Integrating Mobile Devices and Wearable Technology for Optimal Sleep Conditions
Abstract
1. Introduction
2. Materials and Methods
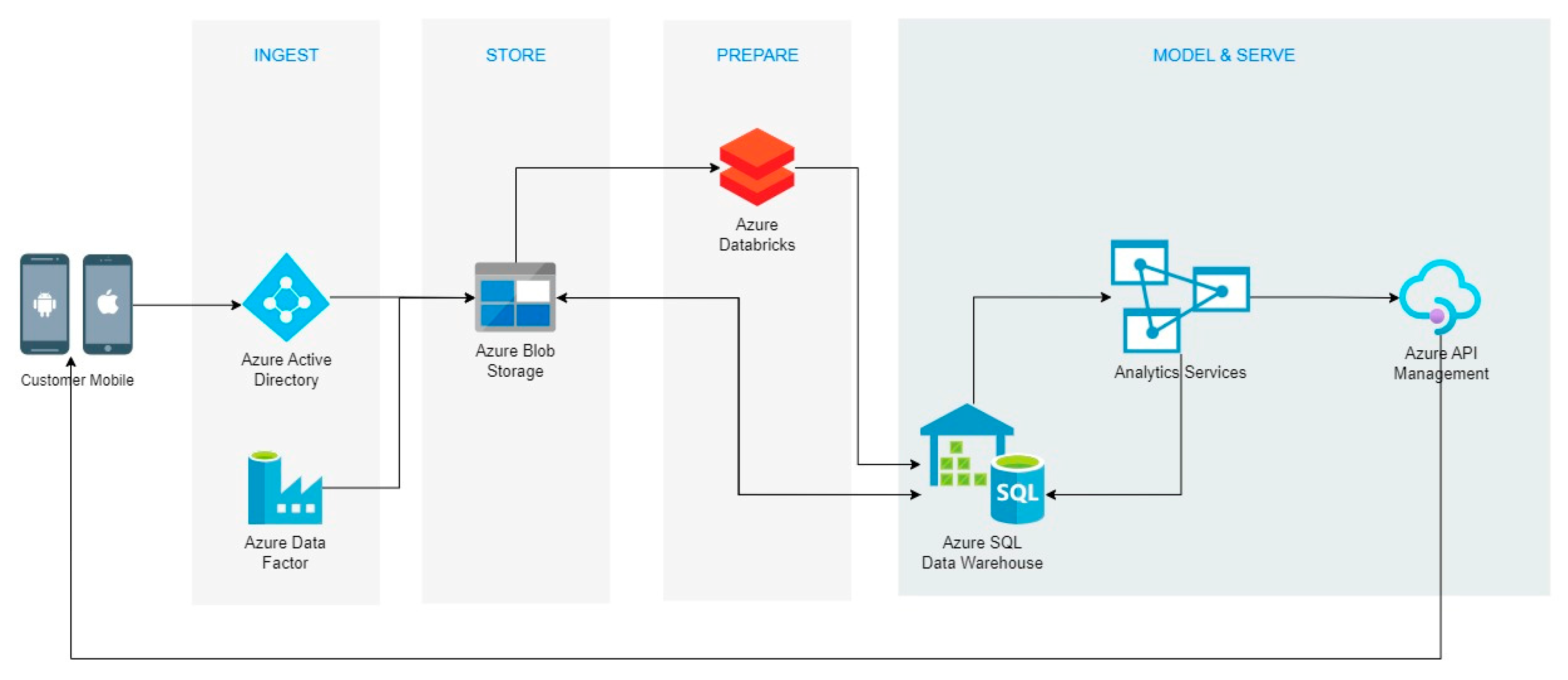
2.1. Cloud System
2.2. Sleep State Analysis Algorithm
2.3. Data Analysis
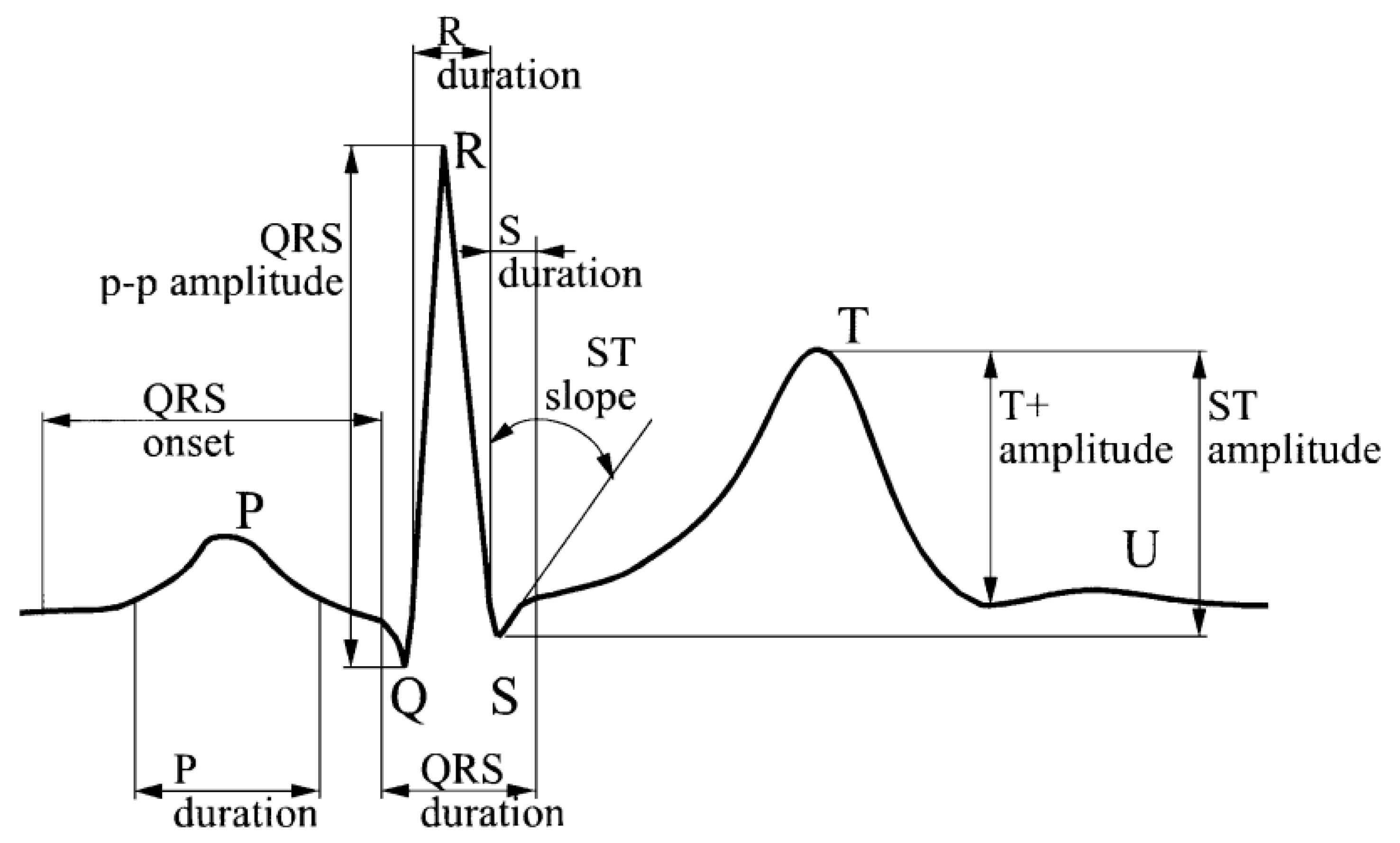
2.4. Brain Wave Analysis
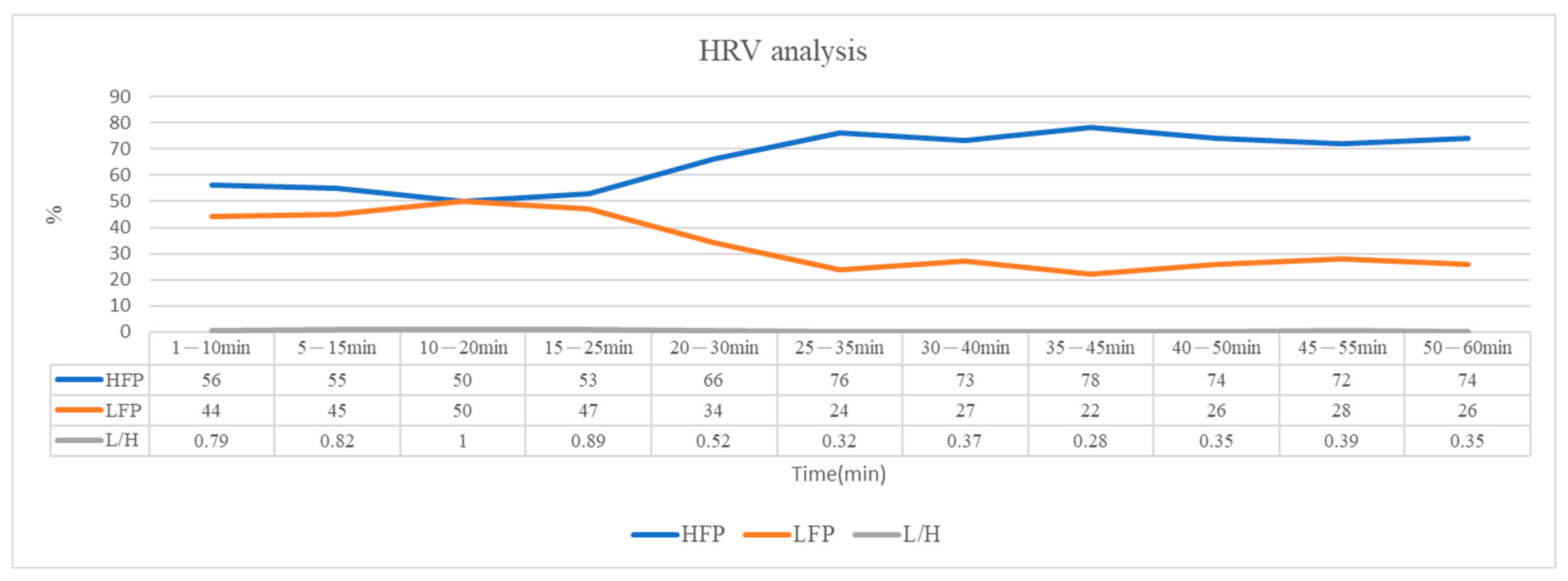
2.5. HRV Analysis
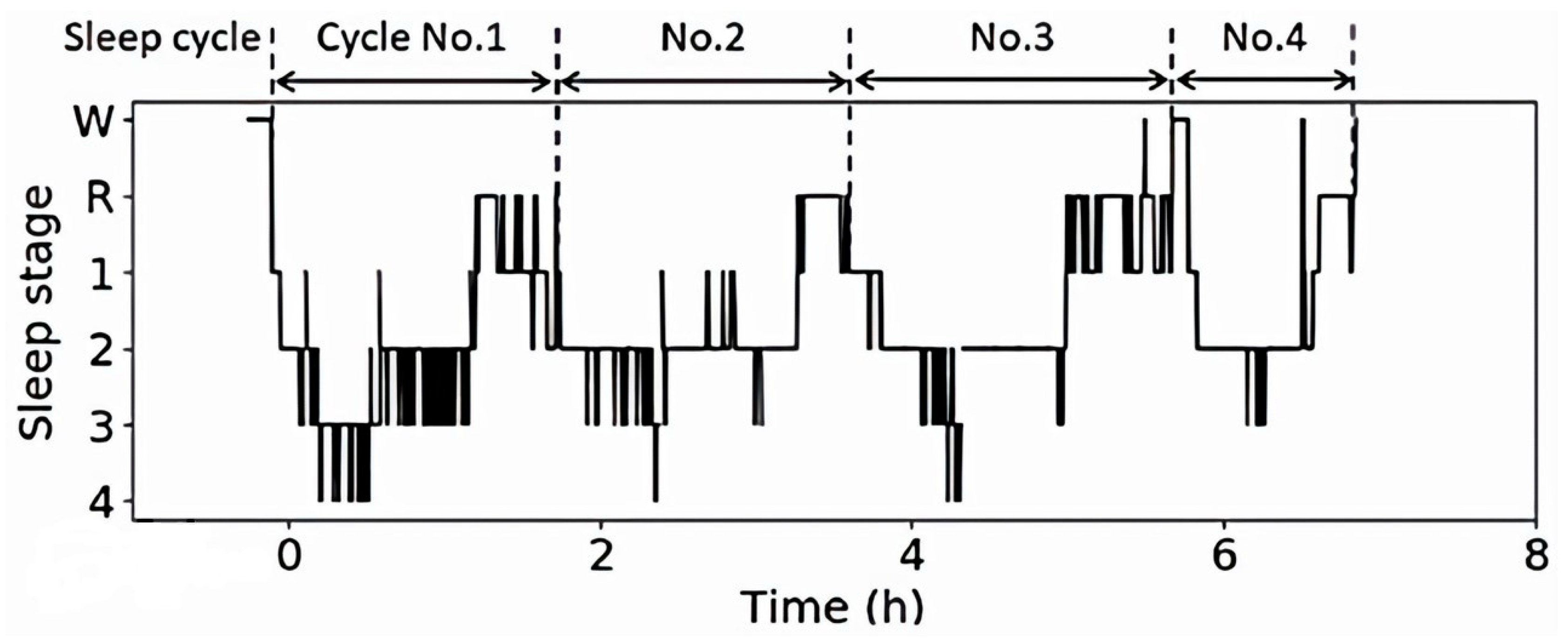
2.6. Sleep Turn-Over Analysis
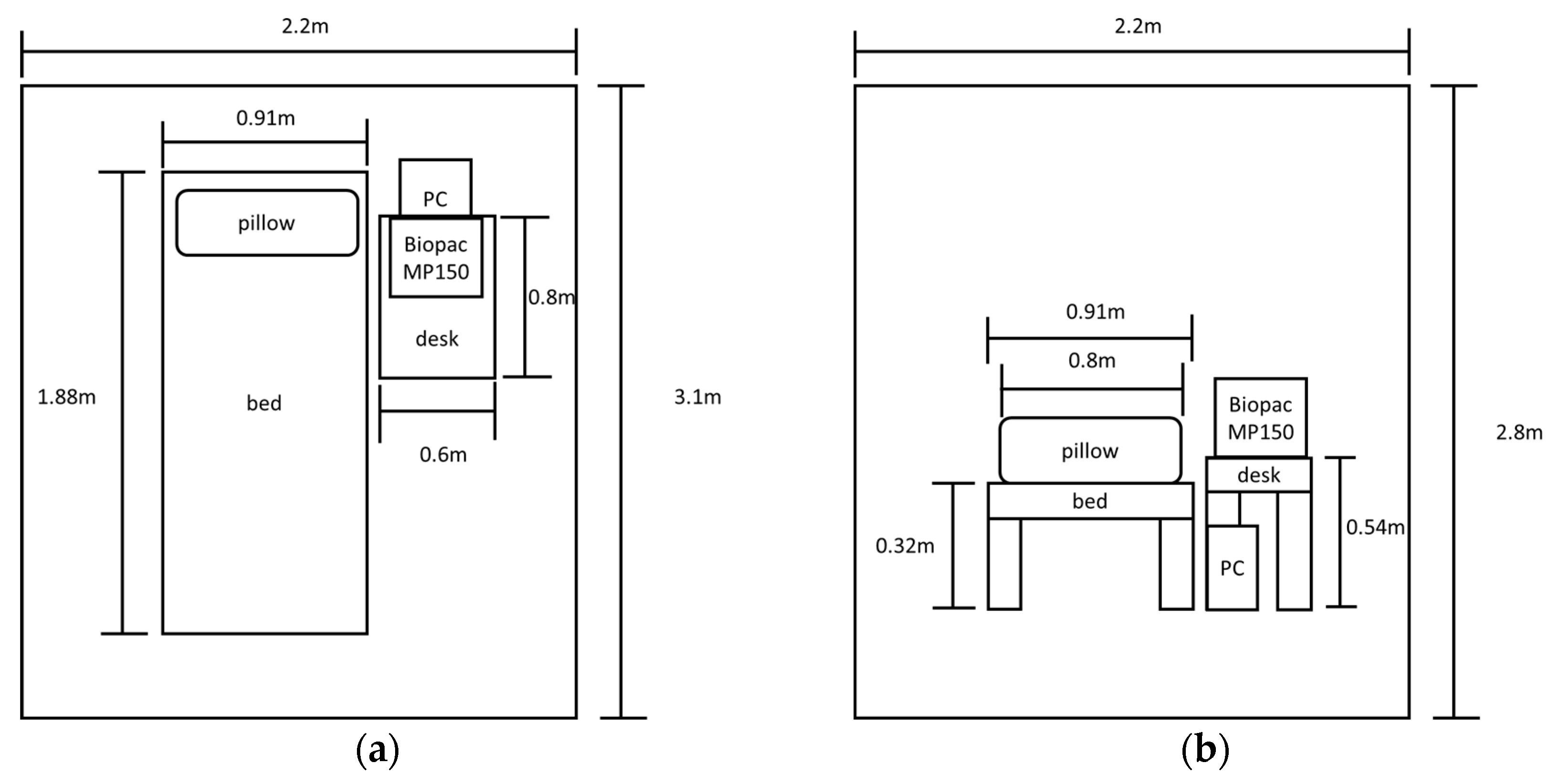
2.7. Experimental Equipment
2.8. Data Identify
2.9. Experiemental Environment
3. Results and Discussion
3.1. Analysis Results and Discussion of Sleep Data
3.2. Cloud Data Analysis System
4. Conclusions
- A novel sleep state algorithm is proffered for interpreting sleep stages using physiological signals from smart wearable devices. The primary objective is to mitigate the reliance on conventional physiological signal acquisition instruments that necessitate direct physical contact with the body.
- Through a comprehensive analysis of cloud-based databases and the uploading of personalized physiological data, our study presents an iterative updating mechanism for personalized and precise sleep algorithms. Each set of individual physiological data is subjected to a secondary analysis compared to the original dataset, facilitating the calibration of individual physiological data algorithms. These refinements consider variations in physiological markers during periods of inactivity as well as the frequency of bodily movements.
- Our research extends beyond prior endeavors in dynamic sleep-inducing illumination [50,56,57,58], aiming to minimize undue physiological data acquisition and re-shape the paradigm of physiological data analysis from smart wearable devices. Prospective work entails utilizing physiological data harnessed by wearable devices to conduct sleep state analyses, enabling refinements to dynamic sleep-inducing illumination, and amplifying users’ sleep efficiency.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, H.; He, L.; Gao, Y.; Gao, X.; Lei, X. Effects of physical activity and sleep quality on well-being: A wrist actigraphy study during the pandemic. Appl. Psychol. Health Well-Being 2021, 13, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Engle-Friedman, M.; Riela, S.; Golan, R.; Ventuneac, A.M.; Davis, C.M.; Jefferson, A.D.; Major, D. The effect of sleep loss on next day effort. J. Sleep Res. 2003, 12, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Vgontzas, A.N.; Zoumakis, E.; Bixler, E.O.; Lin, H.M.; Follett, H.; Kales, A.; Chrousos, G.P. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J. Clin. Endocrinol. Metab. 2004, 89, 2119–2126. [Google Scholar] [CrossRef] [PubMed]
- Haack, M.; Sanchez, E.; Mullington, J.M. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep 2007, 30, 1145–1152. [Google Scholar] [CrossRef]
- Mullington, J.M.; Haack, M.; Toth, M.; Serrador, J.M.; Meier-Ewert, H.K. Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Prog. Cardiovasc. Dis. 2009, 51, 294–302. [Google Scholar]
- Smolensky, M.H.; Di Milia, L.; Ohayon, M.M.; Philip, P. Sleep disorders, medical conditions, and road accident risk. Accid. Anal. Prev. 2011, 43, 533–548. [Google Scholar]
- Ebrahimi, M.H.; Sadeghi, M.; Dehghani, M.; Niiat, K.S. Sleep habits and road traffic accident risk for Iranian occupational drivers. Int. J. Occup. Med. Env. Health 2015, 28, 305–312. [Google Scholar]
- Hystad, S.W.; Nielsen, M.B.; Eid, J. The impact of sleep quality, fatigue and safety climate on the perceptions of accident risk among seafarers. Eur. Rev. Appl. Psychol. 2017, 67, 259–267. [Google Scholar]
- Ohayon, M.; Wickwire, E.M.; Hirshkowitz, M.; Albert, S.M.; Avidan, A.; Daly, F.J.; Dauvilliers, Y.; Ferri, R.; Fung, C.; Gozal, D.; et al. National Sleep Foundation’s sleep quality recommendations: First report. Sleep Health 2017, 3, 6–19. [Google Scholar] [CrossRef]
- Sadeh, A.; Keinan, G.; Daon, K. Effects of stress on sleep: The moderating role of coping style. Health Psychol. 2004, 23, 542. [Google Scholar] [CrossRef]
- Han, K.S.; Kim, L.; Shim, I. Stress and sleep disorder. Exp. Neurobiol. 2012, 21, 141. [Google Scholar] [CrossRef] [PubMed]
- Fullagar, H.H.; Duffield, R.; Skorski, S.; Coutts, A.J.; Julian, R.; Meyer, T. Sleep and recovery in team sport: Current sleep-related issues facing professional team-sport athletes. Int. J. Sports Physiol. Perform. 2015, 10, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Kredlow, M.A.; Capozzoli, M.C.; Hearon, B.A.; Calkins, A.W.; Otto, M.W. The effects of physical activity on sleep: A meta-analytic review. J. Behav. Med. 2015, 38, 427–449. [Google Scholar] [PubMed]
- Bosma, K.J.; Ranieri, V.M. Filtering out the noise: Evaluating the impact of noise and sound reduction strategies on sleep quality for ICU patients. Crit. Care 2009, 13, 151. [Google Scholar] [CrossRef] [PubMed]
- Bion, V.; Lowe, A.S.; Puthucheary, Z.; Montgomery, H. Reducing sound and light exposure to improve sleep on the adult intensive care unit: An inclusive narrative review. J. Intensive Care Soc. 2018, 19, 138–146. [Google Scholar] [CrossRef]
- Jensen, H.I.; Markvart, J.; Holst, R.; Thomsen, T.D.; Larsen, J.W.; Eg, D.M.; Nielsen, L.S. Shift work and quality of sleep: Effect of working in designed dynamic light. Int. Arch. Occup. Environ. Health 2016, 89, 49–61. [Google Scholar] [CrossRef]
- Harper, K. So Tired in the Morning…The Science of Sleep; ChemMatters: Washington, DC, USA, 2015. [Google Scholar]
- Solso, R.L. The Psychology of Art and the Evolution of the Conscious Brain; MIT Press: Cambridge, MA, USA; London, UK, 2003; p. 57. [Google Scholar]
- Berson, D.M.; Dunn, F.A.; Takao, M. Phototransduction by retinal ganglion cells that set the circadian clock. Science 2002, 295, 1070–1073. [Google Scholar] [CrossRef]
- Morin, L.P. The circadian visual system. Brain Res. Rev. 1994, 19, 102–127. [Google Scholar]
- Axelrod, J. The pineal gland: A neurochemical transducer. Science 1974, 184, 1341–1348. [Google Scholar] [CrossRef]
- Chellappa, S.L.; Steiner, R.; Oelhafen, P.; Lang, D.; Götz, T.; Krebs, J.; Cajochen, C. Acute exposure to evening blue-enriched light impacts on human sleep. J. Sleep Res. 2013, 22, 573580. [Google Scholar] [CrossRef]
- Klink, M.E. Risk factors associated with complaints of insomnia in a general adult population: Influence of previous complaints of insomnia. Arch. Intern. Med. 1992, 152, 1634–1637. [Google Scholar] [CrossRef] [PubMed]
- Ohayon, M.M. Epidemiology of insomnia: What we know and what we still need to learn. Sleep Med. Rev. 2002, 6, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Roth, T.; Ancoli-Israel, S. Daytime consequences and correlates of insomnia in the United States: Results of the 1991 National Sleep Foundation Survey. II. Sleep J. Sleep Res. Sleep Med. 1999, 22, S354–S358. [Google Scholar]
- Tonks, A. Children who sleep with light on may damage their sight. BMJ Br. Med. J. 1999, 318, 1369. [Google Scholar] [CrossRef]
- Fonken, L.K.; Nelson, R.J. The effects of light at night on circadian clocks and metabolism. Endocr. Rev. 2014, 35, 648–670. [Google Scholar] [CrossRef]
- Ursino, G.; Coppari, R. Insulin under the influence of light. Swiss Med. Wkly. 2020, 150, w20273. [Google Scholar] [CrossRef] [PubMed]
- Cheung, I.N.; Zee, P.C.; Shalman, D.; Malkani, R.G.; Kang, J.; Reid, K.J. Morning and evening blue-enriched light exposure alters metabolic function in normal weight adults. PLoS ONE 2016, 11, e0155601. [Google Scholar] [CrossRef]
- Park YM, M.; White, A.J.; Jackson, C.L.; Weinberg, C.R.; Sandler, D.P. Association of exposure to artificial light at night while sleeping with risk of obesity in women. JAMA Intern. Med. 2019, 179, 1061–1071. [Google Scholar] [CrossRef]
- Obayashi, K.; Yamagami, Y.; Kurumatani, N.; Saeki, K. Bedroom lighting environment and incident diabetes mellitus: A longitudinal study of the HEIJO-KYO cohort. Sleep Med. 2020, 65, 1–3. [Google Scholar] [CrossRef]
- Kozaki, T.; Kitamura, S.; Higashihara, Y.; Ishibashi, K.; Noguchi, H.; Yasukouchi, A. Effect of color temperature of light sources on slow-wave sleep. J. Physiol. Anthropol. Appl. Hum. Sci. 2005, 24, 183–186. [Google Scholar] [CrossRef]
- Smolders, K.C.; de Kort, Y.A. Investigating daytime effects of correlated colour temperature on experiences, performance, and arousal. J. Environ. Psychol. 2017, 50, 80–93. [Google Scholar] [CrossRef]
- Lu, Y. Design of a Sleep Assistance System Terminalm. In Proceedings of the 2020 IEEE 5th Information Technology and Mechatronics Engineering Conference (ITOEC), Chongqing, China, 12–14 June 2020; IEEE: New York, NY, USA, 2020; pp. 1586–1589. [Google Scholar]
- Saad, W.H.M.; Khoo, C.W.; Ab Rahman, S.I.; Ibrahim, M.M.; Saad, N.H.M. Development of sleep monitoring system for observing the effect of the room ambient toward the quality of sleep. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017; Volume 210, p. 012050. [Google Scholar]
- Sridhar, N.; Shoeb, A.; Stephens, P.; Kharbouch, A.; Ben Shimol, D.; Burkart, J.; Ghoreyshi, A.; Myers, L. Deep learning for automated sleep staging using instantaneous heart rate. NPJ Digit. Med. 2020, 3, 106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Campanella, C.; Aristizabal, S.; Jamrozik, A.; Zhao, J.; Porter, P.; Ly, S.; Bauer, B.A. Impacts of dynamic LED lighting on the well-being and experience of office occupants. Int. J. Environ. Res. Public Health 2020, 17, 7217. [Google Scholar] [CrossRef]
- Jakobsen, G.; Engstrøm, M.; Thronæs, M.; Løhre, E.T.; Kaasa, S.; Fayers, P.; Hjermstad, M.J.; Klepstad, P. Sleep quality in hospitalized patients with advanced cancer: An observational study using self-reports of sleep and actigraphy. Support. Care Cancer 2020, 28, 2015–2023. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.P.; Peng, Y.X. Meta-analysis of differences in sleep quality based on actigraphs between day and night shift workers and the moderating effect of age. J. Occup. Health 2021, 63, e12262. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Liu, X.; Shi, Q.; He, T.; Sun, Z.; Guo, X.; Liu, W.; Bin Sulaiman, O.; Dong, B.; Lee, C. Development trends and perspectives of future sensors and MEMS/NEMS. Micromachines 2019, 11, 7. [Google Scholar] [CrossRef]
- International Data Corporation (IDC). Worldwide Wearables Shipments Surge 94.6% in 3Q 2019 Led by Expanding Hearables Market, Says IDC. Available online: https://www.businesswire.com/news/home/20191209005097/en/Worldwide-Wearables-Shipments-Surge-94.6-in-3Q-2019-Led-by-Expanding-Hearables-Market-Says-IDC (accessed on 30 August 2023).
- Kemp, S. Digital 2020: Global Digital Overview. Datareportal. 2020. Available online: https://datareportal.com/reports/digital-2020-global-digital-overview (accessed on 30 August 2023).
- Aserinsky, E.; Kleitman, N. Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science 1953, 118, 273e4. [Google Scholar] [CrossRef]
- Iber, C.; Ancoli-Israel, S.; Chesson, A.; Quan, S.F. (Eds.) The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specification, 1st ed.; American Academy of Sleep Medicine: Westchester, IL, USA, 2007. [Google Scholar]
- Rechtschaffen, A.; Kales, A. (Eds.) A Manual of Standardized Terminology, Techniques and Scoring System of Sleep Stages in Human Subjects; Brain Information Service/Brain Research Institute, University of California: Los Angeles, CA, USA, 1968. [Google Scholar]
- Sawai, H.; Matsumoto, M.; Koyama, E. The relationship between each length of REM-NREM sleep cycle and sleep stage. In Proceedings of the 2021 IEEE 3rd Global Conference on Life Sciences and Technologies (LifeTech), Nara, Japan, 9–11 March 2021; IEEE: New York, NY, USA, 2021; pp. 171–172. [Google Scholar]
- Maeda, M.; Takajyo, A.; Inoue, K.; Kumamaru, K.; Matsuoka, S. Time-frequency analysis of human sleep EEG and its application to feature extraction about biological rhythm. In Proceedings of the SICE Annual Conference 2007, Takamatsu, Japan, 17–20 September 2007; IEEE: New York, NY, USA, 2007; pp. 1939–1944. [Google Scholar]
- Ek, F.S.; Jakovljević, M. Heart rate variability—A shape analysis of Lorenz plots. Cell. Mol. Biol. Lett. 2002, 7, 160. [Google Scholar]
- Biedenharn, L.C.; Louck, J.D.; Carruthers, P.A. Angular momentum in quantum physics—Theory and application. In Encyclopedia of Mathematics and Its Applications; Addison-Wesley: Reading, MA, USA, 1981. [Google Scholar]
- Chen, C.Y.; Wang, Y.K.; Wang, Z.W. Research on the application of the dynamic assisted sleep light to smart mobile devices. Appl. Sci. 2022, 12, 5191. [Google Scholar] [CrossRef]
- Wichterle, D.; Simek, J.; La Rovere, M.T.; Schwartz, P.J.; Camm, A.J.; Malik, M. Prevalent low-frequency oscillation of heart rate: Novel predictor of mortality after myocardial infarction. Circulation 2004, 110, 1183–1190. [Google Scholar] [CrossRef]
- Wiklund, U.; Hörnsten, R.; Karlsson, M.; Suhr, O.B.; Jensen, S.M. Abnormal heart rate variability and subtle atrial arrhythmia in patients with familial amyloidotic polyneuropathy. Ann. Noninvasive Electrocardiol. 2008, 13, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Mousailidis G, Κ.; Lachanas, V.A.; Vasdeki, A.; Alexopoulos, E.I.; Kaditis, A.G.; Petinaki, E.; Balatsos, N.A.A.; Bizakis, J.G.; Skoulakis, C.E. Urine concentrations changes of cysteinyl leukotrienes in non-obese children with obstructive sleep apnea undergoing adenotonsillectomy. Int. J. Pediatr. Otorhinolaryngol. 2018, 115, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Iqubal MF, B.M.; Lam, Y.Y. Home-based monitoring and alert system for Sleep Apnea patients. In Proceedings of the 2020 IEEE 7th International Conference on Engineering Technologies and Applied Sciences (ICETAS), Kuala Lumpur, Malaysia, 18–20 December 2020; IEEE: New York, NY, USA, 2020; pp. 1–4. [Google Scholar]
- Khurana, S.; Soda, N.; Shiddiky, M.J.; Nayak, R.; Bose, S. Current and future strategies for diagnostic and management of obstructive sleep apnea. Expert Rev. Mol. Diagn. 2021, 21, 1287–1301. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.W.; Chen, C.Y.; Wu, P.J. The Influence of Lighting on Human Circadian Rhythms. In Proceedings of the 2019 16th China International Forum on Solid State Lighting & 2019 International Forum on Wide Bandgap Semiconductors China (SSLChina: IFWS), Shenzhen, China, 25–27 November 2019; IEEE: New York, NY, USA, 2019; pp. 185–188. [Google Scholar]
- Chen, C.Y.; Wang, Y.K. Dynamic color temperature sleep assitant light implemented on the mobile device. In Proceedings of the 2020 Fifth Junior Conference on Lighting (Lighting), Ruse, Bulgaria, 24–26 September 2020; IEEE: New York, NY, USA, 2020; pp. 1–4. [Google Scholar]
- Chen, C.Y.; Chen, H.W. The Effect of dynamic lighting for working shift people on clinical heart rate variability and human slow wave sleep. Appl. Sci. 2022, 12, 2284. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-K.; Chen, C.-Y. Integrating Mobile Devices and Wearable Technology for Optimal Sleep Conditions. Appl. Sci. 2023, 13, 9921. https://doi.org/10.3390/app13179921
Wang Y-K, Chen C-Y. Integrating Mobile Devices and Wearable Technology for Optimal Sleep Conditions. Applied Sciences. 2023; 13(17):9921. https://doi.org/10.3390/app13179921
Chicago/Turabian StyleWang, You-Kwang, and Chien-Yu Chen. 2023. "Integrating Mobile Devices and Wearable Technology for Optimal Sleep Conditions" Applied Sciences 13, no. 17: 9921. https://doi.org/10.3390/app13179921
APA StyleWang, Y.-K., & Chen, C.-Y. (2023). Integrating Mobile Devices and Wearable Technology for Optimal Sleep Conditions. Applied Sciences, 13(17), 9921. https://doi.org/10.3390/app13179921





