Effects of Blue-Light Laser Irradiation on the Enzymatic Activities and Sporulation of Trichoderma atroviride Grown on Rice Husks
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fungal Strain Inoculation and Growing Conditions
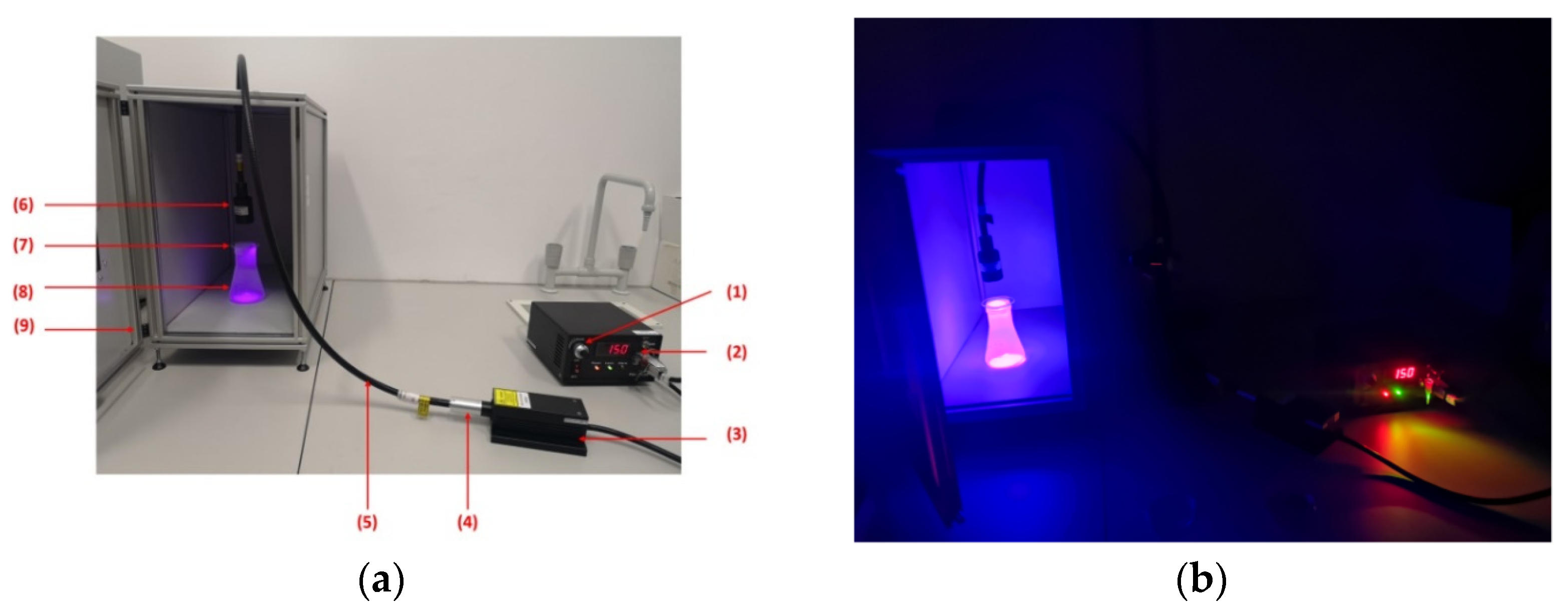
2.3. Experimental Design of Trichoderma Irradiation with Blue–Violet-Light Laser
2.4. Quantitative Analysis of Enzyme Activity
2.5. Total Protein Content
2.6. UV–Vis Spectroscopy
2.7. Determination of the Refractive Index and Dry Mass Content of Supernatant
2.8. Optical Microscopy
2.9. Statistical Analysis
3. Results and Discussion
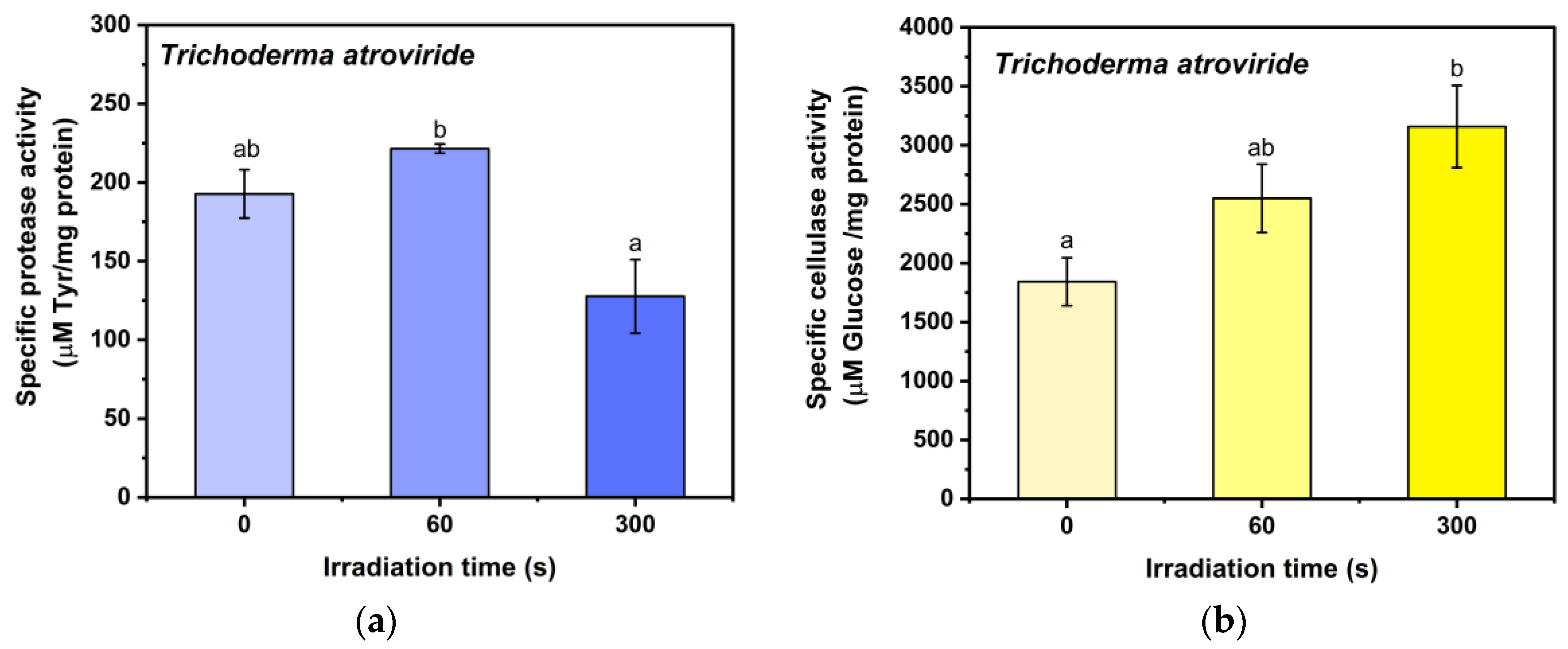
3.1. Protease and Cellulase Activity and Total Protein Content
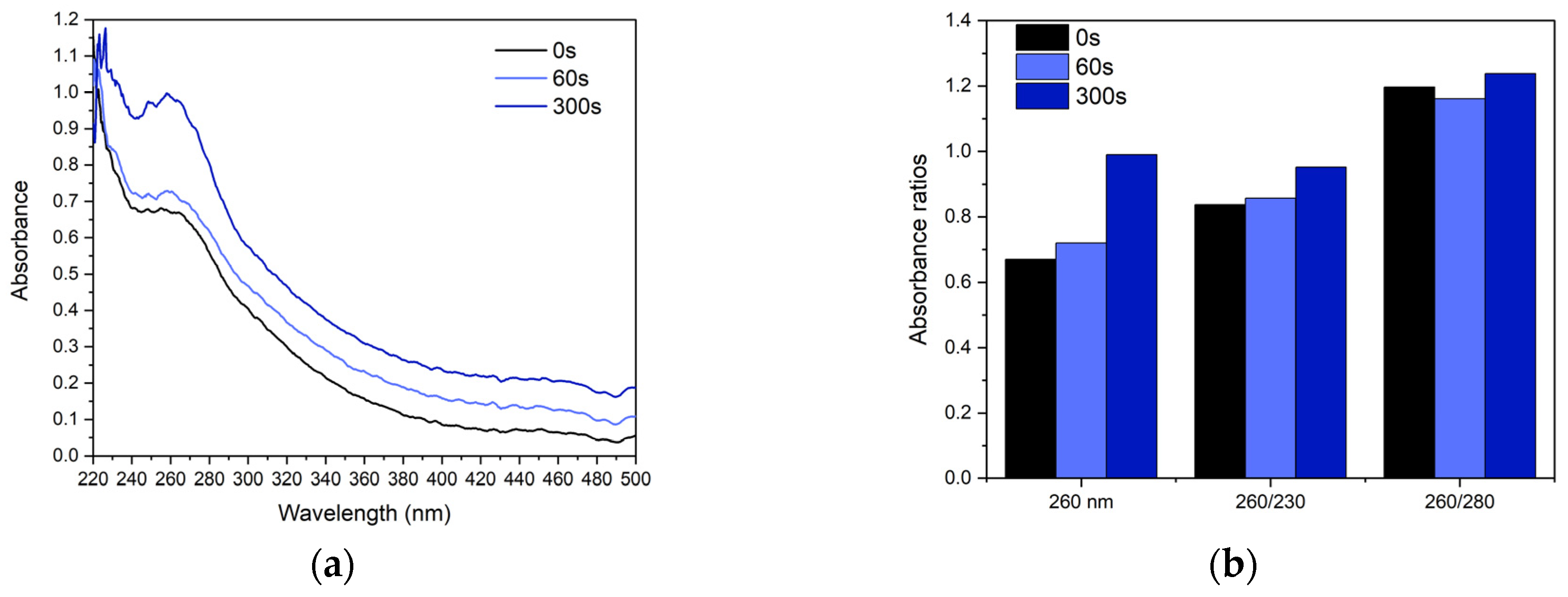
3.2. UV–Vis Spectroscopy
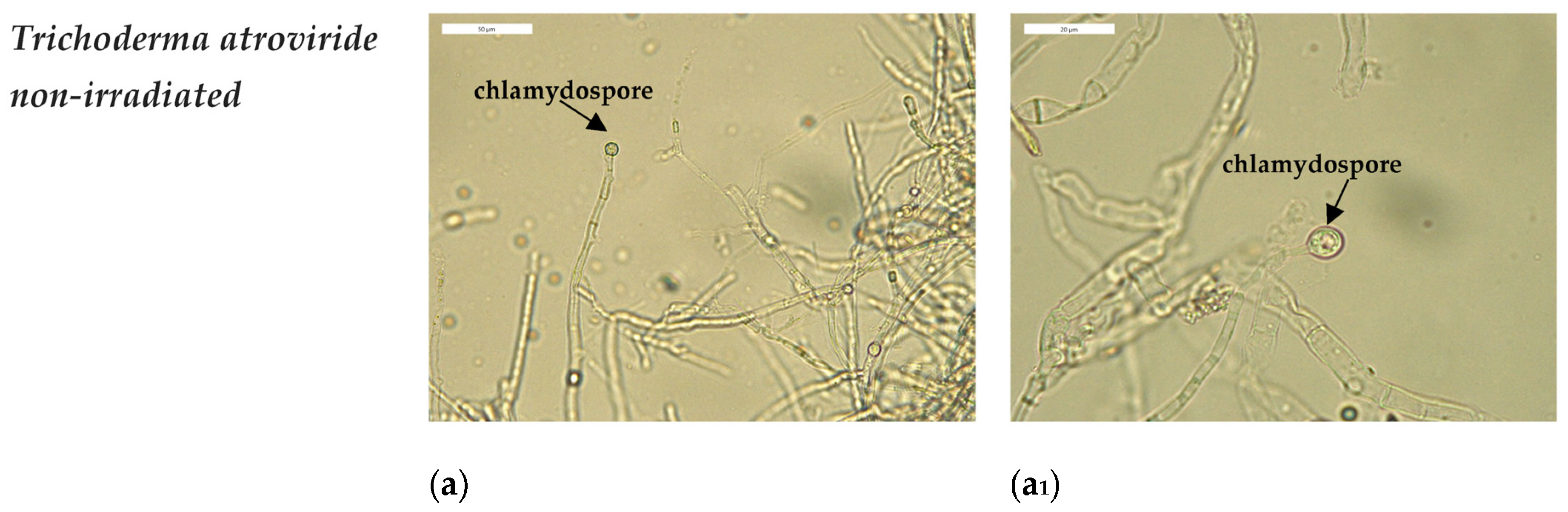
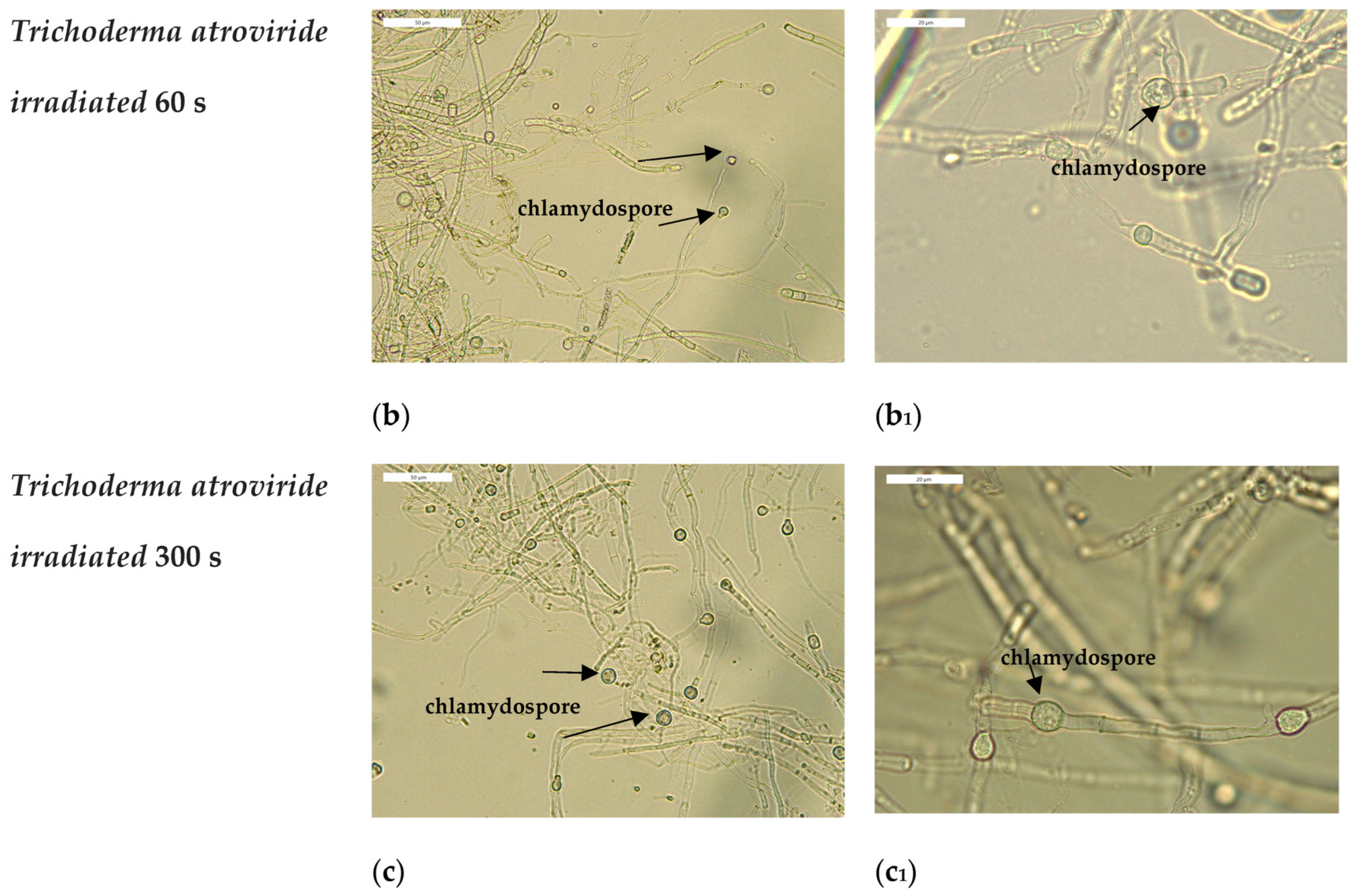
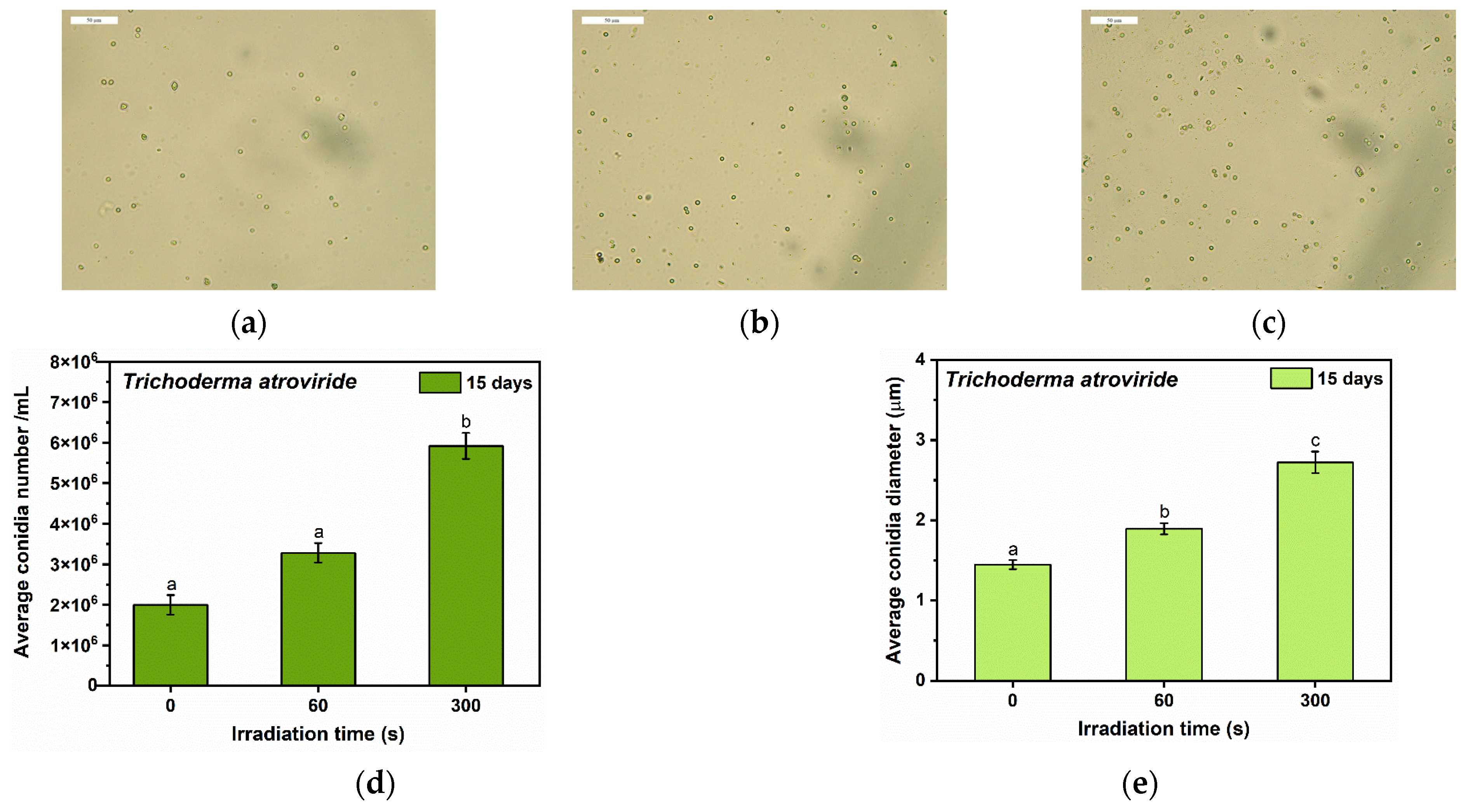
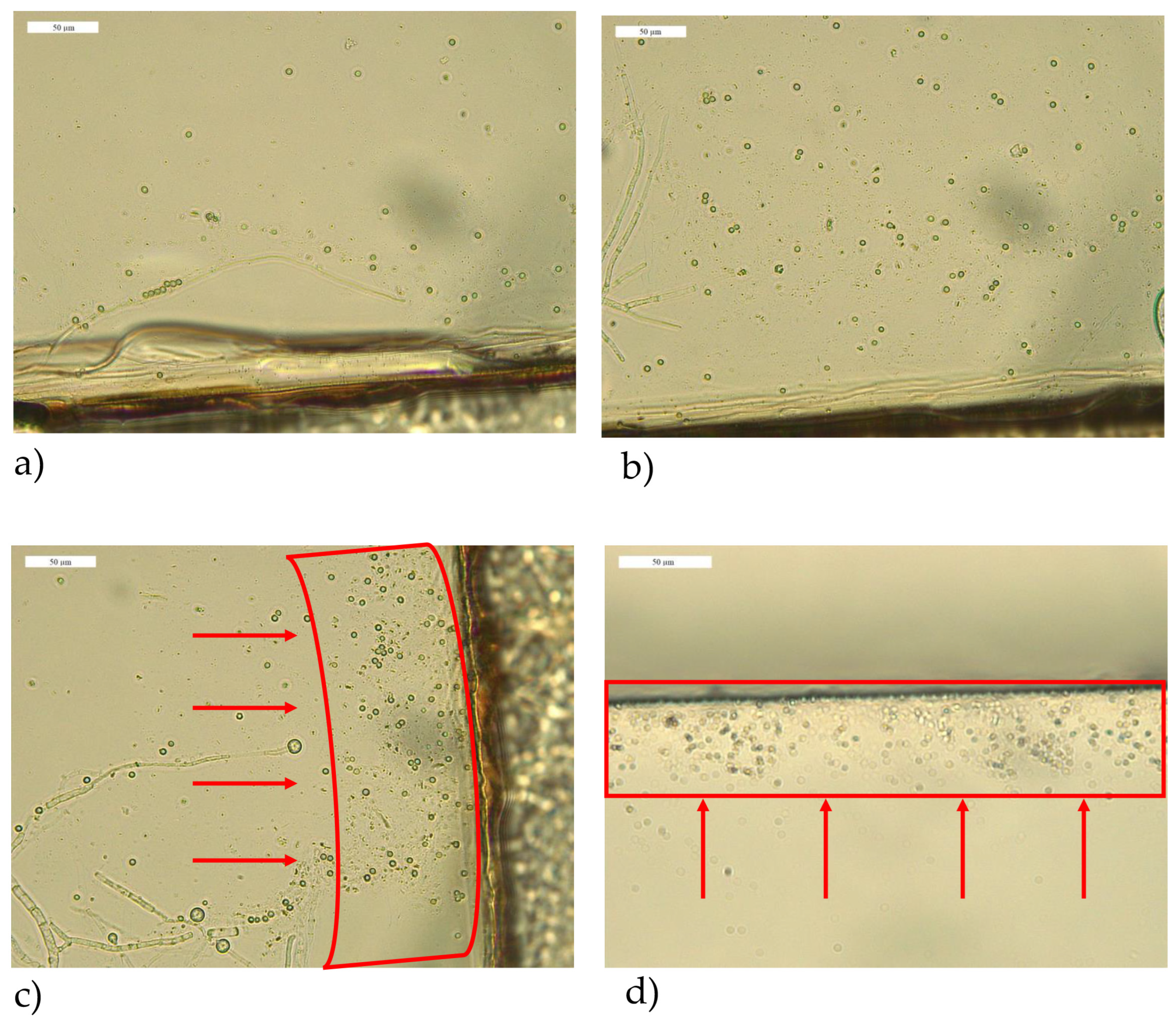
3.3. Optical Microscopy Observations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| 400 nm Laser | |||||||||||||
| Parameters at the exit of the beam radiation from the laser source | |||||||||||||
| Pump Electrical Current (mA) | 0 | 37 | 51 | 74 | 94 | 111 | 133 | 151 * | 170 | 186 | 205 | 227 | 251 |
| Optical Average Power (mW) | 0 | 1 | 30 | 60 | 90 | 120 | 150 | 180 * | 210 | 240 | 270 | 300 | 325 |
| Parameters at the exit from the beam expander | |||||||||||||
| Pump Electrical Current (mA) | 0 | 37 | 51 | 74 | 94 | 111 | 133 | 151 * | 170 | 186 | 205 | 227 | 251 |
| Optical Average Power (mW) | 0 | 1 | 10.14 | 25.85 | 39.85 | 51.8 | 67.58 | 81 * | 97.75 | 106.6 | 119.1 | 134.8 | 152.5 |
| * The underlined numbers represent the values of the laser parameters used in this study. | |||||||||||||
Appendix B
References
- Yu, Z.; Fischer, R. Light sensing and responses in fungi. Nat. Rev. Microbiol. 2019, 17, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Gressel, J.; Galun, E. Morphogenesis in Trichoderma: Photoinduction and RNA. Dev. Biol. 1967, 15, 575–598. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, B.A.; Gressel, J.; Malkin, S. The Quest for Trichoderma Cryptochrome. In Proceedings of the Blue Light Effects in Biological Systems, Marburg, Germany, 20–23 July 1984; pp. 237–249. [Google Scholar]
- Casas-Flores, S.; Rios-Momberg, M.; Bibbins, M.; Ponce-Noyola, P.; Herrera-Estrella, A. BLR-1 and BLR-2, key regulatory elements of photoconidiation and mycelial growth in Trichoderma atroviride. Microbiology 2004, 150, 3561–3569. [Google Scholar] [CrossRef] [PubMed]
- Idnurm, A.; Heitman, J. Light Controls Growth and Development via a Conserved Pathway in the Fungal Kingdom. PLoS Biol. 2005, 3, e95. [Google Scholar] [CrossRef]
- Castellanos, F.; Schmoll, M.; Martínez, P.; Tisch, D.; Kubicek, C.P.; Herrera-Estrella, A.; Esquivel-Naranjo, E.U. Crucial factors of the light perception machinery and their impact on growth and cellulase gene transcription in Trichoderma reesei. Fungal Genet. Biol. 2010, 47, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Casas-Flores, S.; Rios-Momberg, M.; Rosales-Saavedra, T.; Martínez-Hernández, P.; Olmedo-Monfil, V.; Herrera-Estrella, A. Cross talk between a fungal blue-light perception system and the cyclic AMP signaling pathway. Eukaryot. Cell 2006, 5, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Friedl, M.A.; Schmoll, M.; Kubicek, C.P.; Druzhinina, I.S. Photostimulation of Hypocrea atroviridis growth occurs due to a cross-talk of carbon metabolism, blue light receptors and response to oxidative stress. Microbiology 2008, 154, 1229–1241. [Google Scholar] [CrossRef][Green Version]
- Schmoll, M.; Esquivel-Naranjo, E.U.; Herrera-Estrella, A. Trichoderma in the light of day—Physiology and development. Fungal Genet. Biol. 2010, 47, 909–916. [Google Scholar] [CrossRef]
- Schmoll, M. Regulation of plant cell wall degradation by light in Trichoderma. Fungal Biol. Biotechnol. 2018, 5, 10. [Google Scholar] [CrossRef]
- Hitzenhammer, E.; Büschl, C.; Sulyok, M.; Schuhmacher, R.; Kluger, B.; Wischnitzki, E.; Schmoll, M. YPR2 is a regulator of light modulated carbon and secondary metabolism in Trichoderma reesei. BMC Genom. 2019, 20, 211. [Google Scholar] [CrossRef]
- Schalamun, M.; Beier, S.; Hinterdobler, W.; Wanko, N.; Schinnerl, J.; Brecker, L.; Engl, D.E.; Schmoll, M. MAPkinases regulate secondary metabolism, sexual development and light dependent cellulase regulation in Trichoderma reesei. Sci. Rep. 2023, 13, 1912. [Google Scholar] [CrossRef] [PubMed]
- Tisch, D.; Schuster, A.; Schmoll, M. Crossroads between light response and nutrient signalling: ENV1 and PhLP1 act as mutual regulatory pair in Trichoderma reesei. BMC Genom. 2014, 15, 425. [Google Scholar] [CrossRef]
- Tisch, D.; Schmoll, M. Targets of light signalling in Trichoderma reesei. BMC Genom. 2013, 14, 657. [Google Scholar] [CrossRef]
- Schmoll, M.; Schuster, A.; Silva, R.d.N.; Kubicek, C.P. The G-Alpha Protein GNA3 of Hypocrea jecorina (Anamorph Trichoderma reesei) Regulates Cellulase Gene Expression in the Presence of Light. Eukaryot. Cell 2009, 8, 410–420. [Google Scholar] [CrossRef]
- Speckbacher, V.; Ruzsanyi, V.; Wigger, M.; Zeilinger, S. The Trichoderma atroviride Strains P1 and IMI 206040 Differ in Their Light-Response and VOC Production. Molecules 2020, 25, 208. [Google Scholar] [PubMed]
- Bischof, R.H.; Ramoni, J.; Seiboth, B. Cellulases and beyond: The first 70 years of the enzyme producer Trichoderma reesei. Microb. Cell Fact. 2016, 15, 106. [Google Scholar] [CrossRef] [PubMed]
- Schalamun, M.; Schmoll, M. Trichoderma—Genomes and genomics as treasure troves for research towards biology, biotechnology and agriculture. Front. Fungal Biol. 2022, 3, 1002161. [Google Scholar] [CrossRef]
- Rosolen, R.R.; Horta, M.A.C.; de Azevedo, P.H.C.; da Silva, C.C.; Sforca, D.A.; Goldman, G.H.; de Souza, A.P. Whole-genome sequencing and comparative genomic analysis of potential biotechnological strains of Trichoderma harzianum, Trichoderma atroviride, and Trichoderma reesei. Mol. Genet. Genom. 2023, 298, 735–754. [Google Scholar] [CrossRef]
- Shahnaz, E.; Anwar, A.; Banday, S. Chapter 15—Trichoderma spp. as bio-stimulant: Molecular insights. In New and Future Developments in Microbial Biotechnology and Bioengineering; Singh, H.B., Vaishnav, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 337–350. [Google Scholar]
- Velasco-Muñoz, J.F.; Aznar-Sánchez, J.A.; López-Felices, B.; Román-Sánchez, I.M. Circular economy in agriculture. An analysis of the state of research based on the life cycle. Sustain. Prod. Consum. 2022, 34, 257–270. [Google Scholar] [CrossRef]
- Magalhães, A.I., Jr.; de Carvalho, J.C.; de Melo Pereira, G.V.; Karp, S.G.; Câmara, M.C.; Medina, J.D.C.; Soccol, C.R. Lignocellulosic biomass from agro-industrial residues in South America: Current developments and perspectives. Biofuels Bioprod. Biorefin. 2019, 13, 1505–1519. [Google Scholar] [CrossRef]
- Monteiro, A.R.; Battisti, A.P.; Valencia, G.A.; de Andrade, C.J. The production of high-added-value bioproducts from non-conventional biomasses: An overview. Biomass 2023, 3, 123–137. [Google Scholar]
- Sethupathy, S.; Morales, G.M.; Li, Y.; Wang, Y.; Jiang, J.; Sun, J.; Zhu, D. Harnessing microbial wealth for lignocellulose biomass valorization through secretomics: A review. Biotechnol. Biofuels 2021, 14, 154. [Google Scholar] [CrossRef] [PubMed]
- Stappler, E.; Walton, J.D.; Beier, S.; Schmoll, M. Abundance of Secreted Proteins of Trichoderma reesei Is Regulated by Light of Different Intensities. Front. Microbiol. 2017, 8, 2586. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.L.; Hermosa, R.; Lorito, M.; Monte, E. Trichoderma: A multipurpose, plant-beneficial microorganism for eco-sustainable agriculture. Nat. Rev. Microbiol. 2023, 21, 312–326. [Google Scholar] [PubMed]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Del-Val, E.; Larsen, J. Ecological functions of Trichoderma spp. and their secondary metabolites in the rhizosphere: Interactions with plants. FEMS Microbiol. Ecol. 2016, 92, fiw036. [Google Scholar] [PubMed]
- Şesan, T.E.; Oancea, A.O.; Ştefan, L.M.; Mănoiu, V.S.; Ghiurea, M.; Răut, I.; Constantinescu-Aruxandei, D.; Toma, A.; Savin, S.; Bira, A.F. Effects of foliar treatment with a Trichoderma plant biostimulant consortium on Passiflora caerulea L. yield and quality. Microorganisms 2020, 8, 123. [Google Scholar] [PubMed]
- Saxena, A.; Raghuwanshi, R.; Singh, H.B. Elevation of defense network in chilli against Colletotrichum capsici by phyllospheric Trichoderma strain. J. Plant Growth Regul. 2016, 35, 377–389. [Google Scholar]
- Metz, B.; Seidl-Seiboth, V.; Haarmann, T.; Kopchinskiy, A.; Lorenz, P.; Seiboth, B.; Kubicek, C.P. Expression of Biomass-Degrading Enzymes Is a Major Event during Conidium Development in Trichoderma reesei. Eukaryot. Cell 2011, 10, 1527–1535. [Google Scholar] [CrossRef]
- Horwitz, B.A.; Perlman, A.; Gressel, J. Induction of Trichoderma sporulation by nanosecond laser pulses: Evidence against cryptochrome cycling. Photochem. Photobiol. 1990, 51, 99–104. [Google Scholar] [CrossRef]
- Gaderer, R.; Lamdan, N.; Frischmann, A.; Sulyok, M.; Krska, R.; Horwitz, B.; Seidl-Seiboth, V. Sm2, a paralog of the Trichoderma cerato-platanin elicitor Sm1, is also highly important for plant protection conferred by the fungal-root interaction of Trichoderma with maize. BMC Microbiol. 2015, 15, 2. [Google Scholar] [CrossRef]
- Zaidem, A.; Silva, L.; Ferreira, A.; Carvalho, M.; Ragni, M.; Abegão, L.; Pinheiro, P. New biocompatible technique based on the use of a laser to control the whitefly Bemisia tabaci. Photonics 2023, 10, 636. [Google Scholar] [CrossRef]
- Anson, M.L. The estimation of pepsin, trypsin, papain, and cathepsin with hemoglobin. J. Gen. Physiol. 1938, 22, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Folin, O.; Ciocalteu, V. On tyrosine and tryptophane determinations in proteins. J. Biol. Chem. 1927, 73, 627–650. [Google Scholar] [CrossRef]
- Zhang, Y.-H.P.; Hong, J.; Ye, X. Cellulase Assays. Biofuels Methods Mol. Biol. 2009, 581, 213–231. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Copeland, R.A. Methods for Protein Quantitation. In Methods for Protein Analysis; Copeland, R.A., Ed.; Springer: Boston, MA, USA, 1994; pp. 39–58. [Google Scholar]
- Compton, S.J.; Jones, C.G. Mechanism of dye response and interference in the Bradford protein assay. Anal. Biochem. 1985, 151, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Lu, R.; Hu, M. Flavonoids interference in common protein assays: Effect of position and degree of hydroxyl substitution. Anal. Biochem. 2020, 597, 113644. [Google Scholar] [CrossRef]
- Schmoll, M.; Franchi, L.; Kubicek, C.P. Envoy, a PAS/LOV Domain Protein of Hypocrea jecorina (Anamorph Trichoderma reesei), Modulates Cellulase Gene Transcription in Response to Light. Eukaryot. Cell 2005, 4, 1998–2007. [Google Scholar] [CrossRef]
- Askolin, S.; Penttilä, M.; Wösten, H.A.B.; Nakari-Setälä, T. The Trichoderma reesei hydrophobin genes hfb1 and hfb2 have diverse functions in fungal development. FEMS Microbiol. Lett. 2005, 253, 281–288. [Google Scholar] [CrossRef]
- Nakari-Setälä, T.; Aro, N.; IlméN, M.; Muñoz, G.; Kalkkinen, N.; Penttilä, M. Differential Expression of the Vegetative and Spore-Bound Hydrophobins of Trichoderma Reesei Cloning and Characterization of the Hfb2 Gene. Eur. J. Biochem. 1997, 248, 415–423. [Google Scholar] [CrossRef]
- Dubey, M.K.; Jensen, D.F.; Karlsson, M. Hydrophobins are required for conidial hydrophobicity and plant root colonization in the fungal biocontrol agent Clonostachys rosea. BMC Microbiol. 2014, 14, 18. [Google Scholar] [CrossRef]


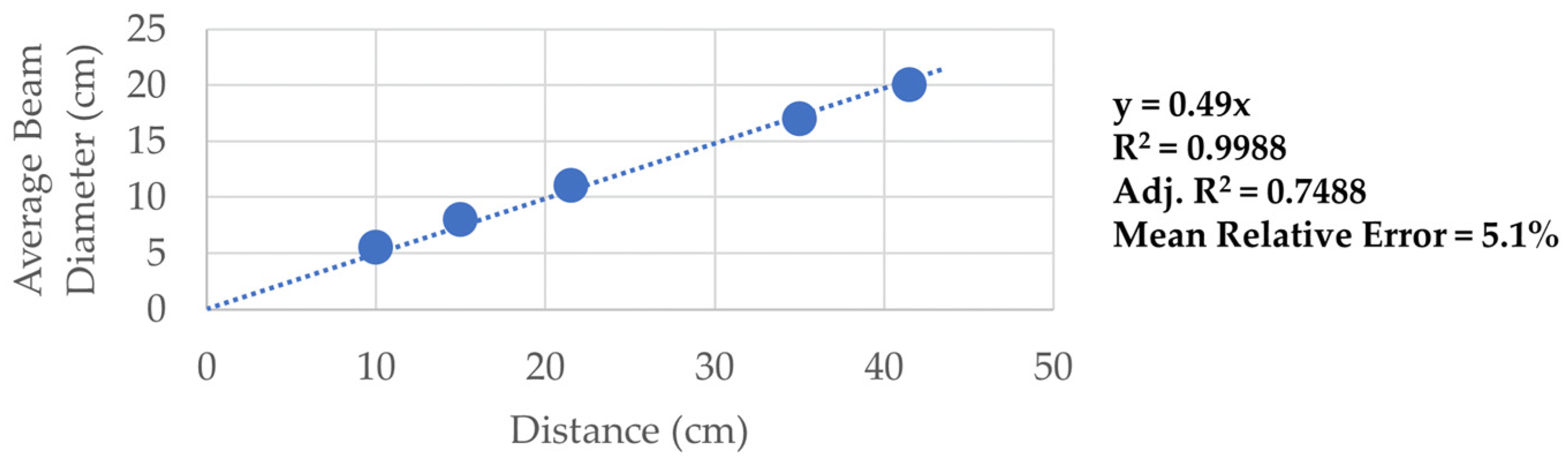
| Distance from Source [cm] | Beam Diameter [cm] | Beam Area [cm2] | Avg. Optical Power [mW] | Optical Intensity [mW/cm2] |
|---|---|---|---|---|
| 10 | 5.5 ± 0.3 | 23.8 ± 1.7 | 81 ± 1.6 | 3.4 ± 0.3 |
| 15 | 8 ± 0.4 | 50.3 ± 3.6 | 81 ± 1.6 | 1.6 ± 0.1 |
| 21.5 | 11 ± 0.6 | 95.0 ± 6.9 | 81 ± 1.6 | 0.9 ± 0.1 |
| 35 | 17 ± 0.9 | 227.0 ± 16.4 | 81 ± 1.6 | 0.4 ± 0.0 |
| 41.5 | 20 ± 1 | 314.2 ± 22.7 | 81 ± 1.6 | 0.3 ± 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bala, I.-A.; Tritean, N.; Enache, A.A.; Trică, B.; Constantinescu-Aruxandei, D.; Oancea, F. Effects of Blue-Light Laser Irradiation on the Enzymatic Activities and Sporulation of Trichoderma atroviride Grown on Rice Husks. Appl. Sci. 2023, 13, 9191. https://doi.org/10.3390/app13169191
Bala I-A, Tritean N, Enache AA, Trică B, Constantinescu-Aruxandei D, Oancea F. Effects of Blue-Light Laser Irradiation on the Enzymatic Activities and Sporulation of Trichoderma atroviride Grown on Rice Husks. Applied Sciences. 2023; 13(16):9191. https://doi.org/10.3390/app13169191
Chicago/Turabian StyleBala, Ioana-Alexandra, Naomi Tritean, Alin Alexandru Enache, Bogdan Trică, Diana Constantinescu-Aruxandei, and Florin Oancea. 2023. "Effects of Blue-Light Laser Irradiation on the Enzymatic Activities and Sporulation of Trichoderma atroviride Grown on Rice Husks" Applied Sciences 13, no. 16: 9191. https://doi.org/10.3390/app13169191
APA StyleBala, I.-A., Tritean, N., Enache, A. A., Trică, B., Constantinescu-Aruxandei, D., & Oancea, F. (2023). Effects of Blue-Light Laser Irradiation on the Enzymatic Activities and Sporulation of Trichoderma atroviride Grown on Rice Husks. Applied Sciences, 13(16), 9191. https://doi.org/10.3390/app13169191








