Antibacterial, Antioxidant, and Cytotoxic Activities. J. Agric. Food Chem. 2014, 62, 3584–3590. [Google Scholar] [CrossRef]
- Deng, M.; Tao, L.; Qiao, Y.; Sun, W.; Xie, S.; Shi, Z.; Qi, C.; Zhang, Y. New cytotoxic secondary metabolites against human pancreatic cancer cells from the Hypericum perforatum endophytic fungus Aspergillus terreus. Fitoterapia 2020, 146, 104685. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Huang, C.; Li, J.; Chen, T.; Tang, J.; Liu, W.; Long, Y. Isolation, Structural Characterization and Antidiabetic Activity of New Diketopiperazine Alkaloids from Mangrove Endophytic Fungus Aspergillus sp. Mar. Drugs 2021, 19, 402. [Google Scholar] [CrossRef]
- Ramírez-Espinosa, J.J.; Rios, M.Y.; López-Martínez, S.; López-Vallejo, F.; Medina-Franco, J.L.; Paoli, P.; Camici, G.; Navarrete-Vázquez, G.; Ortiz-Andrade, R.; Estrada-Soto, S. Antidiabetic activity of some pentacyclic acid triterpenoids, role of PTP–1B: In vitro, in silico, and in vivo approaches. Eur. J. Med. Chem. 2011, 46, 2243–2251. [Google Scholar] [CrossRef]
- Malik, A.; Ardalani, H.; Anam, S.; McNair, L.M.; Kromphardt, K.J.K.; Frandsen, R.J.N.; Franzyk, H.; Staerk, D.; Kongstad, K.T. Antidiabetic xanthones with α-glucosidase inhibitory activities from an endophytic Penicillium canescens. Fitoterapia 2020, 142, 104522. [Google Scholar] [CrossRef]
- Druzian, S.P.; Pinheiro, L.N.; Susin, N.M.B.; Dal Prá, V.; Mazutti, M.A.; Kuhn, R.C.; Terra, L.d.M. Production of metabolites with antioxidant activity by Botryosphaeria dothidea in submerged fermentation. Bioprocess Biosyst. Eng. 2020, 43, 13–20. [Google Scholar] [CrossRef]
- da Silva, A.A.; Polonio, J.C.; Bulla, A.M.; Polli, A.D.; Castro, J.C.; Soares, L.C.; de Oliveira-Junior, V.A.; Vicentini, V.E.P.; de Oliveira, A.J.B.; Gonçalves, J.E.; et al. Antimicrobial and antioxidant activities of secondary metabolites from endophytic fungus Botryosphaeria fabicerciana (MGN23-3) associated to Morus nigra L. Nat. Prod. Res. 2022, 36, 3158–3162. [Google Scholar] [CrossRef]
- Pudjas, N.T.G.; Mubarik, N.R.; Astuti, R.I.; Sudirman, L.I. Antioxidant Activity of Endophytic Bacteria Derived from Hoya multiflora Blume Plant and Their Cellular Activities on Schizosaccharomyces pombe. AYATI J. Biosci. 2022, 29, 214–221. [Google Scholar] [CrossRef]
- Sharma, V.; Singamaneni, V.; Sharma, N.; Kumar, A.; Arora, D.; Kushwaha, M.; Bhushan, S.; Jaglan, S.; Gupta, P. Valproic acid induces three novel cytotoxic secondary metabolites in Diaporthe sp., an endophytic fungus from Datura inoxia Mill. Bioorg. Med. Chem. Lett. 2018, 28, 2217–2221. [Google Scholar] [CrossRef]
- Sun, B.; Jing, R.; Wang, Z.; Tian, L.; Mao, F.; Liu, Y. Diversity and community structure of endophytic Bacillus with antagonistic and antioxidant activity in the fruits of Xisha Wild Noni (Morinda citrifolia L.). Microb. Pathog. 2021, 158, 105065. [Google Scholar] [CrossRef]
- Kumar, V.; Sahai, V.; Bisaria, V.S. High-density spore production of Piriformospora indica, a plant growth-promoting endophyte, by optimization of nutritional and cultural parameters. Bioresour. Technol. 2011, 102, 3169–3175. [Google Scholar] [CrossRef]
- Chandrakar, S.; Gupta, A.K. Actinomycin-Producing Endophytic Streptomyces parvulus Associated with Root of Aloe vera and Optimization of Conditions for Antibiotic Production. Probiotics Antimicrob. Proteins 2019, 11, 1055–1069. [Google Scholar] [CrossRef]
- Deka, D.; Jha, D.K. Optimization of Culture Parameters for Improved Production of Bioactive Metabolite by Endophytic Geosmithia pallida (KU693285) Isolated from Brucea mollis Wall ex. Kurz, An Endangered Medicinal Plant. J. Pure Appl. Microbiol. 2018, 12, 1205–1213. [Google Scholar] [CrossRef]
- Schulz, B.; Boyle, C.; Draeger, S.; Römmert, A.-K.; Krohn, K. Endophytic fungi: A source of novel biologically active secondary metabolites. Mycol. Res. 2002, 106, 996–1004. [Google Scholar] [CrossRef]
- Abdelshafy Mohamad, O.A.; Ma, J.-B.; Liu, Y.-H.; Zhang, D.; Hua, S.; Bhute, S.; Hedlund, B.P.; Li, W.-J.; Li, L. Beneficial Endophytic Bacterial Populations Associated with Medicinal Plant Thymus vulgaris Alleviate Salt Stress and Confer Resistance to Fusarium oxysporum. Front. Plant Sci. 2020, 11, 47. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.K.; Passari, A.K.; Chandra, P.; Leo, V.V.; Kumar, B.; Uthandi, S.; Thankappan, S.; Gupta, V.K.; Singh, B.P. Determination and production of antimicrobial compounds by Aspergillus clavatonanicus strain MJ31, an endophytic fungus from Mirabilis jalapa L. using UPLC-ESI-MS/MS and TD-GC-MS analysis. PLoS ONE 2017, 12, e0186234. [Google Scholar] [CrossRef]
- Alsultan, W.; Vadamalai, G.; Khairulmazmi, A.; Saud, H.M.; Al-Sadi, A.M.; Rashed, O.; Jaaffar, A.K.M.; Nasehi, A. Isolation, identification and characterization of endophytic bacteria antagonistic to Phytophthora palmivora causing black pod of cocoa in Malaysia. Eur. J. Plant Pathol. 2019, 155, 1077–1091. [Google Scholar] [CrossRef]
- Jayakumar, V.; Ramesh Sundar, A.; Viswanathan, R. Biocontrol of Colletotrichum falcatum with volatile metabolites produced by endophytic bacteria and profiling VOCs by headspace SPME coupled with GC–MS. Sugar Tech 2021, 23, 94–107. [Google Scholar] [CrossRef]
- Leylaie, S.; Zafari, D. Antiproliferative and Antimicrobial Activities of Secondary Metabolites and Phylogenetic Study of Endophytic Trichoderma Species from Vinca Plants. Front. Microbiol. 2018, 9, 1484. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Antony, A.R.; Kannan, V.R. Exploration of endophytic microorganisms from selected medicinal plants and their control potential to multi drug resistant pathogens. J. Med. Plants Stud. 2015, 3, 49–57. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).