Abstract
Background: Monitoring the vital signs of delirious patients in an intensive care unit (ICU) is challenging, as they might (un-)intentionally remove devices attached to their bodies. In mock-up scenarios, we systematically assessed whether a motion detector (MD) attached to the bed may help in identifying emergencies. Methods: We recruited 15 employees of the ICU and equipped an ICU bed with an MD (IRON Software GmbH, Grünwald, Germany). Participants were asked to replay 22 mock-up scenes of one-minute duration each: 12 scenes with movements and 10 without movements, of which 5 were emergency scenes (“lying dead-still, with no or very shallow breathing”). Blinded recordings were presented to an evaluation panel consisting of an experienced ICU nurse and a physician, who was asked to assess and rate the presence of motions. Results: Fifteen participants (nine women; 173 ± 7.0 cm; 78 ± 19 kg) joined the study. In total, 286 out of 330 scenes (86.7%) were rated correctly. Ratings were false negative (FN: “no movements detected, but recorded”) in 7 out of 180 motion scenes (3.9%). Ratings were false positive (FP: “movements detected, but not recorded”) in 37 out of 150 scenes (24.7%), more often in men than women (26 out of 60 vs. 11 out of 90, respectively; p < 0.001). Of note, in 16 of these 37 FP-rated scenes, a vibrating mobile phone was identified as a potential confounder. The emergency scenes were correctly rated in 64 of the 75 runs (85.3%); 10 of the 11 FP-rated scenes occurred in male subjects. Conclusions: The MD allowed for identifying motions of test subjects with high sensitivity (96%) and acceptable specificity (75%). Accuracy might increase further if activities are recorded continuously under real-world conditions.
1. Introduction
Intensive care units (ICU) enhance the survival of critically ill patients through constant active monitoring of their vital signs. Heartbeat, blood pressure, respiratory rate, and oxygen saturation are recorded continuously by default [1]. Setting upper and lower limits per vital parameter can then trigger off alarms with different levels of urgency. If the system registers pathological values, the medical staff is alerted by different alarm severity levels. A red alarm signifies a potentially life-threatening situation, prompting the team to respond to the alarm immediately [2]. Monitoring agitated or delirious patients at the ICU is particularly challenging since the attached wires are often not tolerated and may be (un-)intentionally removed by patients. Monitoring is even more difficult in acutely suicidal patients, who deliberately lure for situations with reduced care attention to remove sensors and hurt themselves, e.g., work breaks or shift changes of the responsible nurses [3,4].
If the standard monitoring system is no longer attached, “alarm notifications” are triggered to remind the intensive care staff to check the sensor and reattach the cabling. Such alarms can reiterate multiple times per shift and lead to a phenomenon known as “alarm fatigue”, i.e., the reaction time of the responsible nurse decreases [5,6]. During this unobserved period, no adequate alarm can be triggered, even if medical emergencies occur, such as life-threatening arrhythmias or cardiopulmonary arrest. Although relatively rare, the consequences of these scenarios may be fatal for the patient and legal for the medical staff. More often, these patients lie in their beds, palpate their surroundings with their hands, or prepare to stand up—in other words, they move [7].
Accordingly, the simultaneous detection of patients’ motions could provide information on the ability to move, thus filling an essential gap in the monitoring continuum until the traditional monitoring system is reconnected.
Motion detectors have been repeatedly tested at the ICU for various indications as measuring patients’ activity or mobility levels [8,9,10], assessing patients’ sleep quality [11], or reducing the number of artefact alarms in vital sign monitoring [12]. To date, no study has examined whether motion detectors can be used to identify emergencies. However, detecting immobility following an agitation episode may be highly suspicious for an urgent, vital threat requiring immediate alarm response.
In a proof-of-concept study, we aimed to evaluate whether ICU staff may reliably detect the simulated emergency situation of “lying dead-still” with the help of a noncontact motion detector. We hypothesized that “motionlessness” can be accurately identified, thus enabling correct alerts of medical staff to alarm situations without the traditional monitoring system.
2. Materials and Methods
2.1. Study Population
Between June 2022 and July 2022, 12 examined ICU nurses and three consultants of the ICU (all aged above 18 years) with profound experiences in the care of delirious patients were recruited as test subjects for this pilot study. Pregnant subjects were excluded as lying on the back might not be tolerated.
2.2. Ethical Approval
This study was conducted in accordance with the guidelines for Good Clinical Practice and the principles of the Declaration of Helsinki (2002). The responsible ethics committee of the University Hospital Würzburg, Germany, approved the study (file number: 268/21-am). All subjects provided written informed consent.
2.3. Motion Detector
The Internet of Things (IoT) device used for this study has the dimensions 7 × 9 × 6 cm (length × width × height) and can easily be attached underneath the headboard of the patient’s bed (Figure 1). The IoT device is equipped with a high-definition vibration sensor. It periodically measures acceleration in linear and angular directions and sends the measurement via Wi-Fi to the Orbit IIoT Cloud server. The reporting frequency can be set variably, with the minimum frequency being 1 s, which was used for the current analysis. The detector is provided by Orbit IIoT, a division of IRON Software GmbH (Grünwald bei München, Germany), and has been used mainly in technical areas.
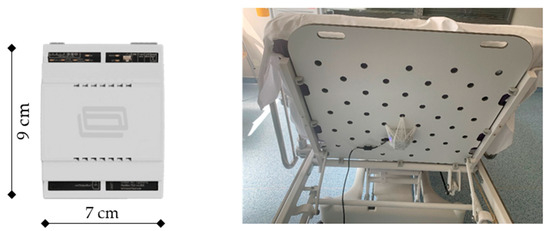
Figure 1.
Orbit IIoT MainBox (left) used at the ICU. The detector is attached underneath the bed with a bandage to allow the greatest possible transmission of vibrations (right). Abbreviations: cm—centimeter.
The sensor measures movements, including rotational movements, in all directions, as illustrated in Figure 2 (X–Y–Z axes corresponding to pitch, roll, and yaw). Sensed movements can be graphically displayed along one axis and combined as a mean square deviation of all lateral accelerations’ sqrt(∂x/∂t2 + ∂y/∂t2 + ∂z/∂t2) or the deviation of angular velocities’ ω = dΘ/dt sqrt(∂φ/∂t2 + ∂ζ/∂t2 + ∂θ/∂t2). Further combining all acceleration and rotational axes is possible using sqrt(∂x/∂t2 + ∂y/∂t2 + ∂z/∂t2 + ∂φ/∂t2 + ∂ζ/∂t2 + ∂θ/∂t2). The mean square deviation, also known as the mean squared error (MSE), is commonly used in the field of machine learning (ML), predictive algorithms, and optimization problems [13]. Due to the combination of all axis alignments, it highlights movements of any direction and may be of interest as a potential additional line within the standard monitor.
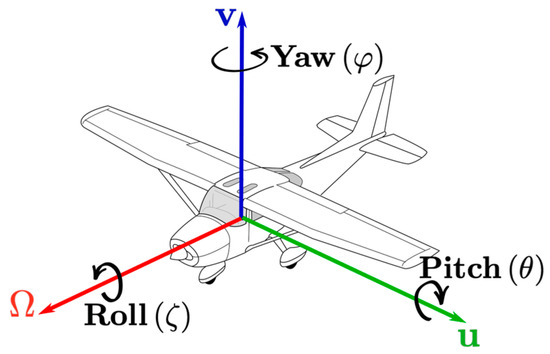
Figure 2.
Illustration of pitch, roll, and yaw.
2.4. Test Procedure
All scenes were categorized beforehand as scenes with or without “motions” based on whether the test subject physically moved during the scene (Table 1). As the impact of the breathing intensity or a vibrating mobile phone on motion detection was unclear and subject to the identification of potential confounders, these scenes were assigned to the category “no motion”.

Table 1.
Description, number, and order of scenes and motion intensities.
Before starting the test session, all 22 scenes were explained to each test subject (Table 1). Each scene had a duration of 1 min and was recorded separately. Subjects were asked to lie on a prepared ICU bed with the motion detector attached out of reach and sight, underneath the bed’s headboard at shoulder height (Figure 1). Test subjects were kept uninformed about where the motion detector was connected exactly. Recordings were stopped between each scene to allow for a short break and to re-explain the subsequent scene (complete list provided in Table 1).
In brief, the test started with the recording of an empty bed. The subject was then asked to get into bed, and the noninvasive monitoring system was connected, including recording of ECG, oxygen saturation, and blood pressure measurement. Afterward, the subject was asked to remove all cables and to perform all other scenes described in Table 1. The scene “lying motionless in bed, not breathing or keeping the breathing very shallow” (defined as the “emergency scene”) was performed 5 times per subject and was used as the primary endpoint. The test subject could improvise one scene (scene number 19, “Joker”), whereby all test subjects chose motions of different intensities (Table 2).

Table 2.
Characteristics of participants.
For the “mobile phone scene,” an iPhone XR 10 was placed below the pillow of each test subject with an activated vibrating function. One study team colleague called the number repeatedly to generate a constant vibration during the 1 min test period. In total, 330 (15 × 22) scenes were anonymized, recorded, and stored live on the Orbit IIoT Cloud-based platform time series database.
2.5. Panel
The graphical results (accelerometer raw x, y, z, and MSE) of each measured scene were provided to the panel in random order (Figure 3 as an example; see also Supplementary file).
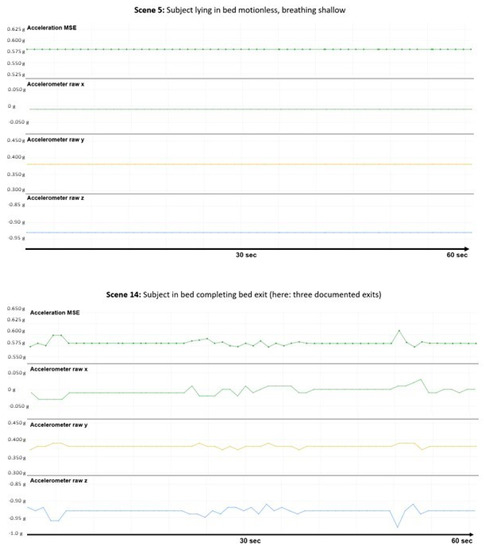
Figure 3.
Visualization of a no motion and a motion scene. Left: Scene #5: no motion, shallow breathing. Right: Scene #1: bed exit (twice or thrice).
The panel consisted of one examined ICU nurse (TF) and one consultant of the ICU (DS). The panel was trained beforehand by graphs recorded from pilot runs of a study team member (GG; Supplementary file). Afterward, the panel reviewed all charts from the test subjects on their own, without the involvement of the study team, and responded to the following questions in the case report form:
- Q1.
- Motions detected or not detected.
- Q2.
- Level of certainty of the response (low, medium, high).
- Q3.
- Intensity of motion (1—no motion, 2—weak motion, 3—strong motion).
- Q4.
- The assumed number of the scene or a possible alternative (see Table 1; all scenes with no motion were counted within one category, i.e., #1, #5, #7, #9, #11, #12, #15, #17, #21, #22).
2.6. Power Analysis
The effectiveness of the motion detector in correctly sensing the above-described emergency “test subject is lying motionless” was entirely unclear before the study. Therefore, only hypothetical estimates were used to calculate case numbers. If the device were useless, the panel’s prediction probability of correctly recognizing the above-described situation would correspond to guessing the correct answer, i.e., the likelihood of 50%. Since using the device at the ICU as a backup sensor would only be justified if the effect size is large, we assumed a high accuracy of the panel decision, i.e., at least 85% for correctly recognizing the emergency mentioned above (motion detection: yes vs. no). Thus, with an assumed effect size of 0.35, an alpha error of 5%, a power of 80%, and using the exact binomial proportion sign test, 13 subjects are required (G-Power statistics 3.1.9.7). With an estimated failure rate of about 10%, 15 subjects were included in this study.
2.7. Endpoints
For endpoint analysis, the panel results of each scene (motion detection: yes vs. no) were compared with the respective motion assignments in Table 1 (motions recorded, yes vs. no).
Primary endpoints: The primary endpoint was the correct identification of the contrived emergency scene by the panel, i.e., “subject is lying dead-still” (scenes #5, #9, #12, 15, and #17).
Secondary endpoints: Detection of “motions” and “motionlessness” in general and correct identification of all other scenes by the panel. We further investigated the influence of potential confounding factors (body weight, height, sex, mobile phone vibration).
2.8. Statistical Analysis
Data are described by count (percent) or median (quartiles), as appropriate. Comparisons between groups were made with Fisher’s exact test, chi-square test, or Mann–Whitney U-test. Diagnostic test performances (sensitivity, specificity, positive and negative predictive values, and Cohen’s kappa statistics with 95% confidence intervals (CIs)) were calculated by comparison of the panel results with the reference (Table 1). The level of concordance was determined with the classification system proposed by Landis and Koch, where a kappa of 0.61–0.80 is regarded as substantial, and a kappa of 0.81–1.00 indicates an almost perfect agreement [14].
A multivariable logistic regression analysis using the forward and backward selection methods included significant univariate predictors (with p-value < 0.05) of false assignments. As the results were similar, only the backward selection results are shown.
A significant group difference was assumed for all test procedures at a (two-sided) p-value of <0.05. Statistical analyses were performed using IBM SPSS Statistics for Windows Version 28.
3. Results
In total, 330 scenes (22 scenes × 15 test subjects) were recorded. Of those, 180 (12 × 15) were classified as “motion” scenes, and 150 (10 × 15) were classified as “no motion” scenes (Table 1). Fifteen participants (nine women; mean body height 173.3 ± 7.0 cm; mean body weight 77.7 ± 19 kg) joined the study. No participant terminated the study early, and no adverse events occurred (Table 2). Subjects’ characteristics and the chosen joker scenes are shown in Table 2, together with the correct and false panel assignments per subject.
The panel assessment converged on whether motions were detected in 319 of the 330 scenes but disagreed in 11 scenes. As any uncertainty would prompt checking the patient’s condition under real-world conditions, they marked all of these 11 scenes as “no motion” scenes. Therefore, those scenes and ratings were not excluded from the analysis. In 10 of the 11 disagreement cases, no motions were recorded.
3.1. Diagnostic Test Performances
The expert panel correctly identified 286 of 330 scenes (86.7%; Table 3 and Table 4). False positive (FP) assignments (motion detection in no motion scenes) occurred in 37 of 150 scenes with no motions (FP rate 24.7%; specificity 75.3%). This finding occurred more often in men than women: in total, 26/60 (43%) vs. 11/90 (12%), p < 0.001 (Table 4). Further, FP occurred more often in scenes with than without a mobile phone: 6/30 (53%) vs. 21/120 (17.5%), p < 0.001 (Table 5). The breathing intensity by contrast (shallow, normal, strong) showed no reliable differences in the number of false assignments (number of FP: 2.2 vs. 2.0 vs. 3.0 FP).

Table 3.
Diagnostic test performances in the total cohort.

Table 4.
Sex differences in diagnostic test performance.

Table 5.
Panel results according to different scenes.
False negative (FN) assignments (no motion detection in motion scenes) occurred mainly in low-intensity motion scenes (scenes #2, #10, #18, #20). In total, 7/180 motion scenes were not recognized correctly (FN rate 3.9%; sensitivity 96%). There was no relevant difference between men and women concerning the number of FN test results: 3/72 (4.2%) vs. 4/108 (3.7%), p = 0.88.
The overall Cohen’s kappa coefficient between the panel and the actual scene was 0.73 (95% confidence interval 0.65; 0.80), indicating substantial agreement. If scenes including a mobile phone were excluded from the analysis, kappa increased to 0.80 (95% CI 0.73; 0.87).
Of note, if the eleven scenes with panel disagreements were excluded, the accuracy in the total cohort would have been similar: correct identification in 276 of 319 scenes (86.5%); 37 FP; 6 FN; Cohen’s kappa 0.72 (95% CI 0.64; 0.80). Again, kappa was higher if the mobile phone scenes were excluded (0.80 (95% CI 0.73; 0.87)).
3.2. Emergency Scene
The emergency scene was correctly identified in 64 out of 75 cases (85.3%; Table 4). Motions were false-positively detected in the remaining 11 out of 75 (14.7%) scenes, whereby 10 of these 11 scenes occurred in male subjects (Table 4). Of note, if the eleven scenes with panel disagreements were excluded, the associations would have been similar: correct identification in 58 out of 69 scenes (84.1%; 11 FP). However, the primary efficacy endpoint would have been missed (expected correct assignments > 85%).
3.3. Other Scenes
Table 5 summarizes the panel results of all 22 scenes performed by the 15 subjects. The scenes with the highest and the second highest number of FP assignments were seen for the vibrating mobile phone scenes (scenes #21 and #22: 9 out of 15 and 7 out of 15 scenes with incorrect assignments), indicating that a vibrating phone is a crucial confounder mimicking physical activity. The scene with the third highest number of FP assignments was found for scene number 1 (no bed occupancy; 5 out of 15 scenes were false positive).
Overall, the median grade of certainty by which the panel detected “motionlessness” was low, whereas the median grade of response certainty for all “motion scenes” was high (p < 0.001). The correct assignment of motion intensity was similar between motion and no motion scenes: 113/150 (75%) vs. 141/180 (78%), p = 0.60. The scene numbers were more often correctly assigned within the no motion scenes: 113/150 (75%) vs. 41/180 (23%), p < 0.001.
3.4. Determinants of False Test Results
In univariate and multivariable logistic regression, male sex and mobile phone scenes were significant predictors of false test results (p < 0.001; Table 6).

Table 6.
Determinants of false test results in logistic regression analysis.
4. Discussion
This proof-of-concept study found that a motion detector, attached under the ICU bed and not on the subject’s body, could help identify an emergency situation even if the classical monitoring modalities are disconnected. Motions were detected with high sensitivity (96%) and reasonable specificity (75%), with an error rate of 13.3% for incorrect assignments. Associations were similar if solely the emergency scene (“motionlessness with no or shallow breathing”) was tested (correct assignments 85.3%; error rate 14.7%).
False alarms of various origins are a constant challenge in the ICU. Alarm fatigue is a consequence of inappropriate alarms, and many concepts have been proposed to overcome false alarms and reduce alarm fatigue [6,15,16,17,18]. If the monitoring modalities are disconnected, as often seen in delirious patients, no false alarms but alarm notifications are given. This reminds the medical staff that the monitoring modalities must be reattached. However, if such alarms are triggered too often, alarm fatigue develops, and the reaction times increase [19,20,21]. During the reaction time, patients are left unmonitored. If motion detectors become part of the monitoring system, detection of patient motions could be used as an indirect confirmation of patients’ vitality until the conventional alarm system has been reattached. In particular, the reliable detection of motionlessness (“zero or near-zero lines”) may indicate the patient’s unconsciousness and trigger immediate high-level alarms, thereby bridging the observational gap created by the detachment of the conventional monitoring system.
While the idea of using motion detectors to monitor ICU patients is not new [8,9,10,11,12,22,23,24,25], their application to monitor potential emergencies has not been investigated so far. Importantly, motion detectors have not been approved medically for this indication and are, therefore, unsuitable as a solitary monitoring solution.
One of the most frequent indications of testing motion detectors at the adult ICU was to measure activity levels, especially in patients with mobility constraints [8,9,10]. As many ICU patients may develop severe critical illness neuropathy due to sedation and immobility, monitoring of the activity level is of prognostic importance [26]. The most common techniques utilized for activity monitoring at the ICU have been wearable accelerometers [10] and imaging techniques [8,9,27]. As wearable devices would probably not be tolerated in patients, who intentionally remove their standard monitoring system, imaging salutation appears more attractive. Reiter et al. tested a noninvasive mobility sensor using an RBG-D (red–blue–green–depth) camera in patients at the ICU [8,9]. Different activities such as lying in bed, sitting and exercising in bed, out-of-bed activities, and walking in an ICU patient room were tested. Automated prediction analysis showed high agreement between the motions detected by the sensor and the expert assessment [8]. Inaccurate estimations occurred mainly in the “lying in bed” and “sitting or exercising in bed” categories. Although the mobility intensity and scene durations were not described, results were fairly comparable to our data, indicating that other motion detectors can also accurately identify motionlessness [8]. Although the information on mobility constraints and exercise capacity of patients at the ICU has prognostic value, measuring their exact activity levels may not be essential during their stay at the ICU, and could also be performed later during rehabilitation. Most importantly, motion detectors are not regularly used for this indication at the ICU.
As motions are a relevant source for the inaccurate reading of vital signs, motion detectors were further tested to reduce the number of false alarms for the conventional monitoring system [12].
In recent years, newer noncontact techniques have been developed and practices introduced to replace the traditional contact-based monitoring systems that concentrate on estimating heart and respiratory rate via, for instance, video-based photoplethysmography or mid-wave infrared (MWIR) thermography [28,29,30]. The detection of motions in this area was performed primarily to reduce or blank out motion artefacts [30]. These techniques were primarily tested and applied in pediatric cohorts, as the significance of contactless monitoring is more meaningful here [31,32,33]. Noncontact monitoring using imaging techniques was also introduced in adult patients with dementia, but the procedure was still susceptible to errors, and safety was not regarded high enough to justify substituting the current standard in clinical practice [34,35].
Imaging techniques have another major disadvantage: continuous video surveillance or video recording with commonly accessible visualization of highly private patient ambience is difficult to justify from an ethical and privacy rights perspective, especially in delirious patients. There are legal concerns from the medical staff regarding video recordings at the ICU, as videotapes could be used to monitor and evaluate the daily work of the co-recorded employees [36]. Although privacy-observing imaging techniques such as thermal imaging or 3D depth cameras are primarily used to detect body movements [22,23], the difference to a real camera might not be detectable for the monitored subject. Thus, noncontact monitoring systems without privacy breaching potential should be the preferred modality in the ICU. Radar measurements to detect patient motions might be an alternative method compliant with data protection regulations [23]. However, the roll-out of stationary radars in an ICU room would imply larger-scale construction modifications, which may limit their use for cost and feasibility reasons.
Any new device comes with the risk of inducing false alarms [37]. According to our concept, motion detector alarms should only be triggered if the traditional system is detached and motionlessness is detected. If motions do not trigger the alarm, the number of false alarms should be low in agitated patients who constantly move around. Accordingly, the number of false negative test results (“motions not detected but recorded”) in our study was very low (3.9%), signifying high sensitivity for detecting motions. Clinically, a false negative test result (as observed in patients falling asleep) indicates that a “false” alarm was activated without an actual emergency. Thus, the walk to check the patient’s condition would have been unnecessary, which again may provoke alarm fatigue with all its consequences [6]. A suitable way to reduce false negative results triggered by motion detectors could be achieved with another promising sensor type.
Noncontact intrinsic optical fiber sensors can be easily installed underneath a patient’s pillow, enabling monitoring of heart rate and breathing frequency [24,25]. They also have a motion-detecting function, primarily used to recognize disruptive effects and blank out “false alarms” [28]. As mentioned, noncontact monitoring of vital signs is not established at the adult ICU and is not in clinical use for legal reasons; especially in moving subjects the error rate is estimated to be high [24,25]. However, when used as a backup monitor, programmed to measure vital signs and motions in patients who detached their conventional monitoring system and are no longer moving, its use could be justified and may exceed the accuracy of our device. With the added ability to monitor heart and respiration rates, the measurement of normal vital signs ranges could be used to block false negative alarms triggered by the motion detector feature.
Even more important could be the prevention of false positives since the (false) detection of motions in an unconscious patient can suppress the alarm function of the detector and thus impede early detection of an emergency. Our study’s amount of false positive results within the no motion scenes was 25% (N = 37/150). However, we identified a vibrating phone as a relevant confounder responsible for 43% (N = 16) of the false positive assignments. As most mobile phones cease the call automatically after 20–30 s, this confounder should not be of major concern in real-world situations. After excluding the vibrating mobile scene, the number of false positives was 14% (21/150). False positive assignments were more frequent in male test subjects. The reason for the sex difference is unclear but might indicate that the sensor susceptibility to triggering alarms has to be adapted individually. Alternatively, some physical characteristics, such as a broader torso or a larger head in men, may impede simulating the “lying dead-still scene” realistically. As the sensor was attached at shoulder height below the ICU bed, minimal breathing-dependent movements may have been transferred more easily to the sensor. In real-world situations in which patients have a true emergency, such as a cardiopulmonary arrest, sex differences are most probably less apparent. False positive assignments were also found for scene #1, i.e., the scene with the bed left empty, indicating that the sensor might detect motions differently if the bed is not occupied and that, so far, unidentified external interference factors might trigger minimal sensor activation. Continuous monitoring under real-world conditions should, however, minimize the error rates. In patients with a life-threatening emergency, the overall activity level may be lower than in the mock-up emergency scenes of our test subjects. Further, an actual emergency lasts longer than the predefined experimental period of 1 min. If the sensor could be programmed to sound alarms after detecting “zero or near-zero lines” for a specific period (e.g., 20–30 s) and after detecting longer episodes of minimal motion intensities, the probability of missing true emergencies may likely reduce.
Other clinical situations in which motion detectors might be helpful are attempted or succeeded bed exits. In our study, the bed exit pattern was very characteristic. This scene was the second most often recognized of all motion scenes (after hitting the bed with fists). There are already motion-sensing mattresses with multiple implemented pressure sensors that are in use for the detection of bed exits and—depending on the added technical features—with multiple other functions such as measurements of respiration, heart rate, sleep pattern, sleep quality, etc. [38]. These mattresses are mainly used for geriatric or nursing home patients. Although smart mattresses may also have other indications at the ICU, such as preventing pressure ulcers [39,40], feasibility and cost issues may limit their use in the ICU. As many patients are not delirious at admission but develop delirium at the ICU [41], affected individuals would need a bed or mattress change, which may be more labor-intensive in an uncooperative patient than the attachment of a portable motion detector underneath the bed or pillow. Further, the robustness and longevity of smart mattresses at ICUs are unclear, as recurrent resuscitations may damage the sensors.
A motion detector that can be attached underneath an ICU bed and solitarily records patients’ motions via acceleration or pressure changes constitutes an approach that is very easy to implement and will be received more readily by medical staff. It further would not change the current care standard but expand it for another monitoring line. Lastly, the additional monitoring of motions in selective patients should neither replace the standard nor discourage the ICU staff from reacting promptly to the alarm notification. However, it has the potential to reliably inform the user whether a particular alarm necessitates a quick response.
5. Limitations
Several limitations of our study need to be considered. First, the current study evaluated only test subjects in mock-up scenarios using a sensor not attached to the routine monitoring system and had not previously been tested for this indication. In addition, motions were detected during some scenes, although the bed remained empty. Therefore, accuracy and the need to adapt the sensor features must be reassessed in actual patients under real-world conditions. All scenes lasted one minute and were recorded and evaluated retrospectively by the panel. However, continuous monitoring lasting longer than 1 min should rather improve the test results, as automated alarming could be further adapted to the needs. Finally, for reasons of practicality, we chose a single-vibration sensor as the test object. Multiple motion sensors combining different (ICU-compatible) functions could further increase diagnostic test accuracy.
6. Conclusions
In conclusion, without direct contact with the test subject, a high-definition vibration sensor detected both motions and motionlessness during mock-up scenarios typical for delirious ICU patients with encouraging accuracy. Thus, assessing patients’ movements with a motion detector as a backup monitor in patients disconnecting their traditional monitoring system can allow for uninterrupted surveillance of an individual’s vitality in the ICU. Since the results are promising, a trial in delirious ICU patients appears feasible and justified.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app13169319/s1, Supplementary file with graphical motion recordings of all scenes (#1-22) captured in all directions (x-, y-, and z-axis, summarized with the mean squared error; MS).
Author Contributions
Conceptualization, G.G.; methodology, G.G. and C.K.; software, C.K. and V.K.; validation, T.F. and D.S.; formal analysis, G.G. and E.v.R., investigation, E.v.R. and G.G.; resources, D.W. and G.G., data curation, E.v.R. and G.G.; writing—original draft preparation, G.G.; writing—review and editing, S.F. and S.S.; visualization, G.G. and E.v.R.; supervision, S.S. and S.F.; project administration, G.G.; funding acquisition, C.K. and V.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by IRON Software GmbH, Grünwald bei München, Germany. This publication was supported by the Open Access Publication Fund of the University of Wuerzburg.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the University of Würzburg (protocol code 268/21; 10 January 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The approval granted by the Ethics committee did not envisage the data to be made publicly available. In case of any inquiries regarding further data analyses, please get in touch with the corresponding author of this study.
Acknowledgments
We thank our colleagues for participating in this study.
Conflicts of Interest
The authors declare no conflict of interest. The funders provided the materials (hardware and software) and set up the handling of the device but had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- McIntosh, N. Intensive care monitoring: Past, present and future. Clin. Med. 2002, 2, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Schmid, F.; Goepfert, M.S.; Reuter, D.A. Patient monitoring alarms in the ICU and in the operating room. Crit. Care 2013, 17, 216. [Google Scholar] [CrossRef]
- Garcia, R.M. Psychiatric Disorders and Suicidality in the Intensive Care Unit. Crit. Care Clin. 2017, 33, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Mills, P.D.; Watts, B.V.; Hemphill, R.R. Suicide attempts and completions on medical-surgical and intensive care units. J. Hosp. Med. 2014, 9, 182–185. [Google Scholar] [CrossRef]
- Drew, B.J.; Harris, P.; Zegre-Hemsey, J.K.; Mammone, T.; Schindler, D.; Salas-Boni, R.; Bai, Y.; Tinoco, A.; Ding, Q.; Hu, X. Insights into the problem of alarm fatigue with physiologic monitor devices: A comprehensive observational study of consecutive intensive care unit patients. PLoS ONE 2014, 9, e110274. [Google Scholar] [CrossRef]
- Jones, K. Alarm fatigue a top patient safety hazard. CMAJ 2014, 186, 178. [Google Scholar] [CrossRef] [PubMed]
- Meagher, D.J.; Moran, M.; Raju, B.; Gibbons, D.; Donnelly, S.; Saunders, J.; Trzepacz, P.T. Motor symptoms in 100 patients with delirium versus control subjects: Comparison of subtyping methods. Psychosomatics 2008, 49, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Reiter, A.; Ma, A.; Rawat, N.; Shrock, C.; Saria, S. Process Monitoring in the Intensive Care Unit: Assessing Patient Mobility Through Activity Analysis with a Non-Invasive Mobility Sensor. Int. Conf. Med. Image Comput. Comput. Assist. Interv. 2016, 9900, 482–490. [Google Scholar] [CrossRef]
- Ma, A.J.; Rawat, N.; Reiter, A.; Shrock, C.; Zhan, A.; Stone, A.; Rabiee, A.; Griffin, S.; Needham, D.M.; Saria, S. Measuring Patient Mobility in the ICU Using a Novel Noninvasive Sensor. Crit. Care Med. 2017, 45, 630–636. [Google Scholar] [CrossRef]
- Verceles, A.C.; Hager, E.R. Use of Accelerometry to Monitor Physical Activity in Critically Ill Subjects: A Systematic Review. Respir. Care 2015, 60, 1330–1336. [Google Scholar] [CrossRef]
- Delaney, L.J.; Currie, M.J.; Huang, H.C.; Litton, E.; Wibrow, B.; Lopez, V.; Haren, F.V. Investigating the application of motion accelerometers as a sleep monitoring technique and the clinical burden of the intensive care environment on sleep quality: Study protocol for a prospective observational study in Australia. BMJ Open 2018, 8, e019704. [Google Scholar] [CrossRef]
- Muroi, C.; Meier, S.; De Luca, V.; Mack, D.J.; Strassle, C.; Schwab, P.; Karlen, W.; Keller, E. Automated False Alarm Reduction in a Real-Life Intensive Care Setting Using Motion Detection. Neurocrit. Care 2020, 32, 419–426. [Google Scholar] [CrossRef]
- Mean Squared Error. In The Concise Encyclopedia of Statistics; Springer: New York, NY, USA, 2008; pp. 337–339. [CrossRef]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Sendelbach, S.; Funk, M. Alarm fatigue: A patient safety concern. AACN Adv. Crit. Care 2013, 24, 378–386. [Google Scholar] [CrossRef]
- Pelter, M.M.; Fidler, R.; Hu, X. Research: Association of Low-Amplitude QRSs with False-Positive Asystole Alarms. Biomed. Instrum. Technol. 2016, 50, 329–335. [Google Scholar] [CrossRef]
- Bonafide, C.P.; Lin, R.; Zander, M.; Graham, C.S.; Paine, C.W.; Rock, W.; Rich, A.; Roberts, K.E.; Fortino, M.; Nadkarni, V.M.; et al. Association between exposure to nonactionable physiologic monitor alarms and response time in a children’s hospital. J. Hosp. Med. 2015, 10, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Ruskin, K.J.; Hueske-Kraus, D. Alarm fatigue: Impacts on patient safety. Curr. Opin. Anaesthesiol. 2015, 28, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Cvach, M. Monitor alarm fatigue: An integrative review. Biomed. Instrum. Technol. 2012, 46, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Bell, L. Alarm fatigue linked to patient’s death. Interview by Laura Wallis. Am. J. Nurs. 2010, 110, 16. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, K.; Weisbrot, M.; Cieloszyk, A.; Medrzycka-Dabrowska, W.; Krupa, S.; Ozga, D. Impact of Alarm Fatigue on the Work of Nurses in an Intensive Care Environment-A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 8409. [Google Scholar] [CrossRef]
- Jakkaew, P.; Onoye, T. Non-Contact Respiration Monitoring and Body Movements Detection for Sleep Using Thermal Imaging. Sensors 2020, 20, 6307. [Google Scholar] [CrossRef]
- He, S.; Han, Z.; Iglesias, C.; Mehta, V.; Bolic, M. A Real-Time Respiration Monitoring and Classification System Using a Depth Camera and Radars. Front. Physiol. 2022, 13, 799621. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Cuevas, A.; Pena, E.R.; Rodriguez-Cobo, L.; Lomer, M.; Higuera, J.M. Low-cost fiber specklegram sensor for noncontact continuous patient monitoring. J. Biomed. Opt. 2017, 22, 37001. [Google Scholar] [CrossRef]
- Ochoa, M.; Algorri, J.F.; Roldan-Varona, P.; Rodriguez-Cobo, L.; Lopez-Higuera, J.M. Recent Advances in Biomedical Photonic Sensors: A Focus on Optical-Fibre-Based Sensing. Sensors 2021, 21, 6469. [Google Scholar] [CrossRef]
- Visser, L.H. Critical illness polyneuropathy and myopathy: Clinical features, risk factors and prognosis. Eur. J. Neurol. 2006, 13, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Davoudi, A.; Shickel, B.; Tighe, P.J.; Bihorac, A.; Rashidi, P. Potentials and Challenges of Pervasive Sensing in the Intensive Care Unit. Front. Digit. Health 2022, 4, 773387. [Google Scholar] [CrossRef]
- Diao, J.A.; Marwaha, J.S.; Kvedar, J.C. Video-based physiologic monitoring: Promising applications for the ICU and beyond. NPJ Digit. Med. 2022, 5, 26. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.B.; David, G.Y.; Zhao, S. Machine learning in intelligent video and automated monitoring. Sci. World J. 2015, 2015, 570145. [Google Scholar] [CrossRef]
- Antink, C.H.; Lyra, S.; Paul, M.; Yu, X.; Leonhardt, S. A Broader Look: Camera-Based Vital Sign Estimation across the Spectrum. Yearb. Med. Inform. 2019, 28, 102–114. [Google Scholar] [CrossRef]
- Cabon, S.; Poree, F.; Simon, A.; Rosec, O.; Pladys, P.; Carrault, G. Video and audio processing in paediatrics: A review. Physiol. Meas. 2019, 40, 02TR02. [Google Scholar] [CrossRef]
- Maurya, L.; Zwiggelaar, R.; Chawla, D.; Mahapatra, P. Non-contact respiratory rate monitoring using thermal and visible imaging: A pilot study on neonates. J. Clin. Monit. Comput. 2023, 37, 815–828. [Google Scholar] [CrossRef] [PubMed]
- Villarroel, M.; Guazzi, A.; Jorge, J.; Davis, S.; Watkinson, P.; Green, G.; Shenvi, A.; McCormick, K.; Tarassenko, L. Continuous non-contact vital sign monitoring in neonatal intensive care unit. Healthc. Technol. Lett. 2014, 1, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Kroll, L.; Bohning, N.; Mussigbrodt, H.; Stahl, M.; Halkin, P.; Liehr, B.; Grunow, C.; Kujumdshieva-Bohning, B.; Freise, C.; Hopfenmuller, W.; et al. Non-contact monitoring of agitation and use of a sheltering device in patients with dementia in emergency departments: A feasibility study. BMC Psychiatry 2020, 20, 165. [Google Scholar] [CrossRef] [PubMed]
- Harford, M.; Catherall, J.; Gerry, S.; Young, J.D.; Watkinson, P. Availability and performance of image-based, non-contact methods of monitoring heart rate, blood pressure, respiratory rate, and oxygen saturation: A systematic review. Physiol. Meas. 2019, 40, 06TR01. [Google Scholar] [CrossRef]
- Glancova, A.; Do, Q.T.; Sanghavi, D.K.; Franco, P.M.; Gopal, N.; Lehman, L.M.; Dong, Y.; Pickering, B.W.; Herasevich, V. Are We Ready for Video Recognition and Computer Vision in the Intensive Care Unit? A Survey. Appl. Clin. Inform. 2021, 12, 120–132. [Google Scholar] [CrossRef]
- Tscholl, D.W.; Handschin, L.; Rossler, J.; Weiss, M.; Spahn, D.R.; Nothiger, C.B. It’s not you, it’s the design—Common problems with patient monitoring reported by anesthesiologists: A mixed qualitative and quantitative study. BMC Anesthesiol. 2019, 19, 87. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.W.; Hsu, Y.L.; Chang, W.Y. Development of a bed-centered telehealth system based on a motion-sensing mattress. J. Clin. Gerontol. Geriatr. 2015, 6, 1–8. [Google Scholar] [CrossRef][Green Version]
- Bai, D.L.; Liu, T.W.; Chou, H.L.; Hsu, Y.L. Relationship between a pressure redistributing foam mattress and pressure injuries: An observational prospective cohort study. PLoS ONE 2020, 15, e0241276. [Google Scholar] [CrossRef]
- Bai, D.; Ho, M.C.; Mathunjwa, B.M.; Hsu, Y.L. Deriving Multiple-Layer Information from a Motion-Sensing Mattress for Precision Care. Sensors 2023, 23, 1736. [Google Scholar] [CrossRef]
- Park, S.Y.; Lee, H.B. Prevention and management of delirium in critically ill adult patients in the intensive care unit: A review based on the 2018 PADIS guidelines. Acute Crit. Care 2019, 34, 117–125. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).