Abstract
As the need for clean and sustainable energy sources grows rapidly, green hydrogen and ammonia have become promising sources of low-carbon energy and important key players in the transition to green energy. However, production and storage problems make it hard to use them widely. The goal of this review paper is to give a complete overview of the latest technology for the manufacture and storage of hydrogen and ammonia. This paper deals with hydrogen and ammonia synthesis and storage. It examines the most recent technological breakthroughs in areas such as electrolysis, reforming, C-ZEROS, HYSATA, DAE, sulfide, and SRBW, as well as novel storage techniques, such as solid-state storage, plasma kinetics, and POWERPASTE. This article examines the history of ammonia production and discusses some of the newer and more sustainable techniques for producing ammonia, such as electrochemical and biological approaches. This study also looks at how artificial intelligence (AI) and additive manufacturing (AM) could be used to revolutionize the way green hydrogen and ammonia are produced, with an emphasis on recent breakthroughs in AI-assisted catalyst design and 3D-printed reactors, as well as considering major investments in the shift to green energy, such as Moroccan government programs, and how they may affect future hydrogen and ammonia production.
1. Introduction
As our global population grows and industrialization accelerates, energy demand continues to soar. However, the conventional energy sources that we rely heavily on, such as fossil fuels, come at a significant cost. The combustion of coal, oil, and natural gas releases greenhouse gases, contributing to climate change and air pollution [1]. It has become clear that we need to prioritize greener energy sources to mitigate these adverse impacts, reduce carbon emissions, and achieve long-term sustainability. Among the various options that have gained attention, hydrogen and ammonia have emerged as promising candidates due to their potential to revolutionize the energy landscape [2]. Hydrogen is a revolutionary energy carrier that has the potential to transform the way we utilize and transition to sustainable energy sources, thereby facilitating sustainable development. This versatile element possesses several key attributes that make it an ideal candidate to address our energy needs in an environmentally friendly way [3]. Hydrogen’s versatility enables its integration with various energy systems. It can be utilized as a standalone fuel or blended with natural gas, offering a smooth transition for existing infrastructure. Additionally, it can be utilized in conjunction with renewable energy sources to store surplus electricity generated during peak production, thereby mitigating the intermittency issues associated with renewables. Forecasts indicate that by 2070, the global demand for hydrogen is projected to exceed 500 million metric tons. Notably, the transportation sector is anticipated to emerge as the primary consumer of hydrogen, as automotive companies increasingly shift away from conventional combustion engines and petroleum-based motor fuels. According to predictions, this sector alone is expected to require approximately 158.2 million metric tons of hydrogen by 2070, as depicted in Figure 1 [4]. The process of utilizing electrolysis to separate water into hydrogen (H2) and oxygen (O2) is commonly referred to as H2O electrolysis. In 2020, this method contributed approximately 0.03% of the hydrogen used in chemical and energy feedstocks.
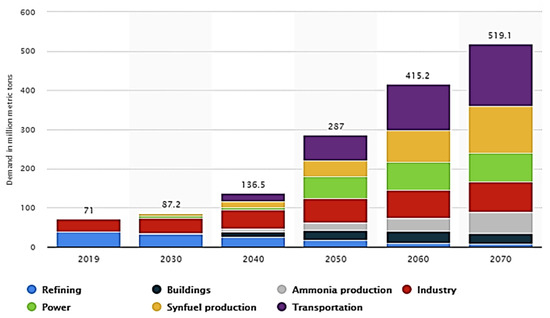
Figure 1.
According to a sustainable growth scenario, the projected global demand for hydrogen by sector, spanning from 2019 to 2070, is presented in million metric tons [4].
In particular, Europe has more than 40% of the world’s installed electrolyzer capacity in the world, with Canada (9%) and China (8%) also making significant contributions, as depicted in Figure 2 [5].
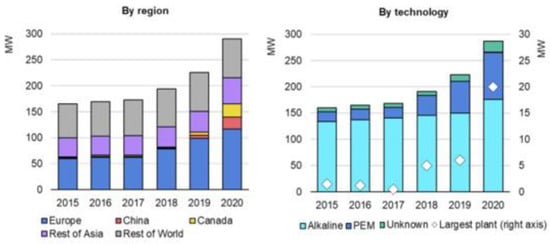
Figure 2.
Global installed electrolysis capacity by technology and region. (Hydrogen projects database 2021) [5].
Ammonia, a key component of the hydrogen economy, also presents significant environmental challenges. Despite being touted as a green fuel during the green revolution of the last century, ammonia continues to be a major energy guzzler in the current era. This is particularly concerning, given that ammonia is a critical building block for the production of fertilizers and is also an emerging energy vector for hydrogen-based systems. In fact, the ammonia market was valued at a staggering 69 billion US dollars in 2021 (Figure 3) [6], underscoring the importance of developing more sustainable methods for its production and use. Ammonia production currently uses 1% of all fossil fuels, resulting in 1% of all carbon dioxide emissions [7]. While this may not seem significant, on a global scale, it is a major contributor to climate change. However, the world is beginning to recognize the need for solutions to address this issue. One potential solution is a new process for ammonia production that could be used worldwide. Ammonia is used in various industries, including food and beverage, paper, leather, rubber, wastewater treatment, medication creation, cold storage, and refrigeration systems, as well as in the printing and cosmetic sectors [8]. However, the agricultural industry is by far the biggest producer and user of ammonia. Currently, ammonia is produced by a fixed process called the Haber–Bosch process. Nevertheless, this process is not environmentally friendly, as it requires significant amounts of energy, with the reactants hydrogen and nitrogen needing to be pressurized to around 150 to 300 times atmospheric pressure, that need sustainable modifications [9]. Hattori et al. (2020) discovered a new catalyst based on CaFH that operates at lower temperatures and pressures to react hydrogen with nitrogen, which is typically captured from the air to produce ammonia (NH3) at 50 °C and can produce the same amount of ammonia in a reaction where the operating conditions are 400 °C and 200 bar [10]. Snyder et al. (2023) introduced a groundbreaking type of metal–organic framework (MOF) that exhibits exceptional capabilities in ammonia separation and extraction from the gas mixture produced through the Haber–Bosch process [11]. This MOF is made up of copper atoms linked by cyclohexane dicarboxylate organic molecules. When exposed to ammonia, the MOF undergoes a transformation into a polymer containing copper and ammonia, boasting an impressively high density of stored ammonia. What is remarkable is that these polymer strands release the bound ammonia at relatively low temperatures, effectively restoring the material back to its original rigid and porous MOF structure.
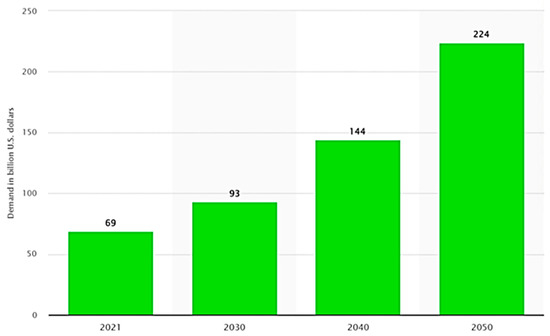
Figure 3.
Estimated global ammonia demand from 2021 to 2050 (in billions of dollars) [6].
The increasing focus on decarbonizing the energy sector has accelerated the adoption of green hydrogen and ammonia technologies, with several countries and companies announcing ambitious targets for their production and use. Armijo et al. (2020) evaluated the feasibility of producing green hydrogen and ammonia from variable solar and wind energy in Chile and Argentina. This study showed that flexible production processes can improve the profitability and sustainability of green hydrogen and ammonia production. It also highlighted the potential for Chile and Argentina to become major producers and exporters of green hydrogen and ammonia and discussed the necessary regulatory and policy frameworks for their development [12]. Salmon et al. (2021) examined the potential of green ammonia as a carrier of renewable energy, particularly for long-distance transportation and energy storage. The article discussed the benefits of green ammonia production, such as its high energy density and ease of storage, and provided an overview of the current status of green ammonia production technologies. The article also highlighted the challenges associated with green ammonia production, including the need for efficient and cost-effective methods for production, storage, and transportation [13]. Kakavand et al. (2023) conducted a techno-economic assessment of green hydrogen and ammonia from wind and solar energy in Iran. The authors evaluated the feasibility of different configurations of electrolysis and ammonia synthesis processes, and analyzed the economic and environmental impacts of the proposed systems. The study concluded that the production of green hydrogen and ammonia from renewable sources can be economically viable and environmentally beneficial in Iran, and recommended further research and development to scale up these technologies [14]. Ourya et al. (2023) investigated the potential of producing green hydrogen in Morocco using a combination of photovoltaic (PV) and wind energy sources. This research presented a techno-economic analysis of the proposed system, evaluating the feasibility and sustainability of the green hydrogen production process. The impact of various parameters, such as weather conditions, energy demand, and capital costs, on the performance of the proposed system was also considered. The results suggested that the proposed system had the potential to produce green hydrogen at a competitive cost while reducing greenhouse gas emissions. This study provided valuable insights into the feasibility of green hydrogen production in Morocco, which could contribute to the country’s energy transition towards a more sustainable future [15]. With the abundance of research and projects in the field, hydrogen and ammonia have emerged as highly promising solutions to achieve net-zero goals in various sectors, including power, transport, heat, and energy storage [16]. It is an opportune time to invest in green hydrogen and ammonia production technologies, as the potential applications of hydrogen expand across multiple industries. From power generation to manufacturing processes in sectors such as steelmaking and cement production, from fuel cells for electric vehicles to heavy transport such as shipping, and from green ammonia production for fertilizers to applications in cleaning products, refrigeration, and electricity grid stabilization, the possibilities for hydrogen utilization are vast. In our comprehensive review, we delve deeply into the latest research and developments in these areas, exploring the transformative potential of green hydrogen and ammonia in shaping the future of energy production. The paper is organized into sections to provide a systematic analysis: Section 2 offers an overview of current hydrogen production technology, while Section 3 investigates existing hydrogen storage systems. Section 4 focuses on conventional ammonia production methods, followed by the exploration of advancements in ammonia manufacturing technology in Section 5. Furthermore, we explore the integration of artificial intelligence (AI) into the manufacturing process, examining how it can enhance efficiency and optimize hydrogen and ammonia production. The benefits of incorporating 3D printing technology, such as reducing material waste and enabling design flexibility, are discussed in Section 8. We also explore how additive manufacturing (AM) can create complex geometries, structures, and catalysts, leading to enhanced efficiency and performance in hydrogen and ammonia generation. Finally, in Section 8, we take a closer look at the significance of ammonia and hydrogen as vital investments in the green energy transition, with a specific focus on the case of Morocco.
2. Different Methods of Hydrogen Generation
Imagine a world where clean and sustainable energy is abundant, where our dependence on fossil fuels is replaced by a greener alternative. This is the vision behind the classification of hydrogen production based on colors, known as the “colors of hydrogen” (Figure 4). It is an innovative approach that allows us to differentiate between different methods of hydrogen production based on their environmental impact. Among these colors, green hydrogen shines the brightest. It is produced through the electrolysis of water using renewable energy sources, such as solar or wind power. This process emits no carbon dioxide and holds the promise of a truly sustainable energy future. On the other end of the spectrum, gray hydrogen represents the traditional and carbon-intensive method of hydrogen production. It is derived from fossil fuels and releases significant amounts of carbon dioxide into the atmosphere. The colors of hydrogen classification open our eyes to the diverse pathways towards a cleaner energy landscape, prompting us to prioritize green hydrogen and encouraging further research and development in this area. By embracing green hydrogen, we can unlock a world of endless possibilities, where sustainable energy powers our industries, propels our transportation, and preserves our planet for generations to come.
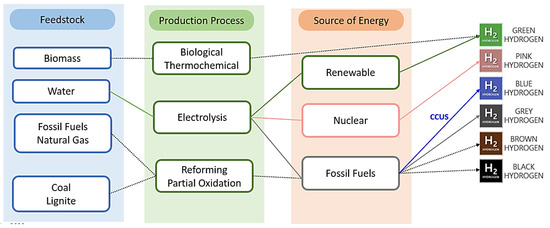
Figure 4.
Classification of hydrogen production.
2.1. Current Hydrogen Production
Hydrogen is one of the most abundant elements in the universe and ranks as the initial element on the periodic table. Extraction of pure hydrogen is a complicated process because we will usually find it bound up with other elements, for example, with oxygen in water with carbon in methane (CH4), ethane (C2H6), or polypropylene (C3H6), etc. We have split up these bounds in Figure 5 [17,18], which shows the numerous technologies used in the manufacture of both sustainable and non-sustainable hydrogen. Figure 6 shows the frequency of using different methods of hydrogen production in the industry, where the steam reforming method from hydrocarbons is the most often employed method of manufacturing in the industry of hydrogen production [19].
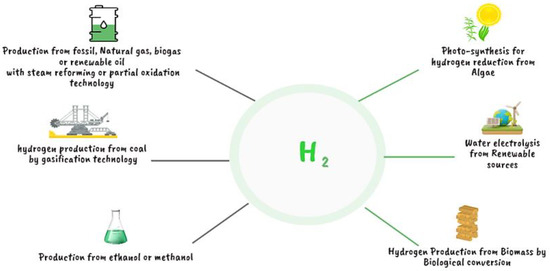
Figure 5.
Current techniques of producing sustainable and non-sustainable hydrogen [17,18].
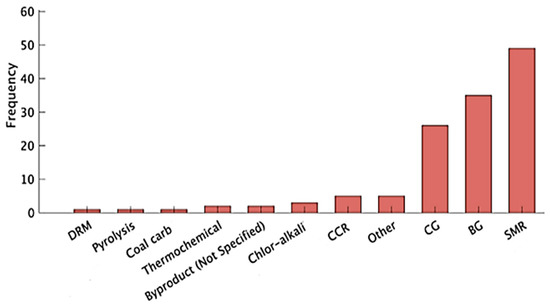
Figure 6.
Hydrogen production methods [19].
The latest reviews [20,21] have covered most types of hydrogen production from renewable sources of energy, as well as hydrogen production from fossil sources, the commercial methods of which are summarized in Table 1. This comparison indicates that not all hydrogen is created equal, but there is a whole rainbow of different ways to make it (gray, turquoise, pink, blue, and green) [22]. This arbitrary color-coding system for how hydrogen is produced despite the multiplicity of production sources. Gray, blue, and green hydrogen remain the most dominant production methods that are attracting scientists and industrialists.

Table 1.
Summary of commercial hydrogen production methods, feedstock/existence, and efficiency.
2.2. Commercial Technologies of Hydrogen Generation
In this section of the review, we provide a concise overview of each method, providing a high-level understanding without delving into intricate details. The purpose is to present the key aspects and characteristics of each approach, highlighting its unique features and distinguishing factors. While not exhaustive, this section offers valuable insights into the specifics of each method, allowing readers to grasp the main principles and concepts behind them [27,28].
- (a)
- Steam Reforming
The endothermic conversion of natural gas and water vapor into hydrogen and carbon monoxide occurs during steam reforming. The heat is frequently provided by the burning of part of the natural feed gas. The process is generally carried out at temperatures that range from 700 to 1000 °C [29].
Equation (1): Steam reforming of hydrocarbon [30]:
CnHm + nH2O ⇌ nCO + ((m/2)+ n) H2
Equation (2): Water–gas shift reaction [31]:
CO + H2O → CO2 + H2
CnHm represents a hydrocarbon with “n” carbon atoms and “m” hydrogen atoms. nH2O represents “n” water molecules. nCO represents “n” molecules of carbon monoxide. Finally, (m/2 + n) H2 represents the total number of hydrogen gas molecules produced, taking into account “m” hydrogen atoms from the hydrocarbon and “n” from water. The second equation represents the reaction between carbon monoxide (CO) and water (H2O) to produce carbon dioxide (CO2) and hydrogen gas (H2). This reaction is known as the water–gas shift reaction (1).
- (b)
- Partial Oxidation
In the process of producing hydrogen by the partial burning of hydrocarbons (natural gas) with the equation for oxygen gas (3), the reaction is exothermic, meaning that heat is released during the reaction. This heat can be utilized to maintain the reactor’s temperature, eliminating the need for external heating. The carbon monoxide generated during the partial oxidation process can then be further transformed into hydrogen gas using another reaction, which is represented by Equation (2). The coefficients “n” and “m” represent the stoichiometric coefficients, indicating the number of molecules of each component involved in the reaction.
Equation (3): Partial oxidation of hydrocarbons:
- (c)
- Proton exchange membrane (PEM) electrolysis
In PEM electrolyzers, an acidic polymer membrane acts as the electrolyte, separating hydrogen from oxygen in water through the application of an electric current. The process involves a negatively charged cathode and a positively charged anode. The reactions involved in PEM electrolysis are as follows.
Equation (4): Anode reaction [32]:
At the anode, water is split into oxygen gas, hydrogen ions (protons), and electrons. The protons are released into the electrolyte, while the electrons flow through an external circuit.
Equation (5): Cathode reaction:
2H+ + 2e− → H2
At the cathode, the protons from the anode combine with the electrons that have traveled through the external circuit. This recombination produces hydrogen gas. These reactions demonstrate the overall process of PEM electrolysis, where water is split into oxygen and hydrogen gas by utilizing electricity. This method allows for the production of “green hydrogen” using renewable energy sources. Although hydrogen produces zero carbon emissions at the point of use, its cleanliness depends on the production pathway and the energy sources used in the production process. Therefore, it is crucial to ensure the origin of hydrogen to consider it as a clean energy.
In recent years, there has been a lot of interest in the various technologies of hydrogen generation to play a significant role in integrating future energy systems and bridging the transition from fossil-based energy to renewable energy. The methods of hydrogen generation can influence its cleanness, cost-effectiveness, and efficacy. All of this is dependent on the feedstocks used to extract the hydrogen, the yield of production, and the available energy resources [33].
2.3. Latest Hydrogen Production Process Advances
Almost 90% of all hydrogen that is produced today is from fossil fuel, called “gray hydrogen.” In order to utilize hydrogen as a clean fuel or energy vector, it is essential to clean up its production process. One approach to achieve this is through blue hydrogen, which is produced from natural gas via steam reforming. Regardless of the nature of the feed gas (such as methane or ethanol), the key factor is the production method, which generates carbon dioxide (CO2) emissions. To prevent these emissions from being released into the atmosphere, capture and storage of CO2 technology should be employed. However, it is important to consider potential leaks along the supply chain. While this method can recover approximately 48% of CO2 emissions, it falls short of fully addressing the issue, as it is estimated that only 90% of CO2 emissions can be effectively reduced [34]. Blue hydrogen has been formally introduced into major economies’ hydrogen programs. Figure 7 showcases the current and projected hydrogen production technologies, offering a visual representation of the various methods currently available and those anticipated for future development.
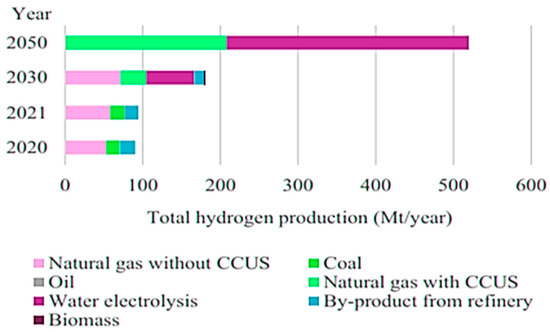
Figure 7.
Current and projected hydrogen production technologies [35].
The following advancements show green hydrogen-generating systems in Figure 8, which are still in the early stages of research for large-scale manufacturing and are exceedingly promising for fulfilling global decarbonization goals.
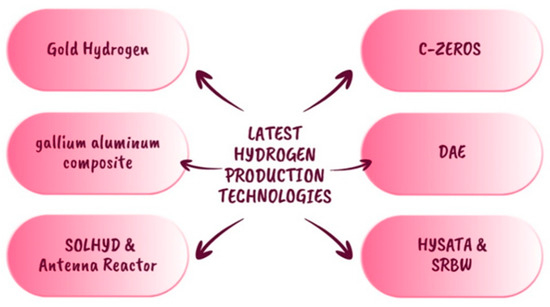
Figure 8.
Recent hydrogen production technologies [36,37,38,39,40,41,42,43].
2.3.1. C-Zero Technology
A new technology dubbed the C-Zero process offers a compromise option for countries that really heavily on exporting fossil fuels while also allowing them to adhere to green energy goals, particularly the creation of hydrogen without carbon dioxide. This technology (Figure 9) utilizes thermocatalysis to transform natural gas into turquoise hydrogen and solid carbon via methane pyrolysis and heat.
CH4(g) + heat → 2H2 + C(s)
This could be a very interesting technology for energy transition [44]. This technology focuses primarily on resolving the problem of CO2 emission, but the leaks of methane during the distribution process remain a problem that should be solved, because this release of methane into the atmosphere is 80 times more powerful in warming plants than CO2. According to the International Energy Agency, 1 ton of methane may be regarded as equal to 28 to 36 tons of CO2 in the atmosphere. There is a recent advanced technology that can detect leaks of methane, but we cannot completely remove them [45]. The disadvantage of this process is that it needs heat to drive its reaction. That heat can be produced in four ways, as follows. (1) Burn a little bit of natural gas to drive the reaction so that this process does not have zero emissions, but the CO2 released in this process would still be a 75% reduction compared to traditional steam methane. (2) Use about a third of the hydrogen produced to drive this reaction. The goal of all recent technologies is to increase the yield of hydrogen production, and using it again is not a good idea. (3) Use electric heating by renewable energy, but the intermittence of this solution can provide a problem. (4) Its climate value depends on providing clean energy for pyrolysis and physical carbon storage, but using a hybrid system that mixes natural gas, hydrogen, and electrical solutions as a source of heat and works with them all at the same time for greater efficiency, especially if there is not much electrical energy available, can resolve this problem [46].
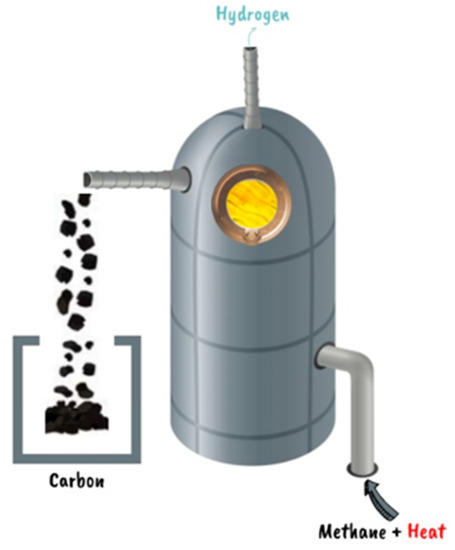
Figure 9.
C-Zero process [44].
Producing hydrogen from natural gas costs about $1.5/kg, while pure hydrogen costs about $5/kg. As a result, 1% of hydrogen in 2021 was produced through electrolysis [47]. With global economies aiming to become carbon neutral, green hydrogen has emerged as a promising alternative; however, production costs must be reduced to make it economically feasible for countries worldwide. The DOE announced the Hydrogen Shot initiative, which seeks to reduce the price of pure hydrogen to $1/kg in 2050 [48]. As such, we still need something new and innovative to properly produce it.
2.3.2. Gold Hydrogen
To reach the Paris Agreement’s objective, according to the idea of the demand for low-carbon hydrogen and 60% of oil and natural gas reserves having to be in the ground [49], a company called Cemvita has developed a new type of hydrogen called gold hydrogen for just $1/kg from depleted and abandoned oil and gas (Figure 10). Petroleum is called “liquid gold” because of its high value in the market and the fact that it is difficult and expensive to extract. “Gold” hydrogen is a new form of carbon-neutral hydrogen recovered from depleted oil reservoirs that are prepared for abandonment. This gold hydrogen is created by employing microbes that can degrade residual oil in oil wells and break it down into hydrogen and carbon dioxide. Gold hydrogen production and usage can not only get us closer to carbon neutrality but can also extend the life of these wells, which were previously nothing more than a big burden [50].
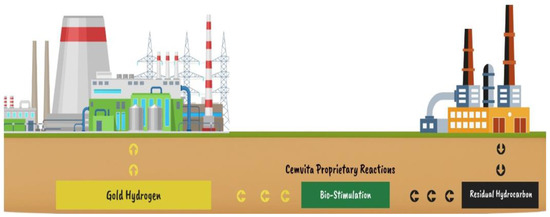
Figure 10.
Gold H2 underground generation [50].
2.3.3. Antenna Reactor
A team from Rice’s Laboratory Nanophotonic has used ammonia to create hydrogen without emitting any carbon gas. They expose ammonia to a novel inexpensive and abundant nanomaterial’s catalyst based on copper–iron capable of converting ammonia into clean hydrogen using only the power of light rather than heat and create a chemical reaction to acquire hydrogen (Figure 11). This process represents a significant advance in the field of sustainable energy generation: the creation of a scalable photocatalyst constructed of low-cost raw materials, making it a low-cost option for hydrogen generation [51]. Conventional ammonia breakdown catalysts rely on high temperatures, which use a lot of energy and can create toxic byproducts. As a result, it is a cleaner and more sustainable hydrogen manufacturing process. The photocatalyst’s usage of transition metals makes it an efficient and effective method for converting ammonia to hydrogen. Because this method may be driven by either sunlight or energy-efficient LEDs, it is both environmentally friendly and cost-effective. Unlike existing thermal catalysts, which are often comprised of precious materials, the novel photocatalyst is built of low-cost raw materials, making it a more accessible and practical alternative for large-scale hydrogen generation [52]. Ultimately, this groundbreaking technology has the potential to transform the field of sustainable energy generation and contribute to a more environmentally friendly future.
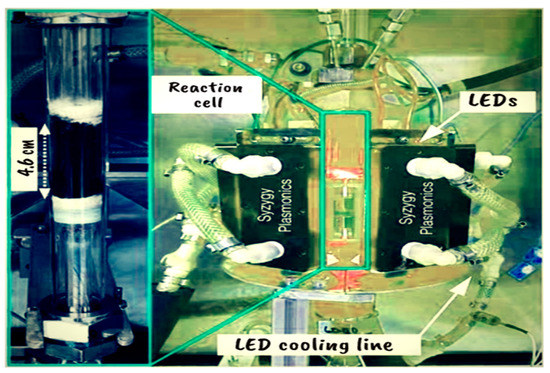
Figure 11.
Experiments of copper–iron plasmonic photocatalysts for hydrogen synthesis from ammonia [51].
2.3.4. HYSATA Technology
The Korean Institute of Science and Technology recently conducted a test with a team of researchers on a novel membrane and electrode module. This module showcases a remarkable 10-fold performance advantage compared to current proton exchange membrane (PEM) technology. The company Hysata has developed this groundbreaking technology, which operates differently from traditional electrolysis cells (Figure 12). Instead of having the anode and cathode electrodes in direct contact with the liquid, this technology utilizes a porous hydrophilic separator placed between the cathode and anode. Moreover, it leverages capillary action to draw up the electrolyte on only one side of the electrode, thereby facilitating the direct production of hydrogen and oxygen without the presence of air bubbles. This innovative approach achieves an impressive efficiency rate of up to 98%, while requiring an energy expenditure of 40.4 kWh/kg. These results surpass those of current commercial electrolytes [53].
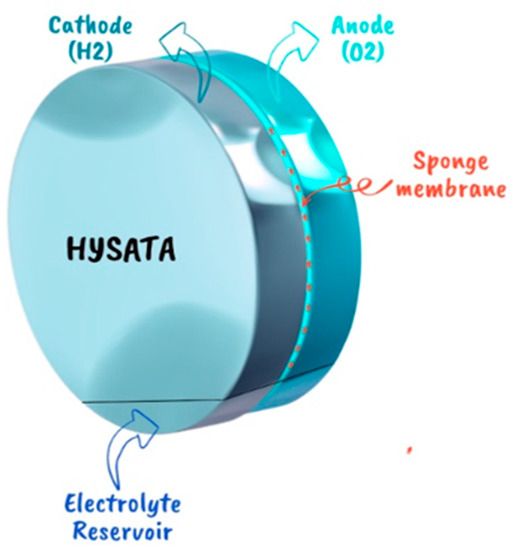
Figure 12.
HYSATA technology [54].
One of the primary challenges associated with electrolyzers is their high consumption of pure water during hydrogen production. However, in many regions of the world, pure water is a scarce resource with limited availability, posing difficulties for electrolyzers that rely on it. To address this issue, the development of a technique that enables the use of abundant saltwater as a viable alternative is crucial. In addition to enhancing the efficiency of the electrolysis process, effective water desalination plays a vital role in prolonging the life span of electrolyzers. By removing impurities, water desalination ensures clean electrodes and facilitates smooth operation of the electrolysis process. This, in turn, optimizes the performance and efficiency of the electrolyzers, leading to increased hydrogen gas production and reduced energy consumption [55].
2.3.5. DAE Technology
DAE technology is a new technology that produces hydrogen directly from air, wherever on Earth, without the need for any other freshwater source. The direct air electrolyzer (DAE) collects and transforms air moisture, even at 4% humidity. This electrolyzer has two flat plates that serve as the anode and cathode, Figure 13. A porous material, such as a melamine sponge or sintered glass foam, is sandwiched between the two plates. This medium is immersed in a hygroscopic ionic solution, which is a chemical capable of absorbing moisture from the air. Connect it to an energy source, expose it to air, and at the cathode and anode, hydrogen and oxygen begin to be released [56].
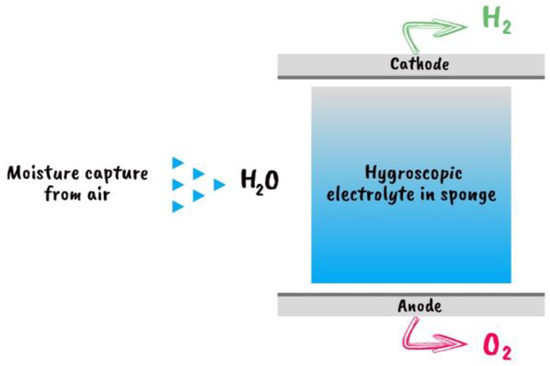
Figure 13.
A schematic depiction of the DAE module’s cross section [56].
2.3.6. Gallium–Aluminum Composite Technology
Amberchan et al. (2022) discovered a novel approach to manufacture hydrogen from water at room temperature by producing a gallium–aluminum composite that can react with water under normal conditions (Figure 14). As the concentration of gallium in the composite grows, so does the production. Hydrogen might be utilized to power fuel cells or internal combustion engines, and could then replace gasoline. The hydrogen is created in an amazingly simple way: water is added to a liquid alloy of aluminum and gallium to make hydrogen, which can then be immediately fed into an engine. The approach causes a chemical reaction in water that divides the oxygen and hydrogen, releasing hydrogen in the process. It may be used with any available water supply, including wastewater, commercial drinks, and even ocean water [57]. Table 2 illustrates the optimal mixing conditions along with different sources of gallium, aluminum and water, providing valuable insights into the best combination for achieving desired results.
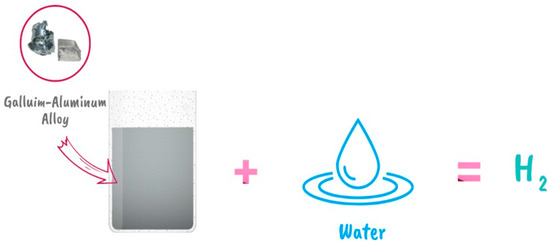
Figure 14.
Water splitting and hydrogen generation using Ga-Al composite [57].

Table 2.
Optimum mixing conditions, as well as varied gallium, aluminum, and water sources [57].
2.3.7. SRBW Technology
RMIT University engineers have devised a technique to improve the efficiency of the electrolysis process used to create hydrogen from water. The engineers were able to dramatically enhance the output of hydrogen generated during the electrolysis process by using sound waves, which might have significant implications for the renewable energy sector. Typically, the electrolysis process necessitates the use of corrosive electrolytes and costly electrodes such as platinum or iridium, both of which may be expensive and ecologically damaging to produce. The RMIT University engineers created a process that includes exposing the water to high-frequency sound waves that cause a vibration, which helps in the breakdown of water molecules into their component atoms. This technology has been demonstrated to be considerably more efficient than typical electrolysis methods, with a net positive energy saving of more than 27%. In general, employing sound waves to produce hydrogen is a viable method that might help in solving the problems posed by current electrolysis methods. This technology may lead to more environmentally friendly and cost-effective ways by reducing the need for pricey and environmentally destructive materials [58].
2.3.8. Solhyde Technology
A startup called Solhyde Technology is developing a hydrogen panel that produces hydrogen from air humidity using sunlight and a photocatalyst. This innovation makes it perfect for decentralized hydrogen generation in homes, enterprises, and communities. The panel works by collecting airborne water molecules and storing them in a tube structure. When the panel’s photocatalyst is activated by sunshine, the stored water is divided into pure hydrogen and oxygen. In comparison to conventional electrolysis methods, this technology has a number of advantages, such as the lack of a requirement for clean water or energy, lower operational costs, and a lower environmental impact. Moreover, it has the ability to produce hydrogen locally and on demand, making it a potentially beneficial technology for use in transportation, energy storage, and other fields. A huge roof would produce 2 to 4 tons of hydrogen annually, whereas 20 hydrogen panels on a small roof at home would produce 240 kg annually [59].
Table 3 provides a concise summary of the advantages and disadvantages associated with the latest advancements in hydrogen production processes. It highlights the key benefits and drawbacks of each method, offering valuable insights into their respective strengths and limitations. By presenting this information in a structured format, the table allows for easy comparison and evaluation of the different approaches, enabling researchers and industry professionals to make informed decisions regarding the most suitable hydrogen production method for their specific needs.

Table 3.
Comparative analysis of hydrogen synthesis technologies.
3. Recent Trends in Hydrogen Storage: Gas, Liquid, Solid
Hydrogen storage is one of the most difficult tasks. Hydrogen is kept in special materials and high-pressure tanks, such as those seen in such vehicles as cars and trains. These tanks are not only huge and expensive to construct, but they are also unsuitable for recycling and long-term storage. Because of this, researchers from all around the world are working to find ways to address these limitations and fully use hydrogen. Hydrogen has the advantage of being able to be kept in a variety of forms, including gaseous, liquid, and sometimes solid, despite the fact that storage poses major difficulties.
3.1. Current Hydrogen Storage
The various types of hydrogen storage systems now in use are depicted in Figure 15 [68,69]. Compressed gas and cryogenic liquid are the two types of hydrogen storage that are most often used. In various shapes, sizes, and capacities, metal or composite cylinders or tanks are used to store compressed hydrogen gas. Often, the gas is compressed to pressures between 350 and 700 bar, requiring a significant amount of energy. High pressures also put a strain on container materials, which can lead to wear and failure over time. Cryogenic liquid hydrogen is stored at very low temperatures (−253 °C) in specially designed containers that minimize heat transmission. Although this method has a higher energy density than compressed gas, cryogenic storage and transportation systems are complicated and costly. Additionally, the danger of asphyxiation and frostbite makes it crucial to handle and store cryogenic liquids carefully. Chemical storage in metal hydrides, chemical hydrides, and ammonia are further hydrogen storage possibilities being considered. These technologies have the potential to offer higher energy densities and lower operating pressures than compressed gas storage, but they also come with their own set of technical and financial difficulties [70].
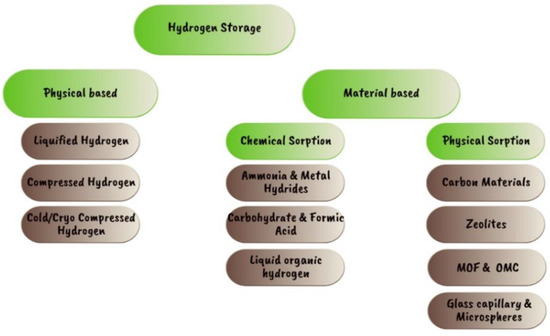
Figure 15.
Current hydrogen storage methods [68,69].
Hydrogen storage containers necessitate the use of robust materials that can resist high pressures while also preventing leaks. In the design of hydrogen storage tanks, materials including metals, polymers, and carbon fibers are frequently employed. Composite materials have played a significant role in the development of the green hydrogen and ammonia industry. One application of composites in this industry is in the construction of pressure vessels used for storing hydrogen and ammonia. These pressure vessels require materials that can withstand high pressures and offer superior resistance to hydrogen and ammonia permeation. Composite materials, such as carbon fiber-reinforced polymers (CFRPs), are ideal for this purpose due to their high strength-to-weight ratio and excellent resistance to permeation [71,72,73,74,75]. One important factor affecting hydrogen’s compatibility with storage materials is its interaction with metals. This interaction can have both chemical and physical effects: embrittlement, wet corrosion, and dry corrosion [76]. Under normal conditions, atmospheric corrosion is a rare chemical reaction that occurs between a dry gas and a metal, resulting in the slow erosion of the thickness of the tank wall [77]. At high temperatures, hydrides may be generated when hydrogen reacts with certain metals. As the temperature decreases below a specific point, known as the “no ductility” temperature, some metals can become more brittle and lose their ductility property [78]. Cryogenic metal storage tanks have been involved in numerous accidents, attributed to cold embrittlement [79]. The results of various investigations that looked into how storage materials interacted with hydrogen are compiled in Table 4. The precise mechanisms causing the phenomenon are still unknown, despite the fact that component failure may be a result. A deep understanding of the storage material’s interactions with hydrogen is required in order to choose suitable materials for storing it.

Table 4.
A hydrogen embrittlement mechanism in several materials and alloys used for hydrogen storage applications [80].
3.2. Latest Hydrogen Storage Technology
Hydrogen storage technology continues to evolve, driven by the increasing demand for clean and sustainable energy solutions. Two notable advancement in this field is the innovative hydrogen storage technology developed by Plasma Kinetics and Leibniz Institute. These technologies described in Figure 16 has the potential to revolutionize various industries and pave the way for a hydrogen-powered future.
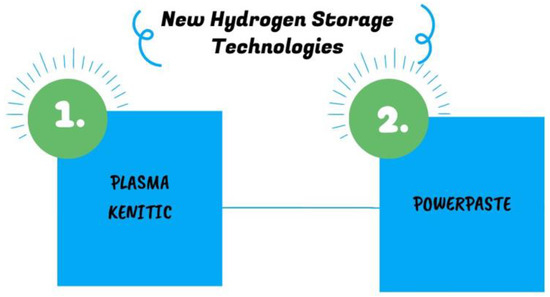
Figure 16.
Modern ways for storing hydrogen [86,87].
3.2.1. Plasma Kinetics
This innovation is the first hydrogen-based energy system to offer carbon-free energy capture, storage, and transport. This technology involves a light-activated nanoscale film that nine to ten times flimsier than human hair but it is able to absorb hydrogen from the air at low temperatures and pressure. This storage process is less expensive than traditional methods, requiring only light to be shone on the film in order to extract hydrogen onto an internal graphite-based structure directly from smokestacks. The technology could capture 99.99% pure hydrogen. Compared to alternative storage technologies, plasma kinetics technology has the following advantages. (1) A dense solid state for storing hydrogen is both secure and nonflammable. This might potentially improve security and lower the risk of accidents or leaks associated with current hydrogen storage methods. (2) In the process of capturing and storing hydrogen, neither pressure nor energy is required. (3) The whole nanophotonic film may be recycled, which could reduce the influence on the environment by minimizing waste. (4) This method can minimize the amount of infrastructure needed for hydrogen storage and distribution, removing the need for pipes and fixed-structure pumping stations. (5) It has quiet operation that reduces noise pollution [86].
3.2.2. POWERPASTE
The Leibniz Institute for Polymer Research Dresden (IPF) in cooperation with Dresden’s Fraunhofer Institute for Manufacturing Technology and Advanced Materials (IFAM) developed new technology based on solid magnesium hydride in the form of a goop called POWERPASTE. This matter has an approximately 10% hydrogen capacity, which means that 1 kg of hydrogen weighs the same as 10 kg of POWERPASTE with a power range of 100 W to 10 kW. The hydrogen is released when POWERPASTE comes into contact with any specific type of water. It can use a variety of different water sources, including tap water and even salt water. This technology provides several advantages: (1) its composition is not toxic and is safe, and it may be transported without any special safety precautions or worries; (2) it has a 5-year storage life, making it a reliable and practical option for storing hydrogen; (3) it is easily recyclable and is produced at a low cost of roughly €2/kg [87]. The parts of a POWERPASTE power supply system are depicted in Figure 17. It has three main parts: a hydrogen generator, a water tank, and a POWERPASTE cartridge.
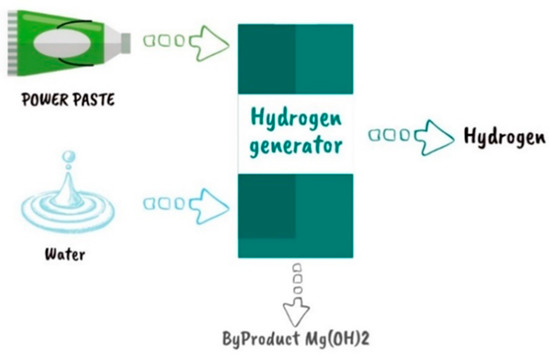
Figure 17.
POWERPASTE-based power system [87].
Table 5 presents a comparison between the plasma kinetics and POWER-PASTE technologies, highlighting their respective characteristics and performance metrics.

Table 5.
Comparison between the plasma kinetics and POWERPASTE technologies. [86,87].
Generating and storing hydrogen on a large scale is a challenge that requires additional study and development. Those technologies will become more widely available and cost-effective in the next few years, making hydrogen a more viable option for large-scale energy generation and storage. Table 6 provides a comprehensive summary of the advantages and disadvantages of the latest hydrogen storage technologies. It offers a concise overview of the strengths and weaknesses associated with each technology, allowing for a quick comparison and evaluation of their respective merits. By examining this table, readers can gain valuable insights into the various aspects that contribute to the effectiveness and feasibility of these storage methods, enabling them to make informed decisions based on their specific requirements and priorities.

Table 6.
Comparative analysis of hydrogen storage technologies.
4. The Hydrogen Highway
Hydrogen, a clean and versatile energy carrier, is increasingly being considered for injection into commercial gas pipelines for transportation purposes. This emerging practice, known as hydrogen blending, holds significant potential for decarbonizing the transportation sector and reducing greenhouse gas emissions. Injection of hydrogen into commercial gas pipelines has gained considerable attention as a key pathway for integrating hydrogen into existing energy infrastructure. This practice allows for the utilization of existing gas pipelines, storage facilities, and distribution networks, making it a cost-effective option for scaling up hydrogen deployment. Several studies and use cases have explored the feasibility, outcomes, and limitations of hydrogen injection into gas pipelines. However, there is significant doubt about whether hydrogen can be safely blended into existing pipelines at proportions high enough to make a significant impact [88]. Research suggests that utilities can only safely blend up to 20% hydrogen with natural gas using today’s pipelines and appliances [89]. The blend limits depend on the design and condition of current pipeline materials, pipeline infrastructure equipment, and applications that utilize natural gas. The HyBlend team is testing pipeline materials in varying concentrations of hydrogen at pressures up to 100 bar to assess their susceptibility to hydrogen effects. However, the article does not mention any specific use cases of hydrogen injection into commercial gas pipelines [88,89]. Further research and collaboration among industries, academia, and policymakers are necessary to address any technical, safety, and regulatory hurdles and unlock the full potential of hydrogen injection into commercial gas pipelines. Looking to the future, hydrogen blending can act as a stepping stone towards the widespread adoption of pure hydrogen as a transportation fuel. It allows for the development of hydrogen infrastructure, including hydrogen production facilities and refueling stations while capitalizing on the existing natural gas distribution network. This gradual approach not only mitigates the upfront costs and infrastructure requirements but also enables a smoother transition for end users and facilitates market acceptance.
As the production of renewable hydrogen expands, the potential for higher blending percentages increases. Renewable hydrogen, produced through processes such as electrolysis powered by renewable energy sources, ensures a carbon-neutral or even carbon-negative energy carrier. By scaling up renewable hydrogen production, the carbon footprint of blended hydrogen transportation can be significantly reduced, contributing to decarbonization goals. Embracing these opportunities will contribute to the ongoing transition towards a sustainable and decarbonized energy future. Blending hydrogen into existing gas infrastructure presents several challenges and uncertainties, presented in Table 7.

Table 7.
Challenges and uncertainties of blending hydrogen.
5. Recent Technologies Used in Ammonia Production
Ammonia is a colorless gas with a strong aroma made up of nitrogen and hydrogen atoms with a molecular weight of 17.03 g/mol and chemical formula NH3. It is highly soluble in water and used in cleaning agents, chemical products, and in the production of fertilizers, which involves the conversion of atmospheric nitrogen into a form that can be used by plants and other organisms. Ammonia can be produced with a chemical interaction between nitrogen gas and hydrogen gas in the presence of a catalyst that accelerates the reaction and increases the ammonia production yield. There are two ways to produce ammonia: conventionally or with sustainable methods. Table 8 presents the characteristics of anhydrous ammonia, providing a comprehensive overview of its properties and attributes.

Table 8.
Anhydrous ammonia characteristics [92].
5.1. Conventional Ammonia Production
In this way, ammonia is produced by the Haber–Bosh process. Hydrogen used in this process is produced using a catalyst at high temperatures and pressures from hydrocarbon sources. Recent developments in catalyst technology have improved the efficiency of the Haber–Bosch process, enabling lower operating temperatures and pressures. The production of ammonia, which remains the most widely used technique, involves the utilization of several catalyst types with unique properties, as illustrated in Figure 18 and Table 9 [93].
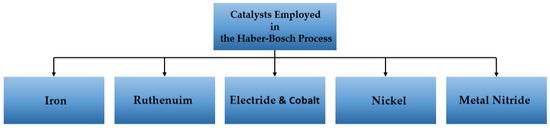
Figure 18.
Catalysts used in the Haber–Bosh process [94].

Table 9.
Ammonia synthesis rate employing various materials as catalysts under various operational parameters [93].
Boron Radical for Ammonia Synthesis
University Paul Sabatier researchers discovered new methods that can give a kinetically and thermodynamically advantageous to produce ammonia at room temperature without metals or hydrogen gas using reactive boron compounds. These boron-centered radicals were produced by combining organic boron halides with a strong reducing agent that transformed molecular nitrogen into borylamines. This, when combined with aqueous acid, yielded ammonium chloride by decomposing the stable three bounds of molecular nitrogen. Figure 19 presents this novel strategy that expands the potential for ammonia production without relying on fossil-based basic sources [98].
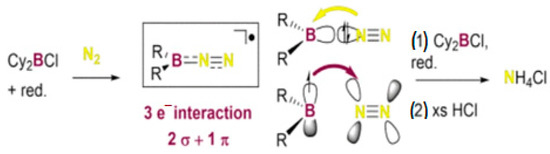
Figure 19.
Boron radical reaction [98].
5.2. Sustainable Ammonia Synthesis Technologies
In recent years, there has been a growing number of companies investing in green ammonia production to discover new technologies and make it more efficient and cost-competitive with traditional ways of production. The future of energy is changing, and green ammonia is playing an important part in this transition by promoting the integration of renewable energy into the energy grid and reducing the carbon footprint of transportation and industry. Figure 20 illustrates the many technologies used to produce green ammonia [99].

Figure 20.
Green ammonia production technologies [99].
5.3. New Ammonia Generation Routes
5.3.1. Electrochemical Ammonia Synthesis
Electrochemical ammonia production is an example of a new way to produce ammonia without the hydrogen production plant but using water as a source of hydrogen. This reaction involves oxidizing water at the anode, which releases protons that move to the cathode side via a solid or liquid electrolyte chosen based on the type of electrochemical cell. This electrolyte is a conductive solution that facilitates the migration of ions between the electrodes when electricity is applied. Under mild conditions, nitrogen interacts with the protons in the cathode to form ammonia. Table 10 highlights several experimental efforts using electrochemical electrolyte ammonia production. Figure 21 displays the different electrolytes employed in the synthesis of ammonia [99].

Table 10.
Electrochemical Electrolyte ammonia production [99].
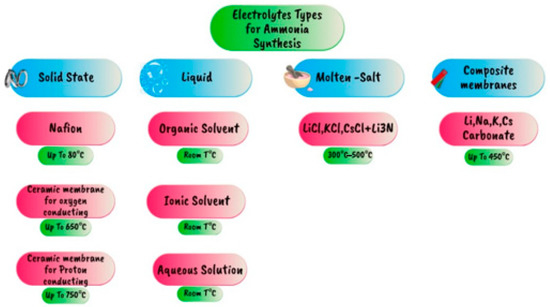
Figure 21.
Different electrolytes used for electrochemical ammonia synthesis [99].
5.3.2. Photocatalytic Ammonia Synthesis
The use of photocatalysts to create sustainable power and avoid global warming is one promising area of research. Electrochemical approaches for ammonia synthesis frequently require the use of costly and sometimes poisonous electrocatalysts, whereas photocatalysts may employ cheap and abundant materials. The semiconductor catalyst converts light energy into chemical energy, which is then utilized to drive the processes required for ammonia generation. Positively charged (h+) holes are left in the valence band by the migration of excited electrons from the valence band to the conduction band, then photoreduction of N2 and photooxidation of water take place simultaneously in the photochemical cell to produce ammonia. If the valence band of a semiconductor is higher than +1.23 V at pH 0 or +0.82 V at pH 7, surface holes (h+) can oxidize water to produce oxygen gas (O2) and protons (H+) via the oxygen evolution reaction (OER), as described in Equation (7). The H+ reduces to H2 on the cathodic side using photogenerated electrons from the conduction band of the semiconductor that contributes to the reduction of N2 to NH3. To obtain considerable H2 production rates, a high-work-potential metal such as Ni, Pd, Pt, or Au must be used as a co-catalyst [100]. Table 11 and Figure 22 and Figure 23 illustrate the catalysts utilized for photocatalytic ammonia production, providing an overview of their properties and effectiveness in facilitating the ammonia synthesis process.
H2O + 4h+→ O2 + 4H+
2H+ + 2e−→H2
N2 + 6H+ + 6e−→2NH3
2N2 + 6H2O→4NH3 + 3O2

Table 11.
Photocatalytic ammonia production catalysts.
Table 11.
Photocatalytic ammonia production catalysts.
| T (°C) | Catalyst | Light Radiation | Ammonia Yield μmol·h−1·g−1 | Ref |
|---|---|---|---|---|
| n/a | Alkynylated N-TiO2 | Xenon lamp | 83.11 | [101] |
| 25 | Fe-doped TiO2 | UV | 400 | [99] |
| n/a | ZnO/CuCo2O4 p-n | UV | 3460 | [102] |
| 25 | TiO2@C/g-C3N4 | Xenon Lamp | 250.6 | [99] |
| 25 | TiO2/Au/a-TiO2 | UV | 13.4 nmol cm−2 h−1 | [103] |
| 360 | K/Ru/TiO2-xHx | UV | 450–495 | [104] |
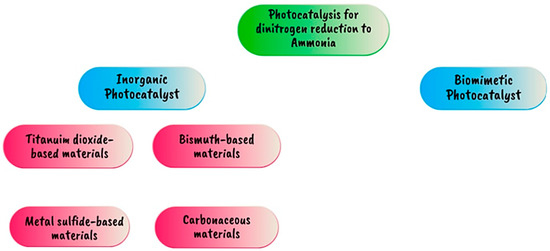
Figure 22.
Photocatalytic ammonia synthesis [100].
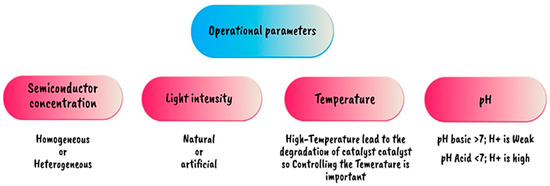
Figure 23.
Operational parameters in the photocatalysis process [105].
The band gap, VB and CB levels, and photocatalyst stability must all be taken into account. Dynamic constraints such as electron transport may make reducing N2 to NH3 challenging.
5.3.3. Nonthermal Plasma Process
This process involves the use of ionized gas containing charged particles called plasma in order to activate the nitrogen and hydrogen and promote the formation of ammonia [106]. This technology has been shown to be a promising transition to lower temperatures and direct ammonia synthesis. The three main variables and factors that influence the nonthermal plasma-assisted ammonia synthesis process are reactor design, operating parameters, and the catalyst, which plays an important role in production yield. Many efforts have been made to develop the catalysts shown in Table 12 to simplify and improve the efficiency of this technology.

Table 12.
Recent catalysts used for nonthermal process synthesis [107,108,109,110].
However, several limitations must still be addressed before these techniques may be deployed in large-scale applications. Olabi et al. [99] summarizes the advantages and disadvantages of several ammonia synthesis methods, despite the fact that all synthesis techniques meet considerable challenges. In comparison to the Haber–Bosh process, biotechnological ammonia is still inefficient for large-scale synthesis. Haber–Bosh technology has been developed during a century of industrial applications around the world. To reduce the current highly significant greenhouse gas emissions associated with the fertilizer industry, it should shift ammonia production to a carbon-neutral process that can be accomplished at any scale and in any part of the world.
6. Recent Trends in Ammonia Storage Technologies
Ammonia has been stored in pressure tanks such as spheres able to hold up to 2000 tons in liquid form since the start of industrial-scale ammonia manufacture nearly a century ago. In the industry, ammonia is stored in tanks with capacity for up to 50,000 tons of ammonia at ambient pressure and −33 °C located in plant sites and distribution hubs. Figure 24 describes many storage options [111,112].
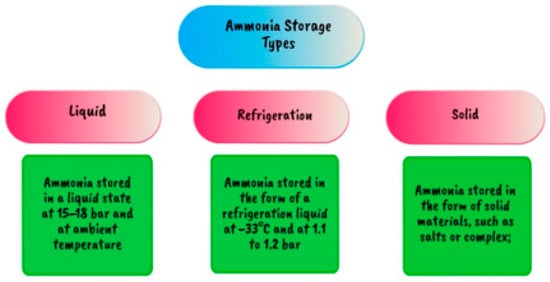
Figure 24.
Various ammonia storage techniques [111,112].
Previously, one-wall tanks were used in some places, but current practice, based on the QRA, recommends the use of DWDI tanks for bulk storage to achieve a risk threshold as low as reasonably practicable (ALARP) [111]. Table 13 presents the most common types of atmospheric ammonia storage tanks and storage facilities [113]. In comparison to hydrogen, which still faces material difficulties for pipeline transportation, ammonia is frequently transported over long distances using carbon steel pipes. To transport ammonia over a distance of 1610 km, it requires only 1119 kJ/kg-H2, which is significantly lower than the 14,814 kJ/kg-H2 required for hydrogen transport [114].

Table 13.
Ammonia storage tank and storage facility types [113].
Table 14 provides a comprehensive comparison of the characteristics of compressed hydrogen, liquid hydrogen, and liquid ammonia, allowing for an assessment of their respective properties and features.

Table 14.
Compressed hydrogen, liquid hydrogen, and liquid ammonia comparisons [115].
Solid-state storage has recently gained attention from researchers and companies as a promising technology for transportation applications by employing metal amine complexes to fix ammonia in solid form to face challenges such as toxicity and transportation. Table 15 describes the proprieties of different metal amines that can be used in this case [116].

Table 15.
The characteristics of various metal amine complexes [116].
Much research in this field is required to improve this way of storage. As a result, future development should concentrate on solid-state storage.
7. Economic Assessment and Future Perspectives of Hydrogen and Ammonia Production
Green hydrogen and ammonia are promising renewable energy solutions that could revolutionize global energy markets and contribute significantly to reducing greenhouse gas emissions. However, making green ammonia economically on a large scale is still a challenge. The economic feasibility of green hydrogen and ammonia technologies is determined by several factors, including initial investment costs, operational expenses, and potential cost savings. The initial investment costs for green hydrogen and ammonia technologies can be high, and this can be a significant barrier to their widespread adoption. However, the operational expenses of these technologies are generally lower than those of traditional fossil fuel-based technologies. Potential cost savings can also be achieved through the use of green hydrogen and ammonia technologies. The cost of green hydrogen and ammonia is an important factor in determining their economic feasibility. According to one study, green hydrogen can be separated from ammonia to sell at about $1.50/kg compared to traditional green hydrogen, which sells for up to $15/kg [117]. The production of green ammonia from clean hydrogen is technically feasible and economically viable, and it could significantly reduce global natural gas demand [118]. However, making green ammonia economically on a large scale is still a challenge, and at current ammonia prices, green ammonia would not be profitable, even when considering a present-day optimistic hydrogen price [119]. The cost of producing hydrogen varies depends on the method used. Making hydrogen from natural gas costs about $1.50 per kilogram, while clean hydrogen costs about $5 per kilogram [120]. The Department of Energy launched a program called Hydrogen Shot, which aims to reduce the cost of clean hydrogen to $1 per kilogram in one decade [120] A techno-economic analysis of a green ammonia production plant in Chile and its subsequent transport to Japan shows that the production of ammonia using hydrogen by means of electrolysis (carried out with solar energy) is economically feasible. Using that ammonia with Kontak’s technology, which Hydrofuel has previously acquired, allows for the production of green NH3 using $0.02/kWh electricity for as low as $220 a ton, whereas fossil fuel-derived NH3 is currently selling at $1500 to $2000 a ton [117]. Here are some details on the economic assessment and future perspectives of hydrogen production:
- The cost of producing hydrogen from renewable electricity could fall 30% by 2030 as a result of declining costs of renewables and the increasing number of policies and projects around the world [121].
- Steam reforming of natural gas is currently the most efficient, low-cost method for hydrogen production [122].
- Hydrogen used for the production of electricity through electrolysis using renewable energy systems is a costlier proposition [123].
- Green hydrogen production systems have been analyzed for their economic feasibility. An optimization model was used to evaluate the levelized cost of green hydrogen (LCOH) for different case studies, including variations in capital expenditure (CAPEX), power purchase agreement (PPA), electrolyzer CAPEX, storage CAPEX, and total CAPEX. The results showed that the LCOH of green hydrogen can be competitive with fossil fuel-based hydrogen production under certain conditions [124].
- A systematic review of various hydrogen production methods found that hydrogen is a low- or zero-carbon energy source that is considered the most promising and potential energy carrier of the future. One study presented an economic assessment to evaluate cost-effectiveness based on different economic indicators, including sensitivity analysis and uncertainty analysis [125].
- An economic assessment of hydrogen technologies participating in California electricity markets found that hydrogen production can be cost-competitive with other energy sources, such as natural gas, in certain markets and applications [126].
The IEA’s Ammonia Technology Roadmap, published in 2021, explores three possible futures for ammonia production and aims to reduce emissions from the industry. The report highlights that the world will need more ammonia with fewer emissions in the future, as an increasingly larger and affluent global population will lead to growth in ammonia demand. The report also notes that ammonia production accounts for around 2% of total final energy consumption and 1.3% of CO2 emissions from the energy system [127,128].
Recent studies have evaluated the feasibility of producing green ammonia, which is produced using renewable energy sources, and blue ammonia, which is produced using fossil fuels with carbon capture and storage [129,130] A techno-economic evaluation of ammonia production via integrated biomass gasification in an existing pulp and paper mill was also conducted [131]. These assessments have shown that green ammonia production can be cost-competitive with fossil-fuel-based ammonia production under certain conditions. In a roadmap to the ammonia economy published in 2020, the authors envisage renewable ammonia being produced in the future at a scale that is significant in terms of global fossil fuel use [132].
8. Recent Progress in Artificial Intelligence and Additive Manufacturing for Green Hydrogen and Ammonia Production
One of the challenges in producing green hydrogen and ammonia is optimizing the production process to maximize efficiency and reduce costs. This is where AI and AM are used. Artificial intelligence (AI), a subset of computer science combining advanced computing and statistics, is the ability of a machine to receive inputs and produce a behavior or reaction similar to that of an intelligent person. While humans have natural cognitive skills and learn through experience, artificial intelligence (AI) is based on data and algorithms [133]. Figure 25 shows the input parameters needed to produce hydrogen using artificial intelligence [134]. AI solutions are designed to enhance rather than replace human decision-making. AI may increase the quantity and quality of information accessible to human decision-makers by analyzing massive volumes of data and delivering insights and recommendations, ultimately leading to better and more informed decisions. Artificial intelligence (AI) tools are increasingly being recognized as a promising approach for modeling and optimizing bioprocesses. These tools have the potential to significantly improve the design and operation of production equipment, as well as predict and prevent equipment failures [135]. Figure 26 presents several AI technologies that have been used in these systems [136,137]. Response surface methodology (RSM) and artificial neural networks (ANNs) have demonstrated their superior predictive accuracy in modeling complex nonlinear bioprocesses, making them more efficient tools for optimizing the design and operation of production equipment. Depending on the manufacturing system, AM may signify a variety of things. can be used to manufacture complex components with higher precision and less waste, reducing material costs and increasing production efficiency [138]. Recent progress in artificial intelligence (AI) and additive manufacturing (AM) has the potential to completely transform the way green hydrogen and ammonia are produced.

Figure 25.
Water splitting via artificial intelligence technology [134].
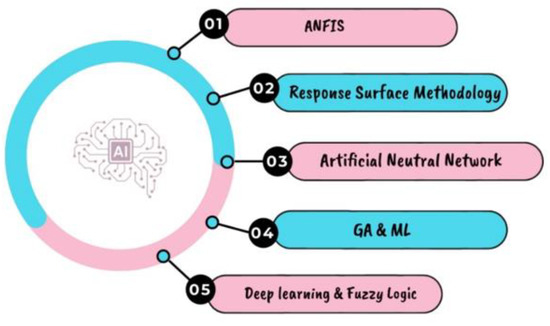
Figure 26.
AI technologies [136,137].
8.1. AI in the Generation of Green Hydrogen and Ammonia
RSM, ANNs, and ML are still the most common AI methods employed in hydrogen and ammonia production. RSM is a statistical approach that uses polynomial regression analysis to generate a second-order model equation that may be used to find a link between the input and output variables of a process [139]. ANNs may be designed to analyze equipment efficiency and detect possible problems before they cause a breakdown [140]. These methods are modeling systems that can aid operators in optimizing the operation of the electrolyte, such as the voltage and current used, in order to maximize hydrogen and ammonia production while minimizing energy consumption costs, and in boosting the operation of the reactor. The nature of the inputs differs depending on the mode of production, which can be either fossil or water splitting. In general, compared to RSM, ANNs have proven to be more efficient in simulating bioprocesses by numerous studies, proving that they can perform effectively even with minimal input. The ANN can then change the process variables in real time to maintain optimal conditions, resulting in increased efficiency and lower energy consumption [141]. In hydrogen and ammonia production systems, ML can also detect quality concerns, estimate energy demand, and optimize energy use. Additionally, these models may be trained to detect and diagnose problems in the manufacturing system, resulting in faster resolution and less downtime. Eventually, this leads to cost reductions in manufacturing and maintenance [142]. Figure 27 describes and depicts the impact of AI in this process [143,144,145].
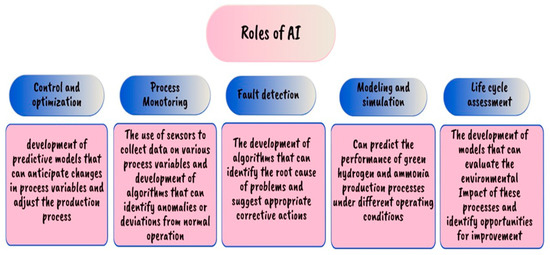
Figure 27.
AI roles [143,144,145].
8.2. AM’s Role in the Production of Green Hydrogen and Ammonia
Additive manufacturing (AM), commonly known as 3D printing, has gained significant attention in recent years as a powerful tool for fabricating complicated geometries with great precision and accuracy. This is especially helpful for making reactors and catalysts. Researchers have used 3D printing to fabricate microreactors with complex geometries for the synthesis of ammonia. These microreactors have shown higher conversion rates and selectivity compared to traditional reactors, which could lead to more efficient and sustainable ammonia production [146]. Additive manufacturing can also be used to fabricate novel catalysts for green hydrogen and ammonia production. For example, researchers have developed 3D-printed nickel foam catalysts for the electrolysis of water to produce hydrogen. These catalysts have shown higher activity and durability compared to traditional catalysts, which could lead to more efficient and cost-effective hydrogen production [147]. Additive manufacturing can also be used to fabricate components for renewable energy systems, such as wind turbines and solar panels, which can provide the electricity needed for green hydrogen and ammonia production. Other researchers have used 3D printing to fabricate wind turbine blades with optimized shapes for increased energy production [148]. As this technology advances, it may become critical in the transition to a more sustainable and carbon-free future. Figure 28 depicts the advanced varieties of AM employed in numerous industries, including energy conversion 3D printing [149].
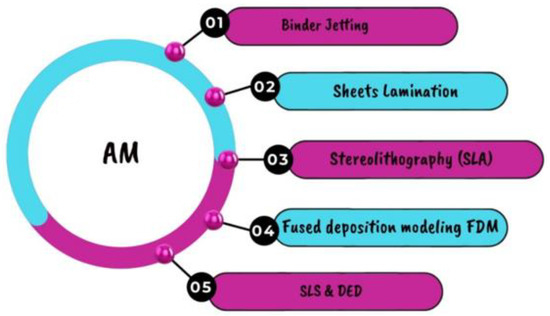
Figure 28.
Techniques of additive manufacturing [149].
Aerosol jet printing [150] and laser powder bed fusion are two forms of AM that are commonly employed in the production of green hydrogen and ammonia. Catalysts are printed onto electrodes and membranes for electrochemical technology using 3D printing [151,152]. The advantages of AI techniques can be summarized as follows. (1) AI automates processes, permitting robots to perform optimization tasks automatically and without the need for human intervention. Moreover, AI technologies reduce the time necessary for data processing, allowing for real-time operation execution. (2) AI technologies have been identified that enhance reasoning, reduce human errors, and remove failures caused by human limits. Despite its capabilities and potential to boost productivity and quality on a variety of levels, AI technologies do have significant limitations, including data. The availability of data is a key barrier to deploying AI systems. The fuel cell sector, for example, has major obstacles in implementing AI solutions due to a limitation of dependable or high-quality data [153]. Additive manufacturing offers various advantages, including the ability to produce complex geometries and structures that would be hard to achieve with traditional manufacturing processes, as well as eliminating waste and reducing the environmental effect of manufacturing. Also, it removes the need for costly tooling and installation costs and enables the creation of components and products on demand, decreasing the need for inventory storage and delivery process lead times. It may employ a variety of materials, including metals, polymers, ceramics, and composites, to create components and products with special characteristics. Overall, the benefits of additive manufacturing make it a powerful tool for a wide range of uses, including prototypes, and complex and unique geometries [154].
9. Green Hydrogen and Ammonia: A Key Investment in the Green Energy Transition
Governments, private organizations, and investors are investing in green energy research and development to promote rapid use of these clean energy sources. Despite its potential for decarbonization in a variety of industries, such as transportation, industry, and power production, the demand for green hydrogen and ammonia is anticipated to increase significantly globally in the coming years, as shown in Figure 29.
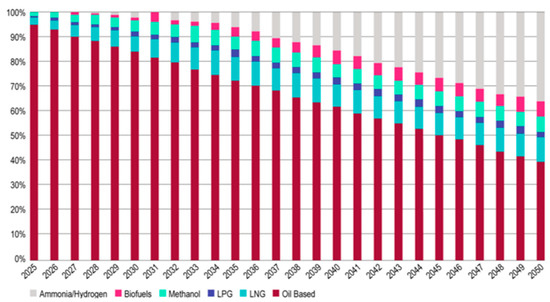
Figure 29.
Future demand for ammonia and hydrogen until 2050 [155].
According to the Hydrogen Council, the world’s yearly demand for hydrogen alone might approach 500–680 million metric tons by 2050 [156]. Take the example of Morocco, which has put forward an ambitious plan to develop green hydrogen and ammonia as part of its strategy efforts to decrease its dependence on petroleum and coal and to advance sustainable economic growth. In 2020, the Moroccan government announced a roadmap to create a green hydrogen sector in the country by 2030, which includes the construction of a sustainable hydrogen manufacturing plant, the creation of a regulatory framework, and the promotion of research and innovation. The government has also announced plans to develop a green ammonia project, which will involve the production of ammonia using renewable energy sources such as wind and solar power. The country is a major producer of phosphate, a key ingredient in fertilizer production that accounts for 85%. The data in Table 16 were taken from statistical databases from reports of the Minister of Energy, Mines, and the Environment. Renewable hydrogen and ammonia can cover nitrogen fertilizer requirements. This provides several benefits to Moroccan producers, such as decreasing the need to import from other countries and encouraging the microeconomy to consume local products in accordance with the economy of the country. Three important axes must be depended on to allow the implementation of the national policy for green hydrogen and ammonia in order to meet local demand and optimize national potential, particularly through exports: (1) technology, which includes technological improvements for cost reductions; (2) investment and supply, which includes investment conditions in the green hydrogen sector and its derivative products; and (3) finding market gaps or unmet needs and creating products or services to fill those gaps, ultimately leading to the creation of new markets [157,158].

Table 16.
Assessment of domestic demand for green hydrogen and its derivatives as a raw material [159].
9.1. Energy Transition: Case of Morocco
9.1.1. Cost Reduction
Morocco will be able to contribute to cost reduction through various actions, such as testing new technologies, R&D activities and innovation, facilitating cost savings along the value chain, notably by deploying projects with a capacity of 1 GW in the medium term [159].
9.1.2. Research and Innovation
Morocco will be able to cooperate with partner countries in the optimization and co-development of green hydrogen technologies and their derivatives in order to mutualize efforts in the field of research and new technologies and contribute to the creation of a regional cluster through the creation of a research and innovation cluster in partnership between the Ministry of Energy, Mines and Environment and IRESEN, MASEN, and other institutions. In terms of public–private partnership, the OCP Group will be a driving force through the first pilot projects for the production of green energy, in particular green ammonia [159].
9.1.3. Local Industrial Integration
The private sector will ensure local industrial integration by developing human and material resources. This integration will improve Morocco’s capacity to participate in the sector’s value chain and will ensure the transfer of knowledge. Good coordination between universities and vocational training institutions and a strong commitment to the socioeconomic world in research and development will be essential to human capital employable by national and international companies in the sector [159].
9.2. Investment
It is necessary to structure and organize the sector in the form of clusters and ecosystems in order to create synergies in the use of infrastructures. This will allow better management of infrastructure development for the establishment of green hydrogen and its derivatives sector. The government, in partnership with local authorities, will ensure the coordination and coordinate and adjust specific regulatory frameworks that can accelerate the deployment of the green hydrogen and derivatives industry while encouraging private investment [159].
9.2.1. Industrial and Infrastructure Cluster
In order to establish synergies in the utilization of infrastructures, the sector must be structured and organized in the form of clusters and ecosystems. This will enable better control of infrastructure development for the construction of the green hydrogen industry and its derivatives. In collaboration with local governments, the government will ensure the coordination and revision of certain regulatory frameworks that can speed the deployment of the green hydrogen sector and its derivatives while promoting private investment [159].
9.2.2. Funding
Direct assistance may be provided in the form of public–private partnerships, direct finance through bilateral or multilateral partnerships, or advantageous tax treatment. Certain tools and initiatives will be designed to help the industry acquire funding. They include financial instruments that reduce risk, such as investment guarantees, federal financial loan guarantees, and export credit guarantees [159].
9.3. Markets and Demand
9.3.1. Exports
The creation of an export industry for green hydrogen and its derivatives will be accomplished primarily through the introduction of synthetic liquid-fueled marine transport, the deployment of appropriate port infrastructure, and the deployment of production, storage, and export infrastructure. Green hydrogen products will be taxed more favorably. The primary consumers of Moroccan green hydrogen products will be Europe and nations striving to cut their GHG emissions by 95% by 2050 [159].
9.3.2. Storage
To boost the utilization of green hydrogen and its derivatives for energy purposes, it will be necessary to develop a storage plan. Morocco will assess the technical feasibility of storing green hydrogen and its derivatives in different forms, particularly in salt cavities. The storage plan will be drawn up on the basis of long-term technical and economic modeling of the national energy system and that of the interconnected countries [159].
9.3.3. Domestic Markets
Green hydrogen products will need to become more competitive in order to gain traction at the national level. Several degrees of involvement will be used: (1) the government will assist businesses by arranging information exchanges and platforms that may serve as a foundation for coordinated planning of various sections of the industry; (2) assistance for R&D, demonstration facilities, and scaling up through grants, research tax credits, infrastructure investment support, and so on; (3) by encouraging industry and private heavy transport firms to employ synthetic fuels, specialized markets will be created and developed; and (4) implementing technical incentives through the imposition of long-term carbon pricing [159].
Morocco boasts an abundance of renewable energy resources, including water, sun, biomass, and wind, which are crucial for producing clean hydrogen. With its 3500 km coastline, the country has the potential to develop impressive wind energy capacities, with wind speeds that can reach up to 10 m/s, which could potentially translate into 135 GW of wind power. Additionally, Morocco has one of the highest rates of solar insolation in the world, with between 3000–3600 h in the Moroccan Sahara. At the end of 2019, Morocco’s renewable energy capacity reached 3685 MW, including 700 MW of solar energy, 1215 MW of wind power, and 1770 MW of hydroelectricity, with a goal of reaching 6000 MW before the end of 2020. The country has been aiming to be a world leader in the hydrogen sector since 2016, with hydrogen being a key part of the equation. Morocco’s potential development in the hydrogen industry will lead to the production of hydrogen derivatives, such as green ammonia, which will be used by Moroccan industries such as OCP to reinforce its position as a leading international player in the fertilizer sector. In partnership with the German Corporation for International Cooperation and the German Moroccan Energy partnership (GIZ-PAREMA), Morocco has set a target of 42% of electricity supply coming from renewables by 2020, moving to 52% by 2030, with plans to add up to 11 GW of solar, wind, and hydropower capacity by 2030. Morocco has already built the Noor solar complex in Ouarzazate, which will have 582 MW of capacity. By aggregating solar and wind energy output in Morocco, a high load factor for hydrogen electrolysis can be achieved, with the potential for hydrogen to be produced in Morocco for around €1/kg, beating expected costs in Europe and cost-competitive with high-carbon-emitting hydrogen production via SMR without CCS. Wind power is a key technology with benefits for the MENA region, and the natural resources in North Africa, particularly Morocco, present a huge opportunity for the region to be a key player in the development of the hydrogen sector [160]. The outlook for green hydrogen and ammonia is highly promising, with these energy sources considered to be essential to the transition to green energy and efforts to combat climate change. The need for renewable energy sources is anticipated to increase quickly as the globe transitions to a low-carbon economy, and green hydrogen and ammonia are seen as key enablers of this transition. The outlook for green hydrogen and ammonia is highly promising, with the potential for significant growth, innovation, and impact in the context of the green energy transition and climate change mitigation. While there are still challenges and barriers to widespread adoption, the commitment and investment by governments, companies, and researchers around the world suggests that green hydrogen and ammonia will be essential in the transition to a future with zero-carbon emissions.
10. Conclusions
This paper presents a comprehensive overview of the latest technological advancements in the field of storage and conversion of hydrogen and ammonia. The areas of focus include electrolysis, reforming, C-Zero, Hysata, DAE, Solhyde, and SRBW, which are all promising methods of energy conversion. Additionally, the article highlights novel storage techniques, such as solid-state storage, plasma kinetics, and POWERPASTE, which offer efficient ways of storing energy. The latest technologies have shown promising results in overcoming challenges of scalability and environmental impact. Similarly, improvements in energy storage technologies, such as solid-state storage, liquid storage, and compressed gas storage, have made them more efficient and environmentally friendly, thereby increasing their viability as energy carriers. AI and additive manufacturing can be used to analyze large datasets and create complex geometries, which can help researchers identify patterns and make predictions about the performance of different process parameters, improve their performance, and reduce their weight. By using this information to optimize production processes, it is possible to increase the efficiency of hydrogen and ammonia production and storage while reducing costs and minimizing waste. These advancements have the potential to significantly reduce the dependence on fossil fuels and accelerate the transition to a cleaner, more sustainable energy future. The purpose of this study is to offer an overview of the Moroccan scheme aimed at boosting capacity, research, and innovation in the green hydrogen and ammonia sectors. Morocco is located at the crossroads of Africa, Europe, and the Middle East, making it an ideal location for the production and distribution of clean hydrogen. The country is also home to abundant renewable energy resources, including solar, wind, and hydro, which can be harnessed to produce green ammonia and hydrogen. These initiatives have positioned Morocco as a leader in renewable energy in Africa and have laid the groundwork for the development of the clean hydrogen sector. If Morocco can successfully develop its clean hydrogen sector, it could have significant economic and environmental benefits. The development of the hydrogen energy sector and ammonia presents a significant opportunity to reshape the economy. By focusing on the production and export of green ammonia and hydrogen, countries can establish a new source of income while simultaneously reducing their reliance on fossil fuels and combating climate change. This transition not only aligns with sustainable practices but also opens doors to new job opportunities and contributes to overall economic growth. The combined impact of these advancements holds immense potential for driving positive change and creating a prosperous, sustainable future for both the country and the global community.
Author Contributions
K.A. investigation, writing—original draft, methodology, writing—review & editing, visualization; M.N. investigation, writing—original draft, methodology, writing—review & editing, visualization, conceptualization, supervision, validation; A.F., D.S. and A.B., validation, project administration, resources. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data have been published in the manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Nomenclature
| AI | artificial intelligence |
| AM | additive manufacturing |
| PEM | proton-exchange membrane |
| SOEC | solid oxide electrolysis cell |
| MOF | metal–organic framework |
| SRBW | surface-reflected bulk wave |
| GHG | greenhouse gas |
| ANN | artificial neutral network |
| GA | genetic algorithm |
| FL | fuzzy logic |
| RSM | response surface methodology |
| IGC | International Code for the Construction and Equipment of Ships Carrying Liquefied Gases in Bulk |
| TSSE | temperature-swap solvent extraction |
| QRA | quantitative risk assessment |
| DWDI | double wall, double integrity |
| NRR | nitrogen reduction reaction |
References
- Wan, C.; Zhou, L.; Xu, S.; Jin, B.; Ge, X.; Qian, X.; Xu, L.; Chen, F.; Zhan, X.; Yang, Y.; et al. Defect engineered mesoporous graphitic carbon nitride modified with AgPd nanoparticles for enhanced photocatalytic hydrogen evolution from formic acid. Chem. Eng. J. 2022, 429, 132388. [Google Scholar] [CrossRef]
- Wan, C.; Liang, Y.; Zhou, L.; Huang, J.; Wang, J.; Chen, F.; Zhan, X.; Cheng, D.-G. Integration of morphology and electronic structure modulation on cobalt phosphide nanosheets to boost photocatalytic hydrogen evolution from ammonia borane hydrolysis. Green Energy Environ. 2022, in press. [Google Scholar] [CrossRef]
- Dincer, I.; Aydin, M.I. New paradigms in sustainable energy systems with hydrogen. Energy Convers. Manag. 2023, 283, 116950. [Google Scholar] [CrossRef]
- Statista. Forecast Global Hydrogen Sector Demand in Sustainable Development Scenario 2019–2070. 2021. Available online: https://www.statista.com/statistics/760001/global-hydrogen-demand-by-sector-sustainable-scenario/ (accessed on 25 June 2023).
- International Energy Agency. Hydrogen Projects Database. 2021. Available online: https://www.iea.org/data-and-statistics/data-product/hydrogen-projects-database (accessed on 25 June 2023).
- Statista. Forecast Demand for Ammonia Worldwide from 2021 to 2050. 2023. Available online: https://www.statista.com/statistics/1345802/forecast-value-global-ammonia-emand/ (accessed on 4 April 2023).
- Cutting Carbon Emissions with a Green Ammonia Production Process. 2023. Available online: https://www.innovationnewsnetwork.com/green-ammonia-process-can-significantly-reduce-carbon-emissions/28787/ (accessed on 4 April 2023).
- Uses of Ammonia. Available online: https://araxchemi.com/en/uses-of-ammonia (accessed on 4 April 2023).
- Kong, J.; Choi, J.; Park, H.S. Advantages and Limitations of Different Electrochemical NH3Production Methods under Ambient Conditions: A Review. Curr. Opin. Electrochem. 2023, 39, 101292. [Google Scholar] [CrossRef]
- Hattori, M.; Iijima, S.; Nakao, T.; Hosono, H.; Hara, M. Solid solution for catalytic ammonia synthesis from nitrogen and hydrogen gases at 50 °C. Nat. Commun. 2020, 11, 2001. [Google Scholar] [CrossRef]
- Snyder, B.E.R.; Turkiewicz, A.B.; Furukawa, H.; Paley, M.V.; Velasquez, E.O.; Dods, M.N.; Long, J.R. A ligand insertion mechanism for cooperative NH3 capture in metal–organic frameworks. Nature 2023, 613, 287–291. [Google Scholar] [CrossRef]
- Armijo, J.; Philibert, C. Flexible production of green hydrogen and ammonia from variable solar and wind energy: Case study of Chile and Argentina. Int. J. Hydrogen Energy 2019, 45, 1541–1558. [Google Scholar] [CrossRef]
- Salmon, N.; Bañares-Alcántara, R. Green ammonia as a spatial energy vector: A review. Sustain. Energy Fuels 2021, 5, 2814–2839. [Google Scholar] [CrossRef]
- Kakavand, A.; Sayadi, S.; Tsatsaronis, G.; Behbahaninia, A. Techno-economic assessment of green hydrogen and ammonia production from wind and solar energy in Iran. Int. J. Hydrogen Energy 2023, 48, 14170–14191. [Google Scholar] [CrossRef]
- Ourya, I.; Nabil, N.; Abderafi, S.; Boutammachte, N.; Rachidi, S. Assessment of green hydrogen production in Morocco, using hybrid renewable sources (PV and wind). Int. J. Hydrogen Energy 2023, in press. [Google Scholar] [CrossRef]
- The Role of Hydrogen and Ammonia in Meeting the Net Zero Challenge? Available online: https://royalsociety.org/-/media/policy/projects/climate-change-science-solutions/climate-science-solutions-hydrogen-ammonia.pdf (accessed on 7 July 2023).
- Ji, M.; Wang, J. Review and comparison of various hydrogen production methods based on costs and life cycle impact assessment indicators. Int. J. Hydrogen Energy 2021, 46, 38612–38635. [Google Scholar] [CrossRef]
- Vecchi, V.; Barera, S.; Bassi, R.; Dall’osto, L. Potential and Challenges of Improving Photosynthesis in Algae. Plants 2020, 9, 67. [Google Scholar] [CrossRef]
- Riera, J.A.; Lima, R.M.; Knio, O.M. A review of hydrogen production and supply chain modeling and optimization. Int. J. Hydrogen Energy 2023, 48, 13731–13755. [Google Scholar] [CrossRef]
- Martínez-Rodríguez, A.; Abánades, A. Comparative Analysis of Energy and Exergy Performance of Hydrogen Production Methods. Entropy 2020, 22, 1286. [Google Scholar] [CrossRef]
- Dermühl, S.; Riedel, U. A comparison of the most promising low-carbon hydrogen production technologies. Fuel 2023, 340, 127478. [Google Scholar] [CrossRef]
- Borowski, P.F.; Karlikowska, B. Clean Hydrogen Is a Challenge for Enterprises in the Era of Low-Emission and Zero-Emission Economy. Energies 2023, 16, 1171. [Google Scholar] [CrossRef]
- Qureshi, F.; Yusuf, M.; Khan, M.A.; Ibrahim, H.; Ekeoma, B.C.; Kamyab, H.; Rahman, M.M.; Nadda, A.K.; Chelliapan, S. A State-of-The-Art Review on the Latest trends in Hydrogen production, storage, and transportation techniques. Fuel 2023, 340, 127574. [Google Scholar] [CrossRef]
- Park, M.-J.; Kim, H.-M.; Gu, Y.-J.; Jeong, D.-W. Optimization of biogas-reforming conditions considering carbon formation, hydrogen production, and energy efficiencies. Energy 2023, 265, 126273. [Google Scholar] [CrossRef]
- Chen, W.-H.; Su, Y.-Q.; Lin, B.-J.; Kuo, J.-K.; Kuo, P.-C. Hydrogen production from partial oxidation and autothermal reforming of methanol from a cold start in sprays. Fuel 2020, 287, 119638. [Google Scholar] [CrossRef]
- Chi, J.; Yu, H. Water electrolysis based on renewable energy for hydrogen production. Chin. J. Catal. 2018, 39, 390–394. [Google Scholar] [CrossRef]
- Chen, W.-H.; Biswas, P.P.; Ong, H.C.; Hoang, A.T.; Nguyen, T.-B.; Dong, C.-D. A critical and systematic review of sustainable hydrogen production from ethanol/bioethanol: Steam reforming, partial oxidation, and autothermal reforming. Fuel 2023, 333, 126526. [Google Scholar] [CrossRef]
- Salehmin, M.N.I.; Husaini, T.; Goh, J.; Sulong, A.B. High-pressure PEM water electrolyser: A review on challenges and mitigation strategies towards green and low-cost hydrogen production. Energy Convers. Manag. 2022, 268, 115985. [Google Scholar] [CrossRef]
- Nabgan, W.; Nabgan, B.; Abdullah, T.A.T.; Ngadi, N.; Jalil, A.A.; Nordin, A.H.; Latif, N.A.F.A.; Othman, N.F.H. Hydrogen Production from Catalytic Polyethylene Terephthalate Waste Reforming Reaction, an overview. Catal. Sustain. Energy 2020, 7, 45–64. [Google Scholar] [CrossRef]
- El-Shafie, M.; Kambara, S.; Hayakawa, Y. Hydrogen production technologies overview. J. Power Energy Eng. 2019, 7, 107–154. [Google Scholar] [CrossRef]
- Chen, W.-H.; Chen, C.-Y. Water gas shift reaction for hydrogen production and carbon dioxide capture: A review. Appl. Energy 2020, 258, 114078. [Google Scholar] [CrossRef]
- Hydrogen Production: Electrolysis. Available online: https://www.energy.gov/eere/fuelcells/hydrogen-production-electrolysis (accessed on 12 July 2023).
- Dawood, F.; Anda, M.; Shafiullah, G.M. Hydrogen Production for Energy: An Overview. Int. J. Hydrogen Energy 2020, 45, 3847–3869. [Google Scholar] [CrossRef]
- Hydrogen’s Hidden Emissions. 2022. Available online: https://www.globalwitness.org/en/campaigns/fossil-gas/shell-hydrogen-true-emissions/ (accessed on 19 June 2023).
- Cho, H.H.; Strezov, V.; Evans, T.J. A review on global warming potential, challenges and opportunities of renewable hydrogen production technologies. Sustain. Mater. Technol. 2023, 35, e00567. [Google Scholar] [CrossRef]
- McCoy, M. C-Zero: Developing a liquid catalyst that could usher in an era of turquoise hydrogen. C&EN Glob. Enterp. 2021, 99, 34–35. [Google Scholar] [CrossRef]
- The Gold Standard or the Energy Transition. Available online: https://www.cemvita.com/gold-hydrogen (accessed on 11 March 2023).
- U of Melbourne Team Demonstrates Direct Hydrogen Production from Air; Direct Air Electrolysis (DAE). 2022. Available online: https://www.greencarcongress.com/2022/09/20220908-dae.html (accessed on 18 April 2023).
- UCSC Chemists Develop Gallium-Aluminum Composite for Rapid Generation of Hydrogen under Ambient Conditions. 2022. Available online: https://www.greencarcongress.com/2022/02/20220222-ucsc.html (accessed on 18 April 2023).
- Green Hydrogen Efficiency Wins. 2021. Available online: https://hysata.com/technology/ (accessed on 18 April 2023).
- Sound Vibrations Turbo Charge Green Hydrogen Production. 2022. Available online: https://techxplore.com/news/2022-12-vibrations-turbo-green-hydrogen-production.html (accessed on 18 April 2023).
- Solhyd Makes Green Hydrogen Accessible to Everyone. 2023. Available online: https://solhyd.org/en/ (accessed on 18 April 2023).
- Rice Lab’s Catalyst Could Be Key for Hydrogen Economy. 2022. Available online: https://www.nanotechnologyworld.org/post/rice-lab-s-catalyst-could-be-key-for-hydrogen-economy (accessed on 18 April 2023).
- Jenkins, S. Methane-Pyrolysis Process Leverages Natural Gas for CO2-Free H2 Generation. 2021. Available online: https://www.chemengonline.com/methane-pyrolysis-process-leverages-natural-gas-for-co2-free-h2-generation/ (accessed on 12 July 2023).
- Methane Emissions Are Driving Climate Change. Here’s How to Reduce Them. Available online: https://www.unep.org/news-and-stories/story/methane-emissions-are-driving-climate-change-heres-how-reduce-them (accessed on 2 March 2023).
- Energy Source Innovation Stream with C-Zero: Decarbonizing Natural Gas through Turquoise Hydrogen Production. Available online: https://www.atlanticcouncil.org/event/energysource-innovation-stream-with-c-zero/ (accessed on 2 March 2023).
- Global Hydrogen Review. 2021. Available online: https://iea.blob.core.windows.net/assets/e57fd1ee-aac7-494d-a351-f2a4024909b4/GlobalHydrogenReview2021.pdf (accessed on 11 March 2023).
- Hydrogen Shot Summit. Available online: https://www.energy.gov/eere/fuelcells/hydrogen-shot-summit (accessed on 11 March 2023).
- Welsby, D.; Price, J.; Pye, S.; Ekins, P. Unextractable fossil fuels in a 1.5 °C world. Nature 2021, 597, 230–234. [Google Scholar] [CrossRef]
- Gold Hydrogen Program Coalition Launches Program for Subsurface Biomanufacturing of Hydrogen. 2022. Available online: https://www.greencarcongress.com/2022/02/20220224-goldh2.html (accessed on 14 April 2023).
- Zhou, L.; Swearer, D.F.; Zhang, C.; Robatjazi, H.; Zhao, H.; Henderson, L.; Dong, L.; Christopher, P.; Carter, E.A.; Nordlander, P.; et al. Quantifying hot carrier and thermal contributions in plasmonic photocatalysis. Science 2018, 362, 69–72. [Google Scholar] [CrossRef]
- Yuan, L.; Zhou, J.; Zhang, M.; Wen, X.; Martirez, J.M.P.; Robatjazi, H.; Zhou, L.; Carter, E.A.; Nordlander, P.; Halas, N.J. Plasmonic Photocatalysis with Chemically and Spatially Specific Antenna–Dual Reactor Complexes. ACS Nano 2022, 16, 17365–17375. [Google Scholar] [CrossRef] [PubMed]
- Hodges, A.; Hoang, A.L.; Tsekouras, G.; Wagner, K.; Lee, C.-Y.; Swiegers, G.F.; Wallace, G.G. A high-performance capillary-fed electrolysis cell promises more cost-competitive renewable hydrogen. Nat. Commun. 2022, 13, 1304. [Google Scholar] [CrossRef] [PubMed]
- Hydrogène: Hysata Annonce un Rendement D’électrolyse de 98%. Available online: https://www.greenunivers.com/2022/03/hydrogene-hysata-annonce-un-rendement-delectrolyse-de-98-284107/ (accessed on 15 April 2023).
- Woods, P.; Bustamante, H.; Aguey-Zinsou, K.-F. The hydrogen economy—Where is the water? Energy Nexus 2022, 7, 100123. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, Y.; Zavabeti, A.; Chen, K.; Guo, Y.; Hu, G.; Fan, X.; Li, G.K. Hydrogen production from the air. Nat. Commun. 2022, 13, 5046. [Google Scholar] [CrossRef]
- Amberchan, G.; Lopez, I.; Ehlke, B.; Barnett, J.; Bao, N.Y.; Allen, A.; Singaram, B.; Oliver, S.R.J. Aluminum Nanoparticles from a Ga–Al Composite for Water Splitting and Hydrogen Generation. ACS Appl. Nano Mater. 2022, 5, 2636–2643. [Google Scholar] [CrossRef]
- Ehrnst, Y.; Sherrell, P.C.; Rezk, A.R.; Yeo, L.Y. Acoustically-Induced Water Frustration for Enhanced Hydrogen Evolution Reaction in Neutral Electrolytes. Adv. Energy Mater. 2023, 13, 2203164. [Google Scholar] [CrossRef]
- Solhyd Project, the Home Solar Panel That Produces Hydrogen. Available online: https://www.sustainabilityenvironment.com/2022/11/14/home-solar-panel-that-produces-hydrogen/?amp=1 (accessed on 12 July 2023).
- Sánchez-Bastardo, N.; Schlögl, R.; Ruland, H. Methane Pyrolysis for CO2-Free H2 Production: A Green Process to Overcome Renewable Energies Unsteadiness. Chem. Ing. Tech. 2020, 92, 1596–1609. [Google Scholar] [CrossRef]
- Decarbonizing Natural Gas & C-Zero. Available online: https://www.eni.com/eninext/en-US/portfolio/c-zero.html (accessed on 19 June 2023).
- Sánchez-Bastardo, N.; Schlögl, R.; Ruland, H. Methane Pyrolysis for Zero-Emission Hydrogen Production: A Potential Bridge Technology from Fossil Fuels to a Renewable and Sustainable Hydrogen Economy. Ind. Eng. Chem. Res. 2021, 60, 11855–11881. [Google Scholar] [CrossRef]
- New Clean Energy Process Converts Methane to Hydrogen with Zero Carbon Dioxide Emissions. Available online: https://www.pnnl.gov/news-media/new-clean-energy-process-converts-methane-hydrogen-zero-carbon-dioxide-emissions (accessed on 19 June 2023).
- Gold Hydrogen’ Is an Untapped Resource in Depleted Oil Wells|WIRED. Available online: https://www.wired.com/story/gold-hydrogen/ (accessed on 19 June 2023).
- Cemvita’s Successful Field Test Demonstrates Gold Hydrogen™ Production in Situ|Business Wire. Available online: https://www.businesswire.com/news/home/20220927005409/en/Cemvita%E2%80%99s-Successful-Field-Test-Demonstrates-Gold-Hydrogen%E2%84%A2-Production-in-Situ (accessed on 23 June 2023).
- Cemvita Launches the Gold Hydrogen Program for Subsurface Biomanufacturing of Hydrogen. Available online: https://www.prnewswire.com/news-releases/cemvita-launches-the-gold-hydrogen-program-for-subsurface-biomanufacturing-of-hydrogen-301483981.html (accessed on 19 June 2023).
- Hydrogen Blending as a Pathway Toward U.S. Decarbonization|News|NREL. Available online: https://www.nrel.gov/news/program/2023/hydrogen-blending-as-a-pathway-toward-u.s.-decarbonization.html (accessed on 19 June 2023).
- Nachtane, M.; Tarfaoui, M.; Abichou, M.A.; Vetcher, A.; Rouway, M.; Aâmir, A.; Mouadili, H.; Laaouidi, H.; Naanani, H. An Overview of the Recent Advances in Composite Materials and Artificial Intelligence for Hydrogen Storage Vessels Design. J. Compos. Sci. 2023, 7, 119. [Google Scholar] [CrossRef]
- Moradi, R.; Groth, K.M. Hydrogen storage and delivery: Review of the state of the art technologies and risk and reliability analysis. Int. J. Hydrogen Energy 2019, 44, 12254–12269. [Google Scholar] [CrossRef]
- Rivard, E.; Trudeau, M.; Zaghib, K. Hydrogen Storage for Mobility: A Review. Materials 2019, 12, 1973. [Google Scholar] [CrossRef]
- Nachtane, M.; Tarfaoui, M.; Mohammed, M.A.; Saifaoui, D.; El Moumen, A. Effects of environmental exposure on the mechanical properties of composite tidal current turbine. Renew. Energy 2020, 156, 1132–1145. [Google Scholar] [CrossRef]
- Nachtane, M.; Tarfaoui, M.; Sassi, S.; El Moumen, A.; Saifaoui, D. An investigation of hygrothermal aging effects on high strain rate behaviour of adhesively bonded composite joints. Compos. Part B Eng. 2019, 172, 111–120. [Google Scholar] [CrossRef]
- Lagdani, O.; Tarfaoui, M.; Rouway, M.; Laaouidi, H.; Sbai, S.J.; Dabachi, M.A.; Aamir, A.; Nachtane, M. Influence of Moisture Diffusion on the Dynamic Compressive Behavior of Glass/Polyester Composite Joints for Marine Engineering Applications. J. Compos. Sci. 2022, 6, 94. [Google Scholar] [CrossRef]
- Tarfaoui, M.; Sassi, S.; Lagdani, O.; Nachtane, M. Strain rate effects on the thermomechanical behavior of glass/polyester composite joints. Polym. Compos. 2022, 43, 36–51. [Google Scholar] [CrossRef]
- Nachtane, M.; Meraghni, F.; Chatzigeorgiou, G.; Harper, L.T.; Pelascini, F. Multiscale viscoplastic modeling of recycled glass fiber-reinforced thermoplastic composites: Experimental and numerical investigations. Compos. Part B Eng. 2022, 242, 110087. [Google Scholar] [CrossRef]
- Compatibility of Hydrogen with Different Materials. Available online: https://hyresponder.eu/wp-content/uploads/2021/06/Lecture-4-slides.pdf (accessed on 7 April 2023).
- Zhang, C.; Li, Y.; Xu, X.; Zhang, M.; Leng, H.; Sun, B. Optimization of Plating Process on Inner Wall of Metal Pipe and Research on Coating Performance. Materials 2023, 16, 2800. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, H.K. Mechanical Properties of Metal Hydrides. Available online: https://apps.dtic.mil/sti/pdfs/ADA141496.pdf (accessed on 12 July 2023).
- Okonkwo, P.C.; Barhoumi, E.M.; Ben Belgacem, I.; Mansir, I.B.; Aliyu, M.; Emori, W.; Uzoma, P.C.; Beitelmal, W.H.; Akyüz, E.; Radwan, A.B.; et al. A focused review of the hydrogen storage tank embrittlement mechanism process. Int. J. Hydrogen Energy 2023, 48, 12935–12948. [Google Scholar] [CrossRef]
- Cryogenic Safety. Available online: http://www.phys.ufl.edu/courses/phy4550-6555c/spring10/lecture-safety-disasters.pdf (accessed on 12 July 2023).
- Koyama, M.; Akiyama, E.; Lee, Y.-K.; Raabe, D.; Tsuzaki, K. Overview of hydrogen embrittlement in high-Mn steels. Int. J. Hydrogen Energy 2017, 42, 12706–12723. [Google Scholar] [CrossRef]
- Djukic, M.B.; Bakic, G.M.; Zeravcic, V.S.; Sedmak, A.; Rajicic, B. Hydrogen Embrittlement of Industrial Components: Prediction, Prevention, and Models. Corrosion 2016, 72, 943–961. [Google Scholar] [CrossRef]
- Safyari, M.; Moshtaghi, M.; Hojo, T.; Akiyama, E. Mechanisms of hydrogen embrittlement in high-strength aluminum alloys containing coherent or incoherent dispersoids. Corros. Sci. 2022, 194, 109895. [Google Scholar] [CrossRef]
- Safyari, M.; Moshtaghi, M.; Kuramoto, S. Environmental hydrogen embrittlement associated with decohesion and void formation at soluble coarse particles in a cold-rolled Al–Cu based alloy. Mater. Sci. Eng. A 2021, 799, 139850. [Google Scholar] [CrossRef]
- Burille, A.; Scheid, A.; Ferreira, D.C.F.; Santana, L.; Kwietniewski, C.E.F. Hydrogen embrittlement of single-phase strain-hardened nickel-based UNS N08830 alloy. Mater. Sci. Eng. A 2021, 803, 140486. [Google Scholar] [CrossRef]
- Plasma Kinetics. Responsible, Renewable Hydrogen Energy Systems. Available online: https://plasmakinetics.com/ (accessed on 27 June 2023).
- POWERPASTE for Off-Grid Power Supply. Available online: https://www.ifam.fraunhofer.de/content/dam/ifam/en/documents/dd/Infobl%C3%A4tter/White_paper_POWERPASTE_final.pdf (accessed on 8 March 2023).
- Gas Utilities Are Promoting Hydrogen, but It Could Be a Dead End for Consumers and the Climate. Available online: https://www.forbes.com/sites/energyinnovation/2022/03/29/gas-utility-hydrogen-proposals-ignore-a-superior-decarbonization-pathway-electrification/?sh=18653b5d76a1 (accessed on 2 July 2023).
- HyBlend: Opportunities for Hydrogen Blending in Natural Gas Pipelines|Department of Energy. Available online: https://www.energy.gov/eere/fuelcells/hyblend-opportunities-hydrogen-blending-natural-gas-pipelines (accessed on 19 June 2023).
- Available online: https://energypost.eu/blending-hydrogen-into-the-gas-network-the-challenges-of-pipeline-fractures-faster-flow-rate-more/ (accessed on 29 June 2023).
- Available online: https://www.cpuc.ca.gov/news-and-updates/all-news/cpuc-issues-independent-study-on-injecting-hydrogen-into-natural-gas-systems (accessed on 29 June 2023).
- Chehade, G.; Dincer, I. Progress in green ammonia production as potential carbon-free fuel. Fuel 2021, 299, 120845. [Google Scholar] [CrossRef]
- Ammonia, PubChem, An official website of the United States Government. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Ammonia (accessed on 12 July 2023).
- Humphreys, J.; Lan, R.; Tao, S. Development and Recent Progress on Ammonia Synthesis Catalysts for Haber–Bosch Process. Adv. Energy Sustain. Res. 2021, 2, 2000043. [Google Scholar] [CrossRef]
- Imamura, K.; Miyahara, S.-I.; Kawano, Y.; Sato, K.; Nakasaka, Y.; Nagaoka, K. Kinetics of ammonia synthesis over Ru/Pr2O3. J. Taiwan Inst. Chem. Eng. 2019, 105, 50–56. [Google Scholar] [CrossRef]
- Gao, W.; Guo, J.; Wang, P.; Wang, Q.; Chang, F.; Pei, Q.; Zhang, W.; Liu, L.; Chen, P. Production of ammonia via a chemical looping process based on metal imides as nitrogen carriers. Nat. Energy 2018, 3, 1067–1075. [Google Scholar] [CrossRef]
- Al Sobhi, S.; Hargreaves, J.S.; Hector, A.L.; Laassiri, S. Citrate-gel preparation and ammonia synthesis activity of compounds in the quaternary (Ni, M) 2 Mo 3 N (M= Cu or Fe) systems. Dalton Trans. 2019, 48, 16786–16792. [Google Scholar] [CrossRef]
- Bennaamane, S.; Rialland, B.; Khrouz, L.; Fustier-Boutignon, M.; Bucher, C.; Clot, E.; Mézailles, N. Ammonia Synthesis at Room Temperature and Atmospheric Pressure from N2: A Boron-Radical Approach. Angew. Chem. Int. Ed. 2023, 135, e202209102. [Google Scholar] [CrossRef]
- Olabi, A.; Abdelkareem, M.A.; Al-Murisi, M.; Shehata, N.; Alami, A.H.; Radwan, A.; Wilberforce, T.; Chae, K.-J.; Sayed, E.T. Recent progress in Green Ammonia: Production, applications, assessment; barriers, and its role in achieving the sustainable development goals. Energy Convers. Manag. 2023, 277, 116594. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, Y.; Shi, R.; Waterhouse, G.I.; Zhang, T. Photocatalytic ammonia synthesis: Recent progress and future. Energychem 2019, 1, 100013. [Google Scholar] [CrossRef]
- Sun, W.; Sun, M.; Meng, X.; Zheng, Y.; Li, Z.; Huang, X.; Humayun, M. Alkynyl carbon functionalized N-TiO2: Ball milling synthesis and investigation of improved photocatalytic activity. J. Alloy. Compd. 2023, 939, 168826. [Google Scholar] [CrossRef]
- Almojil, S.F.; Ali, M.A.; Almohana, A.I.; Alali, A.F.; Almoalimi, K.T.; Althahban, S.; Sharma, K.; Ahmed, A.N. Constructing a ZnO/CuCo2O4 p-n heterojunction photocatalyst for efficiently hexavalent chromium–phenol detoxification and nitrogen fixation. J. Phys. Chem. Solids 2023, 172, 111057. [Google Scholar] [CrossRef]
- Li, C.; Wang, T.; Zhao, Z.; Yang, W.; Li, J.; Li, A.; Yang, Z.; Ozin, G.A.; Gong, J. Promoted Fixation of Molecular Nitrogen with Surface Oxygen Vacancies on Plasmon-Enhanced TiO2 Photoelectrodes. Angew. Chem. Int. Ed. 2018, 57, 5278–5282. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Yu, L.; Li, J.; Zhao, J.; Zhang, L. Energy-confined solar thermal ammonia synthesis with K/Ru/TiO2-xHx. Appl. Catal. B Environ. 2018, 224, 612–620. [Google Scholar] [CrossRef]
- Reza, K.M.; Kurny, A.S.W.; Gulshan, F. Parameters affecting the photocatalytic degradation of dyes using TiO2: A review. Appl. Water Sci. 2017, 7, 1569–1578. [Google Scholar] [CrossRef]
- Meloni, E.; Cafiero, L.; Martino, M.; Palma, V. Structured Catalysts for Non-Thermal Plasma-Assisted Ammonia Synthesis. Energies 2023, 16, 3218. [Google Scholar] [CrossRef]
- Iwamoto, M.; Akiyama, M.; Aihara, K.; Deguchi, T. Ammonia Synthesis on Wool-Like Au, Pt, Pd, Ag, or Cu Electrode Catalysts in Nonthermal Atmospheric-Pressure Plasma of N2 and H2. ACS Catal. 2017, 7, 6924–6929. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, C.-W.; Xu, X.-F.; Liu, B.-W.; Zhang, G.-M.; Liu, Z.-W.; Chen, Q.; Zhang, H.-B. Synergistic Effect of Co–Ni Bimetal on Plasma Catalytic Ammonia Synthesis. Plasma Chem. Plasma Process. 2022, 42, 267–282. [Google Scholar] [CrossRef]
- Ma, Y.; Tian, Y.; Zeng, Y.; Tu, X. Plasma synthesis of ammonia in a tangled wire dielectric barrier discharge reactor: Effect of electrode materials. J. Energy Inst. 2021, 99, 137–144. [Google Scholar] [CrossRef]
- Zhang, T.; Zhou, R.; Zhang, S.; Zhou, R.; Ding, J.; Li FCullen, P.J. Sustainable ammonia synthesis from nitrogen and water by one-step plasma catalysis. Energy Environ. Mater. 2023, 6, e12344. [Google Scholar] [CrossRef]
- Ammonia Storage Tank. Available online: https://ammoniaknowhow.com/ammonia-storage-tanks/ (accessed on 26 March 2023).
- Makepeace, J.W.; He, T.; Weidenthaler, C.; Jensen, T.R.; Chang, F.; Vegge, T.; Ngene, P.; Kojima, Y.; de Jongh, P.E.; Chen, P.; et al. Reversible ammonia-based and liquid organic hydrogen carriers for high-density hydrogen storage: Recent progress. Int. J. Hydrogen Energy 2019, 44, 7746–7767. [Google Scholar] [CrossRef]
- The Safety and Integrity of Ammonia Storage Tank. Available online: https://www.ammoniaknowhow.com/wp-content/uploads/2017/02/2014-Venkat-The-safety-and-integrity-of-ammonia-storage-tanks.pdf (accessed on 7 April 2023).
- Hasan, M.H.; Mahlia, T.M.I.; Mofijur, M.; Fattah, I.M.R.; Handayani, F.; Ong, H.C.; Silitonga, A.S. A Comprehensive Review on the Recent Development of Ammonia as a Renewable Energy Carrier. Energies 2021, 14, 3732. [Google Scholar] [CrossRef]
- Aziz, M.; Wijayanta, A.T.; Nandiyanto, A.B.D. Ammonia as Effective Hydrogen Storage: A Review on Production, Storage and Utilization. Energies 2020, 13, 3062. [Google Scholar] [CrossRef]
- Sonker, M.; Tiwary, S.K.; Shreyash, N.; Bajpai, S.; Ray, M.; Kar, S.K.; Balathanigaimani, M. Ammonia as an alternative fuel for vehicular applications: Paving the way for adsorbed ammonia and direct ammonia fuel cells. J. Clean. Prod. 2022, 376, 133960. [Google Scholar] [CrossRef]
- Green Ammonia and Hydrogen Now Cheaper than Fossil Fuels. Available online: prnewswire.com (accessed on 19 June 2023).
- Saygin, D.; Blanco, H.; Boshell, F.; Cordonnier, J.; Rouwenhorst, K.; Lathwal, P.; Gielen, D. Ammonia Production from Clean Hydrogen and the Implications for Global Natural Gas Demand. Sustainability 2023, 15, 1623. [Google Scholar] [CrossRef]
- Mayer, P.; Ramirez, A.; Pezzella, G.; Winter, B.; Sarathy, M.; Gascon, J.; Bardow, A. Blue vs. Green Ammonia Production: A Techno-Economic and Life Cycle Assessment Perspective. SSRN Electron. J. 2022. [Google Scholar] [CrossRef]
- Available online: https://www.iea.org/reports/the-future-of-hydrogen (accessed on 29 June 2023).
- Kannah, R.Y.; Kavitha, S.; Preethi; Karthikeyan, O.P.; Kumar, G.; Dai-Viet, N.V.; Banu, J.R. Techno-economic assessment of various hydrogen production methods—A review. Bioresour. Technol. 2021, 319, 124175. [Google Scholar] [CrossRef]
- Banerjee, S.; Musa, M.N.; Jaafar, A.B. Economic assessment and prospect of hydrogen generated by OTEC as future fuel. Int. J. Hydrogen Energy 2017, 42, 26–37. [Google Scholar] [CrossRef]
- Díaz, M.T.M.; Oróstica, H.C.; Guajardo, J. Economic Analysis: Green Hydrogen Production Systems. Processes 2023, 11, 1390. [Google Scholar] [CrossRef]
- Zulfhazli AR, K.; Takeda, S.; Managi, S. A systematic review of the techno-economic assessment of various hydrogen production methods of power generation. Front. Sustain. 2022, 3, 943145. [Google Scholar] [CrossRef]
- Eichman, J.; Townsend, A.; Melaina, M. Economic Assessment of Hydrogen Technologies Participating in California Electricity Markets (No. NREL/TP-5400-65856); National Renewable Energy Lab. (NREL): Golden, CO, USA, 2016. [Google Scholar]
- Available online: https://www.iea.org/reports/ammonia-technology-roadmap/executive-summary (accessed on 29 June 2023).
- Available online: https://www.iea.org/reports/ammonia-technology-roadmap (accessed on 29 June 2023).
- Ammonia Technology Roadmap towards More Sustainable Nitrogen Fertiliser Production. 2021. Available online: https://iea.blob.core.windows.net/assets/23c82928-ab51-4725-836b-8efc8ea540d2/Ammonia_Launchpresentation.pdf (accessed on 12 July 2023).
- MacFarlane, D.R.; Cherepanov, P.V.; Choi, J.; Suryanto, B.H.R.; Hodgetts, R.Y.; Bakker, J.M.; Ferrero Vallana, F.M.; Simonov, A.N. A roadmap to the ammonia economy. Joule 2020, 4, 1186–1205. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Van Herle, J.; Maréchal, F.; Desideri, U. Techno-economic comparison of green ammonia production processes. Appl. Energy 2020, 259, 114135. [Google Scholar] [CrossRef]
- Nayak-Luke, R.; Bañares-Alcántara, R.; Wilkinson, I. “Green” Ammonia: Impact of Renewable Energy Intermittency on Plant Sizing and Levelized Cost of Ammonia. Ind. Eng. Chem. Res. 2018, 57, 14607–14616. [Google Scholar] [CrossRef]
- NARUC. Artificial Intelligence for Natural Gas Utilities: A Primer. 2020. Available online: https://pubs.naruc.org/pub/F74D4EC2-155D-0A36-310F-F5B22DC3E286 (accessed on 7 April 2023).
- Bilgiç, G.; ÖZTÜRK, B. Modeling of Artificial Neural Networks for Hydrogen Production via Water Electrolysis. El-Cezeri 2023, 10, 137–146. [Google Scholar] [CrossRef]
- Kheirrouz, M.; Melino, F.; Ancona, M.A. Fault detection and diagnosis methods for green hydrogen production: A review. Int. J. Hydrogen Energy 2022, 47, 27747–27774. [Google Scholar] [CrossRef]
- Nasir, T.; Asmaela, M.; Zeeshana, Q.; Solyalib, D. Applications of machine learning to friction stir welding process optimization. J. Kejuruter. 2020, 32, 171–186. [Google Scholar] [CrossRef]
- Mert, I. Agnostic deep neural network approach to the estimation of hydrogen production for solar-powered systems. Int. J. Hydrogen Energy 2021, 46, 6272–6285. [Google Scholar] [CrossRef]
- Tarfaoui, M.; Nachtane, M.; Goda, I.; Qureshi, Y.; Benyahia, H. 3D Printing to Support the Shortage in Personal Protective Equipment Caused by COVID-19 Pandemic. Materials 2020, 13, 3339. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Y. Introducing Machine Learning Models to Response Surface Methodologies. In Response Surface Methodology in Engineering Science; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Elmolla, E.S.; Chaudhuri, M.; Eltoukhy, M.M. The use of artificial neural network (ANN) for modeling of COD removal from antibiotic aqueous solution by the Fenton process. J. Hazard. Mater. 2010, 179, 127–134. [Google Scholar] [CrossRef]
- Desai, K.M.; Survase, S.A.; Saudagar, P.S.; Lele, S.; Singhal, R.S. Comparison of artificial neural network (ANN) and response surface methodology (RSM) in fermentation media optimization: Case study of fermentative production of scleroglucan. Biochem. Eng. J. 2008, 41, 266–273. [Google Scholar] [CrossRef]
- Forootan, M.M.; Larki, I.; Zahedi, R.; Ahmadi, A. Machine Learning and Deep Learning in Energy Systems: A Review. Sustainability 2022, 14, 4832. [Google Scholar] [CrossRef]
- Ramesh, A.S.; Vigneshwar, S.; Vickram, S.; Manikandan, S.; Subbaiya, R.; Karmegam, N.; Kim, W. Artificial intelligence driven hydrogen and battery technologies—A review. Fuel 2023, 337, 126862. [Google Scholar] [CrossRef]
- Onu, C.E.; Nweke, C.N.; Nwabanne, J.T. Modeling of thermo-chemical pretreatment of yam peel substrate for biogas energy production: RSM, ANN, and ANFIS comparative approach. Appl. Surf. Sci. Adv. 2022, 11, 100299. [Google Scholar] [CrossRef]
- Nishant, R.; Kennedy, M.; Corbett, J. Artificial intelligence for sustainability: Challenges, opportunities, and a research agenda. Int. J. Inf. Manag. 2020, 53, 102104. [Google Scholar] [CrossRef]
- Ibáñez-De-Garayo, A.; Imizcoz, M.; Maisterra, M.; Almazán, F.; Sanz, D.; Bimbela, F.; Cornejo, A.; Pellejero, I.; Gandía, L.M. The 3D-Printing Fabrication of Multichannel Silicone Microreactors for Catalytic Applications. Catalysts 2023, 13, 157. [Google Scholar] [CrossRef]
- Hegde, C.; Yan, Q.; Li, H. 3D printing electro-catalysts for hydrogen production. In Proceedings of the Proceedings of the 3rd International Conference on Progress in Additive Manufacturing (Pro-AM 2018), Singapore, 14–17 May 2018. [Google Scholar] [CrossRef]
- Chirita, A.-P.; Bere, P.-P.; Rdoi, R.I.; Dumitrescu, L. Aspects Regarding the Use of 3D Printing Technology and Composite Materials for Testing and Manufacturing Vertical Axis Wind Turbines. Mater. Plast. 2019, 56, 910–917. [Google Scholar] [CrossRef]
- Bhatia, A.; Sehgal, A.K. Additive manufacturing materials, methods and applications: A review. Mater. Today Proc. 2021, 81, 1060–1067. [Google Scholar] [CrossRef]
- Deiner, L.J.; Reitz, T.L. Inkjet and aerosol jet printing of electrochemical devices for energy conversion and storage. Adv. Eng. Mater. 2017, 19, 1600878. [Google Scholar] [CrossRef]
- Cardoso, R.M.; Kalinke, C.; Rocha, R.G.; dos Santos, P.L.; Rocha, D.P.; Oliveira, P.R.; Janegitz, B.C.; Bonacin, J.A.; Richter, E.M.; Munoz, R.A. Additive-manufactured (3D-printed) electrochemical sensors: A critical review. Anal. Chim. Acta 2020, 1118, 73–91. [Google Scholar] [CrossRef]
- Mandal, M.; Valls, A.; Gangnus, N.; Secanell, M. Analysis of Inkjet Printed Catalyst Coated Membranes for Polymer Electrolyte Electrolyzers. J. Electrochem. Soc. 2018, 165, F543–F552. [Google Scholar] [CrossRef]
- Al-Othman, A.; Tawalbeh, M.; Martis, R.; Dhou, S.; Orhan, M.; Qasim, M.; Olabi, A.G. Artificial intelligence and numerical models in hybrid renewable energy systems with fuel cells: Advances and prospects. Energy Convers. Manag. 2022, 253, 115154. [Google Scholar] [CrossRef]
- Javaid, M.; Haleem, A.; Singh, R.P.; Suman, R.; Rab, S. Role of additive manufacturing applications towards environmental sustainability. Adv. Ind. Eng. Polym. Res. 2021, 4, 312–322. [Google Scholar] [CrossRef]
- Low Carbon-Ammonia. 2022. Available online: https://www.icef.go.jp/pdf/summary/roadmap/icef2022_roadmap_Low-Carbon_Ammonia.pdf (accessed on 15 April 2023).
- Green Hydrogen: A Key Investment for the Energy Transition. Available online: https://blogs.worldbank.org/ppps/green-hydrogen-key-investment-energy-transition (accessed on 15 April 2023).
- INTERNATIONAL ASPECTS OF A POWER-TO-X ROADMAP. 2018. Available online: https://www.frontier-economics.com/media/2642/frontier-int-ptx-roadmap-stc-12-10-18-final-report.pdf (accessed on 7 April 2023).
- POWER-TO-X IN MOROCCO, Driver of Mediterranean Energy Market Integration. 2020. Available online: https://www.fedenerg.ma/wp-content/uploads/2020/06/2020-06-26_IKKEN_2020_IRESEN_PtX_V3.pdf (accessed on 7 April 2023).
- HYDROGÈNE VERT FEUILLE DE ROUTE, Vecteur de Transition Énergétique et de Croissance Durable. 2021. Available online: https://www.mem.gov.ma/Lists/Lst_rapports/Attachments/36/Feuille%20de%20route%20de%20hydrog%C3%A8ne%20vert.pdf (accessed on 1 March 2023).
- The Hydrogen Revolution in EMEA. Available online: https://www.dlapiper.com/en/insights/publications/2021/02/the-hydrogen-revolution-in-emea (accessed on 15 April 2023).
- Hydrogen in the MENA Region: Priorities and Steps Forward. 2023. Available online: https://www.atlanticcouncil.org/blogs/energysource/hydrogen-in-the-mena-region-priorities-and-steps-forward/ (accessed on 25 July 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).