Properties and Stability of Encapsulated Pomegranate Peel Extract Prepared by Co-Crystallization
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Extraction
2.3. Co-Crystallization Procedure
2.4. Characterization of the Product
2.5. Statistical Analysis
3. Results and Discussion
3.1. Powder Properties
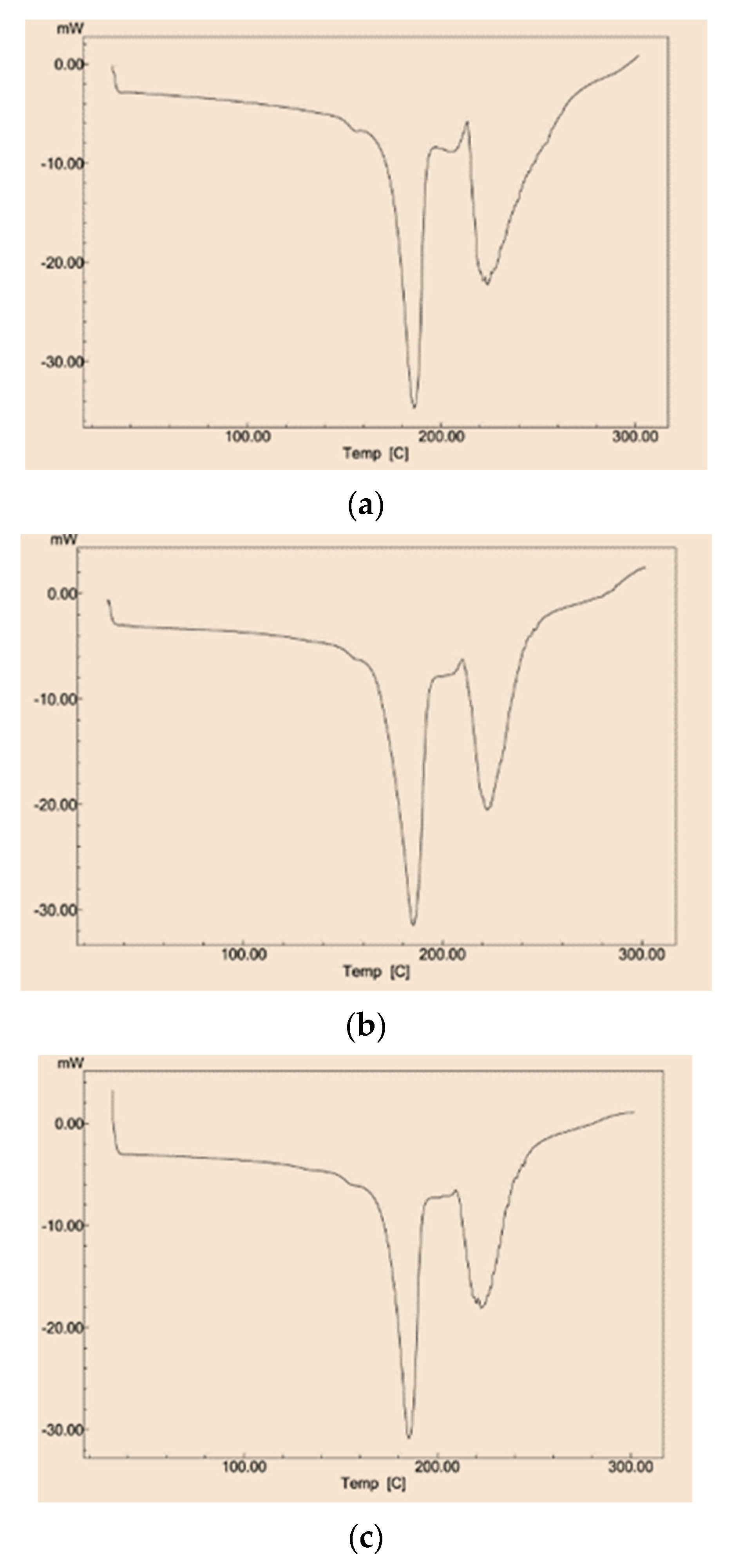
3.2. Differential Scanning Calorimetry
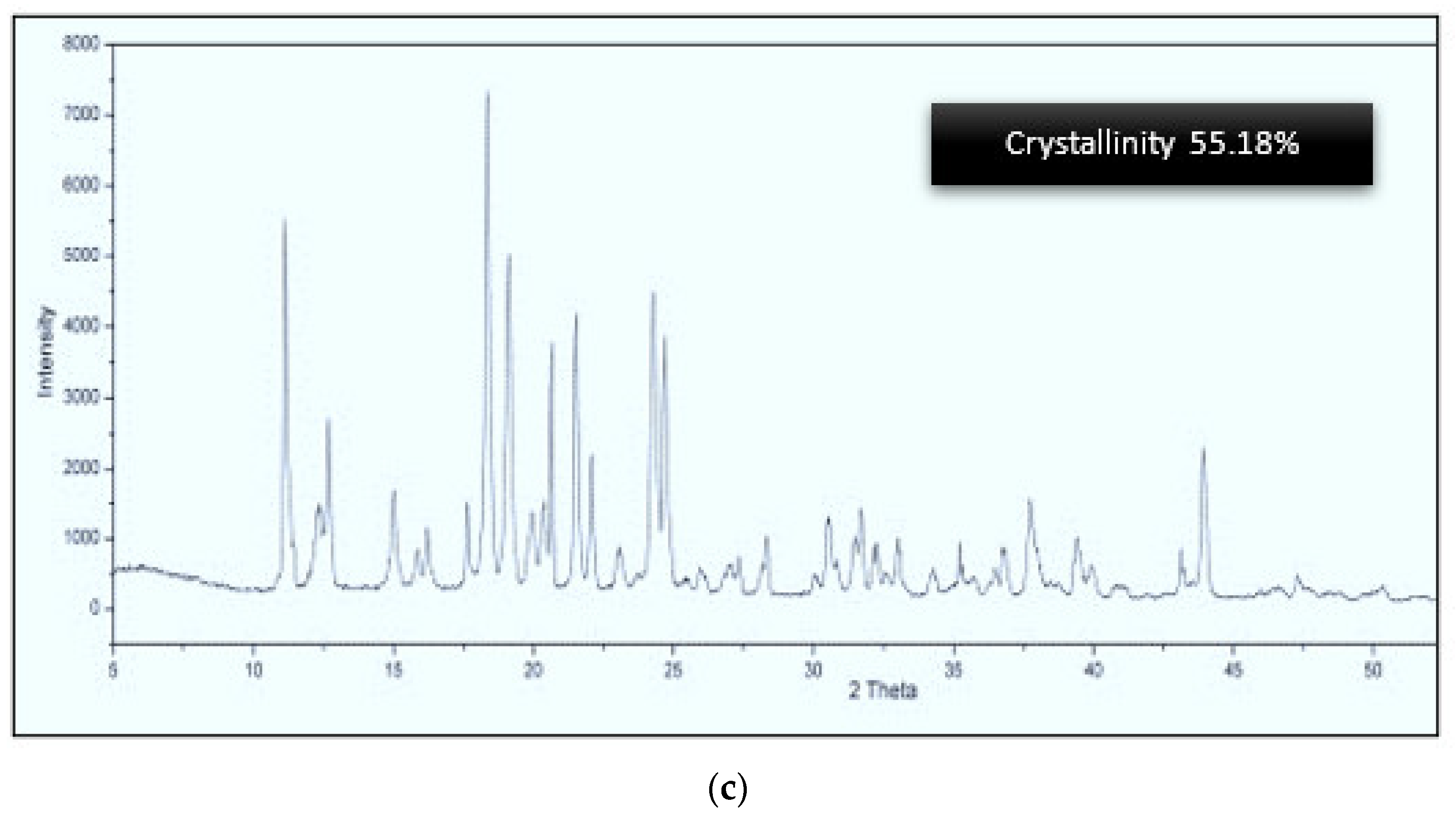
3.3. Degree of Crystallinity with X-ray Scattering
3.4. Morphology with Scanning Electron Microscopy
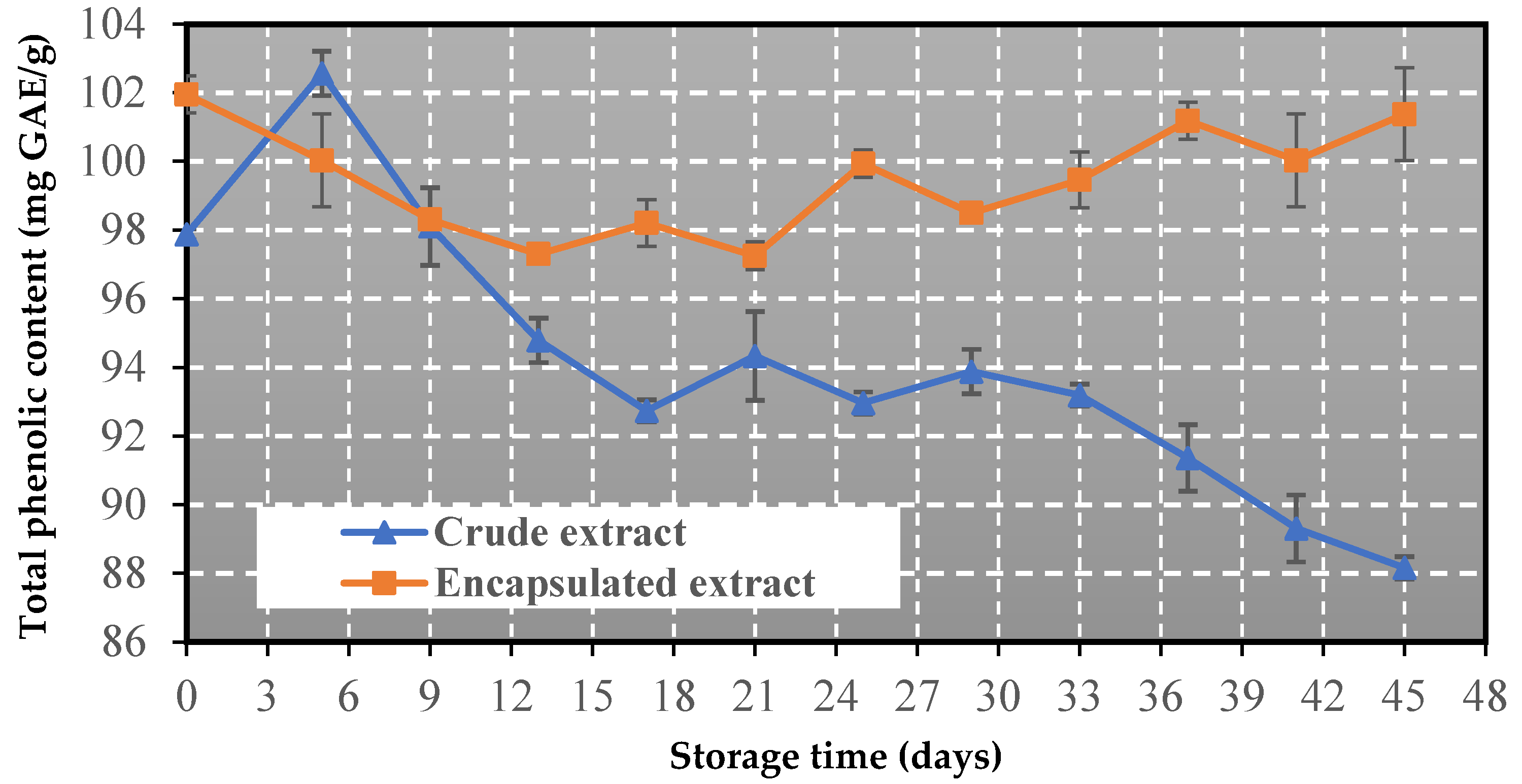
3.5. Change in Total Phenolic Content
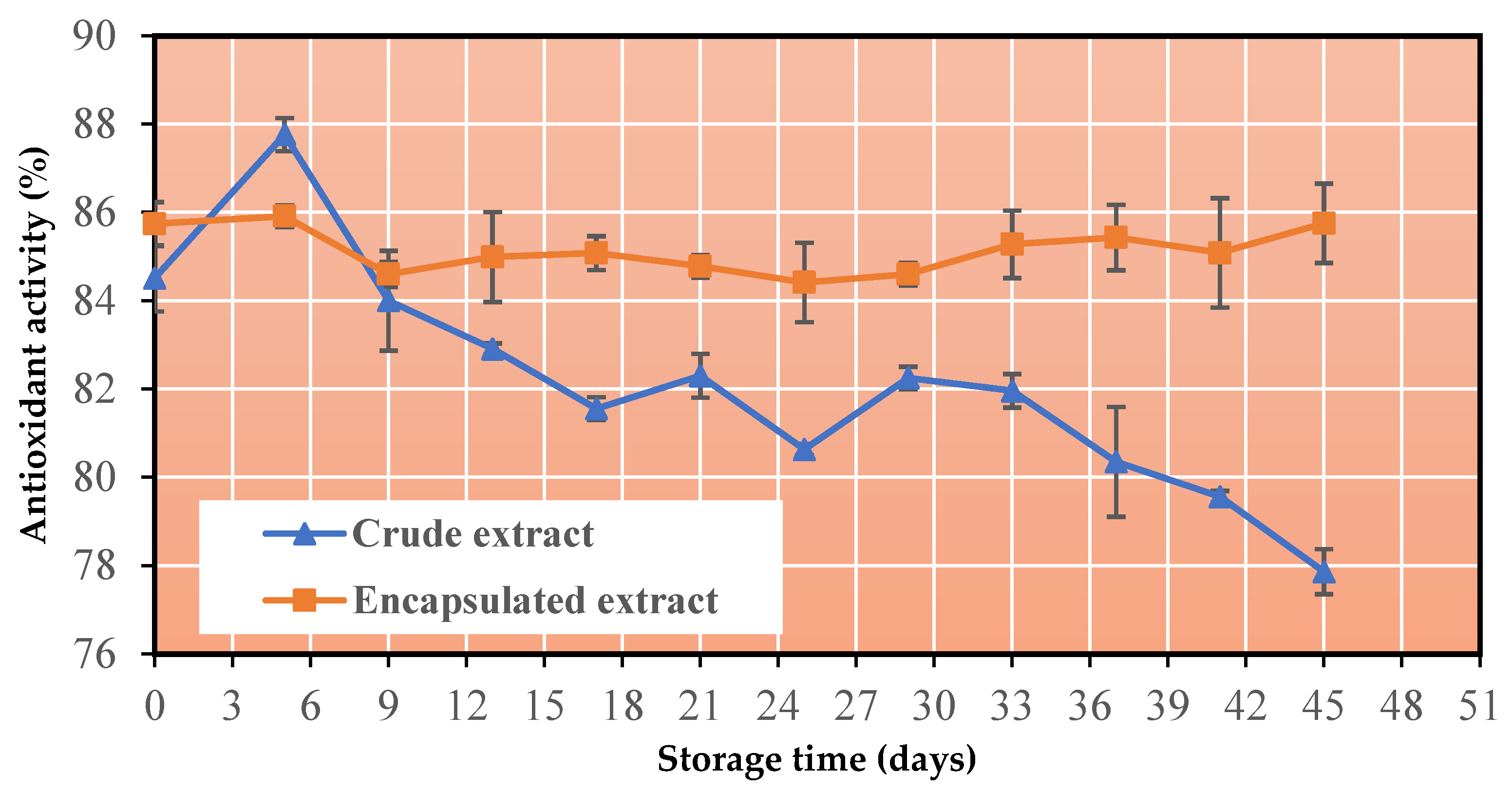
3.6. Change in Antioxidant Capacity
4. Conclusions
- The moisture content of the co-crystallized product is about 0.59%, a value lower than those reported when spray drying was used.
- The moisture absorption rate for the co-crystallized product is about 0.011%, so the powder cannot be considered to be hygroscopic.
- The co-crystallized powder has a high bulk density (0.803 g/cm3) and solubility (61 s).
- The phenolic extract does not crystallize but is still in an amorphous state between gaps in the sucrose crystals.
- The obtained thermographs indicate both the improvement of the thermal stability of the extract by the co-crystallization and its successful encapsulation in the sucrose matrix.
- The co-crystallized powder has an excellent storage stability, as it preserves its phenolic content and antioxidant activity at high levels after storage at 60 °C for 45 days.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Çam, M.; İçyer, N.C.; Erdoğan, F. Pomegranate peel phenolics: Microencapsulation, storage stability and potential ingredient for functional food development. LWT 2014, 55, 117–123. [Google Scholar] [CrossRef]
- Kanatt, S.R.; Chander, R.; Sharma, A. Antioxidant and antimicrobial activity of pomegranate peel extract improves the shelf life of chicken products. Int. J. Food Sci. Techn. 2010, 45, 216–222. [Google Scholar] [CrossRef]
- Çam, M.; Hışıl, Y. Pressurized water extraction of polyphenols from pomegranate peels. Food Chem. 2010, 123, 878–885. [Google Scholar] [CrossRef]
- Kaderides, K.; Goula, A.M.; Adamopoulos, K.G. A process for turning pomegranate peels into a valuable food ingredient using ultrasound-assisted extraction and encapsulation. Innov. Food Sci. Emerg. Technol. 2015, 31, 204–215. [Google Scholar] [CrossRef]
- Kaderides, K.; Papaoikonomou, L.; Serafim, M.; Goula, A.M. Microwave-assisted extraction of phenolics from pomegranate peels: Optimization, kinetics, and comparison with ultrasounds extraction. Chem. Eng. Process 2019, 137, 1–11. [Google Scholar] [CrossRef]
- López-Córdoba, A.; Navarro, A. Physicochemical properties and stability of sucrose/glucose agglomerates obtained by co-crystallization. J. Food Process Eng. 2018, 41, e12901. [Google Scholar] [CrossRef]
- Desai, K.G.H.; Park, H.J. Recent developments in microencapsulation of food ingredients. Dry. Technol. 2005, 23, 1361–1394. [Google Scholar] [CrossRef]
- Karangutkar, A.V.; Ananthanarayan, L. Co-crystallization of Basella rubra extract with sucrose: Characterization of co-crystals and evaluating the storage stability of betacyanin pigments. J. Food Eng. 2020, 271, 109776. [Google Scholar] [CrossRef]
- Sardar, B.R.; Singhal, R.S. Characterization of co-crystallized sucrose entrapped with cardamom oleoresin. J. Food Eng. 2013, 117, 521–529. [Google Scholar] [CrossRef]
- Maulny, A.P.E.; Beckett, S.T.; Mackenzie, G. Physical properties of co-crystalline sugar and honey. J. Food Sci. 2005, 70, E567–E572. [Google Scholar] [CrossRef]
- Khawas, P.; Deka, S.C. Encapsulation of Natural Antioxidant Compounds from Culinary Banana by Cocrystallization. J. Food Process Preserv. 2017, 41, e13033. [Google Scholar] [CrossRef]
- Akbari, M.; Sadeghi Mahoonak, A.; Sarabandi, K.; Ghorbani, A. Physiochemical characterization and antioxidant activities of green tea extract microencapsulated by co-crystallization technique. Food Sci. Technol. 2019, 15, 179–193. [Google Scholar]
- Marpaung, A.M.; Lee, M.; Kartawiria, I.S. The Development of Butterfly pea (Clitoria ternatea) Flower Powder Drink by Co-crystallization. Indones. Food Sci. Technol. J. 2020, 3, 34–37. [Google Scholar] [CrossRef]
- Irigoiti, Y.; Yamul, D.K.; Navarro, A.S. Co-crystallized sucrose with propolis extract as a food ingredient: Powder characterization and antioxidant stability. Food Sci. Technol. 2021, 143, 111164. [Google Scholar] [CrossRef]
- Kaur, P.; Elsayed, A.; Subramanian, J.; Singh, A. Encapsulation of carotenoids with sucrose by co-crystallization: Physicochemical properties, characterization and thermal stability of pigments. Food Sci. Technol. 2021, 140, 110810. [Google Scholar] [CrossRef]
- Tzatsi, P.; Goula, A.M. Encapsulation of Extract from Unused Chokeberries by Spray Drying, Co-crystallization, and Ionic Gelation. Waste Biomass Valorization 2021, 12, 4567–4585. [Google Scholar] [CrossRef]
- Sarabandi, K.; Mohammadi, A. Stabilization of peppermint polyphenols within crystalline sucrose matrix: Fortification of gummy candy as a food model system. J. Food Process. Preserv. 2022, 46, e16720. [Google Scholar] [CrossRef]
- Chezanoglou, E.; Kenanidou, N.; Spyropoulos, C.; Xenitopoulou, D.; Zlati, E.; Goula, A.M. Encapsulation of pomegranate peel extract in sucrose matrix by co-crystallization. Sustain. Chem. Pharm. 2023, 31, 100949. [Google Scholar] [CrossRef]
- Bhandari, B.R.; Hartel, R.W. Co-crystallization of sucrose at high concentration in the presence of glucose and fructose. J. Food Sci. 2002, 67, 1797–1802. [Google Scholar] [CrossRef]
- Deladino, L.; Anbinder, P.S.; Navarro, A.S.; Martino, M.N. Co-crystallization of yerba mate extract (Ilex paraguariensis) and mineral salts within a sucrose matrix. J. Food Eng. 2007, 80, 573–580. [Google Scholar] [CrossRef]
- Deladino, L.; Navarro, A.S.; Martino, M.N. Microstructure of minerals and yerba mate extract co-crystallized with sucrose. J. Food Eng. 2010, 96, 410–415. [Google Scholar] [CrossRef]
- López-Córdoba, A.; Gallo, L.; Bucalá, V.; Martino, M.; Navarro, A. Co-crystallization of zinc sulfate with sucrose: A promissory strategy to render zinc solid dosage forms more palatable. J. Food Eng. 2016, 170, 100–107. [Google Scholar] [CrossRef]
- Nugroho, D.; Sugih, A.K. Determination of process parameters for curcumin–dextrose cocrystallization. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Malang, East Java, Indonesia, 4–5 November 2017. [Google Scholar]
- Bajaj, S.R.; Singhal, R.S. Enhancement of stability of vitamin B12 by co-crystallization: A convenient and palatable form of fortification. J. Food Eng. 2021, 291, 110231. [Google Scholar] [CrossRef]
- Queiroz, M.B.; Sousa, F.R.; da Silva, L.B.; Alves, R.M.V.; Alvim, I.D. Co-crystallized sucrose-soluble fiber matrix: Physicochemical and structural characterization. Food Sci. Technol. 2022, 154, 112685. [Google Scholar] [CrossRef]
- Wang, H.; Gao, S.; Zhang, D.; Wang, Y.; Zhang, Y.; Jiang, S.; Meng, X. Encapsulation of catechin or curcumin in co-crystallized sucrose: Fabrication, characterization and application in beef meatballs. Food Sci. Technol. 2022, 168, 113911. [Google Scholar] [CrossRef]
- Beristain, C.I.; Vazquez, A.; Garcia, H.S.; Vernon-Carter, E.J. Encapsulation of orange peel oil by co-crystallization. Food Sci. Technol. 1996, 29, 645–647. [Google Scholar] [CrossRef]
- Sardar, B.R.; Tarade, K.M.; Singhal, R.S. Stability of active components of cardamom oleoresin in co-crystallized sugar cube during storage. J. Food Eng. 2013, 117, 530–537. [Google Scholar] [CrossRef]
- Federzoni, V.; Alvim, I.D.; Fadini, A.L.; Silva, L.B.D.; Queiroz, M.B. Co-crystallization of paprika oleoresin and storage stability study. Food Sci. Technol. 2019, 39, 182–189. [Google Scholar] [CrossRef]
- Rai, K.; Chhanwal, N.; Shah, N.N.; Singhal, R.S. Encapsulation of ginger oleoresin in co-crystallized sucrose: Development, characterization and storage stability. Food Funct. 2021, 12, 7964–7974. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists; Association of Official Analytical Chemists: Arlington, VA, USA, 2005; Volume II. [Google Scholar]
- Tsali, A.; Goula, A.M. Valorization of grape pomace: Encapsulation and storage stability of its phenolic extract. Powder Technol. 2018, 340, 194–207. [Google Scholar] [CrossRef]
- Goula, A.M.; Adamopoulos, K.G.; Kazakis, N.A. Influence of spray drying conditions on tomato powder properties. Dry. Technol. 2004, 22, 1129–1151. [Google Scholar] [CrossRef]
- Kaderides, K.; Goula, A.M. Development and characterization of a new encapsulating agent from orange juice by-products. Food Res. Int. 2017, 100, 612–622. [Google Scholar] [CrossRef]
- Kaderides, K.; Goula, A.M. Encapsulation of pomegranate peel extract with a new carrier material from orange juice by-products. J. Food Eng. 2019, 253, 1–13. [Google Scholar] [CrossRef]
- Sarabandi, K.; Mahoonak, A.S.; Akbari, M. Physicochemical properties and antioxidant stability of microencapsulated marjoram extract prepared by co-crystallization method. J. Food Process Eng. 2019, 42, e12949. [Google Scholar] [CrossRef]
- Kiritsakis, K.; Goula, A.M.; Adamopoulos, K.G.; Gerasopoulos, D. Valorization of olive leaves: Spray drying of olive leaf extract. Waste Biomass Valorization 2018, 9, 619–633. [Google Scholar] [CrossRef]
- Kurozawa, L.E.; Park, K.J.; Hubinger, M.D. Effect of carrier agents on the physicochemical properties of a spray dried chicken meat protein hydrolysate. J. Food Eng. 2009, 94, 326–333. [Google Scholar] [CrossRef]
- López-Córdoba, A.; Deladino, L.; Agudelo-Mesa, L.; Martino, M. Yerba mate antioxidant powders obtained by co-crystallization: Stability during storage. J. Food Eng. 2014, 124, 158–165. [Google Scholar] [CrossRef]
- Nik, A.B.; Vazifedoost, M.; Didar, Z.; HajirostamLoo, B. The antioxidant and physicochemical properties of microencapsulated bioactive compounds in Securigera securidaca (L.) seed extract by co-crystallization. Food Qual. Saf. 2019, 3, 243–250. [Google Scholar] [CrossRef]
- Tan, S.P.; Tuyen, C.K.; Parks, S.E.; Stathopoulos, C.E.; Roach, P.D. Effects of the spray-drying temperatures on the physiochemical properties of an encapsulated bitter melon aqueous extract powder. Powder Technol. 2015, 281, 65–75. [Google Scholar] [CrossRef]
- Jones, J.R.; Prime, D.; Leaper, M.C.; Richardson, D.J.; Rielly, C.D.; Stapley, A.G. Effect of processing variables and bulk composition on the surface composition of spray dried powders of a model food system. J. Food Eng. 2013, 118, 19–30. [Google Scholar] [CrossRef]
- Kaderides, K.; Mourtzinos, I.; Goula, A.M. Stability of pomegranate peel polyphenols encapsulated in orange juice industry by-product and their incorporation in cookies. Food Chem. 2020, 310, 125849. [Google Scholar] [CrossRef] [PubMed]
- Flores, F.P.; Singh, R.K.; Kong, F. Physical and storage properties of spray-dried blueberry pomace extract with whey protein isolate as wall material. J. Food Eng. 2014, 137, 1–6. [Google Scholar] [CrossRef]
- Laine, P.; Kylli, P.; Heinonen, M.; Jouppila, K. Does Microencapsulation Improve Storage Stability of Cloudberry (Rubus chamaemorus) Ellagitannins? In Water Properties in Food, Health, Pharmaceutical and Biological Systems, 1st ed.; Reid, D.S., Sajjaanantakul, Τ., Lillford, P.J., Charoenrein, S., Eds.; Blackwell Publishing: Ames, IA, USA, 2010; pp. 563–569. [Google Scholar]
- Lei, Z. Monomeric Ellagitannins in Oaks and Sweetgum. Ph.D. Thesis, Virginia Polytechnic Institute and State University, Blacksburg, VA, USA, 25 April 2022. [Google Scholar]
- Haslam, E. Plant Polyphenols: Vegetable Tannins Revisited, 1st ed.; Cambridge University Press: Cambridge, MA, USA, 1998; pp. 90–139. [Google Scholar]
- Xu, L.; Cheng, J.R.; Liu, X.M.; Zhu, M.J. Effect of microencapsulated process on stability of mulberry polyphenol and oxidation property of dried minced pork slices during heat processing and storage. LWT 2019, 100, 62–68. [Google Scholar] [CrossRef]
- Negrão-Murakami, A.N.; Nunes, G.L.; Pinto, S.S.; Murakami, F.S.; Amante, E.R.; Petrus, J.C.C.; Prudêncio, E.S.; Amboni, R.D.M.C. Influence of DE-value of maltodextrin on the physicochemical properties, antioxidant activity, and storage stability of spray dried concentrated mate (Ilex paraguariensis A. St. Hil.). Food Sci. Technol. 2017, 79, 561–567. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Effect of spray drying and storage on the stability of bayberry polyphenols. Food Chem. 2011, 129, 1139–1147. [Google Scholar] [CrossRef]
- Laine, P.; Kylli, P.; Heinonen, M.; Jouppila, K. Storage stability of microencapsulated cloudberry (Rubus chamaemorus) phenolics. J. Agric. Food Chem. 2008, 56, 11251–11261. [Google Scholar] [CrossRef] [PubMed]
- Bors, W.; Michel, C. Chemistry of the antioxidant effect of polyphenols. Ann. N. Y. Acad. Sci. 2002, 957, 57–69. [Google Scholar] [CrossRef]
- Shirode, A.B.; Bharali, D.J.; Nallanthighal, S.; Coon, J.K.; Mousa, S.A.; Reliene, R. Nanoencapsulation of pomegranate bioactive compounds for breast cancer chemoprevention. Int. J. Nanomed. 2015, 10, 475. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chezanoglou, E.; Goula, A.M. Properties and Stability of Encapsulated Pomegranate Peel Extract Prepared by Co-Crystallization. Appl. Sci. 2023, 13, 8680. https://doi.org/10.3390/app13158680
Chezanoglou E, Goula AM. Properties and Stability of Encapsulated Pomegranate Peel Extract Prepared by Co-Crystallization. Applied Sciences. 2023; 13(15):8680. https://doi.org/10.3390/app13158680
Chicago/Turabian StyleChezanoglou, Evangelos, and Athanasia M. Goula. 2023. "Properties and Stability of Encapsulated Pomegranate Peel Extract Prepared by Co-Crystallization" Applied Sciences 13, no. 15: 8680. https://doi.org/10.3390/app13158680
APA StyleChezanoglou, E., & Goula, A. M. (2023). Properties and Stability of Encapsulated Pomegranate Peel Extract Prepared by Co-Crystallization. Applied Sciences, 13(15), 8680. https://doi.org/10.3390/app13158680







