Applicability of Clay/Organic Clay to Environmental Pollutants: Green Way—An Overview
Abstract
1. Introduction
1.1. Background
1.2. Recent Trend of Pollution Control
1.3. Role of Green Chemistry Approaches for Environmental Sustainability
1.4. Strategies to Implement Green Chemistry in Environmental Applications
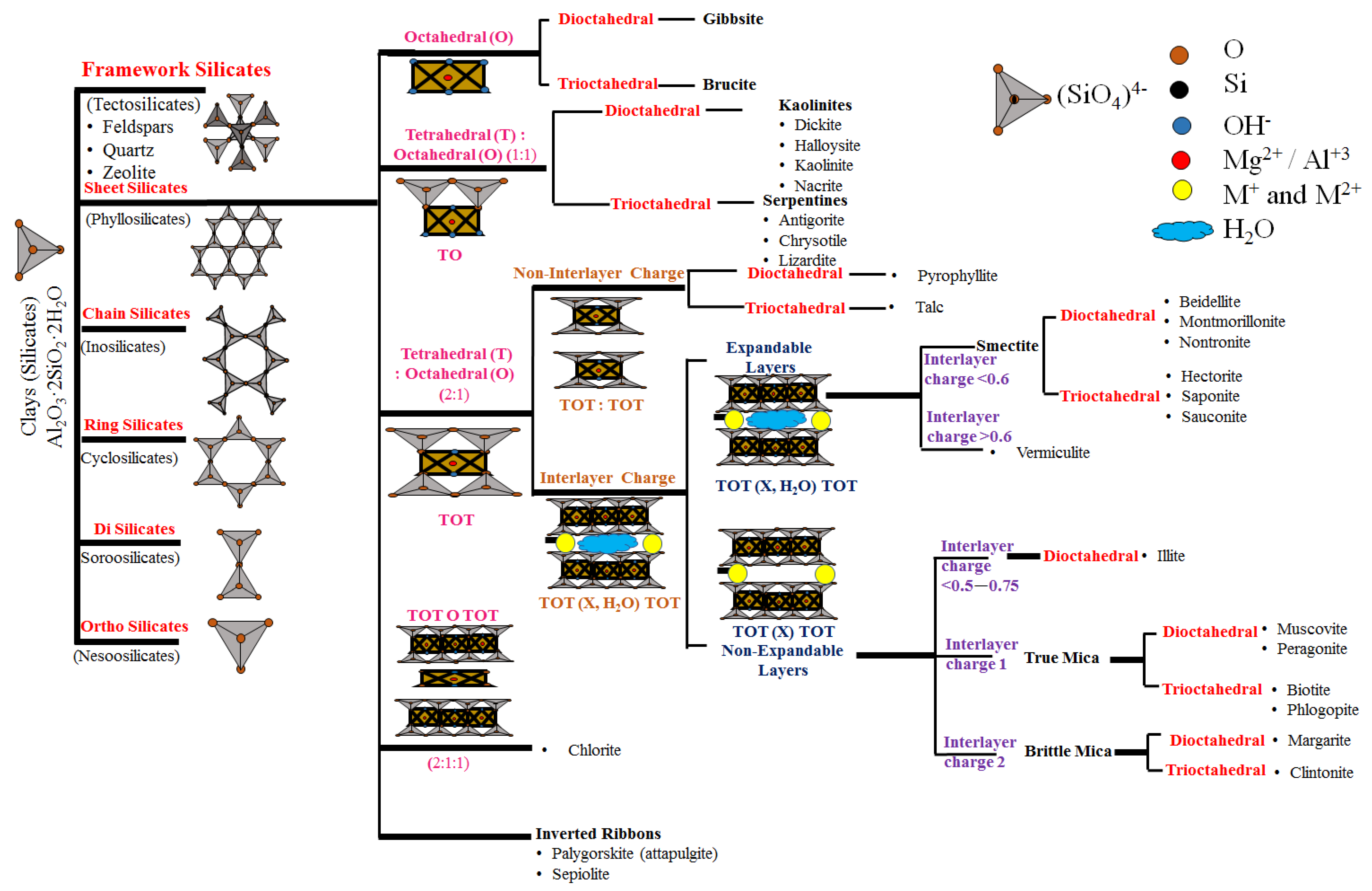
2. Clays and Organoclays
2.1. Classifications of Clays
2.2. Types of Organic Modifiers
2.3. Preparation
2.4. Properties of Clays
2.4.1. Plasticity
2.4.2. CEC
2.4.3. Swelling Capacity
2.4.4. Specific Surface Area
2.4.5. Surface Charge
2.5. Properties of the Modified Clay
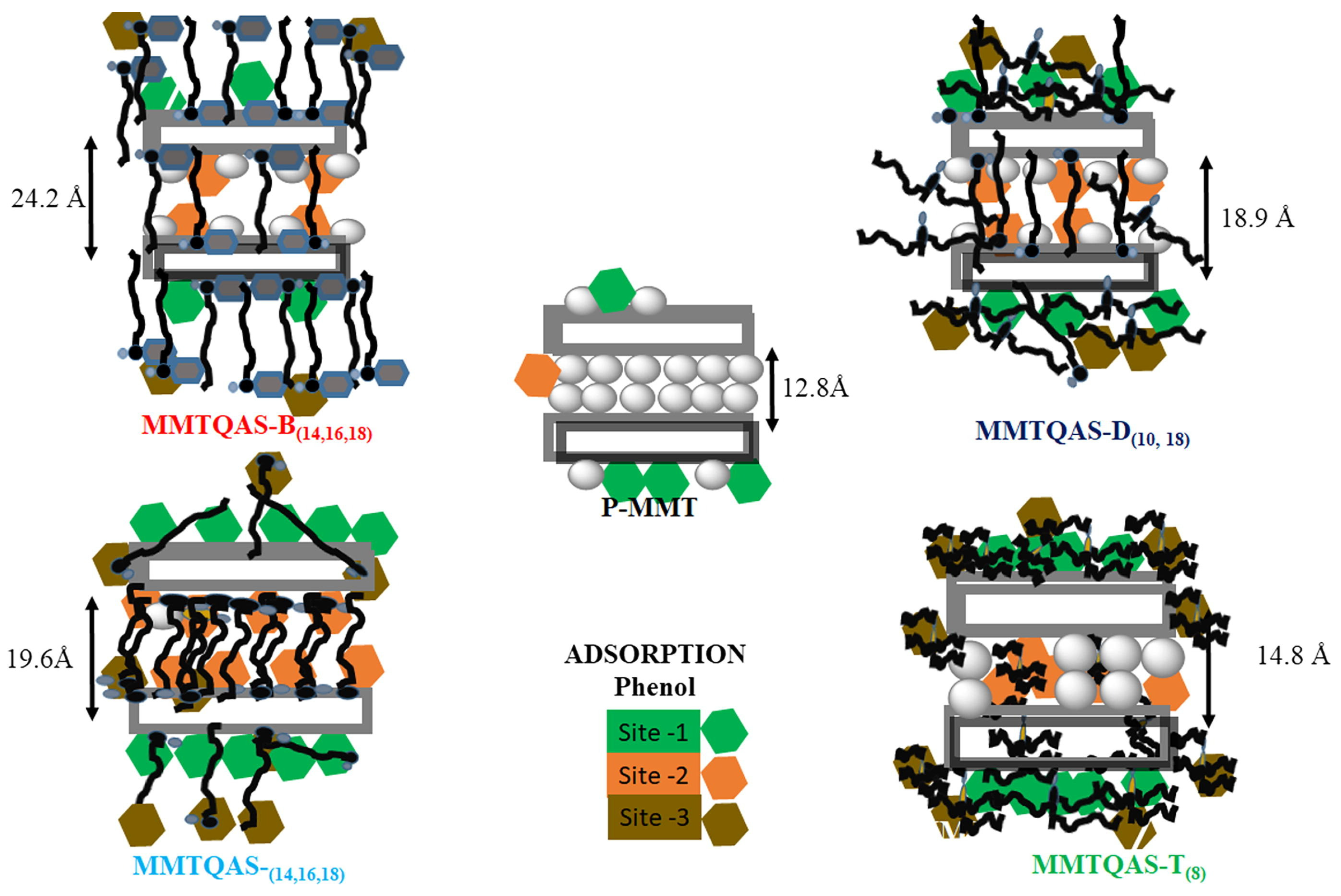
2.5.1. Basal Spacing (d-Space) of Clays
2.5.2. Morphology of the Modified Clay
2.5.3. Hydrophobicity/Hydrophilicity of the Organoclay
2.5.4. Surface Porosity and Volume
2.5.5. Surface Charge (Zeta Potential)
3. Environmental Applications of Organoclays
3.1. Soil Pollution
3.2. Water Pollution
3.3. Air Pollution
4. Concept of Green Chemistry in Organoclay
5. Conclusion and Recommendation
5.1. Current Research Status
5.2. Future Research Directions
- The applicability of clay and modified clay to improve efficiency in soil and air pollution removal. This gap is due to clay’s ease of processing as a water absorber, which is difficult in the air and soil environment but still not negligible;
- Further research is needed to use natural modifiers instead of surfactant molecules or to use biosurfactant as a modifier to aid in a less hazardous synthesis;
- More urgent research is needed to use clay materials to remove microplastics as COVID-19 has increased the use of masks, which are potential water pollutants compared to other organic pollutants;
- In addition, there is high potential for the applicability of clay and organoclay materials to remove and treat emerging contaminants;
- It would also be interesting to determine the role of microbes in breaking down pollutants or converting them to less harmful forms in the presence of clay or organic clay media.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Münzel, T.; Hahad, O.; Daiber, A.; Landrigan, P.J. Soil and Water Pollution and Human Health: What Should Cardiologists Worry About? Cardiovasc. Res. 2023, 119, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Pollution Causes 40 Percent of Deaths Worldwide, Study Finds. Available online: https://www.sciencedaily.com/releases/2007/08/070813162438.htm (accessed on 29 May 2023).
- Ahmed, F.; Ali, I.; Kousar, S.; Ahmed, S. The Environmental Impact of Industrialization and Foreign Direct Investment: Empirical Evidence from Asia-Pacific Region. Environ. Sci. Pollut. Res. 2022, 29, 29778–29792. [Google Scholar] [CrossRef]
- Elehinafe, F.B.; Olomukoro, O.G.; Ayeni, A.O.; Okedere, O.B. A Short Review on Land/Soil Pollution: The Pollutants and the Treatment Techniques (Edit). In Advanced Manufacturing in Biological, Petroleum, and Nanotechnology Processing; Ayeni, A.O., Oladokun, O., Orodu, O.D., Eds.; Springer: Berlin/Heidelberg, Germany, 2022; pp. 267–275. [Google Scholar] [CrossRef]
- Rathi, B.S.; Kumar, P.S.; Vo, D.V.N. Critical Review on Hazardous Pollutants in Water Environment: Occurrence, Monitoring, Fate, Removal Technologies and Risk Assessment. Sci. Total Environ. 2021, 797, 149134. [Google Scholar] [CrossRef] [PubMed]
- Gedda, G.; Balakrishn, K.; Devi, R.U.; Shah, K.J. Introduction to Conventional Wastewater Treatment Technologies: Limitations and Recent Advances. Mater. Res. Found. 2021, 91, 1–36. [Google Scholar] [CrossRef]
- Gandu, B.; Gangagni Rao, A.; Cahan, R. Chapter 22—Air Pollution Control by Using Different Types of Techniques and Sorbents. In Sorbents Materials for Controlling Environmental Pollution; Núñez-Delgado, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 575–594. [Google Scholar] [CrossRef]
- De Gisi, S.; Lofrano, G.; Grassi, M.; Notarnicola, M. Characteristics and Adsorption Capacities of Low-Cost Sorbents for Wastewater Treatment: A Review. Sustain. Mater. Technol. 2016, 9, 10–40. [Google Scholar] [CrossRef]
- Almeida-Naranjo, C.E.; Guerrero, V.H.; Villamar-Ayala, C.A. Emerging Contaminants and Their Removal from Aqueous Media Using Conventional/Non-Conventional Adsorbents: A Glance at the Relationship between Materials, Processes, and Technologies. Water 2023, 15, 1626. [Google Scholar] [CrossRef]
- Worasith, N.; Goodman, B.A. Clay Mineral Products for Improving Environmental Quality. Appl. Clay Sci. 2023, 242, 106980. [Google Scholar] [CrossRef]
- Kasha Patel, Pollution Caused 1 in 6 Deaths Globally for Five Years, Study Says. Available online: https://www.washingtonpost.com/climate-environment/2022/05/17/pollution-caused-1-6-deaths-globally-five-straight-years-study-says/ (accessed on 15 April 2023).
- Kaya, S.I.; Cetinkaya, A.; Ozkan, S.A. Green Analytical Chemistry Approaches on Environmental Analysis. Trends Environ. Anal. Chem. 2022, 33, e00157. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef]
- Mineral Classification. Available online: https://sternberg.fhsu.edu/research-collections/geology/mineral-classification-page.html (accessed on 19 November 2022).
- Kumari, N.; Mohan, C. Basics of Clay Minerals and Their Characteristic Properties. In Clay and Clay Minerals; Nascimento, G.M.D., Ed.; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar] [CrossRef]
- Akisanmi, P. Classification of Clay Minerals. In Mineralogy; René, M., Ed.; IntechOpen: Rijeka, Croatia, 2022. [Google Scholar] [CrossRef]
- Mineralogy. Available online: http://condor.wesleyan.edu/ethomas/ees123/sheet04.htm (accessed on 12 December 2022).
- De Paiva, L.B.; Morales, A.R.; Valenzuela Díaz, F.R. Organoclays: Properties, Preparation and Applications. Appl. Clay Sci. 2008, 42, 8–24. [Google Scholar] [CrossRef]
- Shirzad-Siboni, M.; Khataee, A.; Hassani, A.; Karaca, S. Preparation, Characterization and Application of a CTAB-Modified Nanoclay for the Adsorption of an Herbicide from Aqueous Solutions: Kinetic and Equilibrium Studies. C. R. Chim. 2015, 18, 204–214. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, C.H.; Lin, C.X.; Tong, D.S.; Yu, W.H. Synthesis of Clay Minerals. Appl. Clay Sci. 2010, 50, 1–11. [Google Scholar] [CrossRef]
- Andrade, F.A.; Al-Qureshi, H.A.; Hotza, D. Measuring the Plasticity of Clays: A Review. Appl. Clay Sci. 2011, 51, 1–7. [Google Scholar] [CrossRef]
- Chen, W.L.; Grabowski, R.C.; Goel, S. Clay Swelling: Role of Cations in Stabilizing/Destabilizing Mechanisms. ACS Omega 2022, 7, 3185–3191. [Google Scholar] [CrossRef]
- Komadel, P. Acid Activated Clays: Materials in Continuous Demand. Appl. Clay Sci. 2016, 131, 84–99. [Google Scholar] [CrossRef]
- Al Kausor, M.; Sen Gupta, S.; Bhattacharyya, K.G.; Chakrabortty, D. Montmorillonite and Modified Montmorillonite as Adsorbents for Removal of Water Soluble Organic Dyes: A Review on Current Status of the Art. Inorg. Chem. Commun. 2022, 143, 109686. [Google Scholar] [CrossRef]
- Shah, K.J.; Mishra, M.K.; Shukla, A.D.; Imae, T.; Shah, D.O. Controlling Wettability and Hydrophobicity of Organoclays Modified with Quaternary Ammonium Surfactants. J. Colloid Interface Sci. 2013, 407, 493–499. [Google Scholar] [CrossRef]
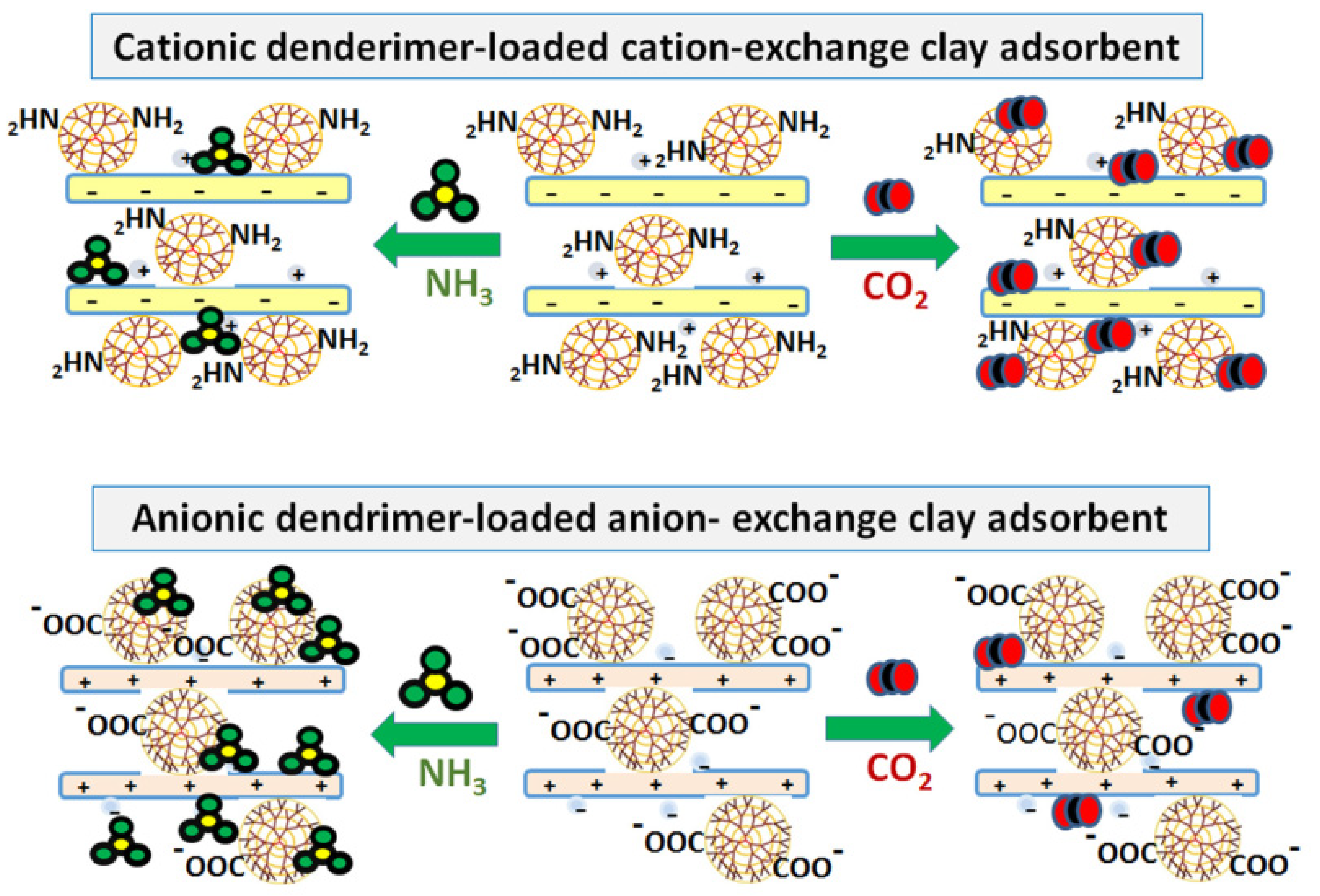
- Shah, K.J.; Imae, T.; Ujihara, M.; Huang, S.J.; Wu, P.H.; Liu, S. Bin Poly(Amido Amine) Dendrimer-Incorporated Organoclays as Efficient Adsorbents for Capture of NH3 and CO2. Chem. Eng. J. 2017, 312, 118–125. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G.; Gupta, S.S. Adsorption of a few heavy metals on natural and modified kaolinite and montmorillonite: A review. Adv. Colloid Interface Sci. 2008, 140, 114–131. [Google Scholar] [CrossRef]
- Ma, Y.; Gu, N.; Gao, J.; Wang, K. Remediation of Anthracene-Contaminated Soil by ClO2 in the Presence of Magnetic Fe3O4-CuO@Montmorillonite as Catalyst. Water Air Soil Pollut. 2016, 227, 303. [Google Scholar] [CrossRef]
- Yang, J.; Yu, K.; Liu, C. Chromium Immobilization in Soil Using Quaternary Ammonium Cations Modified Montmorillonite: Characterization and Mechanism. J. Hazard. Mater. 2017, 321, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, S.; Yang, H. In situ stabilization of some mercury-containing soils using organically modified montmorillonite loading by thiol-based material. J. Soils Sediments 2018, 19, 1767–1774. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Tsang, D.C.W.; Jin, F.; Hou, D. Green Remediation of Cd and Hg Contaminated Soil Using Humic Acid Modified Montmorillonite: Immobilization Performance under Accelerated Ageing Conditions. J. Hazard. Mater. 2020, 387, 122005. [Google Scholar] [CrossRef]
- Qin, C.; Yuan, X.; Xiong, T.; Zen, Y.; Wang, H. Physicochemical Properties, Metal Availability and Bacterial Community Structure in Heavy Metal-Polluted Soil Remediated by Montmorillonite-Based Amendments. Chemosphere 2020, 261, 128010. [Google Scholar] [CrossRef]
- Wu, S.; Yang, Z.; Wang, F.; Jin, X.; Kengara, F.; Xi, K.; Fang, W.; Yang, W.; Zhang, Y. Effect of γ-Fe2O3 Nanoparticles on the Composition of Montmorillonite and Its Sorption Capacity for Pyrene. Sci. Total Environ. 2022, 813, 151893. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Sun, X.; Zhang, W.; Sun, Z.; Na, S.; Chen, Z.; Wang, L.; Yuan, C.; Sun, H. Effect of Fe(III)-modified montmorillonite on arsenic oxidation and anthracene transformation in soil. Sci. Total. Environ. 2022, 814, 151939. [Google Scholar] [CrossRef]
- Li, C.; Li, Y.; Cheng, H.; Jiang, C.; Zheng, L. Remediation of Soil Mercury by Modified Vermiculite-Montmorillonite and Its Effect on the Growth of Brassica chinensis L. Molecules 2022, 27, 5340. [Google Scholar] [CrossRef]
- Liu, C.; Long, L.; Yang, Y.; Zhang, Y.; Wang, J.; Sun, R. The Mechanisms of Iron Modi Fi Ed Montmorillonite in Controlling Mercury Release across Mercury-Contaminated Soil-Air Interface in Greenhouse. Sci. Total Environ. 2022, 812, 152432. [Google Scholar] [CrossRef]
- Huang, Q.; Hu, X.; Yuan, X.; Xiao, T.; Zhang, M. Immobilization of W (VI) and/or Cr (VI) in Soil Treated with Montmorillonite Modified by a Gemini Surfactant and Tetrachloroferrate (FeCl 4−). J. Hazard. Mater. 2022, 425, 127768. [Google Scholar] [CrossRef]
- Jiang, K.; Xiang, A.; Liu, K.; Peng, Q. Potential of Montmorillonite and Humus-like Substances Modified Montmorillonite for Remediation of Pb and Zn-Contaminated Soils. Appl. Clay Sci. 2023, 234, 106853. [Google Scholar] [CrossRef]
- Zhao, C.; Yao, J.; Knudsen, T.; Liu, J.; Zhu, X.; Ma, B.; Li, H.; Cao, Y.; Liu, B. Performance and mechanisms for Cd(II) and As(III) simultaneous adsorption by goethite-loaded montmorillonite in aqueous solution and soil. J. Environ. Manag. 2023, 330, 117163. [Google Scholar] [CrossRef]
- Zhang, H.; Larson, S.; Ballard, J.; Runge, K.A.; Nie, J.; Zhang, Q.; Zhu, X.; Pradhan, N.; Dai, Q.; Ma, Y.; et al. Strontium and Cesium Adsorption on Exopolysaccharide-Modified Clay Minerals. ACS Earth Space Chem. 2023, 7, 936–946. [Google Scholar] [CrossRef]
- Jiang, M.; Wang, K.; Li, G.; Zhao, Q.; Wang, W.; Jiang, J.; Wang, Y. Stabilization of Arsenic, Antimony, and Lead in Contaminated Soil with Montmorillonite Modified by Ferrihydrite: Efficiency and Mechanism. Chem. Eng. J. 2023, 457, 141182. [Google Scholar] [CrossRef]
- Chen, G.; Shah, K.J.; Shi, L.; Chiang, P.C.; You, Z. Red Soil Amelioration and Heavy Metal Immobilization by a Multi-Element Mineral Amendment: Performance and Mechanisms. Environ. Pollut. 2019, 254, 112964. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R. Advances in Application of Natural Clay and Its Composites in Removal of Biological, Organic, and Inorganic Contaminants. Adv. Mater. Sci. Eng. 2011, 2011, 872531. [Google Scholar] [CrossRef]
- Gu, S.; Kang, X.; Wang, L.; Lichtfouse, E.; Wang, C. Clay Mineral Adsorbents for Heavy Metal Removal from Wastewater: A Review. Environ. Chem. Lett. 2019, 17, 629–654. [Google Scholar] [CrossRef]
- Guégan, R. Organoclay applications and limits in the environment. C. R. Chim. 2018, 22, 132–141. [Google Scholar] [CrossRef]
- Shen, T.; Gao, M. Gemini Surfactant Modified Organo-Clays for Removal of Organic Pollutants from Water: A Review. Chem. Eng. J. 2019, 375, 121910. [Google Scholar] [CrossRef]
- Undabeytia, T.; Shuali, U.; Nir, S.; Rubin, B. Applications of Chemically Modified Clay Minerals and Clays to Water Purification and Slow Release Formulations of Herbicides. Minerals 2020, 11, 9. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, W.; Zhao, Y.; Bai, H.; Wen, T.; Kang, S. Removal of Heavy Metals and Dyes by Clay-Based Adsorbents: From Natural Clays to 1D and 2D Nano-Composites. Chem. Eng. J. 2021, 420, 127574. [Google Scholar] [CrossRef]
- Sultana, S.; Karmaker, B.; Galal, A.S.M.S.; Moniruzzaman, U. Environment—Friendly Clay Coagulant Aid for Wastewater Treatment. Appl. Water Sci. 2022, 12, 1–10. [Google Scholar] [CrossRef]
- Ayalew, A.A. A Critical Review on Clay-Based Nanocomposite Particles for Application of Wastewater Treatment. Water Sci. Technol. 2022, 85, 3002–3022. [Google Scholar] [CrossRef] [PubMed]
- Hnamte, M.; Pulikkal, A.K. Clay-Polymer Nanocomposites for Water and Wastewater Treatment: A Comprehensive Review. Chemosphere 2022, 307, 135869. [Google Scholar] [CrossRef] [PubMed]
- Andreo, O.A.; Castelli, C.Z.; Fernanda, M.; Almeida, A.F.; De Meuris, G.C. Adsorption of Synthetic Orange Dye Wastewater in Organoclay. Chem. Eng. 2013, 32, 307–312. [Google Scholar] [CrossRef]
- Nguyen, V.N.; Dieu, T.; Nguyen, C.; Phuong, T.; Thuan, H. Synthesis of Organoclays and Their Application for the Adsorption of Phenolic Compounds from Aqueous Solution. J. Ind. Eng. Chem. 2013, 19, 640–644. [Google Scholar] [CrossRef]
- Park, Y.; Ayoko, G.A.; Kurdi, R.; Horváth, E.; Kristóf, J.; Frost, R.L. Adsorption of Phenolic Compounds by Organoclays: Implications for the Removal of Organic Pollutants from Aqueous Media. J. Colloid Interface Sci. 2013, 406, 196–208. [Google Scholar] [CrossRef]
- Zheng, S.; Sun, Z.; Park, Y.; Ayoko, G.A.; Frost, R.L. Removal of Bisphenol A from Wastewater by Ca-Montmorillonite Modified with Selected Surfactants. Chem. Eng. J. 2013, 234, 416–422. [Google Scholar] [CrossRef]
- Egbon, E.E.; State, E.; State, E. Pharmaceutical Industry Wastewater Treatment Using Organic Surfactant Modified Clay. Civ. Environ. Res. 2013, 3, 173–178. [Google Scholar]
- Ali, D. Treatment of Textile Waste Water with Organoclay. Iran. J. Chem. Chem. Eng. 2013, 32, 67–70. [Google Scholar]
- Park, Y.; Sun, Z.; Ayoko, G.A.; Frost, R.L. Bisphenol A Sorption by Organo-Montmorillonite: Implications for the Removal of Organic Contaminants from Water. Chemosphere 2014, 107, 249–256. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, B.; Wu, T.; Sun, D.; Li, Y. Adsorption Behavior and Mechanism of Chlorophenols onto Organoclays in Aqueous Solution. Colloids Surf. A Physicochem. Eng. Asp. 2015, 484, 118–129. [Google Scholar] [CrossRef]
- Iqbal, M.; Khera, R.A. Adsorption of Copper and Lead in Single and Binary Metal System onto Fumaria Indica Biomass. Chem. Int. 2015, 1, 157–163. [Google Scholar] [CrossRef]
- Yang, S.; Gao, M.; Luo, Z.; Yang, Q. The Characterization of Organo-Montmorillonite Modified with a Novel Aromatic-Containing Gemini Surfactant and Its Comparative Adsorption for 2-Naphthol and Phenol. Chem. Eng. J. 2015, 268, 125–134. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Ramachandran, M. Adsorptive Removal of Basic Dyes from Aqueous Solutions by Surfactant Modified Bentonite Clay (Organoclay): Kinetic and Competitive Adsorption Isotherm. Process Saf. Environ. Prot. 2015, 95, 215–225. [Google Scholar] [CrossRef]
- Makhoukhi, B.; Djab, M.; Didi, M.A. Adsorption of Telon Dyes onto Bis-Imidazolium Modi Fi Ed Bentonite in Aqueous Solutions. J. Environ. Chem. Eng. 2015, 3, 1384–1392. [Google Scholar] [CrossRef]
- Huang, L.; Zhou, Y.; Guo, X.; Chen, Z. Simultaneous Removal of 2, 4-Dichlorophenol and Pb (II) from Aqueous Solution Using Organoclays: Isotherm, Kinetics and Mechanism. J. Ind. Eng. Chem. 2015, 22, 280–287. [Google Scholar] [CrossRef]
- De Faria, E.H.; Nassar, E.J.; Trujillano, R.; Mart, N.; Vicente, M.A.; Rives, V.; Gil, A.; Korili, S.A.; Ciu, K.J. Organically Modi Fi Ed Saponites: SAXS Study of Swelling and Application in Ca Ff Eine Removal. ACS Appl. Mater. Interfaces 2015, 7, 10853–10862. [Google Scholar] [CrossRef]
- Bentahar, Y.; Hurel, C.; Draoui, K.; Khairoun, S.; Marmier, N. Adsorptive Properties of Moroccan Clays for the Removal of Arsenic(V) from Aqueous Solution. Appl. Clay Sci. 2016, 119, 385–392. [Google Scholar] [CrossRef]
- Yang, Q.; Gao, M.; Luo, Z.; Yang, S. Enhanced Removal of Bisphenol A from Aqueous Solution by Organo- Montmorillonites Modified with Novel Gemini Pyridinium Surfactants Containing Long Alkyl Chain. Chem. Eng. J. 2016, 285, 27–38. [Google Scholar] [CrossRef]
- Rathnayake, S.I.; Xi, Y.; Frost, R.L.; Ayoko, G.A. Environmental Applications of Inorganic—Organic Clays for Recalcitrant Organic Pollutants Removal: Bisphenol, A. J. Colloid Interface Sci. 2016, 470, 183–195. [Google Scholar] [CrossRef]
- Tawfik, F.M.; Eshaq, G.; Hefni, H.H.H.; Elmetwally, A.E. Remediation of Distilleries Wastewater Using Chitosan Immobilized Bentonite and Bentonite Based Organoclays. Int. J. Biol. Macromol. 2016, 86, 750–755. [Google Scholar] [CrossRef]
- Nourmoradi, H.; Avazpour, M.; Ghasemian, N.; Heidari, M.; Moradnejadi, K. Surfactant Modified Montmorillonite as a Low Cost Adsorbent for 4-Chlorophenol: Equilibrium, Kinetic and Thermodynamic Study. J. Taiwan Inst. Chem. Eng. 2016, 59, 244–251. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, P.; Wang, J.; Huang, R. Adsorption of Amido Black 10B from Aqueous Solutions onto Zr (IV) Surface-Immobilized Cross-Linked Chitosan/Bentonite Composite. Appl. Surf. Sci. 2016, 369, 558–566. [Google Scholar] [CrossRef]
- Huang, R.; Zhang, L.; Hu, P.; Wang, J. Adsorptive Removal of Congo Red from Aqueous Solutions Using Crosslinked Chitosan and Crosslinked Chitosan Immobilized Bentonite. Int. J. Biol. Macromol. 2016, 86, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Wang, J.; Huang, R. Simultaneous Removal of Cr (VI) and Amido Black 10B (AB10B) from Aqueous Solutions Using Quaternized Chitosan Coated Bentonite. Int. J. Biol. Macromol. 2016, 92, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, T.; Shibayama, T. Removal and Degradation of β-lactam Antibiotics in Water Using Didodecyldimethylammonium Bromide-Modified Montmorillonite Organoclay. J. Hazard. Mater. 2016, 317, 677–685. [Google Scholar] [CrossRef]
- Yang, Q.; Gao, M.; Zang, W. Comparative Study of 2, 4, 6-Trichlorophenol Adsorption by Montmorillonites Functionalized with Surfactants Differing in the Number of Head Group and Alkyl Chain. Colloids Surfaces A Physicochem. Eng. Asp. 2017, 520, 805–816. [Google Scholar] [CrossRef]
- Kahraman, H.T. Development of an Adsorbent via Chitosan Nano-Organoclay Assembly to Remove Hexavalent Chromium from Wastewater. Int. J. Biol. Macromol. 2017, 94, 202–209. [Google Scholar] [CrossRef]
- Ghavami, M.; Zhao, Q.; Javadi, S.; Samuel, J.; Jangam, D.; Jasinski, J.B.; Saraei, N. Change of Organobentonite Interlayer Microstructure Induced by Sorption of Aromatic and Petroleum Hydrocarbons—A Combined Study of Laboratory Characterization and Molecular Dynamics Simulations. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 324–334. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, L.; Xu, Y. Chitosan and Surfactant Co-Modified Montmorillonite: A Multifunctional Adsorbent for Contaminant Removal. Appl. Clay Sci. 2017, 146, 35–42. [Google Scholar] [CrossRef]
- Bahmanpour, H.; Awhadi, S.; Enjili, J.; Mohammad Hosseini, S.; Vanani, H.R.; Eslamian, S.; Ostad-Ali-Askari, K. Optimizing Absorbent Bentonite and Evaluation of Contaminants Removal from Petrochemical Industries Wastewater. Int. J. Constr. Res. Civ. Eng. (IJCRCE) 2017, 3, 34–42. [Google Scholar] [CrossRef]
- Shah, K.J.; Pan, S.-Y.; Shukla, A.D.; Shah, D.O.; Chiang, P.-C. Mechanism of Organic Pollutants Sorption from Aqueous Solution by Cationic Tunable Organoclays. J. Colloid Interface Sci. 2018, 529, 90–99. [Google Scholar] [CrossRef]
- Almasri, D.A.; Rhadfi, T.; Atieh, M.A.; McKay, G.; Ahzi, S. High performance hydroxyiron modified montmorillonite nanoclay adsorbent for arsenite removal. Chem. Eng. J. 2018, 335, 1–12. [Google Scholar] [CrossRef]
- Li, Y.; Hu, X.; Liu, X.; Zhang, Y.; Zhao, Q.; Ning, P.; Tian, S. Adsorption behavior of phenol by reversible surfactant-modified montmorillonite: Mechanism, thermodynamics, and regeneration. Chem. Eng. J. 2018, 334, 1214–1221. [Google Scholar] [CrossRef]
- Xu, Y.; Khan, M.A.; Wang, F.; Xia, M.; Lei, W. Novel Multi Amine-Containing Gemini Surfactant Modi Fi Ed Montmorillonite as Adsorbents for Removal of Phenols. Appl. Clay Sci. 2018, 162, 204–213. [Google Scholar] [CrossRef]
- Dessalegne, M.; Zewge, F.; Mammo, W.; Woldetinsae, G.; Diaz, I. Effective fluoride adsorption by aluminum oxide modified clays: Ethiopian bentonite vs commercial montmorillonite. Bull. Chem. Soc. Ethiop. 2018, 32, 199. [Google Scholar] [CrossRef]
- Belbel, A.; Kharroubi, M.; Janot, J.; Abdessamad, M.; Haouzi, A.; Khaldoun, I.; Balme, S. Preparation and Characterization of Homoionic Montmorillonite Modified with Ionic Liquid: Application in Dye Adsorption. Colloids Surf. A 2018, 558, 219–227. [Google Scholar] [CrossRef]
- Peng, S.; Mao, T.; Zheng, C.; Wu, X.; Wei, Y.; Zeng, Z.; Xiao, R.; Sun, Y. Polyhydroxyl gemini surfactant-modified montmorillonite for efficient removal of methyl orange. Colloids Surf. A Physicochem. Eng. Asp. 2019, 578, 123602. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Taghizadeh, A.; Taghizadeh, M. Surface Modified Montmorillonite with Cationic Surfactants: Preparation, Characterization, and Dye Adsorption from Aqueous Solution. J. Environ. Chem. Eng. 2019, 7, 103243. [Google Scholar] [CrossRef]
- Rahmani, S.; Zeynizadeh, B.; Karami, S. Removal of Cationic Methylene Blue Dye Using Magnetic and Anionic- Cationic Modified Montmorillonite: Kinetic, Isotherm and Thermodynamic Studies. Appl. Clay Sci. 2020, 184, 105391. [Google Scholar] [CrossRef]
- Iriel, A.; Marco-Brown, J.L.; Diljkan, M.; Trinelli, M.A.; Afonso, M.d.S.; Cirelli, A.F. Arsenic Adsorption on Iron-Modified Montmorillonite: Kinetic Equilibrium and Surface Complexes. Environ. Eng. Sci. 2019, 37, 22–32. [Google Scholar] [CrossRef]
- Kameda, T.; Honda, R.; Kumagai, S.; Saito, Y.; Yoshioka, T. Uptake of Heavy Metal Cations by Chitosan-Modified Montmorillonite: Kinetics and Equilibrium Studies. Mater. Chem. Phys. 2019, 236, 121784. [Google Scholar] [CrossRef]
- Zhu, S.; Xia, M.; Chu, Y.; Khan, M.A.; Lei, W.; Wang, F.; Muhmood, T.; Wang, A. Adsorption and Desorption of Pb (II) on L -Lysine Modi Fi Ed Montmorillonite and the Simulation of Interlayer Structure. Appl. Clay Sci. 2019, 169, 40–47. [Google Scholar] [CrossRef]
- Huang, Q.; Li, X.; Ren, S.; Luo, W. Removal of Ethyl, Isobutyl, and Isoamyl Xanthates Using Cationic Gemini Surfactant-Modi Fi Ed Montmorillonites. Colloids Surf. A 2019, 580, 123723. [Google Scholar] [CrossRef]
- Seyedi, Z.; Amooey, A.A.; Amouei, A.; Tashakkorian, H. Pentachlorophenol removal from aqueous solutions using Montmorillonite modified by Silane & Imidazole: Kinetic and isotherm study. J. Environ. Health Sci. Eng. 2019, 17, 989–999. [Google Scholar] [CrossRef]
- Chen, Z.; Tian, H.; Li, H.; Li, J.; Hong, R.; Sheng, F.; Wang, C.; Gu, C. Application of Surfactant Modi Fi Ed Montmorillonite with Different Conformation for Photo-Treatment of per Fl Uorooctanoic Acid by Hydrated Electrons. Chemosphere 2019, 235, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Shin, W.S. Removal of Salicylic and Ibuprofen by Hexadecyltrimethylammonium-Modified Montmorillonite and Zeolite. Minerals 2020, 10, 898. [Google Scholar] [CrossRef]
- Luis, J.; Martín, J.; Orta, M.; Medina-carrasco, S.; Luis, J.; Aparicio, I.; Alonso, E. Simultaneous and Individual Adsorption of Ibuprofen Metabolites by a Modified Montmorillonite. Appl. Clay Sci. 2020, 189, 105529. [Google Scholar] [CrossRef]
- Song, J.; Zhang, S.; Li, G.; Du, Q.; Yang, F. Preparation of Montmorillonite Modi Fi Ed Biochar with Various Temperatures and Their Mechanism for Zn Ion Removal. J. Hazard. Mater. 2020, 391, 121692. [Google Scholar] [CrossRef]
- Liu, S.; Chen, M.; Cao, X.; Li, G.; Zhang, D.; Li, M.; Meng, N. Chromium (VI) Removal from Water Using Cetylpyridinium Chloride (CPC)—Modi Fi Ed Montmorillonite. Sep. Purif. Technol. 2020, 241, 116732. [Google Scholar] [CrossRef]
- Van, H.N.; Van, H.C.; Luu, T.; Khoa, D.; Nguyen, V. The Starch Modified Montmorillonite for the Removal of Pb (II), Cd (II) and Ni (II) Ions from Aqueous Solutions. Arab. J. Chem. 2020, 13, 7212–7223. [Google Scholar] [CrossRef]
- Haghighat, M.H.; Mohammad-Khah, A. Removal of Trihalomethanes from Water using Modified Montmorillonite. Acta Chim. Slov. 2020, 67, 1072–7081. [Google Scholar] [CrossRef] [PubMed]
- El-Kousy, S.M.; El-Shorbagy, H.G.; El-Ghaffar, M.A. Chitosan/montmorillonite composites for fast removal of methylene blue from aqueous solutions. Mater. Chem. Phys. 2020, 254, 123236. [Google Scholar] [CrossRef]
- Bayram, T.; Bucak, S.; Ozturk, D. BR13 Dye Removal Using Sodium Dodecyl Sulfate Modified Montmorillonite: Equilibrium, Thermodynamic, Kinetic and Reusability Studies. Chem. Eng. Process. Process Intensif. 2020, 158, 108186. [Google Scholar] [CrossRef]
- Pormazar, S.M.; Dalvand, A. Adsorption of Direct Red 23 dye from aqueous solution by means of modified montmorillonite nanoclay as a superadsorbent: Mechanism, kinetic and isotherm studies. Korean J. Chem. Eng. 2020, 37, 2192–2201. [Google Scholar] [CrossRef]
- Rahmani, M.; Dadvand, A. Montmorillonite/Xanthan Gum—Sodium Alginate Hybrid. Polym. Bull. 2022, 79, 8241–8267. [Google Scholar] [CrossRef]
- Abu, M.H.; Goda, E.S.; Abdallah, H.M.; Esmail, A.; Gamal, H.; Ro, K. Innovative Bactericidal Adsorbents Containing Modi Fi Ed Xanthan Gum/Montmorillonite Nanocomposites for Wastewater Treatment. Int. J. Biol. Macromol. 2021, 167, 1113–1125. [Google Scholar] [CrossRef]
- Miao, Y.; Peng, W.; Cao, Y.; Chang, L.; Fan, G.; Yu, F. Facile Preparation of Sulfhydryl Modified Montmorillonite Nanosheets Hydrogel and Its Enhancement for Pb (II) Adsorption. Chemosphere 2021, 280, 130727. [Google Scholar] [CrossRef]
- Ali, I.; Kon, T.; Kasianov, V.; Rysev, A.; Panglisch, S.; Mbianda, X.Y.; Habila, M.A.; Almasoud, N. Preparation and Characterization of Nano-Structured Modi Fi Ed Montmorillonite for Dioxidine Antibacterial Drug Removal in Water. J. Mol. Liq. 2021, 331, 115770. [Google Scholar] [CrossRef]
- Imanipoor, J.; Ghafelebashi, A.; Mohammadi, M.; Dinari, M. Fast and Effective Adsorption of Amoxicillin from Aqueous Solutions by L-Methionine Modified Montmorillonite K10. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 611, 125792. [Google Scholar] [CrossRef]
- Xiao, T.; Luo, W.; Wei, J.; Yuan, X.; Huang, Q.; Zou, L. Adsorption of Tungstate Using Cationic Gemini Surfactant-Modified Montmorillonite: Influence of Alkyl Chain Length. Colloids Surf. A Physicochem. Eng. Asp. 2021, 629, 127484. [Google Scholar] [CrossRef]
- Zhang, H.; Xia, M.; Wang, F.; Li, P.; Shi, M. Adsorption Properties and Mechanism of Montmorillonite Modified by Two Gemini Surfactants with Different Chain Lengths for Three Benzotriazole Emerging Contaminants: Experimental and Theoretical Study. Appl. Clay Sci. 2021, 207, 106086. [Google Scholar] [CrossRef]
- Luo, W.; Huang, Q.; Zeng, P.; Cheng, C.; Yuan, X.; Xiao, T. Gemini Surfactant-Modified Montmorillonite with Tetrachloroferrate (FeCl−4) as a Counterion Simultaneously Sequesters Nitrate and Phosphate from Aqueous Solution. J. Hazard. Mater. 2021, 409, 124829. [Google Scholar] [CrossRef] [PubMed]
- Henriques, R.R.; Shiguihara, A.L.; Constantino, V.R.L.; Soares, B.G.; Amim, J. Adsorption behavior of tannic acid on polyethylenimine-modified montmorillonite with different morphologies. Polym. Bull. 2022, 80, 10139–10163. [Google Scholar] [CrossRef]
- Rezala, H.; Boukhatem, H.; Boudechiche, N.; Romero, A. Methyl Orange Adsorption by Modified Montmorillonite Nanomaterials: Characterization, Kinetic, Isotherms and Thermodynamic Studies. Indian J. Chem. Technol. 2023, 30, 85–93. [Google Scholar] [CrossRef]
- Ullah, N.; Ali, Z.; Ullah, S.; Sada, A.; Adalat, B.; Nasrullah, A.; Alsaadi, M.; Ahmad, Z. Synthesis of Activated Carbon-Surfactant Modified Montmorillonite Clay-Alginate Composite Membrane for Methylene Blue Adsorption. Chemosphere 2022, 309, 136623. [Google Scholar] [CrossRef]
- Chen, J.; Lu, J.; Su, L.; Ruan, H.; Zhao, Y.; Lee, C.; Cai, Z.; Wu, Z.; Jiang, Y. Enhanced Adsorption of Methyl Orange by Mongolian Montmorillonite after Aluminum Pillaring. Appl. Sci. 2022, 12, 3182. [Google Scholar] [CrossRef]
- Zhang, H.; Mu, Q.; Yu, X.; Zhou, K.; Chen, X.; Hao, H.; Li, Y. Preparation of Copper Ion Adsorbed Modified Montmorillonite/Cellulose Acetate Porous Composite Fiber Membrane by Centrifugal Spinning. Polymers 2022, 14, 5458. [Google Scholar] [CrossRef] [PubMed]
- Ordinartsev, D.P.; Pechishcheva, N.V.; Estemirova, S.K.; Kim, A.V.; Shunyaev, K.Y. Removal of Cr (VI) from Wastewater by Modified Montmorillonite in Combination with Zero-Valent Iron. Hydrometallurgy 2022, 208, 105813. [Google Scholar] [CrossRef]
- Xu, X.; Li, Y.; Hu, X.; Xie, G.; Xu, H.; Gao, M.; Zhang, X.; Zhang, R.; Tang, C.; Hu, X. Effect of Humic Acid on the Adsorption/Desorption Behaviors of Trivalent Chromium on Calcium Modified Montmorillonite and Kaolinite. Chemistryselect 2022, 7, e202104302. [Google Scholar] [CrossRef]
- Yang, S.; Cheng, Y.; E, T.; Wang, Y.; Liu, L.; Li, Y.; Wang, D.; Qian, J. Construction Si–O–Mo bond via etching method: Enhancing selective adsorption capacity of MoS2/montmorillonite to Pb2+. Mater. Today Chem. 2022, 26, 101056. [Google Scholar] [CrossRef]
- Wei, R.; Mo, Y.; Fu, D.; Liu, H.; Xu, B. Organo-Montmorillonite Modified by Gemini Quaternary Ammonium Surfactants with Different Counterions for Adsorption toward Phenol. Molecules 2023, 28, 2021. [Google Scholar] [CrossRef]
- Xie, H.; Wu, J.; Yu, M.; Yan, H.; Masum, S.; Cai, P. Bisphenol A Adsorption and Transport in Loess and Cationic Surfactant/Hydrophilic Polymer Modified Bentonite Liners. J. Environ. Manag. 2023, 336, 117604. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Meng, Z.; Cao, X.; Liu, Z.; Wu, Z.; Sun, H.; Sun, X.; Li, W. Effect of Double Carbon Chains on Enhanced Removal of Phenol from Wastewater by Amphoteric-Gemini Complex-Modified Bentonite ☆. Environ. Pollut. 2023, 320, 121088. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, R.; Peng, W.; Wang, Y.; Zhang, N.; Duan, Y.; Wang, S.; Liu, L. Adsorption of Cu(II) in aqueous solution by sodium dodecyl benzene sulfonate-modified montmorillonite. J. Chin. Chem. Soc. 2023, 70, 837–847. [Google Scholar] [CrossRef]
- Zeng, Z.; Yu, C.; Liao, R.; Cai, X.; Chen, Z.; Yu, Z. Preparation and Characterization of Sodium Polyacrylate Grafted Montmorillonite Nanocomposite for the Adsorption of Cadmium Ions Form Aqueous Solution. Colloids Surf. A Physicochem. Eng. Asp. 2023, 656, 130389. [Google Scholar] [CrossRef]
- Zadeh, E.N.; Fozooni, S.; Nejad, E.T.; Khaleghi, M. Synthesis of Fe3O4@MCM41 and Kaolinite Coated with Ethyl 2-((3-(Triethoxysilyl)Propylamino)(Phenyl)methyl)-3-Oxobutanoate and their Applications in Heavy Metal Removal and Drug Delivery: Optimization Study Using RSM. Silicon 2023, 15, 4723–4750. [Google Scholar] [CrossRef]
- Dai, X.; Jing, C.; Li, K.; Zhang, X.; Song, D.; Feng, L.; Liu, X.; Ding, H.; Ran, H.; Zhu, K.; et al. Enhanced bifunctional adsorption of anionic and cationic pollutants by MgAl LDH nanosheets modified montmorillonite via acid-salt activation. Appl. Clay Sci. 2023, 233, 106815. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, H.; Xue, P.; Tian, S.; Lv, Z.; Wang, R. High-Performance PVDF Water Treatment Membrane Based on IL-Na + MMT for Simultaneous Removal of Dyes and Oil-Water Emulsions. J. Environ. Chem. Eng. 2023, 11, 109093. [Google Scholar] [CrossRef]
- Lv, Z.; Xue, P.; Xie, T.; Zhao, J.; Tian, S.; Liu, H.; Qi, Y.; Sun, S. High-Performing PVDF Membranes Modified by Na + MMT/Ionic Liquids (ILs) with Different Chain Lengths: Dye Adsorption and Separation from O/W Emulsion. Sep. Purif. Technol. 2023, 305, 122516. [Google Scholar] [CrossRef]
- Moradi, H.; Sabbaghi, S.; Sadat, N.; Chen, P. Removal of Chloride Ion from Drinking Water Using Ag NPs-Modified Bentonite: Characterization and Optimization of Effective Parameters by Response Surface Methodology-Central Composite Design. Environ. Res. 2023, 223, 115484. [Google Scholar] [CrossRef] [PubMed]
- Nousir, S.; Platon, N.; Ghomari, K.; Sergentu, A.; Chieh, T.; Hersant, G.; Bergeron, J.; Roy, R.; Azzouz, A. Correlation between the Hydrophilic Character and Affinity towards Carbon Dioxide of Montmorillonite-Supported Polyalcohols. J. Colloid Interface Sci. 2013, 402, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Azzouz, A.; Nousir, S.; Platon, N.; Ghomari, K.; Chieh, T.; Hersant, G.; Bergeron, J.; Roy, R. Truly Reversible Capture of CO2 by Montmorillonite Intercalated with Soya Oil-Derived Polyglycerols. Int. J. Greenh. Gas Control 2013, 17, 140–147. [Google Scholar] [CrossRef]
- Azzouz, A.; Platon, N.; Nousir, S.; Ghomari, K.; Nistor, D.; Shiao, T.C.; Roy, R. OH-Enriched Organo-Montmorillonites for Potential Applications in Carbon Dioxide Separation and Concentration. Sep. Purif. Technol. 2013, 108, 181–188. [Google Scholar] [CrossRef]
- Roth, E.A.; Agarwal, S.; Gupta, R.K. Nanoclay-Based Solid Sorbents for CO2 Capture. Energy Fuels 2013, 27, 4129–4136. [Google Scholar] [CrossRef]
- Elkhalifah, A.E.; Maitra, S.; Bustam, M.A.; Murugesan, T. Effects of exchanged ammonium cations on structure characteristics and CO2 adsorption capacities of bentonite clay. Appl. Clay Sci. 2013, 83–84, 391–398. [Google Scholar] [CrossRef]
- Stevens, L.; Williams, K.; Yoong, W.; Drage, T.; Snape, C.; Wood, J.; Wang, J. Preparation and CO2 Adsorption of Diamine Modified Montmorillonite via Exfoliation Grafting Route. Chem. Eng. J. 2013, 215–216, 699–708. [Google Scholar] [CrossRef]
- Nousir, S.; Sergentu, A.; Shiao, T.C.; Roy, R.; Azzouz, A. Hybrid Clay Nanomaterials with Improved Affinity for Carbon Dioxide through Chemical Grafting of Amino Groups. J. Environ. Pollut. Remediat. 2014, 2, 58–65. [Google Scholar] [CrossRef][Green Version]
- Shah, K.J.; Imae, T.; Shukla, A. Selective Capture of CO2 by Poly(Amido Amine) Dendrimer-Loaded Organoclays. RSC Adv. 2015, 5, 35985–35992. [Google Scholar] [CrossRef]
- Alhwaige, A.A.; Ishida, H.; Qutubuddin, S. Carbon Aerogels with Excellent CO2 Adsorption Capacity Synthesized from Clay-Reinforced Biobased Chitosan-Polybenzoxazine Nanocomposites. ACS Sustain. Chem. Eng. 2016, 4, 1286–1295. [Google Scholar] [CrossRef]
- Atilhan, M.; Atilhan, S.; Ullah, R.; Anaya, B.; Cagin, T.; Yavuz, C.T.; Aparicio, S. High-Pressure Methane, Carbon Dioxide, and Nitrogen Adsorption on Amine-Impregnated Porous Montmorillonite Nanoclays. J. Chem. Eng. Data 2016, 61, 2749–2760. [Google Scholar] [CrossRef]
- Nousir, S.; Yemelong, G.; Bouguedoura, S.; Chabre, Y.M.; Shiao, T.C.; Roy, R.; Azzouz, A. Improved carbon dioxide storage over clay-supported perhydroxylated glucodendrimer. Can. J. Chem. 2017, 95, 999–1007. [Google Scholar] [CrossRef]
- Pires, J.; Juźków, J.; Pinto, M.L. Amino acid modified montmorillonite clays as sustainable materials for carbon dioxide adsorption and separation. Colloids Surfaces A: Physicochem. Eng. Asp. 2018, 544, 105–110. [Google Scholar] [CrossRef]
- Nousir, S.; Arus, V.-A.; Shiao, T.C.; Bouazizi, N.; Roy, R.; Azzouz, A. Organically modified activated bentonites for the reversible capture of CO2. Microporous Mesoporous Mater. 2019, 290, 109652. [Google Scholar] [CrossRef]
- Arencibia, A.; Pizarro, P.; Sanz, R.; Serrano, D.P. CO2 Adsorption on Amine-Functionalized Clays. Microporous Mesoporous Mater. 2019, 282, 38–47. [Google Scholar] [CrossRef]
- Horri, N.; Sanz, E.S.; Amaya, P.; Raul, A.; Najoua, S.; Srasra, F.; Srasra, E. Effect of Acid Activation on the CO2 Adsorption Capacity of Montmorillonite. Adsorption 2020, 26, 793–811. [Google Scholar] [CrossRef]
- Khajeh, M.; Ghaemi, A. Strontium Hydroxide-Modified Nanoclay Montmorillonite for CO2 Capture: Response Surface Methodology and Adsorption Mechanism. Int. J. Environ. Anal. Chem. 2021, 1–26. [Google Scholar] [CrossRef]
- Penchah, H.R.; Ghaemi, A.; Godarziani, H. Eco-friendly CO2 adsorbent by impregnation of diethanolamine in nanoclay montmorillonite. Environ. Sci. Pollut. Res. 2021, 28, 55754–55770. [Google Scholar] [CrossRef]
- Sun, W.; Li, J.; Li, H.; Jin, B.; Li, Z.; Zhang, T.; Zhu, X. Mechanistic Insights into Ball Milling Enhanced Montmorillonite Modification with Tetramethylammonium for Adsorption of Gaseous Toluene. Chemosphere 2022, 296, 133962. [Google Scholar] [CrossRef]
- Ansari, A.; Shahhosseini, S.; Maleki, A. Eco-Friendly CO2 Adsorption by Activated-Nano- Clay Montmorillonite Promoted with Deep Eutectic Solvent. Sep. Sci. Technol. 2023, 58, 1252–1274. [Google Scholar] [CrossRef]
- Ghosh, P.; Prusty, B.K.; Sandilya, P.; Turlapati, V.Y. Role of Pore Structure Parameters of Clay and Modified Clay Minerals on Their Hydrogen Adsorption at Low- and High-Pressure Conditions. Energy Fuels 2023, 37, 6757–6769. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, T.; Li, J.; Zhu, X. Enhanced Gaseous Acetone Adsorption on Montmorillonite by Ball Milling Generated Si—OH and Interlayer under Synergistic Modification with H2O2 and Tetramethylammonium Bromide. Chemosphere 2023, 321, 138114. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Huang, Y.; Yu, M.; Cheng, H.; Li, Z.; Xu, W. Improved Adsorption Capacity and Applicable Temperature of Gaseous PbCl 2 Capture by Modified Montmorillonite with Combined Thermal Treatment and Acid Activation. Chemosphere 2023, 313, 137466. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, B.; Xi, Y.; Megharaj, M.; Krishnamurti, G.S.R.; Bowman, M.; Rose, H.; Naidu, R. Bioreactive Organoclay: A New Technology for Environmental Remediation. Crit. Rev. Environ. Sci. Technol. 2012, 42, 435–488. [Google Scholar] [CrossRef]


| 1. Quaternary ammonium salts (QAS) Quaternary phosphonium cations Cetyl trimethylammonium Octadecylammonium ions Dodecyltrimethylammonium bromide Tetradecyl trimethyl ammonium bromide Hexadecyl trimethyl ammonium bromide Tetradecyldimethylbenzyl ammonium bromide Hexadecylldimethylbenzyl ammonium bromide Octadecylldimethylbenzyl ammonium bromide Tetra n-octylammonium bromide Dimethyldioctylammonium bromide Dimethyldistearileammonium bromide 2. Polymeric quaternary ammonium salts p-xylylenedichlorides and diamines tetramethyl-ionenes, Epichloridrin-dimethylamine Triethylamine, vinylbenzyl chloride, and lauryl acrylate copolymer Styrene, lauryl acrylate, and vinylbenzyl chloride 4-acetybiphenil Polyethylene-blockpoly(ethylene glycol) copolymers poly(ethylene glycol) Maleic anhydride and pentaerythritol Polyethersulfone membrane Poly(amido amine) (PAMAM) dendrimers 3. Ammonium salts 1-hexadecylamine, 1-octadecylamine, 4. Common gemini surfactants Pyridyl-containing gemini surfactants Aromatic- containing gemini surfactants Imidazoline- containing gemini surfactants hydroxyl- Containing gemini surfactants Imino- containing gemini surfactants Ether- containing genini surfactants | 5. Others Silane materials (3-aminopropyl)-triethoxysilane Alkyloxymethyl dimethyl dodecylammonium chlorides Bis(2-hydroxyethyl)laurylammonium Diethyl [2(methacryloyloxyl)ethyl]ammonium(DEM-M), Quinoline and pyridine groups 2-methylacry-loxyethyl dimethyl hexadecylammonium bromide Linear alcohol ethoxylate p-phenylamino-azo-benzene-3-benzene sodium sulfonate p-amino diphenylamine Benzidine crown-ethers Cryptand imadazolium salts 2-aminopyrimidine Alkylphosponium cations of (4-carboxybutyl)-Triphenylphosphonium bromide Methylene blue cations γ-metacryloxypropyl dimethyl methoxysilane Trifunctionalγ-methacryloxypropyl trimethoxysilane Coupling agents γ-aminopropyl trimethoxysilane Aniline salts AnF, AnCl, AnBr and AnI Aluminum hydroxide Aminopropyl and propyl trimethylammonium histidine 3-(N,N-di-methylhexadecylammonio) propane sulfonate 1,3-bis(hexadecyldimethylammonio)-propane dibromide bis-pyridinium dibromide (hexamethylene bis-pyridinium dibromide, HEMBP) 1,1′-didodecyl-4,4′-trimethylene bispyridinium bromide 1,1′-(butane-1,4-diyl)-bis(3-(tetradecyloxycarbonyl) pyridinium) dibromide Glycol bis-N-tetradecyl nicotinate dibromide bis-N,N,N-hexadecyldimethyl-p-phenylenediammonium dibromide 1,3-bis(dodecyldimethylammonio)-2-hydroxypropane dichloride 1,3-bis(dodecyldimethylammonio)-propane dibromide 1,3-bis(hexadecyldimethylammonio)-2-hydroxypropane dichloride 1,3-bis(octyldimethylammonio)-2-hydroxypropane dichloride |
| Clay | Modifier | Methodology | Application | Efficiency | Reference |
|---|---|---|---|---|---|
| Montmorillonite (China) | Fe3O4-CuO | Coprecipitation | Anthracene Removal | 96.2% | [29] |
| Montmorillonite (China) | Tetramethylammonium (a) Hexadecyltrimethylammonium (b) | Cation exchange | Heavy metal removal (Cr(VI)) | (TCLP) Cr(VI) 16.4% (a) 3.5% (b) | [30] |
| Montmorillonite (China) | trimethylstearylammonium bromide 3-merraptnpropyltrimethylxysilane thiol group | Cation exchange grafting | Heavy metal immobilization | 96.7% (Hg) | [31] |
| Montmorillonite (China) | humic acid | Cation exchange | Heavy metal removal | (TCLP) 97.6% (Cd) 93% (Hg) | [32] |
| Montmorillonite (China) | Magnesium | Cation exchange | Heavy metal removal | Cu 53.8% Pb 76.4% Zn 32.2% Cd 38.2% | [33] |
| Ca-Montmorillonite (China) | γ-Fe2O3 nanoparticles | Magnetic stirred Cation exchange | Pyrene Removal | 843.9 μg g−1 | [34] |
| Montmorillonite (China) | Fe(III) | Cation exchange | As(III) oxidation Anthracene | 90% <99% | [35] |
| Vermiculite-Montmorillonite (China) | MnO2 | Cation exchange | Heavy metal removal | 98.2% (Hg) | [36] |
| Montmorillonite (China) | Iron | Cation exchange | Heavy metal removal | 73% (Hg) | [37] |
| Montmorillonite (China) | butane-1,4-bis(dodecyl dimethyl ammonium bromide) tetrachloroferrate (FeCl4−) | Cation exchange | Heavy metal immobilization | 96.04 ± 0.12% (Cr) 43.91 ± 6.46% (W) | [38] |
| Montmorillonite (China) | Humus-like substances | polyphenol–Maillard reaction | Heavy metal removal | TCLP reduced 13.96% (Pb) 13.18% (Zn) | [39] |
| Montmorillonite (China) | goethite | co-precipitation | Heavy metal Removal | 50.61 mg g−1 (Cd) 57.58 mg g−1 (As) | [40] |
| Montmorillonite (China) | Exopolysaccharides by Rhizobium tropici | Cation exchange | Heavy metal Removal | 256 mg g−1 (Cs) 90.9 mg g−1 (Sr) | [41] |
| Montmorillonite (China) | Ferrihydrite | Ultrasonic combined with co-precipitated | Heavy metal removal | 86.28% (Sb) 94.60% (Pb) could not be detected (As) | [42] |
| Clay | Modifier | Methodology | Application | Efficiency | Reference |
|---|---|---|---|---|---|
| 2013 | |||||
| Bentonite (Sigma Aldrich) | Hexadecyltrimethylammonium bromide | Cation exchange | Dye Removal | 99.5% | [53] |
| Bentonite (Vietnam) | benzylhexadecyldimethylammonium chloride benzylstearyldimethylammonium chloride dimethyldioctadecylammonium bromide | Cation exchange | Phenol Removal | 0.92 mmol g−1 0.70 mmol g−1 0.64 mmol g−1 | [54] |
| Na-montmorillonite (Sigma Aldrich) | Dodecyltrimethylammonium bromide Didodecyldimethylammonium bromide | Cation exchange | Phenol Removal | 80% 99% | [55] |
| Ca-montmorillonite (Arizona) | Dodecyltrimethyl ammonium bromide Hexadecyltrimethyl ammonium bromide | Cation exchange | Phenol removal | 112.36 mg g−1 151.52 mg g−1 | [56] |
| Montmorillonite (Nigeria) | Hexadecyltrimethylammonium bromide | Cation exchange | Phenol Removal | 5.55 ± 1.18 mg L−1 to Below Detectable level | [57] |
| Bentonite (Iran) | Hexadecyltrimethylammonium | Cation exchange | Dye Removal | 50 mg mg−1 | [58] |
| 2014 | |||||
| Montmorillonite (Sigma Aldrich) | Dodecyltrimethylammonium bromide Hexadecyltrimethylammonium bromide Didodecyldimethylammonium bromide | Cation exchange | Phenol Removal | 96.3% 98.9% 99.5% | [59] |
| 2015 | |||||
| Bentonite (China) | dodecyltrimethylammonium bromide cetyltrimethylammonium bromide | Cation exchange | Phenol Removal | 585.8 mg g−1 458.2 mg g−1 | [60] |
| Bentonite (Western Australia) | Arquad® 2HT-75 and Palmic acid | Grafting | Heavy metal removal | Cu 7 mg g−1 Pb 7.5 mg g−1 | [61] |
| Na-montmorillonite (China) | Bis-N,N,N,-hexadecyldimethyl-pphenylenediammonium dibromide | Cation exchange | Phenol Removal | 124 mg g−1 | [62] |
| Bentonite Clay (Germany) | hexadecyltrimenthylammonium chloride | Cation exchange | Dye Removal | 99.99% (399.74 μmol g−1) | [63] |
| Bentonite Clay (Algeria) | Bis-imidazolium salts | Cation exchange | Dye Removal | 108 mg g−1 | [64] |
| Montmorillonite (China) | Dioctadecyldimethylammoniumchloride | Cation exchange | Phenol and Heavy Metal Removal | 93.1% 95.85% | [65] |
| Saponite (Spain) | Hexadecyltrimethylammonium bromide Aminopropyltriethoxysilane | Cation exchange | Caffeine Removal | 33.39 mg g−1 80.54 mg g−1 | [66] |
| 2016 | |||||
| Clay (Morocco) | HNO3 acid treatment | Adsorption | Heavy metal removal | 1.076 mg g−1 As(V) | [67] |
| Na-montmorillonite (China) | 1,10-didodecyl-4,40-trimethylene bispyridinium bromide 1,10-dihexadecyl-4,40-trimethylene bispyridinium bromide | Cation exchange | Phenol Removal | 222.2 mg g−1 208.3 mg g−1 | [68] |
| Montmorillonite STx-1 (Texas) | Cctadecyltrimethylammonium bromide (ODTMA, organic modifier) Hydroxy aluminium (Al13, inorganic modifier) | Cation exchange | Phenol Removal | 109.89 mg g−1 | [69] |
| Bentonite and Chitosan (Egypt) | Trimethylammonium bromide | Cation exchange | Dye Removal | 78% | [70] |
| Montmorillonite (Italy) | Tetradecyl trimethyl ammonium bromide Hexadecyl trimethyl ammonium bromide | Cation exchange | Phenol Removal | 25.9 mg g−1 29.96 mg g−1 | [71] |
| Bentonite (China) | Chitosan | Cation exchange | Dye Removal | 418.4 mg g−1 | [72] |
| Bentonite (China) | Chitosan | Cation exchange | Dye Removal | 500 mg g−1 | [73] |
| Bentonite (China) | N-2-hydroxy-propyl trimethyl ammonium chloride chitosan | Cation exchange | Dye Removal | 847.5 mg g−1 (99.7%) | [74] |
| Montmorillonite (Sigma Aldrich) | Ditetradecyldimethylammonium bromide Dihexadecyldimethylammonium bromide Cetyltrimethylammonium chloride Didodecyldimethylammonium bromide | Cation exchange | Phenol Removal | >98% | [75] |
| 2017 | |||||
| Sodium montmorillonite (China) | Y methyl hexadecyl bis[3-(dimethylhexadecylammonio)ethyl] Ammonium tribromide (16-2-16-2-16) Dimeric surfactants (1, 2-bis (hexadecyldimethylammonio) ethane dibromide, 16-2-16) Cetyl Trimethyl ammonium bromide (CTAB) | Cation exchange | Phenol removal | 322.6 mg g−1 306.7 mg g−1 328.9 mg g−1 | [76] |
| Montmorillonite (USA) | Chitosan | Cation exchange | Heavy Metal Removal | 128.43 mg g−1 (85.4%) | [77] |
| Montmorillonite (USA) | Hexadecyltrimethylammonium bromide | Cation exchange | Phenol Removal | 6.9% | [78] |
| Montmorillonite (China) | Hexadecyltrimethylammonium and Chitosan | Cation exchange | Phenol Cd2+ Conger redCrystal Violet | 11 mg g−1 8.8 mg g−1 325.6 mg g−1 460 mg g−1 | [79] |
| Bentonite (Iran) | Dimethyloctadecylammoniumchloride | Cation exchange | Phenol Removal | 88% | [80] |
| 2018 | |||||
| Montmorillonite (India) | Octadecyl dimethylbenzylammonium bromide c Tetradcyl dimethylbenzylammonium bromide Octadecyl trimethyl ammonium bromide Tetradecyl trimethyl ammonium bromide Tetraoctyl ammonium bromide Dimethyl dioctyl ammonium bromide Dimethyl distearyl ammonium bromide Hexadecyl trimethyl ammonium bromide | Cation exchange | Phenol and nitrobenzene removal | 15.062 mg g−1 (phenol) 31.713 mg g−1 (nitrobenzene) | [81] |
| Montmorillonite (Qatar) | Iron (III) chloride hexahydrate | Cation exchange | Heavy metal Removal | 0.191 mg g−1 | [82] |
| Montmorillonite (China) | (11-Ferrocenylundecyl) trimethyl ammonium bromide (FTMA) | Cation exchange | Phenol Removal | 19.3 mg g−1 | [83] |
| Na-Montmorillonite (China) | Didodecyl di- methyl hydroxypropyl-multi amine bis quaternary ammonium salt | Cation exchange | Phenol Removal 2-CP 2,4,6-TCP | 81.68 mg g−1 336.59 mg g−1 535.49 mg g−1 | [84] |
| Bentonite Montmorillonite (Ethiopia) | Aluminum oxi-hydroxide (AO) | Cation exchange | Fluoride Removal | 28% 45% | [85] |
| Montmorillonite (Algeria) | 1-butyl-3-methylimidazolium chloride | Cation exchange | Dye Removal | 1.918 mg g−1 | [86] |
| 2019 | |||||
| Na-Montmorillonite (China) | dioctadecyl tetrahydroxyethyl dibromopropane diammonium (DTDD) octadecylmethyldihydroxyethyl ammonium bromide (OMDAB) | Cation exchange | Dye Removal | 250.63 mg g−1 91.11 mg g−1 | [87] |
| Montmorillonite (Iran) | cetylpyridinium chloride monohydrate alkyl dimethyl benzyl ammonium chloride | Cation exchange | Dye Removal | 227.3 mg g−1 243.09 mg g−1 | [88] |
| Montmorillonite (Iran) | sodium eicosenoate cetyltrimethylammonium chloride Fe3O4MNPs (magnetic na- noparticles) | irradiated by ultrasound Cation exchange | Dye Removal | 246 mg g−1 | [89] |
| Montmorillonite (Argentina) | Fe(NO3)3·9H2O | Cation exchange | Heavy metal Removal | 6.3 g kg−1 (99%) | [90] |
| Montmorillonite (Janpan) | Chitosan | Cation exchange | Heavy metal Removal | 0.185 mg g−1 | [91] |
| Na-Montmorillonite (China) | L-lysine | Cation exchange | Heavy metal Removal | 48.89 mmol 100 g−1 | [92] |
| Montmorillonite (China) | Gemini surfactant, butane-1,4 bis(dodecyl dimethyl ammonium bromide) | Cation exchange | Ester Removal | 0.6 mmol g−1 | [93] |
| Acid Montmorillonite (Iran) | chloropropyl trimethoxy silane imidazole | Cation exchange | Phenol Removal | 95% | [94] |
| Na-Montmorillonite (China) | Hexadecyltrimethyl ammonium poly-4- Vinylpyridine-co-styrene | Cation exchange | Perfluorooctanoic acid (PFOA) removal | 355.3 mmol kg−1 289.6 mmol kg−1 | [95] |
| 2020 | |||||
| Montmorillonite and zeolite (Korea) | Hexadecyltrimethylammonium | Cation exchange | Phenol Removal | 23.8 mmol kg−1 59.4 mmol kg−1 | [96] |
| Montmorillonite (Spain) | octadecylamine | Cation exchange | Phenol Removal (ibuprofen) | 64 mg g−1 | [97] |
| Montmorillonite (China) | Biochar composites | Cation exchange | Heavy metal Removal | 8.163 mg g−1 | [98] |
| Montmorillonite (China) | Cetyl pyridinium chloride | Cation exchange | Heavy metal Removal | 95.99% (47.83 mg g−1) | [99] |
| Montmorillonite (Viet Nam) | Starch | Cation exchange | Heavy metal Removal | Pb(II) 99% Cd((II) 96.7% N((II) 52.8% | [100] |
| Montmorillonite (Iran) | chlorosulfonic acid | Cation exchange | Trihalomethanes | 87% | [101] |
| Montmorillonite (Egypt) | Chitosan | Cation exchange | Dye Removal | 276.03 mg g−1 | [102] |
| Montmorillonite (Iran) | Sodium dodecyl sulfate | Cation exchange | Dye Removal | 98.24% | [103] |
| Montmorillonite (Iran) | Alum nanoclay | furance | Dye Removal | 2500 mg g−1 | [104] |
| 2021 | |||||
| Montmorillonite (Germany) | Cetyl trimethylammonium bromide | Cation exchange | Dye Removal | 769.23 mg g−1 | [105] |
| Nanoclays Montmorillonite (Egypt) | Xanthan gum with poly(vinylimidazole) | In situ the free radical polymerization pro- cess for grafting and crosslinking xanthan gum | Dye removal | 99.99% (909.1 mg g−1) | [106] |
| Montmorillonite (China) | (3-Mercaptopropyl) trlmethoxysllane | Cation exchange | Heavy metal Removal | 97% (Pb) | [107] |
| Montmorillonite (India) | Nano-structured hetero- nuclear poly-hydroxo complexes cobalt with aluminum | Cation exchange and Calcination | Antibac- terial drug dioxidine Removal | 96.5% | [108] |
| montmorillonite K10 (Iran) | L-methionine | Cation exchange | Phenol Removal | 647.7 mg g−1 | [109] |
| Montmorillonite (China) | Alkyl chain | Cation exchange | Tungstate Removal | 100 mg g−1 | [110] |
| Ca-Montmorillonite (China) | propylbis (dodecyldimethyl) ammonium chloride (12−3−12) propylbis (octade- cyldimethyl) ammonium chloride (18–3-18) | Cation exchange | Benzotriazole micro-pollutants Removal | 21.52 mg g−1 10.52 mg g−1 | [111] |
| Montmorillonite (China) | Butane-1,4-bis (dodecyl dimethyl ammonium bromide) Tetrachloroferrate | Cation exchange | Nitrate Phosphate Removal | 8.77 mg g−1 (N) 28.1 mg g−1 (P) | [112] |
| 2022 | |||||
| Montmorillonite (Germany) | Polyethyleneimine | Cation exchange | Phenol Removal | 790.7 mg g−1 | [113] |
| Na-Montmorillonite (Algeria) | cetyltrimethylammonium bromide hydroxyl aluminium polycati | Cation exchange | Dye Removal | 99% | [114] |
| Montmorillonite (Pakistan) | Sodium benzyl dodycyel sulphate Activated carbon Alginate | Cation exchange | Dye Removal | 1429 mg g−1 | [115] |
| Montmorillonite (China) | Al-intercalated and Al-pillared | Magnetic stirred Calcined and Cation exchange | Dye Removal | 6.23 mg g−1 | [116] |
| Montmorillonite and Cellulose acetate (China) | Acetic acid Sodium dodecyl sulfonate Chitosan | Magnetic stirred Cation exchange | Heavy metal Removal | 46.155 mg g−1 52.381 mg g−1 60.272 mg g−1 (Cu) | [117] |
| Montmorillonite (Russia) | Fe3O4 particles and dodecyldimethylbenzylammonium Chloride | Cation exchange | Heavy metal Removal | 38.8 mg g−1 (Cr) | [118] |
| Ca-Montmorillonite (China) | Humic Acid | Cation exchange | Heavy metal Removal | 70.34% (Cr) | [119] |
| Montmorillonite (China) | MoS2 NaF | hydrothermal method Cation exchange | Heavy metal Removal | 97.09 mg g−1 (Pb) | [120] |
| 2023 | |||||
| Na-Montmorillonite (China) | Gemini quaternary ammonium surfactants | Cation exchange | Phenol Removal | 115.11 mg g−1 | [121] |
| Bentonite (China) | Hexadecyltrimethylammonium chloride and Carboxymethylcellulose | Cation exchange | Phenol Removal | 14.75 mg g−1 | [122] |
| Bentonite (China) | dodecyldimethyl betaine ethylene bis and (tetradecyl dimethyl ammonium chloride) | Cation exchange | Phenol Removal | 1280 mmol g−1 | [123] |
| Montmorillonite (China) | Sodium dodecyl benzene sulfonate | Cation exchange | Heavy metal Removal | 61.53 mg g−1 | [124] |
| Montmorillonite (China) | carboxylate polymer dimethyl vinyl ethoxylsilane | grafting | Heavy metal Removal | 63.49 mg g−1 | [125] |
| Kaolinite Fe3O4@MCM41 (Iran) | Ethyl 2-((3-(triethoxysilyl)propylamino)(phenyl)methyl)-3-oxobutanoate | Cation exchange | Heavy metal Removal | 99.66% (Pb) 93.18% (Cd) 99.88% (Pb) 96.075 (Cd) | [126] |
| Montmorillonite (China) | @MgAl-CO3 LDH | Cation exchange | Dye Removal | 815.998 mg g−1 | [127] |
| Na-Montmorillonite (China) | Polyvinylidene fluoride 1-Hexadecyl-3-methylimidazolium chloride Polyvinylpyrrolidone dimethylacetamide | Cation exchange and Magnetic stirred Degassed | Dye Removal Oil Removal | 90% 99% | [128] |
| Na-Montmorillonite (China) | Polyvinylidene fluoride different chain lengths ionic liquids | Cation exchange | Dye Removal | 99.67% | [129] |
| Bentonite (Iran) | Ag NPs | Cation exchange | Cl-1 Removal | 90% | [130] |
| Clay | Modifier | Methodology | Application | Efficiency | Reference |
|---|---|---|---|---|---|
| Na-Montmorillonite (Boltorn) | Ethylene glycol Trizmabase Pentaerythritol Mannitol Dipentaerythritol Ethoxylated pentaerythritol (D)-(+) Trehalose Polyvinyl alcohol | Dynamic impregnation Cation exchange | CO2 Removal | 13.8 μmol g−1 | [131] |
| Bentonite (Aldrich) | Sodium chloride | Impregnation Cation exchange | CO2 Removal | 16.42 μmol g−1 | [132] |
| Bentonite (Aldrich) | Sodium chloride | Impregnation Cation exchange | CO2 Removal | 14 μmol g−1 | [133] |
| Cloisite (Southern Clay Products) | 3-aminopropyltrimethoxysilane Polyethylenimine | Amine grafting | CO2 Removal | 7.5% | [134] |
| Bentonite (Sudan) | Ammonium cations | Cation exchange | CO2 Removal | 3.15 mmol g−1 | [135] |
| Montmorillonite (United Kingdom) | Diamine | Exfoliation grafting | CO2 Removal | 2.4 mmol g−1 | [136] |
| Na-Montmorillonite | (3aminopropyl)triethoxysilane Ethylene glycol | Nitrogen adsorption desorption isotherm Amino grafting | CO2 Removal | 240–250 mmol g−1 490–500 mmol g−1 | [137] |
| Laponite Hydrotalcite Sericite | poly(amido amine) dendrimers G4.0 and poly(amido amine) dendrimers G4.5 | Cation exchange | CO2 gas adsorption | 0.017 g/g | [138] |
| Chitosan (Aldrich) | Na-MMT | Carbon aerogel | CO2 Removal | 5.72 mmol g−1 | [139] |
| Montmorillonite (Sigma-Aldrich) | Amine | Impregnation Amine grafting | CO2, CH4 and N2 Removal | 7.16 mmol g−1 4.44 mmol g−1 3.86 mmol g−1 | [140] |
| Bentonite (Aldrich) | Perhydroxylated glucodendrimer | Thermal desorption Impregnation | CO2 Removal | 1.5 mmol g−1 | [141] |
| Laponite Hydrotalcite Sericite | poly(amido amine) dendrimers G4.0 and poly(amido amine) dendrimers G4.5 | Cation exchange | CO2 gas and NH3 gas adsorption | 17 mg/g 26 mg/g | [27] |
| Montmorillonite (Portugal) | Amino acid | Cation exchange | CO2 Removal | 0.8 mmol g−1 | [142] |
| Bentonite (Canada) | 3-amino-propyltriethoxysilane (γ-APTES) 3-diethanolamino- propyltriethoxysilane (3-diEtOH-APTES) | acid activation chemical grafting | CO2 Removal | 0.287 mmol g−1 0.279 mmol g−1 | [143] |
| Montmorillonite Bentonite Saponite Sepiolite Palygorskite (Spin) | Grafting with aminopropyl Diethylenetriamine organosilanes Impregnation with polyethyleneimine Double functionalization | grafted and impregnated | CO2 Removal | 60.4 mg g−1 (PEI) 45.7 mg g−1 (PEI) 66.9 mg g−1 (PEI) 61.3 mg g−1 (DT) 67.1 mg g−1 (PEI) | [144] |
| Montmorillonite (Tunisia) | Acid treatment | Cation exchange | CO2 Removal | 67.4 mg g−1 | [145] |
| Nanoclay montmorillonite (Iran) | Strontium hydroxide | Cation exchange | CO2 Removal | 102.21 mg g−1 | [146] |
| Nanoclay Montmorillonite (Iran) | Diethanolamine | Cation exchange | CO2 Removal | 219.9 mg g−1 | [147] |
| Montmorillonite (China) | tetramethylammonium | Cation exchange dry ball milling | gaseous toluene removal | 55.9 mg g−1 | [148] |
| Montmorillonite (Iran) | Choline Chloride-Urea | Cation exchange | CO2 Removal | 252 mg g−1 | [149] |
| Montmorillonite (Iran) | Acid treatment | Cation exchange | H2 Adsorption | 19.98 mg g−1 | [150] |
| Na-Montmorillonite (China) | H2O2 and tetramethylammonium bromide | high energy ball milling | Acetone Adsorption | 51.08 mg g−1 | [151] |
| Montmorillonite (China) | thermal treatment and hydrochloric acid activation | Calcination magnetic stirred | Gaseous PbCl2 Removal | 66.89% | [152] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, J.; Yu, J.; Shah, K.J.; Shah, D.D.; You, Z. Applicability of Clay/Organic Clay to Environmental Pollutants: Green Way—An Overview. Appl. Sci. 2023, 13, 9395. https://doi.org/10.3390/app13169395
Qi J, Yu J, Shah KJ, Shah DD, You Z. Applicability of Clay/Organic Clay to Environmental Pollutants: Green Way—An Overview. Applied Sciences. 2023; 13(16):9395. https://doi.org/10.3390/app13169395
Chicago/Turabian StyleQi, Jingfan, Jiacheng Yu, Kinjal J. Shah, Dhirpal D. Shah, and Zhaoyang You. 2023. "Applicability of Clay/Organic Clay to Environmental Pollutants: Green Way—An Overview" Applied Sciences 13, no. 16: 9395. https://doi.org/10.3390/app13169395
APA StyleQi, J., Yu, J., Shah, K. J., Shah, D. D., & You, Z. (2023). Applicability of Clay/Organic Clay to Environmental Pollutants: Green Way—An Overview. Applied Sciences, 13(16), 9395. https://doi.org/10.3390/app13169395







