Abstract
Using oleanolic acid as a starting compound, a series of new oleanane-type triterpenic derivatives were synthesized via O-acylation (with nicotinic, isonicotinic, and methoxycinnamic acid acyl chlorides), N-amidation (with cyclic- or polyamines), the Mannich reaction (with secondary cyclic amines), and Claisen–Schmidt condensation (with aromatic aldehydes), and their potencies as treatments for immunometabolic disorders were investigated. The compounds were evaluated against α-glucosidase and PTP1B enzymes and LPS-stimulated murine macrophages. It was found that the target compounds are highly effective α-glucosidase inhibitors but lack activity against PTP1B. A leading compound, N-methylpiperazine methylated 2,3-indolo-oleanolic propargyl amide 15, is also a micromolar inhibitor of NO synthesis in LPS-stimulated macrophages and suppresses oxidative bursts in neutrophils with similar efficiency. These results, in addition to its ability to stimulate glucose uptake in rat fibroblasts and improve maltose tolerance in rats, allow us to consider compound 15 a promising prototype drug for the treatment of immunometabolic defects in type 2 diabetes.
1. Introduction
Type 2 diabetes, marked by persistent hyperglycemia, is tied to obesity, insulin resistance, and systemic inflammation (metaflammation) that damages beta cells in the pancreas [1]. The disease contributes significantly to the prevalence of non-communicable diseases globally. Managing it often involves inhibiting α-glucosidase, an enzyme that helps convert complex carbohydrates into glucose. Existing pharmaceutical inhibitors, including acarbose and miglitol, help lower post-meal glucose levels but have drawbacks. They can be contraindicated for patients with gastrointestinal disorders and may not effectively control blood sugar levels. Thus, the pursuit of new compounds to act as α-glucosidase inhibitors for diseases like type 2 diabetes is critical [2]. They could potentially enhance glycemic control, decrease long-term complications, and improve overall disease management [3].
Currently, a large number of discoveries in the field of inhibitors are devoted to the synthesis of N-heterocyclic compounds. Benzimidazoles, thiadiazoles, thiazole, and triazoles are recommended as good inhibitors of α-glucosidase, acetylcholinesterase, and butyrylcholinesterase [4,5,6,7]. Among the various classes of compounds obtained from natural resources, triterpenoids also show potent antidiabetic activity, including α-glucosidase-inhibiting effects [8,9,10,11]. For example, oleanolic acid (OA) derivatives, such as oxime esters, showed inhibitory activities against α-glucosidase and α-amylase [12], a series of benzylidene derivatives exhibited excellent to moderate inhibitory effects, and inhibition kinetics results showed that the leading analogs were reversible and mixed-type inhibitors of α-glucosidase and α-amylase [13,14]. OA indole derivatives with various electron-withdrawing groups possessed anti-α-glucosidase activities and demonstrated the capacity to form ligand-enzyme complexes with α-glucosidase enzymes [15]. However, in the field of metabolic disorders, researchers have not achieved such progress as with CDDO (an OA derivative), a potent anticancer agent which was evaluated in phase I clinical trials for the treatment of advanced solid tumors and lymphoma via the activation of Nrf2 in peripheral blood mononuclear cells and the inhibition of NF-κB and cyclin D1 in tumor biopsies [16]. All this proves that oleanolic acid is a promising scaffold for obtaining new pharmacological agents, and the search for new inhibitors based on it remains an urgent and priority task.
Keeping in mind the importance of these types of compounds, in this study, oleanolic acid derivatives containing nicotinic, isonicotinic, methoxycinnamic, succinic, polyamines, amides, arylidene, propargylaminoalkyl, and azepano fragments were synthesized, and their potencies as antidiabetic agents were investigated. It was found that the majority of the compounds exhibit significant α-glucosidase-inhibiting properties. The N-methylpiperazine methylated 2,3-indolo-oleanolic propargyl amide with the most potential, obtained via a one-pot Cu (I)-catalyzed Mannich reaction, was the most active compound acting as a noncompetitive inhibitor, with a Ki (the dissociation constant of the enzyme-inhibitor complex) value estimated at 3.01 µM. The leading compound demonstrated that it can prevent inflammatory responses and the generation of ROS in immune cells and act synergistically with acarbose to ameliorate hyperglycemia. This exploration can provide alternative therapies while fostering a better understanding of immunometabolic disorders, thereby paving the path for targeted and potent treatments.
2. Materials and Methods
2.1. Chemistry
2.1.1. General
The spectra were recorded at the Center for the Collective Use ‘Chemistry’ at the Ufa Institute of Chemistry of the UFRC RAS and at the RCCU “Agidel” of the UFRC RAS. 1H and 13C NMR spectra were recorded using a “Bruker Avance-III” (Bruker, Billerica, MA, USA, 500 and 125.5 MHz, respectively, δ, ppm, Hz) in CDCl3, internal standard tetramethylsilane. Mass spectra were obtained using an LCMS-2010 EV liquid chromatograph–mass spectrometer (Shimadzu, Kyoto, Japan). Melting points were detected on a microtable «Rapido PHMK05» (Nagema, Dresden, Germany). Optical rotations were measured via a Perkin-Elmer 241 MC polarimeter (PerkinElmer, Waltham, MA, USA) with a tube length of 1 dm. Thin-layer chromatography analyses were performed on Sorbfil plates (Sorbpolimer, Krasnodar, Russia), using the solvent system chloroform/ethyl acetate in a ratio of 40:1. Substances were detected via a 10% of sulfuric acid solution with subsequent heating at 100–120 °C for 2–3 min. All chemicals were of reagent grade (Sigma-Aldrich). Compounds 6, 7 [17], 8 [18], 9 [19], 10 [20], 11–13, 15 and 16 [21]; 18 [22], 19 [23], 20, 24, and 27 [24]; 21, 22, and 25; and 28 [25], 29, and 30 [22] were prepared as described previously. All spectral data are provided in the Supplementary Materials file.
2.1.2. General Procedure for the Synthesis of Compounds 4 and 5
To a solution of compound 2 (1 mmol; 0.44 g) in dry pyridine (15 mL), 3-(3-methoxyphenyl)acryloyl chloride (2 mmol; 0.39 g) or nicotinoyl chloride (2 mmol; 0.28 g) and DMAP (cat.) was added. The reaction mixture was refluxed for 4 h, poured into H2O/H+, filtered, washed with water until neutral, and dried. The residue was purified via Al2O3 column chromatography (eluent petroleum ether-ethyl acetate 20:1→5:1).
- 3,28-Di-O-[3-(3-methoxyphenyl)acrylate]-olean-12(13)-en 4
Beige solid; mp: 114 °C; yield 78%; [α]D20 +17° (c 0.1, CHCl3). 1H NMR (500 MHz, CDCl3) δ 7.64 (m, 2H, H3″, H8″), 7.62 (m, 2H, H3′, H8′), 7.48 (d, J = 8.7 Hz, 2H, H9′, H9″), 6.92 (d, J = 1.5 Hz, 2H, H5′, H5″), 6.86 (d, J = 1.7 Hz, 2H, H7′, H7″), 6.33 (d, J = 2.7 Hz, 1H, H2″), 6.29 (d, J = 2.5 Hz, 1H, H2′), 5.23 (s, 1H, H12), 4.64 (dd, J = 9.2 Hz, 1H, H3), 4.17 (d, J = 11.0 Hz, 1H, H28), 3.83 (2s, 6H), 3.80 (m, 1H, H28), 2.16–1.00 (m, 23H, CH, CH2), 1.18, 0.99, 0.98, 0.94, 0.91, 0.90, 0.88 (7s, 21H, 7CH3). 13C NMR (125.5 MHz, CDCl3) δ 167.50 (C1′), 167.20 (C1″), 161.32 (C6″), 161.27 (C6′), 144.14, 143.96, 143.67 (C13), 131.97, 129.71, 129.68, 127.30, 127.25, 122.86 (C12), 116.35, 115.87, 114.30, 113.47, 113.40, 80.74 (C3), 70.68 (C28), 55.37 (OCH3), 55.32 (C5), 47.55 (C18), 46.27 (C19), 42.60, 41.69, 39.83, 38.34, 37.98, 36.86 (C10), 36.08, 34.05, 33.18, 32.50, 31.55, 30.95 (C20), 28.10, 25.99, 25.65, 23.71, 23.60, 22.37, 18.25, 16.89, 16.82, 16.74, 15.60. MS (APCI) m/z 763.6 [M+H]+ (calcd for C50H66O6, 763.07).
- 3,28-Di-O-[isonicotinate]-olean-12(13)-en 5
Beige solid; mp: 123°C; yield 72%; [α]D20 +5° (c 0.1, CHCl3). 1H NMR (500 MHz, CDCl3) δ 8.80–8.73 (m, 4H, H4″, H6″, H4′, H6′), 7.85–7.80 (m, 4H, H3″, H7″, H3′, H7′), 5.25 (s, 1H, H12), 4.79–4.72 (m, 1H, H3), 4.33 (d, J = 11.0 Hz, 1H, H28), 3.97 (d, J = 11.1 Hz, 1H, H28), 1.02–2.18 (m, 23H, CH, CH2), 1.19, 1.01, 0.99, 0.93, 0.91 (7s, 21H, 7CH3). 13C NMR (125.5 MHz, CDCl3) δ 165.06 (C1″), 164.75 (C1′), 150.67 (C6″, C4″), 150.56 (C6′), 150.51 (C4′), 143.37 (C13), 138.14 (C2′), 137.76 (C2′), 123.07 (C12), 122.86 (C3″, C7″, C3′, C7′), 82.72 (C3), 71.98 (C28), 55.29 (C5), 47.51 (C18), 46.15 (C19), 42.59, 41.69, 40.13, 39.83, 38.25, 38.10, 37.45, 36.85 (C10), 36.24, 34.15, 33.94, 33.13, 32.43, 31.62, 30.91 (C20), 28.20, 26.20, 26.02, 25.62, 23.57, 23.52, 22.45, 21.50, 18.20, 16.94, 16.73, 15.57. MS (APCI) m/z 653.5 [M+ H]+ (calcd for C42H56N2O4, 652.92).
2.1.3. Synthesis of Compound 17
N-Methylpiperazine (1.6 mmol; 0.16 mL), paraformaldehyde (0.3 g), NaOAc (5 mmol; 0.41 g), and CuI (0.05 mmol; 9.5 mg) were added to a solution of compound 11 (1 mmol; 0.56 g) in dry dioxane (10 mL). The reaction mixture was stirred under argon for 10 h at 60 °C. After the reaction, the mixture was diluted with water and extracted with CHCl3 (3 × 20 mL). The combined organic layer was washed with water (3 × 50 mL), dried over CaCl2 and evaporated under reduced pressure. The residue was purified by SiO2 column chromatography (eluent CHCl3–MeOH 100:0→90:10), obtaining compound 17.
- [3,2b]Indolo-N-(4-(piperazin-1-yl)but-2-yn-1-yl)-olean-12(13)-en-28-amide 17
Yellow solid; mp: 139℃; yield 81%; [α]D20 −21° (c 0.1, CHCl3). 1H NMR (500 MHz, CDCl3) δ 7.45–7.00 (m, 4H), 6.12 (br. s, NH), 5.50 (s, 1H, H12), 3.90 and 4.10 (d, 2H, J = 17.2 Hz, NHCH2), 3.30 (d, J = 9.1 Hz, NCH2), 2.80−2.50 (m, 8H, 4CH2), 2.30−1.30 (m, 23H, CH and CH2), 1.32, 1.30, 1.22, 1.20, 0.90, 0.89, 0.81 (7s, 21H, 7CH3). 13C NMR (125.5 MHz, CDCl3) δ 178.03 (CONH), 144.62 (C13), 140.90, 136.16, 128.10, 123.30 (C12), 120.92, 118.81, 117.85, 110.47, 106.50, 81.10 (C≡C), 79.11 (C≡C), 53.07 (2C), 51.32 (2C), 46.91, 46.66, 46.39, 46.24, 42.20, 40.94, 39.50, 38.01, 37.45, 36.82, 34.11, 34.01, 33.01, 32.26, 31.78, 30.97, 30.76, 29.63, 27.32, 25.60, 23.93, 23.62, 23.31, 19.29, 16.63, 15.67. MS (APCI) m/z 663.5 [M+ H]+ (calcd for C44H62N4O, 663.01).
2.1.4. General Procedure for the Synthesis of Compounds 14 and 26
To a solution of compound 3 (1 mmol; 0.53 g), oleanonic acid (1 mmol; 0.45 g), or 21 (1 mmol; 0.54 g) in dry CH2Cl2 (15 mL), (COCl)2 (0.08 mL, 1 mmol), and Et3N (2 drops) were added. The reaction mixture was stirred at room temperature for 2 h, then CH2Cl2 was evaporated to give an intermediate acyl chloride. This residue was dissolved in CH2Cl2 (5 mL) and a CH2Cl2 solution of piperazine (1.3 mmol; 0.11 g), 2-aminopyridine (1.3 mmol; 0.12 g) or ammonia solution (1.3 mmol; 0.02 mL), and Et3N (1 mmol; 0.14 mL) was added in each reaction. The reaction mixture was refluxed for 2 h, the organic layer was diluted with cold water (20 mL) and separated. The aqueous layer was extracted with CH2Cl2 (2 × 15 mL), the combined extracts were washed with 5% HCl (3 × 15 mL), H2O (2 × 15 mL), dried over CaCl2, and the solvent was evaporated in a water jet vacuum. Purification of the crude product was performed using column chromatography on Al2O3 by elution with a mixture of petroleum ether–chloroform (1:0 → 1:1).
- [3,2b]Indolo-N-piperazin-olean-12(13)-en-28-amide 14
Yellow solid; mp: 117 °C; yield 85%; [α]D20 +4° (c 0.1, CHCl3). 1H NMR (500 MHz, CDCl3) δ 7.45–7.00 (m, 4H), 5.38 (s, 1H, H12), 3.15–2.70 (m, 8H, 4CH2), 3.10−1.20 (m, 22H, CH and CH2), 1.30, 1.21, 1.19, 0.95, 0.93, 0.91, 0.81 (7s, 21H, 7CH3). 13C NMR (125.5 MHz, CDCl3) δ 175.34 (C28), 144.17 (C13), 140.91 (C3), 136.12 (C6′), 128.22 (C1′), 122.09 (C12), 120.89 (C4′), 118.82 (C3′), 117.98 (C2′), 110.36 (C5′), 106.81 (C2), 53.27 (2C), 51.63 (2C), 47.59, 46.47, 46.32, 44.60, 43.70, 42.05, 39.26, 38.15, 36.73, 34.01, 33.04, 32.36, 31.01, 30.41, 29.99, 27.99, 25.82, 24.03, 23.44, 23.32, 22.83, 19.31, 16.74, 16.68, 15.59. MS (APCI) m/z 596.5 [M+ H]+ (calcd for C40H57N3O, 595.92).
- 2-[(E)-pyridine-4-ylmethylene]-3-oxo-olean-12(13)-en-28-carboxamide 26
White solid; mp: 166 °C; yield 72%; [α]D20 +49° (c 0.1, CHCl3). 1H NMR (500 MHz, CDCl3) δ 8.71–8.65 (d, J = 6 Hz, 2H), 7.40–7.30 (d, J = 6.2 Hz, 2H), 6.95 (s, 1H), 5.85 (br.s, NH), 5.40 (d, J = 3.4 Hz, 1H, H12), 2.95–1.25 (m, 22H, CH, CH2), 1.24, 1.18, 1.15, 0.90, 0.95, 0.88, 0.87 (7s, 21H, 7CH3). 13C NMR (125.5 MHz, CDCl3) δ 207.09 (C3), 181.01 (C28), 147.92, 145.54, 145.04 (C13), 142.22, 139.08, 133.21, 126.21, 124.69, 122.19 (C12), 53.00, 46.62, 46.49, 45.42, 44.03, 42.69, 42.37, 39.36, 39.13, 36.35, 34.07, 32.97, 32.51, 31.56, 30.73, 29.50, 27.28, 25.72, 25.49, 23.50, 22.68, 20.24, 19.05, 16.59, 15.39. MS (APCI) m/z 543.5 [M+ H]+ (calcd for C36H50N2O2, 542.81).
2.1.5. Synthesis of Compound 23
4-Pyridinecarboxyaldehyde (1.3 mmol; 0.12 mL) and 40% KOH in ethanol (2.5 mL) were added to a solution of compound 19 (1 mmol; 0.53 g) in ethanol (5 mL) under stirring and cooling (from −5 to 10 °C). The mixture was stirred for 24 h at room temperature, pH was adjusted to neutral values with 5% HCl solution, and the mixture was poured into cold water (50 mL). The residue was filtered, washed with water and dried, then purified by column chromatography on Al2O3 using petroleum ether-CHCl3 (1:1 to 1:3) as eluent.
- N-(pyridin-2-yl)-2-[(E)-pyridine-3-ylmethylene]-3-oxo-olean-12(13)-en-28 carboxamide 23
Beige solid; mp: 154–156°C; yield 71%; [α]D20 +23° (c 0.1, CHCl3). 1H NMR (500 MHz, CDCl3) δ 8.63 (d, J = 4.8 Hz, 2H, H4″–H6″), 8.49 (d, J = 4.8 Hz, 2H, H3″- H7″), 8.30 (s, 1H, NH), 8.20–8.25 (m, 2H, H3′–H6′), 7.65 (m, 1H, H4′), 7.25 (s, 1H, H1″), 6.99 (m, 1H, H5′), 5.58 (s, J = 3.21 Hz, 1H, H12), 2.93 (d, J = 15.5 Hz, 1H, H1), 2.82 (d, J = 10.4 Hz, 1H, H18), 2.28 (d, J = 15.5 Hz, 1H, H1), 1.00–2.30 (m, 18H, CH, CH2), 1.23, 1.12, 0.93, 0.92, 0.82, 0.91, 0.69 (7s, 21H, 7CH3). 13C NMR (125.5 MHz, CDCl3) δ 207.41 (C3), 176.75 (C28), 151.43 (C1′), 149.82 (C4″, C6″), 148.69 (C2), 147.49 (C3′), 144.15 (C13), 143.50 (C2″), 138.48 (C5′), 137.96 (C1″), 124.11 (C3″, C7″), 122.89 (C12), 119.61 (C4′), 113.97 (C6′), 52.98 (C5), 47.40, 46.53, 45.38, 45.31, 43.96, 42.20 (C4), 42.10, 39.27, 36.31, 34.09, 32.99, 32.52, 31.48, 30.74, 29.48, 27.39, 25.73, 24.03, 23.78, 23.57, 22.02, 20.19, 16.09, 15.36. MS (APCI) m/z 620.5 [M+H]+ (calcd for C41H53N3O2, 619.89).
2.2. Biological Evaluation
Biological Assays
The experimental procedures for α-Glucosidase assay, PTP1B inhibition assay, cellular assay, animal assay, and statistical analysis are included in Supplementary Materials Data: S2 Biological evaluation.
3. Results and Discussion
3.1. Chemistry
Our previous studies confirm that compounds containing such groups as isonicotinic, methoxycinnamic, succinic, polyamine, arylidene, amide, propargylaminoalkyl, and azepane display cytotoxic and antimicrobial activity [25,26]. Also, we have shown that among the different types of triterpenoids [27,28,29], 2,3-indolo-betulinic acid glycine and L-phenylalanine amides were discovered as lead compounds with IC50 values of 0.04 and 0.05 μM, being 3784- and 4730-fold more active than acarbose [30]. Triterpene type indoles were highlighted as excellent pharmacophore platforms for the development of new anti-diabetes drugs [31]. We have shown that lupane-type C2-furfurylidene, m-iodo-benzylidene, and 3-pyridinylidene derivatives exhibited significantly better activity (up to 700 times higher) than acarbose [32], as well as the chalcone derivatives of platanic acid (20-oxo-lupanes) with furfurylidene and trifluoromethylbenzylidene fragments [33].
Thus, to study the inhibitory activity of α-glucosidase, a series of oleanolic acid derivatives with these pharmacophore groups was obtained.
Triterpenic platforms such as oleanolic acid 1, erythrodiol (ED) 2, 2,3-indolo- and C2-benzylidene derivatives of 3-oxo-OA were successfully used for the synthesis of the desired compounds 4–18 and 19–28 (Scheme 1, Scheme 2 and Scheme 3). Scheme 1 outlines the synthesis of compounds 4–10 by O-acylation and N-amidation of hydroxy and carboxy groups at C3 and C28 positions of OA 1 and ED 2. The reaction of nicotinic, isonicotinic, and methoxycinnamic acid acyl chlorides or succinic anhydride with 1 or 2 led to mono- and bis-esters 4–8. Amides 9 and 10 were obtained from 3-acetoxyoleanolic acid by the reaction of 1 with spermidine or diethyltriamine by the acyl chloride method in the presence of Et3N.
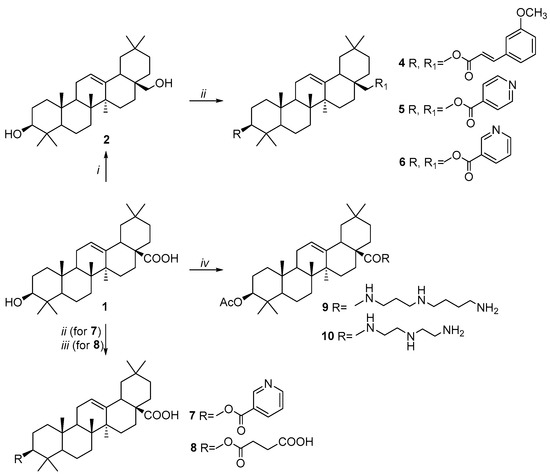
Scheme 1.
Synthesis of OA and ED derivatives 4–10. Reagents and conditions: (i) LiAlH4, THF, reflux; (ii) 3-(3-methoxyphenyl)acryloyl or nicotinoyl chlorides, DMAP, Py, reflux; (iii) succinic anhydride, Py, reflux; (iv) 1. Ac2O, Py, reflux; 2. (COCl)2, CH2Cl2, 25 °C; 3. spermidine or diethyltriamine, Et3N, CH2Cl2, 25 °C.
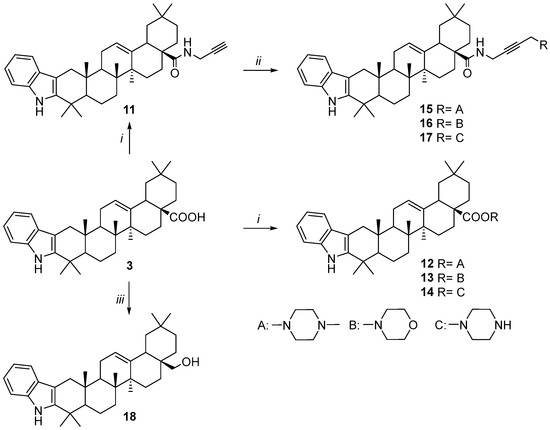
Scheme 2.
Synthesis of 2,3-indolo-oleanolic acid derivatives 12–18. Reagents and conditions: (i) 1. (COCl)2, CH2Cl2, 25 °C; 2. corresponding amine, Et3N, CH2Cl2; 25 °C; (ii) corresponding amine, PFA, NaOAc, CuI, 1,4-dioxane, 60 °C; (iii) LiAlH4, THF, reflux.
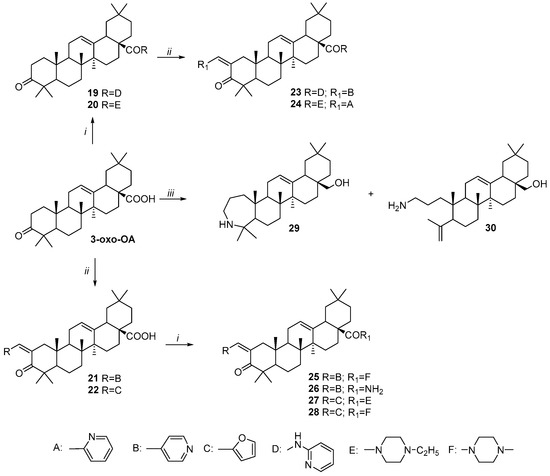
Scheme 3.
Synthesis of A-ring modified OA derivatives 19–30. Reagents and conditions: (i) 1. (COCl)2, CH2Cl2, 25 °C; 2. Corresponding amine, Et3N, CH2Cl2, 25 °C; (ii) corresponding aldehyde, 40% KOH in EtOH, EtOH; (iii) 1. NH2OH·HCl, NaOAc, EtOH, reflux; 2. SOCl2, 1,4-dioxane; 3. LiAlH4 THF, reflux.
Compounds 12–18 were prepared from 2,3-indolo-oleanolic acid 3 [15] (Scheme 2). First, the coupling of 3 with secondary amines by the acyl chloride method led to amides 12–14. The Mannich reaction of alkynylamide 11 with amines in the presence of a catalytic amount of copper iodide and sodium acetate produced propargylaminoalkyl derivatives 15–17.
Scheme 3 describes the Claisen–Schmidt condensation of 3-oxo-OA amide 19 and 20 derivatives with 2- or 4-pyridinecarboxaldehyde or furfural to afford C2-substituted compounds 23–28. Additionally, two other A-ring modified azepano- 29 and seco- 30 derivatives were synthesized [22].
3.2. Biological Evaluation
3.2.1. Enzymatic Screening
Based on our previous results [30], the synthesized compounds were evaluated as α-glucosidase inhibitors. Screening in 100 µM concentration was performed, followed by IC50 evaluation for active compounds. As Table 1 shows, the majority of oleanolic acid derivatives proved to be effective α-glucosidase inhibitors, significantly exceeding the activity of acarbose.

Table 1.
Biological activity of compounds 4–30.
The structure–activity relationship of compounds 4–30 was analyzed according to the experimental data in Table 1. One can see that the enzyme inhibition was increased when the 3,28-dihydroxy-groups of erythrodiol were esterified with m-methoxy-cinnamic acid (compound 4) and isonicotinic acid (compound 5). Compounds 4 and 5 exhibited much better potent inhibitory activity with IC50 values of 2.40 ± 0.47 μM, 4.56 ± 1.1 μM, respectively, which were about 96- to 182-fold higher than that of acarbose (IC50 436 μM). Compound 6 with 3,28-bisnicotinic moieties was not active (IC50 752.6 μM). The replacement of C28 to a free carboxy group in the case of compound 7 was not successful (IC50 >600 μM). OA succinate 8 is more active than 7 with IC50 value 6.50 ± 0.47 μM being 98-fold more efficient than the acarbose. 3β-Acetoxy-OA spermidine amide 9 exhibited significantly better activity (IC50 4.50 ± 0.9 μM) than another long-chain polyamine amide 10 (IC50 > 600 μM).
Modification of 2,3-indolo-OA propargyl amide 11 (IC50 139.1 ± 73.82 μM) to Mannich bases with N-methylpiperazine- 15 and morpholine- 16 moieties led to an increase in activity with IC50 values of 3.01 ± 0.53 μM and 13.91 ± 3.08 μM, respectively, that were about 31- to 145-fold higher than that of acarbose. At the same time, compound 17 with piperazine fragment showed only a weak activity. When the heterocycles, such as N-methylpiperazine and morpholine, were conjugated directly with an oleanane core via amide function at C28 (compounds 12 and 13), the inhibitory activity against α-glucosidase decreased. Meanwhile, piperazine amide 14 exhibited higher activity (IC50 38.3 ± 6.9 μM) than the propargyl containing analogue 17. The derivatives of erythrodiol with indole 18 and seco-amine 30 fragments showed potent activity with IC50 values of 12.37 ± 3.34 μM and 20.5 ± 1 μM, respectively, whereas compound 29 with A-azepano-cycle was inactive.
Among the series of C2-derivatives 21-28 obtained using Claisen–Schmidt condensation, 4-pyridinylideno-OA 21 demonstrated an activity with an IC50 value of 5.56 ± 1.88 μM, being 78-fold more active than acarbose. The enzyme inhibition activity decreased when the carboxyl group of the compound 21 was amidated using pyridine-2-amine up to 23 (IC50 6.6 ± 1.12 μM), N-methyl-piperazine up to 25 (IC50 79.35 ± 14.04 μM), or in the case of carboxamide derivative 26 (IC50 38.84 ± 6.63 μM). The introduction of the furfurylidene fragment (compound 22), as well as the modification of the carboxyl group to N-ethyl- 27 or N-methyl-piperazine amide 28, led to a negative effect on inhibitory activity. Pyridine 19 or N-ethyl-piperazine 20 amides exhibited high activity with an IC50 of 14.2 ± 6.71 μM and 18.88 ± 7.35 μM. Modification of 20 by introducing 2-pyridinylidene- 24 or furfurylidene 27 fragments led to the loss of activity, while the presence of isomeric 4-pyridinylideno fragments increased the activity of 23 (IC50 6.6 ± 1.12 μM) compared to 19 almost twice.
Several studies identified triterpenoid inhibitors of PTP1B, a valuable antidiabetic target [34,35,36]. We have also screened compounds against this enzyme, but no activity was observed up to 100 µM (). This may be attributed to the lack of a 3-hydroxy group, which appears to be a requisite for PTP1B inhibition [37].
3.2.2. Influence on LPS-Stimulated Macrophages
Considering the pivotal role of immunometabolic disorders in type 2 diabetes, target compounds were also profiled using a phenotypic screening approach [38]. Primary peritoneal murine macrophages were stimulated with LPS to induce a pro-inflammatory state. Target compounds were tested for the suppression of LPS-induced nitric oxide (NO) synthesis at a screening concentration of 10 μM. According to the MTT test, seven compounds (compounds 11, 18, 20, 22, 24, 25, and 27) inhibit the formation of NO by LPS-activated peritoneal macrophages in mice. Of these, compound 24 with an IC50 value of 7.8 μM was the most active, but this compound has no activity against a-glucosidase. Compound 20 is also non-cytotoxic, suppresses the synthesis of NO with an IC50 39.11 μM, and inhibits a-glucosidase with an IC50 of 18.88 μM.
Compound 15, the most potent glucosidase inhibitor, also actively suppressed NO synthesis (IC50 4.8 μM), but according to the MTT test, it also reduced cell metabolic activity. Final conclusions about cytotoxicity can be made after the involvement of additional research methods. The MTT test can both overestimate and underestimate the actual cell viability. In particular, such artifacts have been described for triterpene acids [39,40,41]. In general, 2,3-indolo-oleanolic acid derivatives appear to be more active than A-ring modified oleanolic acid derivatives. The anti-inflammatory activity was improved upon the introduction of the N-alkyl-piperazine moiety at C28 carboxyl, as exemplified by compounds 15 and 24.
We observed a lack of correlation between α-glucosidase and NO inhibition. Similar results were reported for betulinic acid derivatives [42]. Compound 15 appeared to be dual active and the most potent one, hence, it was nominated as a lead for further studies. There are a considerable number of studies on the role of intracellular glucosidase enzymes in pancreatic islet metabolism and macrophage immune response [43,44], also see [45], however the precise mechanistic basis of intracellular endoplasmic reticulum glucosidase involvement in NO synthesis remains to be elucidated. Also, multiple other protein targets can be engaged by triterpenoids, and the factor of the various cellular permeabilities of the studied compounds cannot be ruled out.
3.2.3. Mechanism of α-Glucosidase Inhibition by Compound 15
The mechanism of the inhibition of α-1,4-glucosidase of S. cerevisiae by lead compound 15 was elucidated using a kinetic experiment. Results show that 15 behaves as an uncompetitive (mixed-type) inhibitor. The linear correlation of the reaction slope with the concentration of the inhibitor suggests the presence of one allosteric binding site. The inhibition constant Ki was estimated at 3.011 μM (Figure 1).
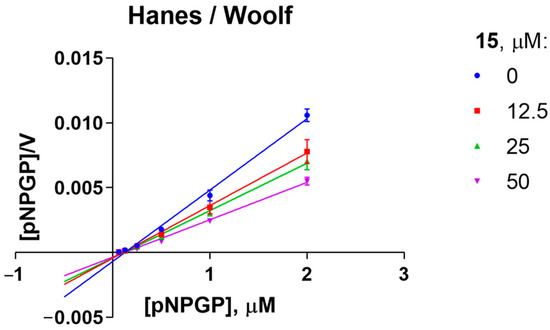
Figure 1.
Hanes–Woolf plot of kinetic experiment on the inhibition of S. cerevisiae α-glucosidase by compound 15. Analysis conditions: [E] 0.25 µM, [S] 1000.0 µM, Km 0.1166 µM.
The studied compounds are inactive against rat intestinal maltase (IC50 for acarbose is 7 μM), i.e., they will not affect the absorption of carbohydrates from the intestine.
3.2.4. Cytotoxic Evaluation of Compound 15 towards Macrophages
To determine whether MTT-based cytotoxicity data for compound 15 is reliable, additional experiments were performed with primary peritoneal macrophages. Neutral red (NR) is a cationic dye whose uptake is known to reflect ATP-dependent lysosomal function. The test with neutral red revealed a similar concentration response as was observed in the NO and MTT assays (Figure 2). MTT and neutral red uptake assays both show viability with CC50 values around 5 μM.
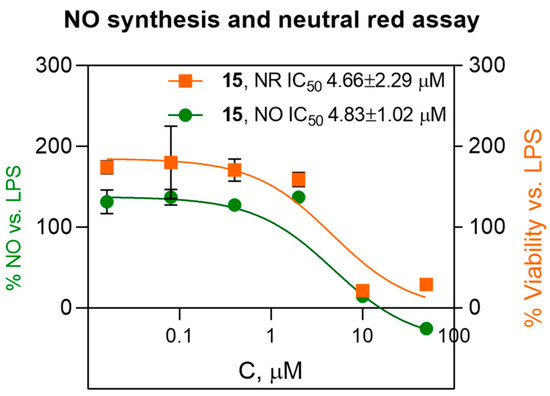
Figure 2.
Compound 15 inhibit NO synthesis and neutral red (NR) viability of LPS-stimulated peritioneal macrophages. Data are shown as mean ± SD (n = 3).
To confirm these findings, we have also assessed the morphological alterations of macrophages treated with 15 or dexamethasone, since this is considered the most reliable method for evaluating cytotoxicity [46]. The final concentration of compound 15 was set at 10 μM, which resulted in a complete loss of apparent viability according to MTT and neutral red uptake, and also a complete suppression of NO synthesis. In the LPS-treated control sample, the cells acquire an elongated fusiform stellate shape, the cells are spread out and are tightly attached to the culture plate (Figure 3). Surprisingly, both compound 15 and dexamethasone did not change the rounded shape of the cells to stellate under LPS stimulation, which may reflect a lack of M1-polarization and explain the suppression of NO synthesis. However, there are cells with nuclei on the periphery, loss of cytoplasm, and vacuolization of the membrane, this may indicate the presence of the first phase of apoptosis. It should be noted that similar features are evident in some of the intact and dexamethasone-treated cells as well. Cell morphology in the presence of 10 μM 15 remains normal and corresponds to the morphological features of intact cells. Final conclusions about cytotoxicity can be made after the involvement of additional research methods.
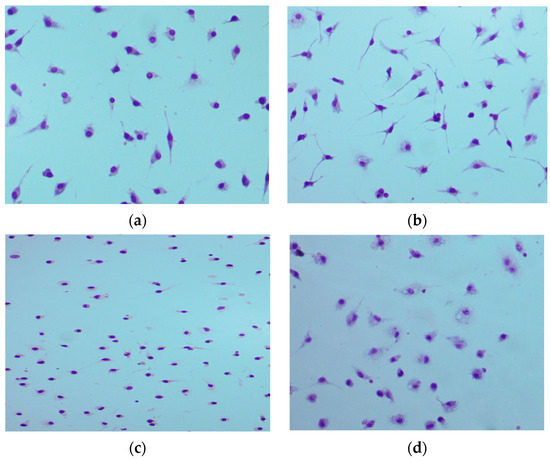
Figure 3.
The effect of compound 15 and dexamethasone on the morphology of mouse peritoneal macrophages. Azur-eosin staining according to Romanovsky; magnification 200× ((a)—Vehicle + 2% DMSO; (b)—LPS + 2% DMSO; (c)—LPS + 10 µM 15; (d)—LPS + 10 µM Dexamethasone).
3.2.5. Oxidative Burst in Neutrophils
Encouraged by the anti-inflammatory activity of 15 along with the intact morphology of macrophages, we tested the influence of compound 15 on the activation of murine neutrophils as well. There are many triterpenoids known to affect the phenotype, immune, and oxidative responses of neutrophils, e.g., celastrol [47] and glutinone [48]. Hence, it was of interest to evaluate the influence of compound 15 on the generation of reactive oxygen species in immune cells. Phagocytosis-induced neutrophil oxidative burst is a convenient model to study this subject. Compound 15 dose-dependently suppressed the zymosan-induced formation of reactive oxygen species in mouse neutrophils with an IC50 of 10 μM. Reducing neutrophil oxidative response has implications for both phagocytosis efficiency and overall oxidative stress in diabetic conditions (Figure 4).
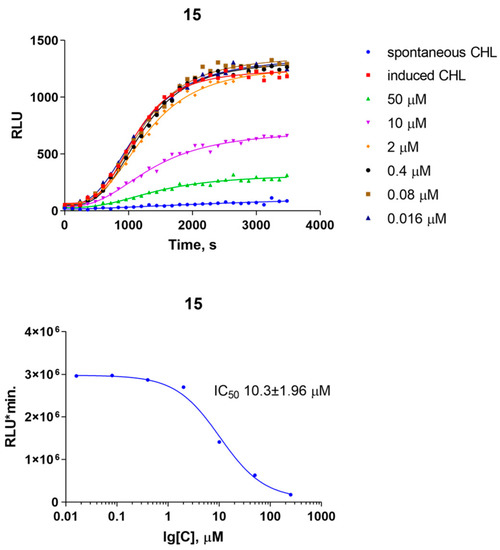
Figure 4.
Compound 15 suppresses the formation of reactive oxygen species during zymosan-induced phagocytosis in mouse neutrophils. CHL—chemiluminescence.
3.2.6. Free Radical Scavenging in Cell-Free System
To discriminate ROS inhibition elicited by lead compounds in cellulo from direct free radical scavenging, we assessed the antiradical activity of lead compounds in a cell-free system. Reactive oxygen species were generated with the hemoglobin-hydrogen peroxide system and detected as luminol-mediated chemiluminescence. Compound 15 scavenges reactive oxygen species in a cell-free model system, but in concentrations an order of magnitude greater than the effective concentrations in the cellular model. Thus, the IC50 for direct antiradical activity is roughly 85.9 μM (see Supplementary Materials), while oxidative burst in neutrophils is inhibited two-fold at 10 μM. This indicates a specific mechanism of action (probably a protein target).
3.2.7. Glucose Uptake in Fibroblasts
To further assess the antidiabetic potential of the lead compound, a cellular model of glucose uptake was employed. Rat neonatal myocardial fibroblasts were cultivated in DMEM containing 25 mM glucose to mimic hyperglycemic conditions. Incubation of fibroblasts with 20 μM of 15 for 24 h led to a significant increase in glucose consumption but, at the same time, to a concomitant drop in viability according to the MTT test (Figure 5).
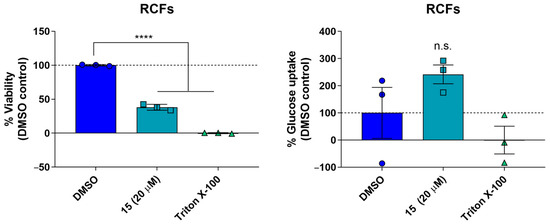
Figure 5.
The compound 15 at a concentration of 20 μM after 24 h of incubation suppresses the viability of neonatal myocardial fibroblasts in rats according to the MTT test, but stimulates glucose uptake (n = 3). Statistical significance according to the one-way ANOVA test with the Dunnet post-test (**** p < 0.0001, n.s.—not significant).
3.2.8. Oral Maltose Tolerance In Vivo
Finally, the antidiabetic potential of compound 15 was evaluated in intact animals. Considering the presumed mode of action, oral maltose tolerance in rats was used. The data obtained are presented as blood glucose levels vs. time and as the areas under the glucose time curves (Figure 6). Compound 15 at a dose of 5 mg/kg prevents hyperglycemia in intact rats after a maltose load of 2 g/kg. Substance 15 at a dose of 5 mg/kg improved maltose tolerance in rats in vivo, reducing the area under the curve relative to the control by 27.3% and by 58.5% when combined with acarbose. Hence, compound 15 is orally active and improves maltose tolerance synergistically with the reference α-glucosidase inhibitor acarbose.
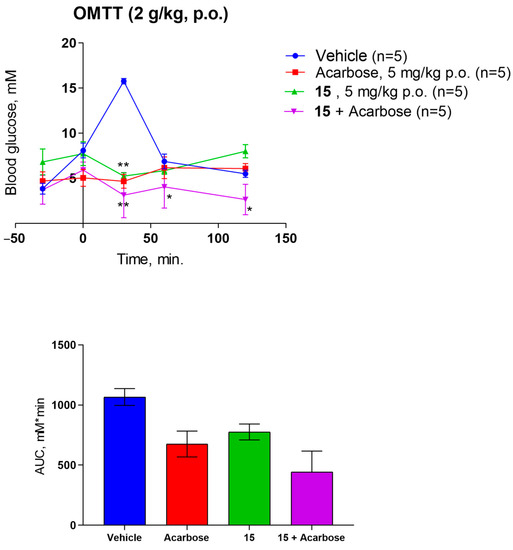
Figure 6.
Antihyperglycemic activity of 15 in rats after maltose challenge. Data shown as mean ± SD. Statistical significance vs. vehicle: two-way ANOVA with Tukey post-test: * p < 0.05, ** p < 0.01.
4. Conclusions
Triterpenoids of natural and semi-synthetic origin are structurally diverse compounds enriched with biological activity. As a development of our previous works, here we report the design, synthesis, and biological evaluation of novel oleanane-type triterpenic acid derivatives against yeast α-glucosidase. We have found that the majority of compounds exhibit significant inhibitory properties. Amide derivatives of 2,3-indolo-oleanolic acid proved to be especially effective, steadily surpassing the activity of the reference drug, acarbose. N-methylpiperazine methylated 2,3-indolo-oleanolic propargyl amide 15 was the most active compound acting as a noncompetitive inhibitor, with Ki estimated at 3.01 µM.
We have found that compound 15 also suppresses LPS-induced NO synthesis in the low micromolar range, representing anti-inflammatory properties. Further cellular studies revealed additional diabetes-related aspects of compound 15 modes of action. It inhibits the production of reactive oxygen species during an oxidative burst in neutrophils with an IC50 of 10 µM. In contrast, the direct radical scavenging activity of compound 15 in a cell-free system is an order of magnitude lower, indicating that a cellular target mediates its action on neutrophils. Rat fibroblasts treated with 20 µM of 15 demonstrate a two-fold increase in glucose uptake. Hence, lead compound 15 might be able to alleviate oxidative stress, inflammation, and hyperglycemia—key pathophysiological alterations of type 2 diabetes (Figure 7).
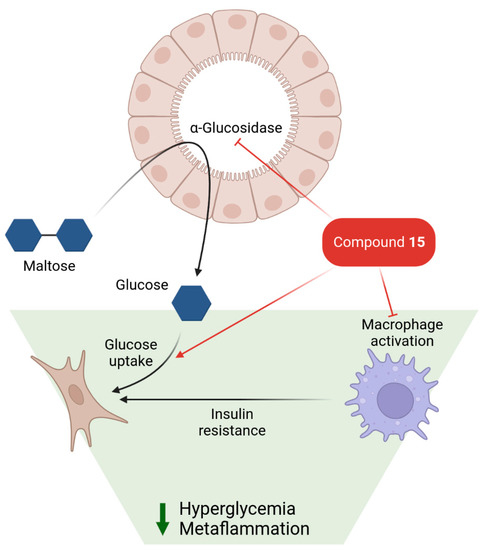
Figure 7.
Proposed mechanism of antidiabetic activity of compound 15.
The antidiabetic potential of 15 was confirmed in vivo using an oral maltose tolerance test. The treatment of rats with 5 mg/kg of 15 prevents hyperglycemia after a maltose load of 2 g/kg. Of note, we observed a synergy when the compound was co-administered with 5 mg/kg of acarbose. In conclusion, we have identified methylpiperazine methylated 2,3-indolo-oleanolic propargyl amide 15 as a promising lead compound for the development of agents against immunometabolic disorders. Future studies are warranted to assess its safety and long-term efficacy in animal models of diabetes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app13169269/s1, Figure S1–S24: NMR, IR, and mass spectra of compounds 4, 5, 14, 17, 23, and 26, (references cited in Supplementary Materials are [49,50,51,52,53,54,55,56,57,58]).
Author Contributions
O.B.K. brought the idea, managed the chemical research and prepared the manuscript; A.V.P. and E.F.K. conducted the chemical experiments and prepared the manuscript; I.P.B. conducted the NMR experiments; D.A.B. and E.V.S. conducted the biological experiments and prepared the manuscript; A.A.S. managed the biological research. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Federal programs No. 1021062311392-9-1.4.1 and 1022040400061-8-1.4.1;1.4.3 (Russia).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Appari, M.; Channon, K.M.; McNeill, E. Metabolic Regulation of Adipose Tissue Macrophage Function in Obesity and Diabetes. Antioxid. Redox Signal. 2018, 29, 297–312. [Google Scholar] [CrossRef]
- Kashtoh, H.; Baek, K.-H. Recent Updates on Phytoconstituent Alpha-Glucosidase Inhibitors: An Approach towards the Treatment of Type Two Diabetes. Plants 2022, 11, 2722. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.L.; Scott, C.A.B.; Lang, Z.; Bethel, M.A.; Tuomilehto, J.; Holman, R.R. Meta-Analysis of the Impact of Alpha-Glucosidase Inhibitors on Incident Diabetes and Cardiovascular Outcomes. Cardiovasc. Diabetol. 2019, 18, 135. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Ullah, H.; Taha, M.; Rahim, F.; Sarfraz, M.; Iqbal, R.; Iqbal, N.; Hussain, R.; Ali Shah, S.A.; Ayub, K.; et al. Synthesis, DFT studies, molecular docking and biological activity evaluation of thiazole-sulfonamide derivatives as potent alzheimer’s inhibitors. Molecules 2023, 28, 559. [Google Scholar] [CrossRef]
- Rahim, F.; Ullah, H.; Hussain, R.; Taha, M.; Khan, S.; Nawaz, M.; Nawaz, F.; Gilani, S.J.; Jumah, M.N.B. Thiadiazole based triazole/hydrazone derivatives: Synthesis, in vitro α-glucosidase inhibitory activity and in silico molecular docking study. J. Mol. Struct. 2023, 1287, 135619. [Google Scholar] [CrossRef]
- Hayat, S.; Ullah, H.; Rahim, F.; Ullah, I.; Taha, M.; Iqbal, N.; Khan, F.; Khan, M.S.; Shah, S.A.A.; Wadood, A.; et al. Synthesis, biological evaluation and molecular docking study of benzimidazole derivatives as α-glucosidase inhibitors and anti-diabetes candidates. J. Mol. Struct. 2023, 1276, 134774. [Google Scholar] [CrossRef]
- Nipun, T.S.; Khatib, A.; Ibrahim, Z.; Ahmed, Q.U.; Redzwan, I.E.; Saiman, M.Z.; Supandi, F.; Primaharinastiti, R.; El-Seedi, H.R. Characterization of α-glucosidase inhibitors from Psychotria malayana jack leaves extract using lc-ms-based multivariate data analysis and in-silico molecular docking. Molecules 2020, 25, 5885. [Google Scholar] [CrossRef]
- Atta-ur-Rahman; Zareen, S.; Choudhary, M.I.; Akhtar, M.N.; Khan, S.N. alpha-Glucosidase inhibitory activity of triterpenoids from Cichorium intybus. J. Nat. Prod. 2008, 71, 903–910. [Google Scholar] [CrossRef]
- Ouyang, J.K.; Dong, L.M.; Xu, Q.L.; Wang, J.; Liu, S.B.; Qian, T.; Yuan, Y.F.; Tan, J.W. Triterpenoids with α-glucosidase inhibitory activity and cytotoxic activity from the leaves of Akebia trifoliata. RSC Adv. 2018, 8, 40483–40489. [Google Scholar] [CrossRef]
- Ding, H.; Hu, X.; Xu, X.; Zhang, G.; Gong, D. Inhibitory mechanism of two allosteric inhibitors, oleanolic acid and ursolic acid on α-glucosidase. Int. J. Biol. Macromol. 2018, 107 Pt B, 1844–1855. [Google Scholar] [CrossRef]
- Khan, A.; Khan, I.; Halim, S.A.; Rehman, N.U.; Karim, N.; Ahmad, W.; Khan, M.; Csuk, R.; Al-Harrasi, A. Anti-diabetic potential of β-boswellic acid and 11-keto-β-boswellic acid: Mechanistic insights from computational and biochemical approaches. Biomed. Pharmacother. 2022, 147, 112669. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.Y.; Ke, J.J.; Zheng, Y.Y.; Li, D.L.; Zhang, K.; Zheng, X.; Wu, J.Y.; Xiong, Z.; Wu, P.P.; Xu, X.T. Synthesis and bioactivities evaluation of oleanolic acid oxime ester derivatives as α-glucosidase and α-amylase inhibitors. J. Enzym. Inhib. Med. Chem. 2022, 37, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Zhu, L.; Chen, Y.; Qin, R.; Mei, Z.; Xu, J.; Yang, G. Synthesis and biological evaluation of oleanolic acid derivative–chalcone conjugates as α-glucosidase inhibitors. RSC Adv. 2014, 4, 10862–10874. [Google Scholar] [CrossRef]
- Ke, J.J.; Lin, J.; Zhang, X.; Wu, X.Z.; Zheng, Y.Y.; Hu, C.M.; Kang, Y.; Zhang, K.; Xiong, Z.; Ma, Z.Q. Synthesis of Benzylidene Analogs of Oleanolic Acid as Potential α-Glucosidase and α-Amylase Inhibitors. Front. Chem. 2022, 10, 911232. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; He, H.; Ma, H.; Tu, B.; Li, J.; Guo, S.; Chen, S.; Cao, N.; Zheng, W.; Tang, X.; et al. Oleanolic acid indole derivatives as novel α-glucosidase inhibitors: Synthesis, biological evaluation, and mechanistic analysis. Bioorg Chem. 2021, 107, 104580. [Google Scholar] [CrossRef]
- Hong, D.S.; Kurzrock, R.; Supko, J.G.; He, X.; Naing, A.; Wheler, J.; Lawrence, D.; Eder, J.P.; Meyer, C.J.; Ferguson, D.A.; et al. A phase I first-in-human trial of bardoxolone methyl in patients with advanced solid tumors and lymphomas. Clin. Cancer Res. 2012, 18, 3396–3406. [Google Scholar] [CrossRef]
- Kazakova, O.B.; Medvedeva, N.I.; Baikova, I.P.; Tolstikov, G.A.; Lopatina, T.V.; Yunusov, M.S.; Zaprutko, L. Synthesis of triterpenoid acylates: Effective reproduction inhibitors of influenza A (H1N1) and papilloma viruses. Russ. J. Bioorg Chem. 2010, 36, 771–778. [Google Scholar] [CrossRef]
- Reyes-Zurita, F.J.; Medina-O’Donnell, M.; Ferrer-Martin, R.M.; Rufino-Palomares, E.E.; Martin-Fonseca, S.; Rivas, F.; Martínez, A.; García-Granados, A.; Pérez-Jiménez, A.; García-Salguero, L.; et al. The oleanolic acid derivative, 3-O-succinyl-28-O-benzyl oleanolate, induces apoptosis in B16-F10 melanoma cells via the mitochondrial apoptotic pathway. RSC Adv. 2016, 6, 93590–93601. [Google Scholar] [CrossRef]
- Spivak, A.Y.; Khalitova, R.R.; Nedopekina, D.A.; Gubaidullin, R.R. Antimicrobial properties of amine- and guanidine-functionalized derivatives of betulinic, ursolic and oleanolic acids: Synthesis and structure/activity evaluation. Steroids 2020, 154, 108530. [Google Scholar] [CrossRef]
- Youqing, S.; Defa, S.; Jianbin, T.; Meihua, S. Oleanolic Acid Derivative as well as Preparation Method and Application Thereof. CN102532246B, 18 June 2014. [Google Scholar]
- Khusnutdinova, E.F.; Petrova, A.V.; Kukovinets, O.S.; Kazakova, O.B. Synthesis and Cytotoxicity of 28-N-Propargylaminoalkylated 2,3-Indolotriterpenic acids. Nat. Prod. Commun. 2018, 13, 665–668. [Google Scholar] [CrossRef]
- Kazakova, O.; Smirnova, I.; Lopatina, T.; Giniyatullina, G.; Petrova, A.; Khusnutdinova, E.; Csuk, R.; Serbian, I.; Loesche, A. Synthesis and cholinesterase inhibiting potential of A-ring azepano- and 3-amino-3,4-seco-triterpenoids. Bioorg Chem. 2020, 101, 104001. [Google Scholar] [CrossRef] [PubMed]
- Kazakova, O.; Rubanik, L.; Smirnova, I.; Poleschuk, N.; Petrova, A.; Kapustina, Y.; Baikova, I.; Tret’yakova, E.; Khusnutdinova, E. Synthesis and in vitro activity of oleanolic acid derivatives against Chlamydia trachomatis and Staphylococcus aureus. Med. Chem. Res. 2021, 30, 1408–1418. [Google Scholar] [CrossRef]
- Kazakova, O.; Mioc, A.; Smirnova, I.; Baikova, I.; Voicu, A.; Vlaia, L.; Macașoi, I.; Mioc, M.; Drăghici, G.; Avram, Ş.; et al. Novel Synthesized N-Ethyl-Piperazinyl-Amides of C2-Substituted Oleanonic and Ursonic Acids Exhibit Cytotoxic Effects through Apoptotic Cell Death Regulation. Int. J. Mol. Sci. 2021, 22, 10967. [Google Scholar] [CrossRef] [PubMed]
- Khusnutdinova, E.; Petrova, A.; Zileeva, Z.; Kuzmina, U.; Zainullina, L.; Vakhitova, Y.; Babkov, D.; Kazakova, O. Novel A-Ring Chalcone Derivatives of Oleanolic and Ursolic Amides with Anti-Proliferative Effect Mediated through ROS-Triggered Apoptosis. Int. J. Mol. Sci. 2021, 22, 9796. [Google Scholar] [CrossRef]
- Kazakova, O.B.; Brunel, J.M.; Khusnutdinova, E.F.; Negrel, S.; Giniyatullina, G.V.; Lopatina, T.V.; Petrova, A.V. A-Ring-Modified Triterpenoids and Their Spermidine–Aldimines with Strong Antibacterial Activity. Molbank 2019, 2019, M1078. [Google Scholar] [CrossRef]
- Smirnova, I.E.; Kazakova, O.B.; Viet, D.Q.; Thuc, N.T.; Linh, T.P.; Huong, D.T.T. Synthesis and evaluation of 29-norcycloartane triterpenoids as α-glucosidase inhibitors. Med. Chem. Res. 2015, 24, 2177–2182. [Google Scholar] [CrossRef]
- Khusnutdinova, E.F.; Smirnova, I.E.; Giniyatullina, G.V.; Medvedeva, N.I.; Yamansarov, E.Y.; Kazakov, D.V.; Kazakova, O.B.; Linh, P.T.; Viet, D.Q.; Huong, D.T. Inhibition of Alpha-Glucosidase by Synthetic Derivatives of Lupane, Oleanane, Ursane and Dammarane Triterpenoids. Nat. Prod. Commun. 2016, 11, 33–35. [Google Scholar] [CrossRef]
- Khusnutdinova, E.F.; Smirnova, I.E.; Kazakova, O.B.; Petrova, A.V.; Ha, N.T.T.; Viet, D.Q. Synthesis and evaluation of 2,3-indolotriterpenoids as new α-glucosidase inhibitors. Med. Chem. Res. 2017, 26, 2737–2742. [Google Scholar] [CrossRef]
- Khusnutdinova, E.F.; Petrova, A.V.; Thu, H.N.T.; Tu, A.L.T.; Thanh, T.N.; Thi, C.B.; Babkov, D.A.; Kazakova, O.B. Structural modifications of 2,3-indolobetulinic acid: Design and synthesis of highly potent α-glucosidase inhibitors. Bioorg Chem. 2019, 88, 102957. [Google Scholar] [CrossRef]
- Wang, J.; Lu, S.; Sheng, R.; Fan, J.; Wu, W.; Guo, R. Structure-Activity Relationships of Natural and Synthetic Indole-Derived Scaffolds as α-Glucosidase Inhibitors: A Mini-Review. Mini Rev. Med. Chem. 2020, 20, 1791–1818. [Google Scholar] [CrossRef]
- Khusnutdinova, E.F.; Ha, N.T.T.; Giniyatullina, G.V.; Anh, L.T.T.; Poptsov, A.I.; Kazakova, O.B. Synthesis and alpha—Inhibitory activity of lupane type C2-benzylidene-triterpenoids. Vietnam. J. Chem. 2021, 59, 612–619. [Google Scholar]
- Khusnutdinova, E.; Galimova, Z.; Lobov, A.; Baikova, I.; Kazakova, O.; Thu, H.N.T.; Tuyen, N.V.; Gatilov, Y.; Csuk, R.; Serbian, I.; et al. Synthesis of messagenin and platanic acid chalcone derivatives and their biological potential. Nat. Prod. Res. 2022, 36, 5189–5198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.N.; Zhang, W.; Hong, D.; Shi, L.; Shen, Q.; Li, J.Y.; Li, J.; Hu, L.H. Oleanolic acid and its derivatives: New inhibitor of protein tyrosine phosphatase 1B with cellular activities. Bioorg. Med. Chem. 2008, 16, 8697–8705. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Espinosa, J.J.; Rios, M.Y.; Paoli, P.; Flores-Morales, V.; Camici, G.; de la Rosa-Lugo, V.; Hidalgo-Figueroa, S.; Navarrete-Vázquez, G.; Estrada-Soto, S. Synthesis of oleanolic acid derivatives: In vitro, in vivo and in silico studies for PTP-1B inhibition. Eur. J. Med. Chem. 2014, 87, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Hong, D.; Zhou, Y.; Zhang, Y.; Shen, Q.; Li, J.Y.; Hu, L.H.; Li, J. Ursolic acid and its derivative inhibit protein tyrosine phosphatase 1B, enhancing insulin receptor phosphorylation and stimulating glucose uptake. Biochim. Et Biophys. Acta 2006, 1760, 1505–1512. [Google Scholar] [CrossRef]
- Zhao, B.T.; Nguyen, D.H.; Le, D.D.; Choi, J.S.; Min, B.S.; Woo, M.H. Protein tyrosine phosphatase 1B inhibitors from natural sources. Arch. Pharm. Res. 2018, 41, 130–161. [Google Scholar] [CrossRef]
- Agrawal, N.K.; Kant, S. Targeting inflammation in diabetes: Newer therapeutic options. World J. Diabetes 2014, 5, 697–710. [Google Scholar] [CrossRef]
- Liu, W.M.; Dalgleish, A.G. MTT assays can underestimate cell numbers. Cancer Chemother. Pharmacol. 2009, 64, 861–862. [Google Scholar] [CrossRef]
- Stepanenko, A.A.; Dmitrenko, V.V. Pitfalls of the MTT assay: Direct and off-target effects of inhibitors can result in over/underestimation of cell viability. Gene 2015, 574, 193–203. [Google Scholar] [CrossRef]
- Es-Saady, D.; Simon, A.; Jayat-Vignoles, C.; Chulia, A.J.; Delage, C. MCF-7 cell cycle arrested at G1 through ursolic acid, and increased reduction of tetrazolium salts. Anticancer. Res. 1996, 16, 481–486. [Google Scholar]
- Gundoju, N.; Bokam, R.; Yalavarthi, N.R.; Azad, R.; Ponnapalli, M.G. Betulinic acid derivatives: A new class of α-glucosidase inhibitors and LPS-stimulated nitric oxide production inhibition on mouse macrophage RAW 264.7 cells. Nat. Prod. Res. 2018, 33, 1462182. [Google Scholar] [CrossRef] [PubMed]
- Mosén, H.; Salehi, A.; Henningsson, R.; Lundquist, I. Nitric oxide inhibits, and carbon monoxide activates, islets acid α-glucoside hydrolase activities in parallel with glucose-stimulated insulin secretion. J. Endocrinol. 2006, 190, 681–693. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Akesson, B.; Henningsson, R.; Salehi, A.; Lundquist, I. Islet constitutive nitric oxide synthase and glucose regulation of insulin release in mice. J. Endocrinol. 1999, 163, 39–48. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tian, G.; Wilcockson, D.; Perry, V.H.; Rudd, P.M.; Dwek, R.A.; Platt, F.M.; Platt, N. Inhibition of α-glucosidases I and II increases the cell surface expression of functional class A macrophage scavenger receptor (SR-A) by extending its half-life. J. Biolog Chem. 2004, 279, 39303–39309. [Google Scholar] [CrossRef] [PubMed]
- Borenfreund, E.; Puerner, J.A. Toxicity determined in vitro by morphological alterations and neutral red absorption. Toxicol. Lett. 1985, 24, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Koehn, C.D.; Yue, Y.; Li, S.; Thiele, G.M.; Hearth-Holmes, M.P.; Mikuls, T.R.; O’Dell, J.R.; Klassen, L.W.; Zhang, Z.; et al. Celastrol Inhibits Inflammatory Stimuli-Induced Neutrophil Extracellular Trap Formation. CMM 2015, 15, 401–410. [Google Scholar] [CrossRef]
- Sharma, K.R. Immunomodulatory Studies on Triterpenoids from Scoparia dulcis Linn. Biochem. Pharmacol. 2015, 4, 1000182. [Google Scholar] [CrossRef]
- Elya, B.; Basah, K.; Mun’im, A.; Yuliastuti, W.; Bangun, A.; Septiana, E.K. Screening of α-Glucosidase Inhibitory Activity from Some Plants of Apocynaceae, Clusiaceae, Euphorbiaceae, and Rubiaceae. J. Biomed. Biotechnol. 2012, 2012, 281078. [Google Scholar] [CrossRef]
- Spasov, A.A.; Babkov, D.A.; Prokhorova, T.Y.; Sturova, E.A.; Muleeva, D.R.; Demidov, M.R.; Osipov, D.V.; Osyanin, V.A.; Klimochkin, Y.N. Synthesis and biological evaluation of 2-acylbenzofuranes as novel α-glucosidase inhibitors with hypoglycemic activity. Chem. Biol. Drug Des. 2017, 90, 1184–1189. [Google Scholar] [CrossRef]
- Fujisawa, T.; Ikegami, H.; Inoue, K.; Kawabata, Y.; Ogihara, T. Effect of two α-glucosidase inhibitors, voglibose and acarbose, on postprandial hyperglycemia correlates with subjective abdominal symptoms. Metabolism 2005, 54, 387–390. [Google Scholar] [CrossRef]
- Lubben, T.; Clampit, J.; Stashko, M.; Trevillyan, J.; Jirousek, M.R. In Vitro Enzymatic Assays of Protein Tyrosine phosphatase 1B. In Current Protocols in Pharmacology; Enna, S.J., Ed.; Wiley: Hoboken, NJ, USA, 2001. [Google Scholar] [CrossRef]
- Song, M.; Park, J.E.; Park, S.G.; Lee, D.H.; Choi, H.K.; Park, B.C.; Ryu, S.E.; Kim, J.H.; Cho, S. NSC-87877, inhibitor of SHP-1/2 PTPs, inhibits dual-specificity phosphatase 26 (DUSP26). Biophys. Res. Commun. 2009, 381, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Villarreal, F.J.; Kim, G.N.N.; Ungab, D.; Printz, M.P.; Dillmann, W.H. Identification of functional angiotensin II receptors on rat cardiac fibroblasts. Circulation 1993, 88, 2849–2861. [Google Scholar] [CrossRef] [PubMed]
- Dubey, R.K.; Gillespie, D.G.; Mi, Z.; Jackson, E.K. Exogenous and Endogenous Adenosine Inhibits Fetal Calf Serum–Induced Growth of Rat Cardiac Fibroblasts: Role of A 2B Receptors. Circulation 1997, 96, 2656–2666. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fan, J.; Cao, L.; Han, T.; Zeng, M.; Xu, Y.; Ling, Z.; Yin, Y. Unique mechanistic insights into the beneficial effects of angiotensin-(1-7) on the prevention of cardiac fibrosis: A metabolomic analysis of primary cardiac fibroblasts. Exp. Cell Res. 2019, 378, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Kopprasch, S.; Pietzsch, J.; Graessler, J. Validation of different chemilumigenic substrates for detecting extracellular generation of reactive oxygen species by phagocytes and endothelial cells. Luminescence 2003, 18, 268–273. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLOS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).