Abstract
Even though most studies consider strict anaerobe Gram-negative bacteria as the main factor associated with peri-implantitis, other studies have identified other microorganisms present in implants related to peri-implant disease that have the ability to reduce the effectiveness of treatment, such as Candida spp., Enterococcus faecalis and Pseudomonas aeruginosa. Therefore, microbiologic diagnosis is important for the success of implant treatment. The main goal of this study was to detect Candida spp., E. faecalis and P. aeruginosa in the peri-implant and periodontal subgingival plaque in the presence or absence of disease and to relate the presence of these microorganisms with demographic data, hygiene habits, the type of implant connection and endodontic treatment. The study population consisted of 20 patients that filled out a questionnaire regarding gender, age, systemic diseases and oral hygiene. The peri-implant and periodontal subgingival plaque from an adjacent tooth, both with and without disease, were analysed for the presence of these three opportunistic pathogens. Microbiological analysis revealed a higher prevalence of E. faecalis in patients with and without periodontal and peri-implant disease. Candida spp. was identified in a higher degree in cases with disease, and P. aeruginosa was mostly detected in peri-implantitis. The detection of these three pathogens suggested a possible means of transmission of infection from adjacent teeth to implants, with the implant design associated with rehabilitation being a primary cause of pathogen growth. Although this study did not relate pathogen growth directly to periodontal disease, the high colony forming unit per millilitre (CFU/mL) values of E. faecalis may reveal an aetiological role of this bacterium in peri-implantitis.
1. Introduction
The success of oral rehabilitation in patients undergoing implant treatment largely depends on the health of the tissues. As the oral cavity is a dynamic system, continuously colonised by interacting and proliferating microorganisms, it is extremely important to understand the microbiota and control causal factors before, during, and after implant placement to prevent the development of peri-implant disease [1,2].
Oral microbiota differ in everyone and, when in balance, these microorganisms do not cause any harm to the oral structure, a phenomenon known as eubiosis, characterised by a mutual beneficial relationship and a defence mechanism against other species [1]. However, alterations in the host’s immune system, pH changes, decreased salivary flow, altered activity of salivary proteins, diet (high carbohydrate consumption), poor oral hygiene, tobacco use, diabetes, prolonged use of oral antibiotics/antimicrobials/antiseptics and genetic factors can lead to microbial imbalances. Under these circumstances, virulent and opportunistic microorganisms in dysbiosis can cause periodontal and peri-implant diseases [1,2,3].
In peri-implant disease, the biofilm has been reported to contain significant amounts of Gram-negative bacteria, such as Porphyromonas gingivalis, Treponema denticola, Tannerella forsythia and Aggregatibacter actinomycetemcomitans, but Candida spp., E. faecalis and P. aeruginosa have also been found in implants with peri-implantitis [4,5,6,7].
Candida species in the oral cavity are commensal and it has been suggested that the subgingival environment can serve as a refuge, where, under favourable conditions, they can transform into opportunistic pathogens and induce oral diseases [8,9,10].
P. aeruginosa is one of the most common microorganisms in healthcare-associated infections, with high mortality rates, especially in severely ill and immunocompromised patients. It is an opportunistic human pathogen characterised by intrinsic resistance to multiple antimicrobial agents. A recent study found significantly higher levels of P. aeruginosa in oral epithelial cells of individuals with periodontitis compared to individuals with a healthy periodontium [11]. In addition, El-Telbany M et al., 2022, isolated P. aeruginosa in 3 out of 30 cases of peri-implantitis, demonstrating that in addition to its difficult elimination when organised in a biofilm, it also showed resistance to 13 out of 16 antibiotics tested, suggesting that resistance to antimicrobials of opportunistic pathogens in peri-implantitis has led to an urgent need to create alternative treatments to antibiotics for the treatment of these infections [12].
E. faecalis are facultative anaerobic Gram-positive cocci. They are rarely found in the oral cavity under healthy conditions, but have a high occurrence in failed endodontic treatments, persistent periapical lesions, chronic periodontitis and have also been detected in cases of peri-implantitis [4].
If peri-implantitis is not diagnosed and treated early, extensive bone loss will occur and compromise implant stability; therefore, microbiological diagnosis is important for a more appropriate and effective treatment.
In Portugal, as far as we know, there are no studies that have evaluated the prevalence of these opportunistic pathogens in implants and adjacent teeth and the implications both in terms of antibiotic prophylaxis and treatment that the presence of these microorganisms represents.
The aimed of this study was to detect Candida spp., E. faecalis and P. aeruginosa in the periodontal and peri-implant subgingival plaque with or without disease presence and to seek a correlation between the presence of these microorganisms and demographic data, hygiene habits, endodontic treatment and implant connection type.
2. Materials and Methods
2.1. Study Characteristics
This was an observational, analytical and cross-sectional study conducted by a single calibrated dentist on patients of the University Clinic at University Institute of Health Sciences—Cooperative for Polytechnic and University Education (IUCS-CESPU) in Gandra who were undergoing oral rehabilitation treatment with implants.
2.1.1. Study Population
The patients who participated in this study were recruited between May and September 2022. Twenty individuals were selected from patients from the University Clinic at IUCS-CESPU in Gandra, Portugal, who were undergoing oral rehabilitation treatment with implants. A questionnaire was used to collect information about demographic data, oral hygiene habits, smoking habits, medical history and dental history, obtained through an examination of the oral cavity. Additionally, a periodontal and peri-implant diagnosis was performed on patients who met the inclusion criteria, according to the “Classification of Periodontal and Peri-implant Diseases 2018” [13]. All participants were informed about the purpose of the research and signed informed consent, and all procedures were carried out with data protection in mind. This study protocol was previously approved by the Ethics Committee of the IUCS-CESPU, following the Helsinki guidelines.
2.1.2. Inclusion Criteria
The study included healthy patients or those with controlled chronic diseases (e.g., hypertension and diabetes) who were over 18 years of age and had implants placed less than 10 years ago and more than 6 months ago.
2.1.3. Exclusion Criteria
Patients with immunological disorders, those undergoing therapy with high-dose steroids, therapeutic levels of fluoride in bone, bisphosphonates, cyclosporine, phenytoin and nifedipine, patients who had received antibiotic treatment in the last 30 days and/or mouthwash with antiseptics in the last 15 days, as well as patients with acute abscesses near the collection areas, were excluded. Patients who had undergone periodontal or peri-implant treatment in the last six months and patients with incomplete or missing clinical information were also excluded.
2.2. Microbiological Examination
2.2.1. Microbial Sampling
A microbiological sample was obtained from the peri-implant and periodontal plaque from the adjacent tooth. Prior to sampling, the supragingival plaque was removed with sterile cotton pellets, and the sample site was isolated with cotton rolls. In each sample site, 3 sterile #30 paper points were inserted into de sulcus/pocket and kept there for 20 s. The 3 paper points from each sample site was transferred to a two screw-capped 2-mL vials containing VMGA III anaerobic transport medium for culture.
2.2.2. Bacterial Culture
Microorganisms were mechanically dispersed from the paper points with a Vortex mixer at the maximal setting for 45 s. The microbial suspension was then 10-fold serially diluted in VMGA I anaerobic dispersion solution. Aliquots of 100 µL from undiluted and of the appropriate dilution (10−1, 10−2 and 10−3) were plated onto CHROMID® CPS Elite (BioMérieux, Marcy-l’Étoile, France), a chromogenic agar medium for the isolation and identification of E. faecalis, CHROMID® Candida (BioMérieux, France), a chromogenic agar medium for the isolation and identification of Candida spp., and another 100 µL for Cetrimide Agar medium (BioMérieux, France), which allows for the isolation and identification of P. aeruginosa. The inoculum deposited in each medium was then spread using a sterile bent rod and all media were incubated in an incubator at 35–37 °C for 24–48 h.
2.2.3. Microbiological Analysis
The isolates from the selective media Cetrimide agar were identified by conventional criteria, including morphology of colonies, production of pigments, Gram stain and positive oxidase test. Colonies isolated in CHROMID® CPS Elite (BioMérieux, France) were identified by the turquoise colour and by Gram staining, a negative catalase test and a positive esculin hydrolysis test. The isolates from CHROMID® Candida (BioMérieux, France) were presumptively identified regarding their colonial morphology and colour, in four species (C. albicans green colour, C. tropicalis steel blue, C. kruzei fuzzy pink and C. glabrata purple). However, as no further identification tests were carried out to confirm these 4 species, we opted for the general designation of Candida spp.
Subsequently, the colony count was performed from the dilution where they were countable, with up to 300 colonies, which were transformed in CFU/mL = number of colonies × total dilution factor/volume of culture plated in mL.
2.2.4. Statistical Analysis
Data analysis was performed using IBM® SPSS® (Statistical Program for Social Sciences), version 29.0 for Windows.
Descriptive statistics were used to estimate frequencies and percentages, mean, median, 1st quartile, 3rd quartile, standard deviation, minimum and maximum. The Shapiro–Wilk test was used to assess the normality of the variables under study, including gender, age, smoking habits, hygiene habits, one or more teeth with endodontic treatment, implant connection type and the use of removable prostheses. Since normality was not observed, non-parametric analyses were chosen. Therefore, to compare the number of CFU/mL of E. faecalis, Candida spp. and P. aeruginosa according to the collection site (tooth or implant), the non-parametric Mann–Whitney test was used. In relation to the implant, to compare the CFU/mL of E. faecalis according to the presence or absence of endodontic treatment and the CFU/mL of Candida spp. according to the use or non-use of an irrigator, the Mann–Whitney test was employed. The non-parametric Kruskal–Wallis test, followed by Dunn’s test with Bonferroni correction for multiple comparisons, was used to compare the CFU/mL of E. faecalis, Candida spp. and P. aeruginosa according to the disease state (mucositis or peri-implantitis) and health, as well as to compare the CFU/mL of E. faecalis, Candida spp. and P. aeruginosa according to the type of implant (Cone morse, internal hexagon, and external hexagon). Spearman’s correlation coefficient was used to assess the relationship between age and the different microorganisms (E. faecalis, Candida spp. and P. aeruginosa). The significance level was set at 0.05.
3. Results
A total of 20 individuals with ages ranging from 26 to 86 years (mean = 52.25; SD = 15.1), 70% of whom were female (n = 14) with a mean age = 53.57, and 30% of whom were male (n = 6) with a mean age = 49.16, agreed to participate in this study. The demographic characteristics, clinical parameters of the study population and collection sites are presented in Table 1 and Table 2, respectively. The analysed data regarding oral hygiene habits showed that 65% (n = 13) brushed their teeth twice a day, with the majority, 85% (n = 17), not using dental floss and 70% (n = 14) not using an irrigator. Regarding smoking habits, 90% (n = 18) were non-smokers, while the remaining 10% (n = 2) had this habit. A total of 90% of the patients (n = 18) did not have removable prostheses, while 10% (n = 2) did. As for the presence of endodontic treatment, 80% (n = 16) had one or more treated teeth. The study population included 25% (n = 5) of patients with edentulous maxillae rehabilitated with prostheses supported by internal connection implants, 25% (n = 5) with single-unit Cone morse connection implants, 20% (n = 4) with single-unit internal connection implants, 15% (n = 3) with single-unit external connection implants and 10% (n = 2) rehabilitated with pontics up to three elements with an internal connection, while 5% (n = 1) were Cone morse implants.

Table 1.
Comparison of CFU/mL of different microorganisms according to the sampling site.

Table 2.
Comparison of E. faecalis CFU/mL according to the presence or absence of endodontic treatment.
In Figure 1, we can observe the distribution of the studied microorganisms. However, it is important to mention that numbers 4, 6, 16, 19 and 20 correspond to implant-supported complete dentures, which means the absence of teeth (which were not analysed).
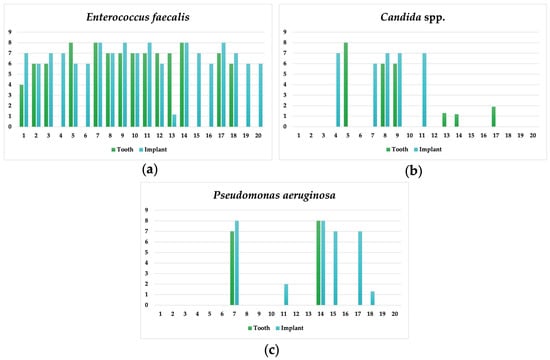
Figure 1.
Individual results for E. faecalis (a), Candida spp. (b) and P. aeruginosa (c) for the implant and adjacent tooth.
For all positive cases of E. faecalis, Candida spp. and P. aeruginosa, their relationship with the periodontal and peri-implant diagnosis was analysed through the following graphs, which showed a higher presence of these three opportunistic pathogens in both periodontal and peri-implant disease (Figure 2).
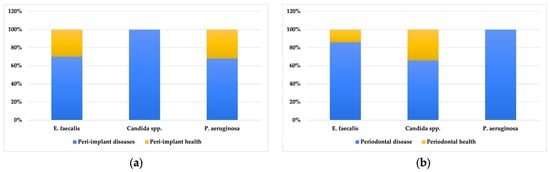
Figure 2.
Percentage distribution of microorganisms for peri-implant disease/health (a) and periodontal health/disease (b) in positive individuals.
All positive cases for the three microorganisms mostly had a diagnosis of periodontal and peri-implant disease. When comparing the presence of microorganisms according to the sampling site (peri-implant or periodontal subgingival plaque—Table 1), it was observed that E. faecalis had equal median values of CFU/mL in the adjacent tooth and the implant, although the values in the implant showed greater variability. Candida spp. had higher median values in the implant (1.00 × 107 [1.00 × 106; 77,500,000.0]), compared to the adjacent tooth (500,040.0 [18.75; 25,750,000.0]), but these differences were not statistically significant. P. aeruginosa also showed a higher CFU/mL in the peri-implant compared to the periodontal subgingival plaque of the adjacent tooth, but these differences did not reach statistical significance.
When comparing the values of E. faecalis in individuals who underwent endodontic treatment with those who did not undergo endodontic treatment, it was found that those who underwent treatment had higher median values of E. faecalis CFU/mL (1.00 × 106 [1.00 × 107; 1.00 × 108]) compared to those who did not undergo treatment (1.00 × 106 [1.00 × 106; 1.00 × 107]). However, these differences are not statistically significant (Table 2).
Through Table 3, we can observe that E. faecalis had higher median values of CFU/mL in individuals with mucositis (1.00 × 107 [1.00 × 107; 1.00 × 108]), compared to individuals with peri-implant health (1.00 × 106 [750,003.75; 32,500,000.0]) and peri-implantitis (5,500,000.0 [1.00 × 106; 32,500,000.0]); however, these differences are not statistically significant.

Table 3.
Comparison of CFU/mL of E. faecalis and Candida spp. in individuals with mucositis, peri-implantitis and peri-implant health.
When analysing the presence or absence of differences in the number of CFU/mL of Candida spp. between individuals who use or do not use an irrigator, it was found that out of the six individuals who were positive for Candida spp., the five individuals who did not use an irrigator had higher median values (1.00 × 107 [1.00 × 107; 1.00 × 107]) compared to the individual who used an irrigator (1.00 × 106 [1.00 × 106; 1.00 × 106])and these differences were statistically significant (p = 0.025) (Table 4).

Table 4.
Comparison of CFU/mL of Candida spp. according to the use or non-use of an irrigator.
The comparison of the number of CFU/mL of the microorganisms among the different types of implant connection was performed using the Kruskal–Wallis test (Table 5), which revealed that individuals with Cone morse implants had significantly higher CFU/mL values of E. faecalis (1.00 × 108 [1.00 × 107; 1.00 × 108]) compared to individuals with external hexagon (1.00 × 107 [1.00 × 107; 1.00 × 107]) and internal hexagon (1.00 × 106 [1.00 × 106; 1.00 × 107]]) implants (H = 10.3; p = 0.007). These differences were observed specifically between Cone morse and internal hexagon implants (p = 0.005). Regarding the number of CFU/mL of Candida spp., all three types of implants showed equal median values. As for P. aeruginosa, individuals with Cone morse implants had higher CFU/mL values compared to internal hexagon and external hexagon implants, respectively, but these differences did not reach statistical significance.

Table 5.
Comparison of CFU/mL of the three microorganisms according to the type of implant connection (Cone morse, internal hexagon and external hexagon).
When we examined the relationship between age and the presence of these three microorganisms (Table 6), a weak to moderate negative correlation was found with E. faecalis, Candida spp. and P. aeruginosa, but this did not reach statistical significance. The older the age, the higher the presence of these microorganisms in both the peri-implant and periodontal subgingival plaque of adjacent teeth.

Table 6.
Spearman correlation between age and the presence of the three analysed microorganisms in the total samples.
In Figure 3, the relationship between the type of rehabilitation and the type of implant connection with the peri-implant diagnosis is presented, where it can be observed that there is a higher number of peri-implantitis cases in implants with internal connection.
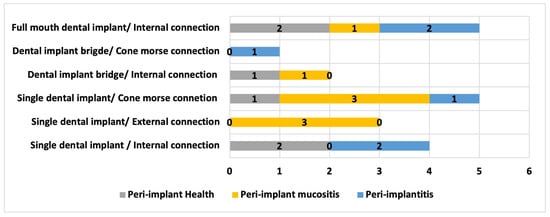
Figure 3.
Distribution of results regarding the type of oral rehabilitation/connection type with peri-implant diagnosis.
4. Discussion
Approximately 10% of titanium implants present with premature failure, mainly due to bacterial infection within the first year of placement [14].
Leonhardt et al., in 2003, evaluated the microflora in peri-implant lesions and demonstrated that facultative anaerobic periodontal pathogens and opportunistic species, such as Staphylococcus spp., Enterococcus spp., Candida spp. and P. aeruginosa, were also found around compromised implants [15]. A recent study also found significantly higher levels of P. aeruginosa in oral epithelial cells of individuals with periodontitis compared to those with a healthy periodontium [11]. In our study, when we correlated the number of CFU/mL of E. faecalis with peri-implant health, peri-implant mucositis and peri-implantitis, the median values were higher in peri-implant mucositis than in peri-implantitis, although these differences were not statistically significant. However, it should be noted that undiagnosed and untreated peri-implant mucositis can progress to peri-implantitis. Based on the Consensus Report of the Sixth European Workshop on Periodontology, Lindhe and Meyle reported an incidence of peri-implant mucositis of up to 80% and incidence of peri-implantitis between 28% and 56% [13]. Several studies have quantified the incidence of peri-implantitis development in patients with a history of periodontitis, indicating that it is about six times more prevalent in patients with periodontitis than in patients without a history of periodontal disease. Other research indicates that teeth can be a source of bacteria in partially edentulous patients who have been rehabilitated with dental implants [16,17]. Regarding our study, we found that the majority of individuals diagnosed with periodontal disease (gingivitis and periodontitis) also had a diagnosis of peri-implant disease in the selected implant (peri-implant mucositis and peri-implantitis).
In our study, when we analysed the number of CFU/mL of Candida spp. among individuals who used an irrigator and those who did not; we found that those who did not use an irrigator had higher median values compared to the individual who used an irrigator, and these differences were statistically significant. These data, although requiring further investigation regarding the usefulness of the irrigator in reducing the colonisation of peri-implant tissues, particularly by Candida species, may indicate an effect of this hygiene method, such as the cleansing action of saliva, preventing the presence of yeast in peri-implant plaque formation.
Alrabiah et al. reported that the subgingival environment can serve as a refuge for various Candida species [8]. In addition to adhering to teeth and oral mucosal surfaces, yeast can also adhere to non-biological surfaces such as titanium implants. While the presence of oral Candida species in the subgingival region plays a role in the aetiopathogenesis of periodontal diseases (such as chronic periodontitis and aggressive periodontitis), the contribution of oral yeast to the occurrence and progression of peri-implant diseases remains uncertain [18]. As in the study by Alrabiah et al., a higher presence of Candida was found in individuals with peri-implantitis compared to those without peri-implantitis. In our study, all cases of peri-implantitis revealed the presence of Candida spp.
Some risk factors associated with an increased oral presence of Candida include smoking and compromised oral hygiene status. These are the same risk factors that have been shown to increase the risk of peri-implant diseases. The results of the present study are in line with the study by Darwazeh et al., which showed a significantly higher presence of Candida in patients with poor oral hygiene [19].
According to Flanagan et al., E. faecalis is present in the majority of endodontic infections and is difficult to eliminate through endodontic treatment, so it can persist in the root canals and the surrounding alveolar bone. This bacterium often remains in the alveolar bone after the extraction of these teeth and can colonise the implant after its placement, which can lead to marginal bone loss and, consequently, implant loss. Although there are few studies linking E. faecalis to peri-implant disease, it appears to play a key role in bone loss around the implant or in peri-implantitis. This author even suggests that E. faecalis can cause infection both individually and in multi-species [20]. When comparing the median CFU/mL values of E. faecalis in individuals with endodontically treated teeth and those without endodontic treatment, it was found that those who underwent endodontic treatment had higher median CFU/mL values of E. faecalis than those who did not. Although these differences were not statistically significant, the role of endodontically treated teeth adjacent to implants should be further analysed to evaluate their role in the colonisation of peri-implant plaque and the development of peri-implantitis.
Various modifications to implant design have been made in recent years to reduce the space between the implant and the prosthetic component to reduce bacterial proliferation, but with limited success. Generally, implants have a polished cervical collar that prevents the adhesion of microorganisms, as the connector region is in contact with soft tissues and not intraosseous [21]. High roughness and hydrophilicity are suggestive of an important role in bacterial adhesion and colonisation on implant surfaces, but they also have benefits for the process of osseointegration [22]. In addition, although studies suggest that there is no significant difference regarding the shape or macrostructure of the implant (external or internal connection), the external connection shows a greater response of the soft tissues due to infiltration [23,24].
In our study, the highest bacterial colonisation by E. faecalis and P. aeruginosa was found in implants with Morse taper connections, while the lowest was associated with implants with internal hexagon connections, contradicting the results of the study by Romanos et al. in 2016, which found that prosthetic components with Cone morse connections had lower bacterial counts, since this type of connection has a frictional locking system that allows for intimate adaptation in the deeper internal portions of the system, reducing micro-movements during loading. However, in the study by Romanos et al., a higher quantity of Prevotella, Selenomonas, Eubacterium and Fusobacterium was detected in the internal connection, and only Ochrobactrum was detected in the Cone morse taper connection, which were not analysed in our study. Khorshidi et al., in 2016, also concluded that, overall, the Cone morse connection seems to have an obvious advantage in terms of microbial sealing capability, although their study mainly focused on the presence of Streptococcus mutans [25].
5. Conclusions
Despite the limitations of this study, it was possible to find some correlations between the presence of Candida spp., E. faecalis and P. aeruginosa in the periodontal and peri-implant plaque and the presence or absence of disease. Although we cannot conclude that these microorganisms can promote periodontal/peri-implant disease, their abundant presence cannot be overlooked, and their aetiological role in peri-implantitis should be further investigated. The relationship of these microorganisms with demographic data, medical history, hygiene habits, implant connection type and endodontic treatment has been established, although with low statistical relevance due to the small sample size. However, a larger study is planned to overcome the limitations.
Author Contributions
Conceptualization, A.M.S. and C.C.; methodology, C.C.; software, M.d.P.G.; validation, J.M.M., M.C. and A.S.S.; formal analysis, M.d.P.G.; investigation, A.M.S.; resources, A.S.S.; data curation, M.C.; writing—original draft preparation, A.M.S.; writing—review and editing, J.M.M.; visualization, M.C.; supervision, C.C.; project administration, C.C.; funding acquisition, J.M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study protocol was previously approved by the Ethics Committee of the University Institute of Health Sciences, IUCS-CESPU (Protocol CE/IUCS/CESPU-17/22), following the Helsinki guidelines.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data that support this study’s findings are available from the corresponding author upon request.
Acknowledgments
We are grateful for the availability and cooperation of the Department of Dental Sciences, the Cooperative for Polytechnic and University Education (Cooperativa de Ensino Superior Politécnico e Universitário (CESPU).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ardila, C.M.; Ramón-Morales, O.M.; Ramón-Morales, C.A. Opportunistic pathogens are associated with deteriorated clinical parameters in peri-implant disease. Oral Dis. 2020, 26, 1284–1291. [Google Scholar] [CrossRef]
- Bäumer, A.; Toekan, S.; Saure, D.; Körner, G. Survival and success of implants in a private periodontal practice: A 10 year retrospective study. BMC Oral Health 2020, 20, 92. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Xuan, S.; Wang, Z. Oral microbiota: A new view of body health. Food Sci. Hum. Wellness 2019, 8, 15. [Google Scholar] [CrossRef]
- FFaveri, M.; Figueiredo, L.C.; Shibli, J.A.; Pérez-Chaparro, P.J.; Feres, M. Microbiological diversity of peri-implantitis biofilms. Adv. Exp. Med. Biol. 2015, 830, 85–96. [Google Scholar] [CrossRef]
- Lafaurie, G.I.; Sabogal, M.A.; Castillo, D.M.; Rincón, M.V.; Gómez, L.A.; Lesmes, Y.A.; Chambrone, L. Microbiome and Microbial Biofilm Profiles of Peri-Implantitis: A Systematic Review. J. Periodontol. 2017, 88, 1066–1089. [Google Scholar] [CrossRef]
- Preethanath, R.S.; AlNahas, N.W.; Bin Huraib, S.M.; Al-Balbeesi, H.O.; Almalik, N.K.; Dalati, M.; Divakar, D.D. Microbiome of dental implants and its clinical aspect. Microb. Pathog. 2017, 106, 20–24. [Google Scholar] [CrossRef]
- Uslu, M.; Sabancı, A.; Eltas, A.; Gomes, V. Peri-Implant Tissue Microbiology: A Review. JOJ Case Stud. 2018, 9, 555756. [Google Scholar] [CrossRef]
- Alrabiah, M.; Alshagroud, R.S.; Alsahhaf, A.; Almojaly, S.A.; Abduljabbar, T.; Javed, F. Presence of Candida species in the subgingival oral biofilm of patients with peri-implantitis. Clin. Implant. Dent. Relat. Res. 2019, 21, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, F. Role of oral yeasts in the etiopathogenesis of peri-implantitis: An evidence-based literature review of clinical studies. Arch. Oral Biol. 2020, 111, 104650. [Google Scholar] [CrossRef] [PubMed]
- De Mendoza, I.L.-I.; Cayero-Garay, A.; Quindós-Andrés, G.; Aguirre-Urizar, J.M. A systematic review on the implication of Candida in peri-implantitis. Int. J. Implant Dent. 2021, 17, 73. [Google Scholar] [CrossRef] [PubMed]
- ElDein, M.A.T.; Yassin, A.S.; El-Tayeb, O.; Kashef, M.T. Chlorhexidine leads to the evolution of antibiotic-resistant Pseudomonas aeruginosa. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 2349–2361. [Google Scholar] [CrossRef] [PubMed]
- El-Telbany, M.; El-Sharaki, A. Antibacterial and anti-biofilm activity of silver nanoparticles on multi-drug resistance pseudomonas aeruginosa isolated from dental-implant. J. Oral Biol. Craniofac. Res. 2022, 12, 199–203. [Google Scholar] [CrossRef]
- Lindhe, J.; Meyle, J.; Group D of European Workshop on Periodontology. Peri-implant diseases: Consensus Report of the Sixth European Workshop on Periodontology. J. Clin. Periodontol. 2008, 35 (Suppl. S8), 282–285. [Google Scholar] [CrossRef] [PubMed]
- Damiati, L.; Eales, M.G.; Nobbs, A.H.; Su, B.; Tsimbouri, P.M.; Salmeron-Sanchez, M.; Dalby, M.J. Impact of surface topography and coating on osteogenesis and bacterial attachment on titanium implants. J. Tissue. Eng. 2018, 9, 2041731418790694. [Google Scholar] [CrossRef] [PubMed]
- Leonhardt, A.; Bergström, C.; Lekholm, U. Microbiologic diagnostics at titanium implants. Clin. Implant Dent. Relat. Res. 2003, 5, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Berger, D.; Rakhamimova, A.; Pollack, A.; Loewy, Z. Oral Biofilms: Development, Control, and Analysis. High-Throughput 2018, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Kuang, X.; Chen, V.; Xu, X. Novel Approaches to the Control of Oral Microbial Biofilms. BioMed Res. Int. 2018, 2018, 6498932. [Google Scholar] [CrossRef] [PubMed]
- Slazhneva, E.; Tikhomirova, E.; Tsarev, V.; Orekhova, L.; Loboda, E.; Atrushkevich, V. Candida species detection in patients with chronic periodontitis: A systematic review and meta-analysis. Clin. Exp. Dent. Res. 2022, 8, 1354–1375. [Google Scholar] [CrossRef]
- Darwazeh, A.M.; Hammad, M.M.; Al-Jamaei, A.A. The relationship between oral hygiene and oral colonization with Candida species in healthy adult subjects. Int. J. Dent. Hyg. 2010, 8, 128–133. [Google Scholar] [CrossRef]
- Flanagan, D. Enterococcus faecalis and Dental Implants. J. Oral Implantol. 2017, 43, 8–11. [Google Scholar] [CrossRef]
- Romanos, G.E.; Biltucci, M.T.; Kokaras, A.; Paster, B.J. Bacterial Composition at the Implant-Abutment Connection under Loading in vivo. Clin. Implant Dent. Relat. Res. 2016, 18, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, A.C.; Brambilla, E.; Azzola, F.; Ottobelli, M.; Pellegrini, G.; Francetti, L.A. Laser microtextured titanium implant surfaces reduce in vitro and in situ oral biofilm formation. PLoS ONE 2018, 13, e0202262. [Google Scholar] [CrossRef] [PubMed]
- Ting, M.; Craig, J.; Balkin, B.E.; Suzuki, J.B. Peri-implantitis: A Comprehensive Overview of Systematic Reviews. J. Oral Implantol. 2018, 44, 225–247. [Google Scholar] [CrossRef] [PubMed]
- González-Martín, O.; Lee, E.; Weisgold, A.; Veltri, M.; Su, H. Contour Management of Implant Restorations for Optimal Emergence Profiles: Guidelines for Immediate and Delayed Provisional Restorations. Int. J. Periodontics Restor. Dent. 2020, 40, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Khorshidi, H.; Raoofi, S.; Moattari, A.; Bagheri, A.; Kalantari, M.H. In Vitro Evaluation of Bacterial Leakage at Implant-Abutment Connection: An 11-Degree Morse Taper Compared to a Butt Joint Connection. Int. J. Biomater. 2016, 2016, 8527849. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).