Abstract
Acute ischemic stroke (AIS) is the loss of neurological function due to a sudden reduction in cerebral blood flow and is a leading cause of disability and death worldwide. The field of radiological imaging has experienced growth in recent years, which could be boosted by the advent of artificial intelligence. One of the latest innovations in artificial intelligence is radiomics, which is based on the fact that a large amount of quantitative data can be extracted from radiological images, from which patterns can be identified and associated with specific pathologies. Since its inception, radiomics has been particularly associated with the field of oncology and has shown promising results in a wide range of clinical situations. The performance of radiomics in non-tumour pathologies has been increasingly explored in recent years, and the results continue to be promising. The aim of this review is to explore the potential applications of radiomics in AIS patients and to theorize how radiomics may change the paradigm for these patients in the coming years.
Keywords:
acute ischemic stroke; AIS; radiomics; artificial intelligence; AI; neuroradiology; neurology 1. Introduction
Acute ischemic stroke (AIS) is the loss of neurological function due to a sudden reduction in cerebral blood flow and is a leading cause of disability and death worldwide [1]. According to the WHO, stroke is the second leading cause of disability and death worldwide after ischemic heart disease, accounting for 11% of all deaths. With improvements in the quality of life and longevity of patients, the incidence of stroke is expected to continue to rise in the coming years [2]. More than 80% of strokes are ischemic in origin [3]. Recent advances in the management of these patients have focused more on their treatment, where endovascular therapies have radically changed the prognosis of these patients [4,5,6]. In recent years, numerous studies have explored the many diagnostic aspects of ischemic stroke. However, since the introduction of cerebral perfusion and diffusion imaging more than a decade ago, technological advances with clinical applicability have been somewhat stagnant [7].
Today, medicine is immersed in a digital world. Specialties such as radiology are growing in demand. In recent years, with the advent of artificial intelligence, applications such as radiomics have emerged to harness this technological revolution and improve diagnostic techniques in the search for personalized clinical management of patients. Since the coining of the term radiomics in 2012 [8], articles on this topic have become increasingly common.
Radiomics is a radiological discipline based on the idea that images are more than just pictures and that there is a lot of data that is invisible to the human eye, but which we can quantify and analyze by looking for data patterns associated with certain pathologies. Initially, radiomics was mainly focused on the field of oncology [9]. All cancer patients have at least one staging CT scan, so the most commonly used imaging technique for feature extraction in these studies was the CT [10]. The aims of these studies have been diverse, ranging from identifying prognostic factors or estimating overall survival to predicting response to treatment. The use of radiomics for gene determination (also called radiogenomics) has also been developed in pathologies such as brain tumours [11], lung cancer [12] and abdominal tumours [13]. In recent years, the use of radiomics has even been extended to other imaging modalities such as MRI, positron emission tomography (PET) based on both CT and MRI, and ultrasound.
In the field of neuroradiology, radiomics features obtained from magnetic resonance imaging (MRI) were used most frequently in the articles. In these articles, radiomics also focused mainly on oncological patients. The objectives ranged from differentiation of high-grade gliomas from brain metastases, prediction of EGFR mutations, or prognostic prediction, to determination of overall survival. In recent years, the use of radiomics has extended to brain pathologies other than tumours. There are articles on the use of radiomic features for prognostic prediction in acute ischemic stroke (AIS) [14], for predicting degenerative diseases [15], or even for predicting aneurysm rupture [16].
The aim of this review is to summarize published articles on the performance of radiomics in AIS patients. In addition, we aim to determine whether radiomics can provide valuable information for the clinical management of AIS patients. We are at a key moment in the evolution of radiology, as we have seen with other technological advances such as diffusion MRI, helical CT or, more recently, dual-energy CT. We need to be at the forefront of this technological evolution so that our patients can benefit.
2. Radiomics
2.1. Radiomics Workflow
The radiomics workflow consists of five consecutive steps (Figure 1):

Figure 1.
Radiomics workflow.
- Obtaining images: Radiomics can be applied to all types of medical imaging. There are published articles on the performance of radiomics in ultrasound, Non-Contrast Enhancement-CT (NCECT), Computed Tomography Angiography (CTA), MRI and PET. In this step, there can be a great deal of heterogeneity when using images acquired in different hospitals or even on different machines within the same hospital. Therefore, the acquired images are subjected to a standardization process in which we try to correct this source of heterogeneity [8].
- Pre-processing: The quality of the images can be improved by using the pre-processing tools. In this step, some image filters are used to reduce noise. The aim is to increase the predictive power of the classifiers [17].
- Segmentation: In this step, the region of interest is selected in the radiological image. The segmentation of this region can be performed in three ways: manual, semi-automatic and automatic. Manual segmentation is the gold standard and the most commonly used method in the studies reviewed in this article. The main advantage of this model is the intervention of an expert radiologist in its performance. The main disadvantage of this model is the time required to manually segment the entire region of interest [18]. Automatic segmentation is based on automatic detection of the region of interest without human intervention. Finally, semi-automatic segmentation is performed under the supervision of an expert radiologist who can edit an initial automatic pre-segmentation. The advantage of this method is the speed of the segmentation and the fact that the human component remains [19]. With today’s increasingly sophisticated segmentation software, semi-automatic 3D segmentation of an area of interest can be performed quickly and comfortably for the radiologist.
- Feature extraction and classification: There is a wealth of numerical data that can be extracted from medical images, known as radiomic features. There are several classes of radiomic features: Histogram features (grey level mean, maximum, minimum, variance and percentiles), texture features (absolute gradient, grey level co-occurrence matrix—GLCM—, grey level run length matrix—GLRLM—, grey level size zone matrix—GLSZM—, grey level distance zone matrix—GLDZM—, Neighborhood Grey Level Difference Matrix—NGTDM—, and Neighborhood Grey Level Dependence Matrix—NGLDM—), model-based features, transform-based features (Fourier, Garbor and Wavelet) and shape-based features (geometric properties of ROIs) [20]. These numerical data are classified by automatic classifiers. These classifiers are capable of recognizing different groups of patterns depending on the objective we set for them. In this way, the classifiers learn the data patterns with a training cohort. Then, with a test cohort, these classifiers are able to classify the data we provide into the pattern that most closely resembles it. In addition to numerical data, automatic classifiers can be used to input clinical data to search for a combined model.
- Data analysis.
2.2. Radiomics in AIS
Articles analyzing the performance of a radiomic model in neurological pathology have become increasingly frequent. The most frequently published articles are about neurological tumour pathology. However, in recent years there has been an increase in the publication of articles about non-tumour neurological pathology.
Regarding the application of radiomics in acute cerebral ischemic pathology, there has been an exponential increase in the publication of articles since 2018 (Figure 2). The objectives of these articles on radiomics in acute ischemic stroke are the prognostic prediction of AIS patients, the detection of early ischemic stroke, prediction of the outcome of revascularization treatment, prediction of complications, prediction of the etiology of AIS, prediction of the time since stroke and differentiation between hemorrhage or contrast extravasation in patients undergoing mechanical thrombectomy (Figure 3) (Table 1).
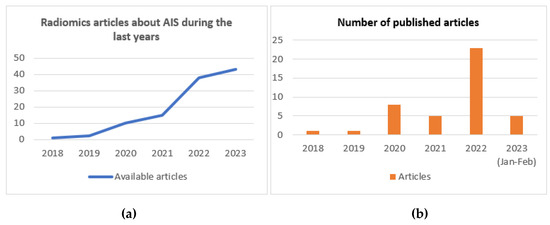
Figure 2.
(a) Radiomics articles about AIS patients since 2018. (b) Radiomics articles about AIS published per year.
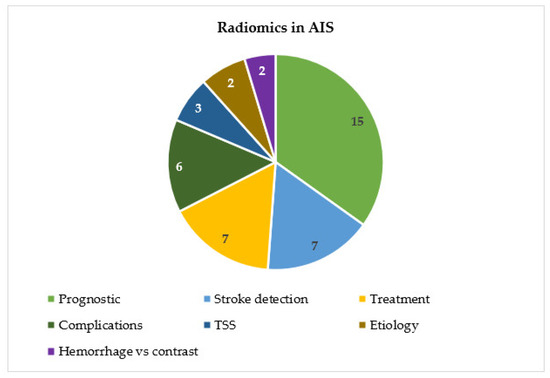
Figure 3.
Distribution of articles by topic.
2.3. Prognostic Prediction
The use of radiomics for the prognostic prediction of AIS patients is the most commonly published. Avery et al. in their study of 829 patients used radiomic features (RFs) obtained from CTA images of patients with anterior circulation Large Vessel Occlusion (LVO). They used masks of the middle cerebral artery (MCA) territory to extract the RF. They used four different input sets: radiomics (only RFs of MCA territory), radiomics + treatment (RF, mTICI score and intravenous thrombolysis), clinical + treatment (clinical variables and treatment) and combined (RF, treatment and clinical variables). The clinical variables included were sex, age and admission NIHSS. The best results were very similar between the clinical + treatment model and the combined model. The area under the curve (AUC) for long-term outcome prediction was 0.81 and 0.82 for the clinical + treatment and combined models, respectively. The AUC for predicting discharge outcome was 0.82 and 0.81 for the clinical + treatment and combined models, respectively. In the internal independent and external test performances, the best results of the different inputs were very similar. There was no significant difference between the best results of the radiomics only model, the radiomics + treatment model, the clinical + treatment model and the combined model. The authors concluded that RFs could help with patients where the collection of clinical information is limited [21].
Other studies of some prognostic predictions have found similar results [22,23,24,25,26,27,28,29,30,31,32,33,34,35]. In these studies, we can see that the performance of radiomics based on different types of images (MRI, CT and CTA) gives good results in predicting prognostic factors such as the mRS scale after AIS or the presence of disability after AIS. Many of these studies compare prediction models based on clinical features, radiomic features and finally evaluate the performance of a combined model by combining clinical and radiomic features. In general, the combined model is the one that performs best.
2.4. Detection of Ischemic Stroke
In the detection of ischemic stroke, articles focus on the early detection of ischemic lesions and the prediction of recurrence. The group of Guan Y et al. showed in their study with 56 patients and 1301 RFs that there is a correlation between RFs and AIS detection (best Acc of 0.7748) [36]. Guo Y et al. also investigated the detection of ischemic stroke (IS) and the prediction of NIHSS and other outcomes using RFs obtained from DSC-PWI in 88 patients. The best AUC they obtained was 0.925 for stroke detection. For the prediction of NIHSS and other prognostic predictions, they obtained an AUC of 0.853 and 0.828, respectively [37]. Zhang et al. also tried to predict early brainstem infarction using an NCECT-based radiomics model. They obtained an AUC of 0.99 and 0.91 in the internal and external validation cohorts, respectively. They concluded that NCECT-based radiomics can detect early brainstem infarction and may be very useful in clinical practice [38].
On the other hand, Zhang R et al. used MRI-based radiomics to predict the ischemic penumbra in their study of 241 patients. They extracted 896 radiomic features from the DWI high signal area using manual segmentation. The AUC obtained was 0.92, and sensitivity, specificity and accuracy were 0.93, 0.75 and 0.82, respectively, in the training cohort. In the validation cohort the AUC, sensitivity, specificity and accuracy were 0.90, 0.88, 0.74 and 0.80, respectively. They concluded that a radiomics model based on MRI images can identify the ischemic penumbra in AIS patients [39].
In ischemic stroke recurrence prediction, Su et al. developed a combined model with some clinical factors (age, sex, number of chronic ischemic lesions, routine laboratory tests, carotid ultrasound, cardiovascular risk factors and medical treatment) and CT-based radiomics to predict future ischemic stroke in patients with silent lacunar infarction. They obtained a C-index of 0.786 and 0.714 in the training and validation cohorts, respectively. They concluded that radiomic features can provide important information for predicting future ischemic stroke in patients with silent lacunar infarction [40]. Tang et al. also developed a radiomics signature based on MRI features of patients with symptomatic intracranial atherosclerotic stenosis (SICAS) to predict stroke recurrence. They obtained an AUC with the combined model of 0.899 and 0.803 in the training and validation cohorts, respectively. They concluded that radiomic features are useful for predicting stroke recurrence in patients with SICAS [41]. Wang et al. also predicted 1-year ischemic stroke recurrence in 1003 AIS patients based on MRI images. They obtained similar results [42].
Many patients without vessel occlusion on CTA images or with inconclusive results on perfusion images require MRI imaging to determine the presence of ischemic lesions. In many cases, MRI images are not available in the first hours of management of these patients. In these situations, radiomics could provide valuable information for the early management of these patients.
2.5. Treatment Predictions
Regarding radiomic models predicting the outcome of reperfusion treatments, most studies focus on predicting the first-pass effect or the TICI scale after thrombectomy. Only one study focuses on predicting recanalization after alteplase treatment.
To predict first-pass recanalization, Hofmeister et al. performed clot segmentation in their study of 156 patients. They used RFs to identify patients with first-pass recanalization by aspiration. They also predicted the total number of passes required to achieve good recanalization with a mechanical thrombectomy device (MTB). They achieved an AUC of 0.88 for predicting patients with first attempt recanalization. They concluded that clot-based radiomics can predict an MTB strategy in patients with AIS [43]. Sarioglu et al. attempted to predict first attempt recanalization in AIS patients using clot-based radiomics features obtained from NCECT. They achieved an accuracy of 0.83 using a combined model with radiomic features and clinical data (ASPECTS scale and gender). They also concluded that clot-based radiomic features can help to estimate successful recanalization in AIS patients [44]. Patel et al. also developed a NCECT/CTA-based radiomic model to predict the first-pass effect in AIS patients with similar results (AUC of 0.832) [45].
In recanalization prediction, Zhang et al. attempted to predict successful recanalization (TICI ≥ 2c) using MRI-based radiomics in 141 AIS patients. They extracted 321 radiomic features from the ischemic lesion and performed a manual segmentation. They obtained an AUC of 0.7442, a sensitivity of 0.7556 and a specificity of 0.7675. They concluded that MRI-based radiomics may be useful in predicting patient response to thrombectomy [46]. Xiong et al. also attempted to predict successful recanalization (TICI ≥ 2b) in AIS patients undergoing stent retrieval. They improved the results of Zhang et al. with an AUC of 0.860 and 0.849 in the training and validation cohorts, respectively, with the combined model. They also conclude that the radiomics model can successfully predict recanalization after stent retrieval [47]. Finally, Van Voorst et al. developed a clot-based radiomics model using the CT images of 699 patients to predict TICI ≥ 2b, first attempt reperfusion, reperfusion within three attempts and mRS scale ≤ 2. They obtained similar results with the clinical, radiomics and morphological features models [48].
In terms of predicting recanalization after treatment with alteplase, Qiu et al. obtained an AUC of 0.85. They concluded that clot-based radiomics was more predictive of recanalization with alteplase than other classic thrombus imaging features (length, volume and permeability) [49].
As we have seen in this section, the performance of radiomic models in predicting the outcome of thrombectomy in patients with AIS is very good. In this situation, radiomics can provide valuable information for the immediate management of these patients, since knowing in advance the risk of achieving inadequate reperfusion with a specific thrombectomy technique may lead the neuroradiologist to change the recanalization strategy in the event of an unsuccessful first pass.
2.6. Prediction of Complications after AIS
There are some studies that have used RFs to predict some complications in patients with AIS. Fu B et al. obtained an AUC of 0.96 in predicting malignant cerebral edema (MCE) using a combined model with RFs from NECT of patients with AIS [50]. Jiang et al. also predicted edema after acute ischemic stroke using MRI-based radiomics in 389 patients, with similar results [51]. Wen et al. developed a radiomic model to predict malignant acute middle cerebral artery infarction (mMCAi) based on CT radiomic features (NCECT and CTA). They obtained an AUC of 0.917 and 0.913 in the training and validation cohorts, respectively. They concluded that radiomic features could be an instrumental tool to predict the risk of mMCAi [52].
On the other hand, Meng et al. attempted to predict hemorrhage transformation in AIS patients based on MRI radiomics features. They extracted 5400 radiomic features from 71 AIS patients. They performed manual segmentation. They obtained an AUC of 911 and an accuracy of 0.894 in predicting hemorrhage transformation with a combined model. They concluded that a combined model with MRI-based radiomics can provide important information to physicians in the diagnosis of AIS patients [53]. Xie et al. also developed a CT-based radiomics model to predict hemorrhagic transformation in AIS patients and achieved similar results (AUC of 0.845–0.750) [54].
Finally, Liu et al. developed a radiomics model based on NCECT to predict hemorrhage expansion in patients with thrombolysis/thrombectomy related hemorrhagic transformation. They concluded that CT-based radiomics is valuable in predicting hemorrhage extension [55].
2.7. Etiology Prediction
In the etiology prediction of AIS using radiomics models, Chen Y et al. tried to differentiate between cardioembolic and atherosclerotic etiology in patients with acute ischemic stroke. They included 82 patients, and they obtained RFs from CTA images. They performed a manual segmentation of the embolic region and extracted 116 RFs. In their results, the best AUC was 0.9018 and the best Acc was 0.8929. They concluded that radiomics can effectively predict the subtype of ischemic stroke and help doctors to correctly manage these patients [56]. Jiang J et al. also studied a clot-based model in 403 AIS patients to identify cardioembolic (CE) stroke before MTB. They achieved an AUC of 0.838 for predicting CE stroke. They concluded that clot-based RFs can reliably predict CE strokes [57].
The current determination of the etiology of AIS is based on a number of clinical, analytical and medical imaging criteria. The management of these patients depends on the etiology of AIS. However, in many cases the etiology determination may be delayed or even undetermined. In this sense, radiomics can provide valuable information to support the diagnosis of patients with cardioembolic or atherothrombotic AIS and thus optimize the management of these patients.
2.8. Time since Stroke Prediction
Chen Y et al. attempted to estimate the time since stroke onset (TSS). They manually outlined the clot on CTA images of 221 patients and obtained 944 RFs. They divided the patients into two different groups: <4.5 h and >4.5 h since stroke onset. They analyzed the performance of the radiomics model and the combined model (radiomics + clinical data). The AUC was 0.803 for the combined model and 0.813–0.803 for the combined model (training and test cohorts). The clinical data used were age, diabetes and atrial fibrillation. They concluded that there was no significant difference between the radiomics and combined models. They also concluded that radiomics based on RFs obtained from CTA images can estimate TSS in patients with AIS [58]. Yao et al. also developed a CT-based radiomic model to classify the onset time of basal ganglia infarcts. They classified the patients into two groups (>4.5 h and <4.5 h). In the validation cohort the results were an AUC of 0.974, sensitivity of 0.951 and specificity of 0.961 [59]. Wen et al. also developed a CT-based radiomics model to predict infarct onset time in patients with MCAO. They obtained similar results with the combined model (AUC of 0.808–0.833) [60]. Finally, Zhang et al. also developed an MRI-based radiomics model to predict TSS with similar results [61].
Determining the time since stroke onset may play an important role in strokes that occur during sleep. In this sense, knowing whether or not a stroke has occurred in time to receive recanalization therapy with alteplase is critical to the management of these patients.
2.9. Differentiation Hemorrhage from Iodinated Contrast Extravasation after Thrombectomy
Chen X et al. in their very interesting study of 101 patients, tried to differentiate intracranial contrast extravasation from intraparenchymal hemorrhage (IPH) after mechanical thrombectomy (MT). They used RFs from non-enhanced CT (NECT) images after MT. They used manual segmentation and extracted 1316 RFs from 166 intraparenchymal areas of hyperattenuation. They obtained very interesting results, with an AUC between 0.848 and 0.826 (training and validation cohorts). The accuracy (ACC), sensitivity (S), specificity (Spec), negative predictive value (NPV) and positive predictive value (PPV) were 0.776, 0.767, 0.789, 0.682 and 0.852, respectively. They concluded that radiomics can effectively differentiate IPH from intracranial contrast extravasation after MT [62]. Yuan Ma et al. also used a CT-based radiomics model to predict bleeding from iodinated extravasation after thrombectomy in AIS patients. They obtained an AUC of 0.972 and 0.926 in the training and validation cohorts, respectively [63].
Differentiating between hemorrhage and contrast extravasation is easy if dual-energy CT is available. However, this technology may not always be available when we need it. In this sense, radiomics could act as an aid to make this distinction in situations where dual-energy CT is not available.

Table 1.
Articles published about radiomics in AIS.
Table 1.
Articles published about radiomics in AIS.
| Author | Year | Type | N | Target | Territory | RF | Type of Imaging | Results | Conclusions |
|---|---|---|---|---|---|---|---|---|---|
| Avery et al. [21] | 2022 | R | 829 | Prognostic prediction | ALVO | 1116 | CTA | AUC between 0.81 and 0.82 | RFs could help in patients with limited clinical information. |
| Cui H et al. [22] | 2018 | R | 70 | Prognostic prediction | NA | 251 | MRI | AUC of 0.821–0983 | The classification model improves when RFs are included. |
| Wang et al. [23] | 2021 | R | 598 | Prognostic prediction | Infarct | 402 | MRI | AUC of 0.80 | A combined nomogram based on MRI has a good performance in prognosis prediction. |
| Gerbasi A et al. [24] | 2022 | R | 164 | Prognostic prediction | ALVO | 107 | MRI | AUC of 0.85 | A combined model can help in the long-term functional outcome prediction. |
| Guo Y et al. [25] | 2022 | R | 56 | Prognostic prediction | NA | 65800 | MRI | AUC of 0.908 and 0.884 Acc of 0.821 and 0.864 | RFs can help to predict functional outcomes in ischemic stroke patients. |
| Guo Y et al. [26] | 2022 | R | 78 | Prognostic prediction | WB | 1674 | MRI | AUC of 0.971 | RFs can predict functional recovery after three months. |
| Jiang L et al. [27] | 2022 | R | 1716 | Prognostic prediction | NA | NA | MRI | AUC of 0.862 | A combined model can predict outcomes of AIS patients. |
| Li et al. [28] | 2023 | R | 102 | Prognostic prediction | MCA | 1389 | MRI | AUC of 0.88 and 0.79 | A combined model can predict a poor prognosis in AIS patients. |
| Ramos et al. [29] | 2022 | R | 3279 | Prognostic prediction | WB | 1260 | CTA | AUC of 0.61 | The combined model obtained better results than the clinical model. |
| Li et al. [30] | 2022 | R | 260 | Prognostic prediction | Infarct | 1936 | MRI | AUC of 0.945 and 0.920 | MRI-based radiomics have high predictive efficiency for the prognostic prediction of AIS patients after thrombectomy. |
| Quan et al. [31] | 2021 | R | 190 | Prognostic prediction | Hyperintensities | 753 | MRI | AUC of 0.926–0.864 | MRI-based radiomics can predict unfavorable outcomes (mRS ≥ 2). |
| Tang et al. [32] | 2020 | R | 168 | Prognostic prediction | Penumbra | 456 | CT/MRI | AUC of 0.886 | Radiomics nomogram adds more value to the current clinical decision-making process. |
| Tolhuisen et al. [33] | 2022 | R | 206 | Prognostic prediction | Infarct | 100 | MRI | AUC of 0.88–0.81 | MRI-based radiomics can provide important information to the functional outcome prediction in AIS patients. |
| Yu et al. [34] | 2022 | R | 148 | Prognostic prediction | Infarct | 4744 | MRI | AUC of 0.902 Acc of 0.831 Sens of 0.739 Spec of 0.902 | A radiomics model based on MRI imaging can predict clinical outcomes in AIS patients. |
| Zhou et al. [35] | 2022 | R | 522 | Prognostic prediction | Ischemic | 1310 | MRI | AUC of 0.868–0.890 | A combined model outperformed individual clinical or radiomics models in predicting AIS outcomes. |
| Guan Y et al. [36] | 2020 | R | 56 | Identification of IS | NA | 1301 | CT/MNR | Best Acc of 0.7748 | There are RFs correlated with acute cerebral infarction. |
| Guo Y et al. [37] | 2022 | R | 88 | Identification of IS | WB | 1674 | MRI | AUC of 0.925 (IS), 0.853 (NIHSS), and 0.828 (outcome prediction) | RF is a potential clinical tool which could help in the diagnosis and outcome prediction before treatment. |
| Zhang et al. [38] | 2023 | R | 355 | Identification of IS | Brainstem | 1781 | CT | AUC of 0.99 and 0.91 | CT-based radiomics can detect early brainstem infarction. |
| Zhang R et al. [39] | 2020 | R | 241 | Identification of ischemic penumbra | DWI hypodensities | 896 | MRI | AUC of 0.92 and 0.90 Sens of 0.93 and 0.88 Spec of 0.75 and 0.74 Acc of 0.82 and 0.80 | MRI-based radiomics can identify ischemic penumbra in AIS patients. |
| Su et al. [40] | 2020 | R | 148 | Stroke prediction | Lacunar lesions | 1209 | CT | C-Index of 0.7864–0.7140 | CT-based radiomics can provide information for the prediction of future ischemic strokes in patients with silent lacunar infarction. |
| Tang et al. [41] | 2022 | R | 156 | Stroke recurrence | Plaque | 402 | MRI | AUC of 0.899–0.803 | MRI-based radiomics provide important information for the prediction of stroke recurrence in SICAS patients. |
| Wang et al. [42] | 2022 | R | 1003 | Stroke recurrence | Infarct | 513 | MRI | AUC of 0.847 | MRI-based radiomics could help to predict 1-year AIS recurrence. |
| Hofmeister et al. [43] | 2020 | R | 156 | Predict MTB strategy | Clot | 9 | CT | AUC of 0.88 | Clot-based RFs can help with the MTB strategy. |
| Sarioglu et al. [44] | 2022 | R | 52 | Predict fist pass effect | Clot | 12 | CT | Acc of 0.83 | Clot-based RFs can estimate recanalization successfully. |
| Patel et al. [45] | 2023 | R | 293 | Predict first pass effect | Clot | 227 | CT/CTA | AUC of 0.832–0.787 Acc of 0.760–0.787 | Clot-based radiomics are potential candidate markers for first pass effect prediction |
| Zhang et al. [46] | 2021 | R | 141 | TICI scale prediction | Infarct | 321 | MRI | AUC of 0.7442 | MRI-based radiomics can provide important information about the patients’ response to thrombectomy. |
| Xiong et al. [47] | 2023 | R | 256 | TICI scale prediction | Clot | 1130 | CT | AUC of 0.860–0.849 | CT-based radiomics can predict the successfully recanalization in AIS patients after stent retrieve therapy |
| Van Voorst et al. [48] | 2022 | R | 699 | Recanalization | Clot | - | CT | - | Clot-based radiomics are independently associated with reperfusion, but the results of the clinical and radiomics model was similar. |
| Qui et al. [49] | 2019 | R | 67 | Recanalization after alteplase | Clot | 326 | CT/CTA | AUC of 0.85 | Clot-based radiomics are more predictive of recanalization with alteplase than other classical clot features. |
| Fu B et al. [50] | 2020 | R | 116 | Predict malignant cerebral edema | MCA | 13 | NECT | AUC of 0.96 | Radiomics could help to predict MCE using NECT. |
| Jiang et al. [51] | 2022 | R | 389 | Predict malignant cerebral edema | Stroke and CSF | 1316 | MRI | AUC 0.83–0.86 Acc 0.85–0.81 | MRI radiomic features can provide information for predicting cerebral edema in AIS patients |
| Wen et al. [52] | 2020 | R | 126 | Predict mMCAi | MCA territory | 396 | CT/CTA | AUC of 0.917–0.913 | CT-based radiomics can be a tool to predict the risk of mMCAi. |
| Meng et al. [53] | 2022 | R | 71 | Predict hemorrhage transformation | Infarct | 5400 | MRI | AUC of 0.911 Acc of 0.894 | A combined model based on MRI radiomic features can predict the hemorrhage transformation in AIS patients. |
| Xie et al. [54] | 2022 | R | 118 | Predict hemorrhage transformation | Infarct | 851 | CT | AUC of 0.845–0.750 | CT-based radiomics could help in the prediction of hemorrhage transformation in AIS patients. |
| Liu et al. [55] | 2021 | R | 104 | Predict hemorrhage expansion | Hemorrhage | 1691 | CT | AUC of 0.91–0.87 Sens of 0.83–0.60 Spec 0.89–0.85 | CT-based radiomics can predict hemorrhage expansion. |
| Chen Y et al. [56] | 2022 | R | 82 | Etiology prediction | NA | 116 | CTA | AUC of 0.9018 Acc of 0.8929 | Radiomics could effectively predict the subtype of ischemic stroke. |
| Jiang J et al. [57] | 2023 | R | 403 | Etiology prediction | Clot | NA | CT | AUC of 0.838 | Clot-based RFs can identify the CE strokes. |
| Cheng Y et al. [58] | 2022 | R | 221 | Time since stroke | Clot | 944 | CTA | AUC of 0.803 AUC of 0.813–0.803 | Radiomics can estimate the TSS in patients with AIS. |
| Yao et al. [59] | 2020 | R | 316 | Time since stroke | Infarct | 295 | CT | AUC of 0.982–0.974 Sens of 0.929–0.951 Spec of 0.959–0.961 | Radiomics is useful in the determination of TSS in basal ganglia infarction. |
| Wen et al. [60] | 2021 | R | 123 | Time since stroke | ASPECTS | 396 | CT/CTA | AUC of 0.808–0.833 | CT-based radiomics can discriminate the TSS in patients with MCAO in the M1 segment. |
| Zhang et al. [61] | 2022 | R | 84 | Time since stroke | - | 4312 | MRI | AUC of 0.754 Acc of 0.788 | MRI-based radiomics could aid in the decision-making for thrombolysis in patients with unknown stroke onset. |
| Chen X et al. [62] | 2022 | R | 101 | Differentiate IPH vs. contrast | NA | 1316 | NECT | AUC of 0.848 and 0.826; Acc of 0.776, S of 0.767, and Spec of 0.789 | RFs can differentiate IPH from contrast extravasation after MT. |
| Ma Y et al. [63] | 2022 | R | 100 | Hemorrhage vs. Iodinated contrast extravasation | Hyperdense area | 1316 | CT | AUC of 0.972 and 0.926 in training and validation cohorts | Combined nomogram based on CT-radiomic features can differentiate between hemorrhage and iodine contrast extravasation. |
3. Discussion
The year 2022 has seen exponential growth in radiomics, which could have been greater or even earlier had it not been for the appearance of COVID-19. Radiomics can be applied to all types of imaging, as we have seen in this review, and it does not require alternative diagnostic procedures to the usual ones for the management of patients with acute ischemic stroke. Of the studies reviewed, most are based on MRI to obtain radiomic features, but we have also seen that CT-based radiomics gives very good results. This, together with the fact that all patients with AIS undergo imaging, means that we have a virtually inexhaustible source of patients, which is likely to increase in the coming years.
The application of radiomics to the management of patients with AIS could represent a major diagnostic advance. In the results we have observed in this review, we have seen that radiomic models perform excellently in a wide variety of clinical situations in patients with AIS. For example, radiomics could be able to predict clinical status at 90 days, identify ischemic lesions not yet detectable by the neuroradiologist’s eye or by perfusion or diffusion techniques, provide a probability of new AIS, predict the outcome of reperfusion techniques, predict complications of AIS, determine the etiology of AIS, determine the time course of patients with AIS of unknown onset, or differentiate hemorrhagic complications from iodinated contrast extravasation. We find the application of radiomics in predicting the outcome of revascularization, predicting complications after AIS, and predicting etiology of AIS particularly interesting.
Radiomics offers a somewhat astonishing range of possibilities, considering the current paradigms of conventional imaging. In particular, the fact that the radiomic analysis of the thrombus may provide information about its etiological origin makes perfect sense, as different clot compositions may imply specific characteristics. Nonetheless, in the author’s opinion, the results of other studies should be interpreted with more caution.
Currently, the determination of the etiology of stroke is a key point in the management of the AIS patient, as the treatment of such a patient is based on the etiology of the AIS. This lies on several laboratory, clinical and imaging parameters, which frequently imply a several days delay in initiating the specific prevention treatment and sometimes cannot provide a specific diagnosis. Therefore, if radiomics can also detect different data patterns depending on the etiology of the thrombus, we could be looking at a technique that could significantly change the clinical management of the AIS patient.
In this respect, it stands to reason that radiomics can offer prognostic information. This could have two possible explanations. On the one hand, if these results were not true, the retrospective design of the studies may have a big influence but can therefore be proven with further prospective studies to rule out any variables that were not analyzed and could act as a bias (such as the different thrombectomy techniques used, the material used, the skill of the neuroradiologist, etc.). This classic drawback of radiomic studies is due to the lack of prospective studies [64], which could be explained by the recent emergence of these radiological techniques in medical research. On the other hand, if the results are surprisingly true, this could be derived by the intrinsic characteristics of the thrombus, by its frailty and susceptibility to fragmentation or its resistance to thrombectomy devices. These characteristics are the ones that seem to be relevant in the, in many ways, opaque radiomic analysis.
In terms of predicting the outcome of revascularization, radiomics can be useful in predicting the number of thrombectomy attempts required to achieve adequate reperfusion. It can also provide valuable information on the final outcome of thrombectomy by predicting the TICI scale.
With regard to the radiomics models reviewed, the studies that used a combined radiomics and clinical model showed better results than the isolated radiomics or clinical models. For this reason, we think that the first steps of radiomics in the routine clinical management of patients with AIS will be in the form of support for clinical criteria currently used for certain diagnoses.
Segmentation is a fundamental point in radiomics, and manual segmentation is still considered the gold standard; however, the major drawback of this method is the waste of time for the neuroradiologist. Segmentation software is now available that allows semi-automated and 3D segmentation to be performed quickly and easily, which we believe will replace the manual method as the gold standard in the coming years [65]. In terms of feature extraction, we found that this varied from study to study. Heterogeneity in imaging acquisition and feature extraction is a classic problem in radiomic studies and one that initiatives such as the Radiomics Quality Score (RQS) are trying to standardize [66].
Finally, in the studies included in this article, we can see that radiomics provides valuable information that can complement the information provided by conventional imaging studies. The future of radiomics is promising, we may be at a tipping point that will change the paradigm of diagnostic imaging, but we believe that prospective studies are needed to demonstrate that radiomic analysis of an image adds specific diagnostic value.
4. Conclusions
Radiomic models can provide valuable information for the management of patients with AIS. The results obtained with radiomic models are very good and encouraging. The wide variety of clinical situations in which radiomics provides valuable information in patients with AIS suggests that we may be looking at a model that will change the paradigm of clinical management of patients with AIS. Further prospective studies are needed to investigate the utility of radiomics in the mentioned fields of knowledge and others that may appear in the future.
Author Contributions
Conceptualization, J.P.-Á. and A.M.M.; methodology, J.M.F., M.S.L. and M.S.-B.; software, J.P.-Á. and A.M.M.; validation, M.B.U. and F.V.H.; formal analysis, A.M.M., M.B.U. and F.V.H.; investigation, J.P.-Á., A.M.M., J.M.F. and R.I.R.; resources, M.S.L. and A.M.M.P.; data curation, J.P.-Á. and J.M.F.; writing—original draft preparation, J.P.-Á. and M.S.L.; writing—review and editing, A.M.M., J.M.F., M.B.U., F.V.H., A.M.M.P., M.R.-Y. and A.B.V.; visualization, M.B.U., F.V.H., J.M.P. and R.I.R.; supervision, A.M.M. and J.M.F.; project administration, A.M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Santiago-Lugo (protocol code 2023/299 approved on 21 June 2023).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
AI: Artificial Intelligence; AIS: Acute Ischemic Stroke; AUC: Area Under Curve; ALVO: Anterior Large Vessel Occlusion; C-Index: Concordance Index; CE: Cardioembolic Stroke; CI: confidence interval; CT: Computed Tomography; CSF: Cerebrospinal Fluid; CTA: Computed Tomography Angiography; DWI: Diffusion Weighted Imaging; DSC-PWI: Dynamic susceptibility contrast perfusion-weighted imaging; Acc: Accuracy; EGFR: Epidermal Grow Factor Receptor; GLCM: grey level co-occurrence matrix; GLDZM: grey level distance zone matrix; GLRLM: grey level run length matrix; GLSZM: grey level size zone matrix; IS: Ischemic Stroke; ICA: Internal Carotid Artery; IPH: Intraparenchymal Hemorrhage; LVO: Large Vessel Occlusion; MCA: middle cerebral artery; MCAO: Middle Cerebral Artery Occlusion; MCE: Malignant Cerebral Edema; mMCAi: Malignant Acute Middle Cerebral Artery Infarction; MRI: Magnetic Resonance Imaging; mRS: modified Rankin Scale; MTB or MT: Mechanical Thrombectomy; NCECT: Non Contrast Enhancing Computed Tomography; NGLDM: Neighborhood Grey Level Dependence Matrix; NGTDM: Neighborhood Grey Level Difference Matrix; NIHSS: National Institutes of Health Stroke Scale; NPV: Negative Predictive Value; OR: Odds Ratio; PET: Positron Emission Tomography; PPV: Positive Predictive Value; RF: radiomic features; ROC: Receiver Operating Characteristic; ROI: Region Of Interest; S: Sensitivity; SICAS: Symptomatic Intracranial Atherosclerotic Stenosis; Spec: Specificity; SVM: Support Vector Machine; TICI: Thrombolysis In Cerebral Infarction; TSS: Time Since Stroke; US: Ultrasound; WB: Whole Brain; WHO: World Health Organization.
References
- Phipps, M.S.; Cronin, C.A. Management of acute ischemic stroke. BMJ 2020, 368, l6983. [Google Scholar] [CrossRef]
- World Health Organization. Global Health Estimates. Available online: https://www.who.int/data/global-health-estimates (accessed on 9 December 2020).
- Saini, V.; Guada, L.; Yavagal, D.R. Global Epidemiology of Stroke and Access to Acute Ischemic Stroke Interventions. Neurology 2021, 97 (Suppl. S2), S6–S16. [Google Scholar] [CrossRef]
- Broderick, J.P.; Palesch, Y.Y.; Demchuk, A.M.; Yeatts, S.D.; Khatri, P.; Hill, M.D.; Jauch, E.C.; Jovin, T.G.; Yan, B.; Silver, F.L.; et al. Endovascular Therapy after Intravenous t-PA versus t-PA Alone for Stroke. N. Engl. J. Med. 2013, 368, 893–903, Erratum in N. Engl. J. Med. 2013, 368, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Kidwell, C.S.; Jahan, R.; Gornbein, J.; Alger, J.R.; Nenov, V.; Ajani, Z.; Feng, L.; Meyer, B.C.; Olson, S.; Schwamm, L.H.; et al. A Trial of Imaging Selection and Endovascular Treatment for Ischemic Stroke. N. Engl. J. Med. 2013, 368, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Fargen, K.M.; Singla, A.; Mocco, J. The New England Journal of Medicine Stroke Trials: What Do They Really Mean? Neurosurgery 2015, 62, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Wintermark, M.; Bogousslavsky, J. Imaging of acute ischemic brain injury: The return of computed tomography. Curr. Opin. Neurol. 2003, 16, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Lambin, P.; Rios-Velazquez, E.; Leijenaar, R.; Carvalho, S.; van Stiphout, R.G.P.M.; Granton, P.; Zegers, C.M.L.; Gillies, R.; Boellard, R.; Dekker, A.; et al. Radiomics: Extracting more information from medical images using advanced feature analysis. Eur. J. Cancer 2012, 48, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef]
- Porto-Álvarez, J.; Barnes, G.T.; Villanueva, A.; García-Figueiras, R.; Baleato-González, S.; Zapico, E.H.; Souto-Bayarri, M. Digital Medical X-ray Imaging, CAD in Lung Cancer and Radiomics in Colorectal Cancer: Past, Present and Future. Appl. Sci. 2023, 13, 2218. [Google Scholar] [CrossRef]
- Singh, G.; Manjila, S.; Sakla, N.; True, A.; Wardeh, A.H.; Beig, N.; Vaysberg, A.; Matthews, J.; Prasanna, P.; Spektor, V. Radiomics and radiogenomics in gliomas: A contemporary update. Br. J. Cancer 2021, 125, 641–657. [Google Scholar] [CrossRef]
- Anagnostopoulos, A.K.; Gaitanis, A.; Gkiozos, I.; Athanasiadis, E.I.; Chatziioannou, S.N.; Syrigos, K.N.; Thanos, D.; Chatziioannou, A.N.; Papanikolaou, N. Radiomics/Radiogenomics in Lung Cancer: Basic Principles and Initial Clinical Results. Cancers 2022, 14, 1657. [Google Scholar] [CrossRef] [PubMed]
- Porto-Álvarez, J.; Cernadas, E.; Martínez, R.A.; Fernández-Delgado, M.; Zapico, E.H.; González-Castro, V.; Baleato-González, S.; García-Figueiras, R.; Antúnez-López, J.R.; Souto-Bayarri, M. CT-Based Radiomics to Predict KRAS Mutation in CRC Patients Using a Machine Learning Algorithm: A Retrospective Study. Biomedicines 2023, 11, 2144. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Xia, T.; Zhang, M.; Xia, N.; Liu, J.; Yang, Y. Radiomics in Stroke Neuroimaging: Techniques, Applications, and Challenges. Aging Dis. 2021, 12, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Rai, C.S.; Jain, J. A Novel Method for Differential Prognosis of Brain Degenerative Diseases Using Radiomics-Based Textural Analysis and Ensemble Learning Classifiers. Comput. Math. Methods Med. 2021, 2021, 7965677. [Google Scholar] [CrossRef] [PubMed]
- Alwalid, O.; Long, X.; Xie, M.; Yang, J.; Cen, C.; Liu, H.; Han, P. CT Angiography-Based Radiomics for Classification of Intracranial Aneurysm Rupture. Front. Neurol. 2021, 12, 619864. [Google Scholar] [CrossRef] [PubMed]
- Demircioğlu, A. The effect of preprocessing filters on predictive performance in radiomics. Eur. Radiol. Exp. 2022, 6, 40. [Google Scholar] [CrossRef] [PubMed]
- Van Timmeren, J.E.; Cester, D.; Tanadini-Lang, S.; Alkadhi, H.; Baessler, B. Radiomics in medical imaging-”how-to” guide and critical reflection. Insights Imaging 2020, 11, 91. [Google Scholar] [CrossRef]
- Parmar, C.; Velazquez, E.R.; Leijenaar, R.; Jermoumi, M.; Carvalho, S.; Mak, R.H.; Mitra, S.; Shankar, B.U.; Kikinis, R.; Haibe-Kains, B.; et al. Robust Radiomics Feature Quantification Using Semiautomatic Volumetric Segmentation. PLoS ONE 2014, 9, e102107. [Google Scholar] [CrossRef]
- Mayerhoefer, M.E.; Materka, A.; Langs, G.; Häggström, I.; Szczypiński, P.; Gibbs, P.; Cook, G. Introduction to Radiomics. J. Nucl. Med. 2020, 61, 488–495. [Google Scholar] [CrossRef]
- Avery, E.W.; Behland, J.; Mak, A.; Haider, S.P.; Zeevi, T.; Sanelli, P.C.; Filippi, C.G.; Malhotra, A.; Matouk, C.C.; Griessenauer, C.J.; et al. CT angiographic radiomics signature for risk stratification in anterior large vessel occlusion stroke. NeuroImage Clin. 2022, 34, 103034. [Google Scholar] [CrossRef]
- Cui, H.; Wang, X.; Bian, Y.; Song, S.; Feng, D.D. Ischemic stroke clinical outcome prediction based on image signature selection from multimodality data. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2018, 2018, 722–725. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sun, Y.; Ge, Y.; Wu, P.-Y.; Lin, J.; Zhao, J.; Song, B. A Clinical-Radiomics Nomogram for Functional Outcome Predictions in Ischemic Stroke. Neurol. Ther. 2021, 10, 819–832. [Google Scholar] [CrossRef] [PubMed]
- Gerbasi, A.; Konduri, P.; Tolhuisen, M.; Cavalcante, F.; Rinkel, L.; Kappelhof, M.; Wolff, L.; Coutinho, J.M.; Emmer, B.J.; Costalat, V.; et al. Prognostic Value of Combined Radiomic Features from Follow-Up DWI and T2-FLAIR in Acute Ischemic Stroke. J. Cardiovasc. Dev. Dis. 2022, 9, 468. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yang, Y.; Cao, F.; Liu, Y.; Li, W.; Yang, C.; Feng, M.; Luo, Y.; Cheng, L.; Li, Q.; et al. Radiomics features of DSC-PWI in time dimension may provide a new chance to identify ischemic stroke. Front. Neurol. 2022, 13, 889090. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yang, Y.; Wang, M.; Luo, Y.; Guo, J.; Cao, F.; Lu, J.; Zeng, X.; Miao, X.; Zaman, A.; et al. The Combination of Whole-Brain Features and Local-Lesion Features in DSC-PWI May Improve Ischemic Stroke Outcome Prediction. Life 2022, 12, 1847. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Miao, Z.; Chen, H.; Geng, W.; Yong, W.; Chen, Y.-C.; Zhang, H.; Duan, S.; Yin, X.; Zhang, Z. Radiomics Analysis of Diffusion-Weighted Imaging and Long-Term Unfavorable Outcomes Risk for Acute Stroke. Stroke 2023, 54, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, M.; Chen, Z.; Lu, F.; Zhao, M.; Zhang, H.; Tong, D. Prognostic value of radiomics-based hyperdense middle cerebral artery sign for patients with acute ischemic stroke after thrombectomy strategy. Front. Neurol. 2023, 13, 1037204. [Google Scholar] [CrossRef] [PubMed]
- Ramos, L.A.; van Os, H.; Hilbert, A.; Olabarriaga, S.D.; van der Lugt, A.; Roos, Y.B.W.E.M.; van Zwam, W.H.; van Walderveen, M.A.A.; Ernst, M.; Zwinderman, A.H.; et al. Combination of Radiological and Clinical Baseline Data for Outcome Prediction of Patients with an Acute Ischemic Stroke. Front. Neurol. 2022, 13, 809343. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Hong, Z.; Wang, Y.; Lu, X. Combining machine learning with radiomics features in predicting outcomes after mechanical thrombectomy in patients with acute ischemic stroke. Comput. Methods Programs Biomed. 2022, 225, 107093. [Google Scholar] [CrossRef]
- Quan, G.; Ban, R.; Ren, J.-L.; Liu, Y.; Wang, W.; Dai, S.; Yuan, T. FLAIR and ADC Image-Based Radiomics Features as Predictive Biomarkers of Unfavorable Outcome in Patients with Acute Ischemic Stroke. Front. Neurosci. 2021, 15, 730879. [Google Scholar] [CrossRef]
- Tang, T.-Y.; Jiao, Y.; Cui, Y.; Zhao, D.-L.; Zhang, Y.; Wang, Z.; Meng, X.-P.; Yin, X.-D.; Yang, Y.-J.; Teng, G.-J.; et al. Penumbra-based radiomics signature as prognostic biomarkers for thrombolysis of acute ischemic stroke patients: A multicenter cohort study. J. Neurol. 2020, 267, 1454–1463. [Google Scholar] [CrossRef] [PubMed]
- Tolhuisen, M.L.; Hoving, J.W.; Koopman, M.S.; Kappelhof, M.; van Voorst, H.; Bruggeman, A.E.; Demchuck, A.M.; Dippel, D.W.J.; Emmer, B.J.; Bracard, S.; et al. Outcome Prediction Based on Automatically Extracted Infarct Core Image Features in Patients with Acute Ischemic Stroke. Diagnostics 2022, 12, 1786. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wang, Z.; Sun, Y.; Bo, W.; Duan, K.; Song, C.; Hu, Y.; Zhou, J.; Mu, Z.; Wu, N. Prognosis of ischemic stroke predicted by machine learning based on multi-modal MRI radiomics. Front. Psychiatry 2023, 13, 1105496. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wu, D.; Yan, S.; Xie, Y.; Zhang, S.; Lv, W.; Qin, Y.; Liu, Y.; Liu, C.; Lu, J.; et al. Feasibility of a Clinical-Radiomics Model to Predict the Outcomes of Acute Ischemic Stroke. Korean J. Radiol. 2022, 23, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Wang, P.; Wang, Q.; Li, P.; Zeng, J.; Qin, P.; Meng, Y. Separability of Acute Cerebral Infarction Lesions in CT Based Radiomics: Toward Artificial Intelligence-Assisted Diagnosis. BioMed Res. Int. 2020, 2020, 8864756. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yang, Y.; Cao, F.; Wang, M.; Luo, Y.; Guo, J.; Liu, Y.; Zeng, X.; Miu, X.; Zaman, A.; et al. A Focus on the Role of DSC-PWI Dynamic Radiomics Features in Diagnosis and Outcome Prediction of Ischemic Stroke. J. Clin. Med. 2022, 11, 5364. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, H.; Zhang, C.; Cao, A.; Lu, Q.; Wu, H.; Zhang, J.; Geng, D. A radiomics feature-based machine learning models to detect brainstem infarction (RMEBI) may enable early diagnosis in non-contrast enhancement CT. Eur. Radiol. 2023, 33, 1004–1014. [Google Scholar] [CrossRef]
- Zhang, R.; Zhu, L.; Zhu, Z.; Ge, Y.; Zhang, Z.; Wang, T. Apparent diffusion coefficient map based radiomics model in identifying the ischemic penumbra in acute ischemic stroke. Ann. Palliat. Med. 2020, 9, 2684–2692. [Google Scholar] [CrossRef]
- Su, J.-H.; Meng, L.-W.; Dong, D.; Zhuo, W.-Y.; Wang, J.-M.; Liu, L.-B.; Qin, Y.; Tian, Y.; Tian, J.; Li, Z.-H. Noninvasive model for predicting future ischemic strokes in patients with silent lacunar infarction using radiomics. BMC Med. Imaging 2020, 20, 77. [Google Scholar] [CrossRef]
- Tang, M.; Gao, J.; Ma, N.; Yan, X.; Zhang, X.; Hu, J.; Zhuo, Z.; Shi, X.; Li, L.; Lei, X.; et al. Radiomics Nomogram for Predicting Stroke Recurrence in Symptomatic Intracranial Atherosclerotic Stenosis. Front. Neurosci. 2022, 16, 851353. [Google Scholar] [CrossRef]
- Wang, H.; Sun, Y.; Zhu, J.; Zhuang, Y.; Song, B. Diffusion-weighted imaging-based radiomics for predicting 1-year ischemic stroke recurrence. Front. Neurol. 2022, 13, 1012896. [Google Scholar] [CrossRef] [PubMed]
- Hofmeister, J.; Bernava, G.; Rosi, A.; Vargas, M.I.; Carrera, E.; Montet, X.; Burgermeister, S.; Poletti, P.-A.; Platon, A.; Lovblad, K.-O.; et al. Clot-Based Radiomics Predict a Mechanical Thrombectomy Strategy for Successful Recanalization in Acute Ischemic Stroke. Stroke 2020, 51, 2488–2494. [Google Scholar] [CrossRef]
- Sarioglu, O.; Sarioglu, F.C.; E Capar, A.; Sokmez, D.F.; Mete, B.D.; Belet, U. Clot-based radiomics features predict first pass effect in acute ischemic stroke. Interv. Neuroradiol. 2022, 28, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Patel, T.R.; Santo, B.A.; Baig, A.A.; Waqas, M.; Monterio, A.; Levy, E.I.; Siddiqui, A.H.; Tutino, V.M. Histologically interpretable clot radiomic features predict treatment outcomes of mechanical thrombectomy for ischemic stroke. Neuroradiology 2023, 65, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Polson, J.; Nael, K.; Salamon, N.; Yoo, B.; Speier, W.; Arnold, C. A Machine Learning Approach to Predict Acute Ischemic Stroke Thrombectomy Reperfusion using Discriminative MR Image Features. In Proceedings of the 2021 IEEE EMBS International Conference on Biomedical and Health Informatics (BHI), Virtual, 27–30 July 2021. [Google Scholar] [CrossRef]
- Xiong, X.; Wang, J.; Ke, J.; Hong, R.; Jiang, S.; Ye, J.; Hu, C. Radiomics-based intracranial thrombus features on preoperative noncontrast CT predicts successful recanalization of mechanical thrombectomy in acute ischemic stroke. Quant. Imaging Med. Surg. 2023, 13, 682–694. [Google Scholar] [CrossRef]
- van Voorst, H.; A E Bruggeman, A.; Yang, W.; Andriessen, J.; Welberg, E.; Dutra, B.G.; Konduri, P.R.; Terreros, N.A.; Hoving, J.W.; Tolhuisen, M.L.; et al. Thrombus radiomics in patients with anterior circulation acute ischemic stroke undergoing endovascular treatment. J. Neurointerv. Surg. 2022, 15, e79–e85. [Google Scholar] [CrossRef]
- Qiu, W.; Kuang, H.; Nair, J.; Assis, Z.; Najm, M.; McDougall, C.; McDougall, B.; Chung, K.; Wilson, A.; Goyal, M.; et al. Radiomics-Based Intracranial Thrombus Features on CT and CTA Predict Recanalization with Intravenous Alteplase in Patients with Acute Ischemic Stroke. Am. J. Neuroradiol. 2019, 40, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.; Qi, S.; Tao, L.; Xu, H.; Kang, Y.; Yao, Y.; Yang, B.; Duan, Y.; Chen, H. Image Patch-Based Net Water Uptake and Radiomics Models Predict Malignant Cerebral Edema After Ischemic Stroke. Front. Neurol. 2020, 11, 609747. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, C.; Wang, S.; Ai, Z.; Shen, T.; Zhang, H.; Duan, S.; Yin, X.; Chen, Y.-C. MRI Radiomics Features From Infarction and Cerebrospinal Fluid for Prediction of Cerebral Edema after Acute Ischemic Stroke. Front. Aging Neurosci. 2022, 14, 782036. [Google Scholar] [CrossRef]
- Wen, X.; Li, Y.; He, X.; Xu, Y.; Shu, Z.; Hu, X.; Chen, J.; Jiang, H.; Gong, X. Prediction of Malignant Acute Middle Cerebral Artery Infarction via Computed Tomography Radiomics. Front. Neurosci. 2020, 14, 708. [Google Scholar] [CrossRef]
- Meng, Y.; Wang, H.; Wu, C.; Liu, X.; Qu, L.; Shi, Y. Prediction Model of Hemorrhage Transformation in Patient with Acute Ischemic Stroke Based on Multiparametric MRI Radiomics and Machine Learning. Brain Sci. 2022, 12, 858. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Li, T.; Ren, Y.; Wang, D.; Tang, W.; Li, J.; Li, K. Radiomics-based infarct features on CT predict hemorrhagic transformation in patients with acute ischemic stroke. Front. Neurosci. 2022, 16, 1002717. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tao, W.; Wang, Z.; Chen, X.; Wu, B.; Liu, M. Radiomics-based prediction of hemorrhage expansion among patients with thrombolysis/thrombectomy related-hemorrhagic transformation using machine learning. Ther. Adv. Neurol. Disord. 2021, 14, 17562864211060029. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; He, Y.; Jiang, Z.; Xie, Y.; Nie, S. Ischemic stroke subtyping method combining convolutional neural network and radiomics. J. X-ray Sci. Technol. 2022, 31, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Wei, J.; Zhu, Y.; Wei, L.; Wei, X.; Tian, H.; Zhang, L.; Wang, T.; Cheng, Y.; Zhao, Q.; et al. Clot-based radiomics model for cardioembolic stroke prediction with CT imaging before recanalization: A multicenter study. Eur. Radiol. 2023, 33, 970–980. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Wan, S.; Wu, W.; Chen, F.; Jiang, J.; Cai, D.; Bao, Z.; Li, Y.; Zhang, L. Computed Tomography Angiography-Based Thrombus Radiomics for Predicting the Time Since Stroke Onset. Acad. Radiol. 2023, 36697269. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Mao, L.; Lv, S.; Ren, Z.; Li, W.; Ren, K. CT radiomics features as a diagnostic tool for classifying basal ganglia infarction onset time. J. Neurol. Sci. 2020, 412, 116730. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Shu, Z.; Li, Y.; Hu, X.; Gong, X. Developing a model for estimating infarction onset time based on computed tomography radiomics in patients with acute middle cerebral artery occlusion. BMC Med. Imaging 2021, 21, 147. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Liu, A.-F.; Man, F.-Y.; Zhang, Y.-Y.; Li, C.; Liu, Y.-E.; Zhou, J.; Zhang, A.-P.; Zhang, Y.-D.; Lv, J.; et al. MRI radiomic features-based machine learning approach to classify ischemic stroke onset time. J. Neurol. 2022, 269, 350–360. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Zhou, Y.; Yang, Y.; Yang, J.; Pang, P.; Wang, Y.; Cheng, J.; Chen, H.; Guo, Y. CT-based radiomics for differentiating intracranial contrast extravasation from intraparenchymal haemorrhage after mechanical thrombectomy. Eur. Radiol. 2022, 32, 4771–4779. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, J.; Zhang, H.; Li, H.; Wang, F.; Lv, P.; Ye, J. A CT-based radiomics nomogram for classification of intraparenchymal hyperdense areas in patients with acute ischemic stroke following mechanical thrombectomy treatment. Front. Neurosci. 2023, 16, 1061745. [Google Scholar] [CrossRef] [PubMed]
- Yip, S.S.F.; Aerts, H.J.W.L. Applications and limitations of radiomics. Phys. Med. Biol. 2016, 61, R150–R166. [Google Scholar] [CrossRef] [PubMed]
- Cardenas, C.E.; Yang, J.; Anderson, B.M.; Court, L.E.; Brock, K.B. Advances in Auto-Segmentation. Semin. Radiat. Oncol. 2019, 29, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Lambin, P.; Leijenaar, R.T.H.; Deist, T.M.; Peerlings, J.; de Jong, E.E.C.; van Timmeren, J.; Sanduleanu, S.; Larue, R.T.H.M.; Even, A.J.G.; Jochems, A.; et al. Radiomics: The bridge between medical imaging and personalized medicine. Nat. Rev. Clin. Oncol. 2017, 14, 749–762. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).